Management of Applications for Import or Export Permits for Controlled Drugs / Substances or Precursors

Download the alternative format
(PDF format, 431 KB, 7 pages)
Organization: Health Canada
Published: 2020-02-XX
Table of Contents:
- Purpose
- Scope
- Communicating with the OCS
- Before filing an application
- Filing an application
- Processing an application
- Unsolicited information
- Table A - service standards for processing application for import/export permit for controlled drugs/substances or precursor
1. Purpose
The purpose of this policy is to outline the manner in which the Office of Controlled Substances (the OCS) manages Import and Export Permit applications for Controlled Drugs and Substances, and Class A and Class B Precursors submitted in accordance with the Controlled Drugs and Substances Act (CDSA), the Food and Drugs Act (FDA), and the following Regulations:
- Narcotic Control Regulations (NCR);
- Benzodiazepines and Other Targeted Substances Regulations (BOTSR);
- Precursor Control Regulations;
- Part G of the Food and Drug Regulations (FDR); and,
- Part J of the FDR.
This policy also outlines the responsibilities and expectations of applicants before and throughout the processing of an application.
2. Scope
This policy applies to the following types of Permit applications:
- Application for Import Permit for Controlled Drugs/Substances or Precursor; and
- Application for Export Permit for Controlled Drugs/Substances or Precursor.
3. Communicating with the OCS
All information dealing with applications identified in the SCOPE section is to be sent via e-mail to hc.permitspermis.sc@canada.ca . The OCS communicates with applicants through generic e-mail accounts for receiving and processing applications. Applicants may contact the Authorization Division's general phone number 613-954-4760 and be redirected to the appropriate administrative section, if there is a need to speak with an OCS representative.
NOTES:
- Generic e-mail accounts are monitored daily on working days, and e-mails are assigned to the appropriate Officer.
- An automatic acknowledgement reply is generated for all e-mails received.
- E-mails which are sent directly to an OCS employee, including CC'ed and BCC'ed, will be deleted without return notification. If an applicant knows the name of the OCS representative who is already working on their application, the OCS representative's name should be identified in the body of any e-mail being sent via the generic e-mail account.
- The OCS may adapt its first in first out approach to the prioritization of applications, on a case-by-case basis.
4. Before filing an application
It is important to be aware of the following information before filling an application:
- It is the applicant's responsibility to be familiar with the legislative and regulatory requirements which govern controlled drugs and substances, and Class A and B Precursors as identified in the PURPOSE section of this policy. Access to the most current version of these documents is available through the Department of Justice website, http://laws-lois.justice.gc.ca/eng/acts/, and http://laws-lois.justice.gc.ca/eng/regulations/.
- The application forms listed in the SCOPE section above are available on the Health Canada website, (identify website name and URL).
5. Filing an application
Permit application forms are provided in electronic PDF fillable format through the Health Canada website and are intended to be completed in the same electronic format. The OCS cannot commit to the service standards outlined in this policy for applications which are completed and submitted in hand written form, or for applications that are not submitted electronically using the most recent version of the application form.
Correctly completed application packages provide benefits to both the applicant and the OCS, some of which include:
- Ensures clearly legible information, reducing the likelihood of errors in data entry;
- Ensures adherence to standard submission content; and
- Enables efficient application processing and faster response times.
All information and data submitted in support of a Permit remains the property of Health Canada.
6. Processing an application
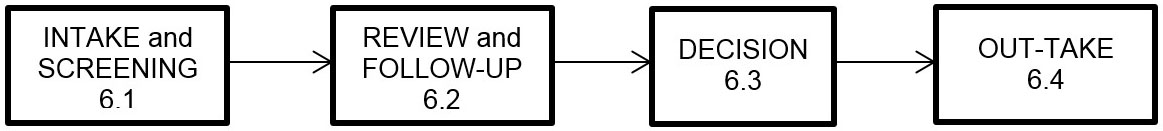
In general, Permit applications are processed through four stages, as illustrated below: INTAKE and SCREENING, REVIEW and FOLLOW-UP, DECISION, and OUT-TAKE. All applications are processed on a first-in first-out basis.
The OCS targets to meet these service standards for the processing of the following types of applications:
- Import Permit applications - within 45 calendar days
- Export Permit applications - within 45 calendar days
NOTE : Whenever a Dealer's Licence or Registration is undergoing an amendment, is subject to other licence related modifications (e.g. change of incorporation), is subject to regulatory restrictions (e.g. licence or registration suspension), or where the applicant has a history of non-compliance, the OCS may not issue the Import/Export Permit, and cannot commit to the service standards outlined in this policy.
Refer to Section 8.0 Table A - Service Standards for Processing Applications for Import/Export Permit for Controlled Drug/Substance or Precursor, for further details regarding service standards.
Important Tip:
It is important that the applicant respond as quickly and as thoroughly as possible to requests for additional information throughout the processing of the application. Response due dates will be identified in every request. The OCS will consider applicant extension requests supported by valid rationales.
Please note that the service standards outlined in this policy are dependent on any additional information submitted with timeliness by the applicant. Failure to respond by the response due date will result in the refusal of the application.
6.1 Intake and screening
All Permit applications are subject to an initial triaging to sort and assign the application, and are recorded for tracking and processing purposes. An in-depth review of the information provided is not performed during INTAKE.
Applications are subsequently screened to determine whether the application form has been correctly completed and all required supporting documentation has been provided, and to identify any problematic issues with the application.
The OCS targets to complete INTAKE and SCREENING within five (5) calendar days of receipt of any type of Permit application.
Incomplete application forms or failure to include all the required supporting documentation with the application will result the application not being processed. The OCS will inform the applicant that the application is incomplete and the reasons why.
Applications that have successfully passed INTAKE and SCREENING will proceed to REVIEW and FOLLOW-UP. The OCS does not notify applicants of the status of their application as it moves through the process steps due to the short processing time.
6.2 Review and follow-up
Permit applications at REVIEW and FOLLOW-UP are subject to a thorough review to verify the content of the information provided. The OCS targets to complete REVIEW and FOLLOW-UP within:
- thirty (30) calendar days of an Import/Export Permit Application successfully completing INTAKE and SCREENING.
Where information deficiencies or questions are identified, the OCS will send a Request for Additional Information to the applicant via e-mail to identify the issues to be addressed.
During REVIEW every effort will be made by the OCS to consolidate identified information deficiencies into as few additional information requests as possible.
It is the responsibility of the applicant to provide the requested information by the response due date. Where a request is made to the applicant for additional information, and the applicant does not respond by the due date, or does not provide a complete response, the OCS may refuse the Permit application. Once refused, a new application may be required to be submitted should the applicant still wish to obtain a permit.
Permit applications that have successfully passed REVIEW and FOLLOW-UP will proceed to DECISION.
6.3 Decision
During DECISION, Permit applications undergo final review and approval.
The OCS targets to issue a decision within five (5) calendar days following the completion of REVIEW and FOLLOW-UP.
Decisions respecting any application will result in one of the following:
- approval and issuance of an Import/Export Permit; or
- refusal to issue and Import/Export Permit.
6.3.1 Approval
Where the decision is to approve the Import or Export Permit, the application will proceed to OUT-TAKE.
6.3.2 Refusal:
Where the decision is to refuse to issue the Import or Export Permit, the OCS will send an Intent to Refuse Notification to the applicant via e-mail setting out the reasons for the proposed refusal. The Intent to Refuse Notification will give the applicant an opportunity to be heard in respect of the proposed refusal.
Where an Intent to Refuse Notification has been sent to an applicant, it is the responsibility of the applicant to respond to the OCS within ten (10) calendar days of the issuance of the Notification, stating whether or not a hearing in respect of the proposed refusal is desired.
Where a hearing in respect of the proposed refusal is desired by the applicant, the OCS will contact the applicant to arrange a mutually acceptable mechanism e.g. in person, by telecom or webinar, date, time and location for the hearing.
Where the applicant does not reply to the Intent to Refuse Notification within the ten (10) calendar days set out in the Notification, the OCS will proceed with the issuance of the refusal. Once refused, a new application must be submitted should the applicant still wish to obtain an Import or Export Permit.
6.4 Out-take
During OUT-TAKE Permits are prepared for the communication of the decision.
The OCS targets to complete OUT-TAKE within five (5) calendar days.
Final Permits are sent to the applicant via mail.
Copies of all correspondence and documentation gathered in support of the Permit application are maintained by the OCS for a period of five (5) years, in accordance with Library and Archives Canada, record retention and disposition frame work.
7. Unsolicited information
Applicants may submit, at any time during the processing of an application, unsolicited information pertaining to any aspect of the submitted application. The OCS reserves the right to accept or not accept the unsolicited information based on the status of the application review. If the information is relevant, it will be added to the application package and processed. If the information is irrelevant, it will be shredded. Before shredding, the applicant will be informed whether the unsolicited information is being accepted or not accepted, together with the reasons why.
Whenever updated information is being submitted, it is the responsibility of the applicant to clearly identify the:
- relevant application that the new information pertains to;
- specific section of the application form to which the updated information applies; and,
- specific information which was originally submitted and how the updated information is to change the original information (e.g. replacement, modification, addition, deletion).
8. Table A
| Application Type | Application Process Stages | Regulatory Decision Outcome | Overall Service Standard Calendar Days |
|||
|---|---|---|---|---|---|---|
| Intake and screening | Review and Follow-up | Decision | Out-take | |||
| Application for Import / Export Permit for Controlled Drugs/Substances or Precursor | 5 Calendar Days | 30 Calendar Days | 5 Calendar Days | 5 Calendar Days | Approval - Permit Issued Refusal - Permit not Issued |
45 |