Cancer Risk Assessment Methodology: A Survey of Current Practices at Health Canada
Download the alternative format
(PDF format, 4.26 MB, 36 pages)
Organization: Health Canada
Date published: November 2021
Cat.: 210342
ISBN: 978-0-660-40495-0
Members of Working Group on Cancer Risk Assessment Methodology
Jane MacAulay, Water Quality Division, Water and Air Quality Bureau, Safe Environments Directorate, HECSB
Ivy Moffat, Water Quality Division, Water and Air Quality Bureau, Safe Environments Directorate, HECSB
Alisa Vespa, Bureau of Metabolism, Oncology, and Reproductive Sciences, Therapeutic Products Directorate, HPFB
Catherine Adcock, Health Effects Division II, Health Evaluation Directorate, PMRA
Ian Aldous, Bureau of Evaluation, Medical Devices Directorate, HPFB
Mohammed Ansari, Marketed Pharmaceuticals Bureau, Marketed Health Products Directorate, HPFB
Alain Beliveau, Bureau of Metabolism, Oncology, and Reproductive Sciences, Therapeutic Products Directorate, HPFB
Vinita Chauhan, Consumer and Clinical Radiation Protection Bureau, Environmental and Radiation Health Sciences Directorate, HECSB
Guosheng Chen, Human Safety Division, Veterinary Drugs Directorate, HPFB
Michelle Deveau, Indoor Air Contaminants Assessment Section, Air Health Science Division, Water and Air Quality Bureau, Safe Environments Directorate, HECSB
John Field, Risk Assessment Bureau, Consumer and Hazardous Product Safety Directorate, HECSB
Jason Fine, Non-Prescription Drugs Evaluation Division, Natural and Non-Prescription Health Products Directorate, HPFB
Zoe Gillespie, Bureau of Chemical Safety, Food Directorate, HPFB
Michael Honeyman, Health Effects Division II, Health Evaluation Directorate, PMRA
Jianli Jiao, New Substances Assessment and Control Bureau, Safe Environments Directorate, HECSB
Dustin Johnson, Office of Clinical Trials, Centre for Regulatory Excellence, Statistics and Trials, Biologic and Radiopharmaceutical Drugs Directorate, HPFB
Steven R Jones, Biologic and Radiopharmaceutical Drugs Directorate, HPFB
Benny Ling, Air Health Effects Assessment Division, Water and Air Quality Bureau, Safe Environments Directorate, HECSB
Luigi Lorusso, Environmental Assessment and Contaminated Sites Division, Chemicals and Environmental Health Management Bureau, Safe Environments Directorate, HECSB
Jocelyn Moore, Risk Assessment Bureau, Consumer and Hazardous Product Safety Directorate, HECSB
Michel Ntemgwa, Non-Prescription Drugs Evaluation Division, Natural and Non-Prescription Health Products Directorate, HPFB
Sanya Petrovic, Environmental Assessment and Contaminated Sites Division, Chemicals and Environmental Health Management Bureau, Safe Environments Directorate, HECSB
Debora Quayle, Radiation Protection Bureau, Environmental and Radiation Health Sciences Directorate, HECSB
Jayadev Raju, Molecular and Applied Toxicology Section, Regulatory Toxicology Research Division, Bureau of Chemical Safety, Food Directorate, HPFB
Tanya Ramsamy, Office of Clinical Trials, Therapeutic Products Directorate, HPFB
Christopher Rowat, Existing Substances Risk Assessment Bureau, Safe Environments Directorate, HECSB
Tim Schrader, Molecular and Applied Toxicology Section, Regulatory Toxicology Research Division, Bureau of Chemical Safety, Food Directorate, HPFB
Janice Weightman, Pre-market Toxicology Assessment Section, Chemical Health Hazard Assessment Division, Bureau of Chemical Safety, Food Directorate, HPFB
Paul White, Environmental Health Science and Research Bureau, Environmental and Radiation Health Sciences Directorate, HECSB
Ruth Wilkins, Consumer and Clinical Radiation Protection Bureau, Environmental and Radiation Health Sciences Directorate, HECSB
Deon Williams, Food Contaminant Toxicology Assessment Section, Chemical Health Hazard Assessment Division, Bureau of Chemical Safety, Food Directorate, HPFB
Table of Contents
- Acronyms and Definitions
- 1. Introduction
- 2. Cancer Risk Assessment at Health Canada
- 3. Information Used to Inform Cancer Risk Assessments
- 4. Methodology Used to Perform Cancer Risk Assessments
- 5. Challenges Encountered When Conducting Cancer Risk Assessments
- 6. Future Needs and Directions
- 6.1. Harmonization
- 6.2. Information Sharing
- 6.3. Evolving Trends in the Assessment of Potential Human Cancer Risk
- 6.3.1. Trend Towards a Reduction in Use of Animal Studies and Incorporation of Alternative Approaches to Assessing Potential Human Cancer Risk
- 6.3.2. Interpretation of Carcinogenicity Dose-Response Data
- 6.3.3. Interpretation and Potential Incorporation of Quantitative Genotoxicity Dose-Response Data into Cancer Risk Assessments
- 7. References
Acronyms and Definitions
- ADI
- Acceptable Daily Intake; the estimate of the amount of a chemical that can be ingested daily over a lifetime without appreciable health risk to the consumer. The ADI is expressed in mg/kg/day and may be applied in the assessment of substances such as food additives, residues of pesticides and residues of veterinary drugs in food.
- AHEAD
- Air Health Effects Assessment Division
- AOP(s)
- Adverse Outcome Pathway(s)
- API
- Active Pharmaceutical Ingredient
- Apical
- An observable outcome in a whole organism, such as a clinical sign or pathologic state, that is indicative of a disease state that can result from exposure to a toxicant
- ATSDR
- Agency for Toxic Substances and Disease Registry
- AQBAT
- Air Quality Benefits Assessment Tool
- BCANS
- Bureau of Cardiology, Allergy and Neurological Sciences
- BCS
- Bureau of Chemical Safety
- BGIVD
- Bureau of Gastroenterology, Infection and Viral Diseases
- BRDD
- Biologic and Radiopharmaceutical Drugs Directorate
- BMC
- Benchmark Concentration
- BMD
- Benchmark Dose
- BMDL10
- Benchmark Dose Lower confidence limit; an estimate of the lower dose which is 95% certain to cause no more than a 10% cancer incidence in rodents
- BMORS
- Bureau of Metabolism, Oncology and Reproductive Sciences
- BPRA
- Bureau of Product Review and Assessment
- CCPSA
- Canada Consumer Product Safety Act
- CCRPB
- Consumer and Clinical Radiation Protection Bureau
- CBE
- Centre for Biologics Evaluation
- CEBS
- Chemical Effects in Biological Systems
- CEHMB
- Chemicals and Environmental Health Management Bureau
- CEPA
- Canadian Environmental Protection Act
- CERB
- Centre for Evaluation of Radiopharmaceuticals and Biotherapeutics
- CMP
- Chemicals Management Plan
- CNSC
- Canadian Nuclear Safety Commission
- CPDB
- Carcinogenic Potency Database
- CHPSD
- Consumer and Hazardous Product Safety Directorate
- CREST-OCT
- Centre for Regulatory Excellence, Statistics and Trials - Office of Clinical Trials
- DINA
- Application for a Drug Identification Number
- DQRACHEM
- Detailed Quantitative Risk Assessment for Chemicals
- EACSD
- Environmental Assessment and Contaminated Sites Division
- EC
- European Commission
- EFSA
- European Food Safety Authority
- EHSRD
- Environmental Health Science and Research Bureau
- ERHSD
- Environmental and Radiation Health Sciences Directorate
- ESRAB
- Existing Substances Risk Assessment Bureau
- FD
- Food Directorate
- Genotoxicity
- A broad term that refers to any deleterious change in the genetic material regardless of the mechanism by which the change is induced (e.g., point mutation, chromosome break).
- HBV
- Health-Based Value
- HECSB
- Healthy Environments and Consumer Safety Branch
- HED
- Health Evaluation Directorate
- HHRA
- Human Health Risk Assessment
- HPFB
- Health Products and Food Branch
- HSDB
- Hazardous Substances Data Bank
- IACAS
- Indoor Air Contaminants Assessment Section
- IARC
- International Agency for Research on Cancer
- IARL
- Indoor Air Reference Levels
- ICH
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- IPCS
- International Programme on Chemical Safety
- IRIS
- Integrated Risk Information System
- ISA
- Integrated Science Assessment (US EPA)
- JECFA
- Joint FAO/WHO Expert Committee on Food Additives
- JRC
- Joint Research Centre; part of the European Commission
- LOAEL
- Lowest Observed Adverse Effect Level
- MAC
- Maximum Acceptable Concentration
- MDD
- Medical Devices Directorate
- MHPD
- Marketed Health Products Directorate
- MOA
- Mode of Action; a biologically plausible sequence of key events leading to an observed effect supported by robust experimental observations and mechanistic data
- MOE
- Margin of Exposure
- MPB
- Marketed Pharmaceuticals Bureau
- MRL
- Minimal Risk Level; an estimate of the daily human exposure to a hazardous substance that is likely to be without appreciable risk of adverse non-cancer health effects over a specified duration of exposure
- Mutagen
- A chemical, physical or biological agent that can introduce permanent, heritable changes to the genome of an exposed organism
- Mutagenic impurity
- An impurity in a pharmaceutical product that has been demonstrated to be mutagenic in an appropriate mutagenicity test model, e.g., bacterial mutagenicity assay
- NDED
- Non-Prescription Drug Evaluation Division
- NDS
- New Drug Submission
- NNHPD
- Natural and Non-Prescription Health Products Directorate
- NOAEL
- No Observed Adverse Effect Level
- NOEL
- No Observed Effect Level
- NRP
- National Radon Program
- NSACB
- New Substances Assessment and Control Bureau
- NTP
- National Toxicology Program
- OCT
- Office of Clinical Trials
- OECD
- Organisation for Economic Co-operation and Development
- PETA
- People for the Ethical Treatment of Animals
- PM2.5
- Fine particulate matter is the name for a range of particles in air that are less than 2.5 microns (µm) in diameter. It is often referred to as PM 2.5.
- PMRA
- Pest Management Regulatory Agency
- POD(s)
- Point(s) of Departure; The point of departure represents a dose derived from observed data that is associated with an extra risk for a specific endpoint. This could be in the form of a LOAEL, NOAEL, or BMD.
- PPARα
- Peroxisome Proliferator-Activation Receptor α
- (Q)SAR
- (Quantitative) Structure-Activity Relationship
- RAB
- Risk Assessment Bureau
- REACH
- Registration, Evaluation, Authorisation and Restriction of Chemicals
- ReCAAP
- Rethinking Carcinogenicity Assessment for Agrochemicals Project
- RfC
- Reference Concentration
- RHI
- Radiation Health Impacts
- RIAQGs
- Residential Indoor Air Quality Guidelines
- RPB
- Radiation Protection Bureau
- SED
- Safe Environments Directorate
- SOT
- Society of Toxicology
- TD50
- Dose giving a 50% tumour incidence at the end of the standard lifespan for the species.
- TDI
- Tolerable Daily Intake; analogous to ADI. The term TDI is used for agents that are not deliberately added, such as contaminants in water.
- TFSRA
- Task Force for Scientific Risk Assessment
- TgRasH2
- A transgenic mouse model
- ToxCast
- Toxicity Forecaster
- ToxNet
- Toxicology Data Network
- ToxTree
- Toxic Hazard Estimation by decision tree approach
- TPD
- Therapeutic Products Directorate
- TRV
- Toxicity Reference Value
- TTC
- Threshold of Toxicological Concern
- US EPA
- United States Environmental Protection Agency
- US FDA
- United States Food and Drug Administration
- VDD
- Veterinary Drugs Directorate
- VICH
- International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products
- WAQB
- Water and Air Quality Bureau
- WHO
- World Health Organization
- WQD
- Water Quality Division
1. Introduction
Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health. As part of fulfilling this mandate, Health Canada regulates and/or establishes guidelines on environmental health risks and product safety including health products (e.g., drugs and medical devices), food, water, air, consumer products (e.g., cosmetics), veterinary drugs, pesticides, and devices and environmental sources that emit radiation. Critical to the evaluation of environmental and product safety is the assessment of human cancer risk resulting from exposure to substances that exhibit mutagenic, genotoxic and/or carcinogenic potential.
The Task Force for Scientific Risk Assessment (TFSRA) was established in 2009 and consists of risk assessors and/or risk managers across various Departmental program areas. The TFSRA develops reports, guidance and other deliverables for the Department through creation of working groups comprised of subject matter experts. The TFSRA addresses a broad range of issues and seeks to enhance the coordination, consistency and coherence of scientific risk assessments across these programs.
The TFSRA created a Cancer Risk Assessment Working Group to better understand the approaches used to conduct cancer risk assessments across Health Canada program areas. Given the breadth of the subject matter, consensus was reached by the Cancer Risk Assessment Working Group to develop this initial explanatory document, with the primary target audience being programs that conduct or use cancer risk assessments, and those wishing to understand the current landscape of cancer risk assessment across Health Canada. This report seeks to outline the approaches used to assess cancer hazard and/or estimate cancer risk across program areas, including if and how genotoxicity, mutagenicity, and carcinogenicity data are used to inform cancer risk assessment methodology. Finally, the Cancer Risk Assessment Working Group was tasked with identifying opportunities for future collaboration and harmonization, where possible. To generate the data required to complete the explanatory document, a survey was created that requested the following information:
- Program area submitting the survey response.
- Legislation/policy initiative supporting the conduct of cancer risk (or hazard) assessments.
- Purpose(s) of conducting cancer risk (or hazard) assessments.
- Are new 'in-house' cancer risk (or hazard) assessments performed and/or are cancer risk (or hazard) assessments from other sources used?
- Cancer risk assessments consulted and/or used for completing the program area's cancer risk assessment (internal and external sources).
- Guidance documents and/or resource materials consulted.
- Sources of data and/or databases consulted.
- Are the results of genotoxicity and mutagenicity studies used when conducting a cancer risk (or hazard) assessment? If yes, how?
- Describe the general approach for performing a cancer risk (or hazard) assessment.
- Describe the challenges that are encountered when performing a cancer risk (or hazard) assessment.
- Outline any other information that may be relevant for interpreting how cancer risk (or hazard) assessments are performed.
The survey was initiated on 27 April 2018 and was completed 16 January 2019. Information was obtained from a number of Health Canada program areas (Figure 1).
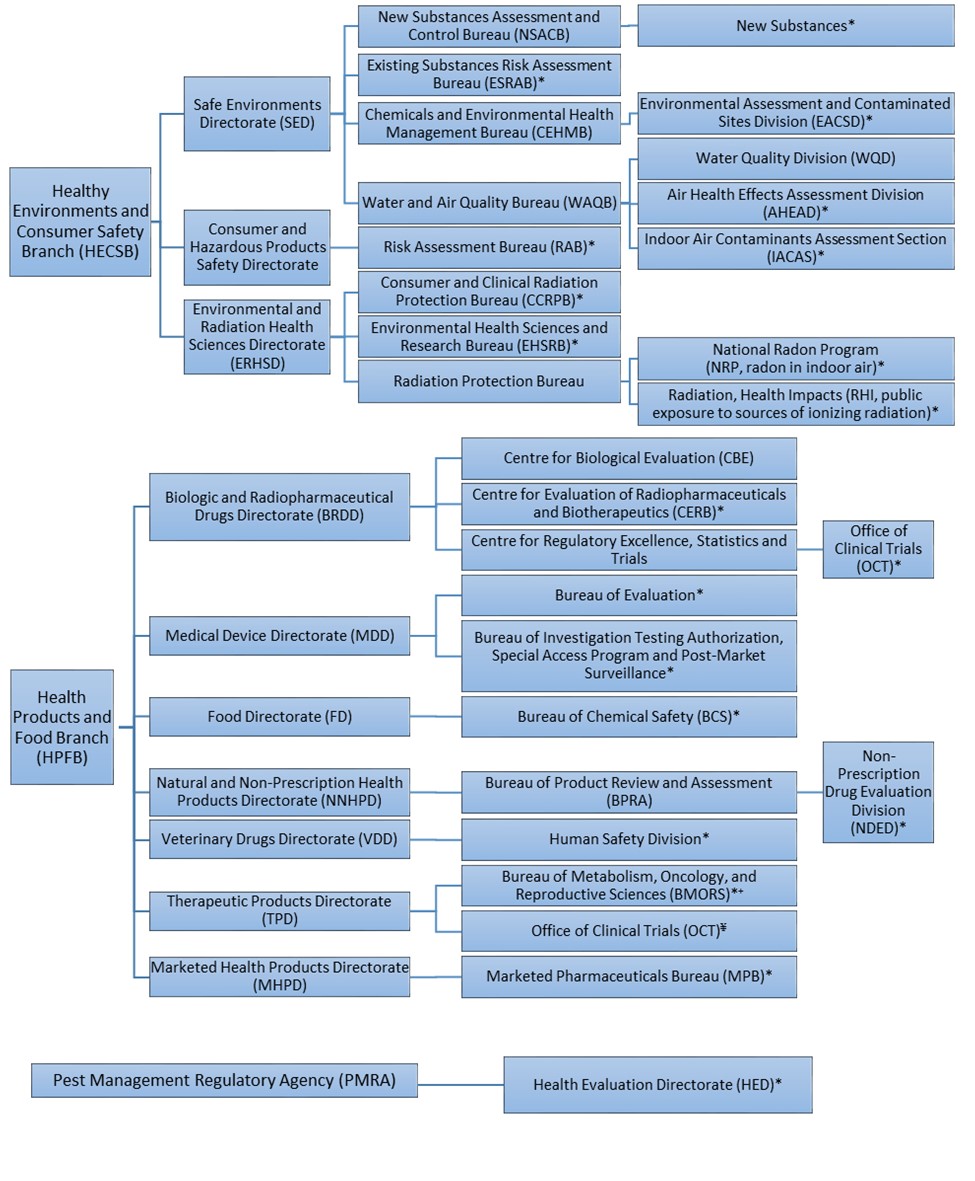
Figure 1. Organizational structure of survey respondents.
Healthy Environments and Consumer Safety Branch (HECSB)
- Safe Environments Directorate (SED)
- New Substances Assessment and Control Bureau (NSACB)
- New Substances*
- Existing Substances Risk Assessment Bureau (ESRAB)*
- Chemicals and Environmental Health Management Bureau (CEHMB)
- Environmental Assessment and Contaminated Sites Division (EACSD)*
- Water and Air Quality Bureau (WAQB)
- Water Quality Division (WQD)
- Air Health Effects Assessment Division (AHEAD)*
- Indoor Air Contaminants Assessment Section (IACAS)*
- New Substances Assessment and Control Bureau (NSACB)
- Consumer and Hazardous Products Safety Directorate (CHPSD)
- Risk Assessment Bureau (RAB)*
- Environmental and Radiation Health Sciences Directorate (ERHSD)
- Consumer and Clinical Radiation Protection Bureau (CCRPB)*
- Environmental Health Sciences and Research Bureau (EHSRB)*
- Radiation Protection Bureau
- National Radon Program (NRP, radon in indoor air)*
- Radiation, Health Impacts (RHI, public exposure to sources of ionizingradiation)*
Health Products and Food Branch (HPFB)
- Biologic and Radiopharmaceutical Drugs Directorate (BRDD)
- Centre for Biologics Evaluation (CBE)
- Centre for Evaluation of Radiopharmaceuticals and Biotherapeutics (CERB)*
- Centre for Regulatory Excellence, Statistics and Trials (CREST)
- Office of Clinical Trials (OCT)*
- Medical Device Directorate (MDD)
- Bureau of Evaluation*
- Bureau of Investigational Testing Authorization, Special Access Programand Post-Market Surveillance*
- Food Directorate (FD)
- Bureau of Chemical Safety (BCS)*
- Natural and Non-Prescription Health Products Directorate (NNHPD)
- Bureau of Product Review and Assessment (BPRA)
- Non-Prescription Drug Evaluation Division (NDED)*
- Bureau of Product Review and Assessment (BPRA)
- Veterinary Drugs Directorate (VDD)
- Human Safety Division*
- Therapeutic Products Directorate (TPD)
- Bureau of Metabolism, Oncology, and Reproductive Sciences (BMORS)*
- Office of Clinical Trials (OCT)¥
- Marketed Health Products Directorate (MHPD)
- Marketed Pharmaceuticals Bureau (MPB)*
Pest Management Agency (PMRA)
- Health Evaluation Directorate (HED)*
Figure 1. Organizational structure of survey respondents. *Program areas that submitted a response to the survey. ¥ Although TPD-OCT was not included in the survey, information was obtained from this program area to ensure they were accurately represented in this report. +Survey responses provided by BMORS are also relevant to the TPD clinical review bureau including Bureau of Gastroenterology, Infection and Viral Diseases (BGIVD) and Bureau of Cardiology, Allergy and Neurological Sciences (BCANS).
2. Cancer Risk Assessment at Health Canada
Cancer risk assessments are conducted or taken into account by all program areas surveyed in some capacity. The content of individual risk assessments and the overall recommendation can vary depending on a number of factors including policy/legislative considerations and the purpose for conducting the cancer risk assessment. Variation can also be attributable to the available information used to inform the assessments, as discussed in section 3.0.
2.1. Policy or legislative considerations
The variety of cancer risk assessments conducted at Health Canada is in large part reflected by the various mandates of the Directorates and Bureaux of which it is comprised. Working Group members were surveyed as to what type of policy or legislative driver directs cancer risk assessments in their respective Directorates and/or Bureaux. Legislative drivers include work under the Food and Drugs Act for representatives from HPFB, the Canadian Environmental Protection Act (CEPA), the Radiation Emitting Devices Act, the Canada Consumer Product Safety Act (CCPSA), the Occupational Health and Safety Act (OHSA), and the Canada Labour Code for representatives from HECSB, and the Pest Control Products Act by PMRA. It should be noted that some of the work of HECSB and the PMRA also falls under the Food and Drugs Act.
2.2. Purpose of conducting cancer risk assessments
The purpose of conducting and using cancer risk assessments across Health Canada varies considerably (Table 1). Respondents from HECSB that conduct cancer risk assessments often look to quantify risks associated with exposure to a carcinogen, or to quantify an exposure level that is associated with acceptableFootnote 1 risk. For example, in the Water and Air Quality Bureau, respondents indicated a need to develop quantitative risk assessments to define a maximum level of a substance that is considered acceptable in indoor air or drinking water. Similarly, the Air Health Effects Division of the Bureau conducts health impact assessments to identify the number of excess health outcomes associated with increased risk due to a change in air pollutant concentration. The Division is also developing an estimate to quantify the excess lung cancer risk of the air pollutant PM2.5 for use in health impact assessments. The process is similar at PMRA; the Health Evaluation Directorate conducts quantitative risk assessments to determine the acceptable uses for a pesticide.
Some evaluations at Health Canada focus on qualitatively identifying whether or not a substance is carcinogenic or presents a risk of carcinogenicity, without the need to derive a safe exposure level. This represents a hazard-based approach. For example, in HPFB, in the evaluation of veterinary drugs, when a substance or any of its metabolites has been found to cause cancer in animals and/or humans, it generally cannot be approved as a drug for use in food-producing animals. Similarly, during the pre-market assessment of food additives in the Bureau of Chemical Safety of the Food Directorate within HPFB, compounds that are genotoxic carcinogens are eliminated from consideration for use in food. However, it should be noted that in the case of post-market contaminants and naturally-occurring toxicants found in food, cancer risk assessments are performed to establish toxicological reference values or health-based guidance values.
For the assessment of small molecule pharmaceuticals, medical devices, biologic and radiopharmaceutical drugs in the Therapeutic Products Directorate, Medical Devices Directorate, Biologics and Radiopharmaceutical Drugs Directorate, and the Marketed Health Products Directorate, the benefit-risk profile of the product is evaluated. As products are approved and/or maintained on the market only in cases where the benefit-risk profile is favourable, both benefits (e.g., drug efficacy) and risks are considered. The cancer risk assessment comprises one aspect of the overall understanding of the benefit-risk profile of the productFootnote 2.
Cancer risk assessments were also reported to be used to determine population health risk and potential impact related to exposure to a substance, to provide guidance for public exposure scenarios, to assess toxicity and establish safe exposure levels to a substance, to verify the safety of a consumer product or cosmetic, and to assess compliance with standards. Cancer risk assessments may also inform the development of general and public health guidance (Table 1).
| PROGRAM | PURPOSE OF CANCER RISK ASSESSMENT |
|---|---|
| Healthy Environments and Consumer Safety Branch (HECSB) | |
| Existing Substances Risk Assessment Bureau (ESRAB) | Performed during the assessment of existing substances included on the Domestic Substances List in order to determine if they meet the definition of "toxic" as per section 64 of CEPA. |
| New Substances Assessment and Control Bureau (NSACB) | Performed as part of the human health risk assessment for new substances notified under the revised New Substances Notification Regulations. |
| Water and Air Quality Bureau (WAQB)- Water Quality | Performed in order to calculate Health-Based Values (HBV) for contaminants in drinking water. These HBVs inform the Maximum Acceptable Concentration (MAC) established in the Guidelines for Canadian Drinking Water Quality. |
| Water and Air Quality Bureau (WAQB) - Outdoor Air | Cancer and carcinogenic potential are considered when conducting health impact assessments (estimate the number of excess health outcomes associated with the increased risk due to a change in air pollutant concentration) and risk assessments (make a causality determination between a particular health endpoint (e.g., cancer) and a source of air pollution), respectively. The Division is also developing an estimate to quantify the excess lung cancer risk of the air pollutant PM2.5 for use in health impact assessments. |
| Water and Air Quality Bureau (WAQB) - Indoor Air | Performed to calculate health-based recommended maximum exposure limits. Residential Indoor Air Quality Guidelines (RIAQGs) typically contain both a short-term and long-term exposure limit. The Indoor Air Reference Levels (IARLs) are screening guidelines for lifetime exposure, and based on risk assessments performed by other Health Canada programs or external organizations. |
| Chemicals and Environmental Health Management Bureau (CEHMB) - Environmental Assessment and Contaminated Sites Division | Performed to ensure that risk-based remediation goals at federal contaminated sites are set at levels that are protective of carcinogenic endpoints. Soil quality guidelines are derived for carcinogenic and non-carcinogenic substances with the purpose of establishing guidelines that are protective of human health and the environment. |
| Risk Assessment Bureau (RAB) | May be conducted in response to a concern about the safety of a particular consumer product, a consumer product class or a cosmetic ingredient. The drivers for risk assessments of consumer products are primarily incidents reported by the public or by industry, and media reports. Risk assessment of cosmetic ingredients may be conducted due to the above, or due to recent scientific information, or actions by other regulatory bodies. |
| Consumer and Clinical Radiation Protection Bureau (CCRPB) | Both ionizing radiation (e.g., x-rays) and ultraviolet radiation are established carcinogens. Cancer risk assessments are rarely conducted since cancer hazard assessments for various forms of ionizing and non-ionizing radiation have already been well established. For devices emitting x-ray and ultraviolet radiation, the program assesses device compliance with established national and international device standards, which are in turn based on international radiation protection principles/guidelines and risk assessment models. Furthermore, the internationally accepted cancer risk models may be applied to compare the relative cancer risk of different radiation exposure scenarios. |
|
Radiation Protection Bureau (RPB) - National Radon Program |
Does not conduct independent assessments of the carcinogenicity of radon because it is a group 1 carcinogen, recognized by the International Agency for Research on Cancer (IARC). Risk of radon-induced cancer for use in assessments has been characterized by international radiation protection organizations based on reviews of best available science; this is used by RPB for developing guidance and encouraging radon reduction measures in public health policy. RPB uses established radon risk models to calculate population-level impacts based on radon distribution across Canada. |
|
Radiation Protection Bureau (RPB) - Radiation Health Impacts |
It is internationally recognized that exposure to ionizing radiation increases the risk of cancer. Risk of radiation-induced cancer for use in assessments has been characterized by international radiation protection organizations based on reviews of best available science. RPB applies and adapts international guidance and best practice for managing the cancer risks posed by various exposure scenarios to the Canadian context. |
| Environmental Health Science and Research Bureau (EHRSB) | Conducts risk assessments for research purposes, and conducts applied research in support of risk assessment modernization. Research is conducted in collaboration with evaluators from a variety of Bureaux. Activities frequently involve interpretation of genetic toxicity assessments in support of mode of action determination and risk assessment modernization. |
| Health Products and Food Branch (HPFB) | |
| Bureau of Metabolism, Oncology and Reproductive Sciences (BMORS)* | New pharmaceutical products are approved for marketing when the product's benefit exceeds any potential harm, with a reasonable degree of certainty. Assessment of carcinogenic potential of the active pharmaceutical ingredient (API), as well as pharmaceutical impurities, contributes to understanding the risk profile of the product. If a new impurity or a higher level of a previously qualified impurity is detected post-marketing, carcinogenic potential may be assessed and potential cancer risk may be estimated. |
| Office of Clinical Trials (OCT) | Qualitative cancer risk assessments are conducted to determine whether the risk to participants of clinical trials is acceptable based on internationally accepted guidelines. |
| Medical Devices Directorate (MDD) | Does not conduct cancer risk assessments. |
| Veterinary Drugs Directorate (VDD) | Carcinogenic risk is considered one important component in the evaluation of veterinary drugs to protect human and animal health and food safety. |
| Bureau of Chemical Safety (BCS) | Cancer risk assessments are used to inform the pre-market safety evaluation of certain foods, food ingredients, substances used in food processing, and food packaging materials. These assessments help to determine whether the food/substance should be permitted for use and at what level. Cancer risk assessments are also employed in post-market risk assessments of chemical contaminants and natural toxicants that may be present in foods and the results help to determine whether risk management is needed. |
| Bureau of Product Review and Assessment (BPRA), Non-Prescription Drug Evaluation Division |
Cancer risk assessments are not performed explicitly in the Non-Prescription Drug Evaluation Division. However, where relevant, genotoxicity and mutagenicity data are assessed in the following cases:
|
| Centre for Biologics Evaluation (CBE) and Centre for Evaluation of Radiopharmaceuticals and Biotherapeutics (CERB) | These review centres evaluate the benefit-risk profile of new biologic drugs (products made from living sources) prior to drug market authorization. Assessment of the carcinogenic potential of a biologic drug substance is performed as part of understanding the risk profile of the product. Given the nature of biological products, it is a product-specific science-based "case-by-case" approach. |
| Centre for Regulatory Excellence, Statistics and Trials - Office of Clinical Trials (CREST-OCT) | Cancer risk assessment, if required, contributes to a benefit-risk analysis of the proposed use of an investigational biologic drug in a clinical trial. Given the nature of biological products, assessment of cancer risk is a product-specific science-based "case-by-case" approach. |
| Marketed Pharmaceuticals Bureau (MPB) | Performed to ensure that the balance of the product associated benefits and harms continues to favour the use of drug or medical device in the post-market setting. |
| Pest Management Regulatory Agency | |
| Health Evaluation Directorate (HED) | Performed during the assessment of new technical active ingredients and the re-evaluation of currently registered active ingredients to ensure risks are acceptable. |
| *While the survey response was provided by BMORS, the approach described is used in other TPD clinical review bureau including BGIVD and BCANS. | |
2.3. Development and use of cancer risk assessments
Although a significant proportion of program areas routinely conduct cancer risk assessments, many rely on risk assessments conducted by other groups within or outside of Health Canada [(e.g., assessments for the Chemicals Management Plan (CMP), other regulatory jurisdictions, international agencies (e.g., US EPA, EFSA, WHO), or on assessments generated by industry (e.g., pharmaceutical companies)] as indicated in Table 2. As such, not all cancer risk assessments used by Health Canada are developed internally and not all program areas conduct new 'in-house' assessments (Table 2).
| PROGRAM AREA | DEVELOPMENT OF NEW IN-HOUSE CANCER RAS | USE OF CANCER RAS CONDUCTED BY OTHERS (E.G., HEALTH CANADA, INTERNATIONAL BODIES, INDUSTRY)* |
|---|---|---|
| HECB-SED-ESRAB | ||
| HECSB-SED-NSACB | ||
| HECSB-SED-WAQB-WQD | ||
| HECSB-SED-WAQB-AHEAD | ||
| HECSB-SED-WAQB-IACAS | ||
| HECSB-SED-EACSD | ||
| HECSB-CHPSD-RAB | ||
| HECSB-ERHSD-CCRPB | ||
| HECSB-ERHSD-RPB-NRP | ||
| HECSB-ERHSD-RPB-RHI | ||
| HPFB-TPD-BMORS | ||
| HPFB-TPD-OCT | ||
| HPFB-MDD | ||
| HPFB-MHPD-MPB | ||
| HPFB-VDD | ||
| HPFB-FD-BCS | ||
| HPFB-NNHPD-NDED | ||
| HPFB-BRDD-CBE/CERB/CREST-OCT | ||
| PMRA-HED | ||
| *A check mark represents cancer risk assessments used 'as is', and does not represent assessments used for the development of a program area's final risk assessment. | ||
3. Information used to inform cancer risk assessments
The survey conducted by the Cancer Risk Assessment Working Group revealed many commonalities, and also many differences in terms of the guidance material consulted and data sources used to inform cancer risk assessments. This information is summarized in the sections that follow.
3.1. Guidance material consulted
Survey results revealed that programs consult many of the same guidance resources when conducting cancer risk assessments (Table 3). The guidance document most frequently cited as a resource is the US EPA's Guidelines for Carcinogen Risk Assessment (2005a) and the accompanying Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens (2005b). Other commonly used guidelines across programs include the Organisation for Economic Co-operation and Development (OECD) Test Guidelines as well as OECD's Guidance Notes for Analysis and Evaluation of Chronic Toxicity and Carcinogenicity Studies (2002), International Programme on Chemical Safety (IPCS) Conceptual Framework for Evaluating a Mode of Action for Chemical Carcinogenesis (2001), and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines relevant to the conduct and assessment of genotoxic and carcinogenic potential of pharmaceuticals and associated impurities and for biologics. Additional guidelines consulted in specific programs include those developed by the World Health Organization (WHO), Joint FAO/WHO Expert Committee on Food Additives (JECFA), the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH), the European Commission's (EC) Joint Research Centre (JRC) and, the US EPA's Preamble to the Integrated Science Assessments (ISA) (2015). The latter is consulted when assessing causality for health endpoints including cancer. Of note, some program areas in the Department (e.g., Environmental Assessment and Contaminated Sites Division, Water Quality Division) have developed their own guidance for external and internal use, respectively.
| GUIDANCE | TYPE OF INFO |
|---|---|
| US EPA Cancer | Carcinogen risk assessment |
| OECD test guidelines | Internationally agreed testing methods |
| IPCS MOA framework | Framework to evaluate a substance's carcinogenic mode of action |
| ICH | Scientific guidelines to assess the safety, quality and efficacy of human medicines |
| VICH | Technical requirements for registration of veterinary medicinal products |
| JECFA | Safety of food additives and contaminants |
| JRC | Supports EU policies with independent scientific evidence |
| US EPA's ISA | Outlines the steps and criteria for developing integrated science assessments |
3.2. Data sources
Of all the sources of data reported to inform cancer risk assessments, the most common are publically available and include in silico tools to perform computational toxicology assessments (Table 4). Some program areas utilize databases and in silico tools that are not open access. This includes proprietary prediction tools such as Leadscope, IQVIA drug exposure data, and data available through the Canadian Institutes for Health Information. In addition, PMRA-HED, HPFB-TPD-BMORS/BCANS/BGIVD/OCT, HPFB-BRDD-CBE/CERB/CREST-OCT, HPFB-NNHPD-NDED, HPFB-MHPD, HPFB-FD-BCS, HPFB-VDD and HECSB-NSACB receive submissions from industry that contain proprietary data to inform the assessment of cancer risk.
| SOURCE | TYPE OF INFORMATION |
|---|---|
| Literature Database | |
| Pubmed | Publications |
| Scopus | Publications |
| National & International reports | |
| NTP's Report on Carcinogens | Evaluates toxicity and carcinogenicity of substances |
| IARC | Publishes monographs for carcinogenic hazards to humans |
| ATSDR | Investigate environmental exposure to substances |
| IRIS assessments | EPA's IRIS program identifies and characterizes the health hazard of chemicals found in the environment |
| EFSA assessments | Assesses risks of chemicals associated with the food chain |
| CNSC | Assesses environmental impacts of nuclear facilities |
| JECFA monographs | |
| Databases: General | |
| CEBS | Compiles individual and summary animal data from the NTP testing program and other depositors into a single electronic repository |
| Carcinogenic Potency Database (CPDB)* | Compiles results of chronic, long-term animal carcinogenicity studies |
| Lhasa Carcinogenicity Database | Compiles results of chronic, long-term animal carcinogenicity studies |
| HSDB** | Toxicology and exposure data of hazardous substances |
| Pubchem | Chemistry and toxicity data of substances |
| ToxCast | High-throughput data for chemical toxicity |
| COSMOS** | Assesses repeated dose toxicity of cosmetic ingredients |
| ToxNet** | Databases that provide toxic effects of substances |
| REACH | Evaluates individual substance registrations from companies; Assesses whether the risks of the substance can be managed. |
| Genomics | Toxicogenomics data that can be used to identify hazards and for analysis of mechanisms of action |
| AOP | Adverse Outcome Pathways (AOPs) for substances |
| Clinical trials | Privately and publicly funded clinical trials |
| Databases: For the safety assessment of pharmaceuticals post-market | |
| Canada vigilance | Adverse drug reaction online database |
| WHO VigiLyze US | Global pharmacovigilance database |
| US FDA Adverse Event Reporting System | Adverse events database |
| Computational toxicology tools | |
| OECD tool kit | Reproducible and transparent chemical hazard assessment |
| ToxTree | Estimates toxic hazard of substances |
| Air Quality Benefits Assessment Tool (AQBAT) | Estimates of human health impacts of changes in Canada's ambient air quality. |
| *: No longer updated; **: No longer available | |
4. Methodology used to perform cancer risk assessments
The Working Group members were surveyed with respect to the methodology used to perform cancer risk assessments. This included an account of approaches used by different program areas, in addition to the use of genotoxicity and carcinogenicity data to inform the cancer risk assessment. As previously outlined in section 2.2 of this report, individual program areas conduct quantitative, qualitative, or both types of cancer risk assessments. Generally speaking, where new 'in house' quantitative cancer risk assessments are conducted, the risk of cancer from exposure to a substance can be calculated (e.g., development of a slope factor or unit risk, estimation of excess cancer risk following exposure), or the margin of exposure (MOE) can be estimated. Further details on these approaches are provided below.
4.1. General approach to assessing cancer risk
When performing a cancer risk assessment, hazard identification is the first step and is performed by all surveyed program areas that conduct de novo cancer risk assessments. Within the context of cancer risk assessments, hazard identification involves evaluation of the collective weight-of-evidence and determination of whether a compound exhibits carcinogenic potential. Following this assessment, the mechanism by which tumour formation occurs is investigated, and any susceptible populations and/or life stages that require additional consideration are identified. Various sources of data are evaluated as applicable and appropriate to the program area, including the results of animal carcinogenicity studies, in vitro and in vivo genotoxicity assays, mechanistic studies, epidemiological stu-dies and, more recently, structure-activity relationship analyses (e.g., (Q)SAR, read-across with chemically similar compounds) and toxicogenomics data. For some program areas surveyed, determination of causality for carcinogenicity is also assessed by considering modified Bradford-Hill criteria.
Human relevance of animal tumour findings was also outlined as a consideration by some program areas. As an example, a number of industrial and therapeutic compounds cause liver tumours in rats via activation of the nuclear receptor known as peroxisome proliferator-activation receptor α (PPARα). A large number of mechanistic studies have demonstrated that the mechanisms leading to tumour formation in rodents via PPARα might not be relevant to humans due to biological differences in downstream responses. Therefore, liver tumour findings in rats and/or mice that exhibit a non-genotoxic PPARα mode of action (MOA) are likely not considered relevant for human cancer risk assessment.
Knowledge of the MOA of a compound is useful to determine the approach for low-dose extrapolation of a substance. This is an important component of the hazard identification process as dose-response at dose levels that are relevant to humans are typically not examined in animal carcinogenicity studies. To this end, if the weight-of-evidence suggests that a compound exhibits a mutagenic MOA, a linear (or non-threshold) dose-response curve is assumed and if a compound exhibits a non-mutagenic MOA, a non-linear (or threshold) dose-response curve is assumed. In cases where the evidence regarding MOA is not clear, a linear dose-response curve is assumed as a conservative default approach.
For compounds that exhibit a non-threshold MOA, the acceptable incremental level of risk across the surveyed program areas ranges from 10-5 to 10-6 (i.e., 1 extra cancer case per 100,000 to 1,000,000 individuals exposed or above background, depending on program area). In TPD, while the acceptable level of risk for a mutagenic impurity in a drug product that exhibits a non-threshold MOA is 10-5, an API that exhibits mutagenic potential is only considered acceptable for human use when the potential benefit is considered to outweigh the risks (e.g., serious or life-threatening disease such as advanced cancer) (Table 5).
Unique to TPD, BRDD, and MHPD of HPFB, is consideration of the cancer risk assessment within the context of a benefit-risk assessment for a pharmaceutical or biologic drug product. A benefit-risk assessment of a drug product is an overall assessment that takes into account potential clinical benefit, potential clinical harms (e.g., adverse events), non-clinical risks (e.g., reproductive toxicity, carcinogenicity), characteristics of the intended patient population (e.g., seriousness of the disease), and the availability of other treatment options. In BRDD and TPD, a favourable outcome of a benefit-risk assessment is required to grant market authorization for a new drug. The assessment of clinical trials in both TPD and BRDD primarily focuses on the risk of the product under review and the acceptability of the proposed risk mitigation for the clinical trial.
Further, in the post-market pharmacovigilance at MHPD, a cancer risk assessment can be initiated when a concern (i.e., safety signal) is detected from one or more of the routinely surveyed sources. Examples of sources of safety signals include, but are not limited to, emerging news reports in the media, intimation of new safety evaluations being undertaken by other regulatory agencies, disproportionality observed in spontaneous reporting of suspected adverse drug reactions, a new study published in the medical literature, new findings from ongoing industry-sponsored studies or safety registries, etc. New safety signals are subsequently prioritized and assessed in detail to determine a causal link (and associated certainty) between a healthcare intervention and cancer. Informed by the current best evidence, these safety signal assessments often lead to various evidence-based risk recommendations to manage and mitigate the risk (e.g., product labelling changes, communication of identified risks to the public and health professionals, education and training, controlled distribution, withdrawal of product market authorization, etc.).
4.2. Margin of exposure (MOE) approach to assessing cancer risk
Consideration of the MOE is another approach used to assess the safety concerns of genotoxic and carcinogenic substances in drugs, foods, and the environment. A MOE is derived by calculating the ratio between an adverse effect level [i.e., the lowest point of departure (POD) for a cancer endpoint] with estimated human exposure. A small MOE is associated with greater concern than a large MOE. For example, an MOE can be a useful tool to understand whether there is a safety concern at a particular level of exposure to a carcinogen (either genotoxic or non-genotoxic MOA). The MOE approach does not provide a specific cancer risk value; instead, it is a practical approach that provides information to help decide whether exposure to a substance is of concern, without further investigation/debate on the shape of the dose-response curve in the low-dose range. Although a quantitative risk estimate is not calculated, the MOA for a carcinogen (i.e., genotoxic or non-genotoxic) can still play an important role in the interpretation of a calculated MOE. Typically, for a genotoxic and carcinogenic substance, if the MOE is ³ 10,000, the level of safety concern is considered to be low.
The survey conducted by the Cancer Risk Assessment Working Group indicated that several programs (e.g., FD-BCS, VDD, SED-ESRAB) use the MOE approach to assess the safety concern of carcinogenic substances, even though these programs may have different ways in selecting the appropriate POD and in interpreting the acceptance of the MOE. For some program areas (e.g., TPD, PMRA-HED), a MOE is only calculated for a substance that exhibits a non-genotoxic MOA.
| PROGRAM | APPROACHES USED |
|---|---|
| ESRAB |
TC/TD05=tumorigenic concentration or tumorigenic dose associated with a 5% increase in the incidence of or mortality due to cancer. |
| NSACB |
|
| WQD |
|
| AHEAD |
|
| IACAS |
|
| EACSD |
|
| RAB |
|
| RPB/CCRPB |
|
| NRP |
|
| RHI |
|
| BMORS |
|
| OCT |
|
| MDD |
|
| VDD |
|
| BCS |
|
| NDED |
|
| CBE, CERB & CREST-OCT |
|
| MPB |
|
| HED |
|
4.3. Use of genotoxicity and mutagenicity data to inform cancer risk assessment methodology
Sixty-eight (68)% of survey respondents use genotoxicity/mutagenicity data to inform their assessments. The major uses (Figure 2) of this data are to inform a substance's carcinogenic MOA (direct or indirect acting genotoxic carcinogen) and to subsequently inform the risk assessment approach (threshold vs non-threshold).
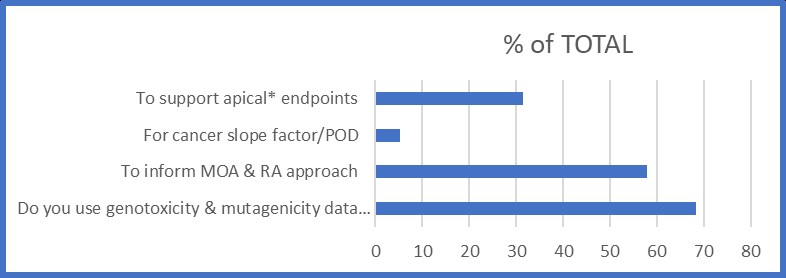
Figure 2. Uses of genotoxicity information
| Uses of genotoxicity information | Percent (%) of TOTAL |
|---|---|
| To support apical* endpoints | 31.6 |
| For cancer slope factor/ POD | 5.3 |
| To inform MOA & RA approach | 57.9 |
| Do you use genotoxicity & mutagenicity data for RA & if so, how? | 68.4 |
Figure 2. Uses of the genotoxicity information. Based on responses from 19 survey respondents across Health Canada. *Apical endpoints refer to observable outcomes in a whole organism, such as a clinical sign or pathologic state, which is indicative of a disease state that can result from exposure to a toxicant
5. Challenges encountered when conducting cancer risk assessments
Several challenges were identified by the Working Group when conducting cancer risk assessments, and are described below. These challenges relate primarily to understanding the dose-response curve for carcinogens, extrapolation of risk from high dose to low dose exposures, and uncertainty in the available data used for risk assessment.
5.1. Determination of the shape of the dose-response curve in the low-dose region
Following hazard identification (refer to section 4.1), an exposure and dose-response assessment can be performed to evaluate the risk(s) to humans at exposure levels of interest, and can involve extrapolation of animal tumour data from high doses (i.e., the dose-range typically evaluated in carcinogenicity studies) to low doses (i.e., the dose-range generally relevant to human exposure). Since the shape of the dose-response curve (i.e., linear versus non-linear) delineates whether a threshold or non-threshold approach should be used for assessing cancer risk, an important component of the hazard identification process is to determine if the totality of the data suggests that the compound exhibits a threshold or non-threshold MOA. For non-genotoxic compounds that exhibit carcinogenic potential in animal carcinogenicity studies, a threshold mechanism of action is assumed in cases where a No Observed Effect Level (NOEL) or No Observed Adverse Effect Level (NOAEL) is identified and/or MOA data is available to support a non-linear approach. Some program areas indicated that it was challenging to determine whether a threshold versus non-threshold approach should be used as the results of studies performed to evaluate the genotoxic potential of the compound are inconclusive. However, in cases where it is not clear, a non-threshold MOA is often assumed as a default approach.
Mutagenic compounds are considered to exhibit a non-threshold dose-response curve, which assumes that even a very low dose of a mutagen is associated with mutagenic effect and therefore poses a carcinogenic risk. More recently, in vitro and in vivo experiments have suggested that a sublinear, or even a threshold, dose-response curve may exist at low dose levels due to the influence of DNA repair mechanisms and cellular metabolism. And, it has been suggested that a NOEL or NOAEL can be established based on the results of low-dose mutagenicity studies, even for potent mutagens such as ethyl methanesulfonate (Muller and Gocke, 2009; ICH M7(R1)). Respondents indicated that it was challenging to determine what level of evidence is required to support a threshold dose-response curve. In addition, some respondents highlighted that the available data is sometimes controversial and inconsistent, making data interpretation challenging.
5.2. How to conduct high-dose to low-dose extrapolation
The shape of the dose-response curve (linear versus non-linear) dictates how a high-dose to low-dose extrapolation is performed. Some respondents expressed uncertainty regarding the relevance of findings in high-dose studies to human cancer risk assessment, and using this data set to extrapolate down to the low-dose level typically associated with human exposure. Similarly, respondents from ERHSD expressed uncertainty around the dose-response relationship at exposure rates and levels consistent with most public exposure scenarios, and the relevance of high dose/high dose-rate radiation exposure scenarios when deriving cancer risk for chronic low-dose exposure scenarios. A lack of high-quality scientific data was also cited as a concern.
A further challenge associated with high-dose to low-dose extrapolation, particularly for non-threshold compounds, is deducing whether the derived cancer risk estimate using linear approaches is too conservative and not appropriate for human cancer risk estimation. In NSACB, carcinogenicity studies are typically not available and the results of genotoxicity studies are used as a surrogate endpoint for carcinogenicity in the cancer risk assessment. The dose-response curve from genotoxicity tests is not determined and linearity is assumed for any genotoxicant, including clastogens. Based on this assessment, the substance is identified as a high-priority or low-priority substance for further consideration.
5.3. Uncertainty
The Working Group identified several challenges related to uncertainty with respect to the data supporting the evaluation of cancer risk and/or uncertainty associated with conducting a cancer risk assessment. They include:
- Uncertainties about historical exposure estimates (particular to radiation exposure).
- Uncertainty regarding how to estimate average daily dose for chemical exposures that are intermittent and/or exposure occurs for less than a lifetime.
- Uncertainty regarding how to estimate excess cancer risk following elevated short-term exposures.
- Lack of information to perform a meaningful cancer risk assessment such as availability of cancer slope factors and relevant human exposure data. This was a particular challenge for New Substances in NSACB.
- Uncertainty regarding the application of the appropriate uncertainty factors when deriving a HBV for threshold carcinogens and assessing the adequacy of the derived HBVs, including those derived for various sub-populations, if warranted.
- Relevance of data derived from different animal species (e.g., species-to-species extrapolation, assessing the relevance of animal tumour findings to humans).
- Uncertainty regarding how in vitro and in vivo test systems reflect human metabolism and/or chemical activation.
- Uncertainty regarding how to incorporate data from (Q)SAR and toxicogenomic studies to human cancer risk assessment.
- Ascertaining if the results of in vitro and in silico studies are sufficient to conclude that a compound does not exhibit mutagenic, genotoxic and/or carcinogenic potential.
- Uncertainty regarding the body of evidence required to conclude that a substance does not exhibit a non-threshold MOA.
- Uncertainty associated with the assessment of carcinogenicity in potentially susceptible age groups (e.g., exposure in children and application of age-dependent adjustment factors to the cancer slope factor or unit risk).
- Uncertainty with derived excess cancer risk estimates due to a lack of data on radio-sensitivity. This challenge was particular to the Radiation Protection Bureau.
- Uncertainties in establishing a causal link between a drug or device and the risk of cancer given the existing evidence. These uncertainties may be related to concerns regarding biologic plausibility, methodological validity of the evidence for causal inference, statistical power of studies, inconsistent study findings, generalisability of the evidence to the Canadian healthcare settings, selective outcome reporting and publication bias.
- Interpretation of the significance of carcinogenic health hazard data relative to environmental exposures.
To manage uncertainty associated with a particular risk assessment, a risk assessor may acknowledge the source(s) of uncertainty in the conclusions of the risk assessment and outline the assumptions that were made in the risk assessment with a rationale. In some cases, it may be appropriate to apply an additional safety or uncertainty factor when deriving a HBV, to require a larger magnitude MOE, or to apply the precautionary principle (e.g., not to incorporate data from (Q)SAR and toxicogenomic studies into a cancer risk assessment until endpoints are better validated).
6. Future needs and directions
One of the goals in developing this explanatory document was to identify needs and opportunities for future collaboration and harmonization, where possible. To this end, Working Group members discussed the needs of their respective areas, and identified some potential areas for further exploration.
6.1. Harmonization
Working Group members discussed opportunities for harmonization. It was generally agreed that standardization of cancer risk assessment methodology at Health Canada would be challenging as different branches and directorates rely on different guidelines for assessing cancer risks under different sets of regulations. Also, risk assessments have varied requirements and serve many different purposes. In some cases, the use of particular guidance documents is defined in law, making their use obligatory for certain program areas. In other cases, the use of particular guidance documents is defined by international convention, making their application more appropriate in the international context. However, in the interest of promoting consistency in approaches to assess carcinogenic risk across the Department where feasible, facilitation of information sharing was identified as a fundamental need. This point is elaborated on in the section that follows.
6.2. Information sharing
Given that all program areas used cancer risk assessments to some extent, a need to allow for efficient information sharing was identified. This need is not restricted to cancer risk assessments, but rather encompasses many areas of risk assessment for contaminants and other substances reviewed by Health Canada. Working Group members expressed a clear need to identify which program areas are currently developing risk assessments for a given substance, or had previously conducted risk assessments on a particular substance. Information sharing would help risk assessors leverage work completed by other program areas in the Department, and enhance consistency in Health Canada's risk assessments.
Some advantages of facilitating the sharing of risk assessments within Health Canada could include enhanced internal consistency, increased efficiency for conducting a risk assessment on a substance of common interest, better informed risk characterization (from understanding risks from other sources of exposure to a substance), and increased opportunities for collaboration between program areas across the Department. It is recommended that a follow-up project be adopted to explore opportunities for information sharing and to facilitate this process across the Department. Additionally, cancer risk assessments could consider data derived from toxicology studies of priority chemicals studied by Health Canada's research scientists. This would provide an opportunity for collaboration between research and regulatory personnel; moreover, for collaborative evaluation of strategies for modernization of risk assessment methodologies.
6.3. Evolving trends in the assessment of potential human cancer risk
The science of risk assessment is continually evolving. Recently, commitments have been made by the US EPA to move away from animal testing which will impact the manner in which cancer risk assessments are performed for environmental (and potentially other) substances. Similarly, changes to the framework regarding how the carcinogenic potential of a pharmaceutical is evaluated are on the horizon. Further, methods to assess cancer risk and interpret genotoxicity dose-response data continue to mature.
6.3.1. Trend towards a reduction in use of animal studies and incorporation of alternative approaches to assessing potential human cancer risk
Recently, the US EPA indicated that it will move away from animal testing, and seek to reduce its requests for and funding of mammal studies by 30 percent by 2025, and "eliminate all mammal study requests and funding by 2035". In addition, the US EPA has indicated that "Any mammal studies requested or funded by the EPA after 2035 will require administrator approval." (US EPA, 2019). Considering that scientific disciplines are seeking to reduce, refine and replace animal testing, it is reasonable to assume that results obtained from alternative toxicological testing methods and strategies will be increasingly incorporated into scientific risk assessment, including cancer risk assessments. Examples of this include toxicogenomics data (see TFSRA report on toxicogenomics (2019)), and inclusion of genotoxicity dose-response data into the cancer risk assessment if appropriate and applicable to the substance under assessment.
At present, the carcinogenic potential of pharmaceuticals is evaluated in a 2-year mouse study and a 2-year rat study, or in a 2-year study in one rodent species (typically the rat) and a short- or medium-term in vivo rodent study (e.g., transgenic mouse models of carcinogenesis such as TgRasH2) (ICH S1A; ICH S1B). Recently, retrospective evaluation of datasets suggests that knowledge of pharmacology and the outcome of various toxicity tests (e.g., genetic toxicology, chronic toxicity studies) may provide sufficient information to predict the outcome of the 2-year rat study in some cases, suggesting that a rat carcinogenicity study may not be needed in these instances (Sistare et al., 2011; Van der Laan et al., 2016). For this reason, a change to current ICH S1 guidance on rodent carcinogenicity testing is being considered to introduce a weight-of-evidence approach to assess human cancer risk where appropriate. In order to define the set of criteria when a 2-year rat study would add value to the assessment of human cancer risk of small molecule pharmaceuticals, or determine if a weight-of-evidence approach could be used in lieu of a 2-year rat study, a prospective study is currently being conducted by the ICH S1 Expert Working Group (for details refer to the Regulatory Notice Document posted on the ICH websiteFootnote 3). The study was launched in August 2013 and is ongoing; the outcome of the study will inform any revisions to the current S1 guidance (status reports are availableFootnote 4). The changes are anticipated to introduce a more integrated approach to the assessment of human cancer risk and facilitate goals to reduce, refine and replace animal testing.
Long-term studies in the mouse and rat are also required to assess the carcinogenic potential of pesticides. Given the US EPA decision to move away from animal testing, a project is underway with the aim of developing criteria for waiving the requirement for one or both of these studies for individual pesticides based on toxicology and exposure data. The Rethinking Carcinogenicity Assessment for Agrochemicals Project (ReCAAP) is led by experts from the People for the Ethical Treatment of Animals (PETA) International Science Consortium, government, and industry who are developing a strategy to move away from a "check-box" approach that includes bioassays to one based on human-relevant mechanisms of disease and other sources of information. Details about this project were presented at the 58th annual Society of Toxicology (SOT) meetingFootnote 5.
6.3.2. Interpretation of carcinogenicity dose-response data
The Working Group identified an opportunity to better characterize emerging approaches for cancer risk assessment. Although the survey identified some important resources used in the interpretation of carcinogenicity data at Health Canada, it did not include a detailed account of how dose-response data are analysed. A follow-up report that would explore various options for the use of MOA information for interpretation of carcinogenicity data would be of value. Such an exploration could scrutinize various options for the use of genotoxicity data. More specifically, such a report could examine how various groups quantify risk; it could include an overview regarding the use of genotoxicity data, and the related reliance on linear low-dose extrapolation (non-threshold) versus non-linear (threshold) approach.
6.3.3. Interpretation and potential incorporation of quantitative genotoxicity dose-response data into cancer risk assessments
In some instances, risk assessors employ genotoxicity data to inform their cancer risk assessments; such data are generally used in a qualitative manner to determine the approach used for interpretation of carcinogenicity dose-response data (i.e., linear non-threshold versus non-linear threshold). However, rather than the current paradigm that recommends the use of genotoxicity data as supportive evidence to determine the approach used to assess cancer hazard and/or risk, there is a growing body of work that is endeavouring to establish a foundation whereby genotoxicity can be treated as a bona fide toxicological endpoint (White et al., 2020). Consequently, risk assessors are now being faced with some difficult questions. For example, in the absence of carcinogenicity data, can quantitative interpretation of in vivo mutagenicity data (e.g., transgenic rodent mutation assay) be used for reliable cancer risk assessment? Alternatively, does linear low-dose extrapolation remain a valid approach to assess carcinogenic risk? A report that examines these issues, and provides some general information for Health Canada's risk assessors could assist inclusion of dose-response data into a cancer risk assessment for relevant groups across the Department as appropriate. The Working Group recommends continued monitoring of the scientific literature regarding quantitative use of in vivo mutagenicity dose-response data for cancer risk assessment and regulatory decision-making.
7. References
7.1. Guidance documents
EFSA. (2019) Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment.
Health Canada. (2000) Science Policy Note: Technical Paper: A Decision Framework for Risk Assessment and Risk Management in the Pest Management Regulatory Agency SPN 2000-01.
Health Canada. (2008) Science Policy Note: The Application of Uncertainty Factors and the Pest Control Products Act Factor in the Human Health Risk Assessment of Pesticides. SPN 2008-01.
Health Canada. (2010a) Federal Contaminated Site Risk Assessment in Canada, Part V: Guidance on Human Health Detailed Quantitative Risk Assessment for Chemicals (DQRACHEM).
Health Canada. (2010b) Federal Contaminated Site Risk Assessment in Canada, Part II: Health Canada Toxicological Reference Values (TRVs) and Chemical-Specific Factors, Version 2.0.
Health Canada. (2013) Federal Contaminated Site Risk Assessment in Canada: Interim Guidance on Human Health Risk Assessment for Short-Term Exposure to Carcinogens at Contaminated Sites.
ICH M7(R1). (2017) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk
ICH M3(R2). (2009) Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals
ICH S1A. (1995) Need for Carcinogenicity Studies of Pharmaceuticals
ICH S1B. (1997) Testing for Carcinogenicity of Pharmaceuticals
ICH S1C(R2). (2008) Dose Selection for Carcinogenicity Studies of Pharmaceuticals
ICH S2(R1). (2011) Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use
ICH S6(R1). (2011) Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals
ICH Q3A(R2). (2006) Impurities in New Drug Substances
ICH Q3B(R2). (2006) Impurities in New Drug Products
ICH Q3C(R6). (2016) Guideline for Residual Solvents
JECFA. (2016) Guidance Document for WHO Monographers and Reviewers Evaluating Veterinary Drug Residues in Food. Joint FAO/WHO Expert Committee on Food Additives.
OECD. (2002) Guidance Notes for Analysis and Evaluation of Chronic Toxicity and Carcinogenicity Studies. OECD Environment, Health and Safety Publications. Series on Testing and Assessment No. 35 and Series on Pesticides No. 14. ENV/JM/MONO(2002)19
US EPA. (2015) Preamble to the Integrated Science Assessments (ISA). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-15/067.
US EPA. (2005a) Guidelines for Carcinogen Risk Assessment.
US EPA. (2005b) Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. Risk Assessment Forum.
VICH GL28. (2005) Studies to Evaluate the Safety of Residues of Veterinary Drugs in Human Food: Carcinogenicity testing. International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH).
VICH GL23 (R). (2014) Studies to Evaluate the Safety of Residues of Veterinary Drugs in Human Food: Genotoxicity testing. International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH).
7.2. Literature references
Annys E., Billington R., Clayton R., Bremm K.D., Graziano M., McKelvie J., Ragan I., Schwarz M., van der Laan J.W., Wood C., Öberg M., Wester P., Woodward K.N. (2014). Advancing the 3Rs in Regulatory Toxicology - Carcinogenicity testing: Scope for Harmonisation and Advancing the 3Rs in Regulated Sectors of the European Union. Regulatory Toxicology and Pharmacology. 69(2): 234-242.
Boobis A., Brown P., Cronin M.T.D., Edwards J., Galli C.L., Goodman J., Jacobs A., Kirkland D., Luijten M., Marsaux C., Martin M., Yang C., Hollnagel H.M. (2017). Origin of the TTC Values for Compounds that are Genotoxic and/or Carcinogenic and an Approach for their Re-Evaluation. Critical Reviews in Toxicology. 47(8): 710-732.
Madia F., Worth A., Corvi R. (2016). Analysis of Carcinogenicity Testing for Regulatory Purposes in the European Union. Review of the Current Demand of in vivo Carcinogenicity Studies Across Sectors. JRC Technical Reports.
Meek, M. E., Boobis, A., Cote, I., Dellarco, V., Fotakis, G., Munn, S., Seed, J., & Vickers, C. (2014). New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. Journal of Applied Toxicology. 34(1): 1-18.
Muller L. and Gocke E. (2009). Considerations Regarding a Permitted Daily Exposure Calculation for Ethyl Methanesulfonate. Toxicology Letters. 190(3): 330-332.
Sistare F.D., Morton D., Alden C., Christensen J., Keller D., Jonghe S.D., Storer R.D., Reddy M.V., Kraynak A., Trela B., Bienvenu J.G., Bjurström S., Bosmans V., Brewster D., Colman K., Dominick M., Evans J., Hailey J.R., Kinter L., Liu M., Mahrt C., Marien D., Myer J., Perry R., Potenta D., Roth A., Sherratt P., Singer T., Slim R., Soper K., Fransson-Steen R., Stoltz J., Turner O., Turnquist S., van Heerden M., Woicke J., DeGeorge J.J. (2011) An Analysis of Pharmaceutical Experience with Decades of Rat Carcinogenicity Testing: Support for a Proposal to Modify Current Regulatory Guidelines. Toxicologic Pathology. 39(4): 716-744.
Toxicogenomics Working Group (2019). Evaluation of the use of toxicogenomics in risk assessment at Health Canada: An Exploratory Document on Current Health Canada Practices for the Use of Toxicogenomics in Risk Assessment. Prepared for the Task Force on Scientific Risk Assessment.
US EPA (2019). Memorandum on the Directive to Prioritize Efforts to Reduce Animal Testing. From Andrew R. Wheeler. September 10, 2019.
Van der Laan, J.W., Kasper P., Silva Lima B., Jones D.R., Pasanen M. (2016). Critical Analysis of Carcinogenicity Study Outcomes. Relationship with Pharmacological Properties. Critical Reviews in Toxicology. 46(7): 587-614.
White, P.A., Long, A.S., Johnson G.E. (2020). Quantitative Interpretation of Genetic Toxicity Dose‐Response Data for Risk Assessment and Regulatory Decision‐Making: Current Status and Emerging Priorities. Environmental and Molecular Mutagenesis. 61: 66-83.
Footnotes
- Footnote 1
-
Throughout this document, the terms 'acceptable' risk, 'acceptable' intake, 'acceptable' level, etc. are used. This terminology reflects what is typically used in regulatory guidance documents and/or in regulations.
- Footnote 2
-
In TPD, the 'benefit-risk assessment' is referred to as a benefit/harm/uncertainty assessment. A drug product is approved when the product's benefit exceeds any potential harm, with a reasonable degree of certainty.
- Footnote 3
-
https://database.ich.org/sites/default/files/S1%28R1%29_EWG_RND.pdf
- Footnote 4
-
https://database.ich.org/sites/default/files/S1_StatusReport_2019_0802.pdf (most recent status report; refer to the ICH website for updates)
- Footnote 5
-
https://www.piscltd.org.uk/wp-content/uploads/2019/03/SOT-2019-Poster_Agchem-Bioassay-Waiver_Final_GMH.pdf
