Annual Report 2019-2020
Table of Contents
- Message from the Chair
- About the Health Canada-PHAC REB
- REB Meetings and Reviews
- Profile of Applications Received in 2019-20
- Application Review Process and Outcomes
- REB Education and Information Sessions
- Secretariat Activities
- Membership
Message from the Chair

On behalf of the Health Canada-PHAC REB, I am pleased to present this report on the REB’s activities for the 2019-20 fiscal year. For nearly 20 years the REB has played an essential role for Health Canada and PHAC in providing ethical oversight of research with humans. The fundamental purpose of research ethics review is to ensure the highest level of protection to human participants in research while encouraging that research to occur. This report has been prepared in the spirit of transparency and accountability to underscore Health Canada and PHAC’s commitment to this principle through its REB.
It has been my privilege to chair the REB since 2015, and prior to that to serve as a regular member of the board. I continue to be inspired by the dedication of the REB members who freely volunteer their time to serve on the board. The thoroughness of their reviews and the quality of the discussions are always of the highest standard. I am also grateful for the relationships that we as a board have been able to develop with many of the researchers who apply to the REB. Ensuring the highest standards in research ethics depends on a partnership between the REB and the researchers, and it is clear that Health Canada and PHAC researchers recognize the value that ethics review brings to their work.
The REB is supported by a very capable Secretariat, which has been managed by Dr. Gregory Huyer since March 2019. I would like to express my sincere thanks to Dr. Peter Monette for his excellent work as interim Manager on top of his regular role as Manager of Bioethics and Science Advice. Under Dr. Huyer’s leadership, the REB Secretariat has implemented a number of operational changes that have improved the REB process for the REB members and applicants alike.
The REB continues to evolve itself as members’ terms run out. I have been amazed at the number and high quality of applicants for open positions; I am confident our new members’ solid backgrounds will only enhance the REB’s expertise.
I also want to recognize the exceptional work of the REB and the Secretariat in response to the COVID-19 pandemic. By late January it was apparent that we were facing an extremely serious situation in Canada and around the world, and the urgent need for research on COVID-19 meant a concomitant need for expedited ethics reviews. The REB members responded without hesitation – despite the impact of the pandemic on their own professional and personal lives – and ensured that urgent protocols were reviewed in as little as 24 to 48 hours, without compromising the thoroughness of their evaluations. I would also like to express my enormous respect for the researchers studying COVID-19, particularly at the National Microbiology Laboratory, who have put so much effort into studying the virus: it is truly an honour for the REB to support the work of such selfless and dedicated scientists.
A unique aspect of the research conducted by Health Canada and PHAC is its often direct contribution to the development of Canadian policy and regulations. Because these policies and regulations affect many, if not all Canadians, it is critical that decision-makers base their decisions on ethically-sound research. The REB takes this responsibility seriously, both in terms of initial review and in following the research progress. I thank all the REB members, the REB Secretariat and the scientists and officials at Health Canada and PHAC for their collective commitment in service of the public good.
Barbara McGillivray, MD, FRCP, FCCMG
Chair, Health Canada-PHAC Research Ethics Board
About the Health Canada-PHAC REB
The Health Canada and Public Health Agency of Canada Research Ethics Board (HC-PHAC REB) is a joint REB for both Health Canada and the Public Health Agency of Canada. The REB provides support and independent ethics review for all proposed and ongoing research involving humans, to ensure the highest ethical standards are being met and that the greatest protection is provided to participants. The REB reviews applications in accordance with the considerations set out in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) as the minimum standard. The REB is also guided by relevant federal laws and regulations, such as the Privacy Act and clinical trial regulations, where applicable.
The HC-PHAC REB reports to the Deputy Minister of Health and the President of PHAC, who jointly appoint REB members, approve REB procedures and authorize research to be initiated or terminated. The REB makes recommendations to the Deputy Minister and President (through their delegated Decisional Authorities) as to whether research projects should be approved, rejected, modified or terminated. The REB also provides ethical oversight of all approved research throughout the duration of the project. Finally, the REB serves various educational functions for Health Canada and PHAC managers and researchers.
The HC-PHAC REB is responsible for the ethics review of all research involving humans that is:
- Carried out by Health Canada or PHAC;
- Performed by Health Canada or PHAC in collaboration with external researchers;
- Carried out on Health Canada or PHAC premises; or
- Conducted under contract to Health Canada or PHAC.
The REB may also review research involving humans that is funded through Health Canada or PHAC grants and contributions.
REB Meetings and Reviews
The full REB meets monthly (except for August), either by teleconference or face-to-face in Ottawa. A minimum of five members is required for quorum (one member each with expertise in ethics and in law, one community member, and two researcher members). All REB members also attend delegated review meetings on a rotating basis. Delegated review meetings are held weekly by teleconference (bi-weekly in July and August) and are attended by the Chair (or Deputy Chair) and one other REB member.
Applications for initial review of research involving humans are normally reviewed at the monthly meetings of the full REB, while delegated review meetings typically evaluate submissions for continuing ethics review (i.e., annual progress reports, amendment requests, adverse event reports and completion/termination reports).
The REB met as a full board 11 times in fiscal year 2019-20:
- 4 face-to-face meetings
- 7 meetings by teleconference
In addition, there were 45 delegated review meetings in 2019-20.
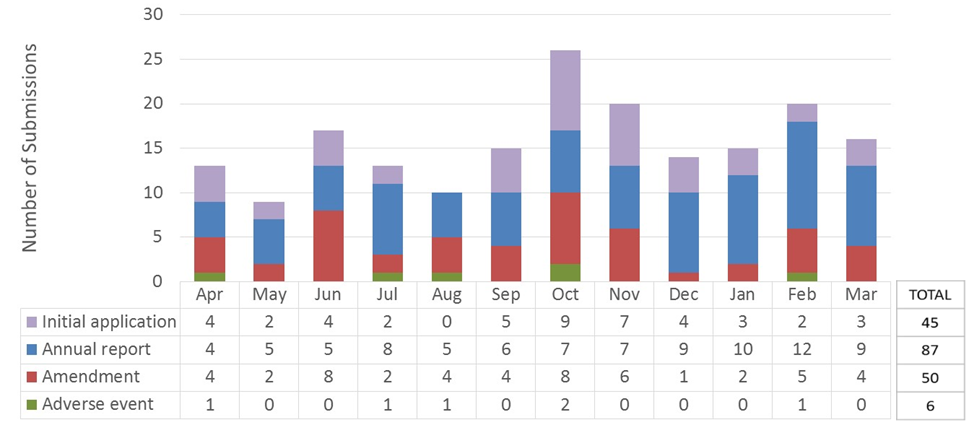
Figure 1 - Text Equivalent
This figure is a bar chart with 12 bars showing the number of submissions reviewed by the REB in the 2019-2020 fiscal year. Each bar corresponds to a month of the year, starting with April and ending with March. Each bar is further subdivided into 4 sections corresponding to the type of submission that was reviewed (initial application, annual progress report, amendment request and adverse event report). The data are as follows:
| Month | Initial applications | Annual reports | Amendments | Adverse events | Total |
|---|---|---|---|---|---|
| April | 4 | 4 | 4 | 1 | 13 |
| May | 2 | 5 | 2 | 0 | 9 |
| June | 4 | 5 | 8 | 0 | 17 |
| July | 2 | 8 | 2 | 1 | 13 |
| August | 0 | 5 | 4 | 1 | 10 |
| September | 5 | 6 | 4 | 0 | 15 |
| October | 9 | 7 | 8 | 2 | 26 |
| November | 7 | 7 | 6 | 0 | 20 |
| December | 4 | 9 | 1 | 0 | 14 |
| January | 3 | 10 | 2 | 0 | 15 |
| February | 2 | 12 | 5 | 1 | 20 |
| March | 3 | 9 | 4 | 0 | 16 |
| Total | 45 | 87 | 50 | 6 | 188 |
The number of open protocols varies throughout the year as the REB approves new applications and closes old protocols. On March 31, 2020, there were 145 open protocols and 22 protocols were closed in 2019-20.
Profile of Applications Received in 2019-20
The REB received 43 applications in 2019-20, 24 from Health Canada and 19 from PHAC. By comparison, 31 applications were received in 2018-19, and 45 in 2017-18. External protocols were led by researchers outside of Health Canada and PHAC, either in collaboration with Health Canada or PHAC researchers or funded by Health Canada or PHAC.
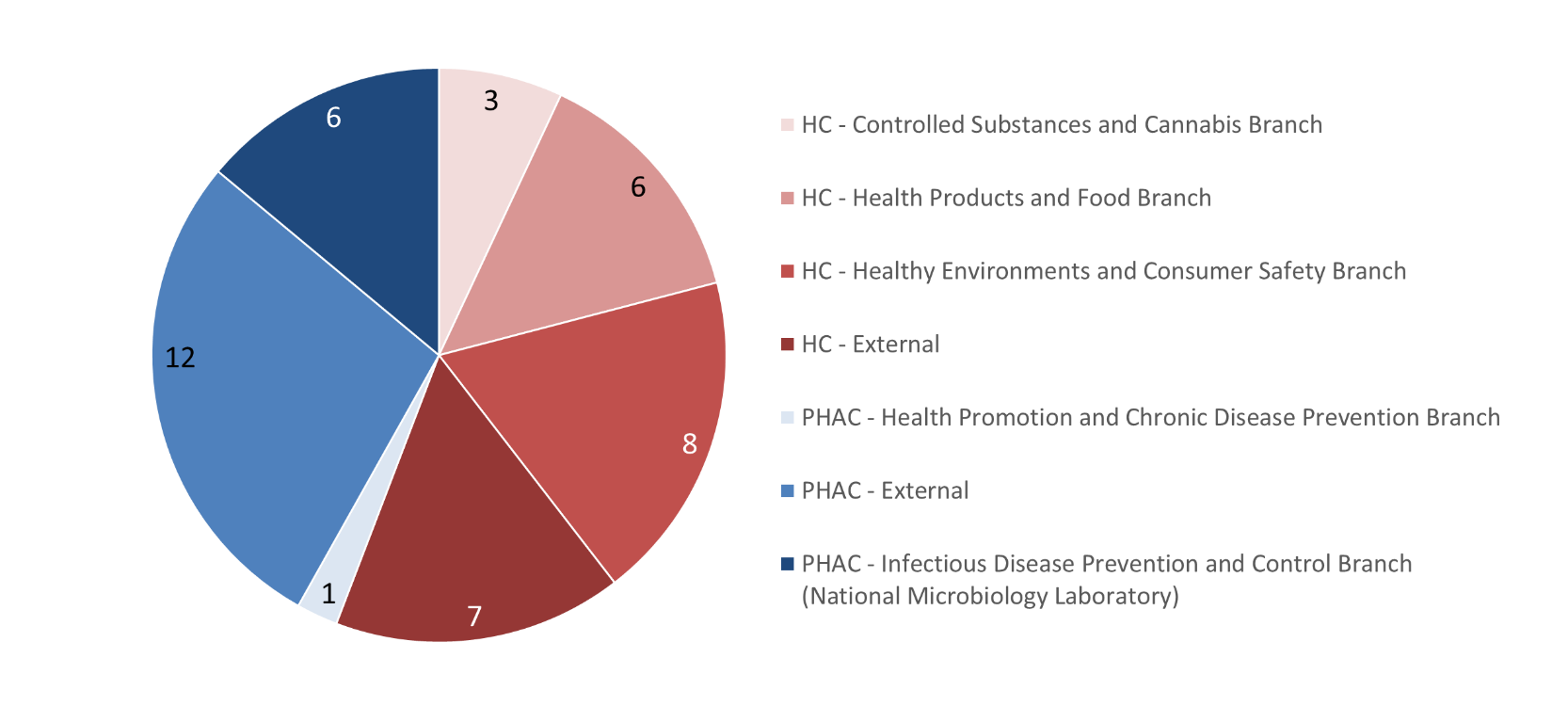
Figure 2 - Text Equivalent
This pie chart illustrates the affiliation of the lead investigator for the REB applications received during the 2019-2020 fiscal year. The data are as follows:
- Among the 24 applications received from Health Canada:
- 3 were from the Controlled Substances and Cannabis branch
- 6 were from the Health Products and Food Branch
- 8 were from the Healthy Environments and Consumer Safety Branch
- 7 were external.
- Among the 19 applications received from PHAC:
- 1 was from the Health Promotion and Chronic Disease Prevention Branch
- 12 were external
- 6 were from the Infectious Disease Prevention and Control Branch (National Microbiology Laboratory).
The protocols received spanned a broad range of research areas. Three studies involved Indigenous populations, and two were related to COVID-19.
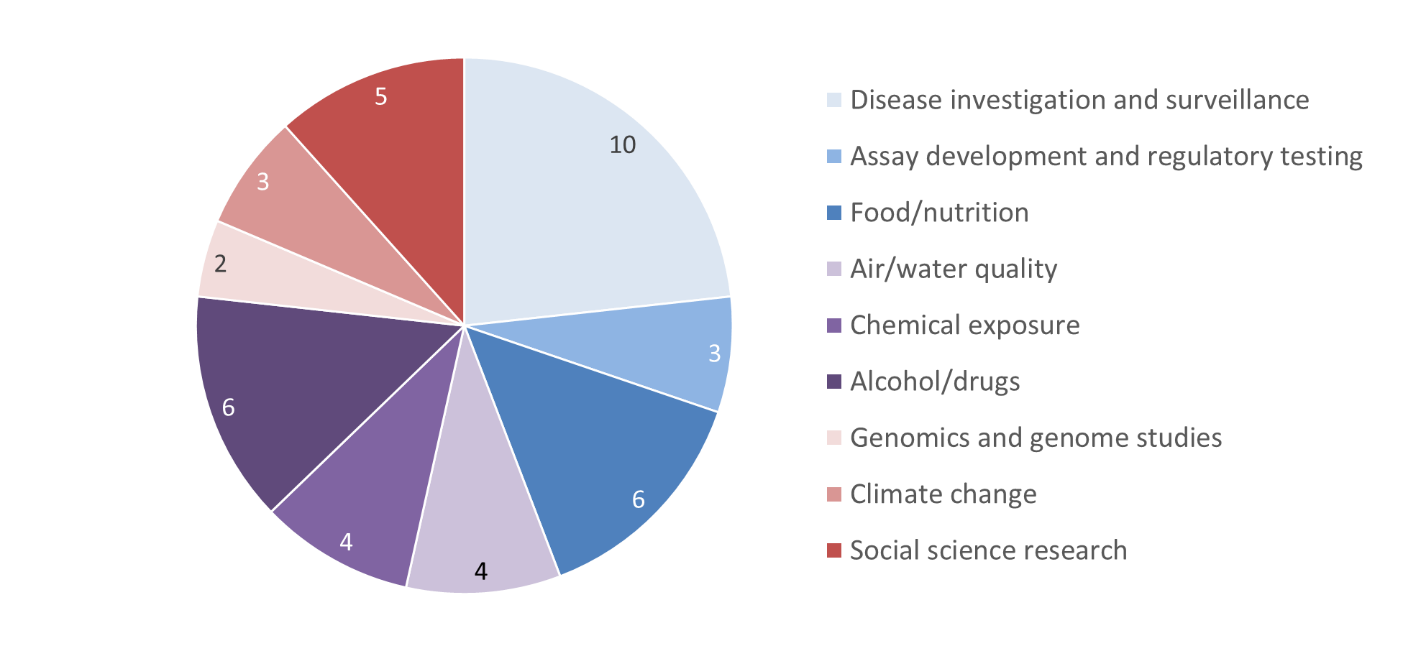
Figure 3 - Text Equivalent
This part chart illustrates the subject areas of the protocols received by the REB for the 2019-2020 fiscal year. The data are as follows:
- 10 were about disease investigation and surveillance
- 3 were about assay development and regulatory testing
- 6 were about food and nutrition
- 4 were about air/water quality
- 4 were about chemical exposure
- 6 were about alcohol and drugs
- 2 were genomics and genome studies
- 3 were about climate change
- 5 were social science research.
Application Review Process and Outcomes
The REB applies a proportionate approach to research ethics review; that is, the level of risk to participants determines the level of scrutiny by the REB. Thus, at the discretion of the REB chair, applications deemed to be minimal risk can be sent to delegated review (and if the risk is determined to be greater than initially thought, subsequently referred to the full board for further review).
Following the review of an application, the REB makes one of the following recommendations:
A: Approved as submitted: The application meets ethics requirements for research involving humans and is approved.
B: Approved with modifications/clarifications: The application meets ethics requirements for research involving humans, but the REB members require modification of the application or clarification or further information to secure approval. The revised material may be reviewed via delegated review or a subset of the members as directed by the REB Chair.
C: Deferral: The application does not meet ethics requirements for research involving humans and is not approved as conditions need to be met. The revised material must be reviewed by the full board via teleconference or face to face, or by email circulation to all REB members.
D. Disapproval: The application fails to meet the ethical standards for approval and revision is unlikely to enable the REB to reach a positive determination.
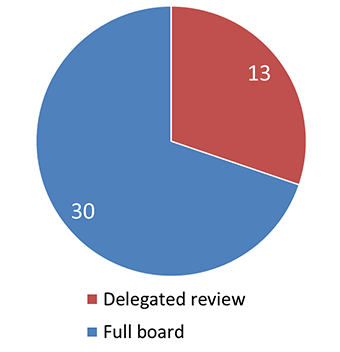
Figure 4 - Text Equivalent
This pie chart illustrates the level of review that initial applications received by the REB. 13 applications were sent to delegated review and 30 applications received full board review.
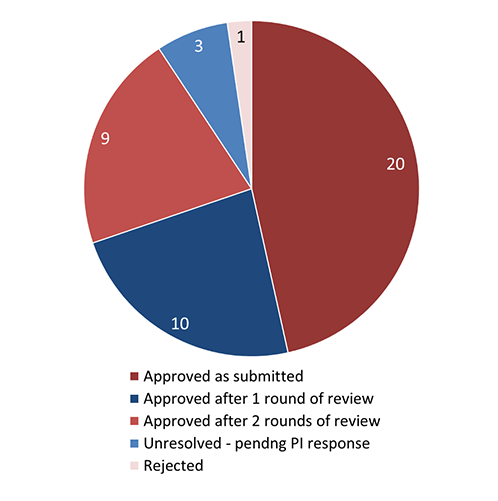
Figure 5 - Text Equivalent
This pie chart illustrates the outcome of the REB review for initial applications. The data are as follows:
- 20 applications were approved as submitted
- 10 applications were approved after 1 round of review
- 9 applications were approved after 2 rounds of review
- 3 applications were unresolved, pending a response from the Principal Investigator
- 1 application was rejected.
Approval times: The time from application submission to approval is a function of the REB Secretariat, the REB and the applicant. To help accelerate the approval time, the REB Secretariat changed the application deadline from approx. one month prior to the REB meeting to three weeks, beginning in January 2020. In the vast majority of cases, the REB Secretariat communicates the REB’s recommendations to the applicant within one week of the REB meeting. The REB also conducted several expedited reviews in March 2020 to ensure the rapid approval of urgent COVID-19 related research.
| - | Average | Std. Dev. | Median |
|---|---|---|---|
| Days with REB | 42 | 18 | 40 |
| Days with applicant | 25 | 47 | 5 |
| Total | 67 | 51 | 51 |
|
|||
The following graph shows the time distribution for each of the 40 applications that were approved or resolved by March 31, 2020
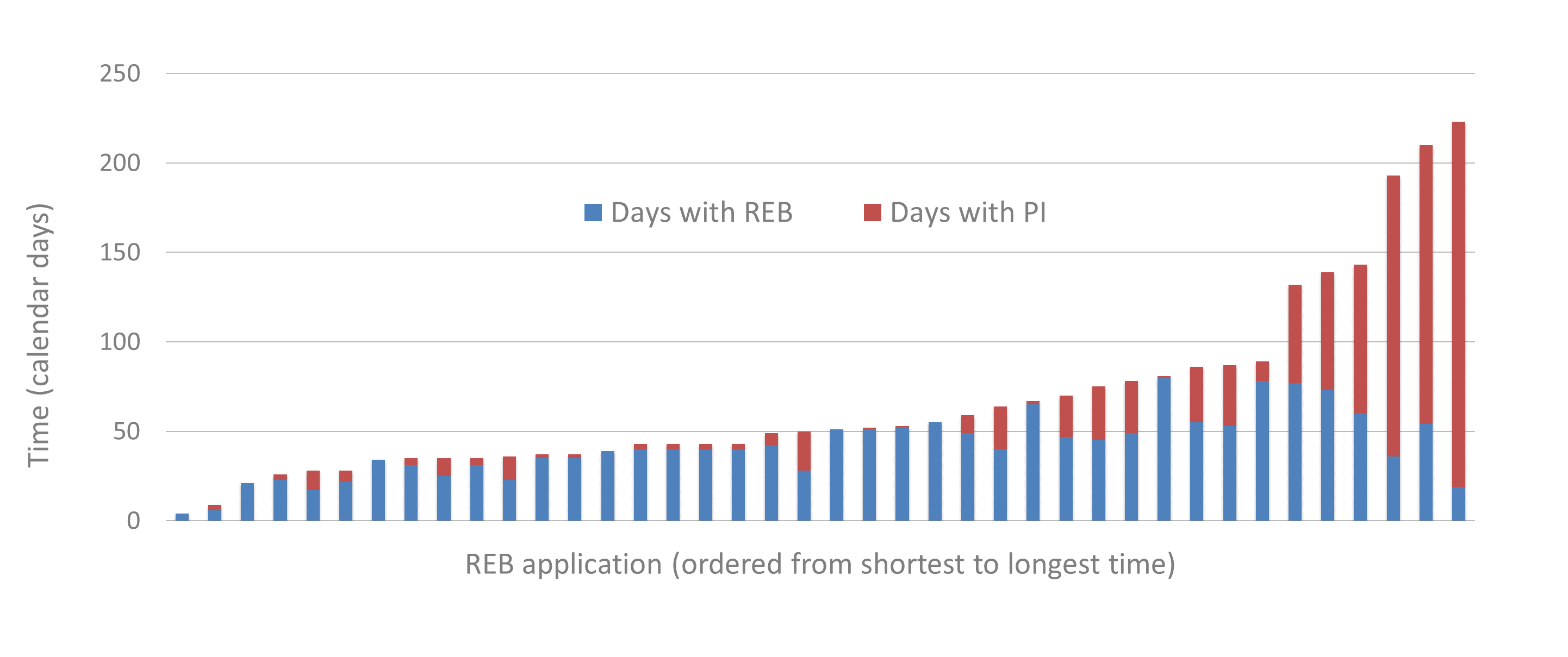
Figure 6 - Text Equivalent
This bar graph contains 40 bars, 1 bar for each application that was approved or resolved by March 31, 2020. The bars are further divided into 2 sections: number of days with the REB and number of days with the principal investigator (PI). The 40 bars are ordered from the shortest to longest amount of time by calendar days.
| Application number | Days with REB | Days with PI | Total |
|---|---|---|---|
| 1 | 4 | 0 | 4 |
| 2 | 6 | 3 | 9 |
| 3 | 21 | 0 | 21 |
| 4 | 23 | 3 | 26 |
| 5 | 17 | 11 | 28 |
| 6 | 22 | 6 | 28 |
| 7 | 34 | 0 | 34 |
| 8 | 31 | 4 | 35 |
| 9 | 25 | 10 | 35 |
| 10 | 31 | 4 | 35 |
| 11 | 23 | 13 | 36 |
| 12 | 35 | 2 | 37 |
| 13 | 35 | 2 | 37 |
| 14 | 39 | 0 | 39 |
| 15 | 40 | 3 | 43 |
| 16 | 40 | 3 | 43 |
| 17 | 40 | 3 | 43 |
| 18 | 40 | 3 | 43 |
| 19 | 42 | 7 | 49 |
| 20 | 28 | 22 | 50 |
| 21 | 51 | 0 | 51 |
| 22 | 51 | 1 | 52 |
| 23 | 52 | 1 | 53 |
| 24 | 55 | 0 | 55 |
| 25 | 49 | 10 | 59 |
| 26 | 40 | 24 | 64 |
| 27 | 65 | 2 | 67 |
| 28 | 47 | 23 | 70 |
| 29 | 45 | 30 | 75 |
| 30 | 49 | 29 | 78 |
| 31 | 80 | 1 | 81 |
| 32 | 55 | 31 | 86 |
| 33 | 53 | 34 | 87 |
| 34 | 78 | 11 | 89 |
| 35 | 77 | 55 | 132 |
| 36 | 73 | 66 | 139 |
| 37 | 60 | 83 | 143 |
| 38 | 36 | 157 | 193 |
| 39 | 54 | 156 | 210 |
| 40 | 19 | 204 | 223 |
REB Education and Information Sessions
As noted in TCPS 2, an important component of REB meetings is the inclusion of educational opportunities that may benefit the overall operation of the REB. To this end, a broad range of educational sessions were organized over the course of the year, as described below. The list also includes a number of presentations from Health Canada and PHAC researchers about their programs of research. These interactions served not only to educate the members about emerging research areas but also to strengthen the relationships between the REB and researchers. The REB would like to thank all the presenters for generously giving of their time to enlighten the REB.
- Privacy management and the role of the Privacy Management Division – Andrea Rousseau and Jeremy Mercer, Health Canada-PHAC Privacy Management Division (April 2019)
- Re-consenting longitudinal cohorts – Dr. Stuart Nicholls, Faculty of Medicine, University of Ottawa (and HC-PHAC REB member) (June 2019)
- Ethical considerations in metagenomics research – Dr. Morag Graham, National Microbiology Laboratory, PHAC (June 2019)
- The Canadian Disaster Research Response Framework (CanDR2) – Marc Lafontaine, Danny Sokolowski and Bill Casley, Healthy Environments and Consumer Safety Branch, Health Canada (June 2019)
- Sex and gender in research – Cindy Moriarty and Jennifer Payne, Strategic Policy Branch, Health Canada (June 2019)
- The Canadian Health Measures Survey – Scott McLean, Ron Gravel and Anie Marcil, Statistics Canada (June 2019)
- Social science research supporting nutrition labelling policies and regulations – Dr. Beth Mansfield, Health Products and Food Branch, Health Canada (June 2019)
- The Maternal-Infant Research on Environmental Chemicals (MIREC) research platform – Dr. Mandy Fisher and Dr. Robin Shutt, Healthy Environments and Consumer Safety Branch, Health Canada (June 2019)
- Canadian Agency for Drugs and Technologies in Health (CADTH) patient engagement activities – Tamara Rader and Andrea Smith, CADTH (June 2019)
- Overview of the Canadian Paediatrics Surveillance Program (CPSP) – Dr. Charlotte Moore-Hepburn, Hospital for Sick Children, and Ms. Melanie Laffin, Canadian Paediatric Society (October 2019)
- Citizen science research – Achla Joshi and Tara Bower, Healthy Environments and Consumer Safety Branch, Health Canada (February 2020)
Secretariat Activities
The Health Canada-PHAC REB is supported by a Secretariat that administers the REB review process. The REB Secretariat works closely with REB members and applicants to ensure a quality and timely review of all research proposals received for ethics review. The Secretariat is based at Health Canada within the Strategic Policy Branch (Science Policy Directorate).
Key Secretariat activities for 2019-20 included the following:
Outreach:
- Dr. Barbara McGillivray (REB Chair) and Melinda Lee Choon (from the REB Secretariat) attended the Canadian Association of Research Ethics Boards (CAREB) annual conference in Winnipeg in April 2019. The conference addressed a number of topics including research with Indigenous people, research with youth, and changes being made to TCPS 2. Dr. McGillivray also spent a day at the National Microbiology Laboratory (NML) to meet with researchers, including to discuss ethical issues associated with metagenomics research.
- Dr. McGillivray and Dr. Gregory Huyer (from the REB Secretariat) participated in a two-day workshop at the NML in Winnipeg in September 2019, along with representatives from the PHAC Office of the Chief Science Officer and the Privacy Management Division. The workshop was a follow-up to the 2018 Research Ethics and Privacy Matters Workshop and included presentations on the REB application and evaluation process, the new PHAC departmental approval form and the new privacy risk assessment process. The workshop was well attended by over 100 NML researchers.
- The REB Secretariat provided ongoing support to the CanDR2 (Canadian Disaster Research Response framework) initiative, with a particular focus on developing a disaster response research protocol that REBs can pre-approve for expedited use. Discussions also focused on strategies to engage other REBs to promote widespread adoption of the “rapid protocol” once complete.
- The REB Secretariat worked with a variety of federal departments (including the National Research Council, Justice Canada, Employment and Social Development Canada, and Indigenous Services Canada) to discuss research ethics issues, provide advice and share best practices.
Operations:
- A new fillable PDF application form for initial ethics review was created to streamline the application process. Detailed instructions for completing the form and preparing a research protocol were also posted on the REB website.
- The REB website received a major refresh to correct out-of-date information and provide clearer information about the REB application and review process, including the privacy risk assessment conducted by the Privacy Management Division.
- The location for in-person REB meetings was changed from an Ottawa hotel to Health Canada/PHAC premises. In addition to significant cost savings, this operational change afforded greater flexibility in planning meetings (including replacing a face-to-face meeting with a teleconference) by eliminating the potential for hotel cancellation penalties. As a result, the application deadline could be reduced from one month before a meeting to three weeks, resulting in a faster turn-around time for applicants.
- A number of internal operational efficiencies were implemented to reduce the use of paper and decrease processing times, with the goal of minimizing any delays in communicating with applicants. Email templates were also updated and streamlined to improve communications and information-sharing with applicants.
- A Microsoft Access database was created to better track REB workflow, increase efficiency by automating more tasks and allow for improved reporting on REB activities.
- The Secretariat supported the Health Canada-PHAC Privacy Management Division in implementing their risk-based approach to privacy assessments. This streamlined approach has reduced the administrative burden for applicants and resulted in faster turnaround times.
Membership
The REB consists of nine regular and nine alternate members with expertise in the following areas:
- Two members with knowledge/expertise in research ethics;
- One member with knowledge/expertise in law;
- One member from Health Canada with knowledge/expertise in Health Canada research;
- One member from PHAC with knowledge/expertise in PHAC research;
- One member external to Health Canada and PHAC with broad knowledge/expertise in both Health Canada and PHAC research;
- One member with broad expertise in public health;
- One member recruited from the community (general population) served by Health Canada and PHAC; and
- One member from the Indigenous community.
Members are appointed by the Deputy Minister of Health and the President of PHAC for a term of three years, renewable once. The terms for three long-time REB members ended in June 2019:
- Katherine Dinner, M.Sc. (Nursing) – Public Health Agency of Canada Researcher
- Robert Kouri, D.C.L. – Ethics
- Donald Sutherland, M.D. – Public Health Community Member
Health Canada and PHAC are especially grateful for their expert contributions to the REB over the years, and they are very much missed by the REB members and Secretariat.
The REB welcomed the following individuals who joined the REB in 2019-20 to fill various vacancies:
- Stephanie Booth, D.Phil. – Public Health Agency of Canada Researcher (regular): Dr. Booth is PHAC’s senior research scientist on prion diseases and has a laboratory at the National Microbiology Laboratory in Winnipeg.
- Guillaume Poliquin, M.D. – Public Health Agency of Canada Researcher (alternate): Dr. Poliquin is the Medical Advisor to the Scientific Director General at the National Microbiology Laboratory. He practices pediatrics in Winnipeg as well as remote communities in northern Manitoba and Nunavut, and he has a research portfolio focused primarily on vaccine research.
- Kue Young, M.D., D.Phil. – Public Health Community Member (regular): Dr. Young is the former Dean of the School of Public Health, University of Alberta. His major research interest is in northern and Indigenous health, and he was inducted as a Member of the Order of Canada for his lifetime contributions to improving the health of Indigenous people.
- Michael Wray Clarke, Ph.D. – Public Health Community Member (alternate): Dr. Clarke has had a long career in public health in both academia (faculty member at Western University) and the federal government (Director at the International Development Research Centre). He also serves on the board of the Middlesex-London Public Health Unit and the Southwest Middlesex Health Centre.
Finally, the REB was pleased to welcome back Marie-Ève Couture Ménard who returned to the REB in December 2019 after a maternity leave.
REB Membership as of March 31, 2020:
- Hanan Abramovici, Ph.D. – Health Canada Researcher
- Stephanie Booth, D.Phil. – Public Health Agency of Canada Researcher
- Michael Wray Clarke, Ph.D. – Public Health Community Member
- Ghislaine Cleret de Langavant, Ph.D. – Ethics
- Marie-Ève Couture Ménard, D.C.L. – Law
- Meredith Curren, Ph.D. – Health Canada Researcher
- Barbara McGillivray, M.D. – Chair and Researcher External to the Health Canada and the Public Health Agency of Canada
- Melanie McPhail, J.D. – Law
- Jean-Frédéric Ménard, LL.B., B.C.L. – Law
- Carla Moore, M.A. – Community Member, Indigenous Population
- Stuart Nicholls, Ph.D. – Researcher External to Health Canada and Public Health Agency of Canada
- Meeka Otway – Community Member, Indigenous Population
- Guillaume Poliquin, M.D. – Public Health Agency of Canada Researcher
- Sandra Romain, Ph.D. – Community Member, General Population
- Aïssata Sako, B.A. – Community Member, General Population
- Nancy Walton, Ph.D. – Ethics
- Kue Young, M.D., D.Phil. – Public Health Community Member
REB Secretariat:
- Gregory Huyer, Ph.D. – Manager
- Melinda Lee Choon – Senior Research Ethics Coordinator
- Suzi Vivolo – Administrator
PHAC liaison to the REB Secretariat:
- Trista Takacs, Ph.D. – Policy Analyst, Office of the Chief Science Officer (April – Dec., 2019)
- Erica Bernier – Policy Analyst, Office of the Chief Science Officer (effective Jan., 2020)