Page 11: Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Vinyl Chloride
Part II. Science and Technical Considerations (continued)
Vinyl chloride has been classified by IARC, the U.S. Department of Health and Human Services and the U.S. EPA as a known human carcinogen (Group 1 carcinogen), with sufficient evidence of cancer in both humans and animals. Health Canada classifies vinyl chloride as a Group 1 carcinogen (carcinogenic to humans). This is consistent with the classifications established by IARC and the U.S. EPA.
The effects of vinyl chloride exposure have been studied in humans and experimental animals, with similar outcomes reported in all species. Liver and neurological effects have been observed consistently in workers as well as several animal species exposed to vinyl chloride over different exposure durations. Neurological effects have been observed only following inhalation exposures; however, liver effects have been reported following both medium- and long-term inhalation and long-term oral exposures. The sensitivity of the liver to vinyl chloride exposure is supported by the mode of action, as outlined in Section 8.5. The key factors supporting the liver as the main target organ for cancer and non-cancer effects from vinyl chloride exposure are the prevalence of mixed-function oxidase activity, specifically CYP2E1, in the liver and the generation of highly reactive metabolites, which have been shown to bind to DNA as a result of vinyl chloride metabolism.
Health Canada has considered both cancer and non-cancer endpoints in deriving a guideline value. The results from both the cancer and non-cancer risk assessment approaches are described below.
The association between occupational exposure to vinyl chloride and the development of ASL is one of the best characterized cases of chemical-induced carcinogenicity in humans. ASL is an extremely rare tumour type in the general population; in fact, primary liver cancer of all types (including ASL) is uncommon in Canada, with an estimated 2,000 new cases for 2012 among an estimated total of 186,400 new cases of all cancers (Canadian Cancer Society's Steering Committee on Cancer Statistics, 2012). With the emergence of vinyl chloride manufacturing, most of the reported ASL cases in North America have been associated with occupational vinyl chloride exposure. The association of vinyl chloride with ASL in numerous epidemiological studies has been supported by findings in rats, mice and hamsters exposed to vinyl chloride through the oral and inhalation routes. Animal data were used for estimating the cancer potency of vinyl chloride in humans, as exposure data deficiencies in the current vinyl chloride occupational exposure studies prevent their use for risk assessment. A PBPK model was used to determine the liver concentration of the active vinyl chloride metabolites, as it allows for a more pharmacologically relevant estimation of the cancer risk than default models incorporating body surface area correction or allometric scaling, which are adjusted for by the model. The most appropriate dose metric generated by the PBPK model was identified to be the amount of vinyl chloride metabolites produced (per litre of liver tissue per day) that interact directly with DNA in both animals and humans.
PBPK modeling allows for the calculation of excess cancer risks based on the estimation of internal dose of vinyl chloride liver metabolites in humans exposed to vinyl chloride through drinking water. PBPK modeling accounted for metabolic differences between animals and humans as well as metabolic differences between high and low exposure levels. Both the Feron et al. (1981) and Til et al. (1991) studies were used for cancer risk assessment, given the appropriate route of exposure used (oral ingestion), the adequate sample sizes and appropriate duration of exposure (lifetime). The external doses from these studies were inputted into the rat PBPK model to determine the daily internal doses of vinyl chloride metabolites generated per litre of liver tissue for several of the reported cancer endpoints in both male and female rats. These daily internal doses were then analysed using the multistage cancer model from BMDS (U.S. EPA, 2010) to determine the most appropriate point of departure to input into the human PBPK model. The cumulative lifetime internal doses of vinyl chloride liver metabolites associated with an excess cancer risk of 10−4, 10−5 and 10−6 for combined liver cancers (neoplastic nodules, HCC and ASL) in male and female rats were determined as the most appropriate inputs into the human PBPK model to estimate the external human doses associated with each risk level for a lifetime (70 years) exposure. The external human doses associated with an excess risk of combined liver cancers of 10−4, 10−5 and 10−6 were estimated as 4.19 × 10−4 , 4.19 × 10−5 and 4.19 × 10−6 mg/kg bw per day, respectively; these doses were derived from an oral human slope factor of 0.24 (mg/kg bw per day)-1.
The cancer risk estimates for combined liver cancers in females from Feron et al. (1981) resulted in the most conservative cancer risk estimate; therefore, combined female liver tumours were chosen as the key endpoint for cancer risk assessment. Although neoplastic nodules may not necessarily progress to malignancy, including this endpoint avoids any potential underestimation of the total cancer risk from vinyl chloride exposure. The combined liver tumour endpoint is also protective of lung angiosarcoma, as the estimated risks for lung angiosarcoma were lower than those for combined liver tumours , and animals displaying this cancer endpoint in the Feron et al. (1981) study also had ASL, suggesting metastases from the liver.
Evidence from animal studies suggests that very young children may be more sensitive to the carcinogenic effects of vinyl chloride due to increased DNA adduct formation and liver tumour incidence observed in animals under five weeks of age exposed to vinyl chloride compared to animals exposed after maturity (see Sections 9.1.6 and 9.2.6). In reviewing cancer potency data from early-life and adult exposures to vinyl chloride, the U.S. EPA (2000b) has suggested that that the full lifetime cancer risk for vinyl chloride can be approximated by adding the risks from exposures in early life and adulthood since, for example, the incidence of angiosarcoma following after short-term, early-life exposure is approximately equal to that following long-term exposure starting after maturity. As a result, the U.S EPA (2000b) concluded that continuous lifetime exposure from birth would approximately double cancer risk, and recommended that if continuous exposure to vinyl chloride were to occur from birth, a twofold increase should be applied to the adult slope factor in order to protect young children who may be more sensitive to vinyl chloride exposure.
Although early-life data in humans is lacking, many of the factors likely to be responsible for early-life sensitivity in animals also pertain to humans. Such factors include rapid cell division during early life as well as dosimetric considerations such as increased water intake per unit body weight and more rapid blood flow to liver. As a result, the approach used in this document is consistent with that of the U.S. EPA, and an additional 2-fold uncertainty factor is applied to protect young children if exposure to vinyl chloride were to occur from birth.
The concentrations of vinyl chloride in drinking water representing a lifetime excess combined liver cancer risk of 10−6 and 10−5 in adult humans exposed to 3.8 L-eq of water daily (see Section 8.4) are 0.08 and 0.8 µg/L, respectively. These concentrations are higher than the values of 0.013 and 0.13 µg/L calculated using traditional linear extrapolation with allometric scaling. Given the animal evidence of early-life sensitivity to vinyl chloride, if lifetime exposure was to occur from birth, then the above concentrations for adults should be divided by two to protect young children whom may be more sensitive to vinyl chloride. Therefore, the resulting health-based values (HBVs) for vinyl chloride in drinking water representing a lifetime excess combined liver cancer risk of 10−6 and 10−5 for early life exposure are 0.04 and 0.4 µg/L, respectively.
The results that incorporate metabolite generation rates (from PBPK modeling) are considered to represent a more reasonable estimate of human risk from exposure to vinyl chloride than simple linear extrapolation with the incorporation of an allometric scaling factor to account for metabolic differences between rats and humans.
The most appropriate study for a non-cancer risk assessment is Til et al. (1991), as it represents a well-controlled study with a large sample size as well as an appropriate duration and route of exposure (orally through the diet). The most appropriate endpoint is liver cell polymorphism, as it represents an effect in the liver that is sensitive to both inhalation and oral exposures to vinyl chloride (ATSDR, 2006). Liver cell polymorphism is not considered a precursor to carcinogenicity, as it is an effect observed in both the nucleus and cytoplasm of the liver cells and therefore is considered to be a toxic effect rather than a carcinogenic effect (Schoental and Magee, 1957, 1959; Afzelius and Schoental, 1967, U.S. EPA, 2000b). Additionally, the lowest-observed-adverse-effect level (LOAEL) and NOAEL for liver cell polymorphism in both sexes of rats from the Til et al. (1991) study represent the lowest doses identified in the literature for any chronic effect resulting from vinyl chloride exposure.
PBPK modeling was used to determine an external human dose corresponding to an external NOAEL of 0.13 mg/kg bw per day for combined moderate and severe liver cell polymorphism in female rats from Til et al. (1991); female rats were found to be more sensitive than males possibly due to a higher internal dose received. Using a rat PBPK model, the internal dose of vinyl chloride metabolites in the liver resulting from exposure to 0.13 mg/kg bw per day was determined as 2.85 mg/L of liver tissue per day. This internal dose was then inputted into the human PBPK model in order to determine a human external dose required (when consuming 1.5 L of drinking water per day for 70 years) to generate the same concentration of liver metabolites; the resulting human external dose corresponding to an internal daily dose 2.85 mg/L of liver metabolites was determined as 0.224 mg/kg bw per day.
Using the human external dose derived above as the most appropriate point of departure, the tolerable daily intake (TDI) for vinyl chloride can be calculated as follows:
Equation 1

where:
- 0.224 mg/kg bw per day is the human external dose for combined moderate and severe liver cell polymorphism derived through PBPK modeling as described above; and
- 25 is the uncertainty factor (×2.5 for interspecies toxicodynamic variability [see below] and ×10 for intraspecies variability).
Equation 1 - Text Description
The interspecies uncertainty factor can be divided in two components: a toxicokinetic (delivered dose) component (4.0) and a toxicodynamic (differential tissue sensitivity) component (2.5) (IPCS, 2005). PBPK modeling accounts for differences in the toxicokinetics between animals and humans; as a result, the toxicokinetic component of the interspecies uncertainty factor (4.0) can be removed from the TDI calculation. As the toxicodynamic variation (relating to tissue sensitivity) between animals and humans for vinyl chloride is not well known, the toxicodynamic portion of the interspecies uncertainty factor was retained for determining the TDI. Retention of the toxicodynamic portion of variation between animals and humans is further supported by the uncertainty surrounding the basic mode of action for non-cancer liver effects as well as the limited evidence of human susceptibility to non-cancer liver effects from occupational exposure to vinyl chloride.
Using the above TDI, a health-based value (HBV) for vinyl chloride in drinking water can be derived as follows:
Equation 2
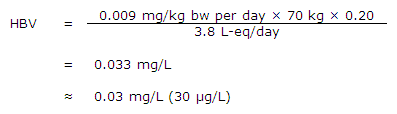
where:
- 0.009 mg/kg bw per day is the TDI as derived above;
- 70 kg is the average body weight of an adult;
- 0.20 is the proportion of the daily intake allocated to drinking water; this is a default value, as there are insufficient data to calculate the actual value; and
- 3.8 L-eq/day is the daily volume of water consumed by an adult, accounting for multiple routes of exposure from showering and bathing, as determined in Section 8.4.
Equation 2 - Text Description
In Section 10.1, the concentrations of vinyl chloride in drinking water associated with a lifetime excess risk of combined liver tumours of 10−6 and 10−5 were determined as 0.08 and 0.8 µg/L, respectively, for exposure during adulthood; if exposure was to occur from birth, these concentrations are to be reduced to 0.04 and 0.4 µg/L, respectively. Using a TDI approach in Section 10.2, a health-based value that is protective of liver cell polymorphism (moderate and severe) was determined to be 30 µg/L. As the cancer risk assessment resulted in a more conservative value for vinyl chloride in drinking water compared with that generated by the non-cancer approach, the cancer risk assessment approach was determined as the most appropriate approach for developing the maximum acceptable concentration (MAC) in drinking water.
The U.S. EPA maximum contaminant level (MCL) for vinyl chloride in drinking water is 0.002 mg/L (2 µg/L) based on liver cancer. The U.S. EPA (2000b) extrapolated its cancer slope factors as well as a non-cancer inhalation reference concentration (RfC) and oral human reference dose (RfD) from the Clewell et al. (1995) PBPK model. For the cancer risk assessment, the U.S. EPA used the data from the Feron et al. (1981) study to derive an a human oral cancer slope factor of 0.72 per mg/kg bw/day for total liver cancers (liver angiosarcoma, hepatocellular carcinoma and neoplastic nodules) resulting from continuous lifetime exposure during adulthood. A twofold increase to 1.4 per mg/kg bw/day is recommended to account for continuous lifetime exposure starting at birth. For the non-cancer assessment, the integration of the rat NOAEL of 0.13 mg/kg bw per day for induction of non-cancer cell polymorphism (Til et al., 1983, 1991) generated a rat target tissue concentration of 3 mg of metabolites/L of liver (U.S. EPA, 2000b). Assuming a consumption of 2 L water per day, 70 kg person, the PBPK-derived human dose given orally that would generate the same target tissue concentration (TTC) equal to 0.09 mg/kg bw per day, resulting in an RfD of 0.003 mg/kg bw per day.
The WHO (2004) drinking water quality guideline of 0.3 µg/L is based on the U.S. EPA (2000b) oral slope factor and a 10−5 cancer risk. A concentration of vinyl chloride in drinking water of 0.5 μg/L was calculated as being associated with an upper-bound excess risk of liver tumours of 10−5 for lifetime exposure beginning at adulthood. However, with uncertainty regarding differences in sensitivity during early life exposure and the assumption by the U.S. EPA (2000b) that continuous lifetime exposure from birth would double cancer risk, the WHO divided the level for adults by a 2-fold uncertainty factor and rounded up to 0.3 µg/L to arrive at its drinking water guideline value.
The California EPA (OEHHA, 2000) established a non-regulatory public health goal (PHG) of 0.05 µg/L for vinyl chloride in drinking water. The PHG is based on an inhalation cancer slope factor of 0.27 (mg/kg bw per day)-1 for lung cancer observed in mice from an inhalation study by Drew et al.(1983). A value of 3 µg/L was calculated based on non-cancer effects of vinyl chloride based on liver cell polymorphism and hepatic cysts in male and female rats. The non-cancer PHG incorporates a 100-fold uncertainty factor and a water exposure rate of 7.1 L/day that includes 5.1 L-equivalents to account for exposure by inhalation and dermal exposure from bathing or showering. California's current drinking water standard (MCL) for vinyl chloride is 0.5 µg/L, which was adopted in 1989.
The Australian Drinking Water Guideline for vinyl chloride of less than 0.0003 mg/L (0.3 µg/L) is based on considerations of health effects (cancer) and the limit of determination (NHMRC, 2004).