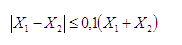Radiation Protection and Quality Standards in Mammography - Safety Procedures for the Installation, Use and Control of Mammographic X-ray Equipment: Safety Code 36
Download the alternative format
(PDF format, 701 KB, 78 pages)
Organization: Health Canada
Published: October 2013
Table of Contents
- Explanatory Notes
- Acknowledgments
- Principal Objectives of the Safety Code
- Section A: Personnel Qualifications and Responsibilities
- 1.0 Qualifications and Responsibilities of Personnel
- 2.0 Procedures for Minimizing Radiation Exposure to Personnel
- 3.0 Procedures for Minimizing Radiation Exposure to Patients
- Section B: Facility and Equipment Requirements
- 1.0 Facility Requirements
- 2.0 Mammographic X-ray Equipment Requirements
- 3.0 Image Processing Systems
- 3.1 Film-Screen Mammography Systems
- 3.2 Digital Mammography Systems
- 3.2.1 Computed Radiography Imaging Plates
- 3.2.2 CR Cassette
- 3.2.3 Breast Tomosynthesis
- 3.2.4 Mammography Review Workstation/Electronic Display Devices
- 3.2.5 Picture Archiving and Communications Systems (PACS)
- 3.2.6 PACS Implementation
- 3.2.7 Integrating the Health Enterprise (IHE) Integration Profiles
- 3.2.8 Computer Aided Detection and Diagnosis (CAD)
- 3.2.9 Telemammography
- 3.2.10 Compression of Digital Images
- 4.0 Test Equipment
- 5.0 Radiation Protection Surveys
- 6.0 Disposal of x-ray Equipment
- Section C: Quality Assurance Program
- Appendix I: Federal/Provincial/Territorial Radiation Safety Agencies
- Appendix II: Dose Limits for Occupational Ionizing Radiation Exposures
- Appendix III: Calculation of Mean Glandular Dose
- Appendix IV: Shielding Guides for Storage of Mammographic Film
- Appendix V: Radiation Measurements Units
- Appendix VI: Glossary
- Reference
Explanatory Notes
This document is one of a series of Safety Codes prepared by Health Canada to set out requirements for the safe use of radiation emitting equipment.
The information in this Safety Code has been prepared to provide specific guidance to owners of mammography equipment, radiologists, mammography radiological technologists, medical physicists, and other personnel concerned with the safety procedures, equipment performance, image quality, radiation protection and the overall quality of a mammography facility.
The scope of this safety code includes mammography technologies such as film/screen, computed radiography (CR), digital radiography (DR) and tomosynthesis. Other breast imaging modalities not employing x-ray radiation are excluding from this code.
This Safety Code replaces Safety Code 33, Radiation Protection in Mammography (HC 1995), and the Canadian Mammography Quality Guidelines (HC 2002).
The personnel requirements, safety procedures, equipment and facility guidelines and quality assurance measures detailed in this Safety Code are primarily for the instruction and guidance of persons employed in Federal Public Service departments and agencies, as well as those under the jurisdiction of the Canada Labour Code. Facilities under provincial or territorial jurisdiction may be subject to requirements under their statutes. The authorities listed in Appendix I should be contacted for details of the regulatory requirements of individual provinces and territories.
The words must and should in this Code have been chosen with purpose. The word must indicates a requirement that is essential to meet the currently accepted standards of protection, while should indicates an advisory recommendation that is highly desirable and is to be implemented where applicable.
In a field in which technology is advancing rapidly and where unexpected and unique problems continually occur, this Code cannot cover all possible situations. Blind adherence to rules cannot substitute for the exercise of sound judgement. Recommendations may be modified in unusual circumstances, but only upon the advice of experts in radiation protection. This Code will be reviewed and revised from time to time, and a particular requirement may be reconsidered at any time, if it becomes necessary to cover an unforeseen situation. Interpretation or elaboration on any point can be obtained by contacting the Consumer and Clinical Radiation Protection Bureau, Health Canada, Ottawa, Ontario K1A 1C1.
Acknowledgments
This Safety Code reflects the work of many individuals. It was prepared and compiled by Ms. Narine Martel of the Medical Imaging Division, Consumer and Clinical Radiation Protection Bureau. The assistance of Mr. Richard Tremblay, Mr. Charles Steiner and the members of the Medical Imaging Division during the preparation of this Code is acknowledged.
Sincere appreciation is expressed for the contributions of the members of the Canadian Mammography Standards and Accreditation Working Group established by the National Committee of the Canadian Breast Cancer Screening Initiative:
Elaine Dever, Canadian Association of Medical Radiation Technologists
Brenda Mitchell, Cancer Care Ontario (formerly)
Tom Butterworth, Canadian Association of Radiologists (formerly)
Andrea Nelson, Canadian Association of Radiologists
Jay Onysko, Public Health Agency of Canada
Rasika Rajapakshe, Canadian College of Physicist in Medicine
Rene Shumak, Cancer Care Ontario
Richard Tremblay, Ministère de la Santé et des Services sociaux du Québec (formerly)
Nancy Wadden, St-Clare's Mercy Hospital, Newfoundland
The contributions of the following organizations, agencies and associations, whose comments and suggestions during the consultation of this document helped in the preparation of this final Code, are gratefully acknowledged:
Alberta College of Medical Diagnostic and Therapeutic Technologists
Association des physiciens et ingénieurs biomédicaux du Québec (APIBQ), Comité de radioprotection
Breast Centre Radiology, Edmonton Alberta
British Columbia Centre for Disease Control
British Columbia Interior Health Authority, Diagnostic Imaging Services
Canadian Association of Radiologists
Federal/Provincial/Territorial Radiation Protection Committee
Health Canada, Medical Devices Bureau
Health Prince Edward Island, Provincial Diagnostic Imaging Services
Horizon Health Network New Brunswick, Saint John Regional Hospital
Horizon Health Network New Brunswick, Upper River Valley Hospital
Imaging Research Program, Sunnybrook Research Institute
Ministère de la Santé et des Services sociaux, Laboratoire de Santé Publique du Québec
Northern Alberta Institute of Technology, School of Health Sciences
Ontario Breast Screening Program, Cancer Care Ontario
Ontario Ministry of Health and Long Term Care
Radiology Consultants Associated, Alberta
Saskatchewan Ministry of Labour Relations and Workplace Safety, Radiation Safety Unit
Saskatchewan Cancer Agency, Screening Program for Breast Cancer
Thunder Bay Regional Health Sciences Centre, Linda Buchan Centre for Breast Screening and Assessment
Vancouver Coastal Health Authority, Department of Radiology
Introduction
Mammography is an effective imaging method used for the detection of breast cancer. It has been proven to detect breast cancer at an early stage and, when followed up with appropriate diagnosis and treatment, to reduce mortality from breast cancer. Over the past 20 years, technological developments have greatly impacted mammography equipment design and performance and have resulted in improvements in image quality and reduction in radiation dose. Mammographic x-ray procedures are one of the most carefully managed radiological procedures. This is necessary in order to ensure optimization of image quality for interpretation of mammograms and to minimize radiation exposure to patients.
Breast cancer is the most frequently diagnosed cancer among Canadian women. In 2013, it is estimated that 23,800 women will be diagnosed with breast cancer. While the incidence of breast cancer in Canada has stabilized since 1999, the rate of breast cancer mortality has fallen by more than 30 percent since 1986. This is likely to be due to improvements in screening and advances in treatment. It is estimated that in 2013 5,000 women will die of breast cancer. Ensuring the quality of mammography, both screening and diagnostic, is an important component in the management of breast cancer. In order to have an effective mammography program, it is essential that mammography be performed to meet rigorous quality requirements. The responsibility for the quality of mammography in Canada is shared among federal, provincial and territorial governments, and the medical professionals who maintain the equipment, carry out the procedure and interpret the mammograms.
The purpose of this document is to provide guidance to mammography facilities, both screening and diagnostic, on radiation protection and quality assurance in mammography. The contents of this document are built upon and harmonized with existing Canadian and international standards pertaining to mammography. This includes the Diagnostic X-ray Equipment Regulations, Part XII of the Radiation Emitting Devices Act, which regulates the design, construction and functioning of mammographic x-ray equipment, the requirements of the Mammography Accreditation Program of the Canadian Association of Radiologists, provincial requirements and recommendations of the International Commission on Radiological Protection (ICRP) and the International Agency on Atomic Energy (IAEA). It should be noted that other provincial or territorial requirements may exist that supersede or add to provisions of this document.
Currently in Canada, mammography is performed using film-screen and digital mammography systems. Both systems utilize low energy x-rays to penetrate breast tissue, but differ in the image receptor, or detector, that is used to create the image. In film-screen mammography, x-ray film is placed in direct contact with a screen. The x-ray photons are captured by the screen which then emits light that exposes the film. The film is then chemically developed to produce the mammogram. Digital mammography systems use detectors with various technologies to produce the mammographic image. These technologies are generally categorized into two groups: direct and indirect conversion detectors. In systems employing direct conversion detectors, x-ray photons incident on the image receptor interact with a photoconducting material which directly converts the x-ray energy into an electrical signal carrying the image information. The electrical signal is processed and displayed almost instantaneously. In systems employing an indirect detector, a scintillator is used to capture the x-ray energy and then convert it to light. Light photons are then converted into an electrical signal. Computed radiography (CR) mammography systems are a type of indirect digital imaging technology. These systems consist of a cassette, an imaging plate containing photostimulable phosphor and an imaging plate reader. The CR cassette is exposed as in film-screen mammography. The latent image is stored in the imaging plate which is then read to produce the mammographic image.
The radiation dose from a properly carried out mammographic examination is very low and is essentially only delivered to the breast tissue. Due to the very low x-ray energies used in mammography, there is very little dose to other tissues. However, any procedure involving exposures to ionizing radiation must be carefully managed as it is presumed that even small doses of radiation may produce some deleterious health effects. Somatic effects may manifest themselves in the exposed individuals and are characterized by observable changes occurring in the body organs of the individuals. Genetic effects may arise in the descendents of exposed individuals. In mammography, the risk of genetic defects in well-conducted examinations is very small, and for post-menopausal women, there is no genetic risk.
Since it is not possible to measure carcinogenic effects at low doses, estimates of the incidences of radiation effects at low doses are based on linear extrapolation from relatively high doses. Due to the uncertainties with respect to radiological risk, a radiation protection risk model assumes that the health risk from radiation exposure is proportional to dose. This is called the linear no-threshold hypothesis. Since the projected effect of a low dose increases the incidence of a deleterious effect only minimally above the naturally occurring level, it is impossible to prove by observation either the validity or falsity of this hypothesis. However, the linear no-threshold hypothesis has been widely adopted in radiological protection and has led to the formulation of the ALARA (As Low As Reasonably Achievable) principle. The ALARA principle is an approach to radiation protection to manage and control exposures to radiation workers and the general public to as low as is reasonable, taking into account social and economic factors.
In mammography, there are four main aspects of radiation protection to be considered. First, patients should not be subjected to unnecessary radiographic procedures. This means that the procedures are ordered with justification, and when the diagnostic information cannot be obtained otherwise. Second, when a procedure is required, it is essential that the patient be protected from excessive radiation exposure during the examination. Third, it is necessary that personnel within the facility be protected from excessive exposure to radiation during the course of their work. Finally, personnel and the general public in the vicinity of such facilities require adequate protection.
While regulatory dose limits have been established for radiation workers and the general public, these limits do not apply to doses received by a patient undergoing medical x-ray procedures. For patients, the risk associated with the exposure to radiation must always be weighed against the clinical benefit of an accurate diagnosis or treatment. There must always be a conscious effort to reduce patient doses to the lowest practical level consistent with optimal quality of diagnostic information. Through close cooperation between medical professionals, technologists, medical physicists, and other support staff it is possible to achieve an effective radiation protection program and maintain a high quality mammography program.
Principal Objectives of the Safety Code
This Safety Code provides guidance to all mammography facilities, both screening and diagnostic, to achieve and maintain effective mammography programs and to ensure radiation protection. The aim of this Safety Code is to provide mammography facilities with the necessary information to achieve the following principal objectives:
- to ensure optimal mammographic image quality while minimizing patient exposure to x-ray radiation;
- to ensure adequate protection of personnel operating mammography equipment; and
- to ensure adequate protection of other personnel and the general public in the vicinity of mammography equipment.
To assist in achieving these objectives, this Safety Code:
- sets out qualifications and responsibilities of the owner, the interpreting radiologists, the mammography radiological technologists, the medical physicist and the information systems specialist;
- presents practices and procedures to minimize doses from mammography equipment to operators and the public;
- presents practices and procedures for minimizing radiation doses to patients while maintaining adequate image quality;
- presents practices and procedures for ensuring the x-ray equipment is used in a safe manner;
- provides information on facility design and shielding requirements;
- specifies minimum standards of construction and performance for mammography equipment;
- supplies information required to implement and operate a quality assurance program for the facility;
- provides a list of acceptance tests and quality control tests for various types of mammography equipment and their accessories; and
- provides a schedule for performing quality control tests.
This Safety Code is composed of three sections:
Section A: Responsibilities and Protection
This section sets out the responsibilities of the owner, the interpreting radiologists, the mammography radiological technologists, the medical physicist and the information systems specialist for the safe installation, operation and control of the equipment, and sets out practices to minimize radiation doses to patients, staff and the public.
Section B: Facility and Equipment Requirements
This section sets out requirements for the facility design and minimum equipment construction and performance standards.
Section C: Quality Assurance Program
This section sets out requirements for quality assurance programs including acceptance testing and quality control procedures.
Section A: Personnel Qualifications and Responsibilities
1.0 Qualifications and Responsibilities of Personnel
This section sets out the qualifications and responsibilities of all personnel involved in mammography. Initial qualifications, continuing experience and education, and re-establishing qualifications are provided and are based upon a 3 year cycle. Although personnel responsibilities are grouped separately, to obtain the optimal level of radiation safety and image quality, it is imperative that full cooperation exists among all concerned parties.
1.1 Owner
The owner is ultimately responsible for the radiation safety of the facility. The owner is defined as the person or group of persons in control of the possession and use of mammography equipment. The owner may be an individual, a corporation, a district, a province or some other entity. It is the responsibility of the owner to ensure that the equipment and the facilities in which such equipment is installed and used meet all applicable radiation safety standards, and that a radiation safety program is developed, implemented and maintained for the facility. The owner may delegate this responsibility to qualified staff. How this responsibility is delegated will depend upon the number of staff members, the nature of the operation and on the number of x-ray equipment owned. In any event, the owner must ensure that one or more qualified persons are designated to carry out the roles of the personnel described below.
The owner has the responsibility of:
- implementing and maintaining an effective diagnostic imaging quality assurance program for the facility, including quality control testing procedures and record keeping;
- ensuring the installation complies with all applicable regulatory requirements;
- consulting with the appropriate government agencies
- when a new facility is being constructed, or modifications of an existing one are planned, to ensure that radiation safety is adequate,
- when mammographic x-ray equipment is purchased to ensure adequate radiation safety, and to register the equipment with the appropriate agency, or
- to set periodic scheduled inspections for the facility. In some jurisdictions, the agency responsible for inspections has the mandate for setting inspection schedules;
- establishing safe working conditions;
- ensuring that
- the equipment functions properly through ongoing maintenance by competent personnel and replacement of outdated or noncompliant equipment,
- the equipment has a Canadian medical device licence,
- safe operating procedures are established and are followed,
- quality control monitoring of mammographic x-ray equipment, image processor and ancillary equipment is carried out,
- technologists are properly trained in the operation of the equipment being used,
- technologists-in-training and inexperienced personnel operate mammographic x-ray equipment only under the direct supervision of a licensed or certified technologist, and
- professional qualifications are maintained;
- declaring who is to be considered a radiation worker where this person may receive a radiation dose in excess of 1/20th of the recommended dose limit for a radiation worker specified in Appendix II;
- keeping records of occupational exposures received by personnel, and investigating any exposure received by personnel in excess of 1/20th of the recommended dose limit;
- keeping records of radiation surveys, including summaries of corrective measures recommended and/or instituted, and organizing participation in a personnel radiation monitoring service, if necessary; and
- ensuring that personnel understand the contents of this Safety Code.
1.2 Interpreting Radiologist
All interpreting radiologists shall meet the following qualifications:
1.2.1 Initial Qualifications
Before beginning to interpret mammograms independently, the interpreting radiologist must:
- possess qualifications required by any relevant federal and provincial/territorial statutes and regulations;
- be certified in Diagnostic Radiology by the Royal College of Physicians and Surgeons of Canada or by the Collège des médecins du Québec, OR be certified in Diagnostic Radiology by another recognized licensing body and comply with provincial licensing requirements;
- have a minimum of 40 credits of documented Continuing Professional Development (CPD) in breast imaging within the Maintenance of Certification (MOCERT) program of the Royal College of Physicians and Surgeons of Canada. It is required that at least half of these credits be in accredited group learning activities or accredited self assessment programs and the remainder can be properly documented non-accredited meetings, readings, videotapes, CD-Roms, etc. At least 15 of the CPD credits must have been acquired within the 3 years immediately prior to the date that the physician qualifies as an interpreting physician. At least half of these 15 credits must be from an accredited activity. Time spent in residency specifically devoted to mammography is acceptable, if documentation from the training program is supplied by the radiologist;
- have interpreted or multi-read a minimum of 480 mammographic examinations within the last year immediately prior to the date that the radiologist qualifies as a mammography interpreting radiologist. This interpretation or multi-reading must be under the direct supervision of an interpreting physician qualified in mammography. It is recommended that a minimum of 1000 mammographic examinations should be interpreted or multi-read, however in certain circumstances this number may be unattainable and in these cases a minimum of 480 mammographic examinations per year will be acceptable. Justification must be provided as to why the target of 1000 mammographic examinations cannot be met;
- have at least 8 hours of training in each mammographic modality(e.g. CR, DR, tomosynthesis) used by the interpreting radiologist. Note that the 8 hours may be part of the 40 hours required in (c), if documentation is supplied.
1.2.2 Continuing Experience and Education
All interpreting radiologists must maintain their qualifications by meeting the following requirements:
- The interpreting radiologist must interpreted or multi-read a minimum of 480 mammographic examinations per year. It is recommended that a minimum of 1000 mammographic examinations should be interpreted or multi-read, however in certain circumstances this number may be unattainable and in these cases a minimum of 480 mammographic examinations per year will be acceptable. Justification must be provided as to why the target of 1000 mammographic examinations cannot be met. For radiologists interpreting screening mammograms, it is recommended that 2000 mammographic examinations be interpreted per year (Coldman et al., 2006).
- Every three years, the interpreting radiologist must have taught or completed at least the equivalent of 15 credits of documented CPD in breast imaging within the MOCERT program of the Royal College of Physicians and Surgeons of Canada. It is required that at least half of these credits be in accredited group learning activities or accredited self assessment programs and the remainder can be properly documented non-accredited meetings, readings, videotapes, CD-Roms, etc. This training must include at least six category I continuing medical education credits in each mammographic modality used by the interpreting physician in his or her practice. Documentation should be available upon request. Units earned through teaching a specific course can be counted only once towards the 15 credits, even if the course is taught multiple times during the three year period; and
- Before an interpreting radiologist may begin independently interpreting mammograms produced by a new mammographic modality, that is, a mammographic modality in which the radiologist has not previously been trained, the interpreting radiologist must have at least 8 hours of training in the new mammographic modality (ex. CR, DR).
1.2.3 Re-establishing Qualifications
Interpreting radiologists who fail to maintain the required continuing experience or continuing education requirements must re-establish their qualifications before resuming the independent interpretation of mammograms, as follows:
- Interpreting radiologists who fail to meet the continuing experience requirements of section 1.2.2 must, within the 6 months immediately prior to resuming independent interpretation;
- interpret or multi-read at least 240 mammographic examinations under the direct supervision of an interpreting radiologist or
- interpret or multi-read a sufficient number of mammographic examinations, under the direct supervision of an interpreting physician, to bring the physician's total up to 960 examinations for the prior 24 months, whichever is less.
- Interpreting physicians who fail to meet the continuing education requirements of section 1.2.2 must obtain a sufficient number of additional document CPD credits in mammography to bring their total up to the required 15 credits in the previous 36 months before resuming independent interpretation.
1.2.4 Interpreting Radiologists Responsibilities
All interpreting radiologists must participate fully in the quality assurance program by:
- communicating with staff any changes in image quality whether they are due to improper positioning, mammographic technique or image processing;
- participating in the collection and maintenance of records concerning outcome data for correlation of positive mammograms to biopsies done and the number of cancers detected; and
- understanding the requirements and recommendations of this Safety Code.
1.3 Mammography Radiological Technologist
1.3.1 Initial Qualifications
All mammography radiological technologists must:
- possess qualifications required by any relevant federal and provincial/territorial statutes and regulations;
- be certified by the Canadian Association of Medical Radiation Technologists or the Ordre des technologues en imagerie medicale, en radio-oncologie et en électrophysiologie médicale du Québec in the discipline of radiological technology and be registered with the provincial/territorial regulatory body for medical radiation technologists in the specialty/discipline of radiological technology, if applicable;
- through professional development programs and specialty courses, such as the CAMRT Certificate in Breast Imaging or courses offered by other organizations, achieve competency to perform breast imaging procedures by acquiring knowledge in:
- breast anatomy and physiology,
- positioning techniques,
- patient management,
- operation of mammography equipment including networking and archival systems,
- image assessment, and
- quality control testing;
- have a minimum of 40 hours supervised clinical experience in the practice of mammography with a minimum of 50 mammographic examinations performed.
1.3.2 Continuing Experience and Education
All practicing mammography radiological technologists must maintain their competence in the practice of breast imaging by meeting the following requirements:
- have 15 hours of continuing professional development in mammography every 3 years or as required by provincial/territorial regulations;
- work the equivalent of at least 390 hours in mammography each year for 3 years, or as required by provincial/territorial regulations; and
- perform a minimum of 480 mammography examinations each year for 3 years. In certain circumstances, this number may be unattainable, in this case justification must be provided as to why the target of 480 mammographic examinations cannot be met.
1.3.3 Re-establishing Qualifications
Mammography radiological technologists who fail to meet the continuing experience requirements must:
- perform a minimum of 50 mammography examinations under the supervision of a qualified mammography radiological technologist; and
- demonstrate competency to operate imaging modalities, especially newly acquired units, through 40 hours of practice supervised by a radiological technologist qualified in mammography. In certain circumstances, 40 hours may not be deemed necessary, however in this case, justification must be provided as to reasons for requiring fewer hours of supervised practice and as to how the adequacy of the level of supervised practice was evaluated.
1.3.4 Mammography Radiological Technologist Responsibilities
Mammography radiological technologists must participate fully in the quality assurance program by:
- ensuring that the optimal level of diagnostic image quality is maintained;
- communicating with staff any changes in image quality;
- reporting any change in equipment performance to the owner;
- performing daily and other routine quality control tests of mammographic x-ray equipment, image processing system (film or digital), and ancillary equipment and keeping records of these tests;
- recognizing the radiation hazards associated with their work and taking measures to minimize them;
- being aware of the consequences of improperly performed mammographic procedures on image quality and patient doses;
- striving to eliminate unnecessary mammographic examinations by reducing the number of retakes, and reducing all patient radiation exposures to the lowest practical values; and
- understanding the requirements and recommendations of this Safety Code.
1.4 Medical Physicist
1.4.1 Initial Qualifications
All medical physicists conducting surveys of mammography facilities and providing oversight of the facility quality assurance program must meet the following requirements:
- possess qualifications required by any relevant federal and provincial/territorial statutes and regulations; and
- be certified in Physics of Mammography by the Canadian College of Physicists in Medicine (CCPM) and be registered with the provincial regulatory body for medical physicists such as the Association des physiciens et ingénieurs biomédicaux du Québec, if applicable.
Requirements of the CCPM for certification in Physics of Mammography include:
- Have a minimum of 15 credits of documented continuing education on relevant matters in mammography within the previous 3 years; and
- Have the experience of conducting surveys of at least 2 mammography facilities and a total of at least 6 mammography units. No more than one survey of a specific facility within a 10 month period or a specific unit within a period of 60 days can be counted towards this requirement. Experience conducting surveys must be acquired under the direct supervision of a medical physicist certified in Physics of Mammography by the CCPM. In certain circumstances this requirement may be unattainable. Exemption may be granted by the CCPM, if acceptable justification is provided.
1.4.2 Continuing Experience and Education
All medical physicists conducting surveys of mammography facilities and providing oversight of the facility quality assurance program must obtain the following continuing experience and education, in accordance with the requirements of the CCPM.
- Continuing Education
- The medical physicist must have taught or completed at least 15 credits of continuing education on relevant matters in mammography during the 3 years following certification or the last renewal of certification by the CCPM. This continuing education shall include hours of training appropriate to each mammographic modality evaluated by the medical physicist during his or her surveys or oversight of quality assurance programs. Units earned through teaching a specific course can be counted only once towards the required 15 hours in a 3 year period, even if the course is taught multiple times during the 3 years.
- Continuing Experience
- The medical physicists must have surveyed at least 2 mammography facilities and a total of at least 6 mammographic units during the 3 years following certification or the last renewal of certification by the CCPM. No more than one survey of a specific facility within a 10 month period or a specific unit within a period of 60 days can be counted towards this requirement. In certain circumstances this requirement may be unattainable. Exemption may be granted by the CCPM, if acceptable justification is provided.
- Before a medical physicist may begin independently performing mammographic surveys of a new mammographic modality, that is, a mammographic modality other than one for which the physicist received training to initially qualify, the physicist must receive at least 8 hours of training in surveying units of the new mammographic modality. Note that the 8 hours may be part of the 15 hours required in (a)(i), if documentation is supplied.
1.4.3 Re-establishing Qualifications
Medical physicists who fail to maintain the required continuing qualifications may not perform surveys without the supervision of a qualified medical physicist. Before independently surveying another facility, medical physicists must re-establish their qualifications in accordance to the requirements of the CCPM as follows:
- medical physicists who fail to meet the continuing educational requirements must obtain a sufficient number of continuing education units to bring their total credits up to the required 15 in the previous 3 years;
- medical physicists who fail to meet the continuing experience requirements must complete a sufficient number of surveys under the direct supervision of a qualified medical physicist to bring their total surveys up to the required 2 facilities and 6 units in the previous 3 years. No more than one survey of a specific unit within a period of 60 days can be counted towards the total mammographic unit survey requirements. In certain circumstances this requirement may be unattainable. Exemption may be granted by the CCPM, if acceptable justification is provided.
1.4.4 Medical Physicist Responsibilities
The medical physicist must:
- verify the safety of an installation at the time of planning and construction, and ensure that the installation complies with all applicable regulatory requirements;
- provide ongoing evaluations of the safety procedures and recommend to the owner the necessary changes to ensure optimum patient and personnel safety, and instruct personnel in proper radiation protection practices;
- participate in the quality assurance program to
- ensure that the quality assurance program is properly implemented and operated,
- verify whether the optimal level of technical image quality is obtained, and
- ensure appropriate quality control monitoring instruments are available and properly calibrated;
- perform the required testing of mammography x-ray equipment, image acquisition and processing system, display monitors and ancillary equipment and document results in accordance to appropriate record keeping procedures;
- provide a complete written report clearly describing survey results (the written report should be available 10 days after the survey and must be available 30 days after the survey);
- provide prompt oral communication of survey results with a responsible individual within the facility (The responsible individual may differ from facility to facility. Prior to a survey, the medical physicist must request the facility to identify the responsible individual to whom survey results are to be communicated.); and
- understand the requirements and recommendations of this Safety Code.
1.5 Information Systems Specialist (ISS)
Facilities performing digital image processing must have access to an individual who is trained and experienced in installation, maintenance and quality control of information technology software and hardware. Depending on the facility, the individual may be on-site or available upon request. The required qualification of this individual will depend highly on the type of facility and the type of equipment. Ideally the individual should have both information technology and radiation technology expertise.
1.5.1 Initial Qualifications
The ISS:
- must possess qualifications required by any relevant federal/provincial/territorial regulations and statutes;
- should be certified according to a recognized standard such as that of the Society of Imaging Informatics in Medicine or the PACS Administrators Registry and Certification Association; and
- should have knowledge in:
- computer basics such as understanding hardware components, types of interfaces; configuration parameters, as well as relevant operating systems and software applications,
- database basics,
- networking concepts such as DICOM, HL7, RIS, and HIS,
- security systems and concepts to ensure confidentiality of patient records,
- medical imaging terminology,
- positioning and viewing characteristics,
- imaging characteristics of various modalities for image acquisition,
- workflow of the facility, and
- federal, provincial, territorial and institutional privacy legislation and policies such as the Personal Information Protection and Electronic Documents Act (PIPEDA).
1.5.2 Continuing Education and Experience
- Continuing Education
- The ISS must have completed at least 15 hours of continuing education on relevant matters in information technology during a period of 3 years. This continuing education shall include hours of training appropriate to the information systems of the facility.
- Continuing Experience
- The ISS should have worked a minimum of 390 hours each year for a period of 3 years, and
- Before an ISS may begin independent work on new information systems, that is, systems other than one for which the individual received training to initially qualify, they must receive at least 8 hours of training on the new information system.
1.5.3 Re-establishing Qualifications
ISSs who fail to maintain the required continuing qualifications may not carry out their duties without the supervision of a qualified ISS. Before carrying out independent work, the ISS must re-establish their qualifications.
- The ISSs who fail to meet the continuing education requirements must obtain a sufficient number of continuing education units to bring their total hours up to the required 15 in the previous 3 years; and
- The ISSs who fail to meet the continuing experience requirements must work under the direct supervision of a qualified ISS to bring their total number of hours worked to 75 hours in the previous 3 years.
1.5.4 Information Systems Specialist Responsibilities
The ISS must:
- ensure confidentiality of patient records;
- understand the policies and procedures in place within the facility;
- understand the importance of and the requirements for an information systems quality assurance program; and
- communicate with staff any changes/upgrades made to the information management equipment hardware or software and the resulting consequences on the operating procedures of the facility.
1.6 Repair and Maintenance Personnel
The repair and maintenance personnel are individuals authorized to perform repairs and maintenance on X-ray generators, control systems, imaging systems and their operating software. Depending on the facility, these individuals may be on-site or available upon request, but in general, this function is sometime contracted to an outside organization, or to the equipment manufacturer.
The repair and maintenance personnel must:
- have knowledge and training in
- repair and maintenance of radiological imaging equipment, and
- radiation protection principles and procedures;
- ensure that, after a repair or maintenance procedure, the equipment meets the required regulatory standards or manufacturer' specifications;
- ensure that all repair and maintenance procedures are properly recorded and communicated to the owner and other appropriate staff;
- report any non-compliance with the established safety procedures to the owner of the equipment;
- review the maintenance procedures periodically and update them to ensure optimum patient and operator safety;
- communicate, if necessary, to staff the need for the appropriate acceptance testing, baseline setting and quality control testing; and
- follow manufacturers' recommendations for the repair and maintenance of equipment.
1.7 Retention of Professional Records
Facilities must maintain records to document the qualifications of all personnel who worked at the facility as interpreting radiologists, mammography radiological technologists, medical physicists and information systems specialists. The retention time must be in accordance with any relevant federal/provincial/territorial statutes and regulations. Records of personnel, including those no longer employed by the facility, should not be discarded as they may be required by the facility to demonstrate the qualifications of all personnel to the accreditation body.
2.0 Procedures for Minimizing Radiation Exposure to Personnel
The required and recommended procedures outlined in this section are primarily directed toward occupational health protection. However, adherence to these will also, in many instances, provide protection to visitors and other individuals in the vicinity of an x-ray facility. The safe work practices and procedures should be regarded as a minimum, to be augmented with additional requirements, when warranted, to cover special circumstances in particular facilities.
To achieve optimal safety, equipment operators must make every reasonable effort to keep exposures to themselves and to other personnel as far below the limits specified in Appendix II as reasonably achievable.
2.1 General Requirements and Recommendations
- An x-ray room must not be used for more than one radiological investigation simultaneously.
- Except for those persons whose presence is essential, all persons must leave the room when the irradiation is carried out.
- Personnel must, at all times, keep as far away from the x-ray beam as practicable.
- Deliberate irradiation of an individual for training purposes or equipment evaluation must never occur.
- All personnel must use available protective devices.
- Operation of the mammography equipment must be controlled from the control panel located behind a protective screen or inside a control booth. The technologist must be shielded when exposures are made.
- Technologists must have a clear view of the patient during every mammographic x-ray examination and must be able to communicate with the patient and/or attendants.
- Mammographic x-ray equipment must be operated only by qualified individuals who are properly trained for the equipment and the procedures being performed.
- All operators of x-ray equipment, together with personnel (i.e., nurses) who routinely participate in radiological procedures, and others, likely to receive a radiation dose in excess of 1/20th of the dose limit to radiation workers specified in Appendix II, must be declared radiation workers and monitor their radiation exposures with the use of a personal dosimeter. In general, personnel only operating mammography equipment are not declared radiation workers as they do not receive radiation doses in excess of 1 mSv. However, personnel who have been declared radiation workers as a result of performance of duties outside of mammography must wear a dosimeter while performing their duties in mammography.
- Personal dosimeters must be worn and stored according to the recommendations of the dosimetry service provider. When a protective apron is worn, the personal dosimeter must be worn under the apron. If eyes or extremities are likely to be exposed to significantly higher doses, additional dosimeters should be worn at those locations on the body. However this is not likely when performing mammography examination.
- Where radiation doses in excess of 1/20th of the effective dose limit for radiation workers specified in Appendix II are regularly received by any one person, appropriate remedial steps must be taken to improve techniques and protective measures.
- All personal dosimetry records must be maintained for the lifetime of the facility.
- A female operator should immediately notify her employer upon knowledge that she is pregnant, in order that appropriate steps may be taken to ensure that her work duties during the remainder of the pregnancy are compatible with the recommended dose limits as stated in Appendix II. Depending on the type of work being performed by the employee, it may not be necessary to remove a pregnant staff member from their duties of operating the x-ray equipment. It is recommended that the decision to remove pregnant workers from their duties include consideration of the radiation exposure risks associated with the employee's duties, as determined by a medical physicist or a radiation safety officer. In general, for the performance of mammographic examinations, there is no need to remove or restrict the duties of mammography equipment operators during pregnancy.
- If a patient escort or other person is called upon to assist, this person must be provided with protective clothing. No person must regularly perform these duties.
- All entrance doors to an x-ray room should be kept closed while a patient is in the room and must be closed while making an x-ray exposure.
- X-ray machines which are energized and ready to produce radiation must not be accessible to the general public.
3.0 Procedures for Minimizing Radiation Exposure to Patients
The largest single contributor of man-made radiation exposure to the population is dental and medical radiography. In total, such use of x-rays accounts for more than 90% of the total man-made radiation dose to the general population.
The risk to the individual patient from a single radiographic examination is very low. However, the risk to a population is increased by increasing the frequency of radiographic examinations and by increasing the number of persons undergoing such examinations. For this reason, it is important to reduce the number of radiographs taken, the number of persons examined radiographically, and the doses associated with the examinations.
To accomplish this reduction, it is essential that patients must only be subjected to necessary radiological examinations and, when a radiological examination is required, patients must be protected from excessive irradiation during the examination.
The required and recommended procedures for the protection of the patient, outlined in this section, are directed toward the health care professionals, radiologist, and technologist. They are intended to provide guidelines for elimination of unnecessary mammographic examinations and for minimizing doses to patients when mammography is necessary.
3.1 Guidelines for the Prescription of Diagnostic Mammography
Unnecessary radiation exposures of patients can be significantly reduced by ensuring that all examinations are clinically justified. This can be done by adhering, as much as possible, to certain basic recommendations. These recommendations are presented below.
- The request for a mammographic x-ray examination of a patient should be based on clinical evaluation of the patient and should be for the purpose of obtaining diagnostic information.
- Mammography examinations should not be performed if there has been no prior clinical examination of the patient.
- It should be determined whether there have been any previous mammographic examinations which would make further examination unnecessary, or allow for the ordering of an abbreviated examination. Relevant previous images or reports should be examined along with a clinical evaluation of the patient.
- When a patient is transferred from one health care professional or hospital to another, any relevant images or reports should accompany the patient and should be reviewed by the consulting health care professionals.
- When prescribing a mammographic x-ray examination, the health care professional should specify precisely the clinical indications and information required.
- The number of radiographic views required in an examination must be kept to the minimum practicable, consistent with the clinical objectives of the examination.
- In prescribing mammographic x-ray examinations of pregnant or possibly pregnant women, full consideration must be taken of the consequences of foetal irradiation. While it is generally accepted that the radiation dose to the ovaries and the foetus is low in mammography, the radiation beam should not irradiate the abdominal area.
- If a mammogram contains the required information, repeat procedures must not be prescribed simply because the radiograph is not of the "best" diagnostic quality.
- Specialized examinations should be undertaken only by, or in close collaboration with a qualified radiologist.
- A patient's clinical records should include details of x-ray examinations carried out.
More specific guidance for the prescription of imaging examinations is available from the Canadian Association of Radiologists (CAR) in their Diagnostic Imaging Referral Guidelines (CAR 2012). These guidelines provide recommendations on the appropriateness of imaging investigations for the purpose of clinical diagnosis and management of specific clinical/diagnostic problems. The objective of these guidelines is to aid the referring physician / health care professional to select the appropriate imaging investigation and thereby reduce unnecessary imaging by eliminating imaging that is not likely to be of diagnostic assistance to a particular patient and by suggesting alternative procedures that do not use ionizing radiation but offer comparable diagnostic testing accuracy.
3.2 Guidelines for Screening Mammography
3.2 Guidelines for Screening Mammography
- Selection of population groups for mammographic screening should be based on the concept that the benefit from the program should outweigh any risks from an increase of radiation dose to the group being targeted by the program.
- Mammographic screening should not be done on pregnant or possibly pregnant women because of the consequences of foetal irradiation. While it is generally accepted that the radiation dose to the ovaries and the foetus is low in mammography, the mammographic x-ray examination should be re-scheduled at a subsequent date.
- Participants having breast implants should follow the same mammographic screening schedule as recommended for women without implants. However, for these participants, there may be a need for the use of special compression, positioning and loading techniques. The personnel of mammographic x-ray facilities must be proficient in performing such procedures. Women with breast implants may be referred to a diagnostic mammography facility.
- The number of mammographic views required in an examination must be kept to the minimum practicable, consistent with screening program objectives.
- Repeat mammographic x-ray examinations should not be prescribed only because a mammogram may not be of the "best" diagnostic quality if the mammogram contains the required diagnostic information.
- The quality of mammograms must be monitored routinely, through a quality assurance program, to ensure that they satisfy diagnostic requirements with minimal patient dose.
- For mobile mammography screening clinics, it is recommended that image processing be performed on site so that technologists can review their films and therefore reduce participant callbacks. However, since it is often difficult to stabilize film processors in mobile mammography screening clinics, additional care must be taken to ensure that image processing is optimized. In the situation where image processing cannot be optimized, batch processing at another location is acceptable.
- Previous mammograms, including baseline mammograms, from screening mammography programs should be available to the radiologist for examination.
3.3 Guidelines for Carrying Out Mammographic X-ray Examinations
Next to elimination of unnecessary x-ray examinations, the most significant factor in reducing dose is ensuring that examinations are performed with good technique. It is possible, for example, to obtain a series of diagnostically acceptable mammograms and have the organ dose vary widely due to the choice of technique and loading factors. It is the responsibility of the technologist, the medical physicist and the radiologist to be aware of this and to know how to carry out a mammographic x-ray examination with the lowest possible radiation exposure to the patient or breast screening participant.
The requirements and recommendations that follow are intended to provide guidance to the technologist, the medical physicist and radiologist in exercising their responsibility towards reduction of patient exposure.
- The mammographic x-ray system must be designed specifically for mammography and the image receptor must be compatible with the system.
- A film-screen combination that provides good quality diagnostic results must be used. Direct exposure film must never be used.
- When retrofitting an existing mammographic x-ray system with a digital image receptor (CR system), the mammographic x-ray system must be calibrated to reflect the sensitivity of the digital image receptor.
- Except in the case of mammography performed within a screening program, the technologist must not perform any examination which has not been prescribed by a health care professional responsible for the patient.
- The dose to the patient must be kept to the lowest practicable value consistent with clinical objectives, and without loss of essential diagnostic information. To achieve this, techniques appropriate to the equipment available should be used and evaluated from time to time in terms of effectiveness.
- Particular care in patient x-ray protection must be taken when mammographic x-ray examinations of pregnant or possibly pregnant women are carried out, even though the radiation dose to the abdomen and fetus are negligible during normal mammographic x-ray examinations.
- Before performing mammographic x-ray examinations, it must be determined whether or not the patient has breast implants. There may be a need to use special procedures when performing mammographic x-ray examinations of patients with breast implants such as extra views, modified positioning, compression and loading techniques. Particular care of compression techniques must be taken since excessive compression of the implants during mammographic x-ray examination may cause rupture of the implant.
- Cassettes should be loaded at least 15 minutes in advance to allow air to escape, thus improving film/screen contact.
- Care must be taken to ensure appropriate breast positioning, including attention to the height and angle of the breast support table, appropriate level of compression and ensuring that no skin folds are present.
- In mammography, it is recommended that the x-ray field normally be the full size of the image receptor, but not larger than the image receptor support, except at the chest wall. The amount of unexposed areas on the films should be minimal so to avoid the need for masking. Collimated-down views are useful in some purposes, but should be considered as a special procedure.
- The use of a thyroid shield is not recommended during mammographic examinations. The radiation dose to the thyroid is very low as it is only due to scattered radiation. In addition, the CAR advises against the use of a thyroid shield as it may obscure important anatomy, interfere with proper positioning of the breast during an examination and affect the quality of the mammographic image (CAR, 2011). Other types of shielding such as a lead apron or gonad shielding offer little extra because mammography machines are designed to ensure patient safety, incorporating internal radiation shielding, which prevents stray radiation.
- The technologist should examine the images after processing in order to verify that the techniques being used are producing diagnostic quality images and that the x-ray equipment is functioning correctly.
- Care must be taken to identify and minimize the occurrence of artefacts. Artefacts may be due to equipment performance, imaging technique, positioning, cleanliness and handling, and patient-related problems. For example, insufficient compression and overly long exposure times may result in motion artefacts. Grid artefacts may also result from longer exposures. Antiperspirant artefacts can occur when patients have applied antiperspirants or creams prior to the mammography examination. Artefacts may also be due to field inhomogeneity, defective detector elements and processing of digital images.
- Full details of the mammographic procedures, including retakes, carried out should be noted on the patient's clinical records.
- While dose limits have been defined for radiation workers and the general population, there is no specific dose level for patients undergoing diagnostic x-rays procedures. However, it is possible to establish diagnostic reference levels in mammography. Appendix III provides a description of the methodology for determining the mean glandular dose.
Section B: Facility and Equipment Requirements
1.0 Facility Requirements
1.1 General Criteria
In the planning of any medical x-ray facility it must be ensured that persons in the vicinity of the facility are not exposed to levels of radiation which surpass the current regulatory exposure limits. Appropriate steps must be taken to ensure adequate shielding is present to meet the following requirements:
- The radiation levels in controlled areas that are occupied routinely by radiation workers must be such that no radiation worker is occupationally exposed to more than 20 mSv per year; and
- The radiation levels in uncontrolled areas must be such that no person receives more than 1mSv per year.
Appendix II provides a detailed description of the regulatory dose limits. For mammography facilities, controlled areas are typically in the immediate areas where the mammographic x-ray equipment is used. The workers in these areas are primarily equipment operators such as medical radiation technologists who are trained in the proper use of the equipment and in radiation protection. Uncontrolled areas are those occupied by individuals such as patients, visitors to the facility, and employees who do not work routinely with or around radiation sources (NCRP 2004).
In general, radiation levels directly beside the image receptor of mammographic x-ray equipment are such that the above limits could be exceeded, depending on the design of the equipment, the techniques used and the total workload. However, because mammographic x-ray equipment uses low x-ray tube voltage, reduction in radiation intensity can be easily accomplished with the presence of a suitable shielding barrier between the patient and the technologist, a suitable combination of distance from the sources of radiation and shielding barriers, and restriction of persons from all areas in which the respective recommended dose limit could be exceeded.
1.2 Design and Plan of Mammography Facilities
In the early stages of designing and planning a mammography facility, three steps should be taken to ensure adequate shielding is in place to provide the necessary level of radiation protection:
- Preparation of facility plans;
- Considerations for room design and layout; and
- Determination of parameters governing shielding requirements.
1.2.1 Preparation of Facility Plan
In order to determine the shielding requirements for an x-ray facility a floor plan must be prepared, clearly identifying the following components:
- The dimensions and shape of the room where the mammography equipment is operated and the physical orientation of the room (a mark indicating north).
- The location where the mammography equipment is planned to be placed and the range of movement of the x-ray tube.
- The location of the control panel.
- The location, use, occupancy level and accessibility of adjacent rooms, as well as rooms above and below the facility.
- The designation of the adjacent rooms, whether to be designated as a controlled or uncontrolled area. Controlled areas, mainly occupied by radiation workers, are subject to the limit of 20 mSv per year, whereas uncontrolled areas, mainly occupied by non-radiation workers, are subject to the limit of 1 mSv per year. In uncontrolled areas, where radiation sensitive populations are present, such as paediatric wards, a constraint level of 0.30 mSv per year should be used.
- The location where image processing is performed, i.e., location of darkrooms, film storage area, location of CR cassettes, CR reader and computer workstations.
- The position of all windows, doors, louvers, etc., that may affect radiation protection requirements.
- The planned and existing materials used to construct the walls, floor, and ceiling, and their thicknesses including additional materials currently being used, or planned for use, as radiation shielding barriers.
- The application of the protective barriers. In mammography, the image receptor assembly acts as the primary protective barrier, therefore floor plans must indicate that the intervening shielding between the mammography equipment and the occupied area will act as secondary barriers to attenuate scattered and leakage radiation.
1.2.2 Considerations for Room Design and Layout
When designing the layout of the mammography facility, the following general recommendations must be considered.
- Mammography rooms, with stationary x-ray equipment, which can be accessed from all areas should be equipped with a self-closing door, and must be identified with warning signs incorporating the x-ray warning symbol and the words "Unauthorized Entry Prohibited". Acceptable forms of the X- ray warning symbol are given in Radiation Emitting Devices Regulations for Diagnostic X-ray Equipment.
- Mobile mammography equipment used routinely in one location must be considered as a fixed installation and the shielding needs for the equipment and room must be determined accordingly.
- The rooms containing the mammography equipment should be designed to provide adequate working space to the equipment operator and to allow for ease of patient movement.
- The mammography equipment should be positioned in the room in such a way that, during an irradiation, no one can enter the room without the knowledge of the equipment operator.
- The control panel, and the viewing window, must have shielding properties such that no radiation worker is exposed to more than 0.4 mSv/week. The ALARA principle requires that additional shielding be specified in the design to further reduce operator exposure, wherever this can reasonably be done.
- Shielding must be constructed to form an unbroken barrier and if lead is used, it should be adequately supported to prevent "creeping".
1.2.3 Determination of Parameters Governing Structural Shielding Requirements
The thickness of the shielding material, such as lead, concrete, or gypsum wallboard, required to reduce radiation levels to the recommended dose limits can be determined through calculations. In general, the radiation exposure to individuals depends primarily on the amount of radiation produced by the source, the distance between the exposed person and the source of the radiation, the amount of time that an individual spends in the irradiated area, and the amount of protective shielding between the individual and the radiation source.
Given that mammography is performed at low operating potentials, permanent mammography facilities may not need shielding in addition to the level of protection provided by typical gypsum wallboard construction. Special consideration must be given to radiation protection when using mobile or temporary mammography equipment. For all types of mammography equipment, a qualified expert must be consulted to ensure that the level of radiation safety of the facility is adequate.
The parameters listed below must be considered for the calculation of barrier thicknesses. Allowance should be made for possible future changes in any one or all of these parameters, including increases in use and occupancy factors, in operating tube voltage and workload, as well as modifications in techniques that may require ancillary equipment.
1. The maximum x-ray Workload, (W) or the workload distribution.
The workload is a measure of the operational time or the amount of use of the x-ray equipment. A workload distribution indicates the workload across a range of operating voltages. The workload and workload spectrum can be determined by recording the operating voltage and current-time product of each irradiation taken in each mammography room over a set period of time (i.e., week). If actual workload values are not available, Table 1 presents estimated total workload in a mammography facility (NCRP 2004).
| Total Workload per patient (mA min/patient) |
Typical Number of Patients (per 40 hour week) |
Total Workload per week (mA min/week) |
|||
|---|---|---|---|---|---|
| Average | Busy | Average | Busy | ||
| Mammography Room | 6.7 | 80 | 160 | 550 | 1,075 |
2. The Occupancy Factor (T)
The occupancy factor is the fraction of time that the area under consideration is occupied by the individual (employee or public) who spends the most time at that location while the x-ray equipment is operating. The following table presents recommended occupancy factors.
| Occupancy Factor | Description |
|---|---|
| T=1 | Administrative offices and receptionist areas, laboratories, pharmacies and other areas fully occupied by an individual, attended waiting rooms, children's indoor play areas, adjacent x-ray rooms, image viewing areas, nurses' stations, x-ray control rooms, living quarters. |
| T=1/2 | Rooms used for patient examinations and treatments. |
| T=1/5 | Corridors, patient rooms, staff lounges, staff rest rooms. |
| T=1/8 | Corridor doors. |
| T=1/20 | Public toilets, unattended vending areas, storage rooms, outdoor areas with seating, unattended waiting rooms, patient holding areas. |
| T=1/40 | Outdoor areas with only transient pedestrian or vehicular traffic, unattended parking lots, vehicular drop off areas (unattended), attics, stairways, unattended elevators, janitor's closets. |
3. The Use Factor (U)
The use factor is the fraction of the workload during which the x-ray beam is pointed in the direction under consideration. In mammography the image-receptor assembly acts as a primary beam stop, and therefore U=0. Only secondary radiation needs to be considered. The use factor for secondary protective barriers is always taken to be 1.
1.3 Shielding Calculations
In mammography rooms, shielding calculations must be made for secondary protective barriers. As the image receptor assembly of mammography equipment acts as a primary protective barrier, additional primary barriers are not needed. Secondary protective barriers are required to provide shielding from scattered and leakage x-rays.
Comprehensive shielding calculations for mammography facilities should only be performed by individuals with current knowledge of structural shielding design and the acceptable methods of performing these calculations. It is recommended that shielding calculations be performed using the methodology presented in the National Council on Radiation Protection and Measurements (NCRP) Report No. 147: Structural Shielding Design for Medical x-ray Imaging Facilities (NCRP 2004). However, it must be noted that the shielding design goals specified in NCRP Report 147 are not adopted in this Safety Code. The shielding design goal values may be lower but must not exceed the limits set out in section B1.1 for controlled and uncontrolled areas. Due to the extensiveness of the information, the methodology of NCRP 147, including equations, tables and figures, is not provided in this Safety Code.
The information outlined in sections B1.1 and B1.2 along with the final plans of the installation must be submitted for review by the appropriate responsible government agency. Radiological facilities that fall under provincial or territorial jurisdiction should contact the responsible agency in their respective province or territory listed in Appendix I.
1.3.1 Shielding of Radiographic Films and CR Cassettes
Film storage containers must be adequately shielded to ensure that excessive exposure of film by X- rays does not occur. Sufficient film shielding must be in place to reduce the radiation level to stored film to less than 0.1 mGy over the storage period of the film. Once films are loaded into cassettes, radiation exposure levels should be less than 0.5 µGy and the resulting increase in the base-plus-fog should be less than 0.05 O.D. Appendix IV provides guidance on the shielding requirements for storage of radiographic film. The shielding requirements presented in Appendix IV are very conservative but will protect films from radiation exposure for most circumstances. Given that CR Cassettes are used more frequently and therefore stored for shorter periods of time, the limit of 0.5 μGy is also considered to provide sufficient shielding for CR cassettes (NCRP 147). In general, CR cassettes are stored behind the transparent shield of the mammography radiological technologists. The transparent shield should have a permanent label indicating the lead equivalence of the panel.
2.0 Mammographic X-ray Equipment Requirements
2.1 Regulatory Requirements for Mammography Equipment
All new, used and refurbished mammographic x-ray equipment, and accessories for such equipment, which are sold, imported or distributed in Canada, must conform to the requirements of the Radiation Emitting Devices Act and the Food and Drugs Act and their promulgated regulations. These are the Radiation Emitting Devices Regulations and the Medical Devices Regulations. The Radiation Emitting Devices Regulations, Schedule II, Part XII (Diagnostic x-ray Equipment) sets out the requirements for information and labelling, construction and performance of mammographic x-ray equipment. The Medical Devices Regulations encompass all other safety and effectiveness considerations. It is the responsibility of the manufacturer or distributor to ensure that their equipment complies with the requirements of these regulations prior to importation and/or sale in Canada. Mammography facilities under provincial or territorial jurisdiction may be subject to requirements specified under their statutes and regulations. In addition, the Canadian Standards Association and provincial electrical utility should be consulted for further information.
The Radiation Emitting Devices Act, the Food and Drugs Act and their promulgated regulations are available on the Department of Justice online source of the consolidated Acts and regulations of Canada (http://laws-lois.justice.gc.ca/eng/acts/R-1/index.html, http://laws-lois.justice.gc.ca/eng/acts/F-27/index.html).
2.2 Equipment Purchasing
The purchase of medical imaging equipment is one of the most significant expenditures of an imaging facility. It is therefore essential to ensure that the desired design and level of performance are being obtained in a cost-effective manner. Below is an outline of the recommended process for purchasing medical imaging equipment.
2.2.1 Needs Analysis
A needs analysis must be performed to identify the type and specifications of equipment required to meet the clinical x-ray imaging needs. When performing a needs analysis, the main points which should be considered are the types of investigations that the facility intends to perform with the equipment, and the level of performance needed from the equipment. Other points which should be addressed are whether the staff of the facility possesses the expertise to use the equipment, whether adequate space is available for installation of the new equipment, and the date on which the equipment must be installed and operational at the facility. All staff members who will be routinely using the equipment should be consulted for input at this stage.
2.2.2 Equipment Specifications
Equipment specifications must be prepared with full knowledge of the clinical needs and operational conditions, as well as manufacturer's specifications, and regulatory requirements. Equipment specifications supplied to the vendor should identify the type of x-ray equipment needed and the types of clinical procedures intended to be performed with the equipment. It should also identify all system components and provide a complete description of the design, construction and performance features of each component. The level of performance should be such that most manufacturers should be able to meet these performance requirements with readily available components and product lines. All relevant requirements stated in this Safety Code and any further requirements as specified by the agency responsible for the facility should also be addressed in the equipment specifications. Any electrical, mechanical and environmental conditions which may affect the performance of the equipment should also be included.
The equipment specifications should also include other relevant information such as the details concerning the equipment installation and calibration by the vendor and the associated deadlines, the type of warranty and service plan needed, and whether training of staff is required from the manufacturer. In general, the equipment specifications must identify all criteria which must be met for acceptance of the equipment.
Testing equipment required to perform daily to monthly quality control procedures, which are not already available, must be purchased at the same time as the x-ray unit.
2.2.3 Analysis of Vendor Quotation and the Purchase Contract
Vendor quotations must be thoroughly reviewed to ensure that the vendor supplied equipment specifications address the identified needs of the facility. The vendor's quotation should include the installation and calibration of the equipment, warrantees, delivery time, maintenance plans, quality control testing equipment, staff training and all other criteria included in the purchaser's equipment specifications.
The purchase contract should set out all items and conditions of the purchase specified in the equipment specifications and vendor's quotation which have been agreed upon by the purchaser and vendor. All conditions for acceptance of the equipment must be clearly specified, as well as, action to be taken if conditions for acceptance are not met. A detailed and concise purchase contract will ensure the delivery of equipment in a timely and cost-effective manner.
2.2.4 Acceptance Testing
Acceptance testing must be performed prior to any clinical use of the equipment. Acceptance testing is a process to verify compliance with the performance specifications of the x-ray equipment as written in the purchase contract. It must also verify that the equipment performance meets the manufacturer's specifications and complies with federal and provincial or territorial regulations. Acceptance testing must be performed by, or under the supervision of, a medical physicist, with in-depth knowledge of the particular type of x-ray equipment and the relevant regulations. This individual must be independent of the manufacturer.
Acceptance testing of a medical x-ray system includes several major steps: They are:
- the verification that delivered components or systems correspond to what was ordered;
- the verification of the system mechanical integrity and stability, including safety mechanisms, automatic patient release, power drives, interlocks;
- the verification that appropriate inspections of electrical installations have been carried out, including electrical safety and line power fluctuation;
- the verification of x-ray performance; and
- the verification of imaging or diagnostic performance.
More detailed information on acceptance testing of mammographic x-ray equipment is available in publications from the International Electrotechnical Commission (IEC 2007).
X-ray performance tests carried out during the acceptance testing should also reflect the requirements described in subsection B2.5. The results from the acceptance testing should be used to establish baseline values and limits of acceptance on operational performance of the x-ray equipment. These baseline values and limits are essential to the quality assurance program.
2.3 Existing Mammography Equipment
Whenever possible, existing mammography equipment should be upgraded to incorporate as many as possible of the safety and performance features required of new medical x-ray equipment, as specified in the Radiation Emitting Devices Regulations, in effect at the time. It should be noted that it is a requirement of the Radiation Emitting Devices Act that replacements for any component or subassembly of mammography equipment, for which a construction or performance standard has been specified in the regulations, must comply with the standards in effect at the time of replacement. In addition, the upgraded components and/or software must be licensed by Health Canada. Information on licensing of mammography equipment, accessories or software is available from the Therapeutic Products Directorate, Medical Devices Bureau of Health Canada (contact information available in Appendix I). The owner of a mammography facility must ensure that any upgrades/changes to the equipment or software meet all applicable federal, provincial and territorial requirements. Any changes/upgrades of equipment affecting image quality and/or radiation dose must undergo acceptance testing by a medical physicist.
2.4 Retrofitting with Digital Imaging Systems
When retrofitting a digital image receptor ( ex. CR system) onto a new or existing mammography system, the owner of the facility must ensure that the digital image receptor meets the requirements of the Radiation Emitting Devices Act and Regulations, as well as the Food and Drugs Act and the Medical Devices Regulations. Furthermore, the mammography system, onto which the digital image receptor is fitted, must meet the requirements of Part XII of the Radiation Emitting Devices Regulations in effect at that time. Digital image receptors must only be installed on x-ray systems which have an automatic exposure control. The system must be calibrated to reflect the sensitivity of the digital image receptor.
2.5 Detailed Mammographic x-ray Equipment Requirements
Mammography must only be performed using radiographic equipment designed specifically for mammography. Radiographic equipment designed for general purpose or special nonmammography procedures are prohibited from use in mammography. This prohibition includes systems that have been modified or equipped with special attachments for mammography.
The information, labelling, construction and function requirements set out in this section are based upon the current Radiation Emitting Devices Regulations, Part XII (Diagnostic X-ray Equipment) and the standard of the International Electrotechnical Commission (IEC), 60601-2-45 (IEC 2011). These requirements are applicable to manufacturers of all new mammography equipment and are presented here in an effort to promote awareness of equipment standards for individuals involved in the acquisition of new mammography equipment. The Radiation Emitting Devices Regulations and the IEC standards applicable to mammography equipment should be referred to for complete and detailed information on equipment standards.
2.5.1 Equipment Information Requirements
- Mammographic x-ray equipment must be accompanied by the following information, which must be provided by the manufacturer:
- installation instructions;
- the address of the manufacturer;
- instructions concerning any radiological safety procedures and additional precautions that are necessary because of unique features of the equipment;
- maintenance instructions necessary to keep the equipment in compliance with Part XII of the Radiation Emitting Devices Regulations;
- quality control procedures to be performed on the equipment, including
- the frequency of performing tests, and
- the acceptance criteria;
- the minimum level of performance required of other equipment necessary to present the images acquired by the equipment for diagnostic purposes;
- for equipment with an integrated digital x-ray image receptor,
- identification of the version of image processing applied to original data, and
- a description of the file transfer format of the images acquired with the unit and of any data associated with the images;
- the instructions for use, including
- the method of inspection and safe use of all compression plates that are provided with the mammographic x-ray equipment,
- the methods for determining and resolving problems with artefacts,
- for equipment with an integrated image receptor,
- the particular handling and maintenance of the x-ray image receptor,
- the procedure for performing quality control of the x-ray image receptor,
- the requirements for image presentation,
- the method for determining and identifying defective detector elements present in the digital x-ray image receptor,
- the method for determining the level of performance of the image receptor following replacement of data originating from defective detector elements, and
- the method for determining whether the level of image homogeneity is acceptable;
- the rated line voltage, the maximum line current and the line voltage regulation for operation of the equipment at the maximum line current;
- the loading factors that constitute the maximum line current condition for the x-ray generator;
- for each x-ray tube assembly, the nominal focal spot sizes and the method of their determination, the cooling curves for the anode and for the x-ray tube housing, the x-ray tube rating charts, and the method by which the focal spot to image receptor distance can be determined;
- the duty cycles, rectification type and the generator rating;
- if the equipment is battery powered, the minimum state of charge necessary for it to operate;
- the operating range of x-ray tube voltages and the maximum deviation for any selected x-ray tube voltage with the range of values;
- if the equipment is not operated exclusively under automatic exposure control mode, the accuracy limits of the controlling timer, the x-ray tube current, and the current time product;
- where the equipment is operated under automatic exposure control, the accuracy limits of that control; and
- the conditions under which the information provided under items (n) to (p) are valid.
2.5.2 Equipment Labeling Requirements
- Mammographic x-ray equipment must display the following information in a manner that is legible, permanent and visible on the specified surfaces:
- on the external surface of the main control panel
- a statement prohibiting unauthorized use and warning that hazardous x-rays are emitted when the equipment is in operation, and
- the x-ray warning symbol, which shall be displayed in two contrasting colours, be clearly visible and identifiable from a distance of 1 m, be at least 2cm high and at least 2 cm wide, bear the words "CAUTION: X-RAYS - ATTENTION: RAYON X", and conform to one of the following diagrams,

- with respect to the x-ray generator, the name of the manufacturer, the model designation, the serial number, the date of manufacture, and the country of manufacture;
- on the external surface of the x-ray tube housing, a visually accessible label indicating, with respect to the x-ray tube assembly, the name of the manufacturer, the model designation, the serial number, the date of installation of the x-ray tube in the x-ray tube housing, the country of manufacture, and the minimum permanent inherent filtration of the x-ray beam emitted from the x-ray tube assembly, expressed in millimetres of aluminium equivalent at a specified x-ray tube voltage;
- on the external surface of the x-ray tube housing, or another suitable structure permanently attached to the x-ray tube housing, an indicator that enables the focal spot to image receptor distance to be determined to within 2 percent of that distance, and if the x-ray tube and the x-ray generator are not located within a common enclosure, marks that clearly indicate the anode and cathode terminals on the x-ray tube housing and on the high-voltage generator; and
- on the external surface of any beam limiting device that adds filtration to the x-ray beam, the total permanent filtration deliverable by the beam limiting device, expressed in millimetres of aluminium equivalent at a specified x-ray tube voltage.
- on the external surface of the main control panel
- All controls, meters, warning lights and other indicators required by Part XII of the Radiation Emitting Devices Regulations must be clearly labelled as to their function.
- Mammography x-ray equipment must have:
- a means, appropriate to its rectification type, to compensate for variation in x-ray tube voltage caused by line voltage fluctuations;
- a visual indicator or audible indicator that warns the operator when the variation in line voltage exceeds the specified limit or a mechanism that, in that event, prevents x-rays from being emitted;
- on the control panel
- a warning light that indicates when the equipment is ready to be energized,
- a second warning light that indicates when x-rays are being emitted,
- a visual indicator showing when the automatic exposure control mode is selected, and
- if the automatic exposure control mode is not selected, controls and visual indicators that enable the operator to select the loading factors before an irradiation;
- a mechanism to initiate and terminate an irradiation;
- an audible signal to indicate the termination of an irradiation;
- a beam limiting device;
- an automatic exposure control; and
- for equipment that operates within the range set out in column 1 of Table 3, radiation filters that result in a measured half-value layer of aluminium, without the compression paddle in place, of not less than
- for each x-ray tube voltage set out in column 2, the corresponding value set out in column 3 of that item, or
- for any other case, the half-value layer obtained by the following formula:

Formula for calculation of the minimum half-value layer of aluminum, measured without the compression paddle in place
The minimum half-value layer of aluminum, measurement without the compression paddle in place, calculated by the equation "HVL (mm of Aluminum) greater than or equal to, X-ray Tube Voltage (kV) divided by 100".
| Column 1 Operating Range for Normal Use (kV) |
Column 2 X-ray Tube Voltage (kV) |
Column 3 Half-Value Layer of Aluminium (mm) |
|---|---|---|
| 50 or less | (a) 30 | 0.30 |
| (b) 40 | 0.40 | |
| (c) 50 | 0.50 |
2. An irradiation switch of mammography equipment must permit the emission of x-rays only when the operator exerts continuous pressure on the switch.
3. Controlling timers of mammography equipment must:
- automatically terminate an irradiation on completion of a preset irradiation time, on attainment of a preset current time product value, or on completion of a present number of x-ray pulses;
- permit the operator to terminate an irradiation at any time;
- automatically reset itself to its original setting or to zero on termination of an irradiation; and
- prevent the initiation of irradiation when the timer is set at zero, at the "off" position or at an unmarked setting.
4. When a support table (including all layers, excluding the grid) is positioned between the patient and the x-ray image receptor, the aluminium equivalence of the support table shall not exceed 0.3 mm, as determined using an x-ray beam that
- is generated at an x-ray tube voltage of 30 kV;
- has a maximum x-ray tube voltage ripple of 10 percent;
- has a half-value layer of aluminium of 0.3 mm; and
Any sensor used in automatic exposure control is considered to be part of the x-ray image receptor.
5. For mammography x-ray equipment,
- the x-ray tube must be securely affixed to and aligned within the x-ray tube housing;
- the radiation filters must be securely affixed to the exit port of the x-ray tube housing or beam limiting device, or both; and
- the x-ray source assembly must maintain its required position or movement without drift or vibration during operation.
6. Mammography equipment that is equipment with automatic exposure control must have
- a means to automatically terminate the irradiation when the current time product exceeds 800 mAs per irradiation; and
- when an irradiation under automatic exposure control terminates because the limits specified in paragraph (a) have been reached, a visual indicator or audible signal that warns the operator of the termination, and a reset control that must be activated manually before another irradiation under automatic exposure control can be made.
7. Mammography equipment must have
- an image receptor supporting device equipped with protective shielding that limits the residual radiation, extending to the patient's chest wall, and at every other edge, extending beyond the X-ray field by at least one percent of the focal spot to image receptor distance;
- a patient support designed such that, in non magnification mode, the maximum distance between the edge of the image reception area that is adjacent to the chest wall of the patient and the adjacent edge of the patient support, when projected on the patient support, is smaller than 5 mm;
- a beam limiting device that limits the size of the X-ray beam such that, in non-magnification mode the X-ray field:
- must not extend more than 2 mm beyond the edge of the patient support that is designed to be adjacent to the chest wall of the patient,
- for non-scanning mammography equipment, must extend beyond the edge of the effective image reception area that is designed to be adjacent to the chest wall of the patient, and
- for non-scanning mammography equipment, must not extend by more than 2% of the direct focal distance beyond all other edges of the effective image reception area;
- a breast compression device that:
- is foot-actuated to start and control the compression,
- permits fine adjustment of motion during the compression,
- permits rapid decompression,
- can be actioned by a means for hands-free (e.g. foot) control of the power-driven compression accessible from both sides of the position of the patient,
- has compression plates that are transparent so that the skin of the patient remains visible when in contact with them, and
- allows the portion of the compression plate in contact with the breast to be brought to within 10mm of the surface of the patient support;
- in situations where there is loss of power, the compression is maintained during biopsy or marking operations but means for manual decompression must be included in the design of the equipment
- an x-ray field indicator that uses light to visually define the x-ray field so that the limits of the x-ray field are visible under the ambient lighting conditions in an x-ray room; and
- a means by which the operator may determine the focal spot to image receptor distance to within 2 percent of that distance.
8. Mammography equipment with a removable fixed-aperture beam limiting device must display on its external surface the dimensions of the image reception area and the focal spot to image receptor distance at which the beam limiting device must be used.
2.5.4 Functioning Requirements
1. Radiation Output Reproducibility
For any combination of x-ray tube voltage, x-ray tube current and irradiation time, or for any selected exposure to the x-ray image receptor, when the line voltage for each measurement is accurate to within one percent of the mean line voltage value of all measurements, and when all variable controls for the loading factors are adjusted to alternate settings and reset to the test setting before each measurement,
- the coefficient of variation of any 10 consecutive air kerma or exposure measurements, taken at the same point along the x-ray beam axis, 4 cm above the patient support, within a period of one hour, must be no greater than 0.05; and
- each of the 10 air kerma or exposure measurements taken under paragraph (a) must be within 15 percent of the mean value of those measurements.
2. Accuracy of Loading Factors
The loading factors set out in column 1 of an item of Table 4 must not deviate from the selected value, for any combination of loading factors, by more than the quantity set out in column 2 of that item.
| Item | Column 1 | Column 2 |
|---|---|---|
| Loading Factor | Maximum Deviation from the Selected Value | |
| 1. | X-ray tube voltage | ±5% |
| 2. | Irradiation time | ±(10% plus 1 ms) |
| 3. | X-ray tube current | ±20% |
| 4. | Current time product | ±(10% plus 0.2 mAs) |
3. Controlling Timer and Automatic Exposure Control
- For mammographic x-ray equipment without an integrated x-ray image receptor, if the automatic exposure control is selected, the variation in optical density set out in subsection (b) must be determined using objects that are made of human-tissue equivalent material and have thicknesses that are representative of the actual range of the body thicknesses of the patients.
- For mammographic x-ray equipment with a film-screen x-ray image receptor, the automatic exposure control device shall perform in such a way that the variation of optical density in the resultant radiograms does not exceed the value of ±0.15 of the mean optical density when the thickness of a breast tissue equivalent material is varied over a range of 2 to 7 cm and the tube voltage and anode filter combinations are varied appropriately for such thickness. If this requirement cannot be met, a technique chart shall be developed showing appropriate loading factors for different breast thicknesses and combinations that must be used so that optical density within ±0.15 of the average under automatic exposure control conditions can be produced.
- For mammographic x-ray equipment with an integrated x-ray image receptor, the performance of the automatic exposure control must be evaluated by jointly assessing image quality, as measured by the contrast to noise ratio in specified conditions, and the dose to the patient, as characterized by the mean glandular dose, and comparing the results to the specifications provided by the manufacturer.
- For mammographic x-ray equipment with an integrated digital x-ray image receptor, the reproducibility of the automatic exposure control must be evaluated by repeatedly imaging a phantom in specified conditions, measuring the variation of one of the following quantities:
- Tube loading (mAs),
- Air kerma in a fixed position between the x-ray source assembly and the x-ray image receptor, or
- The average value of linearised pixel values in a region of interest of the image of the phantom,
Measured value of the selected quantity must not differ by more than ±15% of the mean value of the test loadings or, as appropriate, the manufacturer specification.
4. Radiation Output Linearity
Formula for the calculation of linearity
Linearity, calculated using the equation "absolute value of, open parenthesis, of X subscript 1 minus X subscript 2, closed parentheses, less than or equal to 0.1 multiplied by, open parentheses, X subscript 1 plus X subscript 2, closed parentheses".
- For a selected value of x-ray tube voltage determined in accordance with subsection (b), the quotients of the average air kerma or exposure measurement divided by the indicated current time product, obtained at the applicable settings specified in subsection (c), must not differ by more than 0.10 times their sum as determined by the formula:
where X1 and X2 are the quotients of the average air kerma or exposure measurement divided by the current time product;
- The x-ray tube voltage referred to in subsection (a) must be 30 kV or at the nearest x-ray tube voltage setting available specified for the mammographic x-ray equipment by the manufacturer; and
- The quotients referred to in subsection (a) must be determined at two x-ray current time product settings, over the whole range of current time products selections available, such that:
- The lower value of the first pair shall correspond to the lowest available current time product setting,
- The ratio of the values of the selected current time product settings in each pair shall be as close as possible to 2, but not exceeding 2,
- The higher value of the current time product settings in each pair to be measured shall be used as the lower value of the next pair of current time product settings, and
- The higher value of the last pair shall correspond to the highest available current time product setting and the lower value shall be half or next of half of the value corresponding to the highest available current time product setting.
5. Dosimetric Indications
For all mammographic x-ray equipment with an integrated digital x-ray image receptor, the mean glandular dose must be indicated for each acquired image.
6. Residual Radiation behind Image Receptor
For mammographic x-ray equipment, the residual radiation behind the image receptor supporting device must not exceed an air kerma measurement of 1.0 µGy or an exposure measurement of 0.115 mR per irradiation when the equipment is operated at
- its maximum x-ray field and the minimum focal spot to image receptor distance; and
- its maximum x-ray tube voltage and maximum available current time product.
The air kerma or exposure measurement must be averaged over a detection area that is 100cm2, of which no linear dimensions is greater than 20cm, centred at 5 cm from any accessible surface beyond the image receptor supporting device.
7. Minimum Radiation Output Rate
Mammography equipment without an integrated digital x-ray image receptor must have a minimum rate of radiation output of 7.0 mGy/s or 802 mR/s when the equipment is operated,
- with a molybdenum anode and molybdenum filter;
- without the breast compression device in place between the source and the detector;
- at an x-ray tube voltage of 28 kV in standard mammography mode at any focal spot to image receptor distance; and
- the minimum rate of radiation output must be:
- measured at a position that is 4.0 cm above the patient support and 6.0 cm from the chest wall side on the centre line, and
- averaged over a period of irradiation of 3.0 s.
8. Leakage Radiation in the Loading State
The leakage radiation from the x-ray source assembly of mammographic x-ray equipment must not exceed an air kerma rate of 1.0 mGy/h or an exposure rate of 115 mR/h when the assembly is operated at the nominal x-ray tube conditions of loading that correspond to the maximum specified energy input in one hour.
The rate must be averaged over a detection area of 100 cm2, of which no linear dimension is greater than 20 cm, that is centred at 1 m from the focal spot.
9. Leakage Radiation when not in the Loading State
If high voltage can appear across the x-ray tube of mammographic x-ray equipment, then the radiation emitting from the x-ray source assembly of the equipment must not exceed an air kerma rate of 20.0 µGy/h or an exposure rate of 2.3 mR/h when the equipment is operated with its beam limiting device fully open and the automatic exposure control or the irradiation switch has not been activated.
The rate must be averaged over a detection area of 10 cm2, of which no linear dimension is greater than 5 cm, that is centred at 5 cm from any accessible surface of the x-ray source assembly.
10. Radiation from Other Components
Under any operating condition, the radiation from any component of mammographic x-ray equipment, other than the x-ray source assembly, must not exceed an air kerma rate of 20 µGy/h or an exposure rate of 2.3 mR/h.
The rate must be averaged over a detection area of 10 cm2, of which no linear dimension is greater than 5 cm, that is centred at 5 cm from any accessible surface of the component.
3.0 Image Processing Systems
Image processing includes both film and digital processing of radiological images. Film processing systems have been extensively used in the past. Recently with advances in digital technology, digital image processing systems are being used in many radiological facilities. No matter the type of system used, optimization of image quality at an acceptable dose to the patient is a priority for radiological facilities. This is achieved by ensuring image processing is an integral component of the facility's quality assurance program.
3.1 Film-Screen Mammography Systems
The ability to produce a mammogram of satisfactory diagnostic quality at an acceptable dose to the patient depends on the technique used when performing the examination, the appropriate selection of loading factors, the film-screen employed, the handling and processing of the film, and on the conditions of viewing the image. Good image quality requires proper darkroom techniques, routine processor quality control monitoring, and careful adherence to film and processor manufacturers' instructions.
3.1.1 X-ray Film
X-ray films are sensitive to light, heat, humidity, chemical contamination, mechanical stress and X-radiation. Unexposed film must be stored in such manners that it is protected from stray radiation, chemical fumes and light. The level of optical density from the base material and film fog from all causes must not be greater than 0.30 O.D. Generally, x-ray films should be stored on edge, in an area away from chemical fumes, at temperatures in the range of 10°C to 21°C and humidity between 30% and 60%. The film manufacturers' instructions must be followed. Sealed film packages must be allowed to reach room temperature before opening to prevent condensation on the films. Loaded cassettes must be stored in an area shielded from exposure to radiation. Radiation exposures to stored film must be limited to 0.1 mGy and, for loaded cassettes, to 0.5 µGy. This area is usually in or near the x-ray room. The location of loaded and unexposed cassettes must be clearly marked. The area should be large enough to accommodate the required supply of cassettes needed during the operation of the facility.
3.1.2 Cassette and Screen
Cassettes or screens in poor conditions will impair diagnostic quality. Problems are caused by dirty or damaged screens, warped cassettes, fatigue of foam compression material or closure mechanism, light leaks, and poor film-screen contact. Cassettes should be checked regularly for wear and cleanliness and any damaged cassettes must be replaced. Manufacturers' recommended screen cleaner should be used. To avoid artefacts caused by dirt and dust, the intensifying screens and cassettes must be cleaned at least monthly. The intensifying screens must be inspected with an ultraviolet light to find dust particles. Cleaning tools include a screen cleaner with antistatic solution, lint-free cloths, compressed air, and a camel hair brush. Cassettes and screens must be numbered for identification and matching, both inside the cassette and on the outside of the cassette.
3.1.3 Darkroom
With the exception of daylight automatic image processors not requiring darkrooms, automatic film processors require properly designed darkrooms. While specific details may vary from installation to installation, all darkrooms must include certain basic features listed below. Detailed information on the design of mammography darkrooms is provided by the International Atomic Energy Agency (IAEA, 2009).
- The room must be light-tight. Particular attention must be paid to the door seal and the mounting of the film processor if the film insertion to the processor is done through a wall. The darkroom should incorporate a lockable door or double doors to ensure light-tightness when undeveloped films are being handled. A film strip exposed to an optical density of 1.2 units must not show an increase in optical density greater than 0.05 units in two minutes exposure to the darkroom light environment;
- If the darkroom is adjacent to a radiographic room, the film storage container must be adequately shielded to ensure that excessive exposure of film by x-rays does not occur. Sufficient film shielding must be in place to reduce the radiation level to the film to 0.1 mGy and to the loaded cassettes to 0.5 µGy;
- A warning light should be located outside the darkroom, at the entrance, to indicate when the room is in use. The warning light is not required if the door is locked when it is closed.
- Safelights, fitted with bulbs of intensity not greater than 15 watts, must be provided above the work areas inside the darkroom. The safelight must have filters appropriate to the specifications of the film used and must be positioned at distances greater than 1 metre from work areas to minimize film fogging;
- The processor should be directly exhausted to the outside of the facility. The processor exhaust fan should be operated continuously, even when the processor is turned off. This prevents damage to the processor such as build up of evaporated processing solutions and corrosion of components; and
- The darkroom should be under positive pressure so that dust is not sucked into the room when the door is opened. The number of air changes must be high enough for the processor to operate properly and not create a hazardous situation for personnel.
Cleanliness in the darkroom and of the screens and cassettes is essential. It is important to maintain the cleanest environment possible in order to minimize any artefacts caused by dirt, dust, or improper handling of film. An ultraviolet light should be used to find dust areas around the darkroom. Eating or drinking in the darkroom area must not be permitted. All working surfaces, tops of counters and the floor should be cleaned regularly, at least once a day. Tops of cabinets, vents, light fixtures and any other areas which can collect dust should be cleaned on a regular basis. The ventilation system should be checked to make sure that no dust is carried from it to the inside of the darkroom; any filter should be changed on a regular basis. Except for in an auto-mixer, chemicals should not be mixed inside the darkroom since this operation can result in chemical splashes onto the equipment or working surfaces. Personnel should wear personal protection devices (gloves, masks, etc.) when handling chemicals.
To avoid putting fingerprints on the film and to avoid dirtying the screens, it is important to wash hands frequently with soap that does not leave any residue. Hand lotions and creams may also result in fingerprints on films. Clutter which may collect dust should be eliminated. Corrugated cardboard boxes containing film boxes, chemicals, and other supplies should not be stored or opened inside the darkroom as they will create a lot of dust. The boxes should be opened outside the darkroom, and films and supplies carried inside. Any articles of clothing made of loose fibres or which are static generating, such as wool, silk, some cottons or cotton blend fabrics, should not be worn in the darkroom or should be covered with a laboratory coat.
3.1.4 Film Processing
Improper or careless processing of exposed radiographic films can result in films of poor diagnostic image quality and consequently increase the possibility of wrong diagnosis or requests for repeat x-ray examinations. To achieve full development, the film must be processed in chemically fresh developer, at the correct temperature and for sufficient time to ensure that the silver in exposed silver halide crystals in the film emulsion is completely reduced. If this is not done, the blackening of the film will not be optimum and the tendency will be to increase radiation exposure to achieve proper image density.
Other factors can also affect the quality of the processed film. These include cleanliness of the processing system, film immersion time, and the efficiency of the rinsing. To ensure proper processing of films certain basic procedures must be followed:
- The only acceptable method to monitor the operation of an automated image processor is with the use of a sensitometer to produce repeatable light exposure of the film and with the use of a densitometer to monitor the processed sensitometric film. Processor monitoring must be done each operational day, before processeing patient radiographs, when the processor is started and has stabilized, and at additional times after the processor has been cleaned, or after fresh chemicals have been added.
- Manufacturers' instructions with respect to strength of solution, temperature and time must be followed to ensure optimum development.
- Developing solutions must be replenished as necessary and must be changed or recycled regularly, as required. This should be done often enough to avoid oxidation of the developing solutions. Even unused developer deteriorates with time. Processing chemicals must be protected from freezing. Manufacturers' instructions must be followed in storing chemicals to avoid oxidation. Any chemicals showing signs of oxidation or sedimentation must not be used.
- Fixer must be adequately removed from the processed films. Manufacturers' instructions for film wash must be followed. Fixer retention tests must be done on a regular basis. The fixer is responsible for stopping the development process by removing silver halide crystals remaining on the film. Insufficient washing of films for the removal of fixer will result in staining of films and compromise film storage time.
- Cleanliness is extremely important for reducing film artefacts. The film transport mechanisms of film processors must be cleaned frequently. Abrasive cloths or cleaners should never be used on processors.
- Film processors must be maintained regularly, according to manufacturers' instructions. The accuracy of the processor thermometer should be checked regularly against a non-mercury thermometer. The digital processor thermometer should be accurate to within 0.5°C.
- When film processing volume is less than 50 films per day, it may not be possible to adequately control chemical concentrations and activity. In this situation, flood replenishment should be used to better control chemical concentrations.
- When film processing volume is at least 50 films per day, a volume replenishment system is generally used which replenishes processing solutions each time a film is fed into the processor. Manufacturers' specifications for replenishment of processor solutions should be followed.
X-ray film processing generates silver containing wastes. Silver containing chemicals must not be disposed of directly into the sewer system. These chemicals must be collected and released to the appropriate waste management agency for disposal and/or recycling. The management of silver containing waste must be carried out in accordance to provincial and municipal requirements.
3.1.5 Viewbox
The conditions of viewboxes must be checked regularly along with the conditions under which radiologists and other health care professionals examine mammograms since this may influence diagnostic accuracy. Problems with improper illumination due to the non-uniformity of fluorescent tubes or degradation and discolouration of the viewing surface must be corrected.
3.2 Digital Mammography Systems
An increasing number of Canadian mammography facilities are transitioning from film-screen mammography to digital mammography. Various digital mammography systems are available using different types of detector technologies to produce the digital images. In general, digital mammography equipment are categorized into two groups: Computed Radiography (CR) systems or Digital Radiography (DR) systems.
- CR systems consist of a cassette, a CR imaging plate, which contains a photostimulable storage phosphor, and an imaging plate reader. The CR cassette, loaded with an imaging plate, is positioned in the mammography system, as it is done with film cassettes. Upon X-ray exposure, the imaging plate stores the latent image. The imaging plate is then read and a digital image is produced.
- DR mammography systems are constructed with an integrated digital X-ray detector. There are three main types of integrated digital x-ray detectors used in mammography systems. These are flat plate CsI on a photodiode array, flat plate amorphous selenium on an electrode array and slot scanning photon counting detectors (IAEA, 2011). In DR systems, the electronic signal carrying the image information is directly processed and displayed almost instantaneously.
Quality control testing of digital image systems is essential. Verification of the proper functioning of the x-ray imaging equipment along with appropriate selection of technique and loading factors remains essential for obtaining a satisfactory image at a minimal dose to the patient. For digital systems, specific quality control testing must also be performed on the image acquisition, storage, communication and display systems. In section C of this Safety Code, general quality control tests have been included for digital imaging systems. In addition to these tests, all equipment-specific, manufacturer-specified tests must also be performed. Facilities under provincial or territorial jurisdiction may be subject to other testing requirements.
3.2.1 Computed Radiography Imaging Plates
Computed radiography (CR) imaging plates are reusable and can be exposed, read and erased repeatedly. For this reason, it is necessary to evaluate the conditions of imaging plates on a regular basis. With normal use, the accumulation of dust, dirt, scratches and cracks may reduce image quality. Exposure to chemical agents, such as non-approved imaging plate cleaners, handling with dirty or wet hands or contact with hand lotions are all possible causes of imaging plate damage. It is recommended that a log book be maintained to track the physical conditions of all imaging plates and cassette assemblies. The cleaning frequency depends on patient volume, plate handling, and the frequency at which artefacts are perceived. A weekly visual inspection for dust and dirt must be performed. The imaging plates must be cleaned monthly following manufacturer recommended procedures and using manufacturer recommended cleaners. Cleaner must not be poured directly onto the plates as this may cause staining.
3.2.2 CR Cassette
Under normal conditions of use, dust and dirt can accumulate on cassettes. It is recommended that a log book be maintained to track the physical conditions of all cassettes. In general, a weekly visual inspection for dust and dirt is recommended and monthly cleaning of CR cassettes following manufacturer recommended procedures and using manufacturer recommended cleaners. The outside of the cassette can easily be cleaned with water and soap or a non-aggressive cleaner. The inside must not be cleaned with soap and water, since soap residue may be left on the protective coating after cleaning.
When not in use, CR cassettes, loaded with an imaging plate, must be stored in a location such that the level of radiation exposures is limited to 0.5 µGy.
3.2.3 Breast Tomosynthesis
Digital breast tomosynthesis (DBT) is a new three dimensional (3D) breast imaging technique. DBT equipment acquire multiple projection images of a breast over a range of tomographic angles. The number of images acquired and the tomographic angle varies between manufacturers of DBT equipment. Reconstruction algorithms are then used to produce images of tomographic planes through the breast. Typically plane thicknesses range from 0.5 to 3mm. Images can be viewed on a display monitor of individual planes or of sequential scrolls over all slices through the breast.
DBT has the potential to improve visualization of breast tissues by overcoming limitations of current mammographic techniques resulting from overlapping breast tissue into a single two dimensional image. The dose delivered to the patient during one tomosynthesis scan is comparable to one mammographic exam (Gennaro 2010). The potential benefits of DBT include improvement in screening sensitivity, improvement in lesion size at detection, improvement in characterization, and decrease in recall rates ( Helvie 2010).
For DBT equipment, quality assurance and quality control procedures as well as dosimetry must be performed in accordance with the recommendation of the manufacturer.
3.2.4 Mammography Review Workstation/Electronic Display Devices
In order to realize the full potential of digital mammography it is important to ensure that the electronic display devices on which mammograms are viewed for interpretation provide optimal visualization of breast tissues. The mammography review workstation must meet the following minimum specifications (AAPM 2005, CAR MAP 2011, Van Ongeval 2010):
- There must be at least two monochrome monitors with a minimum spatial resolution of 5 megapixels.
- Display devices must be compliant with the IHE Mammography Image Profile.
- A minimum luminance of 250 cd/m2 is required. (Luminance of 450 cd/m2 is recommended).
- The ratio of the maximum to the minimum luminance must be between 250 and 650 (including ambient light).
- An 8-bit minimum luminance resolution is required.
- The display noise must be as low as possible (preferably 2 - 2.5%).
- Displays must be calibrated using the DICOM greyscale standard display function (NEMA 2011).
A monochrome monitor with a minimum spatial resolution of 3 megapixels should be used for technologist or other health care professional for mammography consultation.
It is important to ensure that display devices undergo acceptance testing and their ongoing performance is verified through routine and annual quality control testing. Detailed information on acceptance and quality control testing of display devices is available from the American Association of Physicists in Medicine (AAPM, 2005). The cleanliness of the display surface must be maintained. Manufacturer recommended cleaners and cleaning procedures must be followed. The performance of the display must be verified using test patterns designed for evaluating various characteristics of display performance. An overall assessment should be made daily prior to clinical use. A weekly visual evaluation must be performed by the technologists and a detailed annual evaluation must be performed by a medical physicist. Section C of this document provides a description of these quality control tests. Attention must be given to reading room viewing conditions when performing quality control tests of display monitors.
3.2.5 Picture Archiving and Communications Systems (PACS)
In digital imaging, a system must be in place to manage patient images so that secure storage and timely retrieval of images is possible. A Picture Archiving and Communications System (PACS) is one such system which is widely used in radiology. A PACS in an imaging facility connects digital image acquisition devices with systems which can store, retrieve and display digital images within and outside the facility. The transition to PACS requires a significant amount of planning, time and resources. Once established, a PACS offers a number of advantages such as improved productivity, widespread, simultaneous access to images and image manipulation. However, attention must be given to ensure that the quality of patient images is maintained and that patient information is not lost or unintentionally altered. Such situations can lead to repeat radiological examinations and misdiagnoses of patients.
3.2.6 PACS Implementation
When deciding whether to implement a PACS, a number of key issues should be addressed. A PACS is a very high capital investment. It requires resources for hardware, software and additional staff such as a PACS administrator and any consultants which may be necessary. Early in the planning stages of a PACS, parties should be consulted from all areas which will be affected by the changes. This should include departmental administrators, PACS specialists, medical physicists, radiologists, technologists, referring physicians and any existing information technology (IT) staff. The information obtained during the consultation should be used to perform an intensive cost/benefit analysis prior to making a decision. Early consulting with all involved parties will facilitate the clinical acceptance of the system. When deciding upon the specifications of a PACS the following key components should be considered.
- Insist on Digital Imaging and Communications in Medicine (DICOM) compliance and require conformance with all necessary capabilities of the equipment. The necessary capabilities depend on the types of equipment which will be connected with the PACS. These capabilities will ultimately translate into a list of Service-Object Pair (SOP) classes and Unique Identifiers (UIDs) to be supported.
- Ensure that all systems can be integrated, based on the desired design of the system architecture. The systems to consider include the Hospital Information System (HIS), the Radiology Information System (RIS), the PACS, image acquisition equipment, printers, and any reporting systems. To ensure ease of integration between systems, equipment should support the applicable profiles of the "Integrating the Healthcare Enterprise" (IHE) Radiology Technical Framework. The IHE is an initiative to promote and support the integration of information systems in the healthcare enterprise to improve the workflow by facilitating communication between systems from different vendors. Information being transferred from one system to another will remove the need to re-enter information independently into each system and thus avoid inconsistencies, redundancies and unavailability of the data (refer to section 3.2.7). When purchasing systems it is important to specify conformance with appropriate IHE profiles.
- Security of patient information must be a priority. Only authorized individuals must be able to access patient data and images. Security measures must be established to control access to patient information as well as to track all activities which are performed on the data. This includes monitoring who accesses information, when the information is accessed, and what changes are made to the information. Authorized system users must understand the importance of keeping system passwords confidential.
- Automated features should be included in the design of the system to facilitate more rapid workflow. For example, a feature should be available which pre-fetches prior studies of the individual to allow for comparison with the current study being interpreted, whenever applicable studies exist.
- The system should adapt to the user. For example, the graphical interface of the PACS client software should adapt to the preferences of the individual signing into the system.
- A system must be in place for quick and efficient error detection and correction. On a regular basis, the systems should ensure agreement between the list of studies planned for a work period, the studies performed at the modalities, and the studies interpreted by the radiologists. This will detect any discrepancies, minimize lost cases and ensure incorrectly filed data is quickly identifiable.
- Fault Tolerance. When working with a digital imaging and reporting system, attention must be given to ensure system availability. Critical patient data must be available whenever needed. This is especially important in operating rooms and emergency departments. When purchasing equipment for imaging or information systems, the vendor must guarantee the level of availability of their system. System down times, for upgrades and maintenance must be well planned so as not to interfere with the workflow of the facility. Depending on the type of facility and the workload, purchasers may require the vendor to guarantee as much as 99.999% availability. It must be determined if it is acceptable for the whole system or parts of the system to be down at any time and the duration of the time. Penalties and conditions for not meeting the uptime and downtime guarantees must be clearly stated and agreed upon with the vendor.
- Disaster Recovery. The facility should establish a disaster recovery plan in case of component failure or catastrophic events. The disaster recovery plan should consist of documented policies and procedures identifying primary and backup people, their responsibilities and a description of the actions necessary to restore operations. A critical component of disaster recovery is the continuous backing up and maintenance of data at an off-site location
- In order for a PACS to work, it should be based upon, and designed to reflect, a proven, effective workflow. Purchasers should ensure the vendor understands the facility workflow and provides a system which does not disrupt the workflow. PACS based upon flawed work flows will carry over all of the existing problems.
- When deciding upon the network and storage requirements of an imaging or information system it is important not to limit the systems to only the current needs of the facility. The system should be scalable to allow for future growth of the system. The system capacity should be based upon the following points:
- the current modalities from which studies are acquired;
- the average number of images per study by modality;
- the number of digitized films to be stored;
- the number of pixels and bit depth of the image;
- projected growth in average procedure data size;
- number of studies performed each year;
- projected procedure growth volume;
- modalities to be added in the future;
- possible use of data compression by a modality or the PACS; and
- other sites/facilities to be added to the system in the future.
3.2.7 Integrating the Health Enterprise (IHE) Integration Profiles
Integrating the Healthcare Enterprise is an initiative whose goal is to make all necessary information about any given patient readily available to a care provider in order to ensure optimal medical care to the patient. IHE defines Integration Profiles that use established information technology (IT) standards to integrate systems from multiple vendors for effective interoperability and efficient workflow in day-to-day work scenarios of health professionals (users), using information from patients' records. (IHE 2007)
An IHE Integration Profile describes precisely how to solve given real-world clinical problems through functional system components, called IHE Actors. These are based on standards such as Digital Imaging and Communication in Medicine (DICOM) and Health Level 7 (HL7). Integration Profiles do not create standards; rather they clearly, carefully define how "Actors", or component devices, use established standards unambiguously in order to be interoperable and work together (e.g. how to acquire and display a digital mammography image).
IHE Mammography Image Profile (MAMMO)
The IHE Mammography Image Profile as applied to the digital mammography modality ensures that the acquired digital mammography images contain all relevant information that is necessary for further image processing, application of computer assisted detection (CAD), storage, review and printing. This profile is absolutely necessary for generating correct digital mammography image content to ensure optimal presentation of images at a mammography review workstation.
Mammography facilities should request support for the MAMMO profile by the digital mammography modality. This will provide the following benefits to the healthcare enterprise:
- Reduce Errors and Enhance Patient Care
- Ensuring proper, consistent creation of patient and technical information
- Ensures that the acquired images contain the necessary data for identifying patient and technology, and that further image processing and review is correct and meaningful, mainly by:
- Scaling of the image so that images from the same patient, acquired on different detectors can be displayed at the same size or printed in true size;
- Storing contrast information at the modality so that contrast adjustments do not degrade the quality of displayed images;
- Clear definition of breast tissue and background air so that if contrast adjustments are made during interpretation of the images, the background blackness will be maintained for optimal viewing of the structure of the breast.
The IHE Mammography Image Profile as applied to the mammography diagnostic review workstation assures the correct display of digital mammography images from any digital mammography modality claiming conformance to the same profile. This profile is absolutely necessary to allow the display of digital mammography images from different vendors in a similar way as it was previously done with analog mammography.
The IHE Mammography Image Profile ensures proper image orientation, justification, contrast display and image sizing. This profile dictates provisions leading to the accurate display of CAD mark and the display of all relevant technical and identification information on image base. The profile also requires that the displays used for image interpretation are correctly calibrated for optimal image review.
The IHE Mammography Image Profile requires that a mammography diagnostic review workstation be able to display mammography images in several standard ways: fit to viewport, true size, same size and view actual pixels. The fit to viewport display is intended to allow up to eight images to be displayed simultaneously on the display pair, primarily for temporal comparison. True size display is useful for percutaneous biopsy, surgical planning or size comparison to prior films. Same size display allows easy comparison of digital mammography images acquired at different resolutions or using different detector sizes. View actual pixels display allows for display of all captured data or "full resolution," which is especially useful when evaluating micro-calcifications or subtle masses.
The IHE Mammography Image Profile, however, does not define hanging protocols or how images of different matrices should be sized relative to each other.
Mammography facilities should request support for the MAMMO profile by the mammography diagnostic review workstation. This will provide the following benefits to the healthcare enterprise:
- Reduce Errors and Enhance Patient Care
- Ensures that images are oriented and marked correctly to avoid inadvertent interpretation errors that are due to improper hanging of an image.
- Ensures that the skin line is detected so that if contrast adjustments are performed for interpretation of the mammogram at the review workstation, the background blackness will be maintained; without this, the background will become lighter as the window width and window level are raised resulting in difficult image interpretation.
- Allows for scaling of the image on the review workstation so that images from the same patient, performed at different times on different detectors can be displayed at the same size or even true size. This allows for evaluation of developing densities and allows the radiologist to evaluate for a change in size of known lesions during temporal comparison with prior digital images or even film mammograms.
- Ensures display of all relevant clinical and technical information. For instance, acquisition dates can be used to display current and prior studies in a meaningful order or technique factors may be used for image quality troubleshooting.
- Ensures that original image presentation and consequent contrast adjustments performed at the review workstation follow the acquisition modality intent. Without this, image presentation and contrast adjustments made at the review workstation may degrade the quality of the displayed image.
- Increase Throughput
- Reduces time to read the images. Images will be displayed on the screen oriented so that the important area is automatically displayed and with the correct orientation and sizing.
- Improve Image Quality
- Improves image display and printing by including relevant data in images.
- Ensures that all technique acquisition parameters are available for review.
- Ensures that the images can be oriented, justified and sized correctly for proper and expeditious interpretation.
- Ensures that presentation images can be used in a consistent manner on different mammography diagnostic review workstations.
- Automatically uses contrast display settings created at the acquisition modality.
- Keeps background brightness unchanged while adjusting the breast tissue contrast keeping background air suppressed.
- Reduce Operational Cost
- Largely eliminates film printing, management and storage costs because the review workstation displays images for soft-copy review, both from the institution's PACS or by exchanging imaging CDs with referring health care professionals.
IHE Portable Data for Imaging (PDI) Integration Profile (IHE 2009)
The IHE Portable Data for Imaging (PDI) Profile enables creating DICOM-compliant image CDs on the modality. Requesting support for the PDI profile by the digital mammography modality will provide the following benefits to your organization:
- Reduce Operational Costs
- Reduces unnecessary films for surgery, referrals to other sites or referring physicians--the modality or review workstation creates digital image CDs to be sent to other image consumers
- Increase Throughput
- Prevents time scanning film priors from other sites--the review workstation supports the import of studies on CD. Reading time may also be decreased significantly by not having to deal with a mixed environment where film and digital images are required to be viewed together.
- Improve Data Availability and Accessibility
- Improves access to data from other sites--the workstation can import patient and diagnostic information on CD from other institutions and clinics
- Improves data access by other care providers (e.g. referring physicians, second-opinion radiologists)--the workstation exports patient and diagnostic information on CD.
3.2.8 Computer Aided Detection and Diagnosis (CAD)
Computer-aided detection (CADe) and computer-aided diagnosis (CADx) are image analysis methods used to assist radiologists when interpreting mammographic images. CADe techniques involve the use of computer algorithms to locate (using distinctive signs such as a triangle, a circle, a square or others) and identify suspicious regions of an image with the ultimate goal of increasing cancer detection. CADx techniques involve the use of computer algorithms to indicate the likelihood that a known lesion is malignant.
The use of computer aided detection and diagnosis systems must be carefully managed in order to assess the resulting effects on the overall quality of the mammography program. Various approaches can be used to asses CADe and CADx systems (Bick 2010). Quality determinants such as specificity, sensitivity, positive predictive value, and overall accuracy should be included in the assessment of computed aided systems for detection and diagnosis.
3.2.9 Telemammography
Telemammography is the electronic transmission of mammographic images from one location to another for the purposes of interpretation and/or consultation. Through telemammography, digital images and patient information can be accessed electronically from multiple sites simultaneously. The benefits of telemammography include more efficient delivery of patient care and the ability to provide radiological services to facilities in remote areas which do not have radiologists available on-site. Since telemammography involves the acquisition and interpretation of patient images at different sites, it is important that policies and procedures be in place at all locations to ensure image quality is optimized and comparable among all facilities accessing patient images. This is especially important when official authenticated written interpretations are made through telemammography. All workstations used for interpretation of telemammography images must be included in the quality assurance program of the facility to ensure performance meets minimum requirements for mammography workstations. All telemammography workstations must meet the same level of performance and undergo the same quality control testing as those of the facility where images are acquired. The relevant workstation quality control tests set out in Section C must be performed at the required frequencies. The information in this section is based on the CAR Standards for Teleradiology (CAR 2008).
- Telemammography Quality Assurance - When used for rendering the official authenticated interpretation of images, the receiving location must conform to the following requirements:
- The entire image data set produced by the digital modality in terms of both image matrix size and pixel bit depth, must be transferred to the PACS / teleradiology system. The DICOM standard must be used. Display software must be used that allows the user to "pan" over the entire image when displayed in its full matrix size.
- Images obtained through user post processing of the original image must not be used for interpretation to the exclusion of the original images themselves. They must only be used to support the interpretation process.
- Only mammograms obtained from digital mammography equipment must be used for interpretation. Mammograms obtained from scanning of film images must only be used for comparison purposes. It is recommended that such devices should enable a minimum spatial resolution of 50 µm and a minimum acquisition of 12 bit grey scale.
- Image Management - Telemammography requires the use of image management for optimal performance. All systems must include the following:
- An integrity-checking mechanism, either in software or using a manual process, to ensure that all transmitted information from the site of origin is received intact by the receiving site.
- Image storage at either the transmitting or receiving site as well as transmission must be arranged such that patient confidentiality is maintained and that the system is secure.
- The provider must ensure that the image quality is the same at the transmitting site and receiving site(s).
- Transmission of Images and Patient Data--Communications protocols and file formats must conform to the current DICOM 3.0 network standards and Canadian IHE standard for all new equipment acquisitions. Conformance with these standards should be considered when upgrading existing equipment.
- Display Capabilities - The radiologists display workstation used for interpretation must meet the requirements of section B3.2.4.
- Patient Database - For images transmitted by telemammography, a database must be available, at both the transmitting and receiving site to serve as a basis for future integrity checking and audits. The data base must include:
- patient name, identification number and date;
- type of examination;
- types of images;
- number of images;
- image acquisition and sending sites (if different); and
- date and time of transmission.
- Security - Telemammography systems must provide network and/or software protocols to protect the confidentiality of the patient's record(s), image(s), interpretation(s) and other data and insure that the system is secure and used only on an as needed basis by those authorized by the patient according to provincial or territorial privacy of information legislation and Canadian Medical Association guidelines.
- Storage of Records - The legal requirements for the storage and retention of images and reports will vary from province to province and the providers of the telemammography service are responsible for adhering to these requirements. Images stored at either site must meet the jurisdictional requirements of the transmitting site. Images interpreted off-site need not be stored at the receiving facility provided that they are stored at the transmitting site. However, if images are retained at the receiving site, the retention period of that jurisdiction must also be met. The policy on record retention must be in writing.
- Documentation - Communication is a critical component of telemammography. Physicians interpreting telemammography examinations must render reports in accordance with the CAR Standards of Communication.
- Quality Control for Telemammography - The images at the receiving site can only be as good as the images captured at the transmission end. It is imperative that a radiologist should visit the acquisition site on a regular basis to ensure that the equipment is functioning properly and that the technologists are adequately supervised and trained. Both the acquisition and reviewing sites must have documented policies and procedures for monitoring and evaluating the effective management, safety, proper performance of imaging, transmitting, receiving and display equipment.
- Quality Improvement - The use of telemammography does not reduce the responsibilities for the management and supervision of radiological medicine. Procedures must be systematically monitored and evaluated as part of the overall quality improvement program of the facility. Monitoring must include the evaluation of the accuracy of the interpretations as well as the appropriateness of the examination. Incidences of complications and adverse events must be reviewed to identify opportunities to improve patient care.
The use of telemammography must be documented. Periodic reviews must be made for the appropriateness, problems and quality of the transmitted data. The data must be collected in a manner which complies with the statutory and regulatory requirements.
3.2.10 Compression of Digital Images
Increasing amounts of data are generated by digital mammography equipment. The costs associated with storage and transmission of this data has resulted in significant interest in compression methods of digital images. There are two types of compression: reversible (also called lossless) compression and irreversible (also called lossy) compression. Using lossless compression, images may be compressed and decompressed and there is no alteration of the original image data. Using lossy compression, decompressed images are modified and pixel values may be different from their original values.
Until scientific studies provide evidence that lossy compression does not compromise accurate diagnosis of patients, there must be no lossy compression of digital mammography images.
4.0 Test Equipment
Consideration must be given to test equipment necessary for ensuring the performance of mammographic equipment and their accessories as well as for ensuring the radiation safety of the facility.
- All equipment used for acceptance and quality control testing must be evaluated for functioning and performance on a regular basis.
- All sensitometric and densitometric equipment, dose meters, tube voltage meters and illuminance/luminance meters must be calibrated according to manufacturers' recommendations. Instruments used to measure air kerma or air kerma rate must be calibrated every 2 years and when the instrument is repaired. The instrument calibration must be traceable to a national standard and calibrated with an accuracy of ±6 percent (95 percent confidence level) in the mammography energy range. For instruments that are not manufacturer-certified for W/Ag or W/Rh target/filter combinations, necessary corrections must be obtained when measuring x-ray beams generated with W/Ag or W/Rh combinations.
- All phantoms and other equipment used for the assessment of image quality, dose and system performance should be checked for damage or any condition which may affect their use.
- Test equipment should be stored away from heat, direct sunlight, and high humidity, and must be operated following manufacturers' recommendations.
5.0 Radiation Protection Surveys
A radiation protection survey is an evaluation, conducted by the regulatory authority, of the radiation safety of a mammography facility. The survey is intended to ensure compliance with the requirements of this Safety Code, to demonstrate that x-ray and auxiliary equipment function properly and according to applicable standards, and that the equipment is installed and used in a way which provides maximum radiation safety for operators, patients and others. It is important to note that radiation protection surveys described in this section are primarily for the instruction and guidance of persons employed in Federal Public Service departments and agencies, as well as those under the jurisdiction of the Canada Labour Code. Facilities under provincial or territorial jurisdiction may be subject to requirements under their statutes. The authorities listed in Appendix I should be contacted for details of the regulatory requirements of individual provinces and territories.
During the investigation, the regulatory authority may request reports of quality control performed by the physicist or by the technologist, safety measures such as protective equipment and shielding are also examined to ensure that they are present and provide the required protection. It is important, therefore, that x-ray facilities are surveyed at regular intervals.
5.1 General Procedures
Routine operation of any new installation or an installation which has undergone modifications should be deferred until a complete survey has been made by an expert. The expert is an individual who is qualified by education and experience to perform advanced or complex procedures in radiation protection that generally are beyond the capabilities of most personnel within the facility. These procedures include evaluation of the facility design to ensure adequate shielding is in place, inspection and evaluation of the performance of x-ray equipment and accessories, and evaluation and recommendation of radiation protection programs. The owner of the facility (or another delegated staff member such as the Radiation Protection/Safety Officer) must contact the appropriate regulatory agency to ascertain inspection and acceptance testing procedures in that jurisdiction. Some jurisdictions may require that the facility be declared in compliance with applicable governmental regulations prior to operations.
For a new facility, it is particularly advantageous to make visual inspections during construction, to ensure compliance with specifications and to identify faulty material or workmanship, since deficiencies can be remedied more economically at this stage than later.
For existing installations, a survey must be carried out after any changes are made which might produce a radiation hazard. This includes alteration of protective barriers, equipment modification and replacement, changes in operating procedures, or increased workloads.
Finally, radiation protection surveys must be carried out at regularly scheduled intervals during routine operations to detect problems due to equipment failure or any long-term trends toward a decrease in the level of radiation safety. Facilities should contact the applicable regulatory authority to establish the survey schedule.
The results of such surveys, including conclusions drawn by the expert, must be submitted to the owner or responsible user in a written report. The written report should be available, 30 days after testing.
All such reports must be retained by the owner or responsible user. For federal facilities, radiation survey reports should be maintained for 5 years and personnel dosimetry records for the lifetime of the facility.
5.2 Survey Report
The survey report must present, in a clear systematic way, details and results of the measurements carried out, as well as the conclusions drawn and recommendations made by the surveyor. Any unusual findings about the equipment itself, the facility or operating procedures, which could affect the safety of operators or other persons in the vicinity of the x-ray facility, must be clearly identified.
The survey report must include the following:
- a sketch of the facility, showing the location of the x-ray equipment and control panel within the facility as well as the nature and occupancy of the areas adjoining the facility;
- identification of the mammography equipment (i.e., the name of the manufacturer, model designation and serial number of the generator, control, x-ray tube assembly, etc. as applicable) and the date, or at least approximate date manufactured;
- observations of the operational conditions (both electrical and mechanical) of the x-ray equipment at the time of the survey;
- the actual or estimated total workload of the facility, as well as the workload apportioned into various x-ray beam directions and procedures used, etc.;
- results of radiation measurements carried out both inside and outside the controlled area under "typical" operating conditions;
- the locations at which the measurements are made;
- an evaluation of the x-ray performance and the imaging or diagnostic performance (this may include performing applicable quality control tests from sections C3.1 to C3.6);
- a summary of typical loading factors used and a measurement of the total filtration in the x-ray beam;
- an assessment of radiological techniques from the point of view of radiation safety and an assessment of the mean glandular dose. Attention must be drawn to any practices which are or could be detrimental to the patient or to personnel working in the facility. Recommendations of improved or safer techniques should be made in such cases;
- a review of the facility's quality assurance program to ensure it exists and is maintained, including quality control testing records; and
- recommendations regarding the need for a follow-up survey.
6.0 Disposal of x-ray Equipment
When x-ray equipment is considered for disposal, an assessment should be made as to whether the equipment can be refurbished and/or recycled. Communication with the manufacturer or supplier of the equipment should be made as to whether the equipment or components of the equipment can be recycled or returned. It must be noted that if the equipment contains any patient information, this information must be adequately removed. Once the decision has been made to dispose of x-ray equipment, an assessment must be made to determine if any equipment components contain hazardous materials. To ensure equipment is not unsafely operated after disposal, it should be made inoperable before disposing. The cables that power the equipment and other electrical connections should be disconnected and removed. It is recommended that mammography facilities, under provincial or territorial jurisdiction contact the responsible agency in their respective province or territory for further information. A listing of these responsible agencies is provided in Appendix I.
Section C: Quality Assurance Program
1.0 Introduction
All mammography facilities must develop and maintain an effective quality assurance program. In mammography, a quality assurance program is defined as the planned and organized actions necessary to provide adequate confidence that mammography equipment and related components reliably produce quality mammograms with minimum dose to patients and staff. A quality assurance program must include quality control procedures for the monitoring and testing of mammographic equipment and related components, and administrative procedures to ensure that monitoring, evaluation and corrective actions are properly performed. The owner of an x-ray facility has the responsibility of establishing a quality assurance program that examines all practices of the facility which affect:
- Information Quality - to ensure all clinical information produced provides for accurate clinical assessment;
- Clinical Efficiency - to ensure all steps leading to accurate diagnosis and intervention are taken and the information is made available in a timely fashion to the patient's physicians or primary medical professionals; and
- Dose - to ensure that the x-ray examination is performed with the lowest possible radiation dose to the patient, staff and others consistent with clinical imaging requirements.
1.1 Goals of the Quality Assurance Program
The ultimate goal of a quality assurance program is to ensure accurate and timely diagnosis and treatment at the minimum dose to the patient and staff. In order to have a successful quality assurance program it is essential that equipment is in proper working condition and all staff members understand the goals of the program and are committed to the implementation of the program through full participation.
Information obtained from mammography equipment must be of utmost quality to ensure accurate diagnosis and treatment. If critical elements are missing or artefacts are added to images, the image is considered to be of poor quality. The consequence of a poor quality mammogram may be incorrect diagnosis resulting in repeat mammography, unnecessary radiation doses to the patient, delayed or improper patient treatment and increased cost.
1.2 Costs-Benefits of the Quality Assurance Program
The initial implementation and the general operation of a quality assurance program will involve cost in both money and time from staff. However, savings from the operation of the program will offset some of these costs. For some facilities, there may be a reduction in the overall operating costs.
1.2.1 Costs of Quality Assurance Program
Some of the costs associated to the quality assurance program are as follow:
- Personnel - The staff will be required to perform new duties, which include generating test images for the x-ray equipment and record keeping;
- Test Equipment - Test equipment to perform quality control tests, such as phantoms, will be required. However, the cost of such equipment is small compared to the cost of the x-ray imaging unit and it may be used for several mammography systems. It would not be necessary to purchase some of the test equipment if the facility decides to have some of the quality control tests performed by an external organization or individual, who would then be responsible for providing their own test equipment;
- Test Images - For film-based systems, and CR and DR systems using laser printers, 2 to 5% of films used by a facility may be required for the performance of sensitometry, phantom imaging, equipment and test imaging; and
- External Organizations - If the facility does not have the capacity to perform internally all quality control tests, it may choose to contract an external organization or individual to perform some of these tests and equipment assessments. In addition, the facility may retain the service of a medical physicist as an advisor during implementation and for consultation during operation of the facility.
1.2.2 Benefits of a Quality Assurance Program
In addition to improved diagnostic quality some of the savings associated with the quality assurance program are as follow:
- Film and Processing Chemicals - For film-based systems, and CR and DR systems using laser printers, a decrease in the number of retakes may result in the reduction in the number of films and processing chemicals used;
- Equipment - The reduction in the number of retakes will lead to a reduction in workloads which in turn will put less stress on mammography equipment and image processors. Problems with equipment may be diagnosed earlier before more serious and costly problems occur thus reducing down time and equipment service costs; and
- Patient Flow - The reduction in the number of repeats, and better image quality will allow efficient use of time for x-ray equipment operators. This will result in better predictability of scheduling and possibly greater patient throughput.
1.3 Implementation of Quality Assurance Program
The implementation of a quality assurance program need not be complicated. It consists of establishing quality control procedures for the equipment along with an administrative methodology to ensure that monitoring, evaluation and corrective actions are properly performed.
1.3.1 Policies and Guidelines Development
One useful step is to develop a series of policies and guidelines where various issues are addressed. The following list presents some of these policies and guidelines. Each facility may require different sets of policies and guidelines depending on the type of work being performed and the organizational structure of the facility. These policies should be established by management with participation from staff. It is recommended that all safety policies, procedures and processes be reviewed by a joint health and safety committee. The policies should be present in the quality assurance (QA) manual. The following information should be readily available to radiology staff:
- Radiology Personnel
- A list of staff and an outline of their duties, authority and responsibilities.
- Policies for Minimizing Radiation Exposure to x-ray Operators and Staff
- Policy for minimizing exposure to pregnant workers.
- Policy for holding patients.
- Policy for the presence of individuals in the x-ray room during procedures.
- Policy for training/orientation program for x-ray equipment operators.
- Policy for the proper use and maintenance of x-ray equipment.
- Policy for personnel radiation dosimetry monitoring.
- Policy for the use of protective devices and radiation protection equipment.
- Policy for the maintenance and testing of radiation protection devices and equipment.
- Policies for Minimizing Radiation Exposure to Patients
- Policy for carrying out x-ray examinations.
- Policy for the radiological examination of pregnant patients.
- Policy for the use of protective devices and radiation protection equipment.
- Policy for patient positioning (positioning manual).
- Policy for exposure loading factors (technique charts).
- Policy for the quality acceptance of radiographic images.
- Policy for a reject analysis of radiographic images.
- Guidelines for Equipment Quality Control Testing
- Guidelines listing all x-ray equipment and system components to be tested.
- Guidelines for all equipment parameters to be measured and the frequency of monitoring (schedule) for each x-ray system and system component.
- Guidelines for the performance standards for each equipment tested and the specific performance tolerance limits expected for each quality control test.
- Guidelines for the measurement of each parameter and recording of the data.
- Guidelines to evaluate the test data and to take the corrective action necessary to maintain equipment optimum performance.
- Guidelines for patient dose measurements (mean glandular dose).
- Guidelines for the calibration and maintenance of radiation measuring equipment and other test equipment.
- Policies for the Acquisition of New x-ray Imaging Equipment
- Policy for a needs analysis.
- Policy for equipment specification writing.
- Policy for equipment acceptance testing.
- Policy for equipment appraisal and replacement.
- Policy for Record Keeping
- Policy for the review of the quality assurance program.
- Policy for the review of the quality control procedures.
- Policy for the retention of records (patient information, quality control test results, survey reports, personal dosimetry records).
- Policy for the creation of digital image CDs (IHE 2009 Radiology Technical Framework Supplement Extensions to the Portable Data for Imaging (PDI) Integration Profile
1.3.2 Establishment of Quality Control Procedures
The following four steps must be included for the establishment of quality control procedures:
- Equipment Operation - It is essential that the mammography equipment and image processing and display equipment function properly before a quality assurance program is implemented. Manufacturers and vendors should provide proper operating characteristics for their equipment. For film-based systems, films and processing must meet manufacturers' speed and contrast values. For CR systems, the imaging system must be properly calibrated with the x-ray systems. This may involve replacement, repair, upgrading or calibration of the equipment;
- Baseline Performance - Baseline performance values of mammography equipment and image processing system must be established after verifying that the equipment functions properly. Images used for determining baseline performance levels should be obtained using the routine technique for a standard compressed breast (ex. mean thickness of 4.2 cm to 4.5 cm). This baseline performance will be used to diagnose any changes in equipment performance. It is important to keep records of equipment operation data and baseline performance measurements. These records will be needed to diagnose any changes in image quality. Baseline values must be determined when new equipment is introduced into the facility, when there are changes in components which affect image quality and patient dose and also when testing equipment is changed;
- Reference Test Image - To evaluate image quality a reference test image is needed. This reference test image is made by using the mammography equipment, image processing system and a quality control phantom and will be used for comparison of quality control test images; and
- Result Evaluation and Action Levels - An effective quality control monitoring program includes not only a quality control testing schedule, data recording and record keeping, but also test result evaluation, such as determination of acceptable or unacceptable limits of equipment operation coupled with a list of corrective actions that may be required. A set of limits should be established which indicates a level of operation outside of which the system or the function should be closely monitored but where no immediate action is required. Another set of limits should also be established where immediate remedial action must be taken.
1.3.3 Establishment of Administrative Procedures
The following administrative procedures must be included in the establishment of an effective quality assurance program.
- Responsibility - Although the owner of the facility is ultimately responsible for the implementation and operation of the quality assurance program, to obtain the optimal level of radiation safety and quality diagnostic information, it is imperative that full cooperation exists among all concerned parties. Staff members may be assigned duties with regard to equipment monitoring, record keeping and general operations of the quality assurance program. It is essential that the level of responsibilities and involvement of the owner and staff be clearly identified, communicated and understood.
- Record Keeping - It is essential that measurements and information gathered for the quality assurance program is clearly documented and readily available for evaluation.
- The medical physics report must be circulated to all mammography staff and retained at the facility.
- As far as practicable, recorded data must be indicated as data points on a control chart when the measurement is made. In this form, trends can be more easily detected. A logbook or other easily identifiable method of recording must be used and records must be kept for a minimum of 3 years.
- Processor quality control charts must be retained in the Quality Control records for 1 year.
- Sensitometric films for the last 30 days of mammographic film processing must be retained.
- One monthly sample quality control phantom film or digital image must be kept for a minimum of 3 years.
- Evaluation of Data - Recorded data must be evaluated immediately and necessary action taken expeditiously.
- Limits of Acceptability of Data - Upper and lower limits of acceptability of recorded data must be determined and documented. When these limits are reached, corrective actions must be taken. For example, they can be the range of acceptable temperatures for the film processor. These limits must be set such that they are just within the range allowable before diagnostically significant image changes are evident. They should not be so restrictive that they exceed the capability of the equipment, or that frequent corrective actions are taken without any evidence of problems. These limits should be reviewed from time to time, especially when major components of the x-ray system are replaced or repaired.
- Testing frequency - Testing frequency must be such that a balance is reached between the cost of testing, disruption to the operation of the facility and the maintenance of quality. The frequency of testing should be increased if the equipment exhibits significant changes between scheduled quality control tests, or if the equipment is used for exceptionally high volume of procedures. Additional testing should be performed if the results of testing fall outside of limits of acceptability for the tests, or after any corrective actions are made. Equipment must be retested after service to any part which may affect the image density, image quality or radiation output from the x-ray tube. The quality control program must not be discontinued if the results indicate relatively stable equipment performance. The purpose of a quality control program is to control quality, and periodic measurement of equipment performance is essential. When there are differences between the manufacturers's recommended testing and the requirements of applicable legislation or policies, the more strict testing frequency should be followed to ensure compliance with legislation/policies and also to ensure fulfilment of equipment warrantee conditions of the manufacturer.
- Corrective Actions - There must be established repair and calibration procedures to deal with significant problems. A decision tree system should be developed to provide guidance to deal with events such as equipment failure and to deal with circumstances when equipment performance deviates beyond the set limits. A list of individuals having the authority to stop operation of an x-ray unit should be established. The decision tree should include the following steps:
- repeat the test to confirm;
- what to do if repeated test confirms performance failure;
- what to do if test fails only marginally;
- what to do if test shows a history of failure; and
- what to do if test fails substantially.
1.4 Mammography Accreditation
Accreditation is a formal process through which a mammography facility can demonstrate that the quality of mammography performed in their facility meets accepted standards. Accreditation requires both self-evaluation by the mammography facilities and external evaluation by a reviewing body. It involves an assessment of personnel qualifications, policies and procedures, equipment design and performance, and the facility's quality assurance program including quality control testing. Through accreditation, a mammography facility can provide additional reassurance in their commitment to quality of care.
In Canada, mammography accreditation programs have been established by provincial organizations and nationally by the Canadian Association of Radiologists. It is strongly recommended that all Canadian mammography facilities are accredited by a recognized standard such as that of the Canadian Association of Radiologists or its equivalent.
2.0 Acceptance Testing
Acceptance testing is a process to verify compliance with the performance specifications of the x-ray equipment as written in the purchase contract and that the equipment performance complies with federal and provincial or territorial regulations. Acceptance testing must be performed prior to any clinical use of the equipment. Acceptance testing must be performed by or under the supervision of, a medical physicist, with in-depth knowledge of the particular type of mammography equipment and the relevant regulations prior to any clinical use of the equipment. The owner must have acceptance testing performed by an individual or organization independent of the manufacturer.
Acceptance testing of a medical x-ray system includes several major steps: They are:
- the verification that delivered components or systems correspond to what was ordered;
- the verification of the system mechanical integrity and stability, including safety mechanisms, automatic patient release, power drives, interlocks;
- the verification that appropriate inspections of electrical installations have been carried out, including electrical safety and line power fluctuation;
- the verification of x-ray performance; and
- the verification of imaging or diagnostic performance.
The results from the acceptance testing should be used to set baseline values and acceptance limits on operational performance of the mammography equipment. These baseline values and limits are essential to the quality assurance program.
2.1 Acceptance Testing Evaluation
Acceptance testing for mammography equipment should evaluate at least the items in Table 5. Not all equipment will be subject to the full set of tests. The type of equipment and its configuration will dictate the sets of tests to be performed.
| Item Under Evaluation for Acceptance Testing | FS | CR | DR |
|---|---|---|---|
| 1.0 Identification | |||
| 1.1 Initial Inspection and Inventory | X | X | X |
| 1.2 Inspection of Documentation | X | X | X |
| 2.0 Visual and Functional Tests | |||
| 2.1 Mechanical Properties | X | X | X |
| 2.2 Safety Systems | X | X | X |
| 3.0. Performance Evaluation - X-ray Generator and Control | |||
| 3.1 Focal Spot Size | X | X | X |
| 3.2 Source-to-image distance | X | X | X |
| 3.3 X-ray Tube Voltage | X | X | X |
| 3.4 Current Time Product | X | X | X |
| 3.5 Loading Time | X | X | X |
| 3.6 Beam Limitation and Indication | X | X | X |
| 3.7 X-ray Beam Filtration | X | X | X |
| 3.8 Automatic Exposure Control | X | X | X |
| 3.9 Radiation Output | X | X | X |
| 3.10 Radiation Leakage | X | X | X |
| 4.0 Performance Evaluation - Compression | |||
| 4.1 Compression force | X | X | X |
| 4.2 Compression force indicator accuracy | X | X | X |
| 5.0 Performance Evaluation - Image Acquisition | |||
| 5.1 Response Function | X | X | |
| 5.2 Noise | X | X | |
| 5.3 Missed Tissue at Chest Wall | X | X | |
| 5.4 Uniformity | X | X | |
| 5.5 Defective Detector Element | X | ||
| 5.6 Uncorrected Defective Detector Elements | X | ||
| 5.7 Inter-Plate Sensitivity | X | ||
| 5.8 Influence of Other Radiation Sources | X | ||
| 5.9 Fading of Latent Image | X | ||
| 5.10 Inter-cassette variation | X | ||
| 5.11 Film/Screen Contact | X | ||
| 5.12 IHE mammography image profile compliance (Acquisition Modality Actor) | X | X | |
| 6.0 Performance Evaluation - Film Processing | |||
| 6.1 Processor Temperature | X | ||
| 6.2 Processing Time | X | ||
| 6.3 Sensitometry | X | ||
| 6.4 Artefacts | X | ||
| 6.5 Darkroom Light | X | ||
| 6.6 Safelight | X | ||
| 7.0 Performance Evaluation - Image Quality | |||
| 7.1 Contrast Detectability | X | X | X |
| 7.2 Spatial Resolution (Modulation Transfer Function (MTF) and Noise Power Spectrum (NPS) for digital systems) | X | X | X |
| 7.3 Irradiation Time | X | X | X |
| 7.4 Geometric Distortion and Artefacts | X | X | X |
| 7.5 Digital Detector Residual Image (Ghost Image) | X | X | |
| 8.0 Performance Evaluation - Dosimetry | |||
| 8.1 Mean Glandular Dose | X | X | X |
| 9.0 Image Presentation - Review Workstations | |||
| 9.1 Ambient light | X | X | |
| 9.2 Contrast Visibility | X | X | |
| 9.3 Resolution | X | X | |
| 9.4 Display Artefacts | X | X | |
| 9.5 Luminance Range | X | X | |
| 9.6 Greyscale Display Function | X | X | |
| 9.7 Luminance Uniformity | X | X | |
| 9.8 IHE mammography image profile compliance (Image Display Actor) | X | X | |
| 10.0 Image Presentation - Viewboxes | |||
| 10.1 Ambient Light | X | X | X |
| 10.2 Luminance | X | X | X |
| 10.3 Light Output Uniformity | X | X | X |
| 10.4 Light Output Homogeneity (between viewboxes) | X | X | X |
| 11.0 Image Presentation - Printers | |||
| 11.1 Geometric Distortion | X | X | |
| 11.2 Contrast Visibility | X | X | |
| 11.3 Resolution | X | X | |
| 11.4 Printer Artefacts | X | X | |
| 11.5 Greyscale Display Function | X | X | |
| 11.6 Optical Density Uniformity | X | X | |
| 12.0 Image Presentation - Film Digitizers | |||
| 12.1 Overall image quality | X | ||
| 12.2 Noise | X | ||
| 12.3 Resolution | X | ||
| 12.4 Optical Density | X | ||
3.0 Quality Control Testing Procedures and Equipment
Quality Control testing must be carried out during routine operation of a mammography facility. This section sets out the required and recommended quality control tests, the associated test equipment, and the testing frequencies.
Quality control testing of a mammography system includes several major steps. They are:
- the verification of the system mechanical integrity and stability, including safety mechanisms, automatic patient release, power drives and interlocks;
- the verification of the performance of ancillary equipment such as imaging processors and display units;
- the verification of x-ray performance; and
- the verification of imaging or diagnostic performance, including assessment of dose.
Quality control tests performed daily to semi-annually are carried out by the mammography radiological technologists. Test equipment required for these tests must be readily available to the individuals responsible for performing those tests. All test equipment must be calibrated and verified to be operating accurately. Individuals performing quality control tests must be trained in the proper operation of the test equipment and in performing the tests. Annual quality control tests must be performed by a qualified medical physicist.
In the following sections, the descriptions of each test indicate whether performance of the test is required or recommended. In addition, not all equipment will be subject to the full set of tests listed in the following sections. The type of imaging system, whether film-based, CR, or DR, to which the quality control tests apply, is identified. The quality control tests and testing procedures provided in this section are based upon existing mammography standards, for film/screen and digital mammography equipment, established by international, national and provincial organizations (IAEA 2009)(IAEA 2011)(CAR MAP 2011)(MSSS 2001)(MSSS 2006). Alternative tests can be performed in place of those specified if it can be shown that the test is capable of verifying the necessary parameter or performance. For some tests, manufacturers' recommended procedures and acceptance criteria are recognized. However, equivalent testing procedures, such as those recommended by a recognized mammography standard setting body may be acceptable. Harmonized testing protocols are desirable as this allows comparison of performance and standardized testing.
3.1 Daily Quality Control Testing
Daily quality control tests must be performed at the beginning of each day that mammography is conducted before commencing patient examinations and processing any patient images.
| Quality Control Procedures Under Evaluation |
FS | CR | DR |
|---|---|---|---|
| Daily Quality Control Tests | |||
| Equipment Warm-up | D1 | D1 | D1 |
| Meters Operation | D2 | D2 | D2 |
| Equipment Conditions | D3 | D3 | D3 |
| Darkroom Cleanliness | D4 | ||
| Cleanliness of Electronic Display Devices and Assessment of Viewing Conditions |
D5 | D5 | D5 |
| Film Processor Function | D6 | ||
| Image Quality Evaluation-Film/Screen Systems | D7 | ||
| Imaging Quality Evaluation - Digital Systems | D8 | D8 | |
| Overall Visual Assessment of Electronic Display Devices | D9 | D9 | |
| Overall Visual Assessment of Printers | D10 | ||
D1. Equipment Warm-up - The manufacturer's recommended warm up procedure must be followed. The warm up procedure must be repeated if the equipment is left idle for an extended period of time. It is important to note that all components of the imaging system which are routinely used must be warmed up, including computer display devices and printers.
D2. Meters Operation - Meters and visual and audible indicators should be checked for proper function.
D3. Equipment Conditions - X-ray equipment conditions should be visually inspected for loose or broken components and cleanliness. The x-ray source assembly should be checked for motion or vibration during operation. Visual inspection should also be conducted of all other components of the imaging system.
D4. Darkroom Cleanliness - In order to maintain the cleanliness of the darkroom, all working surfaces, tops of counters and the floor should be cleaned daily. Dust and debris can more easily be seen using a UV-B lamp.
D5. Cleanliness of Electronic Display Devices and Assessment of Viewing Conditions - A visual inspection for cleanliness should be made of all electronic display devices used for interpretation. Devices must be cleaned as necessary. An assessment must be made of the environment in which mammograms are read. The level of ambient light must be low and consistent, particularly when lightboxes and electronic displays are present in the same room. An assessment should also be made of the temperature, noise and room ergonomics to ensure consistency of the viewing conditions.
D6. Film Processor Function - Film processor function must be evaluated every morning before performing clinical examinations, after the processor has been turned on and has reached the required development temperature, and at other times as required, such as after a replenishment rate change.
- The film processing solution levels must be checked to ensure agreement with the manufacturers' recommended operating levels for the particular processor and film type, for the given number of films processed daily.
- The displayed processor temperature must be checked to ensure agreement with the manufacturers' recommended operating level for the particular processor and film used. If film processing problems are detected during daily sensitometry, it may be necessary to verify the pH of the developing chemicals, the developing time, specific gravity and replenishment rate.
- Sensitometric strip processing must be performed in order to monitor the performance of the film processing system.
- The base plus fog must be within +0.03 of the established operating level.
- The mid-density must be within ±0.15 of the established operating level.
- The density difference must be within ±0.15 of the established operating level.
D7. Image Quality Evaluation - Film/Screen systems. For film/screen systems an image quality evaluation must be performed. A uniform phantom representing average breast thickness should be routinely used to monitor and maintain image density to ensure correct optical density, the absence of excessive artefacts, and a consistent current time product setting. The optical density of the film at the centre of an image of a phantom must be at least 1.40 when exposed under a typical clinical condition. It is strongly recommended that the optical density be greater than 1.60. The optical density of the film at the centre of the phantom image must not change by more than ±0.20 from the established operating levels.
D8. Image Quality Evaluation - Digital Systems - An image quality evaluation must be performed of a flat field image. Using a uniform phantom representing average breast thickness, evaluate images for significant artefacts that could interfere with clinical interpretations. View the "for presentation" image on the acquisition workstation using appropriate window width and level. The images must have a uniform appearance with not significant artefacts. Note that for CR images it is important to monitor clinical images acquired throughout the day to ensure artefacts do not develop due to accumulation of dust.
D9. Overall Visual Assessment of Electronic Display Devices - The performance of electronic display devices used for the interpretation of mammograms must be assessed. The daily quality control tests recommended by the American Association of Physicists in Medicine (AAPM, 2005), including the TG18 test patterns, test procedures and acceptance criteria should be used. The display system must be warmed up prior to testing. Attention must be given to ensure ambient light levels are appropriate (less than 40 lux) and representative of conditions under which clinical images are viewed. A viewing distance of 30 cm is recommended. Displaying the image of a test pattern, an assessment must be made of the general image quality and for the presence of artefacts. The TG18-QC test patterns can be used for this test and should be displayed using the software routinely used to display clinical images.
- Geometric Distortion - The borders of the test pattern must be visible, lines must be straight and the active display area must be centered on the screen.
- Contrast Visibility - All corner patches must be visible and the 5% and 95% pixel value squares must be clearly visible.
- Display Artefacts - There must not be any disturbing artefacts visible.
D10. Overall Visual Assessment of Printers - The performance of printers must be verified whenever they are going to be used. Note that all printers used for printing of clinical images, whether on-site or in a remote facility, must perform this test. Print and inspect the TG18-QC test pattern and inspect for the following:
- Geometric distortion - Borders of the test pattern must be visible and straight;
- Contrast visibility - All corner patches must be visible and the 5% and 95% pixel value squares must be clearly visible; and
- Printer artefacts - There must not be any disturbing artefacts visible.
| Item | Equipment | Systems | Reference |
|---|---|---|---|
| 1 | Uniform phantom representing average breast thickness (ex. PMMA test object thickness 45 ± 0.5 mm) (if needed for manufacturer's recommended warm up procedure) |
FS, DR, CR | D1, D7, D8 |
| 2 | Ultraviolet Light | SF | D4 |
| 3 | Sensitometer (21 steps optical attenuator with densities ranging from approximately 0.00 to 4.80 in steps of 0.15) Accuracy: ± 0.02 log exposure units Reproducibility: ± 0.02 log exposure units |
FS | D6 |
| 4 | Thermometer (non-mercury) | FS | D6 |
| 5 | Densitometer Accuracy: ± 0.02 O.D. at 1.0 O.D. Reproducibility: ± 0.01 O.D. at 1.0 O.D. |
FS | D6, D7 |
| 6 | Test Pattern Image (TG18-QC) | DR, CR | D9, D10 |
| 7 | Viewing box | FS | D10 |
3.2 Weekly Quality Control Testing
| Quality Control Procedures Under Evaluation |
FS | CR | DR |
|---|---|---|---|
| Weekly Quality Control Tests | |||
| Screen Cleanliness and Condition | W1 | ||
| Cassette Cleanliness and Conditions | W2 | W2 | |
| Visual Inspection of Cleanliness of Imaging Systems | W3 | W3 | W3 |
| Darkroom Light Conditions | W4 | ||
| Darkroom Temperature and Humidity Conditions | W5 | ||
| Viewbox Conditions | W6 | W6 | W6 |
| Phantom Image for Film-screen Systems | W7 | ||
| Digital Image Quality Evaluation | W8 | W8 | |
| Electronic Display Device Performance | W9 | W9 | W9 |
| Laser Film Printer Artefacts | W10 | W10 | W10 |
W1. Screen Cleanliness and Condition - Screens should be checked for cleanliness and damage. Manufacturer recommended screen cleaner should be used. An inspection for dust particles should be done with an ultraviolet light.
W2. Cassette Cleanliness and Conditions - Cassettes should be checked for cleanliness, wear, warping, fatigue of foam compression material, closure mechanism, and light leaks. The cassette holder tunnel should be checked for dust and dirt. Cleaning frequency and method should be in accordance to manufacturers' recommendations.
W3. Visual Inspection of Cleanliness of Imaging Systems - Imaging systems must be inspected for dust and dirt on or near the image reception area where they may negatively affect image quality. For CR systems, the imaging plates must be inspected. The imaging plate loading and unloading mechanism must be cleaned and lubricated if necessary. The image receptors for direct digital mammography systems must be kept clean of dust, dirt and other items which may come into contact with them. Laser scanning digitizers must also be checked for cleanliness.
W4. Darkroom Light Conditions - A visual test must be performed in the darkroom to ensure the room is light tight and that other sources of light such as illuminated light switches and computer power supplies do not cause film fogging. Particular attention must be paid to the door seal and the mounting of the film processor if the film insertion to the processor is done through a wall. The assessment of darkroom light conditions should be made after a 10 to 15 minute period of adaptation to the dark conditions with safelights turned off.
W5. Darkroom Temperature and Humidity Conditions - A verification of the darkroom temperature and humidity should be conducted. The temperature should be between 15°C and 23°C and the humidity between 40% and 60%.
W6. Viewbox Conditions - Viewboxes should be inspected visually for cleanliness, viewing area discoloration and improper illumination. Note that this test is also applicable in situations where images acquired from digital mammography systems are printed.
W7. Phantom Image for Film-Screen Systems - A phantom, with image quality evaluation objects, must be used to test the imaging performance of the mammography system. For the RMI-156 mammography phantom, a minimum of the four largest fibers, the three largest speck groups and the three largest masses must be visible.
- The number of test objects of each group type (fibers, specks, and masses) visible in the phantom image must not decrease by more than one half.
- The phantom image background optical density should be between 1.5 OD and 1.9 OD and must not vary by more than ±0.20 from the operating level
- The density difference due to a 4.0 mm acrylic disc must be at least 0.40 and must not vary by more than ±0.05 from the established operating level.
W8. Digital Image Quality Evaluation - For CR and DR systems, an evaluation must be made of the digital image quality. Acquire an image of a uniform phantom representing average breast thickness and a contrast object (typically an acrylic disk of 2.5 cm diameter and 1 mm thick). The following criteria must be met:
- Current-time product. The value of the mAs must not deviate from the established baseline value by more than ±10%;
- Signal difference-to-noise ratio (SDNR). The SDNR is a measure of the difference between a signal and its background divided by the noise. The variation in the SDNR from the established baseline value must be less than ±10%;
- For CR systems: Where the exposure index is a S#, the value of the S# must be within ±10% of the established baseline value; where the exposure index is a SAL, SALlog, or PVIlog number, the value must be within ±5%, ±430, ±580 of the established baseline value respectively; where the exposure index is an EI value, the value must be within 40 units of the established baseline value;
- Images must appear uniform;
- No visible defective detector elements present; and
- No artefacts which could interfere with clinical interpretation.
W9. Electronic Display Device Performance - The performance of all electronic display devices used to view images from digital systems, as well as those obtained through scanning of radiographic films, must be checked. This includes display devices used for acquisition and interpretations of images. For this test, it is recommended that a modified TG18-QC test pattern be used which emulates the images produced by each model of digital mammography equipment in the facility, or which might be interpreted at that workstation (i.e., has the same x-y dimensions, number of bits, and a DICOM header containing appropriate values of all relevant tags). Note that when evaluating the display devices of the interpretation workstations, the viewing conditions must be verified. The following criteria must be met:
- The 5% patch must be visible inside of the 0% patch;
- The 95% patch must be visible inside the 100% patch;
- The 16 luminance patches must be distinct from one another in shade.
- On displays used for interpretation, the letters "QUALITY CONT" must be visible in each of the three regions of the TG18-QC image.
- Images should appear consistently across all interpretation workstations.
- No artefacts upon visual inspection.
W10. Laser Film Printer Artefacts - For mammography facilities printing digital images, the quality of images obtained from the laser film printer must be checked for artefacts. Ensure that the viewbox used to assess printed films has sufficient luminance. Print an image of a uniform test pattern (i.e., TG18-UNL80 test pattern). The following criteria must be met:
- The optical density must be uniform; and
- No disturbing artefacts are present that could interfere with clinical interpretation.
| Item | Equipment | Systems | Reference |
|---|---|---|---|
| 1 | Ultraviolet Light | FS | W1 |
| 2 | Screen Cleaner (as recommended by manufacturer) |
FS | W1 |
| 3 | Hygrometer Thermometer (non-mercury) Accuracy: ± 0.3 °C Reproducibility: ± 0.1 °C |
FS | W5 |
| 4 | RMI 156 Phantom, with image quality evaluation objects | FS | W7 |
| 5 | Viewing box | FS | W7 |
| 6 | Densitometer Accuracy: ± 0.02 O.D. at 1.0 O.D. Reproducibility: ± 0.01 O.D. at 1.0 O.D. |
FS, CR, DR | W7, W10 |
| 7 | Acrylic disk (4 mm thick) | FS | W7 |
| 8 | Acrylic disk (ex. 2.5 cm in diameter and 1mm thick) | CR, DR | W8 |
| 9 | Uniform phantom representing average breast thickness (ex. PMMA of thickness 45 ± 0.5 mm) | CR, DR | W8 |
| 10 | Test Pattern(s) for evaluation of electronic display device performance and laser film printer (ex. TG18-QC, TG-18 UNL80) | CR, DR | W9, W10 |
| 11 | Magnifying lens (4x to 5x magnification) | CR, DR | W9, W10 |
3.3 Monthly Quality Control Testing
| Quality Control Procedures Under Evaluation |
FS | CR | DR |
|---|---|---|---|
| Monthly Quality Control Tests | |||
| Mechanical, Electrical and Overall Safety Inspection | M1 | M1 | M1 |
| Cassette, Screen and Imaging Plate Cleaning | M2 | M2 | |
| Accuracy of Processor Temperature | M3 | ||
| Replenishment rate | M4 | ||
| Extended Full Field Artefacts Evaluation | M5 | M5 | |
| Laser Printer Sensitivity | M6 | M6 | |
M1. Mechanical, Electrical and Overall Safety Inspection - A safety inspection must be performed of the following items:
- mammography acquisition ambient room temperature;
- structural integrity of mammography system components including operator shielding;
- proper system movement;
- performance of indicators, switches and meters;
- proper functioning of interlocks;
- correct image annotation on displayed and printed images;
- other items as appropriate for the specific system and facility.
M2. Cassette, Screen and Imaging Plate Cleaning - Cassettes, screens and imaging plates must be cleaned and inspected for damage. Manufacturer recommended cleaners and cleaning procedures should be used.
M3. Accuracy of Processor Temperature - The accuracy of the processor temperature display should be checked regularly against a non-mercury thermometer. The processor developer temperature should be accurate to within 0.5 °C.
M4. Replenishment Rate - The replenishment rate must be compared with the manufacturers' recommended baseline level for the particular processor and film type, for the given number of films processed daily and for the method of processing.
M5. Extended Full Field Artefacts Evaluation - A flat field image quality evaluation must be performed using all applicable focal spots, filters, and magnification modes. Image a uniform phantom and evaluate for the presence of significant artefacts that could interfere with clinical interpretation. View the "for processing" image under appropriate window width and level settings. The images must have a uniform appearance with no significant artefacts.
M6. Laser Printer Sensitometry - An evaluation must be made of the consistency of the performance of the laser printer. Printing an image of a sensitometry strip, the following criteria must be met:
- Maximum Density Dmax - Dmax is the darkest step. Dmax must be greater than or equal to the established baseline value minus 0.15 OD or 3.50 OD, whichever is less.
- Density Difference, DD - DD is the step closest to an optical density of 2.20 minus the step closest to but not less than 0.45. DD must be within ±0.15 OD of the established baseline value.
- Mid Density, MD - MD is the step closest to but not below an optical density of 1.20 or the working optical density. MD must be within ±0.15 OD of the established baseline value.
- Base plus fog (B +F) - B +F must be less than or equal to the value of the established baseline value plus 0.03 OD.
Note that this test must be performed monthly for dry processing laser printers. However, for wet processing laser printers, this test must be performed each day prior to clinical images.
| Item | Equipment | Systems | Reference |
|---|---|---|---|
| 1 | Thermometer (non-mercury) Accuracy: ± 0.3 °C Reproducibility: ± 0.1 °C |
FS | M1, M3 |
| 2 | Screen Cleaner (as recommended by manufacturer) |
FS | M2 |
| 3 | Ultraviolet Light | FS | M2 |
| 4 | Uniform phantom representing average breast thickness (ex. PMMA test object thickness 45 ± 0.5 mm) | CR, DR | M5 |
| 5 | Densitometer | CR, DR | M6 |
| 6 | Printer sensitometry strip produced by printer or sent from acquisition workstation. | CR, DR | M6 |
3.4 Quarterly Quality Control Testing
| Quality Control Procedures Under Evaluation |
FS | CR | DR |
|---|---|---|---|
| Quarterly Quality Control Tests | |||
| Fixer Retention Analysis | Q1 | ||
| Repeat Analysis | Q2 | Q2 | Q2 |
| Spatial Resolution/Modulation Transfer Function (MTF) Evaluation of CR Equipment | Q3 | ||
| Laser Printer Quality | Q4 | Q4 | Q4 |
| Film Digitizer Performance | Q5 | ||
Q1. Fixer Retention Analysis - Fixer retention tests must be performed to ensure fixer is adequately removed from processed films according to established baseline levels. The level of residual fixer must not exceed 0.05 g/m2.
Q2. Repeat Analysis - For both film/screen and digital mammography systems an analysis must be done of the repeat records to identify and correct any trends or errors. Repeat images are defined as images taken due to inadequate quality. This does not include images taken for quality control purposes, images taken to acquire additional views, or additional images taken to include tissue which could not be imaged due to breast size. Repeat records must be maintained and analysed individually for each mammography system. Facilities must maintain records for every repeat, the reason for the repeat along with any corrective actions, immediately after the repeat image is taken. If images contain some patient diagnostic information, they should be maintained in the patient file. The repeat rate must less than 5 percent and should be less than 2 percent.
Q3. Spatial Resolution/Modulation Transfer Function (MTF) Evaluation of CR Equipment - An evaluation must be made of the spatial resolution of CR mammography systems. Spatial resolution is the ability to resolve objects in a resultant image when the difference in the attenuation between the objects and the background is large compared to noise. Spatial resolution can be evaluated either by imaging an MTF test device and using appropriate software, following manufacturer's testing procedures or using an alternate method deemed acceptable by a qualified physicist. The MTF must be within the manufacturer's specifications and the established baseline levels. This test may also be performed on DR systems, however, spatial resolution is unlikely to vary significantly on a quarterly basis for these systems.
Q4. Laser Film Printer Quality - An evaluation must be made of the quality of printed images. For this test, it is recommended that a modified TG18-QC test pattern be used which emulates the images produced by each model of digital mammography in the facility, or which could be interpreted at this workstation(i.e., has the same x-y dimensions, number of bits, and a DICOM header containing appropriate values of all relevant tags). Annotate the modified TG18-QC test pattern with 5cm horizontal and vertical rulers. Note that images must be printed from both the acquisition and interpretation workstations. Examine the printed images on a viewbox. The following requirements must be met:
- The 5% patch must be visible inside of the 0% patch;
- The 95% patch must be visible inside the 100% patch;
- The finest horizontal and vertical lines pairs must be visible in all four corners;
- Resolution steps (black to white) must be distinct;
- Lines must appear straight and even;
- No artefacts upon visual inspection;
- The measurement of the 5 cm line must be between 5 ± 0.3cm.
Q5. Film Digitizer Performance - An evaluation must be made of the film digitizer performance to ensure that image quality of digitized images is comparable to that of film. The resolution of the digitized images should correspond to the nominal resolution of the digitizer.
| Item | Equipment | Systems | Reference |
|---|---|---|---|
| 1 | Fixer Retention Test Kit | FS | Q1 |
| 2 | MTF test device and software to calculate MTF | CR | Q3 |
| 3 | Test Pattern(s) for evaluation of electronic display device performance and laser film printer (ex. TG18) | CR, DR | Q4, Q5 |
| 4 | Magnifying lens (4X to 5X magnification) | FS,CR, DR | Q4 |
| 5 | Ruler | FS,CR,CR | Q4 |
3.5 Semi-Annual Quality Control Testing
| Quality Control Procedures Under Evaluation |
FS | CR | DR |
|---|---|---|---|
| Semi-Annual Quality Control Tests | |||
| Breast Compression Device | SA1 | SA1 | SA1 |
| Safelight Test for Darkroom Fog | SA2 | ||
| Screen/Film Contact | SA3 | ||
| Interplate Sensitivity Variation of Imaging Plates | SA4 | ||
SA1. Breast Compression Device - The compression device must be evaluated to verify the compression force, alignment of the compression plates, and the accuracy of the indicated breast thickness.
- The variation in the measured and displayed compression force must be within ±2.0kg (±4.5lbs, ±20 N).
- The maximum manual compression force must be less than 300N. The maximum compression force for the initial power drive must be between 11.4 kg (25 lbs, 111 N) and 20.5 kg (45 lbs, 200N). The mammography equipment must be capable of maintaining the compression force to within ± 1 kg ( 2.2 lbs, 9.8N) for at least 1 minute.
- The compression paddle must be flat and parallel to the image receptor surface and must not deflect from parallel by more than 1 cm at any point on the surface of the compression paddle when force is applied. Equipment that is designed not to be flat and parallel must meet manufacturer's specifications and maintenance requirements.
- The precision of the displayed breast thickness must be within ±8 mm of the PMMA slab thickness and should be within ±5 mm of the PMMA slab thickness.
SA2. Safelight Test for Darkroom Fog - An image of a mammographic phantom exposed to a minimum optical density of 1.4 units must not show an increase in optical density greater than 0.05 units in two minutes exposure to the darkroom light environment.
SA3. Screen/Film Contact - All cassettes used in mammography must be tested for screen/film contact using a 16 mesh/cm (40 mesh/in) copper screen. Cassettes with large areas greater than 5mm in diameter of poor contact that are not eliminated by screen cleaning and remain in the same location during subsequent tests must be replaced. Areas of poor contact greater than 2mm at the chest wall edge are not acceptable.
SA4. Interplate Sensitivity Variation of Imaging Plates - For CR equipment, an evaluation must be made of the variation of inter-plate sensitivity.
- The signal to noise ratio variation in a reference region of interest must not exceed ±15% between all clinically used imaging plates.
- The variation in the recorded entrance surface air kerma or tube loading (mAs) must not exceed ±10%.
- The must be no major inhomogeneities on the images.
| Item | Equipment | Systems | Reference |
|---|---|---|---|
| 1 | Compression force test device (conventional analog scale) | FS, CR, DR | SA1 |
| 2 | Bath towels or blocks of foam rubber (specific mass: about 30 mg/cm3) | FS, CR, DR | SA1 |
| 3 | Tape measure | FS, CR, DR | SA1, SA3 |
| 4 | PMMA slabs of uniform thickness | FS, CR, DR | SA1, SA4 |
| 5 | Densitometer Accuracy: ± 0.02 O.D. at 1.0 O.D. Reproducibility: ± 0.01 O.D. at 1.0 O.D. |
FS | SA2 |
| 6 | Stopwatch | FS | SA2 |
| 7 | Film/Screen contact test tool for mammography (40 mesh) | FS | SA3 |
3.6 Annual Quality Control Testing
| Quality Control Procedures Under Evaluation |
FS | CR | DR |
|---|---|---|---|
| Annual Quality Control Tests | |||
| Accuracy of Tube Voltage | A1 | A1 | A1 |
| Reproducibility of Tube Voltage | A2 | A2 | A2 |
| Radiation Output (Air Kerma) Reproducibility and Linearity | A3 | A3 | A3 |
| Normalized Radiation Output | A4 | A4 | A4 |
| X-ray Beam Filtration | A5 | A5 | A5 |
| Collimation | A6 | A6 | A6 |
| Light Field and x-ray Field Alignment | A7 | A7 | A7 |
| Automatic Exposure Control (AEC) | A8 | A8 | A8 |
| Image Receptor Performance | A9 | A9 | A9 |
| Image Quality | A10 | A10 | A10 |
| Dosimetry | A11 | A11 | A11 |
| Viewboxes | A12 | A12 | A12 |
| Electronic Display Device Performance | A13 | A13 | |
| Printers | A14 | A14 | |
| General Preventative Maintenance | A16 | A16 | A16 |
A1. Accuracy of Tube Voltage - At tube voltages commonly used clinically, the x-ray tube voltage must not deviate from the selected value by more than 5%.
A2. Reproducibility of Tube Voltage - At tube voltages commonly used clinically, the coefficient of variation of the kVp must be equal to or less than 0.02, based on 5 measurements at each tube voltage setting. Note however, that if the percentage difference between the first two measurements is not greater than 5%, only two measurements at each tube voltage setting is acceptable.
A3. Radiation Output (Air Kerma) Reproducibility and Linearity - The reproducibility and linearity of the air kerma must be evaluated using manual mode, a Mo/Mo target filter combination and x-ray tube voltage of 28kVp. Select 3 most commonly used mAs settings. The coefficient of variation of any five consecutive air kerma measurements must be no greater than 0.05. Note however, that if the percentage difference between the first two measurements is not greater than 5%, only two measurements at each mAs setting is acceptable. At each mAs setting, calculate the average air kerma value and the output (Y) by dividing each average air kerma value by the corresponding mAs value. For consecutive pairs of mAs settings, calculate the linearity as L=100(Y1-Y2)/(Y1+Y2). The linearity must be less than ±10%.
A4. Normalized Radiation Output - Using the values of output (Y) obtained in test A3, calculate the normalized output by applying an inverse square law correction to obtain the output at 1.0m. The normalized output at 28kVp with a Mo/Mo target filter combination must be greater than 30µGy/mAs.
A5. X-ray Beam Filtration - The first half-value layer must be determined for all commonly used clinical x-ray tube voltage settings and target/filter combinations. The first half-value layer of aluminium, measurement with the compression paddle in place, must be within the range defined by:

Formula for determining the range of values of the first half-value layer of aluminum, measured with the compression paddle in place.
The range of values of the first half-value layer of aluminum, measurement with the compression paddle in place, determined by the equation "X-ray Tube Voltage (kV) divided by 100, plus 0.03, less than or equal to HVL (mm of Aluminum) less than or equal to, X-ray Tube Voltage (kV) divided by 100, plus C".
Where:
C =
- 0.12 for Mo/Mo
- 0.19 for Mo/Rh
- 0.22 for Rh/Rh
- 0.30 for W/Rh
- 0.32 for W/Ag
- 0.25 for W/Al
A6. Collimation - As assessment must be made of the collimation to ensure full exposure of the image receptor and alignment of the compression paddle with the chest wall edge of the image receptor. The x-ray field:
- must completely cover the entire image reception area;
- must extend to the edge of the patient support that is designed to be adjacent to the chest wall of the patient and must not extend beyond this edge by more than 5 mm; and
- must not extend by more than 2 percent of the focal spot to image receptor distance beyond all other edges of the image reception area.
The alignment of the compression paddle must be such that the edge of the compression paddle:
- must not be visible in the image; and
- must not extend beyond the chest wall edge of the image receptor by more than 5mm.
A7. Light Field and X-ray Field Alignment - An assessment should be made of the alignment of the light field and the x-ray field. The separation between the perimeter of the visually defined field and that of the X-ray field must not exceed 2 percent of the focal spot to image receptor distance.
A8. Automatic Exposure Control (AEC) - The performance of the AEC must be evaluated.
- AEC - Reproducibility of AEC and Maximum Exposure Time for Film/Screen Systems - For film/screen mammography equipment, the reproducibility of the AEC must be evaluated. Using a 4.5 cm thickness of PMMA and an appropriate spacer (8 mm thickness, radiolucent U-shaped expanded polystyrene) acquire four exposures. Record the mAs for each exposure. The coefficient of variation of the mAs must not exceed 0.05. The exposure time must be less than or equal to 2 seconds in contact mode and less than or equal to 3 seconds in magnification mode.
- AEC - Constancy of Optical Density for Film/Screen Systems - The optical density of mammography films must be appropriately established and remain constant. The optical density must be at least 1.4 OD and should be in the range of 1.6 OD to 1.9 OD. Using a 4.5 cm thickness of PMMA and an appropriate spacer (8 mm thickness, radiolucent U-shaped expanded polystyrene), acquire an image with the density control at the "0" or "normal" position. Process the film and measure the optical density at a point 40 mm from the chest wall edge, centred laterally. The measured optical density values must be within ± 0.15 OD from the established baseline value.
- AEC - Object Thickness Compensation for Film/Screen Systems - The AEC device must perform in such a way that the variation of optical density in the resultant images does not exceed ± 0.15 of the mean optical density when the thickness of a uniformly attenuating breast tissue equivalent material is varied over a range of 2 to 7 cm and the tube voltage, and anode filter combinations are varied appropriately over the range used clinically in the facility. If this requirement cannot be met, a technique chart must be developed showing appropriate loading factors for different breast thickness and compositions that must be used so that optical density within ±0.15 of the average under automatic exposure control conditions can be produced.
- AEC - Optical Density Control Setting for Film/Screen Systems - The variation in the optical density of images produced by varying the optical density control selector must be in the range of 0.05 OD to 0.25 OD.
- AEC - Reproducibility for Digital Systems - For CR and DR systems, the requirements set out in test A(8)(a) for film -screen systems also apply.
- AEC - Object Thickness Compensation and Maximum Exposure time for Digital Systems - The performance of the AEC must be evaluated by jointly assessing image quality, as measured by the signal difference to noise ratio in specified conditions, and the dose to the patient, as characterized by the mean glandular dose, and comparing them to the established baseline values. Signal difference to noise ratio is determined using uniform PMMA plates of thicknesses over a range of 2 to 7 cm, appropriate spacers and a contrast object (ex. 1 mm thick, 25 mm diameter PMMA disc). Recommended values for signal difference to noise ratio for evaluating the AEC of digital mammography systems is available from the IAEA (IAEA, 2011). While ensuring limitations on mean glandular dose are respected (see A11), the signal difference to noise ratio values must meet established baselines criteria and should meet the recommended values of the IAEA. In contact mode, the exposure time must be less than or equal to 2 seconds for 45 mm of PMMA and less than or equal to 4 seconds for 70 mm of PMMA.
- AEC - Exposure Control Steps for Digital Systems (if available) - The variation in the tube loading (mAs) produced by varying the exposure control steps should provide approximately ±10% to ± 25% variation in mAs per step.
- AEC - Correspondence between AEC sensors - For mammography systems having multiple independent AEC sensors, the performance of the individual sensors should be evaluated. The ability to correctly select each sensor should be verified. The variation in optical density between all AEC sensors should be within 0.20 OD. For CR systems, the variation in mAs between all AEC sensors should be within ± 15%
- AEC - Back Up Timer - The performance of the backup timer must be verified to ensure safe performance of the equipment. For mammography equipment, the current time product must not exceed 800 mAs.
A9. Image Receptor Performance - The performance of the image receptor must be verified against the manufacturer's specifications and the established baseline values.
- Film/Screen Speed Uniformity - The uniformity of radiographic speed of all routinely used image receptors must be evaluated. The difference between the minimum and maximum film optical densities must not exceed 0.25 OD units.
- Phantom Image for Film/Screen Systems - A phantom, with image quality evaluation objects, must be used to the test imaging performance of the mammography system. For the RMI-156 mammography phantom, a minimum of the four largest fibers, the three largest speck groups and the three largest masses must be visible.
- The number of test objects of each group type (fibers, specks, and masses) visible in the phantom image must not decrease by more than one half.
- The phantom image background optical density should between 1.5 OD and 1.9 OD and must not vary by more than ±0.20 from the operating level.
- The density difference due to a 4.0 mm acrylic disc must be at least 0.40 and must not vary by more than ±0.05 from the established operating level.
- Spatial Linearity and Geometric Distortion - For systems with moving parts, such as CR or slot scanning systems, an evaluation of the spatial linearity and geometric distortion must be performed. Using a geometric distortion test tool placed directly on the surface of the patient support, acquire an image using AEC. Place the test tool on the magnification table and acquire a second image. Evaluate the images to ensure that:
- The image size must be within 10% of the manufacturer's stated nominal image size.
- The effective detector element (del) width and length (x and y) dimensions must be within 5% of each other.
- Distances measured using the annotation tool on the workstation must be within 5% of the true size.
- There should be less than 2% deviation from a straight line over a 100 mm length in the centre of the field.
- Response Function and Noise Evaluation - The response of the detector and noise characteristics with varying tube loading (current-time product) must be evaluated. Using manual mode, a uniform test block with a contrast object must be imaged for at least 4 different tube loadings (mAs) covering the range typically used clinically. Record the tube loading. For CR systems without the capability for region of interest (ROI) analysis, the exposure index must also be recorded
Using the unprocessed images, measure the mean pixel value in a region of interest (of approximately 80mm2) located entirely inside the contrast object (A) and, in a reference region of interest located just adjacent to the contrast object, measure the mean pixel value (B) and standard deviation (C). Calculate the signal-difference-to- noise ratio as SDNR = |A-B|/C. For linear systems, plot the mean pixel value inside the contrast object, the variance (i.e. standard deviation squared) and the signal-difference-to-noise-ratio against mAs. For logarithmic systems, it may be necessary to plot the mean pixel value and variance against 1/mAs. Note that for systems where a pixel value offset (Bo) has been applied, this value must be determined and subtracted. Calculate the value of (B-Bo)/mAs for all values for all tube loadings and the overall average value of this quantity.
For linear systems:- Use the plots of mean pixel value (B) and variance C2 to calculate the square of the correlation coefficient (R2). R2 must be greater than or equal to 0.95.
- All values of (B-Bo)/mAs must be within 10% of the mean value of this ratio.
- The value of (B-Bo) must be within ±10% of the established baseline value.
- The value of C must be within ± 5% of the established baseline value.
- The value of SDNR must be within ± 5% of the established baseline value.
- Plot the exposure index against the mAs and calculate R2. R2 must be greater than or equal to 0.95.
- Where the exposure index is a S#, the value of the S# must be within ±10% of the established baseline value.
- Where the exposure index is a SAL, SALlog, or PVIlog number, the value must be within ±5%, ±430, ±580 of the established baseline value respectively.
- Where the exposure index is an EI value, the value must be within 40 units of the established baseline value.
- Image Homogeneity and Artefact Assessment - Using a 45 mm thick uniform test block, covering the entire detector, obtain images at clinical settings for a breast of equivalent thickness, for all target filter combinations used. Record the exposure settings. Using the magnification stand and 25mm PMMA test block, acquire images at clinical settings and relevant target filter combinations. Record the exposure setting. Examine all unprocessed images using window and level settings appropriate for visualization of artefacts. Record the window and level settings.
- There must not be any artefacts of sufficient significance that could interfere with image interpretation (visible dead pixels, missing lines, missing columns).
- There must be no visually distracting structured noise patterns.
- There must be no regions of discernibly different density.
- There must be no regions of unexpected variation in the magnitude of noise. It should be noted that for CR systems, non-uniformity due to the heel effect cannot be removed.
- Detector Element Failure - For direct radiography systems, an inspection must be made of the defective detector elements (bad pixel map) of the digital x-ray image receptor. The number of defective detector elements must be within the manufacturer's specifications.
- Digital Detector Residual/Ghost Image - An evaluation must be made to assess the level of ghosting resulting from a previous image. Place a uniform test block covering the right half of the image receptor. Using manual technique, acquire an image at typical clinical settings for an average breast. Reposition the test block, aligned with and centred along the chest wall edge. As soon as the system is ready, acquire another image. It is important that the elapsed time between images is short (1 minute or shorter) to be consistent with typical times between images in clinical use. Note that for CR systems, this will be the time to process and retrieve the same cassette (imaging plate). Using region of interest analysis tool, determine the mean pixel values A and B and the standard deviation, C, according to the diagram shown. Calculate the signal-difference-to-noise ratio, as SNDR = |(A-B)|/C. The value of SNDR must be less than or equal to 2.0. Alternatively, viewing the second image ("for processing" version) at clinically used window setting, the image of the test block in the first position must not be visible.

Schematic of image receptor and test block set up for the evaluation of digital detector residual ghosting.
Schematic drawing of a grey horizontal rectangle representing the breast support with a second smaller white rectangle side and aligned with the bottom edge of the breast support representing a test block, and a third transparent rectangle, covering the right half of the breast support and bisecting the second rectangle along a line D representing the first position of the test block. Within the second rectangle, to the left of line D, is a circle with the letter A indicated in the middle. Within the second rectangle, to the right of line D, is a circle with the letters B and C indicated in the middle.
A10. Image Quality - The quality of mammographic images must be verified against manufacturer's specifications and established baseline levels.
- Spatial Resolution of Film/Screen Systems - Using a resolution bar pattern test tool, the minimum measured spatial resolution of the mammography system must be:
- 11 line-pairs/mm when a high contrast resolution bar test pattern is oriented with the bars perpendicular to the anode-cathode axis, and
- 13 line-pairs/mm when the bars are parallel to the axis.
- Modulation Transfer Function (MTF) - Digital Systems - A quantitative evaluation must be made of the spatial resolution of digital mammography systems. The MTF is measured by imaging an MTF test device, placed on a 45 mm of PMMA using the technique clinically used for an average breast. Using the "for processing" image and MTF software to calculate the MTF.
- The spatial frequencies at which the MTF falls to 50% and 20% must not be less than the established levels. Values of acceptable frequencies at which the MTF falls to 50% and 20% are available from the IAEA (IAEA 2011).
- The MTF at 2.5, 5 and 7.5 cycles/mm must not change more than 10% from the established baseline values.
The assessment of system spatial resolution through evaluation of the MTF is preferred, however, if this is not possible, the limiting spatial resolution, determined with the use of star or bar resolution pattern may an alternative.
A11. Dosimetry - An assessment must be made of the entrance surface air kerma and the mean glandular dose.
- Entrance Surface Air Kerma - For film-screen mammography equipment, the entrance surface air kerma (without backscatter) must be measured using the established exposure factors selected to expose a phantom equivalent to a standard breast (45 ± 0.5mm). The entrance surface air kerma must be referred to at an optical density of 1.6 to 2.0. For digital mammography equipment, the entrance air kerma (without backscatter) must be measured using the established exposure factors which produce acceptable signal levels (SDNR) for exposing PMMA thicknesses of 20 mm, 45 mm and 70 mm. Measured values of the entrance surface air kerma must be within established baseline levels.
- Standard Breast Mean Glandular Dose (MGD) Calculations -The MGD to a standard breast must not exceed 3.0 mGy and should not exceed 2.5mGy. For digital mammography systems, the MGD must be evaluated for breasts represented by 20 mm, 45 mm and 70 mm of PMMA. Guidance on the estimation of MGD is provided in Appendix III.
Alternatively, breast phantoms such as RMI-156 or NA #18-220 which represent a breast composed of 50 percent fat and 50 percent glandular tissue and compressed to 42 mm thickness, may be used to determine the representative mean glandular dose for a breast of similar composition. The mean glandular dose must not exceed 2.5 mGy and must be within established baseline level
A12. Viewboxes - All viewboxes used for the interpretation of mammograms must be tested for compliance with the following requirements. Ensure all viewboxes have been turned on for a minimum of 30 minutes before obtaining measurements.
- Luminance - The view box luminance should be at least 3,500 nits (cd/m2).
- Light Output Uniformity - The light output from the viewboxes should be uniform to within 10 percent.
- Light Output Homogeneity - The light output homogeneity between all viewboxes used for mammograms should be uniform to within 15 percent of the mean.
- Ambient Light Control - The ambient light within the reading room must be less than 40 lux.
A13. Electronic Display Device Performance - The performance of all electronic display devices, whether part of the interpretation/review workstation or acquisition workstation must be verified. The annual quality control tests recommended by the American Association of Physicists in Medicine (AAPM, 2005) including the TG18 test patterns, test procedures and acceptance criteria should be used. For this test, it is recommended that modified test patterns be used which emulate the images produced by each model of digital mammography in the facility, or which could be interpreted at this workstation(i.e., have the same x-y dimensions, number of bits, and a DICOM header containing appropriate values of all relevant tags). The display system must be warmed up prior to testing. Attention must be given to ensure ambient light levels are appropriate (20 - 40 lux) and representative of conditions under which clinical images are viewed. A viewing distance of 30 cm is recommended.
- The maximum luminance difference between monitors of the same display workstation must be within ±10%.
- Luminance Range - The ratio of maximum luminance to minimum luminance must be greater than or equal to 250 for primary display devices and greater than or equal to 100 for secondary display devices.
- Greyscale Display Function (GSDF) - Digital mammograms must appear consistently on different electronic display devices. Display and measure the luminance at the center of the screen of the TG18 luminance test patterns (TG18-LN12-01 through TG18-LN12-18). Note that this test does not apply to the acquisition workstation display devices. Use software to determine conformance with the DICOM Grayscale Standard Display Function (GSDF). The calculated contrast function must be within ±10% of the GSDF contrast response for primary class displays and within ±20% of the GSDF contrast response for secondary class displays.
- Luminance Uniformity - Contrast visibility must be uniform across all regions of the display device. Displaying TG18 test patterns TG18-UNL10 and TG18-UNL80, measure the display luminance at five locations for each monitor. The maximum luminance deviation, calculated by (Lmax-Lmin)/Lcentre must be less than 0.3 (30%).
A14. Printers - The performance of printers must be evaluated to ensure that the quality of printing is consistent and that the quality of printed images is comparable to that of images on the display monitor. For this test, it is recommended that modified test patterns be used which emulate the images produced by each model of digital mammography system in the facility, or which might be interpreted at that workstation (i.e., has the same x-y dimensions, number of bits, and a DICOM header containing appropriate values of all relevant tags).
- Geometric Distortion - Lines must appear straight and the maximum spatial deviation must not exceed 2%.
- Greyscale Display Function - It must be determined whether a printer conforms to the DICOM GSDF. Printing the TG18-PQC test pattern, measure the optical density of marked regions of the 18 bars. The GSDF is determined by the luminance corresponding to the optical density. Use software to determine conformance with the DICOM GSDF. The calculated contrast function must be within ±10% of the GSDF contrast response.
- Density Uniformity - The optical density must be uniform across all regions of the printed image. The maximum optical density deviation, calculated by (Lmax-Lmin)/Lcentre must be less than 0.1 (10%).
A15. General Preventive Maintenance - Preventive maintenance of the x-ray equipment and accessories is necessary to prolong the life of the equipment. An annual inspection must be conducted for structural integrity, cleanliness, ease of movement of all components and any other procedures recommended by the manufacturers.
| Item | Equipment | Systems | Reference |
|---|---|---|---|
| 1 | Non-invasive x-ray tube voltage meter Accuracy: ± 1.5 kV Reproducibility: ± 0.5 kV |
FS, CR, DR | A1, A2 |
| 2 | Dosemeter Accuracy: ± 5 % Reproducibility: ± 1 % |
FS, CR, DR | A3, A4, A5, A11 |
| 3 | Aluminum filter (> 99.9 % purity) Accuracy: 1 % thickness |
FS, CR, DR | A5 |
| 4 | Metal plate to shield the detector from x-rays (ex.: 1 mm steel, 5 mm Aluminium, > 0.1 mm lead) |
FS, CR, DR | A5 |
| 5 | Ruler(s) or measuring tape | FS, CR, DR | A5, A6, A7 |
| 6 | Two Radiographic Rules | CR, DR | A6 |
| 7 | Phosphorescent screen material (approx. 20 mm x 50 mm) | CR, DR | A6 |
| 8 | Contrast Objects (ex. metallic foil or markers, coins, PMMA disk) | FS, CR, DR | A6, A7, A8 |
| 9 | Multiple sheets of uniform, tissue equivalent attenuator (ex. a set of 10 mm thick PMMA plates covering the complete detector area capable of providing thicknesses of 20, 45 and 70 mm) or uniform phantom representing average breast thickness (ex. PMMA of thickness 45 ± 0.5 mm) |
FS, CR, DR | A6, A8, A9, A10, A11 |
| 10 | Appropriate spacers (ex: radioluscent U shaped rigid expanded polystyrene |
FS, CR, DR | A8, A11 |
| 11 | Stopwatch | FS, CR, DR | A8, A10 |
| 12 | Densitometer Accuracy: ± 0.02 O.D. at 1.0 O.D. Reproducibility: ± 0.01 O.D. at 1.0 O.D |
FS | A8, A9, A10, A14 |
| 13 | Phantom, with image quality evaluation objects (ex. RMI-156 ) | FS | A9, A11 |
| 14 | Magnifying lens (4x to 5x magnification) | FS, CR, DR | A9, A10, A14 |
| 15 | ROI capability or QC software for image analysis | CR, DR | A9 |
| 16 | Geometric Distortion Test Tool | CR | A9 |
| 17 | Spatial Resolution test tool (ex. resolution test pattern up to 20 lp/mm) | FS, CR, DR | A10 |
| 18 | MTF test device and software to calculate MTF | CR, DR | A10 |
| 19 | Light meter (for measurement of luminance and Illuminance) Accuracy: ± 10 % Reproducibility: ± 5 % |
FS, CR, DR | A12, A13 |
| 20 | Test Pattern(s) for evaluation of electronic display device performance and laser film printer (ex. TG18 or SMPTE) | CR, DR | A13, A14 |
| 21 | Transparent Ruler | CR, DR | A14 |
Appendix I: Federal/Provincial/Territorial Radiation Safety Agencies
Federal Government
Consumer and Clinical Radiation Protection Bureau
Health Canada
P.L. 6301A
775 Brookfield Road
Ottawa, Ontario
K1A 1C1
Therapeutic Products Directorate
Medical Devices Bureau
2934 Baseline Road, Tower B
A.L. 3403A
Ottawa, Ontario
K1A 0K9
British Columbia
WorkSafe BC
6951 Westminster Highway
Richmond, British Columbia
V7C 1C6
Alberta
Workplace Policy and Standards Development Branch
Alberta Employment, Immigration and Industry
8th floor, 10808-99th Avenue
Edmonton, Alberta
T5K 0G5
Saskatchewan
Radiation Safety
Ministry of Labour Relations and Workplace Safety
1870 Albert Street
Regina, Saskatchewan
S4P 4W1
Manitoba
Radiation Protection Services
Department of Medical Physics
CancerCare Manitoba
675 McDermot Avenue
Winnipeg, Manitoba
R3E 0V9
Ontario (for issues related to patient and public safety)
Ontario Ministry of Health and Long-Term Care
X-ray Inspection Service
55 St Clair Avenue West, 8th floor
Toronto, Ontario
M4V 2Y7
Ontario (for issues related to worker safety)
Ministry of Labour
Radiation Protection Service
81A Resources Road
Weston, Ontario
M9P 3T1
Quebec
Direction générale de la santé publique
Ministère de la Santé et des Services sociaux
1075, Chemin Ste-Foy, 11e étage
Ste-Foy, Québec
G1S 2M1
New Brunswick
Radiation Protection Services
Department of Health and Wellness
P.O. Box 5100, Carleton Place
3rd Floor
Fredericton, New Brunswick
E3B 5G8
Nova Scotia
Occupational Health and Safety Division
Nova Scotia Department of Environment and Labour
P.O. Box 697
Halifax, Nova Scotia
B3J 2T8
Prince Edward Island
Department of Health and Wellness
Government of Prince Edward Island
P.O. Box 2000
Charlottetown, Prince Edward Island
C1A 7N8
Newfoundland and Labrador
Department of Labour
West Block, 4th floor, Confederation Bldg.
P.O. Box 8700
St. John, Newfoundland
A1B 4J6
Northwest Territories
Occupational Health and Safety
Government of the Northwest Territories
Box 1320
Yellowknife, Northwest Territories
X1A 2L9
Yukon Territory
Occupational Health and Safety
Yukon Workers' Compensation Health and Safety Board
401 Strickland Street
Whitehorse, Yukon Territory
Y1A 5N8
Appendix II: Dose Limits for Occupational Ionizing Radiation Exposures
For the purpose of this Safety Code, individuals may be classified in one of two categories: (1) radiation workers, individuals who are occupationally exposed to x-rays and (2) members of the public. The dose limits are given for both categories in Table AII.1. These dose limits are based on the latest recommendations of the International Commission on Radiological Protection (ICRP) as specified in ICRP Publication 103 (ICRP 2007).
Dose limits for radiation workers apply only to irradiation resulting directly from their occupation and do not include radiation exposure from other sources, such as medical diagnosis and background radiation.
| Applicable Body Organ or Tissue | Radiation Workers | Members of the Public |
|---|---|---|
| Whole Body | 20 mSv effective dose per year averaged average over a defined 5 year period and 50mSv in any single year. | 1mSv effective dose |
| Lens of the eye | 20 mSv equivalent dose per year averaged over a defined 5 year period and 50mSv in any single year. |
15 mSv |
| Skin | 500 mSv equivalent dose | 50 mSv equivalent dose |
| Hands and Feet | 500 mSv equivalent dose | - |
|
||
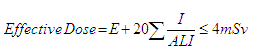
Formula for the calculation of effective dose
Effective dose is equal to E plus 20 times the summation of I divided by ALI less, than or equal to 4mSv
- It is emphasized that any irradiation involves some degree of risk and the levels suggested in this Appendix are maximum values. All doses must be kept as low as reasonably achievable and any unnecessary radiation exposure must be avoided.
- The ICRP does not recommend discrimination in the dose limits between men and women of reproductive capacity, if the dose is received at an approximately regular rate.
- For radiation worker women, once pregnancy has been declared, the foetus must be protected from x-ray exposure for the remainder of the pregnancy. For women who are also radiation workers, an effective dose limit of 4 mSv must be applied, for the remainder of the pregnancy, from all sources of radiation. It is calculated as follows:
where
E is the portion of the effective dose, in millisieverts
- received by a person from sources outside the body; and
- received by and committed to the person from sources inside the body, measured directly or from excreta,
I is the activity in bequerel, of any radionuclide that is taken into the body, excluding the radon progeny and the activity of other radionuclides accounted for in the determination of E, and
ALI, or annual limit on intake, is the activity, in bequerel, of a radionuclide that will deliver an effective dose of 20 mSv during the 50 year period after the radionuclide is taken into the body of a person 18 years or older or during the period beginning at intake and ending at age 70 after it is taken into the body of a person less than 18 years old.
Under the scope of this document, occupational exposures to pregnant workers arise mainly from scattered X-radiation. In this case, the most effective method of monitoring exposures to the foetus is to measure the equivalent dose to the surface of the abdomen using a personal radiation dosimeter.
- For technologists-in-training and students, the recommended dose limits for members of the public should apply.
- ICRP does not recommend different limits for individual organs. For radiation workers, ICRP believes that deterministic effects will be prevented by applying an equivalent dose limit of 500 mSv in a year to all tissues except the lens of the eye, for which it recommends a limit of 20 mSv in a year.
- For the skin, the equivalent dose is averaged over its whole area. In situations where deterministic effects are possible, the recommended equivalent dose limit for the skin is 500 mSv and is averaged over areas of no more than 1 cm2. This limit applies to the skin of the face and hands.
- Some provincial or territorial jurisdictions may have different dose limits for some workers. The appropriate agency, listed in Appendix I, should be consulted to determine the dose limits in effect in a particular jurisdiction.
Appendix III: Calculation of Mean Glandular Dose
Method 1: Mean glandular dose calculation for breasts represented by PMMA thicknesses of 20, 45 and 70 mm.
The mean glandular dose, MGD which is the absorbed dose to the glandular tissues within the breast can be determined using the following formula:
MGD = Ki,t × gt × ct × s
Ki,t is the entrance air kerma (without backscatter) at the upper surface of the PMMA simulating a standard breast of thickness t mm
gt is the air kerma to MGD conversion factor for a breast having 50% fibroglandular tissue and 50% fat composition with a thickness of t mm (provided in Table AIII.1)
ct is the conversion factor for any difference in the breast composition from 50% glandularity of a standard breast with a thickness of t mm (provided in Table AIII.2)
s is the correction factor for the use of target/filter combinations other than Mo/Mo (provided in Tables AIII.3 and AIII.4)
Note that the factors gt and ct depend on the HVL of the x-ray beam measured with the compression paddle in the beam. The measured valued HVL value of the mammography system must be used. Typical measured HVL values for different tube voltage and target/filter combinations are shown in Table AIII.5.
| PMMA thickness (mm) | Equivalent breast thickness (mm) | g-factors (mGy/mGy) HVL (mm Al) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.30 | 0.35 | 0.40 | 0.45 | 0.50 | 0.55 | 0.60 | ||
| 20 | 21 | 0.329 | 0.378 | 0.421 | 0.460 | 0.496 | 0.529 | 0.559 | 0.585 |
| 30 | 32 | 0.222 | 0.261 | 0.294 | 0.326 | 0.357 | 0.388 | 0.419 | 0.448 |
| 40 | 45 | 0.155 | 0.183 | 0.208 | 0.232 | 0.258 | 0.285 | 0.311 | 0.339 |
| 45 | 53 | 0.130 | 0.155 | 0.177 | 0.198 | 0.220 | 0.245 | 0.272 | 0.295 |
| 50 | 60 | 0.112 | 0.135 | 0.154 | 0.172 | 0.192 | 0.214 | 0.236 | 0.261 |
| 60 | 75 | 0.088 | 0.106 | 0.121 | 0.136 | 0.152 | 0.166 | 0.189 | 0.210 |
| 70 | 90 | 0.086 | 0.098 | 0.111 | 0.123 | 0.136 | 0.154 | 0.172 | |
| 80 | 103 | 0.074 | 0.085 | 0.096 | 0.106 | 0.117 | 0.133 | 0.149 | |
| PMMA thickness (mm) | Equivalent breast thickness (mm) | Glandularity of equivalent breast |
c-factors HVL (mm Al) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.30 | 0.35 | 0.40 | 0.45 | 0.50 | 0.55 | 0.60 | |||
| 20 | 21 | 97 | 0.889 | 0.895 | 0.903 | 0.908 | 0.912 | 0.917 | 0.921 |
| 30 | 32 | 67 | 0.940 | 0.943 | 0.945 | 0.946 | 0.949 | 0.952 | 0.953 |
| 40 | 45 | 41 | 1.043 | 1.041 | 1.04 | 1.039 | 1.037 | 1.035 | 1.034 |
| 45 | 53 | 29 | 1.109 | 1.105 | 1.102 | 1.099 | 1.096 | 1.091 | 1.088 |
| 50 | 60 | 20 | 1.164 | 1.16 | 1.151 | 1.15 | 1.144 | 1.139 | 1.134 |
| 60 | 75 | 9 | 1.254 | 1.245 | 1.235 | 1.231 | 1.225 | 1.217 | 1.207 |
| 70 | 90 | 4 | 1.299 | 1.292 | 1.282 | 1.275 | 1.270 | 1.260 | 1.249 |
| 80 | 103 | 3 | 1.307 | 1.299 | 1.292 | 1.287 | 1.283 | 1.273 | 1.262 |
|
|||||||||
| Target Filter Combination | Filter thickness (µm) | S Factor |
|---|---|---|
| Mo/Mo | 30 | 1.000 |
| Mo/Rh | 25 | 1.017 |
| Rh/Rh | 25 | 1.061 |
| W/Rh | 50-60 | 1.042 |
| W/Ag | 50-75 | 1.042 |
| PMMA thickness (mm) | Equivalent breast thickness (mm) | S factor |
|---|---|---|
| 20 | 21 | 1.075 |
| 30 | 32 | 1.104 |
| 40 | 45 | 1.134 |
| 45 | 53 | 1.149 |
| 50 | 60 | 1.160 |
| 60 | 75 | 1.181 |
| 70 | 90 | 1.198 |
| 80 | 103 | 1.208 |
| HVL (mm Al) for target filter combination | |||||
|---|---|---|---|---|---|
| kV | Mo + 30 μm Mo | Mo +25 μm Rh | Rh +25 μm Rh | W +50 μm Rh | W +0.45 μm Al |
| 25 | 0.33 ± .02 | 0.40 ± .02 | 0.38 ± .02 | 0.52 ± .03 | 0.31 ± .03 |
| 28 | 0.36 ± .02 | 0.42 ± .02 | 0.43 ± .02 | 0.54 ± .03 | 0.37 ± .03 |
| 31 | 0.39 ± .02 | 0.44 ± .02 | 0.48 ± .02 | 0.56 ± .03 | 0.42 ± .03 |
| 34 | 0.47 ± .02 | 0.59 ± .03 | 0.47 ± .03 | ||
| 37 | 0.50 ± .02 | 0.51 ± .03 | |||
|
|||||
Method 2: Mean glandular dose calculation for breasts composed of 50% fibroglandular tissue and 50% adipose tissue representedby a 4.2 cm phantom.
The mean glandular dose (in millirad) is determined by multiplying the entrance exposure in air (in roentgens) by a conversion factor which is dependent upon the x-ray tube voltage (kVp) and the half-value layer for a given target filter combinations, using the following formula:
MGD = K × CF
K is the entrance exposure in air in roentgens
CF is the conversion factor in millirads per roentgen (provided in Tables AIII.6, AIII.7 and AIII.8)
| X-Ray Tube Voltage (kVp) | W/Al Target-Filter Combination |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HVL | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | |
| 0.23 | 116 | |||||||||||
| 0.24 | 121 | 124 | ||||||||||
| 0.25 | 126 | 129 | 131 | |||||||||
| 0.26 | 130 | 133 | 135 | 138 | ||||||||
| 0.27 | 135 | 138 | 140 | 142 | 143 | |||||||
| 0.28 | 140 | 142 | 144 | 146 | 147 | 149 | ||||||
| 0.29 | 144 | 146 | 148 | 150 | 151 | 153 | 154 | |||||
| 0.30 | 149 | 151 | 153 | 155 | 156 | 157 | 158 | 159 | 170 | |||
| 0.31 | 154 | 156 | 157 | 159 | 160 | 161 | 162 | 163 | 164 | 175 | ||
| 0.32 | 158 | 160 | 162 | 163 | 164 | 166 | 167 | 168 | 168 | 170 | 171 | 180 |
| 0.33 | 163 | 165 | 166 | 168 | 169 | 170 | 171 | 173 | 173 | 174 | 175 | 185 |
| 0.34 | 168 | 170 | 171 | 172 | 173 | 174 | 175 | 176 | 177 | 178 | 179 | 190 |
| 0.35 | 174 | 175 | 176 | 177 | 178 | 179 | 180 | 181 | 182 | 183 | 194 | |
| 0.36 | 179 | 181 | 182 | 183 | 184 | 185 | 185 | 186 | 187 | 199 | ||
| 0.37 | 185 | 186 | 187 | 188 | 189 | 190 | 191 | 191 | 204 | |||
| 0.38 | 190 | 191 | 192 | 193 | 194 | 195 | 195 | 208 | ||||
| 0.39 | 196 | 197 | 198 | 198 | 199 | 200 | 213 | |||||
| 0.40 | 201 | 202 | 203 | 201 | 204 | 217 | ||||||
| 0.41 | 206 | 207 | 208 | 208 | 221 | |||||||
| 0.42 | 211 | 212 | 212 | 225 | ||||||||
| 0.43 | 215 | 216 | 230 | |||||||||
| 0.44 | 220 | 234 | ||||||||||
| 0.45 | 238 | |||||||||||
|
||||||||||||
| X-Ray Tube Voltage (kVp) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HVL | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 |
| 0.28 | 149 | 151 | 154 | ||||||||
| 0.29 | 154 | 156 | 158 | 159 | |||||||
| 0.30 | 158 | 160 | 162 | 162 | 162 | 163 | |||||
| 0.31 | 163 | 164 | 166 | 166 | 166 | 167 | 167 | ||||
| 0.32 | 167 | 169 | 171 | 171 | 171 | 171 | 172 | 172 | |||
| 0.33 | 171 | 173 | 175 | 176 | 176 | 176 | 176 | 177 | |||
| 0.34 | 176 | 178 | 179 | 179 | 180 | 180 | 180 | 181 | 181 | ||
| 0.35 | 180 | 181 | 183 | 183 | 184 | 185 | 185 | 186 | 187 | ||
| 0.36 | 185 | 186 | 187 | 187 | 188 | 188 | 189 | 190 | 191 | 191 | |
| 0.37 | 189 | 190 | 191 | 191 | 192 | 193 | 193 | 194 | 195 | 195 | |
| 0.38 | 193 | 194 | 196 | 196 | 197 | 197 | 197 | 198 | 199 | 199 | 200 |
| 0.39 | 198 | 199 | 200 | 200 | 201 | 201 | 202 | 202 | 203 | 203 | 204 |
| 0.40 | 202 | 203 | 204 | 204 | 205 | 205 | 206 | 207 | 208 | 208 | 208 |
| 0.41 | 206 | 207 | 208 | 208 | 209 | 209 | 210 | 211 | 212 | 212 | 212 |
| 0.42 | 211 | 211 | 212 | 212 | 213 | 213 | 214 | 215 | 216 | 216 | 217 |
| 0.43 | 215 | 216 | 217 | 217 | 218 | 218 | 219 | 219 | 220 | 220 | 221 |
| 0.44 | 220 | 220 | 221 | 221 | 222 | 222 | 223 | 223 | 224 | 224 | 225 |
| 0.45 | 224 | 224 | 225 | 225 | 226 | 226 | 227 | 227 | 228 | 228 | 229 |
| 0.46 | 228 | 229 | 229 | 230 | 231 | 231 | 232 | 233 | 233 | 234 | |
| 0.47 | 233 | 233 | 234 | 235 | 235 | 236 | 237 | 237 | 238 | ||
| 0.48 | 238 | 238 | 239 | 240 | 240 | 241 | 241 | 242 | 242 | ||
| 0.49 | 242 | 243 | 243 | 244 | 244 | 245 | 245 | 246 | |||
| 0.50 | 247 | 247 | 248 | 248 | 249 | 250 | 251 | ||||
| 0.51 | 251 | 252 | 253 | 254 | 254 | 255 | |||||
| 0.52 | 257 | 257 | 258 | 258 | 259 | ||||||
| 0.53 | 261 | 261 | 262 | 263 | 264 | ||||||
| 0.54 | 265 | 266 | 267 | 268 | |||||||
| 0.55 | 269 | 270 | 271 | 272 | |||||||
| 0.56 | 275 | 276 | 276 | ||||||||
| 0.57 | 279 | 280 | 281 | ||||||||
| 0.58 | 284 | 285 | |||||||||
| 0.59 | 288 | 289 | |||||||||
| 0.60 | 293 | ||||||||||
| X-Ray Tube Voltage (kVp) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HVL | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 |
| 0.28 | 150 | 155 | 159 | ||||||||
| 0.29 | 155 | 160 | 164 | 168 | |||||||
| 0.30 | 160 | 164 | 168 | 172 | 176 | ||||||
| 0.31 | 168 | 168 | 172 | 174 | 180 | 182 | |||||
| 0.32 | 169 | 173 | 177 | 181 | 184 | 186 | 188 | ||||
| 0.33 | 174 | 178 | 181 | 185 | 188 | 190 | 192 | ||||
| 0.34 | 179 | 183 | 186 | 190 | 193 | 195 | 196 | 199 | |||
| 0.35 | 184 | 187 | 190 | 194 | 197 | 199 | 201 | 203 | |||
| 0.36 | 189 | 192 | 195 | 198 | 201 | 204 | 205 | 207 | 209 | ||
| 0.37 | 193 | 196 | 199 | 202 | 205 | 207 | 209 | 211 | 213 | ||
| 0.38 | 198 | 201 | 204 | 207 | 209 | 211 | 213 | 215 | 217 | 219 | 221 |
| 0.39 | 203 | 206 | 208 | 211 | 214 | 216 | 217 | 219 | 221 | 223 | 224 |
| 0.40 | 208 | 211 | 213 | 216 | 218 | 220 | 221 | 223 | 224 | 226 | 228 |
| 0.41 | 213 | 215 | 217 | 220 | 222 | 224 | 225 | 227 | 228 | 230 | 232 |
| 0.42 | 218 | 220 | 222 | 224 | 226 | 228 | 229 | 231 | 232 | 234 | 236 |
| 0.43 | 222 | 224 | 226 | 228 | 230 | 232 | 233 | 235 | 236 | 238 | 240 |
| 0.44 | 227 | 229 | 231 | 233 | 235 | 237 | 238 | 239 | 240 | 242 | 243 |
| 0.45 | 232 | 234 | 235 | 237 | 239 | 241 | 242 | 243 | 244 | 246 | 247 |
| 0.46 | 239 | 241 | 243 | 245 | 246 | 247 | 248 | 250 | 251 | ||
| 0.47 | 247 | 249 | 250 | 251 | 252 | 254 | 255 | ||||
| 0.48 | 251 | 253 | 254 | 255 | 256 | 258 | 259 | ||||
| 0.49 | 257 | 258 | 259 | 260 | 261 | 262 | |||||
| 0.50 | 261 | 262 | 263 | 264 | 265 | 266 | |||||
| 0.51 | 266 | 267 | 268 | 269 | 270 | ||||||
| 0.52 | 270 | 271 | 272 | 273 | 274 | ||||||
| 0.53 | 275 | 276 | 276 | 277 | 278 | ||||||
| 0.54 | 279 | 280 | 280 | 281 | |||||||
| 0.55 | 283 | 284 | 284 | 285 | |||||||
| 0.56 | 288 | 288 | 289 | ||||||||
| 0.57 | 292 | 293 | |||||||||
| 0.58 | 296 | 297 | |||||||||
| 0.59 | 300 | ||||||||||
| 0.60 | 304 | ||||||||||
Appendix IV: Shielding Guides for Storage of Mammographic Film
The following table provides the thicknesses of lead required to reduce the radiation level to film and loaded cassettes to 1.75 µGy (0.2 mR) for a weekly workload of 1000 mA-min at 35 kilovolts peak.
| Distance from x-ray tube to stored films | |||||
|---|---|---|---|---|---|
| 1 m | 2 m | 3 m | 4 m | 5 m | |
| 1 day | 0.4 mm | 0.3 mm | 0.2 mm | 0.2 mm | 0.1 mm |
| 1 week | 0.5 mm | 0.4 mm | 0.4 mm | 0.3 mm | 0.3 mm |
| 1 month | 0.6 mm | 0.5 mm | 0.4 mm | 0.4 mm | 0.4 mm |
| 1 year | 0.7 mm | 0.6 mm | 0.6 mm | 0.6 mm | 0.5 mm |
It should be noted that CR cassettes loaded with an imaging plate typically have a faster rate of use than loaded film cassettes and therefore are stored for shorter periods of time (NCRP 147). For storage of loaded CR cassettes, the manufacturers' specified shielding levels must be followed.
Appendix V: Radiation Measurements Units
Exposure
Following the lead of the International Electrotechnical Commission, the air kerma (in Gray, Gy) replaces the exposure (in Roentgen, R) as the measure of exposure. The relationship between the two units is as follows:
- 1 Gy ~ 115 R
- 1 mGy ~ 115 mR
- 1 R ~ 8.73 mGy
- 1mR ~ 8.73 µGy
Absorbed Dose
The Gray (Gy) replaces the rad (rad) as the unit of absorbed dose. The relationship between the two units is as follows:
- 1 Gy ~ 100 rad
- 1mGy ~ 100 mrad
- 1 rad ~ 10 mGy
- 1 rad ~ 10 mGy
Equivalent Dose
The Sievert (Sv) replaces the rem (rem) as the unit of equivalent dose. The relationship between the two units is as follows:
- 1 Sv ~ 100 rem
- 1 mSv ~ 100 mrem
- 1 rem ~ 10 mSv
- 1mrem ~ 10 µSv
Note: m = milli = 10-3; µ = micro = 10-6
Appendix VI: GlossaryFootnote 1
"absorbed dose"
the mean energy deposited by ionizing radiation to a volume of matter divided by the mass of that volume. The unit of measurement is the gray (Gy)
"air kerma"
the energy deposited per unit mass in air. The unit used to measure air kerma is the gray (Gy). For x-rays with energies less than 300 kilo-electron volts (keV) the magnitude of air kerma and absorbed dose in air are equivalent.
"artefact"
any structure or pattern visible in the image that is not part of the object being imaged
"attenuation"
reduction of a radiation quantity upon passage of the radiation through matter resulting from all types of interactions with this matter
"automatic exposure control"
in an x-ray equipment, mode of operation in which one or more loading factors are controlled automatically in order to obtain, at a pre-selected location, a desired quantity of radiation
"beam limiting device"
device to limit the radiation field
"cassette"
a light-tight case for holding intensifying screens and film or a CR plate
"coefficient of variation"
the ratio of the estimated standard deviation to the mean value of a series of measurements, calculated by using the following equation:

Equation for the calculation of the coefficient of variation
The coefficient of variation, calculated using the formula "C equals S divided by X bar, equals, open square bracket, sum from i equals one to n, open parenthesis, X sub i minus X bar, close parenthesis, squared, all divided by n minus one, close square bracket, to the power of one half.
where
Xi = the value of the ith measurement
= the mean value of the n measurements
S = standard deviation
n = number of measurements
C = the coefficient of variation
"control panel"
part of equipment for the purpose of controlling all, or some, of the functions of the
equipment. The control panel may contain devices for indicating and displaying operating
factors.
"densitometer"
an instrument for measuring the optical density or degree of blackening of film
"effective dose"
measure of dose designed to reflect the amount of radiation detriment. The effective dose is obtained by multiplying the equivalent dose of each tissue or organ by an appropriate tissue weighting factor and summing the products. The unit of measurement is the sievert (Sv).
"equivalent dose"
measure of the dose to a tissue or organ designed to reflect the amount of harm caused to the tissue or organ. The equivalent dose is obtained by multiplying the absorbed dose by a radiation weighting factor to allow for the biological effectiveness of the various types of radiation in causing harm to tissue. The unit of measurement is the sievert (Sv)
"filter"
material or device which modifies the characteristics of the radiation beam as the beam passes through it
"fog"
the unwanted signal added to an image by the exposure of the image receptor to light, radiation or heat
between patient exposures
"half-value layer" or "HVL"
thickness of a specified material, which attenuates, under narrow beam conditions, x-rays with a particular spectrum to an extent such that the air kerma rate, exposure rate or absorbed dose rate is reduced to one half of the value that is measured without the material.
"image receptor"
device, intended to convert x-ray patterns into another form, from which a visible image is obtained either directly or indirectly. The x-ray pattern is the information contained in an x-ray beam in which the distribution of intensity has been modulated by the object passed.
"leakage radiation"
ionizing radiation which has passed through the protective shielding of a radiation source as well as that which, for some types of x-ray generators, has passed through the radiation aperture before and after loading.
"light field"
the area illuminated by light in the plane of the image receptor simulating the radiation field
"loading factor"
factor influencing by its value the x-ray tube load, for example x-ray tube current, loading time, continuous anode input power, x-ray tube voltage and percentage
"mean glandular dose"
mean absorbed dose in the glandular tissue (excluding skin) in a uniformly compressed breast of known tissue composition, using a specified calculation method
"phantom"
a device that simulates some aspect of human anatomy
"PMMA"
polymethyl methacrylate, also known by the generic name acrylic and the trade names Plexiglas,
Acrylate, Lucite and Perspex
"sensitometer"
device permitting the exposure of film in a reproducible manner to different levels of light.
"x-ray tube assembly"
the x-ray tube housing with an x-ray tube installed
"x-ray field"
area on a surface intersected by an x-ray beam within which the radiation intensity exceeds a specific or specified level
Reference
AAPM (2005). American Association of Physicists in Medicine. Assessment of Display Performance for Medical Imaging Systems, AAPM On-Line Report No. 03 (American Association of Physicists in Medicine Task Group 18 Imaging Informatics Subcommittee).
ACR (1999). American College of Radiologists. Mammography Quality Control Manual 1999.
Bick, U., Diekmann, F., Digital Mammography, Medical Radiology - Diagnostic Imaging and Radiation Oncology 2010, 88-102.
CAR (2008) Canadian Association of Radiologists, CAR Standards for Teleradiology
http://www.car.ca/uploads/standards%20guidelines/Standard_Teleradiology_EN.pdf
CAR (2011). CAR Position Statement on the Use of Thyroid Shields. http://www.car.ca/uploads/about/201106_ps_car_thyroid_shield.pdf
CAR (2012). Canadian Association of Radiologists. http://www.car.ca/en/standards-guidelines/guidelines.aspx
CAR MAP (2011). Canadian Association of Radiologists Mammography Accreditation Program. http://www.car.ca/en/accreditation/map.aspx
Coldman, A.J., Major, D., Doyle, G.P., et al. Organized Breast Screening Programs in Canada: Effect of Radiologist Reading Volumes on Outcomes. Radiology 2006;238(3): 809-815. http://radiology.rsnajnls.org/cgi/content/abstract/238/3/809)
D.R. DANCE et al. Additional factors for the estimation of mean glandular breast dose using the UK mammography dosimetry protocols , Physics in medicine and biology, vol. 45, nº 11, November 2000, p. 3237.
Gennaro, G.et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study Eur Radiol (2010) 20: 1545-1553 DOI 10.1007/s00330-009-1699-5
HC (1995). Health Canada. http://www.hc-sc.gc.ca/ewh-semt/pubs/radiation/safety-code_33-securite/index-eng.php
Helvie, M.A. "Digital Mammography Imaging: Breast Tomosynthesis and Advanced Applications", Radiologic Clinics of North America, Volume 48, Issue 5, September 2010, Pages 917-929, ISSN 0033-8389, 10.1016/j.rcl.2010.06.009.
IAEA (2009) International Atomic Energy Agency Human Health Series No.2. Quality Assurance Programme for Screen Film Mammography http://www-pub.iaea.org/MTCD/publications/PDF/Pub1381_web.pdf
IAEA (2011) International Atomic Energy Agency Human Health Series No.17. Quality Assurance Programme for Digital Mammography http://www-pub.iaea.org/books/iaeabooks/8560/Quality-Assurance-Programme-for-Digital-Mammography
ICRP (2007). International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection, ICRP Publication 103, Annals of the ICRP 37 (2-4)1-332.
ICRP (2011). International Commission on Radiological Protection. Statement on Tissue Reactions, April 21, 2011. http://www.icrp.org/docs/ICRP%20Statement%20on%20Tissue%20Reactions.pdf
IEC (2007). International Electrotechnical Commission. Evaluation and routine testing in medical imaging departments - Part 3-2: Acceptance tests - Imaging performance of mammographic x-ray equipment, 2nd ed., IEC 61223-3-2.
IEC (2008). International Electrotechnical Commission. Medical electrical equipment - Part 1-3: General requirements for basic safety and essential performance - Collateral Standard: Radiation protection in diagnostic x-ray, 2nded., IEC 60601-1-3.
IEC (2011). International Electrotechnical Commission. Medical electrical equipment - Part2-45: Particular requirements for the basic safety and essential performance of mammographic x-ray equipment and mammographic stereotactic devices, 3rd. ed.,IEC 60601-2-45.
IHE (2007). Integrating the Healthcare Enterprise Radiology: Mammography User's Handbook Revision 1.0. IHE, Chicago. http://www.ihe.net/Resources/handbook.cfm
IHE (2009). Integrating the Healthcare Enterprise Radiologie : Technical Framework Supplement Extensions to the Portable Data for Imaging (PDI) Integration Profile Revision 1.0. IHE. http://www.ihe.net/technical_framework/index.cfm
MSSS (2001). Manuel de contrôle de la qualité en mammographie - Volume 1 - Technologue en radiologie http://msssa4.msss.gouv.qc.ca/fr/document/publication.nsf/
fb143c75e0c27b69852566aa0064b01c/fe422dad3353003985256ad300698cca?OpenDocument
MSSS (2006). Manuel de contrôle de la qualité pour la mammographie et la biopsie guidée par stéréotaxie (PQDCS) - Volume 2http://msssa4.msss.gouv.qc.ca/fr/document/publication.nsf/
4b1768b3f849519c852568fd0061480d/1b95146d6a98fa1d8525719400538e57?OpenDocument
NCRP (2004). National Council on Radiation Protection and Measurement. Structural Shielding Design for Medical x-ray Imaging Facilities, NCRP Report No. 147 (National Council on Radiation Protection and Measurements, Bethesda, Maryland).
NEMA (2001). National Electrical Manufacturers Association. Digital Imaging and Communications in Medicine (DICOM) Part 14:Grayscale Standard Display Function, National Electrical Manufacturers Association, Roslyn, VA.
Radiation Emitting Devices Act, R. S., C.34.
Radiation Emitting Devices Regulation, C.R.C., c. 1370, s. 3, Part XII Diagnostic X-ray Equipment.
Stanton, L., et al. Dosage evaluation in mammography, Radiology, no 150, 1984, p. 577-584.
Van Engen, R., Van Wouldenberg, S., Bosmans, H., Young, K., Thijssen, M. European Protocol for the Quality Control of the Physical and Technical Aspects of Mammography Screening - Screen-Film Mammography. In: European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, 4th ed. European Commission, Luxembourg, (2006a).
Van Engen, R., Young, K., Bosmans, H., Thijssen, M. European Protocol for the Quality Control of the Physical and Technical Aspects of Mammography Screening - Digital Mammography. In: European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, 4th ed. European Commission, Luxembourg, (2006b). Van Ongelval, C., Bellon, E., Deprez, T., Van Steel, A., Marchal, G., "Digital Workflow, PACS, and Telemammography", Digital Mammography, (BICK, U., DIEKMANN, F., Eds), Springer-Verlag, Berlin-Heidelberg-New York, (2010).
U.Bick, F. Diekmann, Digital Mammography, Medical Radiology - Diagnostic Imaging and Radiation Oncology 2010, 88-102.
WU, X. Breast dosimetry in screen-film mammography, In Barnes, G.T. , and G.T. Frey, Screenfilm Mammography: Imaging Consideration and Medical Physics Responsibilities, Madison (Wisconsin), Medical Physics Publishing, 1991.
WU, X., et al. Normalized average glandular dose in molybdenum target-rhodium filter and
rhodium target-rhodium filter mammography, Radiology, vol. 193, n° 1, October 1994, p. 83-89.