Proposed Approach to the Regulation of Cannabis: Summary of Comments Received During the Public Consultation

Download the alternative format
(PDF format, 561 KB, 40 pages)
Organization: Health Canada
Date published: 2018-03-19
Table of Contents
- 1 Introduction
- 2 Licences, Permits and Authorizations
- 3 Security Clearances
- 4 Cannabis Tracking System
- 5 Cannabis Products
- 6 Packaging and Labelling
- 7 Cannabis for Medical Purposes
- 8 Health Products and Cosmetics with Cannabis
- 9 Other Issues
- Annex A: Details of Proposed Label Content Requirements for Cannabis
- Annex B: Details of Proposed Label Display Requirements for Cannabis
- Annex C: Proposed Health Warning Messages
1 Introduction
In the 2015 Speech from the Throne, the Government of Canada committed to introducing legislation to legalize, strictly regulate, and restrict access to cannabis. On April 13, 2017, the Government of Canada introduced Bill C-45, an Act respecting cannabis and to amend the Controlled Drugs and Substances Act, the Criminal Code and other Acts (the Cannabis Act) in the House of Commons. An overview of the proposed Cannabis Act can be found at: Legislative Background: An Act respecting cannabis and to amend the Controlled Drugs and Substances Act, the Criminal Code and other Acts (Bill c-45).
To support implementation of the proposed Act, regulations would need to be enacted in a range of areas, such as cannabis product standards and packaging and labelling requirements, to ensure that the risks and harms of cannabis are appropriately addressed. The Government of Canada intends to publish final regulations in the Canada Gazette, Part II, as soon as possible following Royal Assent of the proposed Cannabis Act, in order to support timely implementation of the new legal framework.
As a practical matter, provinces and territories have indicated that there must be a sufficient period of time between when the proposed legislation receives Royal Assent and when the new laws come into force, and that a minimum period of 8 to 12 weeks is necessary for the orderly movement of cannabis products from federally-licensed producers to provincially- or territorially-authorized distributors and retailers. The Government of Canada has indicated that it would prioritize an orderly transition to the new legal framework, including by providing for this 8- to 12-week period of time.
1.1 Proposed Approach to the Regulation of Cannabis
On November 21, 2017, Health Canada launched a 60-day public consultation to solicit public input and views on a proposed approach to these regulations. To support the consultation, Health Canada published a consultation paper entitled Proposed Approach to the Regulation of Cannabis. The consultation paper outlined a comprehensive series of regulatory proposals to help achieve the government’s public health and safety goals of restricting youth access to cannabis, minimizing the harms of cannabis use, and preventing criminals and organized crime from profiting from the illegal production of cannabis. The consultation paper made proposals in the following areas:
- Licences, Permits and Authorizations;
- Security Clearances;
- Cannabis Tracking System;
- Cannabis Products;
- Packaging and Labelling;
- Cannabis for Medical Purposes;
- Health Products and Cosmetics with Cannabis; and
- Miscellaneous Issues.
The proposals set out in the consultation paper built upon established regulatory requirements that have long been in place for current producers of cannabis for medical purposes or industrial hemp, adapted as required to reflect that the proposed Cannabis Act was designed in the broader context of legalizing, regulating and restricting access to cannabis. In addition, many of the regulatory proposals reflected the advice provided by the Task Force on Cannabis Legalization and Regulation, in their final report, A Framework for the Legalization and Regulation of Cannabis in Canada.
To meet the Government’s commitment of bringing the proposed Cannabis Act into force in a timely manner, final regulations would need to be published in the Canada Gazette, Part II, as soon as possible following Royal Assent. Draft regulations will not be pre-published. Instead, detailed regulatory proposals set out in the consultation paper were intended to provide industry and stakeholders with as much detail as possible on the proposed regulatory requirements so that their feedback and perspectives could be incorporated into the final regulations.
1.2 Public Consultation
The regulatory consultation was designed to provide any interested person with the opportunity to share their views and perspectives, through both online and written submissions, on how the Government of Canada should regulate cannabis. As well, Health Canada proactively reached out to a number of stakeholder communities most directly affected by the proposed regulations, to ensure they were aware of the proposals and the opportunity to provide their perspectives. Feedback was solicited through four main methods of engagement: online submissions, written submissions, in-person roundtables and web-based discussions.
The online portal generated 3,218 responses and Health Canada received 450 written submissions. The majority of respondents identified themselves as individuals, while the remainder identified themselves as a representative of a group or organization.
A total of seven in-person roundtables were held in Toronto, Montréal, Ottawa, and Vancouver, which included general sessions with interested parties, as well as focused sessions with current licensed producers of cannabis for medical purposes, patients and patient advocates, and prospective licensees. In total, 192 interested parties participated in these in-person roundtables. A further 343 interested parties participated in webinars hosted by Health Canada, including licensed producers, the hemp industry, prospective licensees, and current licensed dealers (i.e. holders of a dealer’s licence under the Narcotic Control Regulations).
In addition, Health Canada held both multilateral and bi-lateral meetings with representatives of all provinces and territories to collect their feedback on the proposed regulatory approach.
In recognition that Indigenous interests in cannabis legalization and regulation go beyond the proposed regulatory approach, broader engagement meetings were underway at the outset of the regulatory consultations. As part of its ongoing, long-term engagement with Indigenous governments and representative organizations on cannabis legalization and regulation, Health Canada held nine bilateral meetings with the Assembly of First Nations, Inuit Tapiriit Kanatami and Inuit Land Claim Holders, and the Métis National Council during the consultation period. Regular meetings and collaborative work will be ongoing throughout 2018 to share information, support the development and delivery of effective public education and communication, and address key areas of interest raised by First Nations, Inuit and Métis across Canada.
In addition, all Modern Treaty Holders, First Nations Provincial-Territorial Organizations, Métis Governing Members, and many other Indigenous representative organizations were invited to provide submissions on the proposed regulatory framework, and on their broader interests related to cannabis legalization and regulation.
1.3 Purpose of this Document
The purpose of this document is to provide a summary of the feedback Health Canada received during the 60-day public comment period, and to outline potential changes to the regulatory proposals that Health Canada is considering in response.
It is recognized that, in some areas, regulated parties require advance notice of regulatory requirements so that they have enough time to manufacture and prepare legally-compliant cannabis products in time for the coming into force of the proposed Cannabis Act. As a result, the purpose of this document is also to provide additional details on proposed regulation in these areas, so that industry has as much information as possible in preparing for the implementation of the proposed new legal framework. That said, it is important to note that the proposed Cannabis Act is still being considered by Parliament. As such, any details provided in the current document should not be interpreted as representing final decisions of the Governor in Council, the Minister of Health, or the Government of Canada.
2 Licences, Permits and Authorizations
2.1 Overview of Proposal
The consultation paper proposed a system of licences, permits, and authorizations intended to:
- allow a range of different activities with cannabis;
- enable a diverse, competitive legal industry comprised of both large and small players in regions across the country;
- reduce the risk that organized crime will infiltrate the legal industry; and
- provide for legal cannabis products that meet high quality standards.
As a result, the paper proposed a system of different types of authorizations based on the activity being undertaken (e.g. cultivation, processing, or sale for medical purposes), and in some cases, the scale of the activity (i.e. “micro” and “standard” scale).
The consultation paper also proposed that the regulations would establish rules and requirements for the different categories of authorized activities that would be proportional to the public health and safety risks posed by each category of activity.
The consultation paper asked stakeholders for views on what an appropriate threshold would be to distinguish between micro- and standard-scale cultivators and processors.
2.2 What We Heard
In general, respondents expressed support for the proposed system of licences and permits, and the corresponding regulatory requirements for each category of licence. The consultation paper asked whether the proposed categories of licences would help achieve the objective of enabling a diverse, competitive legal industry comprised of both large and small players in regions across the country. Of those respondents who answered this question, the majority agreed with the proposal, while a minority disagreed. The hemp industry expressed strong support for the proposed regulatory requirements for licences to cultivate industrial hemp.
There was general support for the proposed requirements for each class of licence. Among those who answered this question, a majority generally agreed that the proposed requirements were proportional to the public health and safety risks posed by each category of licence, while a minority disagreed. Many of those who agreed with the proposed regulatory requirements indicated that Health Canada should be open to making adjustments to the regulatory requirements in the future in order to quickly respond to emerging issues.
The following four topics elicited a high degree of feedback from respondents to the consultation.
- Commercial outdoor cultivation
-
The consultation paper proposed that the regulations permit both outdoor and indoor cultivation of cannabis. A majority of those who commented on outdoor cultivation supported allowing it, while a minority opposed or had concerns. Those who agreed generally felt that outdoor cultivation was the most economical and environmentally sustainable way to grow cannabis. In addition, it was noted that outdoor cultivation would allow producers to take advantage of natural elements including sunlight, water, and soil. However, some respondents questioned whether there is a potential impact on adjacent agricultural crops (including industrial hemp) and whether outdoor cultivation presents a greater risk of theft and diversion. Others also asked how good production practices would be satisfied outdoors and how odour could be managed during flowering. In response to this feedback, further consideration is being given to what measures, if any, are required in the final regulations to address any concerns that were raised with respect to outdoor cultivation.
- Starting material and access to plant genetics
-
Health Canada received feedback from a number of respondents who indicated that access to a broad diversity of cannabis plant genetics used to propagate cannabis (such as seeds, seedlings or cuttings) was necessary to enable the legal market to successfully compete with the current illegal market. These respondents pointed out that currently, there are hundreds, if not thousands, of different strains of cannabis sold on the illegal market and that in order for legal producers to be able to effectively compete, they would need to have access to a broader diversity of cannabis plant genetics. In response to this feedback, consideration is being given to how the regulations could enable the introduction of new plant genetics into the legal system. In doing so, the regulations would need to aim to ensure that organized crime would not benefit from past or ongoing criminal activity with cannabis, and that all cannabis grown by the legal industry, regardless of source, would be subject to the same strict regulatory controls, including pesticide testing and other controls.
- Multiple licences at a single site
-
The consultation paper did not specify whether there would be any restrictions on the ability of a single person to conduct multiple activities at a single site.
In several roundtable meetings, stakeholders stated that allowing a person to hold multiple micro-cultivation or multiple micro-processing licences at a single site would put the system at risk of abuse, such that an individual could combine multiple micro-scale licences together at a single site as a way to avoid the requirements associated with standard-scale licences. In response to this feedback, consideration is being given to how the final regulations would restrict the number of micro-cultivation or micro-processing licences at a single site to avoid this type of scenario.
- Security clearances for major shareholders of privately-held companies
-
The consultation paper proposed that the regulations would require that any shareholder who owned more than 25 percent of a licensed organization (if privately held) or more than 25 percent of a privately held parent company hold a security clearance, as part of a suite of comprehensive proposals to ensure that organized crime are not able to infiltrate the legal industry and use it to further other criminal activities.
While respondents agreed with the government’s objective and supported transparency and scrutiny around shareholders, a number felt that the proposed requirement would be difficult to enforce, and that it would be relatively simple to structure investments and assets to avoid the requirement. In response to this feedback, consideration is being given to alternative options to reduce the risk of criminal organizations establishing a financial relationship with legal cannabis producers in order to further their criminal activities. Such measures could include requiring licence applicants to submit financial information (including information about investors) as part of the licence application process. This information could then be used in determining whether to refuse to issue or renew a licence, should public safety concerns be raised. As well, the regulations could require regular, ongoing reporting of financial information by licensees to help identify suspicious financial relationships or arrangements that may warrant additional regulatory action (including, for example, a licence suspension) or, in the appropriate circumstances, referral to law enforcement for further investigation if needed.
2.3 Thresholds for Micro-cultivation and Micro-processing
As mentioned previously, the consultation paper proposed that the regulations would establish micro-cultivation and micro-processing licences to facilitate the participation of small-scale producers in the legal cannabis industry. The consultation paper did not specifically propose a definition for these micro-scale producers and instead sought feedback on what an appropriate threshold would be to distinguish micro-scale licensees from standard-scale cultivators and processors.
Respondents provided a broad range of opinions and perspectives on how best to define micro-cultivators. Many respondents and industry experts engaged in a thoughtful and extensive debate, during the roundtables hosted by Health Canada, in written submissions, and on social media. Despite the extensive dialogue, no clear consensus emerged on the best means for defining micro-cultivators. The two most popular options for the basis of a definition were the number of plants (approximately 25 percent of respondents) and growing area (or canopy area; approximately 20 percent of respondents). In terms of total size, opinions on the number of plants that could be grown by a micro-cultivator ranged from less than 50 to over 1000 plants. For growing area, opinions ranged from 9 square metres (100 square feet) to 1,858 square metres (20,000 square feet).
While a significant number of respondents provided specific feedback on how a micro-cultivator should be defined, most respondents did not provide similar feedback on how to define a micro-processor. However, it was generally acknowledged that a micro-processor should be large enough to be capable of processing the production of a single micro-cultivator.
As indicated in the consultation paper, the objective of the micro-scale licences is to facilitate the participation of small-scale growers and processors in the legal cannabis industry. In order to be effective, these licence categories must be able to support a viable small business. Establishing a threshold that is too low could jeopardize the sustainability of such businesses, making such ventures uneconomical and potentially create an incentive to produce cannabis outside of the regulated framework. At the same time, the thresholds, and what they mean in terms of expected facility size, number of employees and the quantity of cannabis stored on site, should be low enough to align with the proposed physical security requirements for micro-scale licences. Finally, it is equally important that the thresholds be established in such a manner that there is an opportunity for small businesses to enter the regulated industry at a scale that does not create too great a burden on federal compliance and enforcement resources (e.g. inspections of licensed production facilities).
Based on the government’s objectives, the feedback received during the consultation, and drawing from the experience of U.S. states that have established similar categories of licences for smaller producers, it will be proposed that the final regulations define micro-scale licences as follows:
- Micro-cultivation licence would authorize the cultivation of a plant canopy area of no more than 200 square metres (approximately 2,150 square feet). For a sense of scale and what 200 square metres represents, see Figure 1.
- Micro-processing licence would authorize the processing of no more than 600 kilograms of dried cannabis (or equivalent) per year, or the entire output of a single micro-cultivation licence.

Figure 1 - Text Description
There is an image of a standard, North American sized, hockey rink. The image has all the markings of a hockey rink including two red face-off circles at each end of the rink, the two blue lines and the centre line, which is red. Along the bottom of the rink is a black two-pointed arrow with the text 61 metres, depicting the length of the hockey rink. Along the right hand side of the rink is a black two-pointed arrow with the text 26 metres, depicting the width of the hockey rink. There is also a red face-off circle in the centre of the rink. In the middle of the blue lines, there is a green shaded rectangle with the text "200 square metres" written in it. The green rectangle, which represents the maximum canopy size that would be allowed for a micro-cultivator, covers approximately half the total distance between the blue lines.
3 Security Clearances
3.1 Overview of Proposal
The consultation paper proposed that select personnel associated with certain licences issued under the proposed Cannabis Act be required to hold a valid security clearance issued by the Minister of HealthFootnote 1. The consultation paper proposed that the regulations would enable the Minister to refuse to grant a clearance to individuals with associations to organized crime, or with past convictions for, or an association with, drug trafficking, corruption or violent offences.
The consultation paper acknowledged that there are individuals who have histories of non-violent, lower-risk criminal activity with cannabis (e.g. simple possession of cannabis or smaller-scale cultivation of cannabis plants) who may seek to obtain a security clearance so they can participate in the legal cannabis industry. The consultation paper asked stakeholders whether they felt these individuals should be permitted to hold a security clearance and participate in the legal cannabis industry.
3.2 What We Heard
With respect to the proposed requirements for certain individuals associated with a licensed organization to hold a security clearance, 45 percent of those who answered the question agreed with the proposed requirements and felt that the proposal addressed the positions of greatest risk. Of the 35 percent of respondents who disagreed, opinions were split on whether the proposal was too restrictive and burdensome for the industry (pointing out that similar clearances are not required in the alcohol or tobacco industries) or whether it was too lax and all employees should be subject to the security clearance process.
With respect to whether individuals with histories of non-violent, lower-risk criminal activity should be able to obtain a security clearance and participate in the legal cannabis industry, a strong majority of respondents agreed that they should. Respondents reinforced that allowing certain individuals currently involved in the illegal production of cannabis – and who are not associated with organized crime – to participate in the legal industry is a necessary component of the government’s efforts to displace the illegal market. Many felt that failing to provide an opportunity for these individuals to work in the legal industry could result in them continuing their illegal activities. As well, some respondents highlighted that greater flexibility in the issuance of security clearances would be required to avoid creating a barrier to entering the legal industry for racialized or marginalized communities, who, according to respondents, often have more frequent encounters with law enforcement and the criminal justice system.
Health Canada appreciates the feedback that it has received from respondents on the question of whether individuals with histories of non-violent, lower-risk criminal activity should be able to obtain a security clearance and it will be taken into consideration in the development of the final regulations.
4 Cannabis Tracking System
4.1 Overview of Proposal
The proposed Cannabis Act would authorize the Minister of Health to establish and maintain a national Cannabis Tracking System. The purpose of this system would be to track cannabis throughout the supply chain to help prevent diversion of cannabis into, and out of, the legal market. The consultation paper outlined a series of proposals respecting who would be required to report into the system, what information they would be required to submit, and how often they would be required to report. In addition, the consultation paper proposed that the regulations would provide for the sharing of information collected through the Cannabis Tracking System with provincial and territorial governments for the purpose of administering cannabis-related public health programs or activities.
4.2 What We Heard
Approximately 50 submissions provided comments on this section of the consultation paper. Among those, the majority expressed support for the Cannabis Tracking System. These respondents noted that the ability to monitor cannabis throughout the supply chain would help prevent diversion of cannabis to the illegal market, and could be used to make product recalls more effective, efficient and timely. Among those respondents who expressed concern with the proposed system, many pointed to the potential challenges in implementing the system if it involves major information technology systems, and the potential cost, especially for micro-scale licensees. In response to this feedback, consideration is being given to what, if any, changes are required to the proposed requirements to the Cannabis Tracking System to help it achieve its objective of preventing the diversion of cannabis. In implementing the proposed system, due consideration will be given to minimizing the burden on those required to report, particularly micro-scale licensees and industrial hemp producers.
5 Cannabis Products
5.1 Overview of Proposal
The consultation paper proposed that the regulations would establish rules and standards for the production of cannabis products, with the objectives of:
- providing adults with access to quality-controlled cannabis products of known potency;
- enabling a range of product forms to help the legal industry displace the illegal market;
- reducing the appeal of cannabis products to youth; and
- reducing the risk of accidental consumption of cannabis by young persons.
Proposed rules and standards included a maximum THC concentration for cannabis oil of 30 milligrams of THC per millilitre of oil, which aligns with the current THC limit under the Access to Cannabis for Medical Purposes Regulations (ACMPR). In addition, the consultation paper proposed a limit of 10 milligrams of THC per dose or unit for any cannabis product intended for ingestion. As well, the consultation paper proposed that the regulations would not prohibit the production of single-use forms of dried cannabis products intended for inhalation (such as “heat not burn” vaporization cartridges). It was proposed that such products could not contain more than 1 gram of dried cannabis per unit.
As set out in the proposed Cannabis Act, dried cannabis, cannabis oil, fresh cannabis, cannabis plants, and cannabis seeds could be sold to the public upon coming into force of the proposed Act. The sale of edibles and other cannabis-based products, such as liquid concentrates suitable for vaping, would be permitted within the following year to allow time for the development of specific regulations to address the unique risks posed by these product classes.
5.2 What We Heard
Overall, a strong majority of those who responded to the consultation question regarding cannabis product forms were supportive of the proposal not to restrict the type of product forms that industry would be able to manufacture and sell, within allowed product classes (dried and fresh cannabis, cannabis oil, cannabis plants and seeds).
While the consultation paper was seeking input on product forms, many respondents took the opportunity to urge the government to allow the sale of cannabis edibles and concentrates immediately upon coming into force of the proposed Cannabis Act (rather than enabling their sale within twelve months as set out in the proposed legislation). These respondents cited the need for the legal industry to be able to offer the same diversity of products that are currently available through the illegal market in order to be able to successfully compete, and also suggested that a broader range of products would provide alternatives to smoking cannabis. At the same time, some participants in the consultation acknowledged that additional regulatory provisions would be required to address the unique public health risks associated with cannabis edibles and concentrates, such as quality control, THC limits, portion sizes, and specific packaging and labelling requirements.
A small proportion of respondents felt that the regulations should be more restrictive in the types of cannabis products available through the legal system. These respondents identified a range of different products that they felt should be prohibited, including edibles and candies and solvent-based extracts. As well, some respondents suggested that only dried forms of cannabis should be made legally available, while others felt the opposite and that any product that encourages the smoking of cannabis should be prohibited.
With respect to the proposed THC limit for cannabis oil and the proposed limit on the amount of THC that could be in a single unit or serving, feedback was mixed. In general, most participants in the consultation supported the proposed THC limit of 10 milligrams per unit or serving of a cannabis product intended for ingestion. These respondents viewed the proposal as a prudent safeguard against accidental overconsumption, which would facilitate consumer education and awareness.
There were mixed views on the proposed concentration limit of 30 milligrams of THC per millilitre of cannabis oil. On the one hand, many respondents did not support the proposed limit, suggesting that it was either too low or that there should not be a limit at all. These views reflected the desire of many stakeholders to have the regulations permit the production and sale of concentrates, including liquids suitable for vaping, immediately upon coming into force of the proposed legislation. In contrast, other respondents saw the proposed limit as a prudent safeguard to mitigate against the risk of accidental or overconsumption of a product class primarily intended for ingestion. The feedback received did not include scientific evidence to support the regulations establishing a different, specific THC concentration limit for cannabis oil.
Necessary regulations addressing edibles containing cannabis and cannabis concentrates will be put in place within one year following the coming into force of the proposed Cannabis Act, if it is passed by Parliament. Health Canada plans to consult broadly on these regulations with the provinces and territories, industry, the public health community and other interested stakeholders.
6 Packaging and Labelling
6.1 Overview of Proposal
The consultation paper proposed that the regulations would set requirements for the packaging and labelling of cannabis products that would achieve the government’s public health and safety objectives – in particular protecting the health of young persons by restricting their access to cannabis and by protecting young persons and others from inducements to use cannabis. In addition, the requirements would also help promote informed consumer choice, particularly with respect to the health risks associated with cannabis use, and allow for the safe handling and transportation of cannabis. The paper proposed that all cannabis products would need to be packaged in a manner that is tamper-evident and child-resistant.
As well, the consultation paper proposed that the regulations would set strict limits on the use of colours, graphics, and other special characteristics of packaging to curtail the appeal of products to youth and to ensure that key information would be the most prominently displayed elements. To help consumers make informed decisions and to avoid misuse, products would be required to be labelled with specific information about the product (such as potency), contain mandatory health warnings messages, and be marked with a clearly recognizable standardized cannabis symbol.
6.2 What We Heard
Overall, a clear majority of those who provided views on the proposed packaging and labelling requirements expressed support for the proposals, while a minority disagreed with the proposed requirements. Among those who expressed support, many suggested additional information that they felt should be required to be on the label. Additional information that was not already part of the proposal in the consultation paper included additional information on other cannabinoids and terpenes; growing conditions; whether a product was organic or not; and the origin of the cannabis (name of the cultivator). Beyond information related specifically to the product, there was a subset of respondents who wanted to ensure that the health warning messages covered specific topics, such as cautioning against the use of cannabis while pregnant, or while operating a vehicle.
Among the majority of respondents who generally supported the proposed requirements, opinions were mixed on whether the regulations should require “plain packaging” and restrict the use of colours and branding. Some respondents expressed general support, but felt that some branding should be allowed, while others advocated for strict requirements that would prohibit the display of any branding or logos.
Among the minority of respondents who disagreed with the proposed packaging and labelling requirements, a strong majority indicated the proposal was too strict. Of these respondents, a subset specifically indicated that packaging requirements should allow branding and marketing by producers. These respondents felt that the ability for the legal industry to brand their products was necessary to allow them to differentiate their products from their competitors, including illegal producers operating outside of the legal framework.
Overall, respondents expressed support for the proposed packaging requirements, especially the requirement that all packages be child-resistant. However, a few industry stakeholders felt that the requirement that all packages be opaque was not warranted, and indicated that translucent packages would also meet the government’s objectives and provide consumers with important information (such as how much cannabis oil is left in a bottle).
Health Canada also received feedback from a few industry stakeholders who indicated that the accuracy of weight permitted for fresh or dried cannabis products under the ACMPR (i.e. a tolerance of plus or minus 5%) presented operational challenges with respect to smaller (e.g. 1 gram) package sizes. In response to this feedback, consideration is being given to what measures, if any, are required in the final regulations to address the issue of weight tolerances.
Industry stakeholders were nearly unanimous in stating that, in order to prepare products that are compliant with the packaging and labelling rules in time for the coming into force of the legislation, they needed certainty as soon as possible on what the regulations would require. Based on experience in other areas (including tobacco, food, prescription drugs, and hazardous products), packaging and labelling rules tend to be highly detailed and specific, and industry requires significant lead-time to design, manufacture, and print their packages and labels.
6.3 Detailed Packaging and Labelling Requirements
A primary purpose of the proposed regulatory requirements is to ensure that packaging and labelling are not designed to induce young persons and others to use cannabis. There is extensive evidence to demonstrate that product packaging and labelling has a significant impact on consumer perceptions and behaviour, given its broad reach and exposure at key time points (such as at point of purchase and during use).
It is understood that, in order to provide for the orderly implementation of the proposed Cannabis Act, the legal cannabis industry would need to understand, as soon as possible, what the regulations would require with respect to packaging and labelling. To that end, and to provide industry with as much information as possible, Annexes A and B of this document provide detailed descriptions of label content and labelling requirements that are intended to be proposed for the final regulations. These details include:
- a standardized cannabis symbol that would need to appear on every label, including specific requirements with respect to its size, placement and appearance;
- mandatory health warning messages that would need to appear on every label, including specific requirements with respect to their size, placement and appearance. The warnings, covering six topics, are listed in Annex C of this document. A warning (comprised of a primary and secondary message) would need to appear on every label, and the different warnings would need to be rotated on package labels; and
- requirements with respect to information on THC and CBD content, as well as other information that would be required on each label, including specific requirements with respect to the size, placement and appearance of this information.
The intended proposal is that, consistent with the Task Force on Cannabis Legalization and Regulation’s recommendation to require plain packaging of cannabis products, the regulations would set strict requirements related to the use of branding, logos, and colours. Specifically:
- only one other brand element (in addition to the brand name) could be displayed. This element could include, for example, a slogan or logo. If it is a text element, the font must be no larger than the font of the health warning message, and must be a single, uniform colour. If the brand element is a graphic, image or logo, it would be required to be no larger than the standardized cannabis symbol;
- it would be prohibited to display any other image or graphic;
- label and package backgrounds would need to be a single, uniform colour (inside and outside);
- it would be prohibited to use any fluorescent or metallic colours;
- colours must contrast with the colours of the standardized cannabis symbol and the background of the health warning messages (see Annex B for details);
- labels and packaging could not have any coating (e.g. could not be glossy), embossing (raised or recessed relief images), texture, foil, cut-outs or peel-away labels;
- any over-wrap must be clear; and
- it would be prohibited to include any insert in a package.
With respect to package-specific requirements, the intention is to propose that the regulations would require that cannabis products (other than cannabis plants or cannabis seeds) be packaged in an immediate container that is tamper-evident, child-resistant, prevents contamination and keeps cannabis dry. For clarity, a cannabis product would include cannabis and any cannabis accessory (a thing used in the consumption of the cannabis, such as a rolling paper or gel capsule) that contains the cannabis. These cannabis accessories (for example, the rolling papers or gel capsules) would not be considered a container.
In addition, the intention is to propose that the regulations would require that the immediate container be opaque or translucent. Products could have both an inner and outer package, but every package would need to be labelled in accordance with the proposed requirements.Footnote 2 Finally, the regulations would require licensed processors to ship an informational document developed by Health Canada with every package delivered to a federally-, provincially-, or territorially-licensed distributor or retailer. The document would not be required to be included as an insert in the package, but would be provided to consumers with the sale or delivery of the package. The document would provide adult consumers with health and safety information, such as precautions and directions for use, and would be updated periodically to take into account new information and evidence.
To facilitate the orderly transition from the current packaging and labelling requirements under the ACMPR to the new regulatory requirements, the intention is to propose a transition period for cannabis products sold for medical purposes. Specifically, it is proposed that for six months following the coming into force of the proposed Cannabis Act, all cannabis products sold for medical purposes could be packaged and labelled in accordance with the current rules under the ACMPR. This would mean that for six months following the coming into force of the proposed Cannabis Act, cannabis products delivered to patients would be required to meet either the requirements as they currently exist under the ACMPR or the new requirements under the proposed Cannabis Act.
Health Canada does not intend to propose any transition period for cannabis products sold for non-medical purposes (i.e. those sold to adult consumers through a retail distribution framework overseen by a provincial or territorial government). All of these products would be required to meet the new packaging and labelling requirements immediately upon the coming into force of the proposed Cannabis Act.
There would not be any transitional provisions for the packaging and labelling requirements set out in the proposed Cannabis Act for any cannabis products regardless of whether they are to be sold for medical or non-medical purposes (for example, no packages or labels could be appealing to young persons or contain a celebrity endorsement immediately upon coming into force of the proposed Act).
It is important to note that, in addition to the requirements set out above, there are a number of packaging and labelling requirements set out in the proposed Cannabis Act, as well as in the Annexes to the current report, to which regulated parties would be subject. It is important that all of these requirements be considered in the context of any decisions with respect to packaging and labelling that could be made between the date of publication of this report and the anticipated coming into force of the proposed Cannabis Act and its regulations.
| Current Rules for Medical Cannabis | Proposed Rules for Cannabis | Proposed/Current Rules for Tobacco | Proposed Rules for Vaping | |
|---|---|---|---|---|
| Universal Symbol | REQUIRED (N for narcotic) |
REQUIRED | Not required | REQUIRED |
| Health Warning Message | Not required | REQUIRED (required text in yellow box; font must be largest on the label) |
REQUIRED (graphic and text; must cover 75 percent of package) |
REQUIRED (text; standard style and size font) |
| Product Related Information | REQUIRED (long list, including potency)Table 1 - Footnote 1 |
REQUIRED (long list, including potency)Table 1 - Footnote 1 |
RESTRICTED (short list of allowed info)Table 1 - Footnote 2 |
REQUIRED (short list of required info)Table 1 - Footnote 3 |
| Plain Packaging | ||||
Background Colour |
Not restricted | RESTRICTED (single, uniform colour) |
RESTRICTED (Pantone 448C brown) |
Not restricted |
Font |
Not restricted | RESTRICTED (standard style; size limit; single, uniform colour) |
RESTRICTED (standard style; size limit; Pantone 2C grey) |
Not restricted |
Brand Name |
Allowed, no restrictions | RESTRICTED (size limit; limit of 1 on principal display; single, uniform colour) |
RESTRICTED (standard style; size limit; limited number displayed; specified placement; Pantone 2C grey) |
Allowed, no restrictions |
Brand element (logo) |
Allowed, no restrictions | RESTRICTED (size limit; limit of 1 on principal display) |
PROHIBITED | Allowed, no restrictions |
Other images or graphics |
Allowed, no restrictions | PROHIBITED | PROHIBITED | Allowed, no restrictions |
Coatings, embossing, cut-outs and peel-away |
Allowed, no restrictions | PROHIBITED | PROHIBITED | Allowed, no restrictions |
| Package-specific Rules | ||||
Standard shape, size, material |
Not requiredTable 1 - Footnote 4 | Not requiredTable 1 - Footnote 4 | REQUIREDTable 1 - Footnote 5 | Not required |
Child resistant |
REQUIRED | REQUIRED | Not required | REQUIRED (containers containing nicotine) |
Opaque or translucent |
REQUIRED | REQUIRED | Not required | Not required |
Tamper-evident |
REQUIRED | REQUIRED | Not required | Not required |
Table Notes: |
||||
7 Cannabis for Medical Purposes
7.1 Overview of Proposal
Consistent with the advice of the Task Force on Cannabis Legalization and Regulation, the consultation paper proposed that a distinct system would be maintained to provide patients with reasonable access to cannabis for medical purposes. The proposed regulations would continue to enable individuals who have the support of their healthcare practitioner (including those under 18 years of age) to access cannabis for medical purposes by:
- purchasing from a federally-licensed seller of cannabis for medical purposes;
- cultivating their own cannabis, if over the age of 18 (personal production); or
- designating someone to grow cannabis on their behalf (designated production).
The consultation paper proposed that the medical access regulatory framework would remain substantively the same as it currently exists, with certain adjustments to: create consistency with rules for cannabis for non-medical purposes, improve patient access, and reduce the risk of abuse of the system.
7.2 What We Heard
In general, respondents to the consultation expressed support for the proposed regulatory approach to provide access to cannabis for medical purposes under the proposed Cannabis Act. In particular, respondents were supportive of the proposed adjustments to improve patient access, namely allowing individuals to transfer their valid medical document to a different federally-licensed seller of cannabis for medical purposes, and that registrations would begin on the date of initial registration and not on the date the medical document was signed by their health care practitioner, as is currently the case.
Some participants in the consultation raised specific topics, including recommending that cannabis for medical purposes should be treated like other approved health products and drugs and have some type of Health Canada approval so that it would be eligible for coverage under private health plans; that it should be accessible through other distribution means than the current system of secure ordering online and delivery by mail or by courier, such as through pharmacies; and that cannabis for medical purposes should not have to display mandatory health warning messages.
Health Canada appreciates the feedback that it has received from respondents on the regulatory proposals. Consistent with the advice of the Task Force on Cannabis Legalization and Regulation, the Government of Canada has committed to monitor and evaluate patients’ reasonable access to cannabis for medical purposes during the implementation of the proposed Cannabis Act, and evaluate the medical access framework within five years of the coming into force of the legislation.
As part of their response to the Health Canada consultation, many stakeholders took the opportunity to voice their opinion on a proposal to apply an excise duty to all cannabis products in Canada that was made as part of a separate consultation process conducted by the Department of Finance in the fall of 2017. Most respondents to the Health Canada consultation did not support applying an excise duty to cannabis sold for medical purposes, and also argued that cannabis for medical purposes should be exempt from the federal Goods and Services Tax (GST).
As announced in Budget 2018, the excise duty framework will generally apply to cannabis products that contain THC, the primary psychoactive compound of cannabis. Recognizing the non-addictive, potentially therapeutic role of low-THC cannabis oils, products that contain low amounts of THC will generally not be subject to the excise duty. Pharmaceutical products derived from cannabis will also be exempt, provided that the cannabis product has a Drug Identification Number and can only be acquired through a prescription.
8 Health Products and Cosmetics with Cannabis
8.1 Overview of Proposal
The consultation paper proposed that the regulations would provide for a scientific, evidence-based approach for the approval and oversight of health products containing cannabis that would be regulated under the Food and Drugs Act, including prescription and non-prescription drugs, natural health products, veterinary drugs and veterinary health products, and medical devices. It further proposed that market access would be maintained for previously-approved health products with cannabis, including prescription drugs that have been approved for the treatment of serious conditions. It was also proposed that certain provisions of the proposed Cannabis Act and its regulations would not apply to health products containing cannabis. Finally, the paper indicated that Health Canada would work with provincial and territorial governments and the National Association of Pharmacy Regulatory Authorities (NAPRA) on options to control the sale and display of any potential health products containing cannabis that may not require the oversight of a healthcare practitioner to ensure the government’s objective of restricting youth access to cannabis is achieved.
The consultation paper also proposed that any cosmetics containing cannabis would be subject to all provisions of the proposed Cannabis Act and its regulations.
8.2 What We Heard
In general, a majority of respondents to the consultation expressed support for the proposed approach to the regulation of health products and cosmetics containing cannabis, while a minority of respondents disagreed or were unsure. In particular, stakeholders agreed that any claims made with respect to the therapeutic benefits of cannabis must be evidence-based, verified, and independently validated by Health Canada. Among those who did not support the proposals, opinions were split between those that felt the proposed regulations were too restrictive, and those that felt more regulations are required.
A significant proportion of stakeholders asked questions and sought further details on how the regulations and Health Canada would approach the approval of natural health products and non-prescription drugs containing cannabis. Issues raised included potential limits on the amount of cannabinoids (such as THC or CBD) in different products, where these products could be sold, how they could be promoted, and whether or not pediatric formulations could be marketed.
8.3 Existing approval pathways
As part of the initial regulations required for the orderly implementation of the proposed Cannabis Act, the existing authorizations for health products containing cannabis established by the Food and Drugs Act and its regulations would be maintained. This means that currently-authorized health products will continue to be marketed and remain available to Canadians as they are now. As noted in Budget 2018, Health Canada is undertaking work to evaluate the drug review and approval process so that Canadians in need have access to an array of medicinal options that are safe, effective, and of high quality.
Health Canada would also continue to accept new applications through the current approval process for prescription drugs containing cannabis, medical devices used for consuming cannabis for medical/therapeutic purposes and Natural Health Products (NHPs) and Veterinary Health Products (VHPs) that contain permitted cannabis parts and no more than 10 ppm THC.
For these types of products, the regulations would outline the applicable requirements under the proposed Cannabis Act. For example, it is intended that the regulations would outline licensing requirements for holders of a Drug Establishment Licence that manufacture prescription drugs containing cannabis, similar to those of the existing Dealer’s Licence under the Narcotic Control Regulations. The requirements would focus on security, record keeping, reporting and good production/manufacturing practices under the proposed Cannabis Act, without duplicating requirements under the Food and Drugs Act and its regulations. To support transition to the new licence, current Dealer’s Licences would remain valid during a limited transition period.
Additional consideration is also being given to appropriate promotional controls for prescription drugs under the proposed Cannabis Act. For packaging and labelling, it is proposed that prescription drugs with cannabis only be subject to the requirements of the Food and Drugs Act and its regulations.
As proposed in the discussion paper, medical devices used for consuming cannabis for medical purposes would be subject to certain prohibitions for cannabis accessories under the proposed Cannabis Act. This would include requiring the support of a healthcare practitioner for their sale to young persons. The regulations, and updated policy guidance, would also clarify how medical devices combined with, or for use with, prescription drugs will be overseen.
8.4 Non-Prescription Drugs and Natural Health Products
As a result of ongoing analysis and feedback, it is proposed that further consultation take place with Canadians on the proposed approach for any potential new non-prescription drugs and NHPs containing cannabis. The consultations will focus on the appropriate level of regulatory oversight and evidence requirements to enable the approval of any potential new health products that could be available without the oversight of a physician.
Until these consultations and regulations for these types of products are complete, new applications for health products with cannabis will be limited to prescription drugs, medical devices, or NHPs/VHPs with permitted cannabis parts and no more than 10 ppm THC.
9 Other Issues
During the consultation, Health Canada also heard feedback from a number of respondents concerning the definition of “cannabis accessory” under the proposed Cannabis Act. Currently, the proposed Act would define a cannabis accessory as including “a thing, including rolling papers or wraps, holders, pipes, water bongs and vaporizers, that is represented to be used in the consumption of cannabis or a thing that is represented to be used in the production of cannabis”.
A significant number of respondents suggested that the government clarify the definition of cannabis accessories when dealing with things used in the production of cannabis, so that it would not cover products like fertilizers, pesticides, or gardening supplies. These respondents noted that, with respect to fertilizers and pesticides, these categories of products are already regulated under existing federal legislation (the Fertilizers Act and the Pest Control Products Act). In addition, respondents noted that prohibitions related to cannabis accessories designed to protect youth – for example the requirement that they only be sold behind the counter – should not necessarily apply with respect to things like soil or pots, which could be used in the production of cannabis.
In response to this feedback, consideration is being given to what measures, if any, are required in the final regulations to address stakeholder feedback.
Annex A: Details of Proposed Label Content Requirements for Cannabis
Note: In addition to the requirements set out in this Annex, there are a number of packaging and labelling requirements set out in the proposed Cannabis Act, as well as in Chapter 6 and other Annexes to the current report, to which regulated parties would be subject. It is important that all of these requirements be considered in the context of any decisions with respect to packaging and labelling that could be made between the date of publication of this report and the anticipated coming into force of the proposed Cannabis Act and its regulations.
A1.1 Placement of Information
All cannabis products must be labelled with a health warning message, the standardized cannabis symbol and other required information about the product. All information must appear in both English and French.
The following required elements must appear on the principal display panel:
- Standardized cannabis symbol;
- Health warning message; and
- THC/CBD content.
The following elements can appear on any surface of the package, except for the bottom:
- Other required information; and
- Non-required information.
A1.2 Health Warning Messages
- One health warning message must appear on each package.
- There are 14 health warning messages; these appear in Annex C to this report.
- Each health warning message is comprised of a primary sentence and a secondary sentence. There are 6 primary sentences, and then 1-3 secondary sentences associated with each of the primary sentences.
- The first health warning message listed in Annex C (WARNING: Cannabis smoke is harmful. Harmful chemicals found in tobacco smoke are also found in cannabis smoke.) would only be required to appear on dried cannabis products.
- All health warning messages must be rotated and appear on each package of each brand name with equal frequency in a calendar year.
- The only attributions that could be displayed are: “Health Canada / Santé Canada” for bilingual health warning messages, or “Health Canada” for the English display panel and “Santé Canada” for the French display panel.
A1.3 Other Required Information
It will be proposed that the following general information would be required on all labels:
- Name, telephone number, and email address of the licensed processor;
- Class of cannabis (e.g. dried cannabis);
- Brand name;
- Lot number;
- Recommended storage conditions;
- Packaging date;
- Expiry date or statement that no expiry date has been determined; and
- The statement “KEEP OUT OF REACH OF CHILDREN / TENIR HORS DE LA PORTÉE DES ENFANTS”.
It will be proposed that the following information would also be required, although specific requirements would vary depending on the class of cannabis (fresh or dried cannabis, or cannabis oil) and whether the cannabis is in discrete (i.e. unit) form:
- The net weight (and net volume, for cannabis oil);
- The number of discrete units, if applicable; and
- The percentage (or quantity, in milligrams) of THC and CBD.
The following specific requirements for the different classes of cannabis in non-discrete and discrete forms will be proposed:
A1.3.1 Fresh or Dried Cannabis (not in discrete form)
- the net weight, in grams, of dried cannabis or fresh cannabis;
- the percentage of THC as calculated on a weight-by-weight (w/w) basis, preceded by “THC”;
- the percentage of THC w/w that the dried cannabis or fresh cannabis could yield, taking into account the potential to convert THCA into THC, preceded by “Total THC”;
- the percentage of CBD w/w, preceded by “CBD”;
- the percentage of CBD w/w that the dried cannabis or fresh cannabis could yield, taking into account the potential to convert CBDA into CBD, preceded by “Total CBD”.
A1.3.2 Fresh or Dried Cannabis (in discrete form)
- the net weight, in grams, of dried cannabis or fresh cannabis;
- the number of discrete units;
- the net weight, in grams, of dried cannabis or fresh cannabis in each discrete unit;
- the quantity of THC, in milligrams, in each discrete unit, preceded by “THC per unit”;
- the quantity of THC, in milligrams, that each discrete unit could yield, taking into account the potential to convert THCA into THC, preceded by “Total THC per unit”;
- the quantity of CBD, in milligrams, in each discrete unit, preceded by “CBD per unit” ;
- the quantity of CBD, in milligrams, that each discrete unit could yield, taking into account the potential to convert CBDA into CBD, preceded by “Total CBD per unit quantity of CBD”.
A1.3.3 Cannabis Oil (not in discrete form)
- the net weight, in grams, and net volume, in millilitres, of cannabis oil;
- the carrier oil used;
- the name of any food allergen, within the meaning of subsection B.01.010.1(1) of the Food and Drug Regulations, that is contained in the cannabis oil;
- in the case of cannabis oil that is not intended for ingestion, the warning “DO NOT INGEST / NE PAS INGÉRER”;
- the quantity of THC, in milligrams, in the cannabis oil, preceded by “THC”;
- the quantity of THC, in milligrams, that the cannabis oil could yield, taking into account the potential to convert THCA into THC, preceded by “Total THC”;
- the quantity of CBD, in milligrams, preceded by “CBD”;
- the quantity of CBD, in milligrams, that the cannabis oil could yield, taking into account the potential to convert CBDA into CBD, preceded by “Total CBD”.
A1.3.4 Cannabis Oil (in discrete form)
- the net weight, in grams, and net volume, in mL, of cannabis oil;
- the number of discrete units;
- the net weight, in grams, and net volume, in mL, of cannabis oil in each discrete unit;
- the carrier oil used;
- the name of any food allergen, within the meaning of subsection B.01.010.1(1) of the Food and Drug Regulations, that is contained in the cannabis oil;
- in the case of cannabis oil that is not intended for ingestion, the warning “DO NOT INGEST / NE PAS INGÉRER”
- the quantity of THC, in milligrams, in each discrete unit preceded by “THC per unit”;
- the quantity of THC, in milligrams, that each discrete unit could yield, taking into account the potential to convert THCA into THC, preceded by “Total THC per unit”;
- the quantity of CBD, in milligrams, in each discrete unit, preceded by “CBD per unit”;
- the quantity of CBD, in milligrams, that each discrete unit could yield, taking into account the potential to convert CBDA into CBD, preceded by “Total CBD per unit”.
Annex B: Details of Proposed Label Display Requirements for Cannabis
Note: In addition to the requirements set out in this Annex, there are a number of packaging and labelling requirements set out in the proposed Cannabis Act, as well as in Chapter 6 and other Annexes to the current report, to which regulated parties would be subject. It is important that all of these requirements be considered in the context of any decisions with respect to packaging and labelling that could be made between the date of publication of this report and the anticipated coming into force of the proposed Cannabis Act and its regulations.
B1.1 General Requirements for all Labels
- Text must appear in the same standard sans-serif font style without italics for the health warning messages, THC/CBD content and other required information except for the brand name
B1.2 Standardized Cannabis Symbol
- Minimum size is 1.27 cm (w) x 1.27 cm (h)
- Outset of 2 pointFootnote a (pt) on all sides in white
- Must appear in the upper left 25% of the principal display panel
- The symbol will be available for download from canada.ca/cannabis
- All components of the symbol must be presented as displayed and as a single unit, cannot be altered in any way, except that it can be enlarged provided all elements remain the same proportionally
- The symbol must be oriented in such a manner that its text is readable from left to right when the package is displayed, and is parallel with the base of the package
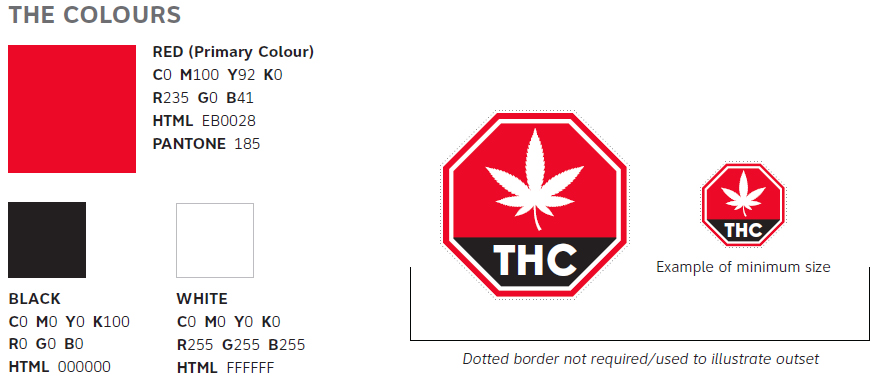
Figure 2 - Text Description
The Colours
There is a red square that illustrates the red colour to be used for the standardized cannabis symbol. The description to the right is as follows:
- RED (Primary Colour)
- C0 M100 Y92 K0
- R235 G0 B41
- HTML EB0028
- PANTONE 185
There is a black square that illustrates the black colour to be used for the standardized cannabis symbol. The description underneath is as follows:
- BLACK
- C0 M0 Y0 K100
- R0 G0 B0
- HTML 000000
There is a white square that illustrates the black colour to be used for the standardized cannabis symbol. The description underneath is as follows:
- WHITE
- C0 M0 Y0 K0
- R255 G255 B255
- HTML FFFFFF
The standardized cannabis symbol is a red octagon. There is a white border on the octagon. Inside the octagon, there is a white cannabis leaf which covers two thirds of the inside of the shape. Underneath the cannabis leaf, one third of the octagon is filled with black and contains the letters THC in a white, bold, uppercase font. There is a dotted line around the octagon to show the outset that is required. The standardized cannabis symbol is show in a large size and a smaller, actual size. The text underneath the smaller size says “Example of minimum size”. Underneath both octagons is a solid line with text underneath. The text reads, “Dotted border not required/used to illustrate outset”.
B1.3 Health Warning Messages
- First sentence of the warning message is to be in bold font in both English and French, sentence case with the word “WARNING” “MISE EN GARDE” in uppercase, bold, followed by a colon
- Second sentence of the warning message is in sentence case with no bold
- Text of the health warning message must be black, standard sans-serif fontFootnote * (example: Calibri) without italics in a 7 pt minimum
- Text must be left justified without hyphenation and must be oriented in such a manner that its text is readable from left to right when the package is displayed, and is parallel with the base of the package
- Leading: 8 pt
- 3 pt spacing between English/ French when English and French health warning messages appear on the same principal display panel
- Text must be surrounded by a 1 pt black stroke border with an inset of 6 pt on all sides
- Text must appear on a yellow background
- The attribution must have the same font attributes as the health warning message except that it can be in a smaller font size (minimum of 6 pt) and must appear in the bottom right corner of the border below the health warning message.
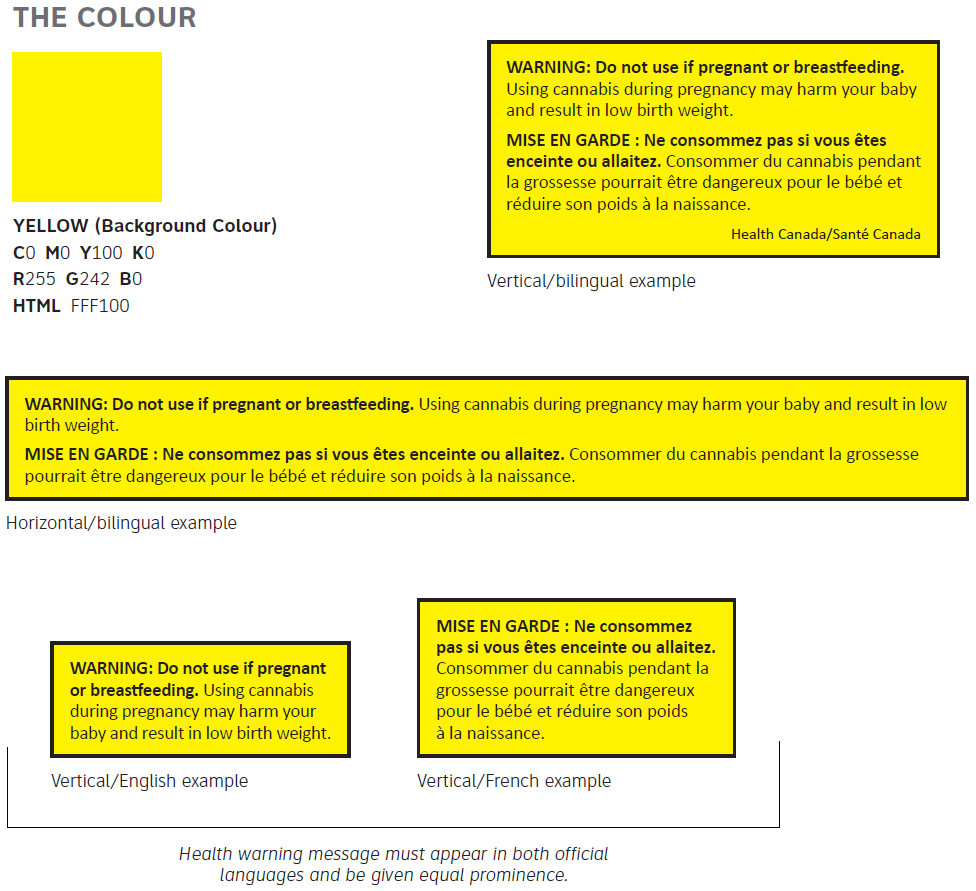
Figure 3 - Text Description
There is a yellow square that illustrates the colour to be used for the background of the health warning message. The description underneath is as follows:
- YELLOW (Background Colour)
- C0 M0 Y100 K0
- R255 G242 B0
- HTML FFF100
-
There is a yellow rectangle that is outlined by a thin black rule. Inside the rectangle the word “Warning” appears in black, bold, uppercase text.
After the word warning, the text “Do not use if pregnant or breastfeeding.” appears in black, bold, mixed-case text. After the last sentence, the text “Using cannabis during pregnancy may harm your baby and result in low birth weight.” appears in black, regular, mixed-case font.
On a new line, the text “Mise en garde” appears in black, bold, uppercase text. After the words mise en garde, the text “Ne consommez pas, si vous êtes enceinte ou allaitez.” appears in black, bold, mixed-case text. After the last sentence, the text “ Consommer du cannabis pendant la grossesse pourrait être dangereux pour le bébé et réduire son poids à la naissance.” appears in black, regular, mixed-case font.
In the bottom right hand corner of the yellow text box are the words “Health Canada / Santé Canada” in black, regular, mixed-case font.
-
There is a yellow rectangle that is outlined by a thin black rule. Inside the rectangle the word “Warning” appears in black, bold, uppercase text. After the word warning, the text “Do not use if pregnant or breastfeeding.” appears in black, bold, mixed-case text. After the last sentence, the text “Using cannabis during pregnancy may harm your baby and result in low birth weight.” appears in black, regular, mixed-case font.
On a new line, the text “Mise en garde” appears in black, bold, uppercase text. After the words “mise en garde”, the text “Ne consommez pas, si vous êtes enceinte ou allaitez.” appears in black, bold, mixed-case text. After the last sentence, the text “Consommer du cannabis pendant la grossesse pourrait être dangereux pour le bébé et réduire son poids à la naissance.” appears in black, regular, mixed-case font.
- There is a yellow rectangle that is outlined by a thin black rule. Inside the rectangle the word “Warning” appears in black, bold, uppercase text. After the word warning, the text “Do not use if pregnant or breastfeeding.” appears in black, bold, mixed-case text. After the last sentence, the text “Using cannabis during pregnancy may harm your baby and result in low birth weight.” appears in black, regular, mixed-case font.
- There is a yellow rectangle that is outlined by a thin black rule. Inside the rectangle the text “Mise en garde” appears in black, bold, uppercase text. After the words mise en garde, the text “Ne consommez pas, si vous êtes enceinte ou allaitez.” appears in black, bold, mixed-case text. After the last sentence, the text “Consommer du cannabis pendant la grossesse pourrait être dangereux pour le bébé et réduire son poids à la naissance.” appears in black, regular, mixed-case font.
B1.4 Other Required Information
Applies to all other required information (except brand name)
- White background
- Outset of 6 pt from the white background on all sides
- Leading: 7 pt
- Black standard sans-serif font (example: Calibri) without italics: 6 pt minimum and must be smaller than the font of the health warning message
Additional requirements for THC/CBD information:
- Text must be in bold
- Outset of 6 pt on all sides between this and any other text
Brand name
- Brand name can be displayed in any font style and font size provided it is equal to or smaller than the health warning message
- Font must be a single, uniform colour—no fluorescent or metallic colours


Figure 4 - Text Description
- 1. There is a rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that required around the text.
- Just outside the dotted rectangle area are the words “BRAND NAME MARQUE NOMINATIVE”. The text appears in green, bold, uppercase font.
- The text on the first line inside the rectangle is “Dried Cannabis” written in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Cannabis séché” written in black, mixed-case font.
- On the next line is the text “Store in a dry place”. This text appears in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Entreposer dans un endroit sec”. This text appears in black, mixed-case font.
- On the next line is “KEEP OUT OF REACH OF CHILDREN” followed by “TENIR HORS DE LA PORTÉE DES ENFANTS”. This text appears in black, uppercase font.
- The text on the following line is “Name of processor | Nom du titulaire d’une licence de transformation”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | name-nom@company-compagnie.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Expiry Date JAN 2020 | Date limite d’utilisation JAN 2020”. It is followed by the text “Packaged on Dec 21 2017 | Emballé le 21 déc. 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the last line is “Net weight 20 g | Poids de net 20 g”. The text appears in black, mixed-case font.
- Within the large rectangle there is a small rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that is required around the text. The text inside the rectangle is on two lines. The first line reads, “THC 5% (Total THC 10% / THC Total 10 %)”. The second line of text reads “CBD 5% (Total CBD 10% / CBD Total 10 %)”. All text is in black, bold, mixed-case font.
- The text underneath the box reads Horizontal/bilingual example in black, mixed-case font.
- 2. There is a rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that required around the text.
- Just outside the dotted rectangle area are the words “BRAND NAME”. The text appears in green, bold, uppercase font.
- The text on the first line inside the rectangle is “Dried Cannabis” written in black, mixed-case font.
- On the next line is the text “Store in a dry place”. This text appears in black, mixed-case font.
- On the next line is “KEEP OUT OF REACH OF CHILDREN”. This text appears in black, uppercase font.
- The text on the following line is “Name of processor”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | name@company.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Expiry Date JAN 2020”. It is followed by the text “Packaged on Dec 21 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the last line is “Net weight 20 g”. The text appears in black, mixed-case font.
- Within the large rectangle there is a small rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that is required around the text. The text inside the rectangle is on two lines. The first line reads, “THC 5% (Total THC 10%)”. The second line of text reads “CBD 5% (Total CBD 10%)”. All text is in black, bold, mixed-case font.
- The text underneath the box reads English example in black, mixed-case font.
- 3. There is a rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that required around the text.
- Just outside the dotted rectangle area are the words “MARQUE NOMINATIVE”. The text appears in green, bold, uppercase font.
- The text on the first line inside the rectangle is “Cannabis séché” written in black, mixed-case font.
- On the next line is the text “Entreposer dans un endroit sec”. This text appears in black, mixed-case font.
- On the next line is “TENIR HORS DE LA PORTÉE DES ENFANTS”. This text appears in black, uppercase font.
- The text on the following line is “Nom du titulaire d’une licence de transformation”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | nom@compagnie.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Date limite d’utilisation JAN 2020”. It is followed by the text “Emballé le 21 déc. 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the last line is “Poids de net 20 g”. The text appears in black, mixed-case font.
- Within the large rectangle there is a small rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that is required around the text. The text inside the rectangle is on two lines. The first line reads, “THC 5 % (THC Total 10 %)”. The second line of text reads “CBD 5 % (CBD Total 10 %)”. All text is in black, bold, mixed-case font.
- The text underneath the box reads French example in black, mixed-case font.
- 4. There is a rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that required around the text.
- Just outside the dotted rectangle area are the words “BRAND NAME MARQUE NOMINATIVE”. The text appears in green, bold, uppercase font.
- The text on the first line inside the rectangle is “Cannabis Oil” written in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Huile de Cannabis” written in black, mixed-case font.
- On the next line is the text “Store in a dry place”. This text appears in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Entreposer dans un endroit sec”. This text appears in black, mixed-case font.
- On the next line is “KEEP OUT OF REACH OF CHILDREN” followed by “TENIR HORS DE LA PORTÉE DES ENFANTS”. This text appears in black, uppercase font.
- The text on the following line is “Name of processor | Nom du titulaire d’une licence de transformation”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | name-nom@company-compagnie.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Expiry Date JAN 2020 | Date limite d’utilisation JAN 2020”. It is followed by the text “Packaged on Dec 21 2017 | Emballé le 21 déc. 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the following line is “Net weight 15 g / Net volume 15 ml” “Poids net de 15 g / Volume net de 15 ml”. The text appears in black, mixed-case font.
- The next line has the text “15 capsules / 15 capsules”. The text appears in black, mixed-case font.
- The following line has the text “1 g per unit / 1 ml per unit” “1 g par unité / 1 ml par unite”. The text appears in black, mixed-case font.
- The next line has the text “Allergen / Allergène” and “Carrier Oil / Huile de base”. The text appears in black, mixed-case font.
- Within the large rectangle there is a small rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that is required around the text. The text inside the rectangle is on two lines. The first line reads, “THC per unit 10 mg / Total THC per unit 10 mg” followed by “THC par unité 10 mg / THC Total par unité 10 mg”. The lines underneath have the text “CBD per unit 5 mg / Total CBD per unit 5 mg” followed by “CBD par unité 5 mg / CBD Total par unité 5 mg”. All text is in black, bold, mixed-case font.
- The text underneath the box reads Horizontal/bilingual example in black, mixed-case font.
- 5. There is a rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that required around the text.
- Just outside the dotted rectangle area are the words “BRAND NAME”. The text appears in green, bold, uppercase font.
- The text on the first line inside the rectangle is “Cannabis Oil” written in black, mixed-case font.
- On the next line is the text “Store in a dry place”. This text appears in black, mixed-case font.
- On the next line is “KEEP OUT OF REACH OF CHILDREN”. This text appears in black, uppercase font.
- The text on the following line is “Name of processor”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | name@company.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Expiry Date JAN 2020”. It is followed by the text “Packaged on Dec 21 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the following line is “Net weight 15 g / Net volume 15 ml”. The text appears in black, mixed-case font.
- The next line has the text “15 capsules”. The text appears in black, mixed-case font.
- The following line has the text “1 g per unit / 1 ml per unit”. The text appears in black, mixed-case font.
- The next line has the text “Allergen” and “Carrier Oil”. The text appears in black, mixed-case font.
- Within the large rectangle there is a small rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that is required around the text. The text inside the rectangle is on two lines. The first line reads, “THC per unit 10 mg / Total THC per unit 10 mg”. The line underneath has the text “CBD per unit 5 mg / Total CBD per unit 5 mg”. All text is in black, bold, mixed-case font.
- The text underneath the box reads English example in black, mixed-case font.
- 6. There is a rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that required around the text.
- Just outside the dotted rectangle area are the words “MARQUE NOMINATIVE”. The text appears in green, bold, uppercase font.
- The text on the first line inside the rectangle is “Huile de Cannabis” written in black, mixed-case font.
- On the next line is the text “Entreposer dans un endroit sec”. This text appears in black, mixed-case font.
- On the next line is “TENIR HORS DE LA PORTÉE DES ENFANTS”. This text appears in black, uppercase font.
- The text on the following line is “Nom du titulaire d’une licence de transformation”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | nom@compagnie.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Date limite d’utilisation JAN 2020”. It is followed by the text “Emballé le 21 déc. 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the following line is “Poids net de 15 g / Volume net de 15 ml”. The text appears in black, mixed-case font.
- The next line has the text “15 capsules”. The text appears in black, mixed-case font.
- The following line has the text “ “1 g par unité / 1 ml par unité”. The text appears in black, mixed-case font.
- The next line has the text “Allergène” and “Huile de base”. The text appears in black, mixed-case font.
- Within the large rectangle there is a small rectangle with a dotted border. The dotted border will not be required to appear on labels, but is used to show the outset that is required around the text. The text inside the rectangle is on two lines. The first line reads, “THC par unité 10 mg / THC Total par unité 10 mg”. The lines underneath have the text “CBD par unité 5 mg / CBD Total par unité 5 mg”. All text is in black, bold, mixed-case font.
- The text underneath the box reads French example in black, mixed-case font.
B1.5 Non-required Information
Brand elementFootnote **
- One brand element, such as a logo, may appear on the principal display panel (Two brand elements are permitted in the case of separate English and French principal display panels)
- If the brand element is a graphic image or logo, it must not be larger than the standardized cannabis symbol
- If it is a text element, the font size must be equal to or smaller than the font size of the health warning message
- No other images or graphics are permitted
Any other information
- The font size of all non-required information must be smaller than the health warning message
- Text must appear in a black or white standard sans-serif font style without italics or bold
B1.6 Examples

Figure 5 - Text Description
- 1. There is an image of a plastic bag with a resealable top and a child resistant fastener. The bag is white.
- On the top left corner is the standardized cannabis symbol. The standardized cannabis symbol is a red octagon. There is a white border on the octagon. Inside the octagon, there is a white cannabis leaf which covers two thirds of the inside of the shape. Underneath the cannabis leaf, one third of the octagon is filled with black and contains the letter THC in a white, bold font. The letters appear in all capital letters.
- On the top right corner is the text “Company Name” written over two lines. The text is purple, bold, uppercase font.
- There is a yellow rectangle that is outlined by a thin black rule. Inside the rectangle the word “Warning” appears in black, bold, uppercase text.
- After the word warning, the text “Do not use if pregnant or breastfeeding.” appears in black, bold, mixed-case text. After the last sentence, the text “Using cannabis during pregnancy may harm your baby and result in low birth weight.” appears in black, regular, mixed-case font.
- On a new line, the text “Mise en garde” appears in black, bold, uppercase text. After the words mise en garde, the text “Ne consommez pas si vous êtes enceinte ou allaitez.” appears in black, bold, mixed-case text. After the last sentence, the text “Consommer du cannabis pendant la grossesse pourrait être dangereux pour le bébé et réduire son poids à la naissance. ” appears in black, regular, mixed-case font.
- Underneath the health warning message, there is the text “BRAND NAME” and “MARQUE NOMINATIVE”. The text appears in green, bold, uppercase font.
- There are two line of text. The first line reads, “THC 5% (Total THC 10% / THC Total 10 %)”. The second line of text reads “CBD 5% (Total CBD 10% / CBD Total 10 %)”. All text is in black, bold, mixed-case font.
- There is text underneath the image that reads “Example of FRONT (principal display panel) with white/plain background and brand/processor name” in black, mixed-case font. The words FRONT and principal display panel appear in bold.
- There is an image of a plastic bag with a resealable top and a child resistant fastener. The bag is white.
- The text on the first line inside the rectangle is “Dried Cannabis” written in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Cannabis séché” written in black, mixed-case font.
- On the next line is the text “Store in a dry place”. This text appears in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Entreposer dans un endroit sec”. This text appears in black, mixed-case font.
- On the next line is “KEEP OUT OF REACH OF CHILDREN” followed by “TENIR HORS DE LA PORTÉE DES ENFANTS”. This text appears in black, uppercase font.
- The text on the following line is “Name of processor | Nom du titulaire d’une licence de transformation”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | name-nom@company-compagnie.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Expiry Date JAN 2020 | Date limite d’utilisation JAN 2020”. It is followed by the text “Packaged on Dec 21 2017 | Emballé le 21 déc. 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the last line line is “Net weight 20 g | Poids de net 20 g”. The text appears in black, mixed-case font.
- Underneath the image of the bag, there is the text “Example of BACK (secondary display panel) with white/plain background” in black, mixed-case font. The words BACK (secondary display panel) appear in bold.
- 2. There is an image of a black cube shaped box.
- On the top left corner is the standardized cannabis symbol. The standardized cannabis symbol is a red octagon. There is a white border on the octagon. Inside the octagon, there is a white cannabis leaf which covers two thirds of the inside of the shape. Underneath the cannabis leaf, one third of the octagon is filled with black and contains the letter THC in a white, bold font. The letters appear in all capital letters.
- On the top right corner is a green, diamond shaped symbol with the words “Company Logo” written inside. The text is white, script font.
- The text is “BRAND NAME” and “MARQUE NOMINATIVE” and it appears in green, bold, uppercase font.
- There is a white rectangle with two lines of text inside. The first line reads, “THC 5% (Total THC 10% / THC Total 10 %)”. The second line of text reads “CBD 5% (Total CBD 10% / CBD Total 10 %)”. All text is in black, bold, mixed-case font.
- There is a yellow rectangle that is outlined by a thin black rule. Inside the rectangle the word “Warning” appears in black, bold, uppercase text.
- After the word warning, the text “Do not use if pregnant or breastfeeding.” appears in black, bold, mixed-case text. After the last sentence, the text “Using cannabis during pregnancy may harm your baby and result in low birth weight.” appears in black, regular, mixed-case font.
- On a new line, the text “Mise en garde” appears in black, bold, uppercase text. After the words mise en garde, the text “Ne consommez pas si vous êtes enceinte ou allaitez.” appears in black, bold, mixed-case text. After the last sentence, the text “ Consommer du cannabis pendant la grossesse pourrait être dangereux pour le bébé et réduire son poids à la naissance.” appears in black, regular, mixed-case font.
- There is text underneath the image that reads “Example of FRONT (principal display panel) with solid coloured background and brand/processor logo” in black, mixed-case font.
- There is an image of a black cube shaped box with a white rectangle on it.
- The text on the first line inside the rectangle is “Dried Cannabis” written in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Cannabis séché” written in black, mixed-case font.
- On the next line is the text “Store in a dry place”. This text appears in black, mixed-case font. Then there is a black, circular, bullet point. After the bullet point there is the text “Entreposer dans un endroit sec”. This text appears in black, mixed-case font.
- On the next line is “KEEP OUT OF REACH OF CHILDREN” followed by “TENIR HORS DE LA PORTÉE DES ENFANTS”. This text appears in black, uppercase font.
- The text on the following line is “Name of processor | Nom du titulaire d’une licence de transformation”. The text appears in black, mixed-case font.
- The text on the next line is “123-456-7890 | name-nom@company-compagnie.ca”. The text appears in black, mixed-case font.
- The text on the next line is “Expiry Date JAN 2020 | Date limite d’utilisation JAN 2020”. It is followed by the text “Packaged on Dec 21 2017 | Emballé le 21 déc. 2017” on the next line. The text appears in black, mixed-case font.
- On the next line is the text “Lot 12345”. This text is in black, mixed-case font.
- The text on the last line line is “Net weight 20 g | Poids de net 20 g”. The text appears in black, mixed-case font.
- Underneath the image of the bag, there is the text “Example of BACK (secondary display panel) with solid coloured background” in black, mixed-case font. The words BACK (secondary display panel) appear in bold.
Annex C: Proposed Health Warning Messages
| Primary Sentence | Secondary Sentence |
|---|---|
WARNING: Cannabis smoke is harmful. |
Harmful chemicals found in tobacco smoke are also found in cannabis smoke. |
WARNING: Do not use if pregnant or breastfeeding. |
Using cannabis during pregnancy may harm your baby and result in low birth weight. |
WARNING: Do not drive or operate machinery after using cannabis. |
More than 4,000 Canadians were injured and 75 died from driving after using cannabis (in 2012). |
WARNING: Cannabis can be addictive. |
Up to half of people who use cannabis on a daily basis have work, social or health problems from using cannabis. |
WARNING: Regular use of cannabis can increase the risk of psychosis and schizophrenia. |
Higher THC content can increase the risk of psychosis and schizophrenia. |
WARNING: Adolescents are at greater risk of harms from cannabis. |
Early and regular use increases the risk of psychosis and schizophrenia. |