Research Ethics Board (REB) Annual Report 2022-23
Table of contents
- Executive summary
- Message from the Chair
- About the Health Canada-PHAC REB
- REB meetings and reviews
- Profile of applications received in 2022-23
- Application review process and outcomes
- REB education and information sessions
- Secretariat activities
- Membership
- References
Executive summary
The Health Canada (HC) and Public Health Agency of Canada (PHAC) Research Ethics Board (REB) is a joint board for both the Department and Agency. The REB provides support and independent ethics review for all proposed and ongoing research involving humans carried out under the auspices of HC and PHAC, to help ensure that the highest ethical standards are being met and the greatest protection is provided to participants.
The REB held 64 meetings in 2022-23 (54 delegated review and 10 full board meetings). All meetings were held by videoconference, except for one full board meeting that was held in person in Ottawa in September (with an option for virtual participation for members unable to travel) - the first in-person gathering in over two-and-a-half years.
The REB received 45 new applications in 2022-23, of which 19 were from HC and 26 from PHAC. Only two studies were considered to be greater than minimal risk and therefore required full board review; all other studies were referred to delegated review. The time from application submission to approval was an average of 41 calendar days (26 days with the REB and 15 with the applicant). The REB also reviewed 147 annual progress reports, 70 amendment requests and 2 adverse event reports in 2022-23 and closed 36 protocols.
In addition to conducting ethics review, the REB members received several educational and informational sessions in 2022-23. These sessions were delivered at the REB meetings in June and September to which the entire membership was invited. There was a high level of participation at both meetings, ensuring that almost all the members were able to benefit from these presentations.
The 2022-23 fiscal year saw an important change to the leadership of the REB. Dr. Glenn Griener assumed the role of Chair on July 1, succeeding Dr. Barbara McGillivray at the completion of her term. Dr. Jean-Frédéric Ménard was also appointed to the position of Deputy Chair, joining Dr. Nancy Walton in this role.
The REB Secretariat's outreach efforts continued this fiscal year with an awareness campaign directed at HC and PHAC staff, accompanied by two learning sessions. The Secretariat also provided targeted support to researchers at the Department and Agency, as well as ethics advice to colleagues at various other federal departments. On the operational side, significant progress was made in documenting and updating the Secretariat's work processes.
Overall, this report demonstrates that the REB effectively delivered on its mandate in 2022-23 in continuing to provide expert research ethics support to HC and PHAC.
Message from the Chair

As the Chair of the Health Canada (HC) and Public Health Agency of Canada (PHAC) Research Ethics Board (REB), I am pleased to present this report of the REB's activities for the 2022-23 fiscal year.
The HC-PHAC REB plays an important role in promoting and maintaining the highest ethical standards in research involving human participants. Its mandate is to review all research conducted under the auspices of the Department and Agency that involves human participants, guided by relevant ethical principles and regulations. Through its work, the REB helps ensure that research participants are adequately protected and that policy makers have access to ethically sound research to support decision-making.
The annual report highlights the Board's accomplishments over the past year, including details on the number and types of proposals reviewed, the broad range of subject areas, and the outcomes of the reviews. It also provides information on the training and education offered to the REB members to support them in fulfilling their duties, and on the REB Secretariat's activities to enhance the research ethics review process.
I want to take this opportunity to thank all members of the Board for their dedication and hard work, often done under tight timeline. Their meticulous attention to the details of each application and commitment to ethical research practices is critical to the success of our mandate.
I would especially like to recognize Dr. Barbara McGillivray, who joined the REB in 2008 and served as Chair from 2015 to 2022. Barb provided exceptional leadership to the board and contributed greatly to advancing the culture of ethical research at the Department and Agency.
I was honoured and humbled to be chosen as Chair following the end of Barb's term and consider myself fortunate to have had the opportunity to work with and learn from her on the REB. I hope to emulate her leadership.
Since becoming Chair, I much more deeply appreciate the excellent support provided to the Board by the Secretariat. Their efforts behind the scenes, communicating with research teams and ensuring that applications are complete, are key to the REB's efficient operations.
Finally, I would like to express my gratitude to the Deputy Minister of Health, the President of PHAC and the HC-PHAC research community for their ongoing support of the REB's work and for their commitment to advancing ethical research practices in Canada.
Dr. Glenn G. Griener
Chair, HC-PHAC Research Ethics Board
About the Health Canada-PHAC REB
The Health Canada (HC) and Public Health Agency of Canada (PHAC) Research Ethics Board (REB) provides support and independent ethics review for all proposed and ongoing research involving humans conducted under the auspices of the Department or Agency, to help ensure the highest ethical standards are being met, and that the greatest protection is provided to participants. The REB reviews applications in accordance with the considerations set out in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2) as the minimum standard. The REB is also guided by relevant federal laws and regulations, such as the Privacy Act and clinical trial regulations, where applicable.
The REB reports to the Deputy Minister of Health and the President of PHAC, who jointly appoint REB members, approve REB procedures, and authorize research to be initiated or terminated. The REB makes recommendations to the Deputy Minister and President (through their delegated Decisional Authorities) as to whether research projects should be approved, rejected, modified, or terminated. The REB also provides ethical oversight of all approved research throughout the duration of the project. Finally, the REB serves various educational functions for Department and Agency managers and researchers.
The REB is responsible for the ethics review of all research involving humans that is:
- Carried out by HC or PHAC;
- Performed by the Department or Agency in collaboration with external researchers;
- Carried out on Department or Agency premises; or
- Conducted under contract to the Department or Agency.
The REB may also review research involving humans that is funded through grants and contributions from the Department or Agency.
The REB is supported by a Secretariat that manages its operations and administers the REB review process. The Secretariat works closely with REB members and applicants to ensure a quality and timely review of all research proposals received for ethics review. The Secretariat is based at Health Canada within the Strategic Policy Branch (Science Policy Directorate).
In the case of applications from PHAC researchers, the REB Secretariat collaborates with the PHAC Office of the Chief Science Officer which administers its approval process and provides additional advice to applicants. The REB Secretariat and the PHAC Office of the Chief Science Officer also work closely together on policies, procedures and guidance related to the REB.
REB meetings and reviews
The Research Ethics Board (REB) meets as required to conduct full board reviews by videoconference. A minimum of five members is required for quorum (one member each with expertise in ethics and in law, one community member, and two researcher members). All REB members also attend delegated review meetings on a rotating basis. Delegated review meetings are held weekly by videoconference (bi-weekly in July and August) and are attended by the Chair (or Deputy Chair) and one other REB member.
Full board meetings are primarily to evaluate initial applications and major amendment requests that represent greater than minimal risk to research participants. By contrast, minimal risk applications are generally sent to delegated review, along with submissions for continuing ethics review (i.e., annual progress reports, amendment requests and adverse event reports). For the review of initial applications, the applicant is normally invited to the REB meeting (whether full board or delegated review) to give a brief presentation about the proposal and to answer any questions from the REB members.
The entire REB also meets up to twice a year in person, and remotely as required, for educational sessions and informational presentations from researchers.
Types and number of REB meetings in 2022-23:
- 10 full board meetings:
- Two meetings were for the review of initial applications, plus two follow-up meetings to review the applicants' responses to the REB's questions and recommendations.
- Two meetings were for the review of major amendment requests.
- One meeting was to provide ethics advice to researchers from Fisheries and Oceans Canada (DFO) for a proposed study.
- Three meetings were held with the entire membership for educational sessions and informational presentations.
- All meetings were held by videoconference, except for one meeting of the entire membership that was held in person in Ottawa.
- 54 delegated review meetings:
- 42 regular weekly meetings
- 12 ad hoc meetings, almost all of which were to review minimal risk initial applications.
The number of open protocols varies throughout the year as the REB approves new applications and closes old protocols. There were 191 open protocols on March 31, 2023 (100 from HC and 91 from PHAC), and 36 protocols were closed in 2022-23.
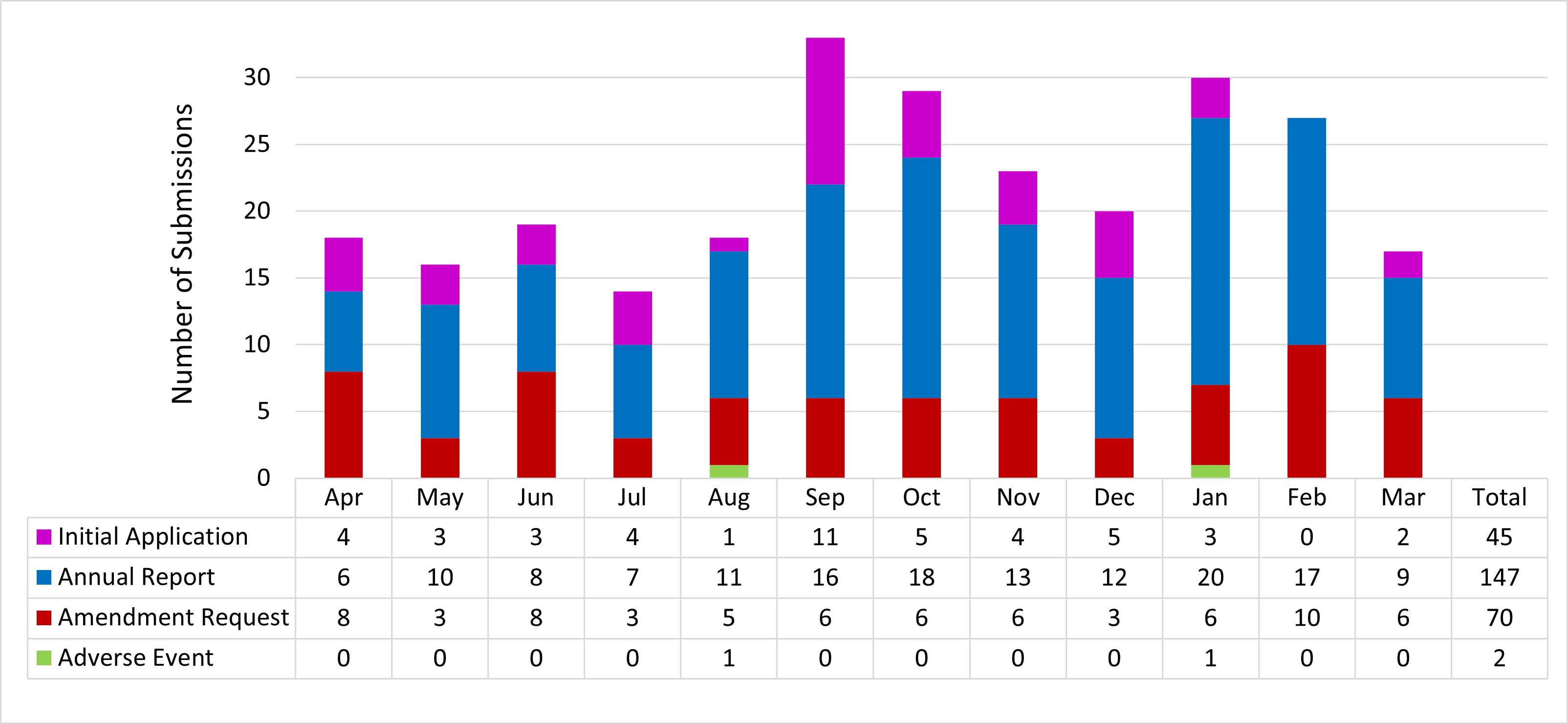
Figure 1: Text description
This figure is a bar chart with 12 bars showing the number of submissions reviewed by the REB in the 2022-2023 fiscal year. Each bar corresponds to a month of the year, starting with April and ending with March. Each bar is further subdivided into 4 sections corresponding to the type of submission that was reviewed (initial application, annual progress report, amendment request and adverse event report). The data are as follows:
| Month | Initial applications | Annual progress reports | Amendment requests | Adverse event reports |
|---|---|---|---|---|
| April | 4 | 6 | 8 | 0 |
| May | 3 | 10 | 3 | 0 |
| June | 3 | 8 | 8 | 0 |
| July | 4 | 7 | 3 | 0 |
| August | 1 | 11 | 5 | 1 |
| September | 11 | 16 | 6 | 0 |
| October | 5 | 18 | 6 | 0 |
| November | 4 | 13 | 6 | 0 |
| December | 5 | 12 | 3 | 0 |
| January | 3 | 20 | 6 | 1 |
| February | 0 | 17 | 10 | 0 |
| March | 2 | 9 | 6 | 0 |
| Total | 45 | 147 | 70 | 2 |
Profile of applications received in 2022-23
The Research Ethics Board (REB) received 45 applications in 2022-23: 19 from Health Canada (HC) and 26 from the Public Health Agency of Canada (PHAC). By comparison, 54 applications were received in 2021-22, and 44 in 2020-21. External protocols were led by researchers outside of the Department and Agency, either in collaboration with HC or PHAC researchers or funded by the Department or Agency.
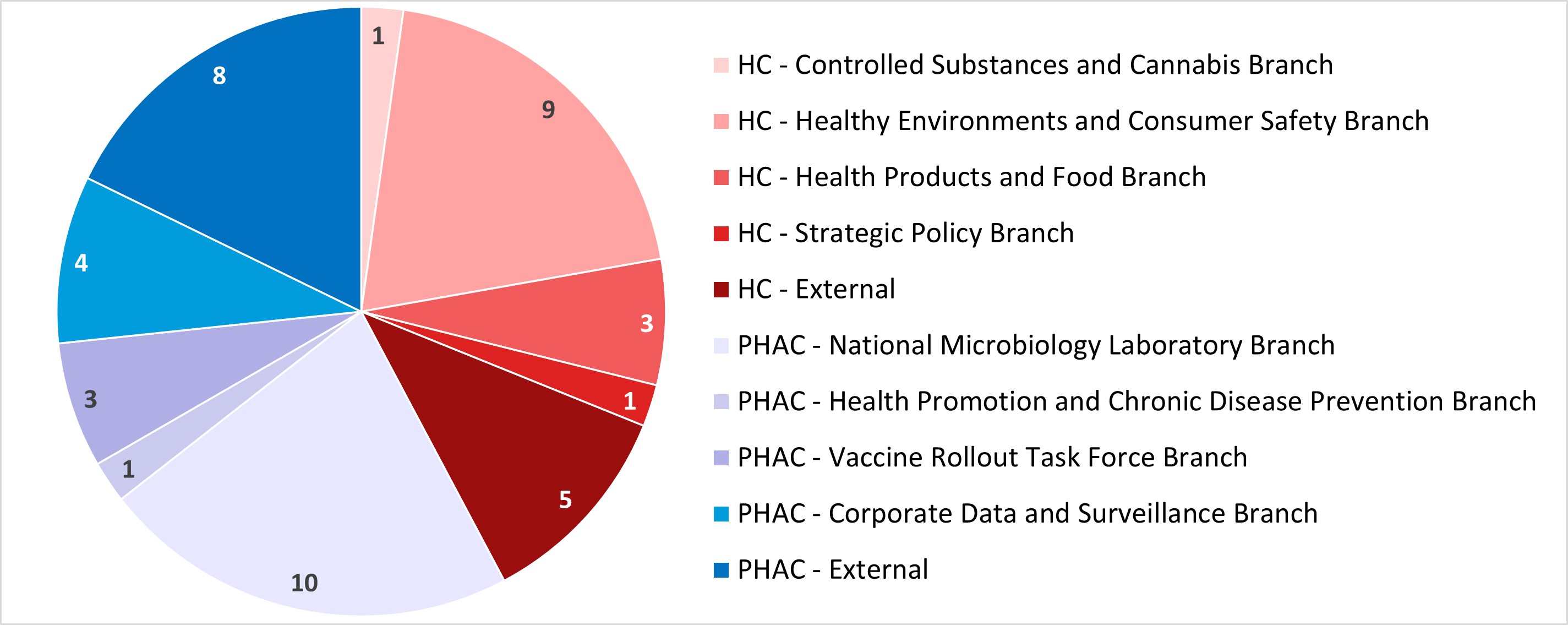
Figure 2: Text description
This pie chart illustrates the affiliation of the lead investigator for the REB applications received during the 2022-2023 fiscal year. The data are as follows:
- Among the 19 applications received from Health Canada:
- 1 was from the Controlled Substances and Cannabis branch
- 9 were from the Healthy Environments and Consumer Safety Branch
- 3 were from the Health Products and Food Branch
- 1 was from the Strategic Policy Branch
- 5 were external
- Among the 26 applications received from PHAC:
- 10 were from the National Microbiology Laboratory Branch
- 1 was from the Health Promotion and Chronic Disease Prevention Branch
- 3 were from the Vaccine Rollout Task Force Branch
- 4 were from the Corporate Data and Surveillance Branch
- 8 were external
The protocols received spanned a broad range of research areas. Four studies focused on Indigenous populations, and seven were related to COVID-19.
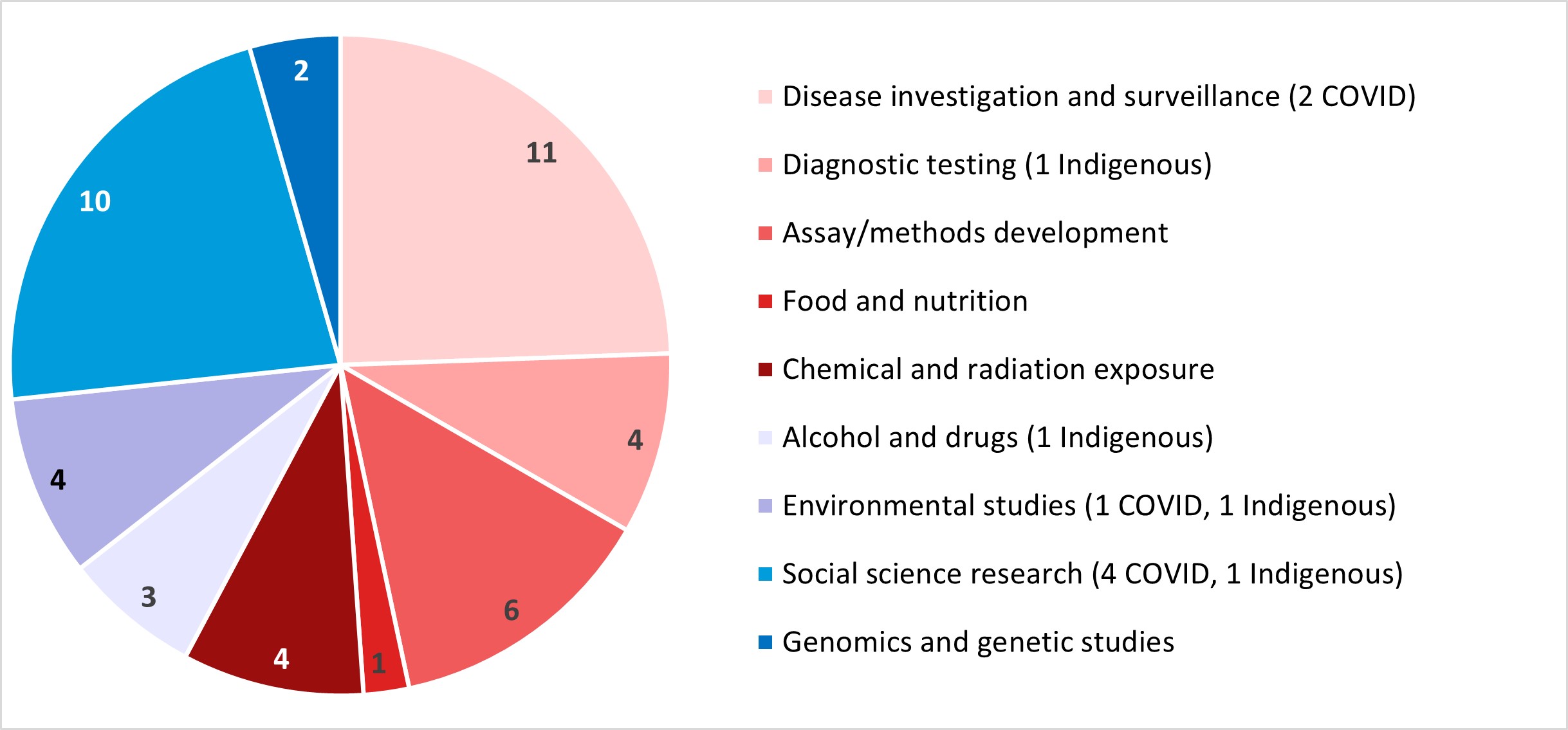
Figure 3: Text description
This pie chart illustrates the subject areas of the protocols received by the REB for the 2022-2023 fiscal year. The data are as follows:
- 11 were about disease investigation and surveillance (2 of which were related to COVID)
- 4 were about diagnostic testing (1 Indigenous)
- 6 were about assay/methods development and regulatory testing
- 1 was about food and nutrition
- 4 were about chemical and radiation exposure
- 3 were about alcohol and drugs (1 Indigenous)
- 4 were environmental studies (1 of which was related to COVID, 1 Indigenous)
- 10 were social science research (4 of which were related to COVID, 1 Indigenous)
- 2 were about genomics and genetic studies
Application review process and outcomes
The Research Ethics Board (REB) applies a proportionate approach to research ethics review; that is, the level of risk to participants determines the level of scrutiny by the REB. Thus, at the discretion of the REB chair, applications deemed to be minimal risk can be sent to delegated review (and if the risk is determined to be greater than initially thought, subsequently referred to the full board for further review).
Following the review of an application, the REB makes one of the following recommendations:
A. Approved as submitted: The application meets ethics requirements for research involving humans and is recommended for approval.
B. Approved with modifications/clarifications: The application meets ethics requirements for research involving humans, but the REB members require modification of the application or clarification or further information to secure approval. The revised material may be reviewed via delegated review or a subset of the members as directed by the REB Chair.
C. Deferral: The application does not meet ethics requirements for research involving humans and is not approved as conditions need to be met. The revised material must be reviewed by the full board via videoconference, or by email circulation to all REB members.
D. Disapproval: The application fails to meet the ethical standards for approval and revision is unlikely to enable the REB to reach a positive determination.

Figure 4: Text description
This pie chart illustrates the level of review that initial applications received by the REB. 43 applications were sent to delegated review and 2 applications received full board review.

Figure 5: Text description
This pie chart illustrates the outcome of the REB review for initial applications. The data are as follows:
- 18 applications were approved as submitted
- 21 applications were approved after 1 round of review
- 3 applications were approved after 2 rounds of review
- 3 applications were unresolved - pending REB review or PI response
Approval times: The time from application submission to approval is a function of the REB Secretariat, the REB and the applicant. The time taken by the applicant to respond to the REB's recommendations and other requests from the Secretariat is beyond the REB's control; thus, the approval time is broken down into days with the REB and days with the applicant. In terms of time with the REB:
- The application deadline is 3 weeks before each pre-scheduled full board meeting. If an application is deemed to represent minimal risk to participants and is sent to delegated review rather than the full board, the time between submission and review is often reduced.
- The REB Secretariat's service standard is to communicate the REB's recommendations to the applicant within one week of the REB meeting. In fact, in many cases this is done within 24 hours.
- The REB will conduct expedited reviews for time-sensitive research projects where the urgency is due to public health emergencies or other circumstances beyond the researchers' control.
Time from submission to approval for the applications received in 2022-23Reference 2
| Average (± SD) | Median | |
|---|---|---|
| Days with REB | 26 ± 13 | 23 |
| Days with applicants | 14 ± 26 | 6 |
| Total | 40 ± 32 | 32 |
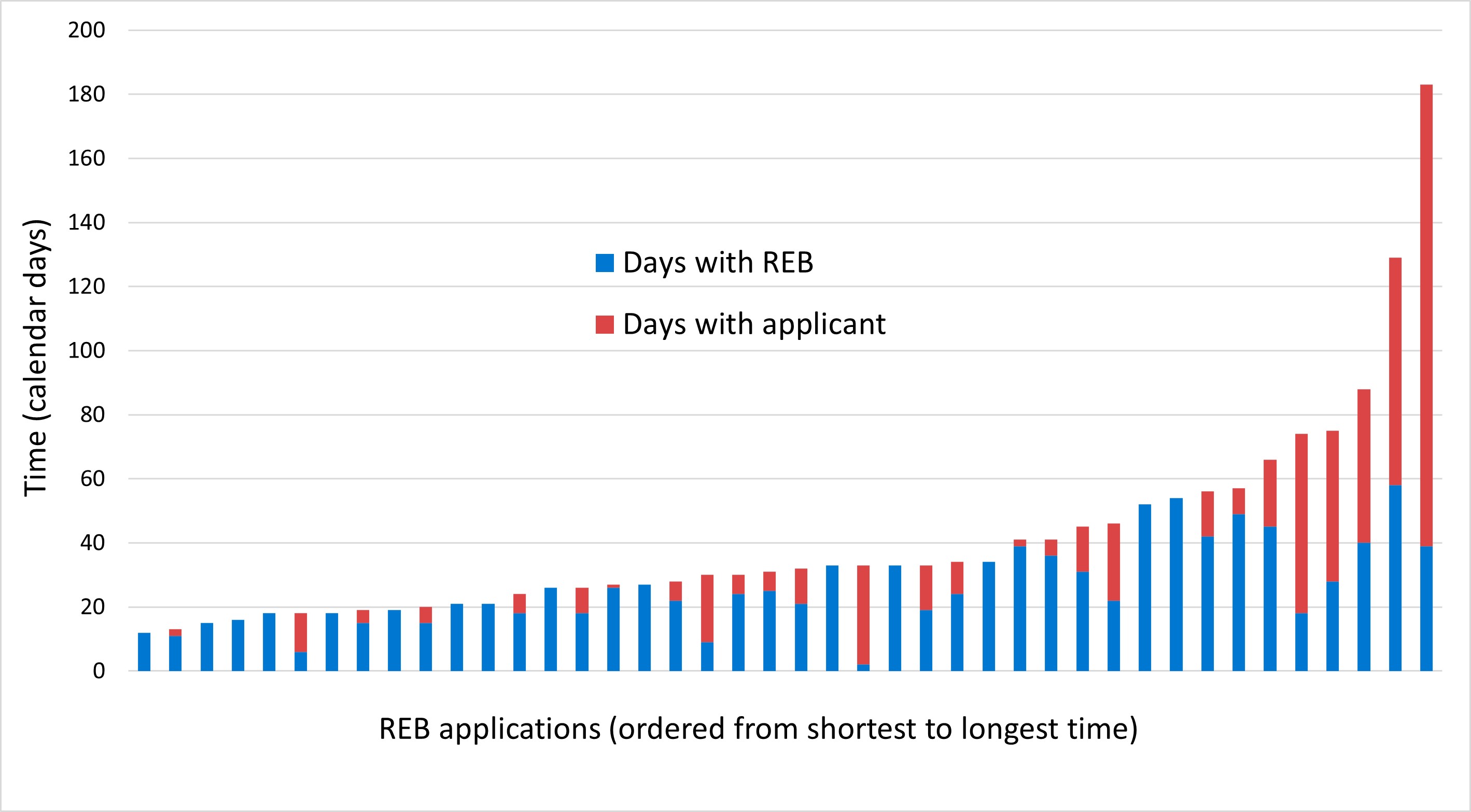
Figure 6: Text description
This bar graph contains 42 bars, 1 bar for each application that was received and approved in the 2022-2023 fiscal year. The bars are further divided into 2 sections: number of days with the REB and number of days with the applicant. The 42 bars are ordered from the shortest to longest amount of time by calendar days.
| Application number | Days with REB | Days with applicant | Total |
|---|---|---|---|
| 1 | 12 | 0 | 12 |
| 2 | 11 | 2 | 13 |
| 3 | 15 | 0 | 15 |
| 4 | 16 | 0 | 16 |
| 5 | 18 | 0 | 18 |
| 6 | 6 | 12 | 18 |
| 7 | 18 | 0 | 18 |
| 8 | 15 | 4 | 19 |
| 9 | 19 | 0 | 19 |
| 10 | 15 | 5 | 20 |
| 11 | 21 | 0 | 21 |
| 12 | 21 | 0 | 21 |
| 13 | 18 | 6 | 24 |
| 14 | 26 | 0 | 26 |
| 15 | 18 | 8 | 26 |
| 16 | 26 | 1 | 27 |
| 17 | 27 | 0 | 27 |
| 18 | 22 | 6 | 28 |
| 19 | 9 | 21 | 30 |
| 20 | 24 | 6 | 30 |
| 21 | 25 | 6 | 31 |
| 22 | 21 | 11 | 32 |
| 23 | 33 | 0 | 33 |
| 24 | 2 | 31 | 33 |
| 25 | 33 | 0 | 33 |
| 26 | 19 | 14 | 33 |
| 27 | 24 | 10 | 34 |
| 28 | 34 | 0 | 34 |
| 29 | 39 | 2 | 41 |
| 30 | 36 | 5 | 41 |
| 31 | 31 | 14 | 45 |
| 32 | 22 | 24 | 46 |
| 33 | 52 | 0 | 52 |
| 34 | 54 | 0 | 54 |
| 35 | 42 | 14 | 56 |
| 36 | 49 | 8 | 57 |
| 37 | 45 | 21 | 66 |
| 38 | 18 | 56 | 74 |
| 39 | 28 | 47 | 75 |
| 40 | 40 | 48 | 88 |
| 41 | 58 | 71 | 129 |
| 42 | 39 | 144 | 183 |
REB education and information sessions
As noted in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2), an important component of Research Ethics Board (REB) meetings is the inclusion of educational opportunities that may benefit the overall operation of the REB. To this end, a broad range of educational sessions were organized over the course of the year, as described below. The list also includes presentations from researchers about their programs of research. These interactions served not only to educate board members about emerging research areas, but also to strengthen the relationships between the REB and researchers. The REB would like to thank all the presenters for generously giving their time to enlighten the board.
- Interdepartmental Indigenous Science, Technology, Engineering and Mathematics (I-STEM) Cluster Partnership - Marc Desjardins and Hasu Ghosh, Science Policy Directorate, Health Canada (June 2022)
- First Nations Data Governance - Jonathan Dewar, CEO, First Nations Information Governance Centre (June 2022)
- Privacy in Research in the Federal Government Context - Madelaine Saginur, HC-PHAC Privacy Management Division (June 2022)
- Diagnostic Research at the PHAC National Microbiology Laboratory - John Kim, Sharon Simon and Christine Mesa, PHAC National Microbiology Laboratory (September 2022)
- Ethical Considerations for Social Media Research - Andrea Zeffiro and Jay Brodeur, Sherman Centre for Digital Scholarship, McMaster University (September 2022)
- Public Opinion Research at HC-PHAC; Use of Market Research Panels - Jared Cohen and Yanik Perigny, Communications and Public Affairs Branch, Health Canada; John Tabone, CEO, Canadian Research Insights Council (September 2022)
- Behavioural Science Research at PHAC - Mark Morrissey, PHAC Office of Behavioural Science (September 2022)
- Scope of Ethics Review and Proposed Updates to TCPS 2 - Erika Kleiderman, Hanan Abdel-Akher and Margaret Blakeney, Tri-Council Secretariat on Responsible Conduct of Research (September 2022)
- Canadian Health Measures Survey: Overview and Launch of Cycle 7 - Jason Deguire, Statistics Canada (September 2022)
- Canadian Paediatric Surveillance Program Overview - Melanie Laffin and Charlotte Moore-Hepburn, Canadian Paediatric Society; Christina Ricci and Jay Onysko, Centre for Surveillance and Applied Research, PHAC (September 2022)
- Sex and Gender-Based Analysis Plus (SGBA+) and Equity, Diversity and Inclusion (EDI) Considerations in Research - Cindy Moriarty and Lorne Holyoak, Health Programs and Strategic Initiatives, Health Canada (September 2022)
Secretariat activities
The Research Ethics Board (REB) Secretariat administers the REB review process and supports the board in fulfilling its mandate. Important elements of the Secretariat's work are to build partnerships and increase awareness about the REB, and to continually improve the board's processes. Key Secretariat activities for 2022-23 included the following:
- The REB Secretariat worked with the Health Canada Communications and Public Affairs Branch to develop and implement a communications strategy to promote awareness of the REB within Health Canada and PHAC.
- The REB Secretariat and the PHAC Office of the Chief Science Officer delivered two general REB learning sessions for employees.
- The REB Secretariat provided advice to a government-wide community of practice in research ethics that was established to help share best practices for conducting research involving humans at federal government departments.
- The REB chair and Secretariat provided ethics advice and support to researchers at other departments including Indigenous Services Canada and Fisheries and Oceans Canada (DFO).
- The REB Secretariat served on a task group to develop a framework to better support citizen science projects at Health Canada.
- The REB Secretariat participated in the annual conferences of the Canadian Association of Research Ethics Boards (CAREB) and Clinical Trials Ontario.
- Dr. Barbara McGillivray (past REB chair) and Dr. Gregory Huyer (Manager, REB Secretariat) gave a guest lecture to students at Carleton University on REBs in the government context.
- The REB Secretariat continued to implement improvements to its operational processes, including development of a structured evaluation grid and improved recordkeeping to better document REB members' recommendations.
Membership
The REB consists of nine regular and nine alternate members with expertise in the following areas:
- Two members with knowledge and expertise in research ethics;
- One member with knowledge and expertise in law;
- One member from Health Canada (HC) with knowledge and expertise in HC research;
- One member from the Public Health Agency of Canada (PHAC) with knowledge and expertise in PHAC research;
- One member external to HC and PHAC with broad knowledge and expertise in both HC and PHAC research;
- One member with broad expertise in public health;
- One member recruited from the community (general population) served by HC and PHAC; and
- One member from the Indigenous community.
Members are appointed by the Deputy Minister of Health and the President of PHAC for a term of three years, renewable once.
Two members left the board in 2022-23:
- Barbara McGillivray - Chair and Researcher External to HC and PHAC
- Kue Young - Public Health Community Member
Health Canada and PHAC are especially grateful for their expert contributions to the REB, and in particular Dr. McGillivray for her efforts and support as chair since 2015, and for her contributions and expertise as a member since 2008. The REB and Secretariat will miss them and wish them all the best in their future endeavours.
The REB welcomed one new member to the REB in 2022-23:
- Diane Lu, MD, Ph.D., MPH, FRCPC - Public Health Community Member (alternate): Dr. Diane Lu is a public health physician and medical advisor in communicable diseases in the Department of National Defence. She also provides clinical care services with Correctional Services Canada. Dr. Lu has adjunct appointments to both the University of Ottawa and Queen's University working with public health and preventive medicine residents. Her current research interests include health surveillance of Canadian Armed Forces members. Dr. Lu holds both an MD and Ph.D. degree from the University of Toronto and was a Postdoctoral Fellow at Harvard University. She also earned a Master's of Public Health from Queen's University and is a Fellow of the Royal College of Physicians of Canada.
The following REB members assumed new roles in 2022-23:
- Glenn Griener (ethics member) - REB Chair (effective July 1, 2022)
- Jean-Frédéric Ménard (law member) - Deputy Chair (effective January 1, 2023)
REB Membership as of March 31, 2023
- Stéphane Ahern, M.D., Ph.D. - Ethics
- Stephanie Booth, D.Phil. - PHAC Researcher
- Michael Wray Clarke, Ph.D. - Public Health Community Member
- Marie-Ève Couture Ménard, D.C.L. - Law
- Meredith Curren, Ph.D. - HC Researcher
- Louis Forti, Ph.D. - HC Researcher
- Glenn Griener, Ph.D. - REB Chair; Ethics
- Janaki Jayanthan, MPH - Community Member, General Population
- Madzouka Kokolo, M.A. - Community Member, General Population
- Diane Lu, MD, Ph.D., MPH, FRCPC - Public Health Community Member
- Jean Levasseur-Moreau, Ph.D. - Community Member, Indigenous Population
- Kathleen Makela, B.A., LL.B. - Community Member, Indigenous Population
- Melanie McPhail, J.D. - Law
- Jean-Frédéric Ménard, LL.B., B.C.L., Ph.D. - Deputy Chair; Law
- Stuart Nicholls, Ph.D. - Researcher External to HC and PHAC
- Sandra Romain, Ph.D. - PHAC Researcher
- Lehana Thabane, Ph.D. - Researcher External to HC and PHAC
- Julie Toole, RM, MHSc - Ethics
- Nancy Walton, Ph.D. - Deputy Chair; Ethics
REB Secretariat
- Gregory Huyer, Ph.D. - Manager
- Maria Pereira - Senior Research Ethics Coordinator (May - September 2022)
- Melinda Lee Choon - Senior Research Ethics Coordinator (effective October 2022)
- Katline Racine - Policy Analyst (April - September 2022)
- Jason Lafontaine - Program Officer (co-op student, January - April 2023)
PHAC liaison to the REB Secretariat
- Sundus Haji-Jama - Policy Analyst, Office of the Chief Science Officer (April - August 2022)
- Trista Takacs, Ph.D. - Policy Analyst, Officer of the Chief Science Officer (effective August 2022)
References
- Reference 1
-
Note that this chart summarizes the submissions reviewed in 2022-23, and not submissions received. For example, 45 initial applications were reviewed in 2022-23, which includes 42 of the 45 received between April 1, 2022, and March 31, 2023, plus 3 applications received at the end of the 2021-22 fiscal year.
- Reference 2
-
"Days with REB" includes time with both the REB Secretariat and the REB. All times are measured in calendar days. In certain cases, additional time was required following REB review for the Privacy Management Division to complete its assessment and for the finalization of contracts or agreements; this additional time was excluded from these calculations.