Hazardous substance assessment - Sodium Hydroxide
Important note: Hazardous substance assessments are technical documents produced by Health Canada as educational and information resources for suppliers of hazardous products under the Hazardous Products Act (HPA) and its regulations. For more information on supplier roles and responsibilities, visit supplier responsibilities.
This hazardous substance assessment was conducted according to the Hazardous Products Regulations (HPR).
Identification
Chemical name:
Sodium hydroxide
CAS #:
1310-73-2
Chemical composition:
NaOH
Synonyms:
Caustic soda, Sodium hydrate, lye.
UN #:
1823 (solid), 1824 (liquid)
Pictogram(s):
Figure 1.
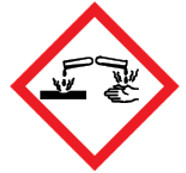
Figure 1 - Text description
The symbol within the pictogram shows a container dripping liquid onto a piece of metal and another container dripping liquid onto a hand. This symbol indicates that hazardous products with this pictogram can
- damage or destroy metal,
- cause irreversible damage to the skin (e.g., burns, blisters, scarring), and/or
- produce tissue damage in the eye or vision loss that is irreversible or not fully reversible within 21 days.
WHMIS classification
Health hazards:
Serious Eye Damage: Category 1
Physical hazards:
Sodium hydroxide does not meet the criteria for classification.
Health hazards
Acute Toxicity (Oral):
No data available
Acute Toxicity (Dermal):
No data available
Acute Toxicity (Inhalation – Gas):
Not applicable
Sodium hydroxide is not a gas.
Acute Toxicity (Inhalation – Vapour):
No data available
Acute Toxicity (Inhalation – Dust and Mist):
No data available
Skin Corrosion / Irritation:
Category 1
Human data: A case report showed that a single exposure can induce a permanent alteration in the epidermal maturation process and cause a hyperkeratotic condition of the skin (based on study summary Footnote 1). Another case report described a human subject that had severe chemical burns after exposure to Sodium hydroxide Footnote 2. A 0.5% Sodium hydroxide solution was shown to be an irritant to humans in a 4-hour human patch test Footnote 3, and in 30 volunteers exposed for 1 hour, with about half the volunteers reacting after 1 hour of treatment Footnote 4. Response was so vigorous that exposure for a greater duration was not undertaken at any site. The available human data meet the classification criteria for Skin Corrosion – Category 1 [8.2.2(1)].
Animal data: In a CORROSITEX in vitro assay, Sodium hydroxide solid was tested as corrosive, Category B1 (substances that produce a large change in pH when they are added to the base buffer, as indicated by a strong color change of the base buffer solution), packing group II (test samples from Category A1 and B1 that produce a detectable color change in the Chemical Detection System (CDS) after more than 3 min, and up to 30 min) Footnote 5. In a study conducted according to the Organisation for Economic Co-operation and Development Test Guideline (OECD TG) 404, Sodium hydroxide showed corrosive effects at concentrations between 5-30% Footnote 6. In a study conducted in rabbits, a 2% solution was corrosive, while 1% was not Footnote 7.
The available human and animal data meet the classification criteria for Skin Corrosion – Category 1 [HPR 8.2.2].
Serious Eye Damage / Eye Irritation:
Category 1
Human data: In a case report, Sodium hydroxide was shown to cause extreme damage in the anterior segment of the eye very quickly Footnote 8. In the worst burns, there is a tendency for the cornea to ulcerate and perforate. In less severe burns, the cornea may become densely vascularized and opaque, resulting in blindness. Another study described the sequelae of alkali burns to the eye as mild-to-severe corneal scarring and vascularization, early corneal slough in the case of massive burns, and early conjunctival slough Footnote 9. Late corneal slough and breakdown can also occur years after the original injury, and may include perforation of the cornea, followed by prolapse of the iris (falling down, or sinking, of the iris) and adherent leukoma (dense, opaque, white opacity of the cornea). Cataracts, inflammation of the uvea (the vascular middle coat of the eye), glaucoma, retinal detachment, inversion of the lower eyelid and the eyelashes, squamous epithelization of the connective tissues forming the eye, adhesion between the eyelid and eyeball, and atrophy of the eye may be later complications. Human data support a Serious Eye Damage - Category 1 classification.
Animal data: A report from the Ecotoxicology and Toxicology of Chemicals Task Force (ECETOC, report No. 48, with data generated in studies carried out according to OECD TG 405) showed that a 10% solution of Sodium hydroxide caused eye damage that was not fully reversible within an observation period of 21 days Footnote 10. In another study, a 3% solution of Sodium hydroxide caused eye damage in rabbits with mean Draize scores over the threshold of 3 for corneal opacity and 1.5 for iritis Footnote 11. Animal data showed that a 1% solution met criteria for Category 2B [HPR 8.3.2(3)] [10,12]. A 2% solution showed corneal opacity and iritis scores below the threshold for Category 1; however, there was no data after 96h and, therefore, no confirmation of reversibility after 21 days Footnote 12. A Smyth & Carpenter study showed a score indicative of serious eye damage Footnote 13. Animal data support classification as Category 1 for solutions above 1%.
The available data meet the classification criteria for Serious Eye Damage - Category 1 [HPR 8.3.2(1)] for Sodium hydroxide solutions above 1% [10,11].
Respiratory Sensitization:
No data available
Skin Sensitization:
Does not meet criteria
In a study in which young healthy adults (with no history of atopy or specific illness) were exposed to different concentrations of Sodium hydroxide then challenged to a 0.125% solution of Sodium hydroxide after 7 days, the irritant response correlated well with the concentration of Sodium hydroxide, but the trial failed to elicit any sensitization Footnote 14.
The available data do not meet the classification criteria for Skin Sensitization.
Germ Cell Mutagenicity:
Does not meet criteria
Sodium hydroxide was negative in an in vitro Ames reversion assay without metabolic activation Footnote 15. In another in vitro study, exposure of Chinese hamster ovary cells to Sodium hydroxide with metabolic activation resulted in chromosomal aberrations in 7.8% of the 400 cells scored Footnote 16. The chromosome aberration frequency increased to 17% along the S9 concentration, but excessive cell toxicity was not seen, and the authors concluded that the mutagenic response was due to the combined effects of pH and metabolic activation, and not to the mutagenicity of Sodium hydroxide.
The available data do not meet the classification criteria for Germ Cell Mutagenicity.
Carcinogenicity:
No data available
Sodium hydroxide has not been reviewed by the International Agency for Research on Cancer (IARC), the National Toxicology Program (NTP), or the American Conference of Governmental Industrial Hygienists (ACGIH).
Reproductive Toxicity:
No data available
Specific Target Organ Toxicity – Single Exposure:
Does not meet criteria
Oral Route of Exposure:
Human data: Death and burns to mouth, pharynx, esophagus, and stomach were reported in humans after ingestion of Sodium hydroxide solutions (autopsy results) Footnote 17, in case reports of burns in oral mucosa Footnote 18, and in a case of surgical treatment following extensive corrosive stricture of esophagus and stomach after ingestion of strong Sodium hydroxide Footnote 19. Effects are secondary to the corrosivity of the substance.
Animal data: In an experimental study where rats were administered a 10% solution, Sodium hydroxide caused severe acute corrosive esophagitis and gastritis, some of which lethal Footnote 20,Footnote 21. In rabbits, corrosive injury to the oesophagus led to the development of oesophageal strictures below saccular dilations Footnote 22. Food packed the dilation and stricture tightly, causing chronic infection. Aspiration of food particles from the stricture area caused pneumonic foci in the lungs. Effects are secondary to the corrosivity of the substance.
Dermal Route of Exposure: No data available.
Inhalation Route of Exposure: In a human case report, severe irritation of respiratory tract following inhalation of 98% Sodium hydroxide manifested as chest pain, breathlessness, and pneumothorax (lung collapse) Footnote 23. Effects were reversible in 48 hours after medical intervention. The authors of this case report postulate that the effects are secondary to the corrosivity of the substance on a distal alveolus (adjacent to the pleura) or on a pre-existing lung lesion.
The available data do not meet the classification criteria for Specific Organ Toxicity – Single Exposure.
Specific Target Organ Toxicity – Repeated Exposure:
Does not meet criteria
Oral Route of Exposure: In an experimental study where rats were administered a 10% solution, Sodium hydroxide caused severe acute corrosive esophagitis and gastritis, some of which lethal Footnote 20. Effects are secondary to the corrosivity of the substance.
Dermal Route of Exposure: No data available.
Inhalation Route of Exposure: In a case report, Sodium hydroxide caused irreversible obstructive airway disease in a worker after long term (for 20 years) regular unprotected exposure (no respiratory protective equipment) Footnote 24. The authors postulated that this outcome was due to a bronchial inflammatory reaction caused by the corrosive effect of Sodium hydroxide over the years. Effects are considered as secondary to the corrosivity of the substance.
The available data do not meet the classification criteria for Specific Organ Toxicity – Repeated Exposure.
Aspiration Hazard:
Does not meet criteria
No human data are available, and Sodium hydroxide is not a liquid hydrocarbon.
Biohazardous Infectious Materials:
Not applicable
Sodium hydroxide is not a microorganism, protein or nucleic acid.
Physical hazards
Explosives:
Not Evaluated*
* Explosives are excluded from the Hazardous Products Act and Regulations. Explosives are regulated under the Explosives Act. For more information, visit Natural Resources Canada.
Flammable Gases:
Not applicable
Sodium hydroxide is not a gas (its boiling point is 1,388 °C) Footnote 20. The classification criteria for Flammable Gases do not apply to this substance.
Aerosols:
Not evaluated
Classification of a hazardous product in the Aerosols hazard class is product dependent.
Oxidizing Gases:
Not applicable
Sodium hydroxide is not a gas (its boiling point is 1,388 ºC) Footnote 20. The classification criteria for Oxidizing Gases do not apply to this substance.
Gases Under Pressure:
Not applicable
Sodium hydroxide is not a gas (its boiling point is 1,388 ºC) Footnote 20. The classification criteria for Gases Under Pressure do not apply to this substance.
Flammable Liquids:
Not applicable
Sodium hydroxide is not a liquid (its melting point is 323 ºC) Footnote 20. The classification criteria for Flammable Liquids do not apply to this substance.
Flammable Solids:
Does not meet criteria
Sodium hydroxide does not react with oxygen, as there are no chemical groups associated with oxidizing properties present in the molecule Footnote 20.
Self-Reactive Substances and Mixtures:
No data available
No data are available to determine whether Sodium hydroxide meets the classification criteria for Self-Reactive Substances and Mixtures.
Pyrophoric Liquids:
Not applicable
Sodium hydroxide is not a liquid (its melting point is 323o C) Footnote 20. The classification criteria for Pyrophoric Liquids do not apply to this substance.
Pyrophoric Solids:
No data available
No data are available to determine whether Sodium hydroxide meets the classification criteria for Pyrophoric Solids.
Self-Heating Substances and Mixtures:
No data available
No data are available to determine whether Sodium hydroxide meets the classification criteria for Self-Heating Substances and Mixtures.
Substances and Mixtures which, in Contact with Water, Emit Flammable Gasses:
Does not meet criteria
Sodium hydroxide is a strong alkaline substance that dissociates completely in water into the Sodium ion (Na+) and hydroxyl ion (OH-).
The available data do not meet the classification criteria for Substances and Mixtures which, in Contact with Water, Emit Flammable Gasses.
Oxidizing Liquids:
Not applicable
Sodium hydroxide is not a liquid (its melting point is 323 °C) Footnote 20. The classification criteria for Oxidizing Liquids do not apply to this substance
Oxidizing Solids:
Does not meet criteria
Sodium hydroxide has no chemical groups associated with oxidizing properties Footnote 20.
The available data do not meet the classification criteria for Oxidizing Solids.
Organic Peroxides:
Not applicable
Sodium hydroxide is not an organic peroxide Footnote 20. The classification criteria for Organic Peroxides do not apply to this substance.
Corrosive to Metals:
Does not meet criteria
Data suggest that Sodium hydroxide is corrosive to several metals (aluminum, zinc and zinc-containing brasses and bronzes, types 1010, 1020, 1075 and 1095 carbon steel, copper, and silicon copper); however, the data are insufficient to classify as Category 1 Footnote 25.
Indeed, Schweitzer's "Corrosion resistance tables" classified the corrosivity of Sodium hydroxide using a threshold of 50 Mils/year (1 Mils is a thousandth of an inch), equivalent of 1.27 mm/year, which is under the 6.25 mm/year threshold for classification.
The available data do not meet the classification criteria for Corrosive to Metals.
Combustible Dusts:
Not applicable
Sodium hydroxide is a white orthorhombic crystal and is hygroscopic Footnote 20.
Simple Asphyxiants:
Not applicable
Sodium hydroxide is not a gas (its boiling point is 1,388 ºC) Footnote 20. The classification criteria for Simple Asphyxiants do not apply to this substance.
Chemicals Under Pressure:
Not evaluated
Classification of a hazardous product in the Chemicals Under Pressure hazard class is product dependent.
Regulatory and other information
Regulatory information:
Hazardous substance assessments are prepared by Health Canada as educational and information resources. Under the HPA, suppliers of hazardous products must, upon the sale or importation of a hazardous product, provide a safety data sheet and label that meet the requirements set out in the HPR.
Other information:
The information and classifications contained in these hazardous substance assessments are based on publicly available sources, such as peer-reviewed literature or reports by international bodies. New information, including proprietary information, could have an impact on the classification of substances or hazardous products containing them. It is the responsibility of the supplier to ensure the accuracy, sufficiency, and reliability of their hazardous product classifications.
Last updated:
2024
Prepared by:
Workplace Hazardous Materials Bureau, Health Canada