Page 13: Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Trihalomethanes
11.0 Classification and assessment
There is insufficient data on each of the individual THMs identified in this document to establish distinct guidelines for each. Rather, the approach chosen is to establish a MAC for THMs as a group, based on Chloroform data, and a separate MAC for BDCM.
As chloroform is the THM present in greatest concentration in drinking water, and the THM for which there are most scientific data available, a guideline developed based on data for this compound should be applicable as a guideline for the THMs identified in this document (chloroform, bromodichloromethane, dibromochloromethane and bromoform). Although not complete, available epidemiological data are consistent with the hypothesis that ingestion of chlorinated drinking water, if not THMs specifically, may be associated with cancers of the bladder and colon (Krasner et al., 1989). Additionally, epidemiological data available since 1993 have associated adverse reproductive outcomes with exposure to THMs, although neither clear evidence of a threshold, nor a dose-response pattern of increasing risk with increasing concentration of total THMs, has been found (Reif et al., 2000).
Chloroform has been classified in Group IIIC in this assessment, possibly carcinogenic to humans based on inadequate evidence for carcinogenicity in humans but limited evidence in experimental animals (Health Canada, 1994). There is compelling mechanistic evidence that both the hepatic and renal tumorigenic responses observed in previous carcinogenicity studies of chloroform (NCI, 1976a; Jorgenson et al., 1985) are mediated by a non-genotoxic mechanism (IPCS, 2000). One of the hypothesized modes of action for chloroform for tumour induction in rodents includes the following requisite precursor steps to cancer: 1) metabolism of chloroform by the target cell population; 2) induction of sustained cytotoxicity by metabolites; and 3) subsequent persistent regenerative cell proliferation (Environment Canada and Health Canada, 2001).
The nature of the vehicle appears to be an important factor in the toxicity and carcinogenicity of chloroform. More marked hepatotoxic effects and increased incidence of liver tumours in rats and mice are observed following administration of chloroform in corn oil compared with drinking water, probably as a result of the major shift in the nature of the caloric intake associated with the former vehicle.
PBPK modelling was performed for chloroform in the 2001 CEPA assessment report in order to estimate doses causing toxicity in specific organs. Basing the calculation of a Canadian drinking water guideline on the PBPK approach would lead to a considerable raising of the MAC. Given the uncertainties surrounding the health effects of THMs in drinking water in humans and that chloroform is used as a surrogate for THMs, it is considered appropriate to use the more conservative tolerable daily intake (TDI) approach for calculating the MAC.Footnote 4
Two key studies were considered in the risk assessment for chloroform: the Heywood et al. (1979) study in dogs and the Larson et al. (1994b) study in mice. The target organ in both studies was the liver. Although the Heywood et al. (1979) study was conducted in a relatively higher mammalian species (dog) and was of a reasonably long duration (7.5 years), it is an older study, used gavage dosing with a toothpaste base in a capsule, and did not cover the full life span of the dog. The Larson et al. (1994b) study, on the other hand, was conducted in a relatively lower mammalian species (mouse), used either corn oil vehicle (which may have influenced the pharmacokinetics and toxicity of the test compound) by gavage or drinking water given ad libitum, and was of short duration (3 weeks), which is insufficient for proper assessment of a lifetime exposure.
The NOAEL for treatment-related changes in the liver (cytolethality and regenerative hyperplasia) established in the mouse study (Larson et al., 1994b) with chloroform in corn oil vehicle was 10 mg/kg bw per day (corrected to 7 mg/kg bw per day due to 5 days per week dosing). In the same study (Larson et al., 1994b), however, mice treated with chloroform administered via drinking water had no treatment-related changes up to a dose of 329 mg/kg bw per day. In the Heywood et al. (1979) dog study, treatment-related liver changes (fatty cysts) were observed at the lowest administered dose (LOAEL = 15 mg/kg bw per day corrected to 13 mg/kg bw per day due to 6 days per week dosing), and no NOAEL was established.
The Heywood et al. (1979) dog study was chosen as the most appropriate study for risk assessment, due to its long duration and the possible influence of the corn oil vehicle on the effects observed in the short-term Larson et al. (1994b) mouse study. The TDI is calculated as follows:
TDI = [13 mg/kg bw per day] / 2100 ≈ 0.0062 mg/kg bw per day
where:
- 13 mg/kg bw per day is the LOAEL in the Heywood et al. (1979) dog study, corrected from 15 mg/kg bw per day to 13 mg/kg bw per day due to 6 days per week dosing,
- 2100 is the uncertainty factor (×10 for intraspecies variation; ×10 for interspecies variation; ×7 for less-than-lifetime exposure; ×3 for use of a LOAEL instead of a NOAEL). A moderate uncertainty factor of 7 was chosen for the less-than-lifetime exposure because 7.5 years was considered a reasonably long duration in the dog's life span. A modest uncertainty factor of 3 was used for the use of a LOAEL, because of the subtle nature of the endpoint (fatty cysts) observed in the dog study. Further support for this uncertainty factor is given by the absence of effects seen in the liver at a considerably higher dose of up to 329 mg/kg bw per day when chloroform was applied in drinking water in what appeared to be a more sensitive species (mouse).
The health-based target for THMs (based on chloroform) can be calculated as follows:
[6.2 µg/kg bw per day × 70 kg × 0.80] /4.11 Leq/d ≈80 µg/L (rounded)
where:
- 6.2 µg/kg bw per day is the TDI, as derived above,
- 70 kg is the average adult body weight,
- 0.80 is the allocation factor based on CEPA estimates (Environment Canada and Health Canada, 2001), and considering that chloroform is an important DBP in treated water. Of all media of exposure, drinking water is the main source of exposure to chloroform.
- 4.11 Leq/d is the total exposure contribution from drinking water (see section on Multiroute Exposure through Drinking Water).
BDCM is used as an indicator of the presence of brominated THMs, but the MAC developed applies to the level of BDCM in drinking water.
Genotoxicity studies indicate that BDCM is weakly mutagenic, probably as a result of glutathione conjugation. Carcinogenicity studies show that, BDCM administered to rats by gavage in corn oil for 102 weeks at doses ranging from 50 to 100 mg/kg bw per day, resulted in increased incidences of renal tubular cell adenomas and adenocarcinomas affecting both sexes and a markedly increased incidence of large intestinal tumours (combined adenomas and carcinomas) in both sexes. In mice, BDCM administered by gavage in corn oil for 102 weeks at dose levels of 0, 25, or 50 mg/kg bw per day or 0, 75, or 150 mg/kg bw per day in males and females, respectively, caused renal cytomegaly and hepatic fatty metamorphosis, increased incidences of renal tubular adenomas and carcinomas in males, and an increased incidence of combined hepatocellular adenomas and carcinomas in females. These carcinogenicity studies are supported by epidemiological studies showing an apparent association between the THM group of compounds and colorectal cancer in humans.
BDCM has been classified in Group II -- probably carcinogenic to humans, with sufficient evidence in animals and inadequate evidence in humans (Health Canada, 1994). Among the four THMs commonly found in drinking water, BDCM appears to be the most potent rodent carcinogen. BDCM caused tumours at lower doses and at more target sites than for any of the other THMs (IPCS, 2000).
The tumours of the large intestines (combined adenomatous polyps and carcinomas) in rats were chosen for the cancer risk assessment, as they occurred with the highest frequency and affected both sexes in the study, and because of the apparent epidemiological association of this group of compounds (THMs) with colorectal cancer in humans. Furthermore, these tumours appear most likely to be associated with a mutagenic mechanism, as they were not associated with underlying cytotoxicity or other non-epigenetic mechanism. The combined large intestinal tumours had high unit risk value, equal to or higher than the unit risks for the other tumour types (kidney and liver) identified in carcinogenicity studies with this compound.
Cancer risks have been estimated on the basis of the results of the only adequate carcinogenesis bioassay in F344/N rats, which was conducted by the NTP in 1987. It should be noted, however, that the compound was administered by gavage in corn oil in this bioassay and that quantitative risks may be overestimated. Although there has been one carcinogenesis bioassay in which BDCM was administered in a more appropriate vehicle (i.e., drinking water) (Tumasonis et al., 1985), it was considered inadequate for quantitative risk estimation, based on the limitations mentioned in the Chronic Toxicity/Carcinogenicity section. Moreover, the increases in adenomas and adenocarcinomas in the kidney of male mice and in hepatocellular adenomas and carcinomas in female mice in the NTP bioassay have not been used for quantitative estimation of the cancer risks, because these increases were confined to one sex and because of the possible contribution of the corn oil vehicle to the induction of liver tumours in mice.
Based on the tumours that were significantly increased in F344/N rats in the NTP (1987) bioassay (i.e., intestinal adenomatous polyps and adenocarcinomas; renal tubular cell adenomas and adenocarcinomas), unit risks were calculated using the linearized multistage [LMS] method of Howe (1995). An allometric scaling factor was applied to the final unit risks, assuming a rat weighs 0.35 kg, a mouse weighs 0.03 kg, and a human weighs 70 kg. The Kaplan-Meier mortality-adjusted data were not used, since using these data generally resulted in a worse fit while not appreciably changing the unit risk. The raw incidence data were used instead.
The multistage model was first fit to the bioassay data. The multistage model is given by
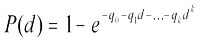
where d is dose, k is the number of dose groups in the study (excluding control), P(d) is the probability of the animal developing a tumour at dose d, and qi > 0, I = 0,...,k are parameters to be estimated.
The unit risk is defined as the increase in excess risk per unit dose, where excess risk is given by
P(d) - P(0) / 1 - P(0)
The unit risk is applicable at very low doses, presumably in the range where humans will be exposed. For a small dose, d, the excess risk can be shown to be approximately equal to q1d. Thus, when the background P(0) is small, q1 represents the slope (i.e., change in risk per increase of unit dose) of the dose-response curve in the low-dose region. In practice, the upper 95% confidence limit on q1 is used and is denoted by q1Footnote *. This is the unit risk for the LMS method.
A chi-square lack of fit test was performed for the model fits. The degrees of freedom for this test are equal to k minus the number of qis whose estimates are non-zero. A P-value less than 0.05 indicates a significant lack of fit. Some models exhibited a significant lack of fit, but since only three dose groups were present (including control), removing the highest dose group is unadvisable. Unit risks and lack of fit P-values are displayed in Appendix 1 of Health Canada (2003b).
The allometric scaling factor is given by (0.35/70)1/4 or (0.03/70)1/4, where 0.35 kg is the body weight of a rat, 0.03 kg is the body weight of a mouse, and 70 kg is the body weight of a human. The "raw" unit risks are divided by this factor to obtain the "converted" unit risks in Table 2 of Health Canada (2003b). Using the LMS model for the tumours that were significantly increased in F344/N rats in the NTP (1987) bioassay, the estimated calculated unit lifetime human cancer risks associated with the ingestion of 1 µg/L BDCM in drinking water range from 2.06 × 10-7Footnote * (based on combined adenomatous polyps and carcinomas of the large intestine in females rats) to 6.33 × 10-7Footnote * (based on combined adenomatous polyps and carcinomas of the large intestine in males rats).
In the context of drinking water guidelines, Health Canada has defined the term "essentially negligible" as a range from one new cancer above background per 100,000 people to one new cancer above background per 1 million people (i.e., 10-5 to 10-6) over a lifetime. The estimated concentrations corresponding to lifetime human cancer risks of 10-5, 10-6, and 10-7 for these tumour types, based on the model described above and the calculated unit lifetime human cancer risks, are as follows:
| Lifetime risk | Concentrations in drinking water (µg/L) |
|---|---|
| 10-5 | 15.8-48.5 |
| 10-6 | 1.6-4.9 |
| 10-7 | 0.2-0.5 |
Using the most conservative concentration in drinking water estimated for a 10-5 lifetime human cancer risk, a health-based target of 16 µg/L (rounded) is derived.
DBCM has been classified in Group IIID, possibly carcinogenic to humans based on limited evidence for carcinogenicity in one species of experimental animals and no data in humans (Health Canada, 1994). An expert panel convened in 2002 by Health Canada to assess the toxicological and epidemiological evidence for the THMs for the purpose of drafting an updated Canadian drinking water guideline concluded that there was insufficient information available to calculate a drinking water guideline for DBCM (Health Canada, 2003b).
Bromoform has been classified in Group IIID, possibly carcinogenic to humans based on limited evidence for carcinogenicity in one species of experimental animals and no data in humans (Health Canada, 1994). An expert panel convened in 2002 by Health Canada to assess the toxicological and epidemiological evidence for the THMs for the purpose of drafting an updated Canadian drinking water guideline concluded that there was insufficient information available to calculate a drinking water guideline for bromoform (Health Canada, 2003b).