Guidelines for Canadian Drinking Water Quality: Guideline Technical Document - 1,4-Dioxane
Download in PDF format
(1.07 MB, 63 pages)
Organization: Health Canada or Public Health Agency of Canada
Date published: March 2021
Cat.: H144-80/2021E-PDF
ISBN: 978-0-660-37417-8
Pub.: 200436
Table of Contents
- Part I. Overview and Application
- 1.0 Guideline value
- 2.0 Executive summary
- 3.0 Application of the guideline
- Part II. Science and Technical Considerations
- 4.0 Identity, use and sources in the environment
- 5.0 Exposure
- 6.0 Analytical methods
- 7.0 Treatment technology system considerations.
- 8.0 Kinetics and metabolism
- 9.0 Health effects
- 10.0 Classification and assessment
- 11.0 Rationale
- 12.0 References
- Appendix A: List of abbreviations
Part I. Overview and Application
1.0 Guideline value
A maximum acceptable concentration (MAC) of 0.050 mg/L (50 µg/L) is established for 1,4-dioxane in drinking water.
2.0 Executive summary
1,4-Dioxane is a synthetic chemical that is not found naturally in the environment. It is produced in Canada and imported from other countries, primarily to be used as an industrial and commercial solvent. It can also be present as a contaminant in cosmetics, food additives, and food packaging materials, or on food crops treated with pesticides containing 1,4-dioxane. Its release to the environment is mainly from chemical waste disposal practices, leaks from landfills, or wastewater discharges. Because of its chemical properties, 1,4-dioxane travels rapidly, partitioning from soil to groundwater sources.
This guideline technical document reviews and assesses all identified health risks associated with 1,4-dioxane in drinking water. It incorporates available studies and approaches and takes into consideration the availability of appropriate treatment technology. Based on this review, the guideline for 1,4-dioxane in drinking water is a maximum concentration of 0.050 mg/L (50 μg/L).
2.1 Health effects
The International Agency for Research on Cancer (IARC) classified 1,4-dioxane as “possibly carcinogenic to humans” (group 2B) based on sufficient evidence in experimental animals and inadequate evidence in humans.
The MAC of 0.050 mg/L is based on studies of liver effects in rats that occur before the development of cancer, and is protective of both cancer and non-cancer health effects of 1,4-dioxane. Studies in humans are limited to the non-cancer health risks associated with exposure via inhalation, which affects the liver and kidneys, and support the observations in experimental animal studies.
The most severe health effect associated with exposure to 1,4-dioxane in animals is cancer. Science indicates that 1,4-dioxane only causes cancer above a certain level of exposure. As the non-cancer health effects on the liver are the most sensitive health effects and are precursors of the cancer effects, they are deemed appropriate as the basis for a MAC that is protective of both cancer and non-cancer health effects.
2.2 Exposure
The primary sources of exposure to 1,4-dioxane are inhalation of outdoor air or vapours during cleaning activities, ingestion of contaminated food and drinking water, and dermal contact with consumer products. 1,4-Dioxane is generally not detected in water supplies in Canada. In some cases, it has been found in groundwater located near landfills and industrial sites as it can migrate rapidly in the subsurface.
Although skin contact and inhalation are potential routes of exposure to 1,4-dioxane, the intake of 1,4-dioxane from drinking water through these routes (e.g., while bathing or showering) is not significant and is not considered in this assessment.
2.3 Analysis and treatment considerations
Because of its chemical properties, analysis of 1,4-dioxane can be challenging. Therefore, appropriate sample preparation methods are needed for 1,4-dioxane to be measured in drinking water at levels well below the MAC.
Since the physical and chemical properties of 1,4-dioxane make it difficult to remove using conventional drinking water treatment at the municipal level, alternative treatment technologies, such as advanced oxidation processes and, to a lesser extent, synthetic adsorbents, need to be considered. These alternative technologies are capable of effectively removing 1,4-dioxane, achieving treated water concentrations below the MAC. Recent research also indicates that reverse osmosis membranes may be capable of removing a large proportion of 1,4-dioxane from water.
At the residential level, there are no certified residential treatment units for the reduction of 1,4-dioxane from drinking water. However, available data suggest that 1,4-dioxane may be effectively removed by reverse osmosis at the point of use.
3.0 Application of the guideline
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority in the affected jurisdiction.
The main use of 1,4-dioxane has historically been in industrial applications as a stabilizer of 1,1,1-trichloroethane (TCA). It commonly co-occurs in groundwater contaminated with the chlorinated solvent TCA and its degradation product 1,1-dichloroethene (1,1-DCE) as well trichloroethylene (TCE) at sites with long operating histories where both TCA and TCE were used. 1,4-Dioxane also occurs as a by-product in the production of ethoxylated surfactants and polyethylene terephthalate plastics, and it is used directly in pharmaceutical and other industries. Landfills and solvent recycling facilities are among the most common sources of 1,4-dioxane contamination in groundwater. Effluents from industrial facilities and wastewater treatment plants have also been found to be sources of 1,4-dioxane in surface water.
Due to the chemically persistent nature of 1,4-dioxane, the impact of release events (such as historical waste disposal practices) are typically long-lasting on the receiving environment. Since 1,4-dioxane is resistant to natural degradation and other attenuation processes once it enters the subsurface, it can reach drinking water wells through the migration of a contaminated groundwater plume.
3.1 Monitoring
Utilities should characterize their source water to determine the concentration of 1,4-dioxane. Semi-annual monitoring should be conducted for sources that are known to be impacted by industrial wastes, landfill leachate, wastewater effluent and/or sources that contain chlorinated solvents. Utilities with baseline data indicating that 1,4-dioxane is not present in source water may conduct less frequent monitoring.
Drinking water systems can treat source water using specific treatment processes (i.e., advanced oxidation processes) to remove 1,4-dioxane from drinking water. 1,4-Dioxane is not effectively treated by technologies usually employed for volatile organic compounds (VOCs). Therefore, these treatment systems should be carefully designed and maintained to ensure that they are effective for treating 1,4-dioxane. When treatment is in place for 1,4-dioxane, compliance monitoring of the treated water should be conducted semi-annually and in conjunction with monitoring of the source water to confirm the efficacy of treatment. Drinking water samples should be collected after treatment and prior to distribution (typically at the entry point to the distribution system). The operational monitoring frequency will depend on the treatment technology the utility employs.
Part II. Science and Technical Considerations
4.0 Identity, use and sources in the environment
The chemical 1,4-dioxane (C4H8O2; Chemical Abstracts Services Registry No. 123-91-1) is a cyclic ether with a molecular mass of 88.1 g/mol. 1,4-Dioxane is a flammable and colourless liquid that is miscible in water, does not bind well to soils, and resists hydrolyzation in nature (ATSDR, 2012; US EPA, 2013). 1,4-Dioxane has a reported odour threshold of 24 parts per million (ppm) in air and 230 ppm in water (Amoore and Hautale, 1983), a unit-less log octanol-water partition coefficient (Kow) of -0.27 (Hansch et al., 1995), a vapour pressure of 38.1 mmHg at 25°C (Daubert and Danner, 1985), and a Henry's law constant of 4.80 × 10-6 atm-m3/molecule at 25°C (Park et al., 1987).
Information submitted under section 71 of the Canadian Environmental Protection Act, 1999 indicates that 10 000 to 100 000 kg of 1,4-dioxane were manufactured in Canada in 2006 and that between 10 000 and 100 000 kg were imported in the same year and used by Canadian companies (Environment Canada, 2008).
1,4-Dioxane is primarily used as an industrial and commercial solvent that reduces the harsh nature of certain compounds (to reduce the risk of irritation) and enhances their foaming capabilities. More specifically, 1,4-dioxane is used in the preparation of lacquers, coatings, plastics, varnishes, polishes, waxes, and adhesives, as well as in pharmaceuticals, polyurethane materials for medical devices, and cleaning and detergent preparations. Residual 1,4-dioxane is present as a contaminant that forms as a by-product during the sulphonation reaction with alcohol ethoxylates in certain cosmetics, food additives, and food packaging materials, or on food crops treated with pesticides that contain 1,4-dioxane (Environment Canada and Health Canada, 2010; US EPA, 2014, 2015a). It may also be used in laboratory settings as a reagent and as a solvent in the production of brominated fire retardants. Historically, the main use of 1,4-dioxane was as a stabilizer in chlorinated solvents (e.g., TCA), accounting for approximately 90% of its use (US EPA, 2015a). The use of TCA was phased out under the 1995 Montreal Protocol (UNEP, 2000); thus the use of 1,4-dioxane as a stabilizer is no longer significant.
Natural sources of 1,4-dioxane have not been identified (Environment Canada and Health Canada, 2010). The largest sources of 1,4-dioxane in drinking water in the United States (U.S.) are wastewater discharge, unintended spills, leaks, and historical disposal practices of its host solvent TCA (Water Research Foundation, 2014). Indeed, 1,4-dioxane is often co-detected with chlorinated solvents; 93.7% of 1,4-dioxane detections in groundwater wells at United States Air Force installations (n = 5788 at 49 installations) had co-detection with TCE and/or TCA (Anderson et al., 2012). As well, in a study of over 2 000 sites in California where groundwater had been impacted by chlorinated solvents and/or 1,4-dioxane, 95% of 1,4-dioxane detections had co-detection with one or more chlorinated solvents (Adamson et al., 2014). 1,4-Dioxane contamination in groundwater most commonly originates from landfills and solvent recycling facilities; mean concentrations of 1,4-dioxane in landfill leachate in the U.S. ranged from 118 ppb in municipal landfills to 466 ppb at hazardous waste disposal sites (as described in Mohr et al., 2010). Additional anthropogenic sources include direct production/processing or unintentional by-product formation as a result of ethoxylation reactions during the development of ethoxylated polymers for industrial and consumer applications (Robinson and Ciurczak, 1980; NICNAS, 1998; Black et al., 2001). The National Pollutant Release Inventory (NPRI) collects information from Canadian industrial, commercial and institutional facilities on their releases (to air, water and land), disposals, and transfers of pollutants and other substances of concern, including 1,4-dioxane.
On-site releases of 1,4-dioxane to water bodies totalled 7 400 kg in 2011, 3 900 kg in 2012, 3 200 kg in 2013, 3 100 kg in 2014, and 4 300 kg in 2015 (NPRI, 2016). On-site releases to air totalled 13 000 kg in 2011, 888 kg in 2012, 934 kg in 2013, 64 kg in 2014, and 1 100 kg in 2015. No releases to land were reported.
4.1 Environmental fate
1,4-Dioxane may enter the environment through air, soil and water. In air, 1,4-dioxane remains a vapour and is degraded through reactions with photochemically produced hydroxyl radicals, resulting in an estimated half-life of 35 h (HSDB, 2015). Due to its high vapour pressure, 1,4-dioxane may volatilize from dry soil. In soil and water, 1,4-dioxane is relatively resistant to biodegradation (US EPA, 2013). In water, 1,4-dioxane is miscible (solubility = 1,000 mg/mL) and does not sorb strongly to organic material (log Koc= 1.23; log Kow = -0.27). As a result, 1,4-dioxane is highly mobile in wet soil and will readily leach into lower soil horizons and groundwater (Mohr, 2010; US EPA 2009; 2013). Based on a Henry's Law constant of 4.8×10-6 atm-m3/mole, moderate volatilization may occur in surface water with half-lives in a model river and lake of 5 and 56 days, respectively (US EPA, 2013). Hydrolysis and photolysis in surface waters are minimal therefore these are not expected to be important environmental fate processes. In groundwater, 1,4-dioxane is not well attenuated with reported half-lives ranging from 2 to 5 years (Adamson et al., 2015). Because 1,4-dioxane is relatively resistant to adsorption and biodegradation, it generally migrates substantially further in groundwater than many organic contaminants including other chlorinated solvents (Mohr, 2001, 2010; Zenker et al., 2003). For example, at the Gloucester landfill in Ottawa, Ontario, 1,4-dioxane led a TCA plume by 500 feet (Zenker et al., 2003).
5.0 Exposure
The primary sources of human exposure to 1,4-dioxane are inhalation of outdoor air or vapours during cleaning activities, ingestion of contaminated food and drinking water, and dermal contact with consumer products. An exposure assessment for 1,4-dioxane was previously conducted (Environment Canada and Health Canada, 2010) based on exposure estimates for environmental media and consumer products; however, Canadian data for all exposure sources are either limited or unavailable. Thus, a default allocation factor for 1,4-dioxane in drinking water of 0.2 was applied.
5.1 Water
Data regarding 1,4-dioxane concentrations in drinking water from Prince Edward Island, Newfoundland and Labrador, Nova Scotia, New Brunswick, Manitoba, Saskatchewan, Alberta, British Columbia, Yukon, Northwest Territories, and Nunavut are not currently available. Data from 111 regions in Ontario for 2013-2015 indicated that 1,4-dioxane was below the detection limit (DL) of (0.02 µg/L) in 78% of the samples, and concentrations ranged from less than DL to 1.60 µg/L (Ontario Ministry of the Environment, 2016). The mean and median concentrations were 0.06 and 0.02 µg/L, respectively (95% confidence interval on the mean of 0.05-0.07 µg/L). Data from surface water and groundwater samples collected from 24 sites in Québec for 2010-2016 indicated that 1,4-dioxane was below the DL in all samples (between 0.03 and 0.5 µg/L) (Ministère du Développement durable, de l’Environnement et de la Lutte aux Changements Climatiques du Québec, 2016).
Surface water concentrations of 1,4-dioxane in eastern Canada (New Brunswick, Nova Scotia and Newfoundland and Labrador), as measured in 101 samples by the National Water Quality Monitoring Office of Environment Canada, were all below the DL of 0.5 µg/L (CCME, 2008). The screening assessment on 1,4-dioxane (Environment Canada and Health Canada, 2010) reported that 1,4-dioxane was not detected in 42 raw water samples and 42 treated drinking water samples from a municipal water treatment plant located in the Great Lakes region; the reported DL was 10 µg/L. 1,4-Dioxane was detected in 13% of groundwater samples collected up to 200 m away from a laboratory waste disposal site in Ottawa, Ontario, at concentrations ranging from 300 µg/L to 2 000 µg/L, with a DL of 150 µg/L (Lesage et al., 1990). In groundwater samples near various landfill sites in Canada, 1,4-dioxane concentrations of less than 1 µg/L were reported in 1983-1986, whereas 1,4-dioxane concentrations in groundwater samples beneath landfills were as high as 500 µg/L in 1986 (European Commission, 2002; CCME, 2008). In an evaluation of 1,4-dioxane levels across 21 wellfields in the Kitchener-Waterloo region, 1,4-dioxane was detected at concentrations greater than 30 µg/L in one wellfield (Greenbrook wellfield) and was detected (concentration not specified) at three other wellfields (Stantec, 2014). The 1,4-dioxane detected at the Greenbrook wellfield originated from a region south of the wellfield that includes a former landfill (a historic industrial and waste disposal site), industrial sites, a fire station, and a city works yard (Region of Waterloo, 2005). 1,4-Dioxane was detected (concentrations not reported) in groundwater in the Greater Napanee region near a landfill that accepted domestic, commercial, and non-hazardous solid industrial waste (Environmental Review Tribunal of Ontario, 2015); it was detected at concentrations ranging from 1.3 µg/L to 10 µg/L at nine wells in the region almost five years after the landfill’s closure in 2011 (Environmental Review Tribunal of Ontario, 2015; Waste Management, 2016a, 2016b). In general, the 1,4-dioxane concentrations in source and drinking water are below the DLs; however, concentrations of up to 2 000 µg/L have been observed in groundwater samples near waste disposal sites.
1,4-Dioxane was included in the United States Environmental Protection Agency’s (US EPA) third Unregulated Contaminant Monitoring Rule (UCMR3) survey that included monitoring of over 5 000 drinking water supplies (US EPA, 2012a). 1,4-Dioxane was detected above the minimum reporting level of 0.07 µg/L in 22% of public water systems tested (7% > 0.35 µg/L; 0% > 35 µg/L) (US EPA, 2017). Additional analysis of the UCMR3 data conducted by Adamson et al. (2017) found that the detection frequency of 1,4-dioxane in surface water was only slightly lower than in groundwater. However, groundwater concentrations were higher than those in surface water and contributed to a higher number of systems where 1,4-dioxane was greater than 0.35 μg/L. Other studies have also found the presence of 1,4-dioxane in surface water and the effluents of wastewater treatment plants (Simonich et al., 2013; Sun et al., 2016). Sun et al. (2016) reported 1,4-dioxane concentrations up to 436 μg/L downstream of a wastewater treatment plant discharge.
5.2 Food
Data from Japan and the U.S. suggest that 1,4-dioxane is present in several food groups (Nishimura et al., 2005). No studies measuring 1,4-dioxane in foods in Canada were identified. Conservative estimates of 1,4-dioxane exposure via food were calculated in the 2010 screening assessment for 1,4-dioxane (Environment Canada and Health Canada, 2010). The assessment assumed that 1,4-dioxane was present as an impurity in four permitted food additives (polysorbate 60, 65, and 80, and polyethylene glycol) at the maximum level permitted by the food-grade specifications for these food additives (10 mg 1,4-dioxane per kg food additive) (U.S. Pharmacopeial Convention, 2008). Children aged 1-4 are estimated to have the highest 1,4-dioxane exposure from food additives (~0.335 µg/kg body weight [bw] per day). The estimates from this analysis are considered conservative; since it was based on the maximum level of use and the maximum residue limit of 1,4-dioxane in permitted food additives, it assumed that no alternatives permitted for the same technical effect were used, and it did not account for losses due to volatility as a result of the low boiling point of 1,4-dioxane.
Food intake for infants (<6 months) is primarily through breast milk or infant formula, neither of which has been tested for 1,4-dioxane levels. A physiologically based pharmacokinetic (PBPK) model examining lactational transfer of 1,4-dioxane among occupationally exposed women predicted significant lactational transfer (18% of inhaled 1,4-dioxane) despite an experimental milk/blood partition coefficient of 0.89, indicating that more 1,4-dioxane would be expected to be present in the blood than in milk at steady-state concentrations (Fisher et al., 1997). Environment Canada and Health Canada (2010) presented an upper-bounding estimate of 1.07 µg/kg bw per day for formula-fed infants based on intake from water in the amount required to reconstitute the formula.
5.3 Air
No information was found regarding 1,4-dioxane concentrations in air in Canada. Fellin and Otson (1997) estimated 1,4-dioxane concentrations of 0.646 µg/m3 in ambient air and 0.685 µg/m3 in indoor air in Canada. Studies conducted in the U.S. in 1984 reported 1,4-dioxane concentrations of up to 4.2 µg/m3 in indoor air and of up to 4.6 µg/m3 in outdoor air, with median concentrations of up to 0.26 µg/m3 and 0.27 µg/m3 in indoor and outdoor air, respectively (Pellizzari et al., 1986). These levels are consistent with other reports of 1,4-dioxane concentrations in U.S. air samples. (Harkov et al., 1984; Shah and Singh, 1988; Brown et al., 1994).
5.4 Consumer products
Consumer exposure to 1,4-dioxane occurs through inhalation or dermal contact with products containing ethoxylated surfactants, including personal care products and soaps or detergents. 1,4-Dioxane has been found in various consumer products up to a maximum of 45.5 mg/kg in hair shampoo, 0.14 mg/kg in hair conditioner, 7.5 mg/kg in hand soap, and 15.7 mg/kg in body wash, but it was not detected in laundry detergent (DL <5 mg/kg; Scalia and Menegatti, 1991; Fuh et al., 2005; Makino et al., 2006; Tanabe and Kawata, 2008; Tahara et al., 2013). Black et al. (2001) summarized a U.S. Food and Drug Administration-led survey of cosmetic products containing 1,4-dioxane and reported concentrations of up to 279 mg/kg in cosmetic products and in excess of 85 mg/kg in children’s shampoo. 1,4-Dioxane can penetrate and be absorbed through skin after topical application or exposure (Marzulli et al., 1981); however, the volatile nature of 1,4-dioxane causes most of it to evaporate before skin contact and to continue to evaporate once applied, thus minimizing contact time.
Environment Canada and Health Canada (2010) evaluated the risk of exposure to several consumer products for various age groups. Women were considered the most highly exposed demographic due to the use of cosmetics and other consumer products. The estimated aggregate intake of 1,4-dioxane for daily use of hair shampoo, hair conditioner, body wash, and body moisturizer was 1.2 µg/kg bw per day for women, primarily via inhalation of volatilized 1,4-dioxane. The exposure analysis found the intake of 1,4-dioxane in children aged 0-6 months (via daily use of skin moisturizers, hair shampoo, and body wash) to be minimal (estimated aggregate intake of 4.2 × 10-5 mg/kg bw per day). Additionally, exposure to household cleaning products in women, including dishwashing liquids and detergents, was also found to be minimal (estimated aggregate intake of 2.9 × 10-4 mg/kg bw per day).
5.5 Soil
No information is available regarding the levels of 1,4-dioxane in soil in Canada. A report from Golder Associates (1987) stated that 1,4-dioxane was not detected in soils in background urban areas in Canada (DL 100 µg/kg), consistent with its poor sorption with soil (Section 4.1).
5.6 Multi-route exposure through drinking water
To assess the overall exposure to 1,4-dioxane in drinking water, the relative contribution of dermal and inhalation routes of exposure during bathing and showering was assessed through a two-tier multi-route exposure assessment approach (Krishnan, 2004; Krishnan and Carrier, 2008). For each route of exposure, the first tier determines whether the contribution of the exposure route is significant and the second tier determines the contribution from the exposure route expressed in litre equivalents (L-eq) per day. A route of exposure is considered to be significant if it contributes at least 10% of the drinking water consumption level (i.e., 10% of 1.5 L).
For dermal exposure, the tier 1 goal of 0.15 L-eq is associated with a skin permeability coefficient (Kp) for VOCs of 0.024 cm/h (Krishnan and Carrier, 2008). Using the log Kow and molecular weight for 1,4-dioxane, the Kp was estimated as 0.013 cm/h; given that this value is less than the tier 1 goal, exposure via dermal absorption is not considered significant and no further calculation of the L-eq contribution is required.
For inhalation exposure, the tier 1 goal of 0.15 L-eq is associated with an air to water concentration ratio (Fair:water) value of 0.00063, which is based on an exposure time of 0.5 h, a ventilation rate of 675 L/h for adults, and an absorption fraction of 0.7. Using the Henry’s law constant of 2.41 × 10-4 obtained from the US EPA’s EPI Suite Program (US EPA, 2000a), the Fair:water value for 1,4-dioxane was estimated as 0.00015; given that this value is less than the tier 1 goal, exposure via inhalation is not considered significant and no further calculation of the L-eq contribution is required.
Since the criteria for tier 1 were not met for both routes of exposure, exposure to 1,4-dioxane via dermal absorption or inhalation from bathing or showering is not considered significant.
6.0 Analytical methods
Analysis of 1,4-dioxane in water can be challenging due to its high affinity for water (Isaacson et al., 2006; Li et al., 2011; Sun et al., 2016). Analytical methods available for the determination of 1,4-dioxane in water include gas chromatography (GC) with flame ionization detection (FID) or mass spectrometry (MS). Liquid-liquid extraction and solid-phase extraction (SPE) are the most common sample preparation methods used to achieve reporting limits below 1 µg/L. Other extraction methods such as solid-phase micro-extraction, frozen micro-extraction, vacuum distillation and elevated heat or extended time purge and trap are also appropriate preparation methods for measuring low-level 1,4-dioxane concentrations (Draper et al., 2000; Strout et al., 2004; Isaacson et al., 2006; Li et al., 2011; Sun et al., 2016).
The US EPA has developed several methods for analyzing 1,4-dioxane in source and drinking water. These methods can be used to measure 1,4-dioxane in water at levels well below the MAC (US EPA, 1996, 2000b, 2008, 2015b). It should be noted that some of these methods were developed for analyzing a suite of VOCs and other organic compounds. However, since 1,4-dioxane has different chemical properties than VOCs, these methods often have high DLs and long extraction times, and they require large sample and solvent volumes for 1,4-dioxane analysis (Isaacson et al., 2006; Li et al., 2011).
There are no statistical data available for 1,4-dioxane to determine the practical quantitation limit achievable by a wide variety of laboratories. For reporting purposes, laboratories typically use a minimum reporting level (MRL) to indicate the lowest concentration of an analyte that can be determined with an acceptable level of accuracy and precision. The US EPA has defined the lowest concentration minimum reporting limit (LCMRL) as the lowest spiking concentration at which recovery of between 50% and 150% is expected 99% of the time by a single analyst. The methods discussed below employ different extraction and measurement techniques with large variations in the detection and reporting limits. The responsible authorities should discuss the method being used by the laboratory and ensure that the appropriate method detection limit (MDL) or MRL is being achieved in order to adequately assess whether 1,4-dioxane is below the MAC.
The US EPA (2008, 2015b) has developed two standardized analytical methods for analyzing 1,4-dioxane in drinking water. In EPA Method 522, the sample is spiked with an isotopically labelled surrogate standard followed by extraction using SPE. The extract is dried and injected onto a high-resolution GC column interfaced with a mass spectrometer operated in selected ion monitoring (SIM) mode. The MDL for this method is 0.020 μg/L. Two single laboratory LCMRLs of 0.036 μg/L and 0.047 μg/L were determined using this method and reagent water (US EPA, 2008). Monitoring for 1,4-dioxane was included in the US EPA’s UCMR 3. This rule stipulates that using Method 522 an MRL of 0.07 µg/L must be achieved by the laboratories conducting the analyses (US EPA, 2012a). This MRL value was determined by using LCMRL data from multiple laboratories (US EPA, 2012b).
EPA Method 541 was developed more recently and requires sample spiking with two surrogate analytes followed by extraction using SPE cartridges. The cartridges are dried and eluted with 5% methanol in dichloromethane followed by direct analysis by GC/MS in SIM mode of detection. The single laboratory LCMRL for this method is 0.074 μg/L (US EPA, 2015b).
The US EPA has also developed several standardized methods for the analysis of volatile and semi-volatile organics (including 1,4-dioxane) in various matrices. EPA methods 8015C and 8260B determine the concentration of 1,4-dioxane in surface water or groundwater using either direct injection of aqueous samples or sample preparation using azeotropic distillation (EPA Method 5031) followed by analysis using GC/FID (EPA Method 8015C) or GC/MS (EPA Method 8260B). The MDLs are 15 μg/L and 12 µg/L for methods 8015C and 8260B, respectively, when azeotropic distillation is used for sample preparation. No MRL data were reported for either method (US EPA, 1996, 2000b). An advantage of these methods is that they can be used for a broad list of VOCs as well as 1,4-dioxane, which may be useful for sites where co-contaminants are present.
Although 1,4-dioxane is not listed as an analyte, other EPA methods such as 8270D (based on liquid-liquid extraction) and GC/MS have been modified and used to analyze 1,4-dioxane in source water. A summary of modified US EPA methods to include 1,4-dioxane as an analyte, as well as additional methods that have been reported in the literature, is available in Sun et al. (2016).
7.0 Treatment technology system considerations
The chemical structure of 1,4-dioxane, a cyclic organic molecule with two opposed ether linkages, makes it resistant to hydrolysis and biodegradation in the environment. In addition, based on the physical and chemical properties of 1,4-dioxane discussed in Section 4.0 (high solubility in water, low Henry’s Law constant and low adsorptive capacity) it can be challenging to remove from water using common drinking water treatment technologies (US EPA, 2006; Mohr et al., 2010; Stepien et al., 2013; Water Research Foundation, 2014; DiGuiseppi et al., 2016). Therefore, more advanced (i.e., more complex) technologies are required to remove 1,4-dioxane. This highlights the importance of establishing source-water protection strategies and a source-to-tap approach to preventing or minimizing the occurrence 1,4-dioxane in drinking water supplies (CCME, 2004).
7.1 Municipal scale
Since higher concentrations of 1,4-dioxane are found primarily in groundwater, treatment technology data reported in the literature are from groundwater remediation sites (Mohr, 2001; US EPA, 2006; Mohr et al., 2010; Woodard et al., 2014) or drinking water systems using groundwater (Rocarro et al., 2012; Civardi et al., 2014; Collins et al., 2014). Data indicate that technologies used to treat groundwater for drinking water supplies, such as chlorination, ultraviolet (UV) irradiation and other chemical oxidation techniques such as permanganate, hydrogen peroxide (H2 O2) and ozone (O3) are not individually effective for removing 1,4-dioxane (Zenker et al., 2003, US EPA, 2011). In addition, since 1,4-dioxane is often found in groundwater supplies where VOCs such as TCA are present, utilities should be aware that treatment technologies commonly used for removal of VOCs (such as air stripping and granular activated carbon [GAC]) have limited effectiveness for 1,4-dioxane removal (0-35%) (Bowman et al., 2003; Mohr et al., 2010; Rocarro et al., 2012). Although the data are limited, studies have also shown that 1,4-dioxane can be present in surface water supplies (Sun et al., 2016; US EPA, 2017), and there is evidence that traditional surface water treatment techniques such as conventional filtration (i.e., coagulation, sedimentation and filtration) are not effective for removing 1,4-dioxane (Stepien et al., 2013). Treated wastewater used for direct and indirect potable water reuse applications can also be a source of 1,4-dioxane in drinking water systems (Rodriguez et al., 2009; Yangali-Quintanilla et al., 2010; Liang et al., 2011; Orange County Water District, 2015).
Given that traditional treatment technologies used at both groundwater and surface water treatment plants have limited ability to remove 1,4-dioxane, utilities will likely need to consider alternative treatment technologies to remove it from drinking water. Treatment technologies using advanced oxidation processes (AOPs) are considered the most effective treatment methods when using either H2O2/O3 or UV/H2O2 (Zenker et al., 2003, Mohr et al., 2010; US EPA, 2011). Synthetic adsorbents have also been shown to be effective in a limited number of full-scale applications (Woodard et al., 2104). These technologies are typically capable of achieving treated water concentrations below 10 µg/L and often to below 3 µg/L. Pilot-scale testing indicates that reverse osmosis (RO) membranes may also be capable of removing approximately 90% of 1,4-dioxane (Schoonenberg-Kegel et al., 2010; Liang et al., 2011, Metropolitan Water District of Southern California, 2012). Selection of the most appropriate treatment technology for 1,4-dioxane removal will depend on the concentration of 1,4-dioxane in the source water, the overall water chemistry, the process selected and other water quality objectives. Bench- and pilot-scale testing prior to design and installation of a treatment system is recommended. Treatability studies have been reported in the literature and provide useful information on additional considerations when selecting a technology for 1,4-dioxane removal (Civardi et al., 2012; Rocarro et al., 2012).
A non-treatment option is to blend water from a contaminated source with one that has low or no 1,4-dioxane. This ensures that the water being delivered to the consumer has a final concentration below the MAC. Attention must be given to the water quality of a new source prior to making any changes to an existing supply. Characterization of the water quality must be carried out to ensure that changes in water quality resulting from control options are assessed and that potential impacts on the existing treatment processes and distribution system are determined.
7.1.1 Advanced oxidation processes
AOPs have been employed for removing contaminants that are resistant to traditional chemical oxidation treatment processes. They include the use of appropriate combinations of chemical oxidants (e.g., O3, H2O2 and/or UV) to generate highly reactive hydroxyl radicals, which rapidly and non-selectively oxidize organic contaminants. AOPs using a combination of H2O2/O3 or UV/H2O2 are reported to be the most effective methods for treating 1,4-dioxane in drinking water, routinely achieving greater than 99% reduction (Mohr, 2001; Zenker et al., 2003; US EPA, 2006; Mohr et al., 2010; US EPA, 2011; Water Research Foundation, 2014; DiGuiseppi et al., 2016). However, degradation of 1,4-dioxane using AOPs is a function of oxidant dose, and effectiveness will vary between systems depending on a variety of factors, including water quality and operating conditions.
Water quality parameters, such as organic matter, turbidity, alkalinity, iron, sulphide, nitrate, nitrite and ammonia play an important role in AOPs because they are hydroxyl radical “scavengers,” which can reduce the effectiveness of oxidation of the contaminant of interest or impede light transmittance for UV treatment. Consideration also needs to be given to the potential formation of bromate for systems where O3 is being applied and bromide is present in the source water (Ikehata et al., 2016). More information on the formation of bromate through ozonation processes and other sources of bromate in drinking water systems can be obtained from the guideline technical document for bromate (Health Canada, 2016).
7.1.1.1 Hydrogen peroxide and UV
UV combined with H2O2 (UV/H2O2) is a two-step oxidation process. In the first step UV light photolyzes hydrogen peroxide into hydroxyl radicals through a series of chain reactions. The radicals then react with the contaminant (e.g., 1,4-dioxane) and other inorganic and organic constituents in the water. Systems can be designed using either low-pressure high output lamps or medium pressure lamps with the addition of H2O2. Consideration needs to be given to optimizing the H2O2 dose and quenching the excess H2O2 following the AOP process, which is typically achieved using chemical additions (free chlorine, sulphur-based reducing agents) or GAC (US EPA, 2011). Key design and operating considerations for UV/ H2O2systems include H2O2 dose, UV lamp type and intensity, reactor contact time, and pH and temperature. Water quality parameters such as turbidity, iron, hardness and nitrate that can interfere with UV light transmittance are also important. The hydroxyl radicals generated in the UV/ H2O2 process are nonselective and thus can be consumed by organic and inorganic scavenging compounds (Maurino et al., 1997).
UV/H2O2 is an effective technology for treating 1,4-dioxane in groundwater (greater than 99% reduction) and numerous full- and pilot-scale applications have been reported (Mohr, 2001; US EPA, 2006, Mohr et al., 2010; Rocarro et al., 2012; Civardi et al., 2014; Collins et al., 2014; DiGuiseppi et al., 2016). Early work investigating the use of UV/H2O2for the removal of 1,4-dioxane from water demonstrated that advanced oxidation of 1,4-dioxane follows pseudo first-order kinetics. The authors noted the formation of primary products such as formaldehyde, methoxyacetic acid and a number of esters (Stefan and Bolton, 1998).
Pilot- and full-scale UV/H2O2 treatment systems have demonstrated that achieving treated water concentrations below 10 µg/L (and in many cases below 3 µg/L) is feasible (US EPA 2006; Civardi et al., 2012; Rocarro et al., 2012; Collins et al., 2014). Rocarro et al. (2012) presented data from a pilot-scale low-pressure high output (LPHO) UV/H2O2 system treating a groundwater supply with a concentration of 8 µg/L of 1,4-dioxane. At flow rates of 150 gallons per minute (gpm), 1.8 ppm (mg/L) of H2O2 and an electrical energy dose (EED) of 0.059 kWh/m3, 95% destruction of 1,4-dioxane was observed. Greater removal of 1,4-dioxane to levels below the DL of the study (0.25 µg/L) was achieved at slightly higher EED values (0.13-0.24 kWh/m3) (Rocarro et al., 2012). A full-scale LPHO UV/H2O2 system designed to treat 2.2 million gallons per day of groundwater with an influent 1,4-dioxane concentration of 300 µg/L was reported by Civardi et al. (2014). During initial commissioning, the system was operated at 400 gpm with an H2O2 dose of 18 mg/L and an EED of 0.82 kWh/kgal. The system achieved greater than 99.95% reduction of 1,4-dioxane (influent concentration 140 µg/L) to achieve a treated water concentration below the DL of 0.07 µg/L (Civardi et al., 2014).
Higher concentrations of 1,4-dioxane typically found at contaminated sites have also been successfully treated using UV/H2O2. The US EPA (2006) reported on a full-scale UV/H2O2 groundwater treatment system using H2O2 and a multiple-chamber UV system consisting of 22 lamps with a 5-second exposure to the water (no H2O2 dose or UV energy data provided). The system reduced an influent 1,4-dioxane concentration of 4 000 µg/L down to concentrations ranging from 1 µg/L to 10 µg/L.
7.1.1.2 Hydrogen peroxide and ozone
In H2O2/O3 systems, H2O2 is used in conjunction with O3 to enhance the formation of hydroxyl radicals. H2O2 is fed as an aqueous solution in which the deprotonated form of H2O2 (HO2-) reacts with O3 to form hydroxyl radicals (von Gunten, 2003). The typically applied ratio of H2O2/O3 is between 0.2 and 3.0; it is a function of disinfection requirements, bromide concentration, contaminant concentration, and other water quality parameters. Major by-products formed by the H2O2/O3 process are expected to be similar to those formed by ozonation alone. Both O3 and AOP processes form bromate in the presence of bromide. Utilities considering H2O2/O3 for treatment of 1,4-dioxane should have a good understanding of the sources and concentration of bromide in their source waters and the seasonal variability of water quality parameters that may affect the formation of bromate or other disinfectant by-products (Health Canada, 2016). Different configurations of H2O2/O3 systems are possible. In some cases, H2O2 is added during the second stage of operation (i.e., peroxone process) by injecting it into the second chamber of an O3 contactor (US EPA, 2011). This configuration allows the utility to obtain disinfection credits for ozonation while achieving the benefit of AOP for the destruction of micropollutants. An alternative configuration is a trademarked system in which H2O2 is injected into the water stream first, followed by high-pressure injection of O3. The O3 is injected at various locations along the in-line reactor flow path to minimize the creation of bromate (Mohr et al., 2010). Quenching of excess H2O2 needs to be conducted at the end of the treatment process.
Full-scale H2O2/O3 systems are capable of treating influent concentrations of 4.6 to 320 µg/L to achieve treated water concentrations below 10 µg/L and often below 3 µg/L (Bowman et al., 2003; US EPA 2006; Mohr et al., 2010; DiGuiseppi et al., 2016). The US EPA (2006) data from five full-scale H2O2/O3 treatment systems showed that four of the systems were capable of achieving treated water concentrations below 1 µg/L (operational data not provided). Bowman et al. (2003) reported data from a 500 gpm system employing three O3 injectors with 8-inch static mixers and dosing of 3.1 mg/L of O3 and 6.9 mg/L of H2O2. The system was capable of removing a concentration of 4.1 µg/L of 1,4-dioxane to below the DL of 0.95 µg/L. Full- and pilot-scale data at other sites have demonstrated that higher concentrations of 1,4-dioxane (300 to 7 000 µg/L) can be lowered to less than 10 µg/L by increasing the number of reaction vessels and the O3 and H2O2doses (US EPA, 2006; Mohr et al., 2010).
7.1.2 Adsorption
Given the low organic partitioning coefficient of 1,4-dioxane and its hydrophilicity, it is not expected to be efficiently removed using powdered or granulated activated carbon (Summers et al., 2014). Although bench-scale studies have shown moderate removals (50-67% removal) of 1,4-dioxane in columns (McGuire et al., 1978; Zenker et al., 2003) full-scale data have demonstrated that 1,4-dioxane is poorly removed by GAC (Roccaro et al., 2012). Complete breakthrough of 1,4-dioxane was observed after 1 500 bed volumes while other organic contaminants did not break through until 20 000 bed volumes (Rocarro et al., 2012). Similarly, Schoonenberg-Kegel et al. (2010) found that a GAC column with 0.7 L of carbon, a hydraulic loading rate of 14 L/h and an empty bed contact time of 3 min was only capable of removing 18% (influent concentration of 2 000 µg/L) of 1,4-dioxane following 1 200 bed volumes. Overall, it is likely that under most conditions it is impractical to use activated carbon processes to achieve low levels of 1,4-dioxane in treated water (DiGuiseppi et al., 2016).
In research conducted into the use of alternative adsorptive media for removing 1,4-dioxane from water, Woodard et al. (2014) demonstrated that a synthetic adsorption media was effective at removing 1,4-dioxane from water over a wide range of concentrations and operating conditions. The media is characterized as a carbonaceous adsorbent with a high surface area, high porosity, and higher hydrophobicity than traditional activated carbon material. Regeneration is conducted on site using low-pressure steam, microwave radiation, solvents or hot gases. Two full-scale applications were reported in this study. A 15 gpm system composed of multiple synthetic media vessels in series operated in the upflow mode were capable of reducing influent 1,4-dioxane concentrations of 20-60 µg/L down to less than 0.2 µg/L. The authors noted that the conditions to completely regenerate the media required adjustment following start-up. A larger 100 gpm system comprising two 70 ft3 beds of synthetic media was capable of reducing very high influent 1,4-dioxane concentrations (2 000 to 40 000 µg/L) to less than 3.2 µg/L. Regeneration was required every 2-3 days.
7.1.3 Membrane filtration
The two driving mechanisms for removing contaminants with membranes are the nominal pore size for physical removal and the membrane material that may provide functional rejection due to chemical interactions. Low-pressure membranes such as microfiltration and ultrafiltration are not capable of rejecting 1,4-dioxane, since their pore sizes are larger than the size of the 1,4-dioxane molecule (Liang et al., 2011). Limited bench- and pilot-scale studies have demonstrated that the small molecular size of this contaminant is the key consideration when selecting a particular membrane for treatment of 1,4-dioxane (Yangali-Quintanilla et al. 2010; US EPA, 2011).
RO and to a much lesser extent nanofiltration (NF) may be effective for 1,4-dioxane removal. Pilot testing of a thin film composite polyamide membrane indicated that greater than 96% removal of 1,4-dioxane can be achieved using RO. Schoonenberg-Kegel et al. (2010) demonstrated that a 4-in. spiral wound membrane element with a flow of 1 500 L/h and a permeate flux of 20 L/m2h was capable of rejecting an influent concentration of 2 000 µg/L down to 80 µg/L. After pilot-scale testing, Liang et al. (2011) reported 88-94% removal of an influent 1,4-dioxane concentration of 8 µg/L using spiral-wound membrane elements. The system consisted of a total of 21 elements (two parallel series with 14 and 7 elements) operated at a feed flow rate of 17.5 gpm and 84% recovery. To date, full-scale data demonstrating effective removal of 1,4-dioxane in drinking water systems are not available in the literature. However, data from an indirect potable water re-use project where one stage of a comprehensive treatment train employed RO indicated an average removal of approximately 90% of 1,4-dioxane. Influent concentrations ranged from non-detectable to 3.2 µg/L (Orange County Water District, 2015).
Lower removal was observed in a bench-scale study of aromatic polyamide NF membranes. This study found that NF membranes with a molecular weight cut-off (MWCO) of 200 daltons were capable of rejecting 46-48% of 1,4-dioxane (influent concentration of 5-18 µg/L), compared with negligible removal using NF membranes with a MWCO of 300 daltons (Yangali-Quintanilla et al., 2010). Depending on the influent concentration of 1,4-dioxane, NF may not be sufficient to adequately lower the 1,4-dioxane concentration in treated water.
7.1.4 Biological degradation
The use of engineered biological treatment (bioreactors) for 1,4-dioxane removal from water has been investigated, and while biological processes in water treatment are generally ineffective for 1,4-dioxane, co-metabolism has been shown to provide some removal (Stanfill et al., 2004; Zenker et al., 2004; Masuda et al., 2012; Cordone et al., 2016). Limited studies have been conducted on the use of biological degradation for 1,4-dioxane removal, and its applicability for full-scale drinking water systems has not been proven.
Zenker et al. (2004) performed experiments using a laboratory-scale trickling filter to biodegrade cyclic ethers. It was found that in the presence of tetrahydrofuran (THF) as a growth substrate, the reactor was likely able to co-metabolically biodegrade 1,4-dioxane influent concentrations ranging from 200 µg/L to 1 250 µg/L by 93-97%. Cordone et al. (2016) recently reported data from a full-scale aerobic fixed film biological treatment system designed to treat heavily contaminated groundwater and landfill leachate containing both 1,4-dioxane and THF. The system was partially seeded with media from a pilot plant, but a microbial population based on indigenous bacteria from the groundwater was found to be sufficiently effective on its own. The system was capable of co-metabolically degrading greater than 98% of 1,4-dioxane at influent concentrations of up to 25 000 µg/L and THF of up to 60 000 µg/L. During later phases of the system’s operation, blending of the water supply resulted in lower influent concentrations of 1,4-dioxane (1 326 µg/L). The average treated water concentration was 93 µg/L (92% removal). The authors noted that abrupt increases in flow rate and lower water temperature (<23°C) resulted in higher 1,4-dioxane concentrations in treated water.
A laboratory-scale study has shown that co-metabolic degradation may also be effective for lower 1,4-dioxane concentrations that are more representative of drinking water sources. McElroy et al. (2015) investigated the co-metabolic degradation of 1,4-dioxane using bacteria grown on an isobutane. Pure cultures of bacteria that are available commercially were capable of degrading an initial 1,4-dioxane concentration of 100 µg/L to below 0.35 µg/L after incubation for 3 h at 30oC. The authors suggested that seeding of biologically active filters with the appropriate bacteria could be used as part of a treatment system for 1,4-dioxane removal.
7.1.5 Emerging technologies
Alternative AOP processes (including H2O2 and ferrous iron, photocatalytic oxidation, and electro-peroxone) may also be effective for oxidizing 1,4-dioxane (Coleman et al., 2007; Son et al., 2009; Mohr et al., 2010; Chitra et al., 2012; Merayo et al., 2014; Sekar et al., 2014; Wang et al., 2015). However, these technologies have not been implemented for the full-scale treatment of drinking water.
The H2O2 and ferrous iron process (referred to as the Fenton process) involves catalyzing hydrogen peroxide with ferrous iron to produce hydroxyl radicals. Bench-scale studies have shown that this process can rapidly oxidize 1,4-dioxane under acidic conditions. Identified by-products include ethylene glycol, glycolic acid and formic acid (Merayo et al., 2014). The use of UV light to catalyze titanium dioxide to produce hydroxyl radicals has also been investigated for the removal of 1,4-dioxane (Coleman et al., 2007). Pilot-scale testing for remediation of a contaminated groundwater demonstrated a decrease in 1,4-dioxane concentration from 150 µg/L to less than 1.9 µg/L (Mohr et al., 2010). Electrolysis with ozonation to drive the generation of hydroxyl radicals from H2O2/O3 reactions was also found to be effective for the rapid oxidation of 1,4-dioxane (Wang et al., 2015). The authors noted that this process enhances the degradation kinetics of 1,4-dioxane and consumes less energy than ozonation and electrolysis alone.
7.2 Residential scale
In cases where 1,4-dioxane removal is desired at the household level, for example, when a household obtains its drinking water from a private well, a residential drinking water treatment unit may be an option for decreasing 1,4-dioxane concentrations. Before a treatment unit is installed, the water should be tested to determine the general water chemistry and 1,4-dioxane concentration in the source water.
To verify that a treatment unit is effective, water entering and leaving the treatment unit should be sampled periodically and submitted to an accredited laboratory for analysis. Units can lose removal capacity through use and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer’s recommendations and service it when required. Systems classified as residential scale may have a rated capacity to treat volumes greater than that needed for a single residence, and thus, may also be used in small systems.
Health Canada does not recommend specific brands of drinking water treatment units, but it strongly recommends that consumers use units that have been certified by an accredited certification body as meeting the appropriate NSF International Standard/American National Standard (NSF/ANSI) for drinking water treatment units. The purpose of these standards is to establish minimum requirements for the materials, design and construction of drinking water treatment units that can be tested by a third party. This ensures that materials in the unit do not leach contaminants into the drinking water (i.e., material safety). In addition, the standards include performance requirements that specify the removal that must be achieved for specific contaminants (e.g., reduction claim) that may be present in water supplies. Certification organizations (i.e., third party) provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). Accredited organizations in Canada include (SCC, 2020):
- CSA Group;
- NSF International;
- Water Quality Association;
- UL LLC;
- Bureau de Normalisation du Québec (available in French only);
- International Association of Plumbing and Mechanical Officials; and
- Truesdail Laboratories Inc.
An up-to-date list of accredited certification organizations can be obtained from the SCC.
The technologies that are expected to be effective for 1,4-dioxane at the residential-scale are:
- reverse osmosis; and
- adsorption (e.g., activated carbon)
Currently, 1,4-dioxane is not included in the performance requirements (e.g., reduction claims) of NSF/ANSI standards. However, use of a treatment unit that is certified to NSF/ANSI Standard 58 Reverse Osmosis Drinking Water Treatment Systems will ensure that the material safety of the unit has been tested. (NSF/ANSI, 2019 a, b).
Water that has been treated using RO may be corrosive to internal plumbing components. Therefore, these units should be installed only at the point-of-use (e.g., kitchen tap). Also, as large quantities of influent water are needed to obtain the required volume of treated water, these units are generally not practical for point-of-entry installation.
Although 1,4-dioxane is not well removed under larger-scale (i.e., municipal-scale) flow rates and contact times, one study reported that GAC has been effective at removing low concentrations of 1,4-dioxane (10–20 µg/L) in systems with low flow rates typical of domestic wells (Mohr, 2012). The author noted that potentially numerous adaptations to typical GAC treatment systems were needed to achieve low levels of 1,-4-dioxane. Since this is a complex process, this type of treatment may only be applicable for small systems with technical staff to operate it.
8.0 Kinetics and metabolism
Data on the kinetics and metabolism of 1,4-dioxane in humans are limited. Based on extensive descriptions of 1,4-dioxane absorption, distribution, metabolism, and elimination in rats via oral, inhalation, and intravenous routes of exposure, it is recognized that 1,4-dioxane is rapidly absorbed and metabolized. Regardless of the route of administration, the primary excretion route for 1,4-dioxane is urinary excretion of β-hydroxyethoxyacetic acid (HEAA), which is a metabolite of 1,4-dioxane. 1,4-Dioxane exhibits non-linear toxicokinetics; saturation of metabolism has been observed in rats and two human studies have shown that urinary excretion of HEAA decreases with increased inhalation dose (Young et al., 1976, 1977). The following sections summarize relevant elements of the toxicokinetics of 1,4-dioxane.
8.1 Absorption
Gastrointestinal absorption was rapid and nearly complete in male Sprague-Dawley rats administered 10, 100, or 1,000 mg/kg bw per day of radiolabelled-1,4-dioxane via oral gavage in a single dose or for 17 consecutive daily doses (Young et al., 1978a, 1978b); less than 2% of the administered dose was recovered in the feces up to 72 h and 480 h post-exposure in the single exposure and repeat-dose exposure studies, respectively. In another study, male F344/DuCrj specific pathogen free (SPF) rats were administered a single oral dose of 65 mg/kg bw deuterated 1,4-dioxane dissolved in water (Take et al., 2012). The dose was selected to be equal to the daily dose level in drinking water that induced peritoneal mesotheliomas in a previous study (Kasai et al., 2009). Blood 1,4-dioxane concentrations rose rapidly, peaking at 100 µg/mL 60 min after exposure, then decreasing to undetectable levels at 420 min. Exhaled breath was not analyzed and the absorbed dose was not calculated.
Absorption of 1,4-dioxane in humans has only been reported following inhalation exposure (Young et al., 1976, 1977; Goen et al., 2016). In a study of five workers in a 1,4-dioxane plant exposed to a time-weighted average (TWA) of 1.6 ppm 1,4-dioxane in air for 7.5 h, Young et al. (1976) calculated an absorbed dose of 0.37 mg/kg for a 70 kg person based on assumed pulmonary absorption of 100%. In another study, four male volunteers were exposed to 50 ppm 1,4-dioxane vapours in air chambers for 6 h (Young et al., 1977). Plasma concentrations of 1,4-dioxane rapidly rose within the first 2 h and slowed down between 3 and 6 h. The concentration of HEAA peaked 1 h post-exposure and reached undetectable levels by 4 h post-exposure. Based on measurements of 1,4-dioxane and HEAA in the urine, the authors calculated that the mean absorbed dose was 5.4 mg/kg bw at a mean rate of 76.1 mg/h. Goen et al. (2016) exposed 18 healthy volunteers to 20 ppm 1,4-dioxane for 8 h. Levels of 1,4-dioxane in blood were detected at 4 h and 8.75 h time points; however, sufficient data points were not taken to identify any trend in 1,4-dioxane plasma uptake. The authors observed elevated plasma 1,4-dioxane levels in volunteers who had undergone higher levels of physical stress, reflective of the increased inhalation rate during physical activity. The authors did not calculate absorbed dose.
Young et al. (1978a, 1978b) exposed four male Sprague Dawley rats to 50 ppm 1,4-dioxane vapours for 6 h (head only). The plasma 1,4-dioxane concentration peaked at the 6 h time point and decreased thereafter until it was no longer detected at 5 h post-exposure. Based on the measured 1,4-dioxane and HEAA in the urine, the mean absorbed dose of 1,4-dioxane was estimated to be 71.9 mg/kg. In another study, male F344/DuCrj SPF rats were exposed to 250 ppm 1,4-dioxane vapours by inhalation in whole-body chambers for 360 min (Take et al., 2012). Blood concentration of 1,4-dioxane increased from time 0 until 180 min and remained constant until 360 min, peaking at 22 µg/mL. Blood concentrations declined after the end of the exposure period until 1,4-dioxane was no longer detected at 780 min. Absorbed dose was not calculated.
No data were found regarding the dermal absorption of 1,4-dioxane in humans; however, a lethal case of intoxication with 1,4-dioxane was reported where the patient came into extensive dermal contact with 1,4-dioxane combined with exposure via inhalation of vapours (Johnstone, 1959). Additionally, 1,4-dioxane has been shown to rapidly penetrate excised human skin in vitro under both occluded and unoccluded skin conditions (Bronaugh, 1982; Dennerlein et al., 2013, 2015). Bronaugh (1982) estimated that 0.3-3.2% of the applied dose can be absorbed, depending on the level of occlusion, and noted that the percentage absorbed was low due to the rapid evaporation of 1,4-dioxane.
Dermal absorption in the forearm skin of monkeys was also reported to be low (Marzulli et al., 1981). Radiolabelled 1,4-dioxane in methanol or lotion was applied to the unoccluded skin of Rhesus monkeys for 24 h. Based on radiotracer recovery in urine up to 5 days post-exposure, the dermal absorption of 1,4-dioxane was estimated to be less than 4%.
8.2 Distribution
No data were found regarding the distribution of 1,4-dioxane in humans via any route of exposure.
Following oral exposure, Take et al. (2012) detected radiolabelled-1,4-dioxane in all tested tissues with concentrations peaking at 60 min post-exposure and declining until no longer detectable at 720 min, except for blood, which declined until the concentration was no longer detectable at 420 min (see Section 8.1). Peak concentrations of radiolabelled-1,4-dioxane in the lung, liver, kidney, brain and abdominal fat were approximately 215, 185, 180, 175, and 85 µg/g tissue, respectively. The concentration of 1,4-dioxane in the lung, liver, kidney and brain at all collection points was higher than that in the abdominal fat, which the authors suggested was attributed to lower blood:abdominal fat partition coefficients than in the other tissues.
Take et al. (2012) also investigated the distribution of radiolabelled-1,4-dioxane in rats following combined exposures, wherein rats were administered a single gavage dose of 65 mg/kg bw D-1,4-dioxane followed immediately by whole-body exposure to 250 ppm of 1,4-dioxane vapours for 360 min; the oral dose of 1,4-dioxane was deuterated to be able to assess the contribution of each route of exposure. 1,4-Dioxane was detected in all tested tissues, and levels of 1,4-dioxane and D-1,4-dioxane in all tissues increased in a pattern similar to that observed in the single oral exposure study. Peak levels were higher after the combined exposure than the single exposure.
8.3 Metabolism
Major metabolite: The major urinary metabolite of 1,4-dioxane is reported to be HEAA (Young et al., 1976; Braun and Young, 1977; USDA, 2010), which can be reversibly converted to 1,4-dioxane-2-one under acidic conditions. Acidic conditions are often used in analytical assays, which explains why some studies have identified 1,4-dioxane-2-one as the major urinary metabolite (Woo et al., 1977a, 1977b, 1977c). Nearly all of 1,4-dioxane is metabolized to HEAA, as evidenced by the observation of an over 3 000:1 HEAA:1,4-dioxane ratio in the urine from rats exposed to 50 ppm 1,4-dioxane vapours for 6 h (Young et al., 1978a, 1978b).
HEAA has also been detected in urine from humans exposed to 1,4-dioxane by inhalation (Young et al., 1976, 1977; Goen et al., 2016). Workers in a 1,4-dioxane plant exposed to a TWA of 1.6 ppm for 7.5 h had HEAA and 1,4-dioxane in the urine at a ratio of 118:1 (Young et al., 1976). Similarly >99% of inhaled 1,4-dioxane was excreted as HEAA in healthy volunteers exposed to 50 ppm 1,4-dioxane for 6 h (Young et al., 1977). Goen et al. (2016) estimated the urinary excretion of HEAA to be approximately 53% of the inhaled dose in volunteers exposed to 20 ppm 1,4-dioxane for 8 h.
Enzymes: Several P450 enzymes have been shown to be involved in 1,4-dioxane metabolism. Male Sprague Dawley rats were exposed to 1,4-dioxane in drinking water at a concentration of 1.5% for 10 days or via gavage at a concentration of 2 000 mg/kg bw per day for 2 days (Nannelli et al., 2005). Both treatments resulted in increased cytochrome P450 (CYP) CYP2B1, CYP2B2, CYP2C11, and CYP2E1 enzyme activity in the liver microsomes and increased activity of CYP2E1 alone in the nasal mucosa and kidney. None of the evaluated cytochrome P450 enzymes were altered in the pulmonary mucosa. Gavage administration also resulted in increased CYP3A activity in the liver. Additionally, pretreatment with known inducers of cytochrome P450 enzymes (phenobarbital and polychlorinated biphenyls) significantly increased the total amount of 1,4-dioxane metabolite excretion and reduced the peak excretion time in male Sprague Dawley rats, whereas pretreatment with an inducer of cytochrome P488 enzymes (3-methylcholanthrene) had no effect (Woo et al., 1977c).
Proposed metabolic pathways: There are three proposed metabolic pathways between 1,4-dioxane and HEAA formation (Woo et al., 1977a).
(1) 1,4-Dioxane may be oxidized by a cytochrome P450 enzyme at one of the oxane oxygens, resulting in decyclization and the formation of diethylene glycol, which may be further oxidized to HEAA.
(2) The cytochrome P450 enzyme may add an oxygen atom to one of the carbon atoms in 1,4-dioxane, resulting in the formation of 1,4-dioxane-2-one.
(3) A hydroxyl group may be added to a carbon atom of 1,4-dioxane, resulting in 1,4-dioxane-2-ol, which can be further oxidized to form HEAA.
Support for the first pathway comes from a study that showed that HEAA was the major metabolite in urine collected from rats injected with diethylene glycol (DEG) (Woo et al., 1977a). However, DEG, which is found as a minor metabolite of 1,4-dioxane following single gavage of 1,000 mg/kg bw per day in male rats (Braun and Young, 1977) and is absent in urine from 1,4-dioxane exposed rodents, has been shown to be excreted at roughly equivalent levels as HEAA following administration of 2 000 mg/kg bw per day DEG in male rats (Besenhofer et al., 2010), which adds uncertainty to the evidence for this pathway. There is no experimental evidence to support the latter two pathways (Woo et al., 1977c; ATSDR, 2012).
Kinetics: 1,4-dioxane metabolism is a saturable process. Sprague Dawley rats administered 1,4-dioxane by a single intravenous injection of 3, 10, 30, 100, 300, or 1,000 mg/kg bw showed a shift from linear to non-linear, saturable metabolism with increasing doses, saturating at plasma 1,4-dioxane concentrations of between 30 and 100 µg/mL (Young et al., 1978a). In the same study, the authors showed that as the oral doses of 1,4-dioxane increased, the percentage of urinary excretion of 1,4-dioxane decreased and the percentage of 1,4-dioxane exhaled increased, indicating that the metabolism of 1,4-dioxane had reached a maximum, allowing the parent compound to circulate in the blood and be eliminated by evaporation. In another paper by the same authors (Young et al., 1978b) the metabolic saturation was estimated at plasma levels of 100 µg/mL. Based on the slow elimination rate of 1,4-dioxane in the plasma following single oral exposures to increasing doses of 1,4-dioxane (2, 10, 100, or 1,000 mg/kg) in rats, Kociba et al. (1975) concluded that, at oral doses above 10 mg/kg, 1,4-dioxane had saturated either metabolic or excretory mechanisms. Similar results were demonstrated in intravenous exposures to 3, 10, 30, 100, 300, or 1,000 mg/kg bw of 1,4-dioxane (Kociba et al., 1975). The kinetics of mouse 1,4-dioxane metabolism has only been described in one study (Sweeney et al., 2008). The authors suggested that the metabolic saturation in mice occurred between 245 and 2 230 mg/kg bw per day, indicative of a higher rate of metabolism than rats. More specifically, the authors reported a disproportional increase in 1,4-dioxane’s area under the curve with increasing administered dose, along with a decrease in the HEAA area under the curve:1,4-dioxane dose ratio with increasing administered dose in male B6C3F1 mice that were administered a single oral dose of 0, 24, 245, or 2 230 mg/kg bw per day.
It has also been suggested that 1,4-dioxane induces its own metabolism at very high doses. 1,4-Dioxane administration for three days in male and female mice (500 and 1,000 mg/kg bw per day) resulted in significant increases in levels of cytochrome P450 enzymes and in microsomal protein content (Mungikar and Pawar, 1978). Furthermore, in a 13-week inhalation exposure study, male and female F344 rats were exposed to 0, 100, 200, 400, 1 600, or 3 200 ppm 1,4-dioxane. Plasma 1,4-dioxane concentrations increased linearly with increasing concentrations; plasma concentrations reached 730 and 1,054 µg/mL in male and female rats, respectively (Kasai et al., 2008). The authors suggested that the repeated inhalation exposure enhanced the induction of cytochrome P450 enzymes, including CYP2E1. Physiologically based pharmacokinetic models (PBPK) models (discussed in Section 8.5) demonstrated that the metabolic profile and rate constants in primary human hepatocytes were similar to those from primary hepatocytes from rats and mice (Sweeney et al., 2008).
8.4 Excretion
Urinary excretion is the predominant excretory pathway following oral exposure to 1,4-dioxane; however, as the dose increases, the proportion eliminated in expired air increases. Following single oral administration of 1,4-dioxane in rats of 10, 100, or 1,000 mg/kg bw, urinary recovery of 1,4-dioxane decreased from 99% to 86% to 76% along with a concomitant increase in 1,4-dioxane in expired air from <1% to 4.7% to 25% (Young et al., 1978a, 1978b). Similarly, in Sprague Dawley rats exposed to repeated daily gavage of 10 and 1,000 mg/kg bw per day, urinary recovery of 1,4-dioxane decreased from 99% to 82%, while the percentage in expired air increased from 1.3% to 8.9% (Young et al., 1978a, 1978b).
1,4-Dioxane and its metabolite, HEAA, have been detected in human and rat urine following inhalation exposures. The urine of workers exposed to a TWA of 1.6 ppm for 7.5 h contained 414 µmol HEAA/L urine, corresponding to 99% of the inhaled dose, and 3.5 µmol 1,4-dioxane/L urine (Young et al., 1976), suggestive of rapid metabolism. In volunteers exposed to 50 ppm 1,4-dioxane for 6 h, the elimination half-life from plasma and urine was 59 and 43 min, respectively (Young et al., 1977). Goen et al. (2016) calculated the mean level of HEAA in the urine from these studies to be 487 mg HEAA/L urine in the healthy volunteers exposed to 50 ppm and 1 165 mg HEAA/L urine in the workers exposed to a TWA of 1.6 ppm for 7.5 h, suggesting that the percentage of urinary excreted HEAA decreases as the inhaled dose increases. In another study in healthy volunteers exposed to 20 ppm 1,4-dioxane for 8 h, 53% and 0.2-0.3% of the inhaled dose was excreted in the urine after 24 h as HEAA and 1,4-dioxane, respectively (Goen et al., 2016). Exhaled breath and feces were not analyzed in any study. The primary route of 1,4-dioxane elimination was the urine in Sprague Dawley rats exposed to 50 ppm 1,4-dioxane vapours for 6 h, primarily in the form of the HEAA metabolite (Young et al., 1978a, 1978b).
Data from intravenous injection studies suggest a dose-related shift in the elimination of 1,4-dioxane, possibly due to metabolic saturation (Young et al., 1978a, 1978b). For example, elimination of 1,4-dioxane from plasma in male Sprague Dawley rats administered 3, 10, 30, 100, 300, or 1,000 mg/kg bw 1,4-dioxane by intravenous injection followed linear kinetics with a half-life of 1.1 h at the two lowest doses. However, elimination from plasma at high doses occurred more slowly, suggestive of a saturable process in the elimination of 1,4-dioxane; urinary recovery of 1,4-dioxane increased from 4% to 11%, urinary HEAA levels decreased from 92% to 60%, and levels in expired air increased from 1% to 27%.
8.5 Physiologically based pharmacokinetic models
PBPK models have been described for 1,4-dioxane in rats (Leung and Paustenbach, 1990; Reitz et al., 1990; Sweeney et al., 2008; Takano et al., 2010), mice (Reitz et al., 1990) and humans (Leung and Paustenbach, 1990; Reitz et al., 1990; Fisher et al., 1997; Sweeney et al., 2008; Takano et al., 2010). The majority of the available models are based on the Young et al. (1978a, 1978b) studies that investigated the disposition of 1,4-dioxane in adult male Sprague Dawley rats following intravenous, inhalation, and single and multiple oral gavage exposures. The only human data adequate for PBPK modelling were from the Young et al. (1977) study that investigated adult male volunteers exposed to 50 ppm 1,4-dioxane vapours for 6 h. The US EPA (2013) evaluated the existing PBPK models for their ability to predict observations made in experimental studies in rats and humans, including revised and recalibrated versions of the models. Following extensive evaluation, they concluded the use of these models was not superior to default approaches for dose extrapolation between species. A brief description of the existing models is provided below.
Leung and Paustenbach (1990) and Reitz et al. (1990) developed PBPK models for 1,4-dioxane and HEAA in rats and humans based on an earlier model for styrene (Ramsey and Andersen, 1984). Assuming that mouse parameters are the same as rat parameters, Reitz et al. (1990) additionally developed a murine PBPK model. These models attempt to estimate the concentration of 1,4-dioxane in the blood and tissue compartments, as well as the levels of HEAA metabolite formed, following inhalation or oral exposures to 1,4-dioxane. As human and rat pharmacokinetic data were used in the optimization of the model parameters, species extrapolation to humans should be possible. However, comparison of the model simulations against data from other studies was not presented. Moreover, the Reitz et al. (1990) model adjusted some parameter values beyond measured values to attain adequate fit to human observations. More specifically, Reitz et al. (1990) doubled the measured blood:air partition coefficient and substituted the tissue:air partition coefficient with the liver:air value, which increases the uncertainty regarding these parameter values and the utility of the model for extrapolation. The US EPA re-evaluated the ability of the Reitz et al. (1990) model to predict observations in studies of humans by replacing the substituted parameter values employed by Reitz et al. (1990) with several options for biologically plausible parameter values, as well as measured parameter values from the models published by Leung and Paustenbach (1990) and Sweeney et al. (1990). The recalibrations did not result in an adequate fit to the experimental observations (US EPA, 2013).
Fisher et al. (1997) developed a model for VOCs in general (including 1,4-dioxane) in lactating women. The model is similar to the Leung and Paustenbach (1990) and Reitz et al. (1990) models with the addition of elimination in breast milk. This model attempts to estimate the concentration of 1,4-dioxane in breast milk but no attempts were made to validate the results.
Sweeney et al. (2008) developed a PBPK model for 1,4-dioxane and HEAA in mice, rats, and humans using data generated in mice and rats. The authors conducted validation exercises to test the ability of their model to predict observations in experimental studies of rat and human exposures to 1,4-dioxane; the model could not provide an adequate fit for low-dose data in rats, and the human model could not replicate the experimental inhalation human data.
Takano et al. (2010) developed a PBPK model for 1,4-dioxane in rats and humans using a combination of in vivo studies in rats administered 1,4-dioxane intraperitoneally, in vitro studies of rat and human liver microsomes, and in silico estimations of partition coefficients. This model attempts to estimate the concentration of 1,4-dioxane in blood following oral exposure; however, the study authors only conducted validation exercises using a single dose (500 mg/kg bw) and route of exposure (oral administration) in rats. The ability of the model to predict other routes of exposure is unknown. Human data were used in the optimization of model parameters, so technically this model could be used to extrapolate to humans; however, the study authors did not compare the human model to the available published human data.
9.0 Health effects
9.1 Effects in humans
The epidemiological database on 1,4-dioxane is limited to studies on the health risks associated with exposure via inhalation. No oral studies in humans were found. There is inadequate evidence of carcinogenicity in humans; however, its toxicity at high concentrations is apparent.
9.1.1 Acute and short-term toxicity
Two case reports of occupational poisonings with 1,4-dioxane have been published. Barber (1934) described five deaths that occurred among factory workers exposed to high concentrations of 1,4-dioxane (concentration not specified) via inhalation within two weeks of exposure, although dermal exposure may have also occurred. Post-mortem findings showed extensive lesions in the kidneys, which the author suggested were the cause of death, and in the liver, which were reported as compatible with recovery. Johnstone (1959) described the death of a worker exposed to 1,4-dioxane in the air for one week. Air concentrations were 208-650 ppm 1,4-dioxane with a mean value of 470 ppm. Post-mortem examination showed histopathological changes in the liver, kidney, and brain, including hepatic necrosis, hemorrhagic necrosis of the kidney cortex, and perivascular widening in the brain.
Short-term inhalation of very high concentrations of 1,4-dioxane (200-5 500 ppm) has resulted in nose and throat irritation (Yant et al., 1930; Wirth and Klimmer 1936; Silverman et al., 1946), eye irritation (Yant et al., 1930; Silverman et al., 1946; Young et al., 1977), and vertigo (Yant et al., 1930). Additionally, two studies reported no symptoms after exposure to 1,000-2 000 ppm 1,4-dioxane vapours for 3-5 min (Fairley, 1934) or to 20 ppm 1,4-dioxane for 2 h (Ernstgard et al., 2006).
9.1.2 Subchronic and chronic toxicity and carcinogenicity
Thiess et al. (1976) conducted a cross-sectional study of 74 German workers in a 1,4-dioxane production plant to determine the association between 1,4-dioxane exposure and risks to health, including death and chromosomal aberrations. The air concentration at the time of the study was 0.06-0.69 ppm 1,4-dioxane and a simulation of pre-1969 exposure conditions yielded results of 0.06-7.2 ppm 1,4-dioxane. The authors reported elevated levels of serum transaminase in 16/47 workers; however, this result is also consistent with chronic consumption of 80 grams of alcohol per day, which was reported by the workers. When the workers were compared with the general German population, no statistically significant effects were found in any studied parameter, including age-specific mortality and cancer.
Buffler et al. (1978) conducted a mortality study on employees at a chemical manufacturing and processing plant in Texas to determine if there is an association between 1,4-dioxane exposure and death. The study group totalled 165 employees from both the manufacturing (100 workers) and processing (65 workers) plants whose exposure levels were less than 25 ppm. When the group was compared with the general Texan population, no significant increase in cancer-related or all-cause mortality was found. The conclusions are limited, however, by the small cohort size and the short mean exposure duration (<5 years) and latency period (<10 years).
9.1.3 Developmental and reproductive toxicity
No human studies on the reproductive or developmental toxicity of exposure to 1,4-dioxane were identified; however, occupational exposure to the chemicals used in the silk-screening process and the electronics industry (known to include 1,4-dioxane but not specifically measured for) in Russia was found to be associated with elevated rates of spontaneous abortion and stillbirth (NIOSH, 1988).
9.2 Effects on experimental animals
As outlined below, in experimental animals, cancer is the most severe long-term health effect associated with exposure to 1,4-dioxane. Additionally, the liver, kidney and respiratory tract have been identified as major target organs for 1,4-dioxane toxicity, with reported effects including degeneration and necrosis. The multi-route exposure assessment (see Section 5.6) determined that human exposures to 1,4-dioxane via dermal and inhalation routes from bathing and showering are not expected to be significant. Therefore, the following section will primarily discuss effects that occur following oral exposure.
9.2.1 Acute and short-term exposures
Experimental animals exposed to acute and short-term, high, oral doses of 1,4-dioxane (between 2 500 and 11,000 mg/kg) exhibited central nervous system depression, such as staggered gait, narcosis, paralysis, and coma (de Navasquez, 1935; Schrenk and Yant, 1936; Laug et al., 1939; Nelson, 1951; Kanada et al., 1994), as well as hepatic and renal degeneration and necrosis (De Navasquez, 1935; Schrenk and Yant, 1936; Kesten et al., 1939; Laug et al., 1939; David, 1964; Kitchin and Brown, 1990; JBRC, 1998) and histopathological lesions in the nasal cavity and brain (JBRC, 1998). Increased mortality was observed in many studies (DeNavasquez et al., 1935; Kesten, 1939; Laug et al., 1939; Smyth, 1941; Pozzani et al., 1959; BASF, 1973; GDCH, 1991; Mirkova, 1994; JBRC, 1998;). The lowest oral LD50 values of 1 270 mg/kg, 2 000 mg/kg, 2 000 mg/kg, 4 500 mg/kg, and 5 170 mg/kg were reported in the guinea pig, rabbit, cat, mouse, and rat, respectively (BASF, 1973; GDCH, 1991; Mirkova, 1994; Patty, 1994; DeRosa et al., 1996).
9.2.2 Subchronic exposure
9.2.2.1 Hepatic effects
Hepatic effects of exposure to 1,4-dioxane have been observed in rats and mice, including non-neoplastic lesions and changes in liver weight at ≥126 mg/kg bw per day in the drinking water (Fairley, 1934; Stott et al., 1981; Kano et al., 2008).
The lowest-observable-adverse-effect level (LOAEL) for histopathological alterations is based upon the presence of non-neoplastic lesions in the liver, including single cell necrosis, centrilobular swelling (hypertrophy), and vacuolitic changes in male and female F344/DuCrj rats at ≥126 and ≥756 mg/kg bw per day, respectively (Kano et al., 2008). In this study, male and female F344/DuCrj rats (10/sex/group) were exposed to 0, 640, 1 600, 4 000, 10 000, or 25 000 ppm 1,4-dioxane in drinking water for 13 weeks. The authors calculated the average daily doses to be 0, 52, 126, 274, 657, or 1 554 mg/kg bw per day in males and 0, 83, 185, 427, 756, or 1 614 mg/kg bw per day in females.
Other hepatic histopathological alterations observed following oral 1,4-dioxane exposure in rats include centrilobular swelling in male Sprague Dawley rats exposed to 1,000 mg/kg bw per day (Stott et al., 1981) and hepatocellular degeneration in rats exposed to 1 900 mg/kg bw per day (Fairley, 1934). In the former study, male Sprague Dawley rats (4-6/group) were exposed to an average daily dose of 0, 10, or 1,000 mg/kg bw per day in drinking water for 11 weeks (Stott et al., 1981). While the methods section of the study by Stott et al. (1981) describes the high dose as 100 mg/kg bw per day, the rest of the article describes the high dose as 1,000 mg/kg bw per day. In the latter study by Fairley (1934), rats (n = 6; strain unspecified) were exposed to 1,4-dioxane in their drinking water for up to 67 days at a concentration of 5% by volume. Using reference body weights and drinking water ingestion rates for rats, the US EPA (2013) calculated this dose to be approximately equivalent to 1 900 mg/kg bw per day. No controls were included in this study.
Histopathological alterations were additionally reported in the livers of mice exposed to 1,4-dioxane in the drinking water (Fairley, 1934; Kano et al., 2008). Hepatocellular degeneration was observed in mice exposed to 3 300 mg/kg bw per day (Fairley, 1934) and single cell necrosis and centrilobular swelling were observed in male and female Crj:BDF1 mice exposed to ≥585 and ≥898 mg/kg bw per day, respectively (Kano et al., 2008). In the former study by Fairley (1934) mice (n = 6; unspecified strain) were exposed to 1,4-dioxane in drinking water for up to 67 days at a concentration of 5% by volume (using reference body weights and drinking water intakes for mice, the US EPA [2013] determined this to be an average daily dose of 3 300 mg/kg bw per day). In the latter study by Kano et al. (2008), male and female Crj:BDF1 mice (10/sex/group) were exposed to 0, 640, 1 600, 4 000, 10 000, or 25 000 ppm 1,4-dioxane in the drinking water for 13 weeks. The authors calculated the average daily dose to be 0, 86, 231, 585, 882, or 1 570 mg/kg bw per day in males and 0, 170, 387, 898, 1 620 or 2 669 mg/kg bw per day in females.
Changes in liver weight were also observed by Stott et al. (1981) and Kano et al. (2008). More specifically, Stott et al. (1981) reported a significant increase in liver to body weight ratio in male Sprague Dawley rats exposed to 1,000 mg/kg bw per day. Kano et al. (2008) reported a significant increase in relative liver weights in male and female F344/DuCrj rats exposed to ≥657 and ≥185 mg/kg bw per day, respectively, and a significant decrease in relative liver weight in female Crj:BDF1 mice exposed to 2 669 mg/kg bw per day.
Significant changes in serum enzyme activity, including serum aspartate aminotransferase and alanine aminotransferase (ALT) were observed in male and female F344/DuCrj rats exposed to doses of 1 554 and 1 614 mg/kg bw per day, respectively, and in male and female Crj:BDF1 mice exposed to 1 570 mg/kg bw per day and ≥1 620 mg/kg bw per day, respectively. A greater than two-fold increase in ALT enzyme activity was observed in female mice exposed to 2 669 mg/kg bw per day (Kano et al., 2008).
9.2.2.2 Renal effects
Histopathological alterations and/or increases in kidney weight have been observed in rats and mice subchronically exposed to 1,4-dioxane in the drinking water at doses of ≥185 mg/kg bw per day (Fairley, 1934; Kano et al., 2008).
Male and female F344/DuCrj rats had a significant increase in histopathological changes in the kidneys at doses of ≥657 and ≥756 mg/kg bw per day in males and females, respectively, including hydropic changes (reversible cellular swelling/vacuolar changes due to the inability to maintain ionic and fluid equilibrium) and/or nuclear enlargement of the proximal tubule (Kano et al., 2008). Additionally, non-neoplastic lesions in the renal cortex (such as degeneration, necrosis, hemorrhages and vascular congestion) were reported in rats and mice exposed to 1 900 and 3 300 mg/kg bw per day, respectively (Fairley, 1934).
Increases in kidney weight and size were also reported. Fairley (1934) reported kidney enlargement in rats and mice at doses of 1 900 and 3 300 mg/kg bw per day, respectively. Kano et al. (2008) reported a significant increase in relative kidney weights in male and female F344/DuCrj rats given doses of ≥274 and ≥185 mg/kg bw per day, respectively, and in male and female Crj:BDF1 mice at doses of 1 570 and 2 669 mg/kg bw per day, respectively.
9.2.2.3 Respiratory tract effects
Histopathological alterations and/or increases in lung weight have been observed in rats and mice sub-chronically exposed to 1,4-dioxane in drinking water at a dose of ≥126 mg/kg bw per day (Kano et al., 2008).
In male and female F344/DuCrj rats, non-neoplastic lesions, including nuclear enlargement of respiratory and olfactory epithelium of the nasal cavity, of tracheal epithelium, and of bronchial epithelium, were observed at doses of ≥126 and ≥185 mg/kg bw per day in males and females, respectively (Kano et al., 2008). In male and female Crj:BDF1 mice, non-neoplastic lesions—including nuclear enlargement of respiratory and olfactory epithelia in the nasal cavity, vacuolar changes in the olfactory nerve, nuclear enlargement of tracheal epithelium, and nuclear enlargement and degeneration of bronchial epithelium—were observed at doses of ≥585 and ≥387 mg/kg bw per day in males and females, respectively (Kano et al., 2008). The researchers also reported increases in lung weight in high-dose male and female rats and mice.
9.2.2.4 Other effects
Additional effects observed following sub-chronic oral exposure to 1,4-dioxane include gastrointestinal effects, neurological effects and increased mortality. Acute gastroenteritis was observed in rats exposed to 1 900 mg/kg bw per day (Fairley, 1934). Vacuolar changes were reported in the brains of male and female F344/DuCrj rats exposed to 1 554 and 1 614 mg/kg bw per day, respectively (Kano et al., 2008). Significantly reduced dopamine and serotonin content was observed in the hypothalamus of male Sprague Dawley rats (5/group) administered 1,050 mg/kg 1,4-dioxane by oral gavage (no vehicle included; controls were exposed to nothing) (Kanada et al., 1994). Fairley (1934) reported that mortality was high in rats and low in mice.
No significant change in clinical endpoints or increase in the development of early putative pre-neoplastic lesions (placental glutathione S transferase positive foci) were observed in male F344 rats (6/group) administered 1,4-dioxane-2-one (a synthesized metabolite of 1,4- dioxane), by oral gavage for 3 days per week for 12 weeks (Koissi et al., 2012). The dose of 1,4-dioxane-2-one was 0.02 g per rat per day for the first week and 0.04 g per rat per day for the subsequent 11 weeks (the dose of 1,4-dioxane necessary to yield these doses of the metabolite was not specified). Based on the body weights of the rats at the start of the study (125 g), this was calculated to be an average of 131 mg/kg bw per day. It is important to note that the calculated equilibrium constant for 1,4-dioxane-2-one favours the presence of HEAA or its deprotinated anion over 1,4-dioxane-2-one, such that 1,4-dioxane-2-one would account for a maximum of 1.4% of the sum of the metabolites (Koissi et al., 2012).
9.2.3 Long-term exposure and carcinogenicity
Several oral carcinogenicity bioassays have been conducted for 1,4-dioxane in mice, rats, and guinea pigs (Argus et al., 1965, 1973; Hoch-Ligeti and Argus, 1970; Hoch-Ligeti et al., 1970; King et al., 1973; Kociba et al., 1974; NTP, 1978; Yamazaki et al., 1994; JBRC, 1998; Yamamoto et al., 1998a, 1998b; Kano et al., 2009). The majority of the studies were conducted with drinking water as the exposure medium. The primary target organs for cancer in the oral studies were the liver and the nasal cavity. The liver, kidney, and nasal cavity were the most sensitive target organs in the chronic studies (the no-observed-adverse-effect level [NOAEL] in male rats was 9.6-11 mg/kg bw per day for increased incidence of histopathological alterations in the liver).
9.2.3.1 Hepatic effects
Hepatic effects have been observed in rats and mice following long-term exposure to 1,4-dioxane, including histopathological alterations and increased liver weight at doses of ≥55-94 mg/kg bw per day in the drinking water (Argus et al., 1965; Kociba et al., 1974; NTP, 1978; Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009).
Kociba et al. (1974) examined hepatic histopathological alterations in male and female Sherman rats exposed to 0, 0.01%, 0.1%, or 1.0% 1,4-dioxane in the drinking water for 716 days. These doses are equivalent to 0, 9.6 (range 8.3-12), 94 (range 59-113), or 1,015 (range 914-1 229) mg/kg bw per day for males and 0, 19 (range 18-20), 148 (range 130-168), or 1 599 (range 1 416-2 149) mg/kg bw per day for females, as calculated by the study authors. Histopathological alterations, including hepatocellular degeneration and necrosis, were observed at doses exceeding 94 mg/kg bw per day in males and 148 mg/kg bw per day in females (Kociba et al., 1974). Quantitative data for these histopathological alterations from the laboratory report for this study were provided by the Dow Chemical Company and published in Dourson et al. (2014). The NOAEL and LOAEL from this study are 9.6 and 94 mg/kg bw per day, respectively, based on hepatic degeneration and necrosis in male rats (Kociba et al., 1974). The study with the lowest LOAEL for histopathological alterations in rats (LOAEL of 55 mg/kg bw per day; NOAEL of 11 mg/kg bw per day) was a 2-year drinking water study with 1,4-dioxane conducted by the Japan Bioassay Research Centre (JBRC) and reported in conference proceedings (Yamazaki et al., 1994), a laboratory report (JBRC, 1998), and as a peer-reviewed publication (Kano et al., 2009). In this study, male and female F344/DuCrj rats (50/sex/dose) were exposed to 0, 200, 1 000 or 5 000 ppm 1,4-dioxane in the drinking water for 104 weeks. These doses are equivalent to 0, 11±1, 55±3, or 274±18 mg/kg bw per day in males and 0, 18±3, 83±14, or 429±69 mg/kg bw per day in females, as calculated by Kano et al. (2009). Non-neoplastic lesions, including spongiosis and hyperplasia, were observed in the livers of male and female rats at doses of ≥55 and ≥83 mg/kg bw per day, respectively. Additionally, hepatocellular hypertrophy, necrosis, and foci development were identified in male and female rats in a re-read of the translations of the JBRC laboratory reports conducted by Dourson et al. (2017). The JBRC study also reported a significant increase in fatty changes in the livers of male mice exposed to 191 mg/kg bw per day and angiectasis (gross dilatation of blood vessels) with exposure to 677 mg/kg bw per day. In this study, male and female Crj:BDC1 mice (50/sex/group) were exposed to 0, 500, 2 000, or 8 000 ppm 1,4-dioxane in drinking water for 104 weeks. These doses are equivalent to 0, 49±5, 191±21, and 677±74 mg/kg bw per day in males and 0, 66±10, 278±40, or 964±88 mg/kg bw per day in females, as calculated by Kano et al. (2009). In addition, Dourson et al. (2017) reported hypertrophy and necrosis in male mice exposed to ≥191 mg/kg bw per day.
Other studies also reported histopathological alterations in the liver at higher doses, including enlarged hyperchromic nuclei at 857 mg/kg bw per day in male Wistar rats (Argus et al., 1965) and hepatocytomegaly at 640 mg/kg bw per day in female Osborne-Mendel rats (NTP, 1978). Argus et al. (1965) exposed male Wistar rats (n = 26) to 1% 1,4-dioxane in the drinking water for 63 weeks. A group of nine untreated rats served as controls. Using the reported drinking water intake of 30 mL/day and a reference body weight of 0.35 kg for rats (Health Canada, 1994), the dose is equivalent to 857 mg/kg bw per day. The National Cancer Institute (NCI) of the National Toxicology Program) (NTP, 1978) exposed male and female Osborne-Mendel rats (35/sex/dose) to 0, 0.5%, or 1% 1,4-dioxane in the drinking water for 110 weeks. These doses are equivalent to average daily doses of 0, 240 (range 130-380), or 530 (range 290-780) mg/kg bw per day in males and 0, 350 (range 200-580), or 640 (range 500-940) mg/kg bw per day in females, as calculated by the study authors. Additionally, hepatocellular hypertrophy, necrosis, inflammation, and a depletion of glycogen were identified at doses exceeding 720 and 860 mg/kg bw per day in male and female B6C3F1 mice, respectively, in a re-examination of mouse liver slides from a 90-week drinking water study conducted by the NCI (NTP, 1978) and published by Dourson et al. (2014). Note that at the time of the original study, the NCI typically recorded only the most severe diagnosis on a given slide. The NCI exposed male and female B6C3F1 mice (50/sex/dose) to 0, 0.5%, or 1% 1,4-dioxane in the drinking water for 90 weeks. These doses are equivalent to average daily doses of 0, 720 (range 530-990), or 830 (range 680-1 150) mg/kg bw per day in males and 0, 380 (range 180-620), or 860 (range 450-1 560) mg/kg bw per day in females. Kupffer cell hyperplasia in male and female mice and bile duct hyperplasia were also noted in a few exposed animals (Dourson et al., 2014).
Increases in liver weight were additionally reported by Kociba et al. (1974) and the JBRC (JBRC, 1998; Kano et al., 2009) studies described above. Kociba et al. (1974) reported a significant increase in absolute and relative liver weights in male and female Sherman rats exposed to 1 015 and 1 599 mg/kg bw per day 1,4-dioxane in the drinking water, respectively, and Kano et al. (2009) reported a significant increase in relative liver weight in male and female F344/DuCrj rats exposed to doses of ≥55 and ≥429 mg/kg bw per day 1,4-dioxane in the drinking water, respectively.
Clinical pathology parameters following oral long-term exposure to 1,4-dioxane were only investigated in the JBRC study (1998). Exposure to 1,4-dioxane induced a significant increase in serum ALT (3.8-fold to 15.2-fold over controls), alkaline phosphatase (2.3-fold to 4.2-fold), and aspartate aminotransferase (2.6-fold to 14.2-fold) activity in male and female F344/DuCrj rats at 274 and 428 mg/kg bw per day, respectively, and in male and female Crj:BDF1 mice at ≥191 and 278 mg/kg bw per day, respectively. Serum gamma-glutamyltransferase (GGT) activity was additionally increased (9.5-fold to 17.5-fold over controls) in male and female rats at the same doses. Exposure to 1,4-dioxane was also found to induce significant increases in total bilirubin and cholesterol in female rats, a decrease in triglycerides in male and female mice, and a decrease in glucose in male and female rats and mice.
9.2.3.2 Renal effects
Histopathological alterations in the kidney have been observed in rats and mice exposed to 1,4-dioxane in drinking water at doses exceeding 55-94 mg/kg bw per day (Argus et al., 1965, 1973; Hoch-Ligeti and Argus, 1970; Kociba et al., 1974; NTP, 1978; JBRC, 1998; Kano et al., 2009).
The studies that reported renal effects at the lowest doses were Kociba et al. (1974) and the JBRC studies (JBRC, 1998; Kano et al., 2009) described above. Kociba et al. (1974) reported kidney damage in male and female Sherman rats at doses of ≥94 and ≥148 mg/kg bw per day, respectively. The JBRC reported nuclear enlargement of the kidney proximal tubules at doses exceeding 55 mg/kg bw per day in male and 83 mg/kg bw per day in female F344/DuCrj rats and in male Crj:BDF1 mice at 677 mg/kg bw per day (JBRC, 1998; Kano et al., 2009). In addition, non-neoplastic lesions (including vacuolar degeneration and focal tubular epithelial regeneration in the proximal cortical tubules) were observed in male and female Osborne-Mendel rats exposed to 1,4-dioxane in drinking water at doses of ≥240 and ≥350 mg/kg bw per day, respectively (NTP, 1978). Glomerulonephritis, thickening of the glomerular capsule, and/or pyelonephritis were observed in the kidneys of many male Wistar rats (exact number not specified) exposed to 857 mg/kg bw per day of 1,4-dioxane in the drinking water (Argus et al., 1965) and in some male Charles River CD rats (exact number not specified) at unspecified doses of 1,4-dioxane (Hoch-Ligeti et al., 1970; Argus et al., 1973). In the latter study, Argus et al. (1973) and Hoch-Ligeti et al. (1970) exposed male Charles River CD rats (30/group) to 0, 0.75%, 1.0%, 1.4%, or 1.8% 1,4-dioxane in the drinking water for 13 months; the average daily dose was calculated by IRIS (US EPA, 2013) as being 0, 400, 600, 800, or 1,000 mg/kg bw per day.
9.2.3.3 Respiratory tract effects
Respiratory tract effects of chronic exposure to 1,4-dioxane have been observed in rats, mice, and guinea pigs, including histopathological alterations in the nasal cavity and respiratory tract and increased lung weight at doses of ≥55 mg/kg bw per day in the drinking water (Argus et al., 1965, 1973; Hoch-Ligeti and Argus, 1970; Hoch-Ligeti et al., 1970; NTP, 1978; Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009).
The lowest LOAEL for respiratory tract effects of 55 mg/kg bw per day was reported in male F344/DuCrj rats, along with a NOAEL of 11 mg/kg bw per day (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). Non-neoplastic lesions were observed in the nasal cavity (nuclear enlargement and atrophy of the olfactory epithelium, squamous cell metaplasia of the respiratory epithelium, hydropic change and sclerosis in the lamina propria, adhesion, inflammation, and/or proliferation of the nasal gland) in males at doses exceeding 55 mg/kg bw per day and in females at doses of ≥83 mg/kg bw per day. Other studies that reported histopathological alterations in the respiratory tract of rats include Argus et al. (1965), who reported severe bronchitis with epithelial hyperplasia, marked peri-bronchial infiltration, and abscesses in the lungs in male Wistar rats exposed to 857 mg/kg bw per day, Argus et al. (1973) and Hoch-Ligeti et al. (1973), who reported one rat at 800 mg/kg bw per day that showed early peripheral adenomatous change of the alveolar epithelium and another rat with papillary hyperplasia of the bronchial epithelium, and the NCI (NTP, 1978), which reported increased incidence of pneumonia in female Osborne-Mendel rats exposed to 830 mg/kg bw per day 1,4-dioxane in drinking water.
Additional histopathological alterations were reported in the respiratory tract of mice and guinea pigs exposed to 1,4-dioxane. The JBRC studies reported non-neoplastic lesions in the respiratory tract epithelium of male and female Crj:BDF1 mice at exposures of 677 and 964 mg/kg bw per day, respectively, including nuclear enlargement of nasal respiratory epithelium, atrophy of nasal olfactory epithelium, inflammation of the nasal cavity, atrophy of the tracheal epithelium, atrophy of the lung/bronchial epithelium, and accumulation of foamy cells in the lung for both males and females, and nuclear enlargement of tracheal epithelium only in males (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). Some changes were also observed at 191 and 278 mg/kg bw per day in male and female mice, respectively, including nuclear enlargement of the nasal olfactory epithelium and nuclear enlargement of the bronchial epithelium in females only (JBRC, 1998; Kano et al., 2009). The NCI (NTP, 1978) reported non-neoplastic lesions (including pneumonia) in male and female B6C3F1 mice at doses of ≥720 and ≥380 mg/kg bw per day, respectively, and rhinitis in females at doses of ≥380 mg/kg bw per day. Hoch-Ligeti and Argus (1970) reported bronchial epithelial hyperplasia and mononuclear infiltration in the lung following exposure to 0.5-2% 1,4-dioxane in the drinking water. In this study, Hoch-Ligeti and Argus (1970) exposed male guinea pigs (22/group) to 1,4-dioxane in the drinking water for 23-28 months. A group of 10 untreated guinea pigs served as controls. The doses varied from 0.5% to 2% (regulated so that normal growth was maintained), corresponding to 588-635 g of 1,4-dioxane over the study period, as reported by the study authors (approximately equivalent to 1,000-1,080 mg/kg bw per day based on a reference body weight of 0.84 for guinea pigs [Health Canada, 1994] over 700 days). Moreover, relative and absolute lung weights were increased in male and female Crj:BDF1 mice exposed to doses of 677 mg/kg bw per day and of ≥278 mg/kg bw per day, respectively (JBRC, 1998; Kano et al., 2009).
9.2.3.4 Other effects
In addition to hepatic, renal, and respiratory tract effects, other effects have also been reported following long-term exposure to 1,4-dioxane in drinking water, including gastrointestinal effects, body weight changes, and reduced survival. Gastric ulcers were observed in the stomachs of male Osborne-Mendel rats exposed to doses of ≥240 mg/kg bw per day (NTP, 1978). Significantly reduced body weights and survival rates were observed in male and female Sherman rats exposed to 1 015 and 1 599 mg/kg bw per day, respectively (Kociba et al., 1974). Body weight gain decreased in male and female rats exposed to 530 and 640 mg/kg bw per day, respectively; mortality increased at doses of ≥350 and ≥720 mg/kg bw per day in male and female Osborne-Mendel rats, respectively; and body weight gain decreased in male and female rats exposed to 530 and 640 mg/kg bw per day, respectively (NTP, 1978). In Crj:BDF1 mice, a significant decrease in body weight was observed for both sexes at doses of ≥191 and ≥278 mg/kg bw per day, respectively (JBRC, 1998; Kano et al., 2009).A significant decrease in survival rate was observed in male and female F344/DuCrj rats exposed to 274 and 428 mg/kg bw per day 1,4-dioxane, respectively (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). The deaths were attributed to tumours in the nasal passage and peritoneal mesotheliomas of males and in the nasal passage and livers of the females (discussed below). A significant decrease in growth rates was also observed for both sexes at the same doses (JBRC, 1998; Kano et al., 2009). In B6C3F1 mice, mortality was significantly increased in females only, beginning after 80 weeks of exposure (NTP, 1978).
9.2.3.5 Cancer
Numerous studies have shown that exposure to 1,4-dioxane is associated with increased incidence of tumours in the liver, kidney, nasal cavity, peritoneum and mammary gland.
Hepatocellular adenomas and carcinomas have been observed in rats, mice, and guinea pigs chronically exposed to 1,4-dioxane in the drinking water (Argus et al., 1965, 1973; Hoch-Ligeti and Argus, 1970; Hoch-Ligeti et al., 1970; Kociba et al., 1974; NTP, 1978; Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). A significant increase in the incidence of hepatocellular carcinomas was observed in male Charles River CD rats exposed to 800 and 1,000 mg/kg bw per day (Hoch-Ligeti et al., 1970; Argus et al., 1973), in male Wistar rats exposed to 857 mg/kg bw per day (Argus et al., 1965), in male and female Sherman rats exposed to 1,015 and 1 599 mg/kg bw per day, respectively (Kociba et al., 1974), in male and female F344/DuCrj rats exposed to 274 and 428 mg/kg bw per day, respectively (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009), in male and female B6C3F1 mice exposed to doses of ≥720 and ≥380 mg/kg bw per day, respectively (NTP, 1978), and in male and female Crj:BDF1 mice exposed to ≥191 and ≥66 mg/kg bw per day, respectively (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). The high incidence of hepatic tumours in the latter study was thought to be responsible for the low survival rates in female mice (~40% survival at 278 mg/kg bw per day and ~20% survival at 964 mg/kg bw per day) according to correspondence between the US EPA and Yamazaki in 2006, as reported in the US EPA (2013). In considering the weight of evidence surrounding the development of liver tumours in rats and mice orally exposed to 1,4-dioxane, a large degree of uncertainty exists regarding the liver tumour occurrence in female mice (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009) at ≥66 mg/kg bw per day. More specifically, liver tumours were generally reported at higher doses (LOAELs of 274-1599 mg/kg bw per day) in the other chronic studies described above. The absence of non-cancer histopathological changes and the concomitant increase in liver enzymes in the JBRC studies despite the presence of both endpoints in the sub-chronic studies from the same group (described in sections 9.2.2.1 and 9.2.2.5) lend credence to the uncertainty surrounding the development of tumours at this low dose.
Hepatocellular adenomas were observed in female Osborne-Mendel rats and in male and female B6C3F1 mice (NTP, 1978) and in male and female F344/DuCrj rats (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). Hepatocellular adenomas were also significantly increased in male and female Crj:BDF1 mice; however, the incidence of adenomas was greater in the medium- and low-dose groups of the male and female mice, respectively, than in the high-dose groups, which is not reflective of a dose-response behaviour (JBRC, 1998; Kano et al., 2009). Please note that Kano et al. (2009) stated that the hepatic hyperplasia reported in their earlier conference proceeding (Yamakazi et al., 1994) were reclassified as hepatocellular adenomas and altered hepatocellular foci; however, the JBRC laboratory report (1998) does not show any dose-related hyperplasia or foci (Dourson et al., 2017). Hepatocellular adenomas were observed in 2 of 22 male guinea pigs exposed to 857 mg/kg bw per day (Hoch-Ligeti and Argus, 1970). Liver nodules were observed in male Charles River CD rats and male Wistar rats exposed to 1,4-dioxane, as described by Argus et al. (1965, 1973) and Hoch-Ligeti et al. (1970).
Tumours in kidneys were only reported in two drinking water studies. Hoch-Ligeti and Argus (1970) reported kidney adenomas in one guinea pig exposed to approximately 1,000 mg/kg bw per day, and Argus et al. (1965) reported early transitional cell carcinoma in one Wistar rat exposed to approximately 857 mg/kg bw per day 1,4-dioxane.
Tumours in the respiratory tract have been reported in rats exposed to 1,4-dioxane in drinking water (Argus et al., 1965; Kociba et al., 1974; NTP, 1978; Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). A significant increase in squamous cell carcinoma of the nasal cavity was observed in male and female Osborne-Mendel rats exposed to ≥240 and ≥350 mg/kg bw per day, respectively (NTP, 1978). Re-examination of the nasal tissue sections from this study determined that, based on the location of the tumours in the nasal cavity, the tumours resulted from inhalation of water droplets (Goldsworthy et al., 1991). Nasal cavity tumours were reported in 1-2 male Charles River CD rats exposed to ≥400 mg/kg bw per day, as well as adenomatous areas in two of these rats (Hoch-Ligeti et al., 1970; Argus et al., 1973). The authors noted that one control rat had a subcutaneous fibroma at the back of the nose. Kociba et al. (1974) observed an increased incidence of cholangiomas and squamous cell carcinomas of the nasal cavities in male and female Sherman rats exposed to 1,015 and 1 599 mg/kg bw per day, respectively. In addition, the significant decrease in survival of male and female F344/DuCrj rats exposed to 274 and 428 mg/kg bw per day, respectively, was attributed to tumours in the nasal passage, along with mesotheliomas in males (described below) and tumours in the livers in females (JBRC, 1998; Kano et al., 2009).
Other tumour sites have also been reported following oral exposure to 1,4-dioxane, including carcinoma of the gallbladder in two male guinea pigs (Hoch-Ligeti and Argus, 1970), mesotheliomas of the tunica vaginalis of the testis/epididymis in male Osborne-Mendel rats (NTP, 1978), mesotheliomas of the peritoneum in male F344/DuCrj rats (JBRC, 1998; Kano et al., 2009), adenomas of the mammary gland and fibroadenomas in male and female F344/DuCrj rats (JBRC, 1998; Kano et al., 2009), as well as leukemia with infiltration of the liver and spleen in male Wistar rats (Argus et al., 1965).
No significant increase in the incidence of tumours was identified in A/J mice or mice carrying the human gene c-Ha-ras (two cancer-prone transgenic rodent models) exposed to 1,4-dioxane (Stoner et al., 1986; Yamamoto et al., 1998a, 1998b). Stoner et al. (1986) administered male and female A/J mice (16/sex/group) a total cumulative dose of 24 000 mg/kg bw 1,4-dioxane by oral gavage three times per week for 8 weeks (equivalent to an amortized dose of 1,000 mg/kg bw per day). Yamamoto et al. (1998a, 1998b) exposed male and female c-Ha-ras mice to 0 (10/sex), 0.5% (15/sex), or 1.0% (15/sex) 1,4-dioxane in the drinking water for 26 weeks (equivalent to a dose of 1,000 and 2 000 mg/kg bw per day, respectively, using reference values for mouse body weight and water intakes from Health Canada, 1994). Both studies included 1,4-dioxane as part of large studies to investigate rapid carcinogenicity testing in each respective species prone to develop lung tumours.
1,4-Dioxane did not exhibit initiation activity in an initiation study conducted by Bull et al. (1986). Briefly, female SENCAR mice (40/group) received a single dose of 0 or 1,000 mg/kg bw 1,4-dioxane by oral gavage (vehicle not specified). Two weeks later, 12-O-tetradecanoylphorbol-13-acetate (TPA) (1.0 µg) in acetone was applied to the shaved backs of the mice three times per week for 20 weeks. In addition to negative results in the initiation study, single administration of 1,4-dioxane did not induce any tumours in the absence of TPA promotion. Moreover, 1,4-dioxane was negative for initiation activity following topical application or subcutaneous injection of 1,000 mg/kg bw per day 1,4-dioxane under the same promotion study regime (Bull et al., 1986).
Conversely, 1,4-dioxane exhibited promotion activity in a study conducted by Lundberg et al. (1987), in which partially hepatectomized male Sprague Dawley rats (n = 8 for treatment; n = 19 for controls) were administered 30 mg/kg bw diethylnitrosamine (DEN) followed by 0, 100, or 1,000 mg/kg bw per day 1,4-dioxane (with 25 ppm butylated hydroxytoluene as a stabilizer) by oral gavage in saline vehicle for 5 days per week for 7 weeks. An additional group of rats received 1,4-dioxane in the absence of the promotional inducer DEN. There was no increase in the number or volume of GGT-positive foci in the liver in the absence of DEN promotion; however, an increase in the number and total volume of GGT-positive foci was observed in the 1,000 mg/kg bw per day dose group, as well as histopathological changes, including enlarged, foamy hepatocytes in the midzonal region (Lundberg et al., 1987). Moreover, 1,4-dioxane was positive for promotion of skin tumours in male and female Swiss-Webster mice administered 50 µg of dimethylbenzanthracene one week prior to the topical application of a solution of 1,4-dioxane in acetone (concentration not specified) three times per week for 60 weeks (King et al., 1973). Acetone-vehicle- and croton-oil-treated mice were used as negative and positive controls, respectively. An additional group of rats was administered 1,4-dioxane in the absence of promotion. No significant increase in the incidence of tumour formation or lesions was observed in the absence of initiation; however, a significant increase in the percentage of mice with skin tumours was observed after 10 weeks of promotion treatment, as well as significant mortality. Additional tumours were observed in the lungs and kidneys. Limitations of this study include the presentation of only interim results (no final results), unequal exposures for the treatment and control groups, high mortality in the dermal exposure study, and no control for 1,4-dioxane alone without initiation.
9.2.4 Genotoxicity
Based on the weight of evidence provided below, 1,4-dioxane is not genotoxic at low doses. The genotoxicity of 1,4-dioxane has been investigated in several in vitro and in vivo test systems. In the vast majority of in vitro system assays, 1,4-dioxane was not genotoxic. In the few cases where genotoxicity was observed, it was accompanied by cytotoxicity. Likewise, 1,4-dioxane was negative for in vivo mutations and DNA repair, recognizing mixed results in micronucleus assays.
9.2.4.1 In vitro findings
DNA mutation: Assays for mutagenicity of 1,4-dioxane in bacteria and yeast were universally negative, with or without exogenous metabolic activation. These include the reverse mutation assay (Ames) in Salmonella typhimurium strains TA98, TA100, TA1530, TA1535, TA1537, and TA1538, with and without metabolic activation (Stott et al., 1981; Haworth, 1983; Nestmann et al., 1984; Khudoley et al., 1987; Morita and Hayashi, 1998); the reverse mutation assay in Eschericia coli strains WP2 uvrA and WP2, with and without metabolic activation (Morita and Hayashi, 1998); the MutatTox assay in Photobacterium phosphoreum, without metabolic activation (Kwan et al., 1990); and in point mutation assays in Saccharomyces cerevisiae strain D61M (Zimmermann et al., 1985). Negative results have also been demonstrated in mammalian cells in vitro for the forward mutation assay in mouse lymphoma L5178Y tk cells (McGregor et al., 1991; Morita and Hayashi, 1998).
Cytogenic studies: Negative results have been reported for micronucleus formation in Chinese hamster ovary (CHO) cells, with and without metabolic activation (Morita and Hayashi, 1998), chromosomal aberrations in CHO cells, with and without metabolic activation (Galloway et al., 1987; Morita and Hayashi, 1998), sister chromatid exchange in CHO cells, with and without metabolic activation (Galloway et al., 1987; Morita and Hayashi, 1998), covalent binding to calf thymus DNA, with and without metabolic activation (Woo et al., 1977b), and in a colorimetric aneuploidy assay without metabolic activation in Saccaromyces cerevisieae (Zimmermann et al., 1985). Galloway et al. (1987) also reported weakly positive results for sister chromatid exchange in CHO cells without metabolic activation.
DNA damage and repair: Largely negative results have been reported for 1,4-dioxane-induced DNA damage and repair. Positive results were reported for single-strand breaks measured by alkaline elution in primary rat hepatocytes; however, this occurred at cytotoxic doses (Sina et al., 1983). DNA repair was not detected following exposure to 1,4-dioxane in the differential DNA repair test in a DNA repair-deficient strain of E. coli (K-12 uvrB/recA; Hellmér and Bolcsfoldi, 1992) or by quantitative autoradiography in rat primary hepatocytes (Goldsworthy et al., 1991).
Other assays: 1,4-dioxane was positive in a cell transformation assay in BALB/3T3 mouse embryonic fibroblasts; however, this occurred at cytotoxic doses (Sheu et al., 1988).
9.2.4.2 In vivo findings
DNA mutation: Exposure to 1,4-dioxane (35 000-50 000 ppm) did not induce mutations in fruit flies (Drosophila melanogaster), as measured by the sex-linked recessive lethal mutagenicity test (a forward mutation assay), when 1,4-dioxane was administered in the feed for three days or administered by injection (Yoon et al., 1985).
The administration of 1,4-dioxane to gpt delta transgenic F344 rats in drinking water for 16 weeks was associated with a significant increase in mutations in the gtp transgene, as well as a significant change in mutational spectra, a change in the expression of some DNA repair and maintenance genes, and an increase in GST-P positive foci in livers at 5000 ppm 1,4-dioxane (equal to 440.2 mg/kg bw-day). In contrast, no increase was seen in Spi- deletion mutations or 8-OHdG adducts, indicative of oxidative DNA damage, in the treated rats (Gi et al., 2018). None of the measured parameters were statistically altered by administration of 1,4-dioxane at doses of 200 ppm and lower. Gi et al. (2018) concluded that 1,4-dioxane is a genotoxic hepatocarcinogen but present evidence for a threshold in 1,4-dioxane’s carcinogenic response. Additional studies are needed to confirm the Gi et al. (2018) study results, the significant (three-fold) increase in mutation frequency (and supporting changes) was only seen at one drinking water concentration (5000 ppm) in one experiment.
DNA damage: Mixed results have been reported for 1,4-dioxane’s ability to induce DNA and chromosomal damage in the form of micronucleus formation in mice and rats, single-strand breaks in rats, DNA alkylation in rats, and meiotic nondisjunction. Positive results were seen at high to very high doses.
No evidence of micronucleus formation was observed in the peripheral blood of male CD-1 mice (NOAEL 3,000 mg/kg bw per day; Morita and Hayashi, 1998), in the bone marrow of male CBA mice (NOAEL 3,600 mg/kg bw per day; Tinwell and Ashby, 1994), C57BL6 mice (NOAEL 3,600 mg/kg bw per day; Tinwell and Ashby, 1994), B6C3F1 mice (NOAEL 2 000-4 000 mg/kg bw per day; McFee et al., 1994), or BALBc mice (NOAEL 5 000 mg/kg bw per day; Mirkova, 1994) administered 1,4-dioxane via oral gavage or in the peripheral blood of male CD-1 mice administered up to 3,000 mg/kg bw per day 1,4-dioxane by intraperitoneal injection of 2 000-5 000 mg/kg bw per day (Morita, 1994). In addition, no increase in DNA strand breakage (Comet tails) were seen in the stomach, colon, liver, kidney, urinary bladder, lung, brain, and bone marrow of the mice at 3, 8 and 24 hr time points in which doses of up to 3200 mg/kg 1,4-dioxane were adminis (Sasaki et al., 2000).
Positive results for micronucleus formation were observed in the bone marrow of male CD-1 mice (Roy et al., 2005) and male and female C57BL6 mice (Mirkova, 1994), in the hepatocytes of male CD-1 mice (Morita and Hayashi, 1998; Roy et al., 2005), and in the liver of F344 rats (Suzuki et al., 1995) at oral 1,4-dioxane doses as low as 900 mg/kg bw per day. The increase in micronuclei in Roy et al. (2005) was paralleled by a reduction in polychromatic erythrocyte/ normochromatic erythrocyte (PCE/NCE) ratio, which is a measure of the cytotoxicity of bone marrow cells,and an indicator that the chemical reached the target tissue Roy et al. (2005) also reported a significant dose-related decrease in BRdU incorporation indicative of DNA replication/cell division that occurred in the liver of the young 1-4-dioxane-treated mice. This would be considered a type of toxic effect similar to the PCE:NCE ratio. Of the other positive studies (Mirkova, 1994; Suzuki et al., 1995; Morita and Hayashi, 1998; Roy et al., 2005), none measured for cytotoxic effects and two were conducted at doses above 1 500 mg/kg bw per day 1,4-dioxane (Morita and Hayashi, 1998; Roy et al., 2005), which is the limit dose for in vivo micronucleus studies (OECD, 2014). In addition, a report of a significant induction of micronuclei in the liver of 1,4-dioxane-treated rats (1000-3000 mg/kg) using three different approaches as well as a lack of induced micronuclei in the bone marrow erythrocytes and no increase in CD59 negative cells, an indicator of Pig-a mutations, in peripheral blood erythrocytes; These results suggested that 1,4-dioxane is clastogenic in the liver but not genotoxic in the bone marrow of rats (Itoh and Hattori, 2019).
No significant increase in hepatic DNA alkylation was observed in male Sprague Dawley rats administered a single oral dose of 1,000 mg/kg 1,4-dioxane (Stott et al., 1981). Conversely, an increase in single-strand breaks was observed in female Sprague Dawley rats administered 2 550 mg/kg 1,4-dioxane by oral gavage (Kitchin and Brown, 1990), and a significant increased incidence of meiotic nondisjunction was observed in oocytes of female fruit flies orally administered 1,4-dioxane prior to mating (Munoz and Mazar Barnett, 2002).
DNA repair was not detected in hepatocytes or nasal epithelial cells recovered from male F344 rats following a single gavage administration of 1,000 mg/kg bw per day or continuous administration of 1 500-3,000 mg/kg bw per day 1,4-dioxane in drinking water for 1-2 weeks (Goldsworthy et al., 1991), nor were they detected in hepatocytes from male Sprague Dawley rats administered 1,000 mg/kg bw per day 1,4-dioxane in drinking water for 11 weeks (Stott et al., 1981).
More mixed results have been reported for 1,4-dioxane’s ability to induce cell proliferation via replicative DNA synthesis. 1,4-Dioxane did not induce replicative DNA synthesis in hepatocytes from male F344 rats (Goldsworthy et al., 1991) and male Sprague Dawley rats (Stott et al., 1981) administered a single dose of 1,000 mg/kg bw per day 1,4-dioxane by oral gavage and in nasal epithelial cells of male F344 rats administered 1 500 mg/kg bw per day 1,4-dioxane in drinking water for 2 weeks (Goldsworthy et al., 1991). 1,4-Dioxane significantly induced replicative DNA synthesis in hepatocytes from male F344 rats (Miyagawa et al., 1999) and Sprague Dawley rats (Stott et al., 1981) administered 1 000 and 1 500 mg/kg bw per day 1,4-dioxane via oral gavage and in male F344 rats (Goldsworthy et al., 1991) administered a single dose of 1 000 mg/kg bw per day 1,4-dioxane in drinking water for two weeks. Additionally, equivocal results were obtained in hepatocytes from male F344 rats administered 1 000-2 000 mg/kg bw per day (Uno et al., 1994; Miyagawa et al., 1999). 1,4-Dioxane was also able to induce RNA synthesis in hepatocytes from male Sprague Dawley rats following intravenous injection of 1,000 mg/kg bw per day (Kurl et al., 1981).
In summary, studies on 1,4-dioxane induced DNA damage in vivo are equivocal and positive results were seen at higher doses.
9.2.5 Reproductive and developmental toxicity
Only two studies were found investigating reproductive or developmental effects of 1,4-dioxane. Giavini et al. (1985) administered pregnant Sprague Dawley rats (18-20/dose group) 0, 0.25, 0.5, or 1.0 mL/kg bw per day 1,4-dioxane in water by oral gavage on days 6-15 of gestation. These doses are equivalent to 0, 257.5, 515, or 1 030 mg/kg bw per day based on 1,4-dioxane’s density of 1.03 g/mL. The dams were sacrificed on gestational day 21. Fetal birth weight and ossification of sternebrae were significantly reduced in the 1030 mg/kg bw per day dose group. No difference was observed in the number of implantations, fetuses, or resorptions compared with controls. The NOAEL and LOAEL for this study are 515 and 1,030 mg/kg bw per day, respectively, based on reduced fetal birth weight and delayed ossification of sternebrae (Giavini et al., 1985). Lane et al. (1982) conducted a three-generation study investigating the reproductive and developmental toxicity of TCA in ICR Swiss mice when administered in drinking water, in which 1,4-dioxane was used as a solvent for TCA. As a result, the vehicle-control group exposed to 3% 1,4-dioxane (equivalent to a dose of 6 000 mg/kg bw per day using reference values for mouse body weight and water intake from Health Canada, 1994) had a significant increase in the ratio of dead:live fetuses in the F1 generation compared with water controls, but the exposure had no effect on litter size, postnatal body weight, survival indices, or visceral/skeletal malformations (Lane et al., 1982). Additionally, a non-dose-dependent increase in testis mineralization was observed in male Crj:BDF1 mice at a dose of ≥191 mg/kg bw per day (JBRC, 1998), which was not observed in rats exposed to 1,4-dioxane up to 1,025 mg/kg bw per day (Kociba et al., 1974; JBRC, 1998).
9.3 Mode of action
1,4-Dioxane has been shown to cause tumours in multiple sites in multiple species (of both sexes) following oral exposure. Effects appear mostly in the liver; however, tumours have also been reported in the kidney, nasal cavity, and mammary glands, along with mesotheliomas of the tunica vaginalis in males. Studies in both rats and mice found liver toxicity to be the most sensitive endpoint of concern; thus, this section focuses on the mode of action (MOA) evidence for liver tumours. This analysis proposes MOAs and then provides the weight of evidence behind each MOA.Using a MOA analysis, the weight of evidence supports a non-genotoxic MOA, with 1,4-dioxane inducing liver tumours through a cytotoxicity and regenerative proliferation-induced MOA.
9.3.1 Proposed MOA no. 1: genotoxicity-induced carcinogenicity
The available data with respect to genotoxicity and cancer were re-evaluated considering the modified Bradford Hill criteria of dose-response temporal concordance, consistency and specificity, and biological plausibility (Meek et al., 2014).
1,4-Dioxane-induced tumours and pre-neoplastic lesions are reported at doses lower than those at which genotoxicity occurs in vivo; micronucleus formation was observed at higher doses (≥900 mg/kg bw per day) than those producing hepatic lesions (between 9.6 and 94 mg/kg bw per day; corresponding to the NOAEL and LOAEL in Kociba et al., 1974) and liver tumours (≥240 mg/kg bw per day; the LOAEL in the NCI study [NTP, 1978]). Therefore, the condition of dose concordance is not met. Insufficient data are available to definitively comment on the temporal concordance of these data, although since genotoxicity studies are often conducted over shorter durations than studies investigating cancer endpoints, it is often the case that temporal concordance is met for this MOA.
The genotoxicity of 1,4-dioxane was investigated using various endpoints, including DNA mutations, clastogenicity, micronucleus formation, and DNA damage or repair, which yielded mainly negative and few positive findings (see Section 9.2.4). Although different testing methods, dosing regimen, rodent strains, and target tissues were investigated, and although the overall genotoxicity was mostly negative, the positive findings were at doses that exceeded the limit dose of 2 000 mg/kg bw per day, indicating that the observed effects at low doses are not due to direct acting genotoxicity. By contrast, positive results were also found below this limit dose in two oral studies (Mirkova, 1994; Suzuki et al., 1995) which suggests that 1,4-dioxane may have some genotoxic potential. However, the DNA damage observed at high doses cannot explain the tumours occurring at lower doses. Therefore, high doses of 1,4-dioxane may induce genotoxicity due to cytotoxicity, but it does not appear to be a key event leading to tumour formation. On the other hand, proliferation induction, which is supported by the positive results in replicative DNA synthesis tests, can be seen as markers of cell proliferation and part of the regenerative hyperplasia MOA. In support of this conclusion, the Health Council of the Netherlands (2015) concluded that 1,4-dioxane acts via a non-stochastic genotoxic mechanism following a thorough evaluation of the genotoxicity and carcinogenicity of 1,4-dioxane. The evaluation was performed by the Subcommittee on Classifying Carcinogenic Substances of the Dutch Expert Committee on Occupational Safety of the Health Council. The committee’s evaluation and recommendation is based on scientific data published since 2015 and addresses comments from public review.
Moreover, structural analysis using the computer automated structural evaluator (CASE) structure activity method was also used to predict whether 1,4-dioxane would be a non-genotoxic carcinogen (Rosenkranz and Klopman, 1992). More specifically, the analysis predicted that 1,4-dioxane would not induce mutagenicity in Salmonella, chromosomal aberrations in Chinese hamster ovary cells, or unscheduled DNA synthesis in rat hepatocytes; however, it was predicted to be an inducer of sister chromatid exchange in Chinese hamster ovary cells and micronuclei in the bone marrow of rats. Moreover, according to the in vivo mutagenicity (micronucleus) alerts by the Instituto Superiore di Sanita (ISS) of Italy (Benigni and Bossa, 2008; OECD, 2015), 1,4-dioxane is a potential hydrogen bond acceptor. Therefore, there is a possibility that it interacts with DNA and/or protein via non-covalent bonding, such as DNA intercalation.
Additionally, in a DNA microarray study to discriminate between genotoxic and non-genotoxic carcinogens, 1,4-dioxane was classified as a non-genotoxic carcinogen (van Delft et al., 2004). In this study, gene expression profiling was conducted on human hepatoma HepG2 cells exposed to a training set of nine genotoxic and seven non-genotoxic carcinogens.
In summary, the genotoxic MOA was not able to satisfy the conditions of dose concordance, consistency and specificity, and biological plausibility of the modified Bradford Hill criteria for a plausible MOA (Meek et al., 2014). This analysis indicates that the pattern of genotoxicity is inconsistent with a MOA where genotoxicity is an early, direct and influential key event in the carcinogenic MOA. Similar conclusions were reached by the governments of Canada (Environment Canada and Health Canada, 2010) and Australia (NICNAS, 1998), by the European Union (European Commission, 2002), and by the US EPA (2013).
9.3.2 Proposed MOA no. 2: regenerative proliferation-induced carcinogenicity
The lack of conclusive genotoxicity data and the cytotoxicity observed at doses that induce tumours support the notion that 1,4-dioxane acts via a non-genotoxic MOA. Additionally, doses above those causing a saturation of metabolic capacity led to the formation of liver tumours with prolonged exposure, as discussed above (NICNAS, 1998; Environment Canada and Health Canada, 2010; US EPA, 2013). The available data from in vivo studies point to a regenerative proliferation MOA. The proposed key events describing this MOA for 1,4-dioxane include: (1) accumulation of parent compound, (2) liver cell hypertrophy and necrosis, (3) DNA synthesis, (4) regenerative cell proliferation, and (5) promotion of endogenously initiated tumours. A thorough presentation and analysis of the data supporting these key events has been published by Dourson et al. (2014) and is summarized below.
Key event 1: accumulation of parent compound. 1,4-Dioxane is extensively metabolized in experimental animals and humans via cytochrome P450 (although which specific P450 is still unclear) as the major pathway (Kociba et al., 1975; Young et al., 1978a; Sweeney et al., 2008). Young et al. (1978a) reported that in rats, 1,4-dioxane metabolism is capacity-limited, transitioning from linear first-order pharmacokinetics to nonlinear Michaelis-Menten kinetics as doses increase. Since similar metabolic pathways are presumed to be active in humans and rats, capacity-limited metabolism can be inferred in humans. This transition appears to occur at plasma 1,4-dioxane concentrations ranging from 30 µg/mL to 100 µg/mL in rats (Young et al., 1978a). At doses yielding plasma concentrations below this range (up to oral doses of 10 mg/kg body weight), 1,4-dioxane is rapidly metabolized, and exposure for as long as 2 years generally results in no observable adverse effects in rats (Kociba et al., 1974, 1975). At doses approaching or exceeding metabolic capacity, the accumulation of parent compound and target organ toxicity has been observed (Kociba et al., 1975; Young et al., 1978a). Please note that 1,4-dioxane’s kinetics and metabolic pathways have not been investigated in the mouse.
Although the mechanism by which 1,4-dioxane causes hepatotoxicity has not been fully elucidated, it is likely that the parent compound is the toxic moiety and not a metabolite. Evidence supporting the parent compound toxicity includes the absence of additional liver toxicity following phenobarbital-exposure-induced 1,4-dioxane metabolism (Nannelli et al., 2005) and the lack of effect on the covalent binding of 1,4-dioxane to macromolecules in subcellular fractions of hepatocytes from rats treated with CYP450 inducers before exposure to 1,4-dioxane (Woo et al., 1977b). Toxicity is also more evident at doses that saturate metabolic clearance, as described more fully below. Dourson et al. (2014) further notes that typical human exposures to 1,4-dioxane are unlikely to saturate P450 metabolic enzymes or to activate non-P450 enzyme pathways that would generate additional toxic metabolites.
Key event 2: liver cell hypertrophy and necrosis. Liver cell hypertrophy and necrosis have been identified as key precursor events leading to regenerative cell proliferation and liver tumours following short-term and chronic exposure to high doses of 1,4-dioxane. Liver lesions (characterized by centrilobular swelling and single cell necrosis) have been shown to occur following 5-14 days of exposure to very high doses of 1,4-dioxane (Kesten et al., 1939; JBRC, 1998) and following 11 to 13 weeks of exposure to doses of 1,4-dioxane exceeding the metabolic capacity in rats (Stott et al., 1981; Kano et al., 2008; Kasai et al., 2008, 2009). Liver degeneration was reported following a single oral exposure or following repeated exposure over 3-10 days to very high doses of 1,4-dioxane (Schrenk and Yant, 1936; Laug et al., 1939). Hepatocellular hypertrophy was reported at doses as low as 55 mg/kg bw per day in rats and 191 mg/kg bw per day in mice exposed to 1,4-dioxane for 2 years (Kano et al., 2009), and hepatocellular necrosis was reported at doses as low as 94 mg/kg bw per day in rats (Kociba et al., 1974) and 191 mg/kg bw per day in mice exposed to 1,4-dioxane for 2 years (Kano et al., 2009).
Key event 3: DNA synthesis. As discussed above, 1,4-dioxane does not cause DNA repair activity in in vitro or in vivo assays and has weak genotoxic potential at high doses (often in the presence of cytotoxicity); however, of the five studies investigating DNA synthesis (all in rats), three were positive. For example, at doses of ≥1,000 mg/kg bw per day, 1,4-dioxane induced DNA synthesis in hepatocytes (Stott et al., 1981; Goldsworthy et al., 1991; Uno et al., 1994; Miyagawa et al., 1999), which is indicative of cell proliferation. These DNA synthesis studies provide evidence that 1,4-dioxane promotes cell proliferation through cytotoxicity.
Key event 4: regenerative cell proliferation. Temporal and dose-response analysis supports the development of hepatocellular hyperplasia following DNA synthesis and preceding tumour development in rats and, to a lesser extent, in mice. In a 2-year bioassay, Kociba et al. (1974) reported that male and female rats receiving mean daily doses of ≥94 mg/kg bw per day and of ≥148 mg/kg bw per day, respectively, showed evidence of hepatic regeneration as indicated by hepatocellular hyperplastic nodule formation. Stott et al. (1981) reported that hepatocytes from Sprague Dawley rats receiving a tumourigenic dose of 1,4-dioxane (1,000 mg/kg bw per day) for 11 weeks showed no evidence of DNA alkylation or DNA repair activity; however, increased levels of DNA synthesis (an indicator of cell proliferation) were observed. Goldsworthy et al. (1991) found that although a single dose of 1,000 mg/kg 1,4-dioxane caused no increase in hepatocyte labelling index (proportion of hepatocytes actively synthesizing DNA) or liver/body weight ratios at 24 or 48 h following treatment (indicating a lack of mitogenic activity or regenerative cell proliferation in response to hepatotoxicity), continuous exposure to 1.0% 1,4-dioxane in drinking water for up to two weeks did cause a two-fold increase in the hepatic labelling index, suggesting a potential role for cell proliferation in the carcinogenicity of 1,4-dioxane. In two studies by the same group of investigators, one (Uno et al., 1994) reported that single gavage doses of 1,4-dioxane up to 2 000 mg/kg in male F344 rats failed to induce replicative DNA synthesis in hepatocytes, suggesting that 1,4-dioxane may only induce liver cancer in initiated cells. However, in a subsequent study (using the same experimental protocol) it was reported that 1,4-dioxane did increase replicative DNA synthesis (thus inducing hepatocyte proliferation) (Miyagawa et al. 1999).
Key event 5: tumour promotion. The final key event in the hypothesized MOA for 1,4-dioxane is tumour promotion, for which evidence is limited. Tumour promotion studies in mouse skin and rat liver suggest that 1,4-dioxane may enhance the growth of previously initiated cells (King et al., 1973; Lundberg et al., 1987); however, definitive conclusions cannot be drawn from these studies due to deficiencies in their design and/or execution. King et al. (1973) observed a lack of direct carcinogenic and initiation activity (via the absence of confirmed skin tumours and lesions) in mice dermally exposed to 1,4-dioxane; however, evidence of promoting activity was observed in rats and mice initiated with dimethylbenzanthracene one week prior to oral exposure to 1,4-dioxane. This evidence is of limited reliability due to the reasons outlined in Section 9.2.3.5. Lundberg et al. (1987) reported tumour promotion (via significant increase of GGT-positive foci and lipid accumulation) in the rat liver after initiation with diethylnitrosamine and partial hepatectomy with subsequent administration of 1 000 mg/kg 1,4-dioxane (5 days/week over 7 weeks), suggesting that toxicity may be an important contributor to the observed pre-neoplastic lesions.
9.3.3 Confidence in the proposed MOA no. 2 regenerative proliferation-induced carcinogenicity
The key events for this MOA have been evaluated based on the modified Bradford Hill criteria (Meek et al., 2003) of dose and temporal concordance, consistency and essentiality of key events, analogy, and biological concordance. Dose and temporal concordance are evident upon consideration of multiple studies across different durations. Increased 1,4-dioxane doses were associated with increased tumour incidence in mice and rats, and the key events are observed at doses below or similar to those associated with cancer. The sequence of key events is logical, and the key events and adverse outcomes occur in an expected order. More specifically, histopathological changes indicative of hypertrophy and necrosis are observed following short-term studies and are further observed in chronic bioassays preceding the development of tumours. These key events have been observed in repeated chronic experiments in different laboratories (NCI, 1978; Kano et al., 2009) as evidence for consistency. The 1,4-dioxane exposures also induce nasal toxicity, at all time-points and in a dose-related manner that precedes nasal tumour development. Essentiality of these key events is more difficult to ascertain, since none of the studies were designed to test this essentiality directly (by knocking out one key event or another). However, support for the proposed MOA is found by analogy to other solvents that cause liver tumours in both rats and mice. Moreover, all key events in the rodent MOA are concordant and plausible in humans, although limited data are available to provide support. The regenerative proliferation-induced carcinogenic MOA meets many of the Bradford Hill criteria (specifically dose and temporal concordance, consistency, analogy and biological concordance), while the alternate MOA (genotoxicity) does not.
Further evidence for 1,4-dioxane’s regenerative proliferation MOA has recently been provided by the Centre for Toxicology Excellence in Risk Assessment, which conducted a detailed MOA analysis for 1,4-dioxane-induced liver tumours for the Alliance for Risk Assessment (Dourson et al., 2014, 2017). This analysis included a re-read of the mouse liver slides from the NCI study (NTP, 1978) and analysis of the translated laboratory reports from the Japan Bioassay Research Center studies (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). More specifically, the reanalyses were initiated in order to clarify the absence of non-cancer toxicity in the two mouse studies (NTP, 1998; Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). The results of the analyses showed additional non-cancer toxicity in mice from the NCI study (NTP, 1978) that were not reported in the original study (which reported only the most severe effects: i.e., cancer), including hypertrophy, necrosis, inflammation, and hyperplastic hepatocellular foci, which are consistent with the regenerative hyperplasia-induced liver tumours (Dourson et al., 2014, 2017). However, the information from the JBRC reports confirmed the absence of liver non-cancer toxicity at tumourigenic doses in mice reported in the published studies (Yamazaki et al., 1994; Kano et al., 2009), including the change in classification of hyperplasia and other endpoints (discussed in Section 9.2.3.5). As described in Dourson et al. (2017), the absence of liver non-cancer toxicity was unexpected because the sub-chronic (13-week) mouse study from the same laboratory (Kano et al., 2008) reported extensive liver non-cancer histopathology at similar sub-chronic-to-chronic adjusted doses. Moreover, the chronic study also reported increases in liver enzymes associated with adverse hepatic effects (Hall et al., 2012). These results from the re-read and re-analysis were determined to support the modified Bradford Hill criteria for strength, consistency, analogy, biological plausibility, and coherence. Moreover, the dose and temporal concordance for key events leading to liver tumour formation and the severity of the events was met in all datasets except one (female mice at the lowest dose in the JBRC studies), which appears to be due in part to a change in classification of some endpoints in this study (Dourson et al., 2017).
Insufficient data exist to allow for the assessment of the MOA for other tumour types, including those occurring in the nasal cavity, mammary gland, and mesotheliomas of the tunica vaginalis. However, Dourson et al. (2014) conducted a brief analysis of the relevance of each tumour type: mesotheliomas of the tunica vaginalis are common to only male F344 rats, are exceedingly rare in humans, and have been shown elsewhere to be irrelevant to humans (Haber et al., 2009); mammary gland tumours occur at high doses and are uniformly benign; for nasal tumours a regenerative hyperplasia MOA is reasonable, given the evidence of nasal toxicity by histology preceding tumours in both sexes of rats and mice. Dourson et al. (2014) noted that for all tumour types, the response is not as severe as that for the liver.
10.0 Classification and assessment
Based primarily on evidence in experimental animals, 1,4-dioxane has been classified according to its carcinogenicity by national and international bodies. The International Agency for Research on Cancer (IARC, 1999) has classified it as “possibly carcinogenic to humans” (group 2B) based on sufficient evidence in experimental animals and inadequate evidence in humans. The National Toxicology Program (NTP, 2011) has classified it as “reasonably anticipated to be a human carcinogen” based on sufficient evidence in animals and inadequate evidence in humans. The US EPA (2013) has classified it as “likely to be carcinogenic to humans” based on sufficient evidence in animals (including hepatic tumours in multiple species and strains, as well as peritoneal mesotheliomas, mammary gland, and nasal tumours) and inadequate evidence of carcinogenicity in humans.
Analysis of the weight of evidence indicates that at low doses, 1,4-dioxane is not a mutagen and does not cause DNA repair or initiation; however, 1,4-dioxane appears to promote tumours, stimulate DNA synthesis, and evoke cancer following saturation of 1,4-dioxane metabolism or elimination. Analysis supports a non-genotoxic MOA involving cytotoxicity followed by regenerative hyperplasia and stimulation of endogenously formed mutations. Since 1,4-dioxane acts through a non-genotoxic MOA and demonstrates dose-related non-linear kinetics, a non-linear (threshold) risk assessment approach is considered appropriate.
Despite the paucity of epidemiological studies investigating the effect of 1,4-dioxane exposure in humans, the primary non-cancer health effects associated with occupational exposure are reported to be on the liver and kidney, including hemorrhagic nephritis and centrilobular necrosis of the liver. Liver and kidney effects have also been reported in experimental animals after both acute and chronic exposure.
Liver toxicity associated with oral exposure to 1,4-dioxane has been identified as the most sensitive endpoint of concern; effects include hepatocellular degradation, foci, hyperplasia, hypertrophy, inflammation, and necrosis. Hepatocellular degeneration, necrosis, and hyperplastic nodules were observed in male rats at doses of 94 mg/kg bw per day and above (LOAEL; NOAEL 9.6 mg/kg bw per day) (Kociba et al., 1974), and spongiosis and hyperplasia of the liver were noted in male rats at 55 mg/kg bw per day and above (LOAEL; NOAEL 11 mg/kg bw per day) (Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009). Hepatocellular hyperplasia, necrosis, and hepatocellular foci were additionally identified in rat liver, and necrosis was found in mouse liver in the reanalysis of the JBRC liver slides provided by the Toxicology Excellence for Risk Assessment Center as part of the Alliance for Risk Assessment reanalysis described in Section 9.4 (Dourson et al., 2014; Dourson et al., 2017). Kidney toxicity in male rats was also observed at the same doses (Kociba et al., 1974; Yamazaki et al., 1994; JBRC, 1998; Kano et al., 2009); however, these effects do not occur at the same frequency or severity as the liver effects. Hepatocellular hypertrophy, necrosis, inflammation, and hyperplasia were observed in female rats at 350 mg/kg bw per day and above (LOAEL; no NOAEL) (NTP, 1978). No data exist on how life stage affects liver toxicity or other health effects associated with 1,4-dioxane exposure.
Hepatocellular necrosis in rats from the Kociba et al. (1974) study was selected for calculation of the health-based value (HBV). This was a well-designed study: it was two years in duration; it used both male and female rats, which included three dose levels plus a control group; there were many animals in each group (60 animals/sex/dose); it included a comprehensive statistical analysis, histopathological evaluations, blood samples, and body and organ weights; it identified a NOAEL and LOAEL for the critical effect. Liver toxicity was selected as the key endpoint for the basis of an HBV, given that it is considered to be protective of future liver tumours and is deemed to have a threshold, as suggested by the MOA analysis.
Benchmark dose (BMD) modelling was performed separately for male or female rats alone and for male and female rats combined (using the hepatocellular necrosis incidence data provided by the Dow Chemical Company to Dourson et al., 2014) at a 10% and 5% increased incidence of adverse effects over background rates (benchmark response [BMR]). Of the models that provided a reasonable fit (goodness of fit p-value > 0.1, BMD/BMDL [benchmark dose lower confidence limit] ratio < 5, and visual inspection of the curve), the LogProbit model provided the best fit (i.e., lowest Akaike Information Criterion [AIC]) for female rats alone and for male and female rats combined, and the dichotomous model provided the best fit for male rats alone. Estimated BMD/BMDL values in males were 37.7/24.7 mg/kg bw per day at a BMR of 10% and 16.2/ 9.6 mg/kg bw per day at a BMR of 5%; in females, the values were 80.1/34.3 mg/kg bw per day at a BMR of 10% and 35.2/11.4 mg/kg bw per day at a BMR of 5%; and in males and females combined, the values were 47.8/21.0 mg/kg bw per day at a BMR of 10% and 16.8/5.4 mg/kg bw per day at a BMR of 5%. A BMR of 5% was selected upon weighing statistical and biological considerations. More specifically, the study design (e.g., sample size of 60/dose group/sex) provides sufficient power to support the selection of a BMR of 5%. Moreover, the 5% BMR falls near the low end of the observable range, and its selection is strengthened by the similarity between the BMD and BMDL values. Also, the selection of such a response level is considered most appropriate given that the endpoint of concern is cancer. As the lower 95% confidence limit on the BMD for a 5% response (BMDL5) of 5.4 mg/kg bw per day for male and female rats combined is lower than the corresponding values in males or females alone, this has been selected as the point-of-departure for the calculation of the HBV.
The tolerable daily intake (TDI) for 1,4-dioxane is calculated as follows:

Text Description
The TDI for 1,4-dioxane is 0.0054 mg/kg bw per day. This is calculated by dividing the BMDL5 of 5.4 mg/kg bw per day by the uncertainty factor (UF) 1000.
where:
- 5.4 mg/kg bw/day is the BMDL5 for hepatocellular necrosis using the combined incidence data from male and female rats (Kociba et al., 1974; Dourson et al., 2014); and
- 1,000 is the uncertainty factor: ×10 for interspecies variability, ×10 for intraspecies variability and ×10 for database deficiencies, including poor characterization of reproductive and developmental toxicity, as well as inadequate characterization of effects in a second species (mice).
Using this TDI, the HBV for 1,4-dioxane in drinking water for non-cancer effects is derived as follows:
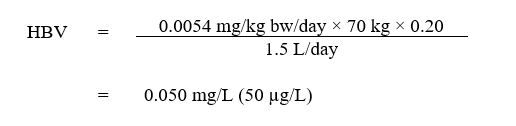
Text Description
The HBV for 1,4-dioxane is 0.050 mg/L. This is calculated by multiplying the TDI (0.0054 mg/kg bw per day) by 70 kg, then by the allocation factor (AF) for drinking water of 0.2, then dividing this product by the 1.5 L/day.
where:
- 0.0054 mg/kg bw/day is the TDI, as derived above;
- 70 kg is the average body weight of an adult;
- 0.20 is the default allocation factor for drinking water; the proportion of exposure to 1,4-dioxane from drinking water, as opposed to other environmental media (i.e., food, air, soil, consumer products); and
- 1.5 L/day is the total daily exposure contribution from drinking water for an adult.
10.1 International considerations
This section presents drinking water guidelines and standards for 1,4-dioxane from other national and international organizations. Variations in these limits can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights, and allocation factors.
The World Health Organization (WHO, 2005) has established a guideline value of 0.05 mg/L using both a linear extrapolation for estimating cancer risk and a TDI approach, assuming that 1,4-dioxane is not genotoxic to humans at low doses. The linear extrapolation employed a linearized multistage model for estimating cancer risk based on hepatic tumours in rats (Yamazaki et al., 1994) at a 10-5 cancer risk level, which resulted in a value of 0.054 mg/L. The TDI approach was conducted using a NOAEL of 16 mg/kg bw per day for hepatocellular tumours in rats (Yamazaki et al., 1994) (note that this NOAEL was calculated by WHO using default rat body weight and water consumption calculations before the publication of average daily doses by the authors in Kano et al., 2009) and a composite uncertainty factor of 1,000 (10 for interspecies variability, 10 for intraspecies variability, and 10 for non-genotoxic carcinogenicity), which resulted in a rounded guideline value of 0.05 mg/L.
The US EPA has established a non-enforceable health advisory of 0.035 mg/L in 1987, but it has not developed an enforceable Maximum Contaminant Level (MCL) for 1,4-dioxane. The US EPA derived a Reference Dose of 0.03 mg/kg bw per day based on a NOAEL of 9.6 mg/kg bw per day for liver and kidney degeneration, applying an uncertainty factor of 300 (10 each for inter- and intra-species variation and 3 for database deficiencies), using the results of the Kociba et al. (1974) study (US EPA, 2013). Additionally, an oral cancer slope factor of 0.1 mg/kg bw per day was calculated using the linear multistage model for carcinogenesis, based on BMD modelling of hepatocellular adenoma and carcinoma in female BDF1 mice in a 2-year drinking water study with a benchmark response of 50% (Kano et al., 2009). The California EPA has also not set a MCL but has a drinking water notification (CalEPA, 2014) level of 1 µg/L, based on the US EPA oral cancer slope factor. ATSDR (2012) calculated a chronic oral Minimal Risk Level of 0.1 mg/kg bw per day, based on a NOAEL of 9.6 mg/kg bw per day for liver effects in male rats from the Kociba et al. (1974) study.
11.0 Rationale
1,4-Dioxane is a synthetic chemical used as a solvent in multiple industrial applications. Its presence in the environment is mainly due to chemical waste disposal practices, leaks from landfills, or industrial and municipal wastewater discharges. Because of its physico-chemical properties, it can travel fast through soil and reach groundwater sources. Canadians can be exposed to 1,4-dioxane in indoor and outdoor air, food, drinking water, and consumer products. Although skin contact and inhalation are potential routes of exposure to 1,4-dioxane, its intake proportion from drinking water through these routes was assessed and is not considered to be significant.
The IARC classified 1,4-dioxane as “possibly carcinogenic to humans” (group 2B) based on sufficient evidence in experimental animals and inadequate evidence in humans.
In humans, data on 1,4-dioxane are limited to studies on the health risks associated with exposure via inhalation. The primary non-cancer health effects associated with exposure to 1,4-dioxane are reported to be on the liver and kidney; no studies have looked at the ability of 1,4-dioxane to cause cancer in humans. In animals, the most severe health effect associated with exposure to 1,4-dioxane is cancer. Additionally, the liver, kidney, and respiratory tract have been identified as major target organs for 1,4-dioxane toxicity.
Analysis of the weight of evidence indicates that at low doses 1,4-dioxane is not a mutagen but promotes tumours through non-genotoxic mechanisms, which is supported by a MOA analysis indicating the progression from non-cancer to cancer effects after exposure to 1,4-dioxane. Thus, the assessment of 1,4-dioxane in drinking water considers the cancer and non-cancer effects together using a threshold approach. Liver effects that are early events of cancer are the most sensitive endpoints for both cancer and non-cancer toxicity associated with oral exposure to 1,4-dioxane.
A MAC of 0.050 mg/L is established for 1,4-dioxane in drinking water. This MAC is based on studies of early events of liver cancers in rats, which is deemed protective of both cancer and non-cancer health effects. It can be reliably measured by available analytical methods and is achievable by municipal and residential scale treatment technologies.
As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area and recommend any change to the guideline that is deemed necessary.
12.0 References
Adamson, D.T., Mahendra, S., Walker Jr., K.L.W., Rauch, S.R., Sengupta, S. and Newell, C.J. (2014). A multisite survey to identify the scale of the 1,4-dioxane problem at contaminated groundwater sites. Environ. Sci. Technol. Lett., 1: 254-258.
Adamson, D.T., Anderson, R.H., Mahendra, S., and Newell, C. J. (2015). Evidence of 1,4-dioxane attenuation at groundwater sites contaminated with chlorinated solvents and 1,4-dioxane. Environ. Sci. Technol., 49: 6510−6518.
Adamson, D.T., Piña E.A., Cartwright, A.E., Rauch, S.R., Anderson, R.H., Mohr, T., and Connor, J.A. (2017). 1,4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule. Science Total Environ., 596-597: 236-245.Amoore, J.E. and Hautala, E. (1983). Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatiles for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3(6): 272-290.
Anderson, R.H., Anderson, J.K. and Bower, P.A. (2012). Co-occurrence of 1,4-dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US Air Force installations: Fact or fiction. Integr. Environ. Assess. Manag., 8(4): 731-737.
Argus, M.F., Arcos, J.C. and Hoch-Ligeti, C. (1965). Studies on the carcinogenic activity of protein-denaturing agents: Hepatocarcinogenicity of dioxane. J. Natl. Cancer Inst., 35(6): 949-958.
Argus, M.F., Sohal, R.S., Bryant, G.M., Hoch-Ligeti, C. and Arcos, J.C. (1973). Dose-response and ultrastructural alterations in dioxane carcinogenesis. influence of methylcholanthrene on acute toxicity. Eur. J. Cancer, 9(4): 237-240, IN1-IN3,241-243.
ATSDR (2012). Toxicological profile for 1,4-dioxane. Agency for Toxic Substances and Disease Registry, Public Health Service, US Department of Health and Human Services, Atlanta, Georgia.
Barber, H. (1934). Haemorrhagic nephritis and necrosis of the liver from dioxan poisoning. Guy's Hospital Reports, 84: 267-280.
BASF (1973). Unpublished report. May 4. [cited in European Commission, 2002].
Benigni, R. and Bossa, C. (2008). Structure alerts for carcinogenicity, and the salmonella assay system: A novel insight through the chemical relational databases technology. Mutat.Res., 659: 248-261.
Besenhofer, L.M., Adegboyega, R.A., Bartels, M., Filary, M.J., Perala, A.W., McLaren, M.C. and McMartin, K.E. (2012). Inhibition of metabolism of diethylene glycol prevents target organ toxicity in rats. Toxicol. Sci., 117(1): 25-35.
Black, R.E., Hurley, F.J. and Havery, D.C. (2001). Occurrence of 1,4-dioxane in cosmetic raw materials and finished cosmetic products. J. AOAC Int., 84(3): 666-670.
Bowman, R. (2003). Ozone-peroxide advanced oxidation water treatment system for treatment of chlorinated solvents and 1,4-dioxane. Paper presented at the Groundwater Association of California 9th Symposium on Groundwater Contaminants.
Braun, W.H. and Young, J.D. (1977). Identification of beta-hydroxyethoxyacetic acid as the major urinary metabolite of 1,4-dioxane in the rat. Toxicol. Appl. Pharmacol., 39(1): 33-38.
Bronaugh, R. (1982). Percutaneous absorption of cosmetic ingredients. In: Frost P, editor. Principles of cosmetics for the dermatologist. Minneapolis (MN): University of Minnesota Press. pp. 277-284.
Brown, S.K., Sim, M.R., Abramson, M.J. and Gray, C.N. (1994). Concentrations of volatile organic compounds in indoor air - a review. Indoor Air, 4: 123.
Buffler, P.A., Wood, S.M., Suarez, L. and Kilian, D.J. (1978). Mortality follow-up of workers exposed to 1,4-dioxane. J. Occup. Med., 20(4): 255-259.
Bull, R.J., Robinson, M. and Laurie, R.D. (1986). Association of carcinoma yield with early papilloma development in SENCAR mice. Environ. Health Perspect., 68: 11-17.
CalEPA (2014). 1,4-Dioxane groundwater information sheet. State Water Resources Control Board, Division of Water Quality, California Environmental Protection Agency, California.
CCME (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Canadian Council of Ministers of the Environment. Winnipeg, Manitoba. Available at: www.ccme.ca/files/Resources/water/source_tap/mba_guidance_doc_e.pdf.
CCME (2008). Canadian water quality guidelines for the protection of aquatic life: 1,4-dioxane. Canadian Council of Ministers of the Environment, Report No. 1299, Winnipeg, Canada.
Chitra, S., Paramasivan, K., Cheralathan, M. and Sinha, P.K. (2012). Degradation of 1,4-dioxane using advanced oxidation processes. Environ. Sci. Pollut. Res., 19: 871-878.
Civardi, J., Prosser, A., Gray, J.M., Marie, J. and Greising, K. (2014). Treatment of 1,4-dioxane using advance oxidation at Artesian Water Company’s Llangollen Wellfield. In: Proceedings of the American Water Works Association Water Quality and Technology Conference, New Orleans, Louisiana.
Coleman, H.M., Vimonses, V., Leslie, G. and Amal, R. (2007). Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes. J. Haz. Mat., 146: 496-501.
Collins, J., Biggs, J., Maseeh, G., Dettmer, J. and Cotton, C. (2014). Staying ahead of the curve: Tucson Water’s management and treatment of 1,4-dioxane. In: Proceedings of the American Water Works Association Water Quality and Technology Conference, New Orleans, Louisiana.
Cordone, L., Carlson, C., Plaehn, W., Shangraw, T. and Wilmouth. (2016). Case study and retrospective: aerobic fixed film biological treatment process for 1,4-dioxane at the Lowry Landfill Superfund Site. Remediation, 27(1): 159-172.
Daubert, T.E. and Danner, R.P. (1985). 1,4-Dioxane. In: Physical and thermodynamic properties of pure chemicals. 1,4-Dioxane. Taylor & Francis, New York, New York.
David, H. (1964). Electron-microscopic findings in dioxan-dependent nephrosis in rat kidneys. Beitr. Pathol. Anat., 130: 187-212.
de Navasquez, S. (1935). Experimental tubular necrosis of the kidneys accompanied by liver changes due to dioxane poisoning. J. Hyg., 35(4): 540-548.
Dennerlein, K., Schneider, D., Göen, T., Schaller, H.D., Drexler, H. and Korinth, G. (2013). Studies on percutaneous penetration of chemicals - Impact of storage conditions for excised human skin. Toxicol. In Vitro, 27(2): 708-713.
Dennerlein, K., Jäger, T., Göen, T., Kilo, S., Schaller, K.H., Drexler, H. and Korinth, G. (2015). Evaluation of the effect of skin cleaning procedures on the dermal absorption of chemicals. Toxicol. In Vitro, 29(5): 828-833.
DeRosa, C.T., Wilbur, S., Holler, J., Richter, P. and Stevens, Y.W. (1996). Health evaluation of 1,4-dioxane. Toxicol. Ind. Health, 12(1): 1-43.
DiGuiseppi, W., Walecka-Hutchinson, C. and Hutton, J. (2016).1,4-dioxane treatment technologies. Remediation, 27(1): 71-92.
Dourson, M., Reichard, J., Nance, P., Burleigh-Flayer, H., Parker, A., Vincent, M. and McConnell, E.E. (2014). Mode of action analysis for liver tumors from oral 1,4-dioxane exposures and evidence-based dose response assessment. Regul. Toxicol. Pharmacol., 68(3): 387-401.
Dourson, M., Higginbotham, J., Crum, J., Burleigh-Flayer, H., Nance, P., Forsberg, N., Lafranconi, M., Sassi, B. and Wambaugh, D. (2017). Update: Mode of action (MOA) for liver tumors induced by oral exposure to 1,4-dioxane. Regul. Toxicol. Pharmacol., 88:45-55. Available at: https://doi.org/10.1016/j.yrtph.2017.02.025
Draper, W.M., Dhoot, J.S., Remoy, J.W. and Perera, S.K. (2000). Trace level determination of 1,4-dioxane in water by isotopic dilution GC and GC-MS. Analyst, 125: 1403-1408.
Environment Canada (2008). Data for batch 7 substances collected under the Canadian Environmental Protection Act. Ottawa, Ontario.
Environment Canada and Health Canada (2010). Screening assessment for the challenge 1,4-dioxane. Ottawa, Ontario.
Environmental Review Tribunal of Ontario (2015). Proceedings of the Environmental Review Tribunal of Ontario: Concerned Citizens Committee of Tyendinaga and Environs v. Ontario (Environment and Climate Change) case no. 12-033. Proceeding commenced under section 41 of the Environmental Bill of Rights, 1993, S.O. 1993, c.28, as amended. Belleville and Tyendinaga Township, Ontario, Canada.
Ernstgard, L., Iregren, A., Sjogren, B. and Johanson, G. (2006). Acute effects of exposure to vapours of dioxane in humans. Hum. Exp. Toxicol., 25(12): 723-729.
European Commission (2002). European Union risk assessment report 1,4-dioxane. Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau, EINECS No: 204-661-8, The Netherlands.
Fairley, A. (1934). The toxicity to animals of 1:4 dioxan. J. Hyg., 34(4): 486-501.
Fellin, P. and Otson, R. (1997). Emissions of VPOC from residences in the Metropolitan Toronto area. In: Proceedings of the 90th Annual Meeting of the Air & Waste Management Association; 1997 June 8-13; Pittsburgh, Pennsylvania. [as cited in Environment Canada and Health Canada, 2010].
Fisher, J., Mahle, D., Bankston, L., Greene, R. and Gearhart, J. (1997). Lactational transfer of volatile chemicals in breast milk. Am. Ind. Hyg. Assoc. J., 58(6): 425-431.
Fuh, C.B., Lai, M., Tsai, H.Y. and Chang, C.M. (2005). Impurity analysis of 1,4-dioxane in nonionic surfactants and cosmetics using headspace solid-phase microextraction coupled with gas chromatography and gas chromatography-mass spectrometry. J. Chromatogr. A, 1071(1-2): 141-145.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B., Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., Rimpo, J., Margolin, B.H., Resnick, M.A., Anderson, B. and Zeiger, E. (1987). Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: Evaluations of 108 chemicals. Environ. Mol. Mutagen., 10(SUPPL. 10): 1-175.
GDCH (1991). 1,4-Dioxane BUA report 80. German Chemical Society [Gessellschaft Deutscher Chemiker]. Committee on Existing Chemicals of Environmental Relevance, Beratergremium für Umweltrelevante Alstoffe [BUA]. ISBN 3-7776-0600-6. Pp. 1-70.
Giavini, E., Vismara, C. and Broccia, M.L. (1985). Teratogenesis study of dioxane in rats. Toxicol. Lett., 26(1): 85-88.
Gi, M., Fujioka, M., Kakehashi, A., Okuno, T., Masumura, K., Nohmi, T., Matsumoto, M., Omori, M., Wanibuchi, H. and Fukushima, S. (2018). In vivo positive mutagenicity of 1,4-dioxane and quantitative analysis of its mutagenicity and carcinogenicity in rats. Arch. Toxicol., 92(10):3207-3221.
Goen, T., von Helden, F., Eckert, E., Knecht, U., Drexler, H. and Walter, D. (2016). Metabolism and toxicokinetics of 1,4-dioxane in humans after inhalational exposure at rest and under physical stress. Arch. Toxicol., 90: 1315-1324.
Golder Associates (1987). Testing of specific organic compounds in soils in background urban areas. Golder Associates, Oakville, Ontario. [as cited in Environment Canada and Health Canada, 2010].
Goldsworthy, T.L., Monticello, T.M., Morgan, K.T., Bermudez, E., Wilson, D.M., Jackh, R. and Butterworth, B.E. (1991). Examination of potential mechanisms of carcinogenicity of 1,4-dioxane in rat nasal epithelial cells and hepatocytes. Arch. Toxicol., 65(1): 1-9.
Haber, L.T., Maier, A., Kroner, O.L. and Kohrman, M.J. (2009). Assessment of human relevance and mode of action for tunica vaginalis mesotheliomas resulting from oral exposure to acrylamide. Regul. Toxicol. Pharmacol., 53(2): 134-149.
Hall, A.P., Elcome, C.R., Foster, J.R., Harada, T., Kaufman, W., Knippel, A., Küttler, K., Malarky, D.E., Maronpot, R.R., Nishikawa, A., Nolte, T., Schulte, A., Strauss, V. and York, M.J. (2012). Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes-conclusions from the 3rd International ESTP Expert Workshop. Toxicol Pathol. 40(7): 971-994.
Hansch, C., Leo, A. and Hoekman, D.H. (1995). Exploring QSAR: Hydrophobic, electronic, and steric constants, ACS professional reference book, Volume 2 of Exploring QSAR. Albert Leo (ed.). American Chemical Society.
Harkov, R., Kebbekus, B., Bozzelli, J.W. and Lioy, P.J. (1984). Measurement of selected volatile organic compounds at three locations in New Jersey during the summer season. J. Air Pollut. Control Assoc., 33(12): 1177.
Haworth, S. (1983). Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen., 5(SUPPL. 1): 3-142.
Health Canada (1994). Canadian Environmental Protection Act. Human health risk assessment for priority
substances. Minister of Supply and Services Canada, Ottawa, Ontario.
Health Canada (2016). Guidelines for Canadian drinking water quality: Guideline technical document — Bromate. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
Health Council of the Netherlands (2015). 1,4-Dioxane - re-evaluation of the carcinogenicity and genotoxicity. The Health Council of the Netherlands (Gezondheidsraad), Subcommittee on the Classification of Carcinogenic Substances of the Dutch Expert Committee on Occupational Safety, Report No. 2015/26, The Hague, the Netherlands.
Hellmér, L. and Bolcsfoldi, G. (1992). An evaluation of the E. coli K-12 uvrB/recA DNA repair host-mediated assay. I. in vitro sensitivity of the bacteria to 61 compounds. Mutat. Res. Environ. Mutagen. Relat. Subj., 272(2): 145-160.
Hoch-Ligeti, C. and Argus, M.F. (1970). Effect of carcinogens on the lung of guinea pigs. In: Morphology of experimental respiratory carcinogenesis: Proceedings of a Biology Division, Oak Ridge National Laboratory, conference held in Gatlinburg, Tennessee, May 13-16, 1970. 21st. United States Atomic Energy Comission, Division of Technical Information, Oak Ridge, Tennessee, pp. 267-279.
Hoch-Ligeti, C., Argus, M.F. and Arcos, J.C. (1970). Induction of carcinomas in the nasal cavity of rats by dioxane. Br. J. Cancer, 24(1): 165-167.
HSDB (2015). 1,4-Dioxane. Hazardous substances database. Hazardous Substances Data Bank. Available at : http://toxnet.nlm.nih.gov
IARC (1999). IARC monographs on the evaluation of carcinogenic risks to humans. Re-evaluation of some organic chemicals, hydrazine, and hydrogen peroxide. International Agency for Research on Cancer, World Health Organization, Volume 71, Lyon, France.
Ikehata, K., Wang-Staley, L., Qu, Xiaoyan, Q. and Li, Y. (2016). Treatment of groundwater contaminated with 1,4-dioxane, tetrahydrofuran, and chlorinated volatile organic compounds using advance oxidation processes. Ozone: Sci. Eng., 38(6): 413-424.
Isaacson, C., Mohr, T.K. and Field, J.A. (2006). Quantitative determination of 1,4-dioxane and tetrahydrofuran in groundwater by solid-phase extraction GC/MS/MS. Environ. Sci. Technol., 40(23): 7305-7311.
Itoh, S., and Hattori, C. (2019). In vivo genotoxicity of 1,4-dioxane evaluated by liver and bone marrow micronucleus tests and Pig-a assay in rats. Mutat. Res. Genet. Toxicol. Mutagen., 837:8-14.
JBRC (1998). Laboratory report: Two-year studies of 1,4-dioxane in F344 rats and BDF1 mice (drinking water). Japan Bioassay Research Center, Kanagawa, Japan.
Johnstone, R.T. (1959). Death due to dioxane? AMA Arch. Ind. Health., 20: 445-447.
Kanada, M., Miyagawa, M., Sato, M., Hasegawa, H. and Honma, T. (1994). Neurochemical profile of effects of 28 neurotoxic chemicals on the central nervous system in rats. (1) effects of oral administration on brain contents of biogenic amines and metabolites. Ind. Health, 32(3): 145-164.
Kano, H., Umeda, Y., Saito, M., Senoh, H., Ohbayashi, H., Aiso, S., Yamazaki, K., Nagano, K. and Fukushima, S. (2008). Thirteen-week oral toxicity of 1,4-dioxane in rats and mice. J. Toxicol. Sci., 33(2): 141-153.
Kano, H., Umeda, Y., Kasai, T., Sasaki, T., Matsumoto, M., Yamazaki, K., Nagano, K., Arito, H. and Fukushima, S. (2009). Carcinogenicity studies of 1,4-dioxane administered in drinking-water to rats and mice for 2 years. Food Chem. Toxicol., 47(11): 2776-2784.
Kasai, T., Saito, M., Senoh, H., Umeda, Y., Aiso, S., Ohbayashi, H., Nishizawa, T., Nagano, K. and Fukushima, S. (2008). Thirteen-week inhalation toxicity of 1,4-dioxane in rats. Inhal. Toxicol., 20(10): 961-971.
Kasai, T., Kano, H., Umeda, Y., Sasaki, T., Ikawa, N., Nishizawa, T., Nagano, K., Arito, H., Nagashima, H. and Fukushima, S. (2009). Two-year inhalation study of carcinogenicity and chronic toxicity of 1,4-dioxane in male rats. Inhal. Toxicol., 21(11): 889-897.
Kesten, H.D., Mulinos, M.G. and Pomerantz, L. (1939). Pathologic effects of certain glycols and related compounds. Arch. Pathol., 27: 447-465.
Khudoley, V.V., Mizgireuv, I. and Pliss, G.B. (1987). The study of mutagenic activity of carcinogens and other chemical agents with Salmonella typhimurium assays: Testing of 126 compounds. Arch. Geschwulstforsch., 57(6): 453-462.
King, M.E., Shefner, A.M. and Bates, R.R. (1973). Carcinogenesis bioassay of chlorinated dibenzodioxins and related chemicals. Environ. Health Perspect., 5: 163-170.
Kitchin, K.T. and Brown, J.L. (1990). Is 1,4-dioxane a genotoxic carcinogen? Cancer Lett., 53(1): 67-71.
Kociba, R.J., McCollister, S.B., Park, C., Torkelson, T.R. and Gehring, P.J. (1974). 1,4-Dioxane. I. results of a 2-year ingestion study in rats. Toxicol. Appl. Pharmacol., 30(2): 275-286.
Kociba, R.J., Torkelson, T.R., Young, J.D. and Gehring, P.J. (1975). 1,4-Dioxane: Correlation of the results of chronic ingestion and inhalation studies with its dose-dependent fate in rats. In: Proceedings of the 6th annual Conference on Environmental Toxicology. Aerospace Medical Research Laboratory, Aerospace Medical Division, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio, p. 345.
Koissi, N., Shah, N.H., Ginevan, B., Eck, W.S., Roebuck, B.D. and Fishbein, J.C. (2012). Lactone metabolite common to the carcinogens dioxane, diethylene glycol, and N-nitrosomorpholine: Aqueous chemistry and failure to mediate liver carcinogenesis in the F344 rat. Chem. Res. Toxicol., 25(5): 1022-1028.
Krishnan, K. (2004). Development of a two tier approach for evaluating the relevance of multi-route exposures in establishing drinking water goals for volatile organic chemicals. Montreal, Quebec. Final report submitted to the Water Quality and Health Bureau, Safe Environment Programs, Health Canada, Ottawa, Ontario.
Krishnan, K. and Carrier, R. (2008). Approaches for evaluating the relevance of multiroute exposures in establishing guideline values for drinking water contaminants. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev., 26(3): 300-316.
Kurl, R.N., Poellinger, L., Lund, J. and Gustafsson, J. (1981). Effects of dioxane on RNA synthesis in the rat liver. Arch. Toxicol., 49(1): 29-33.
Kwan, K.K., Dutka, B.J., Rao, S.S. and Liu, D. (1990). Mutatox test: A new test for monitoring environmental genotoxic agents. Environ. Pollut., 65(4): 323-332.
Lane, R.W., Riddle, B.L. and Borzelleca, J.F. (1982). Effects of 1,2-dichloroethane and l,l, I-trichloroethane in drinking water on reproduction and development in mice. Toxicol. Appl. Pharmacol. 63: 409-412.
Laug, E.P., Calvery, H.O., Morris, H.J. and Woodard, G. (1939). The toxicology of some glycols and derivatives. J. Ind. Hyg. Toxicol., 21(6): 173-201.
Lesage, S., Jackson, R.E., Priddle, M.W. and Riemann, P.G. (1990). Occurrence and fate of organic solvent residues in anoxic groundwater at the Gloucester landfill, Canada. Environ. Sci. Technol., 24(4): 559-566.
Leung, H.W. and Paustenbach, D.J. (1990). Cancer risk assessment for dioxane based upon a physiologically-based pharmacokinetic approach. Toxicol. Lett., 51(2): 147.
Li, M., Conlon, P., Fiorenza, S., Vitale, R.J. and Alvarez, P.J.J. (2011). Rapid analysis of 1,4-dioxane in groundwater by frozen microextraction with gas chromatography/mass spectrometry. Ground Water Monit. Remed., 31(4): 70-76.
Liang, S., Morton, R., Tang, C-C., Barry, J., Knapp, T. and Smal, N. (2011). Pilot study of advanced water treatment processes to purify secondary effluent. In: Proceedings of the American Water Works Association Water Quality and Technology Conference, Phoenix, Arizona.
Lundberg, I., Hogberg, J., Kronevi, T. and Holmberg, B. (1987). Three industrial solvents investigated for tumor promoting activity in the rat liver. Cancer Lett., 36(1): 29-33.
Makino, R., Kawasaki, H., Kishimoto, A., Gamo, M. and Nakanishi, J. (2006). Estimating health risk from exposure to 1,4-dioxane in Japan. Environ. Sci., 13(1): 43-58.
Marzulli, F.N., Anjo, D.M. and Maibach, H.I. (1981). In vivo skin penetration studies of 2,4-toluenediamine, 2,4-diaminoanisole, 2-nitro-p-phenylenediamine, p-dioxane and N-nitrosodiethanolamine in cosmetics. Food Cosmet. Toxicol., 19(6): 743-747.
Masuda, H., McClay, K., Steffan, R. and Zylstra, G. (2012). Biodegradation of tetrahydrofuran and 1,4-dioxane by soluble diiron monooxygenase-containing bacteria. Environ. Sci. Technol, 41(21): 7330-7336.
Maurino, V., Calza, P., Minero, C., Pelizzetti, E. and Vincenti, M. (1997). Light-assisted 1,4-dioxane degradation. Chemosphere; 35(11): 2675.
McElroy, A., Hyman, M., Smith, C. and Knappe, D. (2015). Biodegradation of 1,4-dioxane at drinking water-relevant concentrations. In: Proceedings of the American Water Works Association Water Quality and Technology Conference, Salt Lake City, Utah.
McFee, A.F., Abbott, M.G., Gulati, D.K. and Shelby, M.D. (1994). Results of mouse bone marrow micronucleus studies on 1,4-dioxane. Mutat. Res., 322(2): 145-148.
McGregor, D.B., Brown, A.G., Howgate, S., McBride, D., Riach, C. and Caspary, W.J. (1991). Responses of the L5178Y mouse lymphoma cell forward mutation assay. V: 27 coded chemicals. Environ. Mol. Mutagen., 17(3): 196-219.
McGuire, M.J., Suffet, I.M. and Radziul, J.V. (1978). Assessment of unit processes for the removal of trace organic compounds from drinking water. J. Am. Water Works, 70(10): 565-572.
Meek, M.E., Palermo, C.M., Bachman, A.N., North, C.M. and Lewis, R.J. (2014). Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J. Appl. Toxicol., 34(6): 595-606.
Merayo, N., Hermosilla, D., Cortijo, L. and Blanco, A. (2014) Optimization of the Fenton treatment of 1,4-dioxane and on-line FTIR monitoring of the reaction. J. Haz. Mat., 268: 102-109.
Mirkova, E.T. (1994). Activity of the rodent carcinogen 1,4-dioxane in the mouse bone marrow micronucleus assay. Mutat. Res., 322(2): 142-144.
Miyagawa, M., Shirotori, T., Tsuchitani, M. and Yoshikawa, K. (1999). Repeat-assessment of 1,4-dioxane in a rat-hepatocyte replicative DNA synthesis (RDS) test: Evidence for stimulus of hepatocyte proliferation. Exp. Toxicol. Pathol., 51(6): 555-558.
Mohr, T.K.G. (2001). 1,4-Dioxane and other solvent stabilizers white paper. Santa Clara Valley Water District of California. San Jose, California.
Mohr, T.K, Stickney, J.A, and DiGuiseppi, W.H. (2010). Environmental investigation and remediation: 1,4-dioxane and other solvent stabilizers. Boca Raton, Florida. CRC Press.
Mohr, T.K. (2012). 1,4-Dioxane occurrences and treatment options in private wells. US EPA Technology News and Trends Newsletter. EPA 542-N-12-003 Issue No. 59, June. Available at: https://clu-in.org/download/ newsltrs/tnandt0612.pdf
Morita, T. and Hayashi, M. (1998). 1,4-Dioxane is not mutagenic in five in vitro assays and mouse peripheral blood micronucleus assay, but is in mouse liver micronucleus assay. Environ. Mol. Mutagen., 32(3): 269-280.
Mungikar, A.M. and Pawar, S.S. (1978). Induction of the hepatic microsomal mixed function oxidase system in mice by p-dioxane. Bull. Environ. Contam. Toxicol., 20: 797-804.
Munoz, E.R. and Mazar Barnett, B. (2002). The rodent carcinogens 1,4-dioxane and thiourea induce meiotic non-disjunction in drosophila melanogaster females. Mutat. Res., 517(1-2): 231-238.
Metropolitan Water District of Southern California (2012). Joint water purification pilot program: pilot study of advanced treatment processes to recycle JWPCP secondary effluent. Metropolitan Water District of Southern California, September 28, 2012. Available at: www.mwdh3o.com/PDF_About_Your_Water/Pilot_Study.pdf
Ministère du Développement durable, de l’Environnement et de la Lutte aux Changements Climatiques (2016). Personal communication with C. Robert.
Nannelli, A., De Rubertis, A., Longo, V. and Gervasi, P.G. (2005). Effects of dioxane on cytochrome P450 enzymes in liver, kidney, lung and nasal mucosa of rat. Arch. Toxicol., 79(2): 74-82.
Nelson, N. (1951). Solvent toxicity with particular reference to certain octyl alcohols and dioxanes. Med. Bull., 11(2): 226-238.
Nestmann, E.R., Otson, R., Kowbel, D.J., Bothwell, P.D. and Harrington, T.R. (1984). Mutagenicity in a modified Salmonella assay of fabric-protecting products containing 1,1,1-trichloroethane. Environ. Mutagen., 6(1): 71-80.
NICNAS (1998). 1,4-Dioxane priority existing chemical no. 7, full public report. National Industrial Chemicals Notification and Assessment Scheme, Commonwealth of Australia, Canberra, Australia.
NIOSH (1997). Pocket guide to chemical hazards. National Institute for Occupational Safety and Health, U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Cincinnati, Ohio.
Nishimura, T., Iizuka, S., Kibune, N., Ando, M. and Magara, Y. (2005). Study of 1,4-dioxane intake in the total diet. J. Health Sci., 51(4): 514.
NPRI (2016). National Pollutant Release Inventory. Environment Canada, Gatineau, Quebec. Available at: http://ec.gc.ca/inrp-npri/donnees-data/index.cfm?do=query&lang=en
NSF/ANSI (2019a). Standard 58: Reverse osmosis drinking water treatment systems. NSF International/American National Standards Institute. NSF International, Ann Arbor, Michigan.
NSF/ANSI (2019b). Standard 53: Drinking water treatment units - health effects. NSF International/American National Standards Institute. NSF International, Ann Arbor, Michigan.
NTP (1978). Bioassay of 1,4-dioxane for possible carcinogenicity. National Toxicology Program. Natl. Cancer. Inst. Carcinog. Tech. Rep. Ser., 80: 1-123.
NTP (2011). 1,4-Dioxane. National toxicology program report on carcinogens, 12: 176-178.
OECD (2014). Test no. 474: Mammalian erythrocyte micronucleus test. OECD guidelines for the testing of chemicals, section 4. Health effects. Organisation for Economic Co-operation and Development, TG 474.
OECD (2015). The OECD (Q)SAR toolbox v3.3.5 - in vivo mutagenicity (micronucleus) alerts profiler by the Instituto Superiore di Sanita (ISS). Organisation for Economic Co-operation and Development.
Ontario Ministry of the Environment (2016). Personal communication with S. Deshpande.
Orange County Water District (2015). Groundwater replenishment system 2014 annual report. Orange County Water District, California Regional Water Quality Control Board, Santa Ana Region.
Park, J.H., Hussam, A., Couasnon, P., Fritz, D. and Carr, P.W. (1987). Experimental reexamination of selected partition coefficients from Rohrschneider's data set. Anal. Chem., 59(15): 1970-1976.
Patty (1994). Patty’s industrial hygiene and toxicology, Toxicology. Volume II. 4th Edition. George D. Clayton and Florence E. Clayton, Eds. Wiley. University of Minnesota, Minneapolis, Minnesota.
Pellizzari, E.D., Hartwell, T.D., Perritt, H.R., Sparacino, C.M., Sheldon, L.S., Zelon, H.S. and Whitmore, R.W. (1986). Comparison of indoor and outdoor residential levels of volatile organic chemicals in five U.S. geographical areas. Environ. Int., 12: 619.
Pozzani, U.C., Weil, C.S. and Carpenter, C.P. (1959). The toxicological basis of threshold limit values. 5: The experimental inhalation of vapor mixtures by rats, with notes upon the relationship between single dose inhalation and single dose oral data. Am. Ind. Hyg. Assoc. J., 20: 364-369.
Ramsey, J.C. and Andersen, M.E. (1984). A physiologically based description of the inhalation pharmacokinetics of styrene in rats and humans. Toxicol. Appl. Pharmacol., 73(1): 159-175.
Region of Waterloo (2005). Greenbrook wellfield assessment, report no. E-05-017. Water Services, Transportation and Environmental Services of the Region of Waterloo. Kitchener-Waterloo, Ontario, Canada.
Reitz, R.H., McCroskey, P.S., Park, C.N., Andersen, M.E. and Gargas, M.L. (1990). Development of a physiologically based pharmacokinetic model for risk assessment with 1,4-dioxane. Toxicol. Appl. Pharmacol., 105(1): 37-54.
Robinson, J.J. and Ciurczak, E.W. (1980). Direct gas chromatographic determination of 1,4-dioxane in ethoxylated surfactants. J. Soc. Cosmet. Chem., 31: 329.
Roccaro, J., Brandt, P., Meyerdierks, S., Rosenfeldt, E. and Royce, A. (2012). Advanced oxidation for 1,4-dioxane and VOC removal from drinking water: results of a demonstration-scale pilot study. In: Proceedings of the American Water Works Association Water Quality and Technology Conference, Toronto, Canada.
Rodriguez, C., Van Buynder, P., Lugg, R., Blair, P., Devine, B., Cook, A. and Weinstein, P. (2009). Indirect potable water reuse: a sustainable water supply alternative. Int. J. Environ. Res. Public Health, 6: 1174-1209.
Rosenkranz, H.S. and Klopman, G. (1992). 1,4-Dioxane: Prediction of in vivo clastogenicity. Mutat. Res., 280(4): 245-251.
Roy, S.K., Thilagar, A.K. and Eastmond, D.A. (2005). Chromosome breakage is primarily responsible for the micronuclei induced by 1,4-dioxane in the bone marrow and liver of young CD-1 mice. Mutat. Res., 586(1): 28-37.
Sasaki, Y.F., Sekihashi, K., Izumiyama, F., Nishidate, E., Saga, A., Ishida, K., and Tsuda, S. (2000). The comet assay with multiple mouse organs: comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and U.S. NTP Carcinogenicity Database. Crit. Rev. Toxicol.,30(6):629-799.
Scalia, S. and Menegatti, E. (1991). Assay of 1,4-dioxane in commercial cosmetic products by HPLC. Farmaco, 46(11): 1365-1370.
SCC (2020). Directory of accredited product, process and service certification bodies. Standards Council of Canada, Ottawa, Ontario. Available at: www.scc.ca/en/accreditation/product-process-and-service-certification/directory-of-accredited-clients
Schoonenberg-Kegel, F. S., Rietman, B. M. and Verliefde, A. R. D. (2010). Reverse osmosis followed by activated carbon filtration for efficient removal of organic micropollutants from river bank filtrate. Water Sci. Technol., 61(10): 2603.
Schrenk, H.H. and Yant, W.P. (1936). Toxicity of dioxan. J. Ind. Hyg.Toxicol., 18(7): 448-460.
Sekar, R. and DiChristina, T.J. (2014). Microbially driven Fenton reaction for degradation of the widespread environmental contaminant 1,4-dioxane. Environ. Sci. Technol., 48: 12858-12867.
Shah, J.J. and Singh, H.B. (1988). Distribution of volatile organic chemicals in outdoor and indoor air: A national VOCs data base. Environ. Sci. Technol., 22(12): 1381-1388.
Sheu, C.W., Moreland, F.M., Lee, J.K. and Dunkel, V.C. (1988). In vitro BALB/3T3 cell transformation assay of nonoxynol-9 and 1,4-dioxane. Environ. Mol. Mutagen., 11(1): 41-48.
Silverman, L., Schulte, H.F. and First, M.W. (1946). Further studies on sensory response to certain industrial solvent vapors. J. Ind. Hyg. Toxicol., 28(6): 262-266.
Simonich, S.M., Sun, P., Casteel, K., Dyer, S., Wernery, D., Garber, K., Carr, G., and Federle, T. (2013). Probabilistic analysis of risks to U.S. drinking water intakes from 1,4-dioxane in domestic wastewater treatment plant effluents. Integr. Environ. Assess. Manag., 9(4): 554-559.
Sina, J.F., Bean, C.L., Dysart, G.R., Taylor, V.I. and Bradley, M.O. (1983). Evaluation of the alkaline elution/rat hepatocyte assay as a predictor of carcinogenic/mutagenic potential. Mutat. Res. Environ. Mutagen. Relat. Subj., 113(5): 357-391.
Smyth, H.F., Jr, Seaton, J. and Fischer, L. (1941). The single dose toxicity of some glycols and derivatives. J. Ind. Hyg.Toxicol., 23: 259-268.
Son, H-S., Im, J-K. and Zoh, K-D. (2009). A Fenton-like degradation mechanism for 1,4-dioxane using zero-valent iron (FeO) and UV light. Water Res., 43: 1457-1463.
Stanfill, J.C., Koon, J., Shangraw, T. and Bollmann, D. (2004). 1,4-Dioxane bio-degradation bench study at the Lowry Landfill Superfund Site. In: Proceedings of the Water Environment Federation, 10th Annual Industrial Wastes Technical and Regulatory Conference, WEF/A&WMA Industrial Wastes, 14: 420-433.
Stantec (2014). Water supply master plan update. Regional Municipality of Waterloo, Transportation and Environmental Services Department, prepared by Stantec Consulting Ltd., Project No. 1611 11001, Kitchener, Ontario.
Stefan, M. I. and Bolton, J. R. (1998). Mechanism of the degradation of 1,4-dioxane in dilute aqueous solution using the UV/hydrogen peroxide process. Environ. Sci. Technol., 32(11): 1588-1595.
Stepien, D.K., Diehl, P., Helm, J., Thoms, A. and Putmann, W. (2014). Fate of 1,4-dioxane in the aquatic environment: from sewage to drinking water. Water Res., 48(1): 406-419.
Stoner, G.D., Conran, P.B., Greisiger, E.A., Stober, J., Morgan, M. and Pereira, M.A. (1986). Comparison of two routes of chemical administration on the lung adenoma response in strain A/J mice. Toxicol. Appl. Pharmacol., 82(1): 19-31.
Stott, W.T., Quast, J.F. and Watanabe, P.G. (1981). Differentiation of the mechanisms of oncogenicity of 1,4-dioxane and 1,3-hexachlorobutadiene in the rat. Toxicol. Appl. Pharmacol., 60(2): 287-300.
Strout, K., Hedin, C., Zimmerman, M. and Smith, T. (2004). Vacuum distillation unit interlaboratory study evaluation. Paper presented at the 20th Annual National Environmental Monitoring Conference, July 19-22, Independent Laboratory Institute, Washington, DC.
Summers, S.R., Kennedy, A.M., Knappe, D.R.U., Reiner, A.M., Fotta, M.E., Mastropole, a.J., Corwin, C.J. and Roccaro, J. (2014). Evaluation of available scale-up approaches for the design of GAC contactors. Water Research Foundation, Denver, Colorado.
Sun, M., Lopez-Velandia, C. and Knappe, D.R.U. (2016). Determination of 1,4-dioxane in the Cape Fear River Watershed by heated purge-and-trap preconcentration and gas chromatography-mass spectrometry. Environ. Sci. Technol., 50: 2246-2254.
Suzuki M., Noguchi T., Noda K., Fukuda K. and Matsushima T. (1995). Rat liver micronucleus test with organic solvents (Japanese). 24th JEMS, Osaka, Japan, p. 131 (Abstract). [cited in: Morita and Hayashi (1998)].
Sweeney, L.M., Thrall, K.D., Poet, T.S., Corley, R.A., Weber, T.J., Locey, B.J., Clarkson, J., Sager, S. and Gargas, M.L. (2008). Physiologically based pharmacokinetic modeling of 1,4-dioxane in rats, mice, and humans. Toxicol. Sci., 101(1): 32-50.
Tahara, M., Obama, T. and Ikarashi, Y. (2013). Development of analytical method for determination of 1,4-dioxane in cleansing products. Int. J. Cosmet. Sci., 35(6): 575-580.
Takano, R., Murayama, N., Horiuchi, K., Kitajima, M., Shono, F. and Yamazaki, H. (2010). Blood concentrations of 1,4-dioxane in humans after oral administration extrapolated from in vivo rat pharmacokinetics, in vitro human metabolism, and physiologically based pharmacokinetic modeling. J. Health Sci., 56(5): 557-565.
Take, M., Ohnishi, M., Yamamoto, S., Matsumoto, M., Nagano, K. and Fukushima, S. (2012). Distribution of 1,4-dioxane by combined inhalation plus oral exposure routes in rats. Int. J. Environ. Anal. Chem., 92(15): 1715-1728.
Tanabe, A. and Kawata, K. (2008). Determination of 1,4-dioxane in household detergents and cleaners. J. AOAC Int., 91(2): 439-444.
Thiess, A.M., Tress, E. and Fleig, E. (1976). Examination results of persons exposed to dioxan [Arbeitsmedizinische Untersuchungsergebnisse Von Dioxan Exponier Ten Mitarbeitern]. Arbeitsmedizin, Sozialmedizin, Präventivmedizin, 11(2): 36-46.
Tinwell, H. and Ashby, J. (1994). Activity of 1,4-dioxane in mouse bone marrow micronucleus assays. Mutat. Res., 322(2): 148-150.
UNEP (2000). The Montreal Protocol on substances that deplete the ozone layer as either adjusted and/or amended in London 1990, Copenhagen 1992, Vienna 1995, Montreal 1997, Beijing 1999. Ozone Secretariat, United Nations Environment Programme, Nairobi, Kenya.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T. and Yoshikawa, K. (1994). An in vivo-in vitro replicative DNA synthesis (RDS) test using rat hepatocytes as an early prediction assay for nongenotoxic hepatocarcinogens screening of 22 known positives and 25 noncarcinogens. Mutat. Res. Genet. Toxicol., 320(3): 189-205.
USDA (2010). Studies on metabolism of 1,4-dioxane. United States Department of the Army, 87-XE-08WR-09, Aberdeen Proving Ground, Maryland.
US EPA (1996). Method 8260B: Volatile organic compounds by gas chromatography/mass spectrometry (GC/MS), revision 2. United States Environmental Protection Agency, Washington, D.C.
US EPA (2000a). Estimation programs interface suite for Microsoft Windows, version 3.11.
US EPA (2000b). Method 8015C: Nonhalogenated organics by gas chromatography, revision 3. United States Environmental Protection Agency, Washington, D.C.
US EPA (2006) Treatment technologies for 1,4-dioxane: fundamentals and field applications. United States Environmental Protection Agency, Washington, D.C. EPA-542-R-06-009.
US EPA (2008). Method 522: Determination of 1,4-dioxane in drinking water by solid phase extraction (SPE) and gas chromatography/mass spectrometry (GC/MS) with selected ion monitoring (SIM). EPA/600-R-08/101. National Exposure Research Laboratory, Office of Research and Development, United States Environmental Protection Agency, Cincinnati, Ohio.
US EPA (2009). IRIS toxicological review and summary documents for 1,4-dioxane. United States Environmental Protection Agency, External Review Draft, Washington, D.C.
US EPA (2012a). UCMR 3 laboratory approval requirements and information document, version 2.0. Technical Support Center, Standards and Risk Management Division, Office of Ground Water and Drinking Water, U.S. Environmental Protection Agency, Cincinatti, Ohio.
US EPA (2012b). 40 CFR Parts 141 and 142. Revisions to the unregulated contaminant monitoring regulation (UCMR 3) for public water systems. Federal Register, 77(85): 26084-26085.
US EPA (2013). Toxicological review of 1,4-dioxane (with inhalation update) (CAS no. 123-91-1) in support of summary information on the integrated risk information system (IRIS). United States Environmental Protection Agency, EPA/635/R-11/003F, Washington, D.C.
US EPA (2014). Technical fact sheet - 1,4-dioxane. United States Environmental Protection Agency, EPA-505-F-14-011.
US EPA (2015a). TSCA work plan chemical problem formulation and initial assessment. 1,4-Dioxane. CASRN: 123-91-1. Office of Chemical Safety and Pollution Prevention, United States Environmental Protection Agency, EPA Document # 740-R1-5003.
US EPA (2015b). Method 541: Determination of 1-butanol, 1,4-dioxane, 2-methoxyethanol, and 2-propen-1-ol in drinking water by solid phase extraction and gas chromatography/mass spectrometry. EPA 815-R-15-011. Office of Water, United States Environmental Protection Agency, Cincinnati, Ohio.
US EPA (2017). Unregulated contaminant monitoring rule 3 (UCMR3) occurrence data. Available at: www.epa.gov/dwucmr/occurrence-data-unregulated-contaminant-monitoring-rule#3
U.S. Pharmacopeial Convention (2008). Food chemicals codex, 6th edition. Rockville, Maryland.
van Delft, J.H.M., van Agen, E., van Breda, S.G.J., Herwijnen, M.H., Staal, Y.C.M. and Kleinjans, J.C.S. (2004). Discrimination of genotoxic from non-genotoxic carcinogens by gene expression profiling. Carcinogenesis, 25(7): 1265-1276.
von Gunten, U. (2003). Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res., 37: 1443-1467.
Wang, H., Bakheet, B., Yuan, S., Li, X., Yu, G., Murayama, S. and Wang, Y. (2015). Kinetics and energy efficiency for the degradation of 1,4-dioxane by electro-peroxone process. J. Haz. Mat., 294: 90-98.
Waste Management (2016a). WM notice to residents, WM Richmond Landfill, Town of Greater Napanee. Communication dated January 4. Available at: http://brec-currentops.wm.com/about-us/reports.jsp
Waste Management (2016b). WM notice to interested parties, WM Richmond Landfill, Town of Greater Napanee. Communications dated March 24, April 7 and May 25. Available at: http://brec-currentops.wm.com/about-us/reports.jsp
Water Research Foundation (2014). 1,4-Dioxane white paper. Denver, Colorado.
WHO (2005). 1,4-Dioxane in drinking water. Background document for development of WHO guidelines for drinking-water quality. WHO/SDE/WSH/05.08/120. World Health Organization, Geneva, Switzerland.
Wirth, W. and Klimmer, O. (1936). [On the toxicology of organic solvents. 1,4 dioxane (diethylene dioxide)]. Archiv Fuer Gewerbepathologie Und Gewerbehygiene, 17: 192-206.
Woo, Y., Arcos, J.C., Argus, M.F., Griffin, G.W. and Nishiyama, K. (1977a). Structural identification of p-dioxane-2-one as the major urinary metabolite of p-dioxane. Naunyn Schmiedebergs Arch. Pharmacol., 299(3): 283-287.
Woo, Y., Argus, M.F. and Arcos, J.C. (1977b). Tissue and subcellular distribution of 3H-dioxane in the rat and apparent lack of microsome-catalyzed covalent binding in the target tissue. Life Sci., 21(10): 1447-1456.
Woo, Y., Argus, M.F. and Arcos, J.C. (1977c). Metabolism in vivo of dioxane: Effect of inducers and inhibitors of hepatic mixed-function oxidases. Biochem. Pharmacol., 26(16): 1539-1542.
Woodard, S., Mohr, T. and Nickelsen, M.G. (2014). Synthetic media: a promising treatment technology for 1,4-dioxane. Remediation, 24(4): 27-40.
Yamamoto, S., Urano, K., Koizumi, H., Wakana, S., Hioki, K., Mitsumori, K., Kurokawa, Y., Hayashi, Y. and Nomura, T. (1998a). Validation of transgenic mice carrying the human prototype c-Ha-ras gene as a bioassay model for rapid carcinogenicity testing. Environ. Health Perspect., 106(SUPPL. 1): 57-69.
Yamamoto, S., Urano, K. and Nomura, T. (1998b). Validation of transgenic mice harboring the human prototype c-Ha-ras gene as a bioassay model for rapid carcinogenicity testing. Toxicol. Lett., 102-103: 473-478.
Yamazaki, K., Ohno, H., Asakura, M., Narumi, A., Ohbayashi, H., Fujita, H., Ohnishi, M., Katagiri, T., Senoh, H., Yamanouchi, K., Nakayama, E., Yamamoto, S., Noguchi, T., Nagano, K., Enomoto, M. and Sakabe, H. (1994). Two-year toxicological and carcinogenesis studies of 1,4-dioxane in F344 rats and BDF1 mice. In: Proceedings of the Second Asia-Pacific Symposium on Environmental and Occupational Health 22-24 July, 1993. Sumino, K., Sato, S. and Shinkokai, N.G. (eds.). Kobe University School of Medicine, International Center for Medical Research, Kobe, Japan, pp. 193-198.
Yangali-Quintanilla, V., Maeng, S.K., Fujioka, T., Kennedy, M. and Amy, G. (2010) Proposing nanofiltration as acceptable barrier for organic contaminants in water reuse. J. Membr. Sci., 362: 334.
Yant, W.P., Schrenk, H.H., Waite, C.P. and Patty, F.A. (1930). Acute response of guinea pigs to vapors of some new commercial organic compounds: VI. Dioxan. Public Health Rep., 45: 2023-2032.
Yoon, J.S., Mason, J.M., Valencia, R., Woodruff, R.C. and Zimmering, S. (1985). Chemical mutagenesis testing in drosophila. IV. results of 45 coded compounds tested for the national toxicology program. Environ. Mutagen., 7(3): 349-367.
Young, J.D., Braun, W.H., Gehring, P.J., Horvath, B.S. and Daniel, R.L. (1976). 1,4-Dioxane and beta-hydroxyethoxyacetic acid excretion in urine of humans exposed to dioxane vapors. Toxicol. Appl. Pharmacol., 38(3): 643-646.
Young, J.D., Braun, W.H., Rampy, L.W., Chenoweth, M.B. and Blau, G.E. (1977). Pharmacokinetics of 1,4-dioxane in humans. J. Toxicol. Environ. Health, 3(3): 507-520.
Young, J.D., Braun, W.H. and Gehring, P.J. (1978a). The dose-dependent fate of 1,4-dioxane in rats. J. Environ. Pathol. Toxicol., 2(2): 263-282.
Young, J.D., Braun, W.H. and Gehring, P.J. (1978b). Dose-dependent fate of 1,4-dioxane in rats. J. Toxicol. Environ. Health, 4(5-6): 709-726.
Zenker, M., Borden, R.C. and Barlaz, M.A. (2003). Occurrence and treatment of 1,4-dioxane in aqueous environments. Environ. Eng. Sci., 20: 423-431.
Zenker, M., Borden, R. and Barlaz, M.A. (2004). Biodegradation of 1,4-dioxane using trickling filter. J. Environ. Eng., 130(9): 926-931.
Zimmermann, F.K., Mayer, V.W., Scheel, I. and Resnick, M.A. (1985). Acetone, methyl ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic solvents are strong inducers of aneuploidy in saccharomyces cerevisiae. Mutat. Res. Fundam. Mol. Mech. Mutagen., 149(3): 339-351.
Appendix A: List of abbreviations
- 1,1-DCE
- 1,1-dichloroethene
- ALT
- alanine aminotransferase
- ANSI
- American National Standards Institute
- AOP
- advanced oxidation process
- BMD
- benchmark dose
- BMDL
- lower confidence limit on the benchmark dose
- BMDL5
- lower 95% confidence limit on the benchmark dose for a 5% response
- bw
- body weight
- CYP
- cytochrome P450 enzyme
- DEG
- diethylene glycol
- DEN
- diethylnitrosamine
- DL
- detection limit
- EED
- electrical energy dose
- FID
- flame ionization detection
- GAC
- granular activated carbon
- GC
- gas chromatography
- GGT
- gamma-gluramyltransferase
- gpm
- gallons per minute
- HBV
- health-based value
- HEAA
- β-hydroxyethoxy acetic acid
- JBRC
- Japan Bioassay Research Centre
- LCMRL
- lowest concentration minimum reporting limit
- L-eq
- litre equivalents
- LOAEL
- lowest-observable-adverse-effect level
- LPHO
- low-pressure high output
- MAC
- maximum acceptable concentration
- MCL
- Maximum Contaminant Level
- MDL
- method detection limit
- MOA
- mode of action
- MRL
- minimum reporting level
- MS
- mass spectrometry
- NCI
- National Cancer Institute
- NF
- nanofiltration
- NOAEL
- no-observed-adverse-effect level
- NPRI
- National Pollutant Release Inventory
- NSF
- International Standard
- PBPK
- physiologically-based pharmacokinetic
- ppm
- parts per million
- RO
- reverse osmosis
- SIM
- selected ion monitoring
- SPE
- solid-phase extraction
- TCA
- 1,1,1-trichloroethane
- TCE
- trichloroethylene
- TDI
- tolerable daily intake
- THF
- tetrahydrofuran
- TWA
- time-weighted average
- UCMR3
- Unregulated Contaminant Monitoring Rule
- U.S.
- United States
- US EPA
- United States Environmental Protection Agency
- UV
- ultraviolet
- VOCs
- volatile organic compounds
