Research Ethics Board (REB) Annual Report 2021-22
Table of contents
- Executive summary
- Message from the Chair
- About the Health Canada-PHAC REB
- REB meetings and reviews
- Profile of applications received in 2021-22
- Application review process and outcomes
- REB education and information sessions
- Secretariat activities
- Membership
- References
Executive summary
The Health Canada (HC) and Public Health Agency of Canada (PHAC) Research Ethics Board (REB) is a joint board for both the Department and the Agency.
The REB provides support and independent ethics review for all proposed and ongoing research involving humans carried out under the auspices of Health Canada and PHAC, to help ensure that the highest ethical standards are being met and the greatest protection is provided to participants.
Due to the ongoing COVID-19 pandemic, there were no in-person REB meetings in 2021-22; instead, all meetings (8 full board and 64 delegated review) were held by videoconference. Otherwise, the impact of the pandemic on the REB's activities was reduced as compared to 2020-21. In particular, the REB received fewer requests in 2021-22 to conduct expedited reviews, and only 10 of the 54 applications (18.5%) were related to COVID-19, as compared to 19 out of 44 (43%) in the previous fiscal year.
The REB received 54 new applications in 2021-22, of which 30 were from Health Canada and 24 from PHAC. The vast majority of initial applications (46 out of 54, or 85%) were deemed to be minimal risk and therefore reviewed at delegated review meetings, compared to the previous year in which 34 out of 44 applications (77%) were considered minimal risk. The time from application submission to approval was an average of 42 calendar days (23 days with the REB and 19 days with the applicant). The REB also reviewed 124 annual progress reports, 79 amendment requests and 2 adverse event reports in 2021-22, and 28 protocols were closed.
In addition to conducting ethics reviews, the REB received a number of education and information sessions in 2021-22. While there were fewer sessions than in previous years because of the pandemic, the shift to videoconference meetings increased participation by making the sessions more easily accessible to the entire membership. In terms of membership, two members left the REB in 2021-22 and three new members were welcomed to the board.
The REB Secretariat continued its outreach efforts among Health Canada and PHAC staff, including training sessions, targeted consultations and participation in the Health Canada Science Forum. Additional refinements were also made to the operational improvements from the prior year, including revisions to the REB website, forms and templates for correspondence with applicants. The combination of a robust operational base and a strong cadre of REB members has enabled the board to deliver effectively on its mandate and ensures that it can continue to adapt to an ever-evolving research landscape.
Message from the Chair

On behalf of the Health Canada (HC) and Public Health Agency of Canada (PHAC) Research Ethics Board (REB), it is my pleasure to report on our REB's activities for the 2021-22 fiscal year.
This report is intended for a diverse audience, beginning with the Deputy Minister of Health and the President of PHAC to whom the REB reports, and extending to researchers and other staff at Health Canada and PHAC as well as to the general public. This report supports the Department and Agency's commitment to transparency and accountability, and I hope it provides the reader with a better understanding of the role and operation of the REB.
This has been a notable year in many respects, beginning with the fact that 2022 marks the 20th anniversary of the REB. While there have been many changes over the past 20 years, there have been two essential constants: the REB's commitment to supporting the highest degree of protection to human participants in Health Canada and PHAC research and the dedication of board members to carrying out this critical responsibility. For this, I would like to extend my sincere thanks to all the REB members, past and present.
The past year has been marked by the ongoing COVID-19 pandemic, the most obvious impact being the inability to meet in person for the second year in a row. The board has adapted exceptionally well to virtual meetings (save for the occasional refrain of "you're on mute"); nevertheless, I hope the coming year affords the opportunity to safely renew old acquaintances and meet new members who joined during the pandemic in person.
The 2021-22 fiscal year also saw a shift in the REB's work. While there was still a need for some expedited reviews for time-sensitive COVID-related studies, there were fewer requests than in 2020-21. In addition, there were fewer COVID-related studies in both relative and absolute terms, suggesting that researchers were returning to projects that were delayed or put on hold during the first year of the pandemic. As always, I am grateful for the REB's responsiveness to urgent review requests and their expert reviews no matter the topic of the proposal.
On a personal note, this year is also notable as it marks the end of my term on the REB, following a one-year extension to provide continuity during the COVID-19 pandemic. It has been my distinct honour and privilege to serve on the board, beginning in 2008 as one of the researchers external to Health Canada and PHAC, and then assuming the position of chair in 2015. I am grateful for the wonderful relationships I've built with the REB members, researchers and Secretariat staff and I will greatly miss the regular interactions.
As I prepare to hand the reins to my successor, I can say with full confidence that the REB is as sound and capable as I have ever seen it over the past 14 years. This is due both to the exceptional calibre of the REB members and to the outstanding support afforded to the REB by the Secretariat.
Congratulations to the REB on its first 20 years, and here's to continued success in the next 20 years and beyond.
Respectfully submitted,
Barbara McGillivray, MD, FRCP, FCCMG
Chair, Health Canada-PHAC Research Ethics Board
About the Health Canada-PHAC REB
The Health Canada (HC) and Public Health Agency of Canada (PHAC) Research Ethics Board (REB) provides support and independent ethics review for all proposed and ongoing research involving humans conducted under the auspices of the Department or Agency, to ensure the highest ethical standards are being met, and that the greatest protection is provided to participants. The REB reviews applications in accordance with the considerations set out in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) as the minimum standard. The REB is also guided by relevant federal laws and regulations, such as the Privacy Act and clinical trial regulations, where applicable.
The REB reports to the Deputy Minister of Health Canada and the President of PHAC, who jointly appoint REB members, approve REB procedures and authorize research to be initiated or terminated. The REB makes recommendations to the Deputy Minister and President (through their delegated Decisional Authorities) as to whether research projects should be approved, rejected, modified or terminated. The REB also provides ethical oversight of all approved research throughout the duration of the project. Finally, the REB serves various educational functions for Department and Agency managers and researchers.
The REB is responsible for the ethics review of all research involving humans that is:
- Carried out by Health Canada or PHAC;
- Performed by the Department or Agency in collaboration with external researchers;
- Carried out on Department or Agency premises; or
- Conducted under contract to the Department or Agency.
The REB may also review research involving humans that is funded through the Department or Agency grants and contributions.
The REB is supported by a Secretariat that manages its operations and administers the REB review process. The Secretariat works closely with REB members and applicants to ensure a quality and timely review of all research proposals received for ethics review. The Secretariat is based at Health Canada within the Strategic Policy Branch (Science Policy Directorate).
In the case of applications from PHAC researchers, the REB Secretariat collaborates with the PHAC Office of the Chief Science Officer which administers its approval process and provides additional advice to applicants. The REB Secretariat and the PHAC Office of the Chief Science Officer also work closely together on policies, procedures and guidance related to the REB.
REB meetings and reviews
The full Research Ethics Board (REB) typically meets monthly, either remotely or face-to-face in Ottawa. A minimum of five members is required for quorum (one member each with expertise in ethics and in law, one community member, and two researcher members). All REB members also attend delegated review meetings on a rotating basis. Delegated review meetings are held weekly by videoconference (bi-weekly in July and August) and are attended by the Chair (or Deputy Chair) and one other REB member.
Applications for initial review of research involving humans are generally reviewed at the monthly meetings of the full REB, while delegated review meetings typically evaluate submissions for continuing ethics review (i.e., annual progress reports, amendment requests, adverse event reports and completion/termination reports). Initial applications that represent minimal risk to participants may also be sent to delegated review. For the review of initial applications, the applicant is invited to the REB meeting to give a brief presentation of their proposal and to answer any questions from the REB members.
Types and number of REB meetings in 2021-22:
- 8 full board meetings:
- all meetings were regular monthly meetings (no ad hoc meetings)
- all meetings were held by videoconference due to the COVID-19 pandemic
- 64 delegated review meetings:
- 41 regular weekly meetings
- 23 ad hoc meetings, 21 of which were to review minimal risk initial applications
The number of open protocols varies throughout the year as the REB approves new applications and closes old protocols. There were 183 open protocols on March 31, 2022, and 28 protocols were closed in 2021-22.

Figure 1: Text description
This figure is a bar chart with 12 bars showing the number of submissions reviewed by the REB in the 2021-2022 fiscal year. Each bar corresponds to a month of the year, starting with April and ending with March. Each bar is further subdivided into 4 sections corresponding to the type of submission that was reviewed (initial application, annual progress report, amendment request and adverse event report). The data are as follows:
| Month | Initial applications | Annual progress reports | Amendment requests | Adverse event reports |
|---|---|---|---|---|
| April | 3 | 10 | 6 | 0 |
| May | 4 | 7 | 8 | 0 |
| June | 10 | 9 | 9 | 0 |
| July | 5 | 7 | 3 | 1 |
| August | 2 | 4 | 4 | 0 |
| September | 3 | 12 | 7 | 0 |
| October | 4 | 13 | 6 | 0 |
| November | 5 | 15 | 4 | 0 |
| December | 4 | 7 | 6 | 0 |
| January | 4 | 21 | 9 | 0 |
| February | 9 | 6 | 4 | 1 |
| March | 2 | 13 | 13 | 0 |
| Total | 55 | 124 | 79 | 2 |
Profile of applications received in 2021-22
The Research Ethics Board (REB) received 54 applications in 2021-22, 30 from Health Canada (HC) and 24 from the Public Health Agency of Canada (PHAC). By comparison, 44 applications were received in 2020-21, and 42 in 2019-20. External protocols were led by researchers outside of the Department and Agency, either in collaboration with HC or PHAC researchers or funded by the Department or Agency.
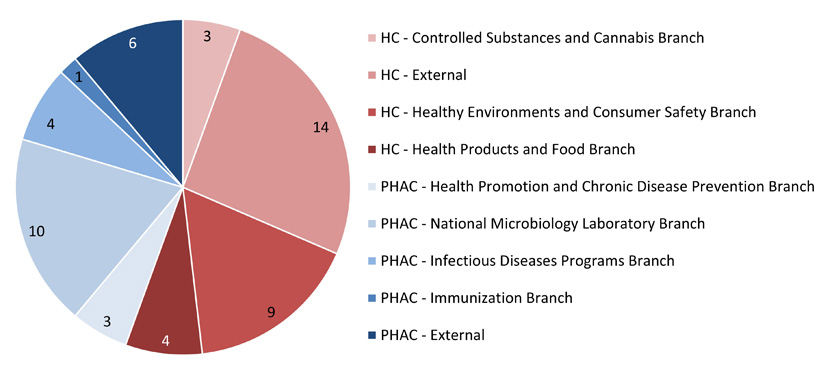
Figure 2: Text description
This pie chart illustrates the affiliation of the lead investigator for the REB applications received during the 2021-2022 fiscal year. The data are as follows:
- Among the 30 applications received from Health Canada:
- 3 were from the Controlled Substances and Cannabis Branch
- 9 were from the Healthy Environments and Consumer Safety Branch
- 4 were from the Health Products and Food Branch
- 14 were external.
- Among the 24 applications received from PHAC:
- 3 were from the Health Promotion and Chronic Disease Prevention Branch
- 10 were from the National Microbiology Laboratory Branch
- 4 were from the Infectious Diseases Programs Branch
- 1 was from the Immunization Branch
- 6 were external
The protocols received spanned a broad range of research areas. Two studies focused on Indigenous populations, and 10 were related to COVID-19.
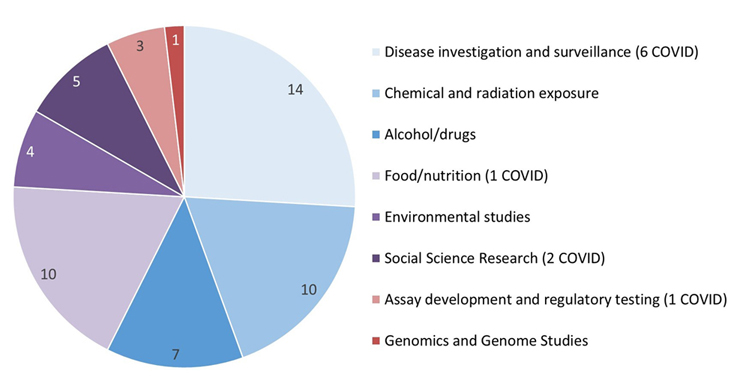
Figure 3: Text description
This pie chart illustrates the subject areas of the protocols received by the REB for the 2021-2022 fiscal year. The data are as follows:
- 14 were about disease investigation and surveillance (6 of which were related to COVID)
- 10 were about chemical and radiation exposure
- 7 were about alcohol and drugs
- 10 were about food and nutrition (1 of which was related to COVID)
- 4 were environmental studies
- 5 were social science research (2 of which were related to COVID)
- 3 were about assay development and regulatory testing (1 of which was related to COVID)
- 1 was about genomics and genome studies
Application review process and outcomes
The Research Ethics Board (REB) applies a proportionate approach to research ethics review; that is, the level of risk to participants determines the level of scrutiny by the REB. Thus, at the discretion of the REB chair, applications deemed to be minimal risk can be sent to delegated review (and if the risk is determined to be greater than initially thought, subsequently referred to the full board for further review).
Following the review of an application, the REB makes one of the following recommendations:
- A. Approved as submitted: The application meets ethics requirements for research involving humans and is recommended for approval.
- B. Approved with modifications/clarifications: The application meets ethics requirements for research involving humans, but the REB members require modification of the application or clarification or further information to secure approval. The revised material may be reviewed via delegated review or a subset of the members as directed by the REB Chair.
- C. Deferral: The application does not meet ethics requirements for research involving humans and is not approved as conditions need to be met. The revised material must be reviewed by the full board via videoconference or in person, or by email circulation to all REB members.
- D. Disapproval: The application fails to meet the ethical standards for approval and revision is unlikely to enable the REB to reach a positive determination.
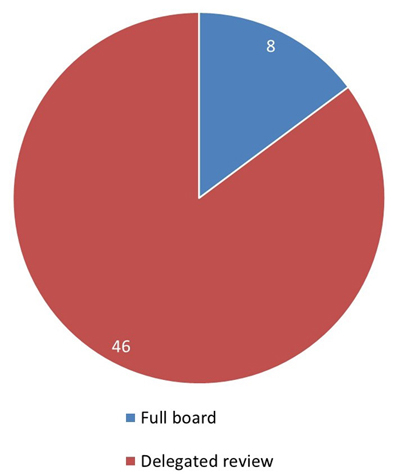
Figure 4: Text description
This pie chart illustrates the level of review that initial applications received by the REB. 46 applications were sent to delegated review and 8 applications received full board review.
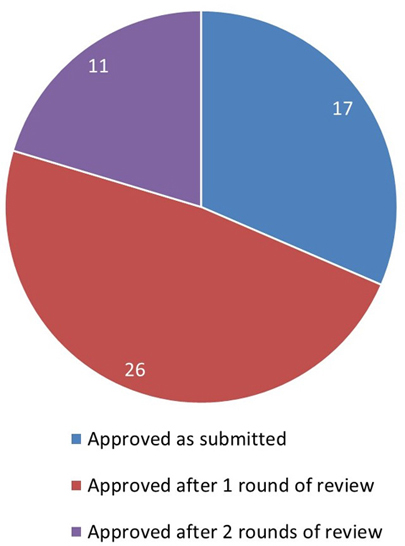
Figure 5: Text description
This pie chart illustrates the outcome of the REB review for initial applications. The data are as follows:
- 17 applications were approved as submitted
- 26 applications were approved after 1 round of review
- 11 applications were approved after 2 rounds of review
Approval times: The time from application submission to approval is a function of the REB Secretariat, the REB and the applicant. The time taken by the applicant to respond to the REB's recommendations and other requests from the Secretariat is beyond the REB's control; thus, the approval time is broken down into days with the REB and days with the applicant. In terms of time with the REB:
- The application deadline is 3 weeks before each monthly REB meeting. If an application is deemed to represent minimal risk to participants and is sent to delegated review rather than the full board, the time between submission and review is often reduced.
- The REB Secretariat's service standard is to communicate the REB's recommendations to the applicant within one week of the REB meeting. In fact, in many cases this was done within 24 hours.
- The REB will conduct expedited reviews for time-sensitive research projects where the urgency is due to public health emergencies or other circumstances beyond the researchers' control. While there was a need for some expedited reviews in 2021-22, it was greatly reduced as compared to 2020-21 in which one third of applications required expedited review.
| N/A | All applications (n = 54) |
Applications sent to delegated review (n = 46) |
Applications reviewed by the full board (n = 8) |
|||
|---|---|---|---|---|---|---|
| Average (± SD) | Median | Average (± SD) | Median | Average (± SD) | Median | |
| Days with REB | 23 (± 11) | 21 | 23 (± 11) | 20 | 28 (± 8) | 29 |
| Days with applicant | 19 (± 27) | 7 | 16 (± 23) | 7 | 44 (± 41) | 32 |
| Total | 42 (± 33) | 30 | 38 (± 29) | 27 | 71 (± 47) | 57 |

Figure 6: Text description
This bar graph contains 54 bars, 1 bar for each application that was received in the 2021-2022 fiscal year. The bars are further divided into 2 sections: number of days with the REB and number of days with the applicant. The 54 bars are ordered from the shortest to longest amount of time by calendar days.
| Application number | Days with REB | Days with applicant | Total |
|---|---|---|---|
| 1 | 7 | 0 | 7 |
| 2 | 7 | 4 | 11 |
| 3 | 12 | 0 | 12 |
| 4 | 16 | 0 | 16 |
| 5 | 18 | 0 | 18 |
| 6 | 17 | 1 | 18 |
| 7 | 18 | 0 | 18 |
| 8 | 18 | 0 | 18 |
| 9 | 19 | 0 | 19 |
| 10 | 20 | 0 | 20 |
| 11 | 14 | 6 | 20 |
| 12 | 12 | 8 | 20 |
| 13 | 19 | 1 | 20 |
| 14 | 21 | 0 | 21 |
| 15 | 14 | 7 | 21 |
| 16 | 17 | 4 | 21 |
| 17 | 6 | 16 | 22 |
| 18 | 22 | 0 | 22 |
| 19 | 23 | 0 | 23 |
| 20 | 8 | 17 | 25 |
| 21 | 14 | 11 | 25 |
| 22 | 23 | 3 | 26 |
| 23 | 21 | 5 | 26 |
| 24 | 20 | 7 | 27 |
| 25 | 15 | 12 | 27 |
| 26 | 20 | 8 | 28 |
| 27 | 20 | 9 | 29 |
| 28 | 16 | 14 | 30 |
| 29 | 28 | 2 | 30 |
| 30 | 16 | 14 | 30 |
| 31 | 23 | 7 | 30 |
| 32 | 22 | 8 | 30 |
| 33 | 26 | 7 | 33 |
| 34 | 31 | 2 | 33 |
| 35 | 33 | 0 | 33 |
| 36 | 29 | 7 | 36 |
| 37 | 21 | 17 | 38 |
| 38 | 38 | 0 | 38 |
| 39 | 19 | 24 | 43 |
| 40 | 49 | 6 | 55 |
| 41 | 47 | 10 | 57 |
| 42 | 44 | 16 | 60 |
| 43 | 16 | 49 | 65 |
| 44 | 23 | 43 | 66 |
| 45 | 37 | 34 | 71 |
| 46 | 29 | 45 | 74 |
| 47 | 22 | 55 | 77 |
| 48 | 28 | 50 | 78 |
| 49 | 29 | 61 | 90 |
| 50 | 10 | 85 | 95 |
| 51 | 49 | 63 | 112 |
| 52 | 35 | 78 | 113 |
| 53 | 36 | 104 | 140 |
| 54 | 52 | 96 | 148 |
REB education and information sessions
As noted in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2), an important component of Research Ethics Board (REB) meetings is the inclusion of educational opportunities that may benefit the overall operation of the REB. To this end, a broad range of educational sessions were organized over the course of the year, as described below. The list also includes presentations from researchers about their programs of research. These interactions served not only to educate board members about emerging research areas, but also to strengthen the relationships between the REB and researchers. The REB would like to thank all the presenters for generously giving their time to enlighten the board.
- Northern research considerations – Jamal Shirley, Acting Director, Innovation and Research, Nunavut Research Institute, and Dr. Gwen Healey Akearok, Executive and Scientific Director, Quajigiartit Health Research Centre (June 2021)
- Canadian Health Measures Survey (CHMS): overview and update on activities – Sylvain Tremblay, Anie Marcil and Jason Deguire, Statistics Canada (June 2021)
- Canadian COVID-19 Antibody and Health Survey (CCAHS) update on activities – Peter Jiao and Erik Dorff, Statistics Canada (June 2021)
- Overview of COVID-19 research at the PHAC National Microbiology Laboratory (NML) – Dr. Guillaume Poliquin, Acting Vice President, NML (June 2021)
- Update on Health Canada's initiative to promote Citizen Science – Tara Bower and Achla Joshi, Office of Science Policy, Liaison and Coordination, Health Canada (November 2021)
- Research involving humans at Correctional Service Canada (CSC) – Olivia Varsaneux and James Worthington, CSC (November 2021)
Secretariat activities
The Research Ethics Board (REB) Secretariat administers the REB review process and supports the board in fulfilling its mandate. Important elements of the Secretariat's work are to build partnerships and increase awareness about the REB, and to continually improve the board's processes. Key Secretariat activities for 2021-22 included the following:
- The REB Secretariat provided an overview of the REB process to staff at Health Canada responsible for administering grants and contributions to researchers, as well as a general REB learning session for PHAC employees.
- Dr. Barbara McGillivray (REB chair) participated in a panel presentation and discussion about the REB that highlighted Health Canada research reviewed by the REB at the annual Health Canada Science Forum in February 2022.
- The REB Secretariat participated in engagement sessions on clinical trial oversight and implementation in the context of COVID-19, led by Health Canada's Office of Clinical Trials.
- The REB Secretariat provided advice to a government-wide community of practice in research ethics that was established to help share best practices for conducting research involving humans at federal government departments.
- The REB chair and Secretariat provided ethics advice and support to researchers at other departments including Indigenous Services Canada, Department of Fisheries and Oceans, and Agriculture and Agri-Food Canada.
- The REB Secretariat served on a task group to develop a framework to better support citizen science projects at Health Canada.
- The REB chair and Secretariat provided input to the Tri-Council Secretariat on Responsible Conduct of Research regarding proposed changes to the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2).
- The REB website was modified to add a simplified overview page and to consolidate all information related to the consent process.
- Ongoing improvements were made to the REB Secretariat's operations to improve efficiency and clarity, including updates and revisions to the REB application and continuing review forms, email templates and internal work processes.
Membership
The REB consists of nine regular and nine alternate members with expertise in the following areas:
- Two members with knowledge/expertise in research ethics;
- One member with knowledge/expertise in law;
- One member from Health Canada with knowledge/expertise in Health Canada research;
- One member from the Public Health Agency of Canada (PHAC) with knowledge/expertise in PHAC research;
- One member external to Health Canada and PHAC with broad knowledge/expertise in both Health Canada and PHAC research;
- One member with broad expertise in public health;
- One member recruited from the community (general population) served by Health Canada and PHAC; and
- One member from the Indigenous community.
Members are appointed by the Deputy Minister of Health and the President of PHAC for a term of three years, renewable once. Two REB members left the board in 2021-22:
- Meeka Otway – Community Member, Indigenous Population
- Guillaume Poliquin – PHAC Researcher
Health Canada and PHAC are especially grateful for their expert contributions to the REB over the years, and they are very much missed by the REB members and Secretariat.
The following REB members assumed new roles in 2021-22 due to changes in employment:
- Sandra Romain – PHAC Researcher (formerly Community Member, General Population)
- Madzouka Kokolo – Community Member, General Population (formerly Health Canada Researcher)
The REB welcomed the following individuals who joined the REB in 2021-22 to fill various vacancies:
- Julie Toole, RM, MHSc – Ethics (alternate): Julie Toole has worked as a Registered Midwife in Toronto, where her practice focused largely on serving uninsured clients and the urban Indigenous community. She currently works as a Quality & Risk Management Specialist at the Association of Ontario Midwives. Her particular areas of interest include management of adverse events, outbreaks and pandemics, clinical ethics, privacy and mental health; clinical areas of interest include equity, informed choice and prenatal genetic screening. Ms. Toole currently co-leads two ethics task forces, one focused on ethical issues related to the COVID-19 pandemic and the other on the creation of a code of ethics for midwives in Ontario. She supervises both midwifery undergraduate students and graduate students in the fields of bioethics and public health.
- Jean Levasseur-Moreau, Ph.D. – Community Member, Indigenous Population (regular): Jean Levasseur-Moreau is an Innu from the community of Essipit, where he grew up. During his doctoral studies, he was involved in the Indigenous Student Association as well as being a regional representative for Indspire, an organization that provides scholarships to Indigenous students in need. He has worked in the field of governance at the First Nations of Quebec and Labrador Health and Social Services Commission, where he had the privilege of visiting almost all First Nations communities in Quebec and witnessing the major issues related to health. He is currently working for the Police Ethics Commissioner of the Ministry of Public Security as an Indigenous liaison to promote access to justice for Indigenous people and to offer culturally sensitive services. He is also a member of the Human Research Ethics Committee of the Université du Québec en Abitibi-Témiscamingue.
- Louis Forti, B.Sc. (Hons), D.E.S.S., M.Sc., Ph.D. – Health Canada researcher (alternate): Louis Forti's academic research activities focused on investigating the effect of physical exercise on inflammation in older persons and various projects on diabetes. He has worked as Regulatory Affairs Officer for the Natural and Non-prescription Health Products Directorate at Health Canada where he reviewed non-prescription drugs to ensure they complied with the Plain Language Labelling Regulations. He currently works as a Scientific Evaluator at Health Canada's Natural and Non-prescription Health Products Directorate. He routinely conducts critical reviews of disinfectant drug submissions for efficacy and safety to support regulatory decision-making.
REB membership as of March 31, 2022
- Stéphane Ahern, M.D., Ph.D. – Ethics
- Stephanie Booth, D.Phil. – PHAC Researcher
- Michael Wray Clarke, Ph.D. – Public Health Community Member
- Marie-Ève Couture Ménard, D.C.L. – Law
- Meredith Curren, Ph.D. – Health Canada Researcher
- Louis Forti, Ph.D. – Health Canada Researcher
- Glenn Griener, Ph.D. – Ethics
- Janaki Jayanthan, MPH – Community Member, General Population
- Madzouka Kokolo, M.A. – Community Member, General Population
- Jean Levasseur-Moreau, Ph.D. – Community Member, Indigenous Population
- Kathleen Makela, B.A., LL.B. – Community Member, Indigenous Population
- Barbara McGillivray, M.D. – Chair and Researcher External to Health Canada and PHAC
- Melanie McPhail, J.D. – Law
- Jean-Frédéric Ménard, LL.B., B.C.L. – Law
- Stuart Nicholls, Ph.D. – Researcher External to Health Canada and PHAC
- Sandra Romain, Ph.D. – PHAC Researcher
- Lehana Thabane, Ph.D. – Researcher External to Health Canada and PHAC
- Julie Toole, RM, MHSc – Ethics
- Nancy Walton, Ph.D. – Ethics
- Kue Young, M.D., D.Phil. – Public Health Community Member
REB Secretariat
- Gregory Huyer, Ph.D. – Manager
- Sandra Dyal – Senior Research Ethics Coordinator (effective May 2021)
- Katline Racine – Policy Analyst (effective January 2022)
- Gabriella Hilkes – Junior Analyst (co-op student)
PHAC liaison to the REB Secretariat
- Trista Takacs, Ph.D. – Policy Analyst, Officer of the Chief Science Officer (April – June, 2021)
- Erica Bernier – Policy Analyst, Office of the Chief Science Officer (July – September, 2021)
- Sundus Haji-Jama – Policy Analyst, Office of the Chief Science Officer (effective November 2021)