What We Heard Report – Stakeholder Consultation on the Naming of Biologic Drugs

Download the alternative format
(PDF format, 595 KB, 8 pages)
Organization: Health Canada
Type: Report
Date published: 2019-02-14
Introduction
Health Canada and the Institute for Safe Medication Practices (ISMP) Canada conducted an online consultation on the naming of biologic drugs from January 18 to February 9, 2018. With the entry of biosimilars into the Canadian market, the number of biologic drugs sharing a non-proprietary (common) name is increasing. The objectives of the consultation were (1) to seek stakeholder views on whether Health Canada’s current approach to biologics naming is adequate or, if not, which alternate proposed option would be most appropriate to distinguish among biologics that share the same non-proprietary name, and (2) to better understand the impact of implementing each proposed option on external stakeholders.
The target audience for the consultation included healthcare providers, consumers, drug manufacturers and their representative associations. Additionally, input was sought from information technology providers and insurers.
Three options for the naming of biologic drugs (including biosimilars) were proposed:
- Option 1 – Continue the current Canadian drug identification and naming approach [status quo]
- Biosimilars, reference biologics, and innovator biologics that share the same non-proprietary name can be distinguished by their unique brand names or Drug Identification Numbers (DINs), however, in some settings only the non-proprietary name is used.
- Option 2 – Use of the brand name with the non-proprietary name to distinguish among biologics
- Both brand and non-proprietary names would be used, so biosimilars, reference biologics, and innovator biologics that share the same non-proprietary name would be distinguished by their unique brand names. Guidelines would be provided on the importance of using both the brand name and non-proprietary name throughout the medication use process and in adverse reaction reporting.
- Option 3 – Implement a 4-letter suffix appended to the non-proprietary name
- All biologic drugs, including biosimilars, reference biologics and innovator biologics, would receive a unique, meaningless 4-letter suffix appended to the non-proprietary name. Products sharing the same non-proprietary name would be distinguished by the suffix. Guidelines would be developed to align with the United States Food and Drug Administration (FDA)’s suffix-based naming convention as much as possible.
For all 3 options, all biologic drugs, including biosimilars, will continue to have a unique DIN.
Respondents’ comments were broad and diverse. This report summarizes the key messages, perceptions, and suggestions heard from consultation participants.
Who provided feedback?
A total of 362 responses were received. 79% of the respondents were healthcare providers, with pharmacists and pharmacy-related organizations representing 62% of respondents. There were also responses from pharmaceutical manufacturers, insurers, information technology providers, consumers and organizations representing consumers, as well as other organizations, such as advocacy and educational groups.
The breakdown of responses by stakeholder groups is shown in Figure 1 below.
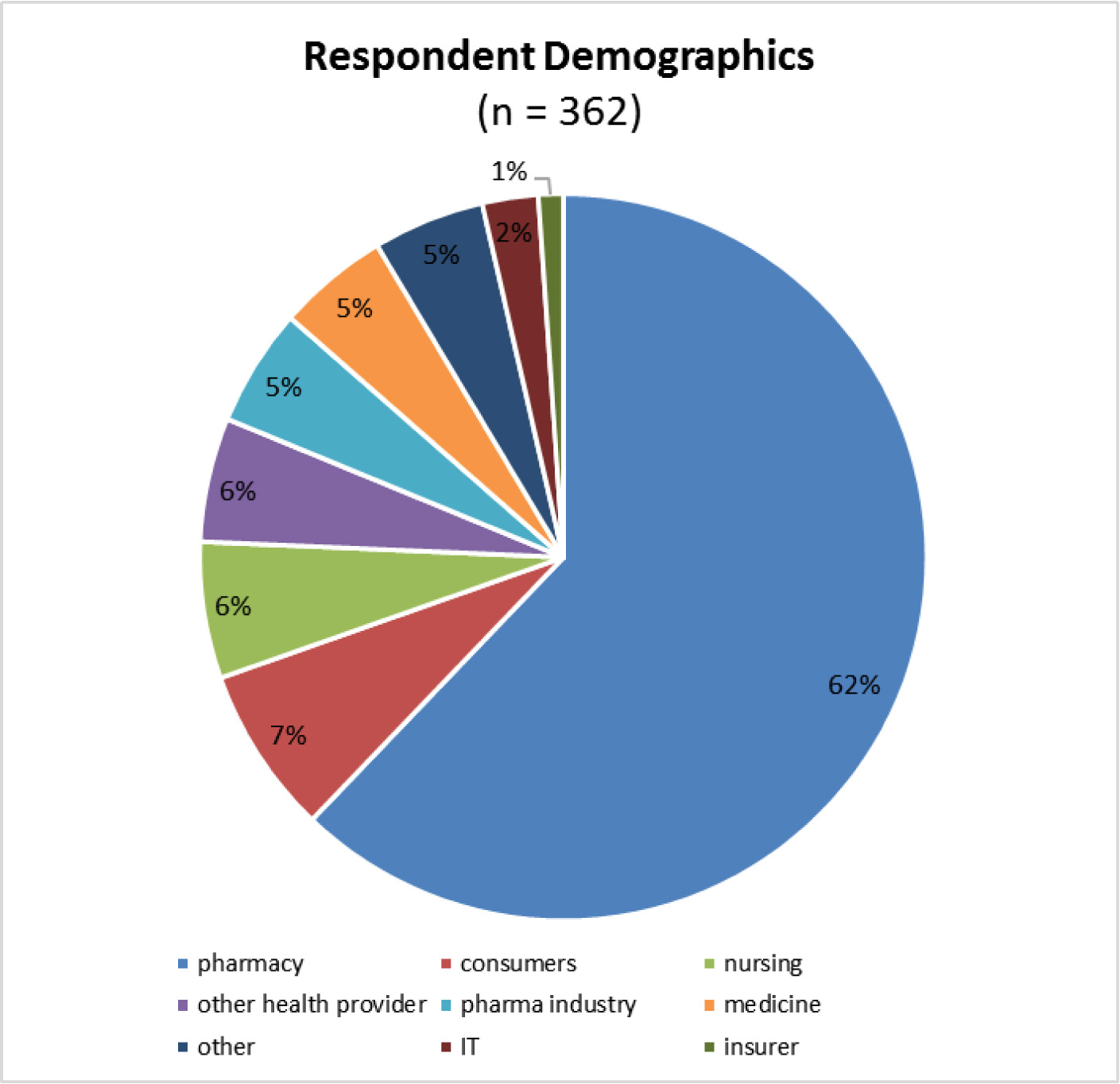
- Figure 1 footnote *
Note: the numbers below reflect unique submissions from each self-identified stakeholder; submissions were received from individual stakeholders and from associations or organizations.
Text equivalent – Figure 1
| Respondent Demographics (n = 362) |
Percentage (%) |
|---|---|
| pharmacy | 62% |
| consumers | 7% |
| nursing | 6% |
| other health provider | 6% |
| pharma industry | 5% |
| medicine | 5% |
| other | 5% |
| IT | 2% |
| insurer | 1% |
What did the stakeholders say?
Respondents were asked to rate each of the three proposed options as preferred, acceptable, or not acceptable, and to provide comments in support of their views. Respondents were also asked whether the options are compatible within their current practice or environment, and if not, to identify what changes would be needed to implement each option.
Subsequently, respondents were asked two additional questions:
- (1) If a suffix were to be appended to non-proprietary names, should previously authorized biosimilars, biologics and innovator biologics be renamed to conform to the new nomenclature?
- (2) Should any other options or factors be considered when developing a naming policy to distinguish between biologic drugs that share the same non-proprietary name?
| Option | Preferred | Acceptable | Total Preferred + Acceptable |
Not Acceptable |
|---|---|---|---|---|
| 1. Status quo | 9% | 21% | 30% | 70% |
| 2. Brand + non-proprietary names | 48% | 27% | 75% | 25% |
| 3. Suffix | 34% | 17% | 51% | 49% |
A summary of frequently heard comments from respondents is provided for each option and the two additional questions.
Option 1: Continue the current Canadian drug identification and naming approach [status quo]
In general, respondents were supportive of change from the status quo. 70% of respondents indicated that the current Canadian drug identification and naming approach was not acceptable. However, some pharmaceutical manufacturers and industry associations preferred this naming option.
- Summary of Stakeholder Comments on Option 1:
- Many respondents commented on the risk of selecting the wrong biologic drug when only the non-proprietary name of the biologic is used, which may lead to patients receiving a drug for an indication that was not authorized, imprecise reporting of adverse drug reactions, and financial implications for patients reliant on reimbursement of a specific product.
- The status quo approach does not align with developments in other jurisdictions for improving monitoring of adverse events.
- Patients may have difficulty knowing if their product has been switched.
- Concerns that the use of the non-proprietary name alone may encourage healthcare providers or patients to mistakenly consider biologics to be interchangeable.
Option 2: Use of the brand name with the non-proprietary name to distinguish among biologics
This was the most favoured and highest ranked naming option, with 75% of respondents rating it as preferred or acceptable. 75% of respondents indicated that this naming option is compatible with their current practice or environment.
Overall, public drug plans, the majority of pharmacists, physicians, nurses, information technology providers, individual consumers/patients, and some pharmaceutical companies rated this option as preferred or acceptable.
- Summary of Stakeholder Comments on Option 2:
- Respondents who favoured this option commented that it would clearly identify products for prescribing, dispensing and tracking of adverse events and reduce the likelihood of confusion and errors for healthcare providers and patients; other respondents commented that shared non-proprietary names among products may lead to presumed interchangeability or product selection errors.
- Many respondents commented that option 2 is intuitive since brand names are already in use and are more memorable than the proposed suffix approach.
- Many healthcare professionals and information technology providers commented that option 2 could be easily implemented, although other respondents commented that changes to information systems in some settings would be needed to accommodate both names.
- Some respondents commented that use of the brand name to differentiate among products is already the standard approach in some practice environments; other respondents commented that education and a change in practice would be needed as brand names are not commonly used or encouraged for prescribing or dispensing.
Option 3 – Implement a 4-Letter Suffix Appended to the Non-Proprietary Name:
This was the second-ranked naming option, with 51% of respondents rating it as preferred or acceptable. 61% of respondents indicated that this naming option is compatible with their current practice or environment.
This option was preferred by organizations representing consumers/patients, private insurers, and educational and advocacy groups, as well as some pharmaceutical companies. A minority of pharmacists, physicians, nurses, individual patients, and information technology providers preferred this approach. About half of the respondents who preferred option 3 also rated option 2 as acceptable.
Although suffixes that are devoid of meaning were proposed in alignment with the US FDA approach, many of the respondents who preferred option 3 commented that they would prefer meaningful suffixes for memorability and to avoid confusion.
- Summary of Stakeholder Comments on Option 3:
- Respondents who favoured this approach commented that option 3 would identify products for prescribing, dispensing and tracking of adverse events and reduce the likelihood of error for prescribers, pharmacists and patients; other respondents commented that suffixes would be cumbersome and unnecessary and that the lack of meaning would make suffixes difficult to remember and lead to confusion and medication errors.
- Respondents who favoured option 3 commented that non-proprietary names that are differentiated by a suffix would clearly identify that the products are not the same nor interchangeable; other respondents commented that the suffix may be more confusing than helpful as drugs with different suffixes may have the same indications and clinicians and patients could assume that different suffixes indicate clinically meaningful differences between a biosimilar and its reference product.
- Many respondents commented that suffixes are not intuitive; their complexity and lack of meaning may lead to their omission by prescribers, and as a result dispensers would need to follow up to know which product was intended.
- Respondents who favoured option 3 noted its consistency with the US FDA approach; other respondents noted that suffixes would be inconsistent with the European Medicines Agency and Australian Therapeutic Goods Administration approaches to drug naming.
- Some respondents commented that option 3 could be easily implemented since the current practice of using the non-proprietary name in information systems would not need to change; other respondents commented that education and a change in practice would be required and that option 3 would be difficult to implement due to the need to update databases and reconfigure software and labelling.
Renaming of Previously Authorized Biologic Drugs:
Respondents were asked to provide their views on whether previously authorized biologics should be renamed if a suffix-based nomenclature were adopted. Over 80% of respondents indicated that previously authorized and innovator biologics should be renamed if suffixes were to be appended to non-proprietary names. There was strong support for renaming if suffixes were adopted, both from respondents who preferred a suffix and from respondents who preferred options 1 or 2. Many respondents commented on the need for a consistent, standardized naming convention for all biologics to avoid confusion, medication errors and compromised patient safety.
- Summary of Stakeholder Comments in Renaming:
- Numerous changes would be required to implement renaming of previously authorized biologics including updates to computer software for drug information and decision support tools, order entry, smart pumps, inventory control, third-party payer databases, changes to product labels, etc. Supply chains and stock management would also be impacted.
- Many respondents commented on the cost implications of renaming due to the time and labour required to implement the necessary changes and the challenges of implementing this approach in some electronic systems.
- Requests for staggered implementation over a multi-year transition period.
- Some respondents expressed concern that manufacturers of previously authorized biologics may use the lack of a suffix for marketing advantage if these products are not renamed.
Other Options or Factors to Consider:
Respondents were asked to suggest other options or factors that should be considered when developing a naming policy.
- Summary of Stakeholder Suggestions:
Please note: the summary provides only suggestions not provided elsewhere in the report.
- Global naming approach:
- Wait for a recommendation from the World Health Organization for international consistency
- Other Naming Alternatives:
- Suffix with an abbreviated version of the manufacturer name
- Assign numerical suffixes sequentially according to market entry
- Incorporate random letters into the non-proprietary name to avoid name truncation
- Suggestions for Pharmacovigilance:
- Need for more education for adverse event reporting
- Need for complementary information about the drug (e.g., brand name, DIN, lot number, and expiry date) for drug identification in adverse events reports
- If a naming convention change is made, there should be an analysis after implementation to assess the impact on correct attribution of adverse events
Conclusion
We would like to thank all respondents for completing the questionnaire. Health Canada reviewed and considered all comments to inform the development of a naming convention for biologic drugs.
Contact us
Office of Policy and International Collaboration
Biologics and Genetic Therapies Directorate
Health Products and Food Branch
Health Canada
Building #6, 100 Eglantine Driveway
Tunney’s Pasture
Ottawa, ON K1A 0K9
Address Locator: 0601B
Email: hc.bgtd.opic-bpci.dpbtg.sc@canada.ca