Lingua franca – Chapter 1: Processes
On this page
- Daguerreotype
- Salt print
- Albumen
- Ambrotype
- Pannotype
- Tintype
- Lantern slide
- Collodion negative
- Carbon print
- Platinum/Palladium
- Gelatin silver developing-out print (fibre-based)
- Cellulose nitrate negative
- Autochrome plate
- Cellulose acetate negative
- Colour transparency on plastic film
- Chromogenic print
- Dye destruction print
- Dye diffusion print


Processes
Daguerreotype
Dates of major use: 1840-1865
Inventor: Louis Jacques Mandé Daguerre (1839)
A daguerreotype is an image formed on a copper plate coated with a thin layer of silver, which can appear to be a positive or a negative depending on the angle it is viewed. They were often hand-coloured. Because the silver-coated plates of daguerreotypes were extremely fragile, they were always protected under glass.

Salt print
Dates of major use: 1840-1860s
Inventor: William Henry Fox Talbot (1839)
A salted paper photograph is a positive printed from a negative on a chloride and silver nitrate photosensitized paper support. To produce the image, a negative is placed in contact with the paper. Following exposure to light, an image appears and must then be fixed. By using ordinary paper, prints have a matte surface and warm image tones, from deep red to purple, which is achieved depending on the sizing of the paper. Characteristics are visible paper fibres and fading.

Albumen
Dates of major use: 1850-1895
Inventor: Louis Désiré Blanquart-Évrard (1850)
An albumen is a positive photograph on a paper support. The silver particles are suspended within the albumen layer, which is made of egg whites and sensitized with silver nitrate. The sensitized paper is placed in contact with a negative and exposed to light, which results in a contact print by printingout. The final processing steps are fixing and washing. Gold toning was introduced at a later date. The image tones range in colour from brown to purple to bluish black, depending on the processing. Characteristics include some visible paper fibres as well as overall small cracks and fading into an overall yellow discolouration.

Ambrotype
Dates of major use: 1855-1865
Inventor: Scott Archer (1852), J. Ambrose Cutting (1854)
An ambrotype is a collodion emulsion on a glass support. This negative on glass is underexposed and treated with a chemical solution that results in a silver image with a whitish tone rather than a brown tone.
To make the ambrotype a positive, a dark material such as paper, velvet or applied lacquer is placed against the back. These images are also housed in cases similar to those used for daguerreotypes and tintypes.

Pannotype
Dates of major use: 1853-1880
Inventor: Wülff and Company
A pannotype is made by transferring a direct positive collodion emulsion from glass onto fabric. This fabric support can be a waxed textile, a piece of patent leather or a black oilcloth. The term “pannus” stems from Latin meaning “cloth.” It gained popularity because of the support’s ability to withstand breaking. Particularly, they would not be damaged in the mail like photographic supports such as metal (daguerreotypes) or glass (ambrotypes).

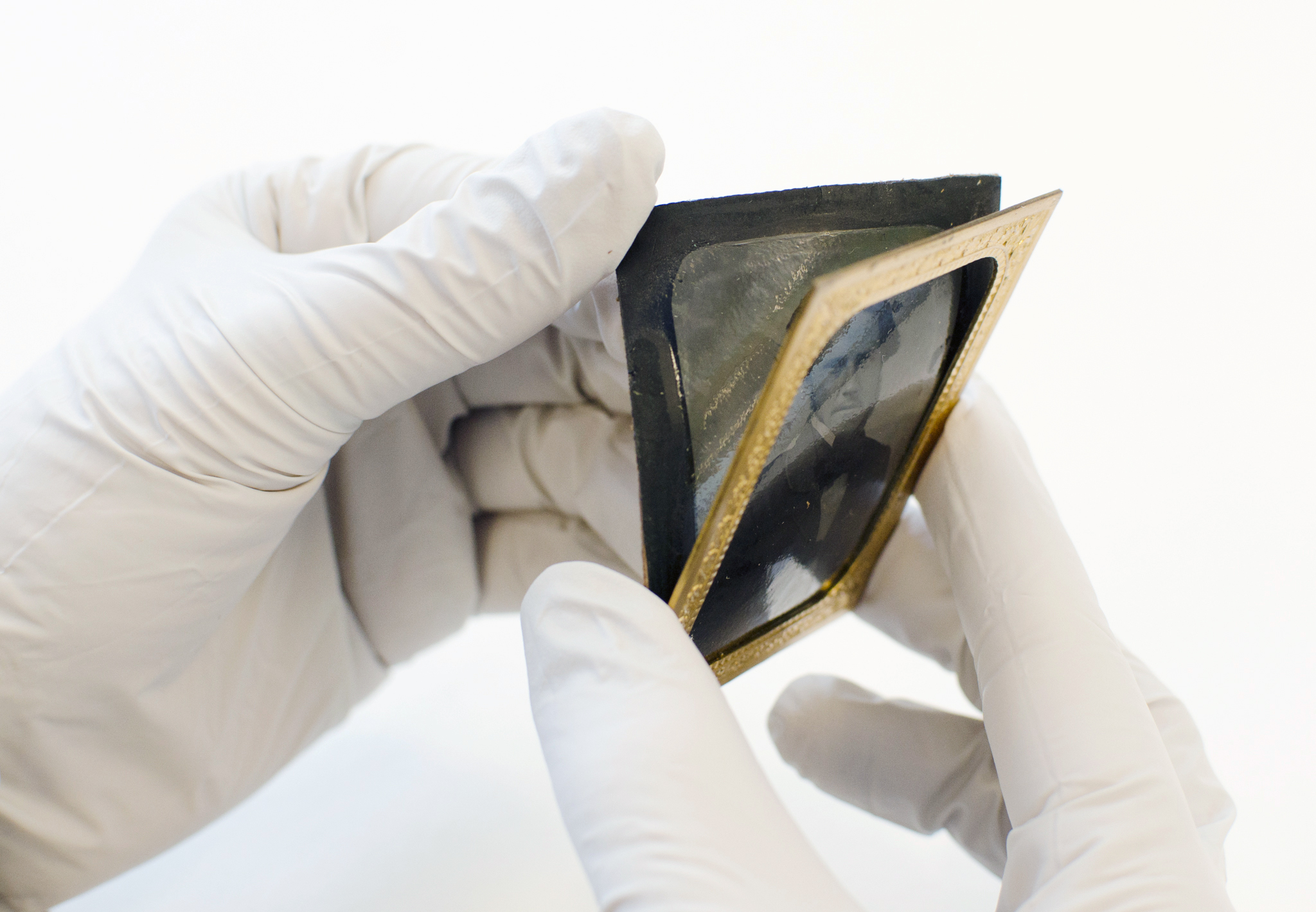
Tintype
Dates of major use: 1855-1860s
Inventor: Adolphe-Alexandre Martin (1853), Hamilton A. Smith (1856)
A tintype is a monochromatic direct positive image that is formed on a thin metal plate covered with a black varnish. They were often hand-coloured. Tintypes can be presented in paper mounts to be slid into albums or protected in American cases under glass.
Lantern slide
Dates of major use: 1850-1950
Inventor: Christian Huygens (1629-1695), Langenheim Bros (1850)
A lantern slide is a transparency of a positive photograph on glass. It is viewed either by projection or by transmitted light. These images can be made using albumen, collodion or gelatin. To view lantern slides, a magic lantern was used.

Collodion negative
Dates of major use: 1851-1890
Inventor: Frederick Scott Archer (1851)
A collodion negative is a negative made on glass. It is coated with cellulose nitrate, which makes it sensitive to light. The emulsion may be uneven or absent along the edges. This may result in a non-image area. It may also be missing varnish along the corners if it was coated unevenly, which could lead to areas of silver mirroring. The glass itself could also be hand cut, and thus have uneven edges. Overall, the tones are warm, ranging from creamy white to light and dark beige.

Carbon print
Dates of major use: 1870s-1900s
Inventor: Louis-Alphonse Poitevin (1855)
Carbon printing, called the "unalterable powder process" at the time, is a non-silver photographic process invented by Louis-Alphonse Poitevin in 1855. Its principle is based on the action of potassium bichromate, which hardens gelatin after exposure to light. A sheet of paper is coated with a mix of bichromated gelatin and pigment. It is then exposed to light in contact with a negative. The image is obtained in warm water. Areas exposed to light are hardened and become insoluble, while, in areas that have not been exposed, the gelatin and the pigments dissolve in the water. The invention of the "carbon transfer" in 1860 helped improve the rendering of mid-tones. The image layer is removed from its original support before being transferred to a second support. To tackle the problem of the image being reversed during a single transfer, photographers then developed the double transfer technique, which allowed them to reproduce the true orientation of the image.

Platinum/Palladium
Dates of major use: 1880 -1930s
Inventor: William Willis (1873)
A platinum (or palladium) print is a photograph on an uncoated paper support. The paper is sensitized with salts of platinum and/or palladium and light-sensitive iron salts. The papers are exposed in contact with the negative, the sensitized paper is then developed in potassium oxalate (or sodium citrate) and immersed in a series of clearing baths, and finally washed in water. Characteristically, these images have a wide range of mid-tone density values ranging from cool grey-black to sepia.

Gelatin silver developing-out print (fibre-based)
Dates of major use: 1880-present
Inventors: Photographic industries
Fibre-based gelatin silver prints are composed of four layers: paper base, baryta, gelatin binder, and an overcoat of a protective gelatin. This paper is placed into a developing solution, hence developing-out print (DOP). Exposed to light, the paper produces a latent image. Then it is placed into a developer bath, where the image becomes visible. Finally, the fibre-based gelatin silver prints are toned in gold, platinum, selenium and other sulfide toners for image stability. See Silver mirroring.
Generally, they have black to gray tones that result in a neutral colouration. The highlights can be either bright white or warm cream in tone. The surface can range from high gloss to matte. In addition, the texture can be smooth to highly textured depending on the paper stock.

Cellulose nitrate negative
Dates of major use: 1888-1951
Inventor: Hannibal Goodwin (1887), George Eastman (1889)
Cellulose nitrate was the first transparent flexible plasticized base that was commercially available. A major drawback is that it decomposes over time and transforms into brittle shards. It is extremely flammable and therefore dangerous to store in a photographic collection.
There are three ways to identify nitrate film-based negatives: by inspecting edge printing and notch codes for dating information, by testing the materials (polarization, diphenylamine test, burn test, float test) and by looking for nitrate film deterioration.
This nitrate negative bears an inscription: No. 7 Draft, "C" Battery, R.C.H.A. It is dated between 1914-1918, which falls within the 1888-1951 date range for the use of nitrate film.

Autochrome plate
Dates of major use: 1907-1935
Inventor: Auguste and Louis Lumière (1903)
The autochrome plate patented in 1904 by the Lumière brothers was designed to be viewed using handheld devices or displayed using carbon arc lamps. It was made with miniscule grains of potato starch dyed red-orange, green and blue-violet on a glass plate. Lampblack was added to fill in the gaps between the grains. It was then laminated and pressed. A black-and-white gelatin emulsion was added as the lightsensitive layer. The small particles of colour can be noticed while viewing.

Cellulose acetate negative
Dates of major use: 1925-1950
Inventor: Film manufacturers
Cellulose acetate film was used as a safe replacement for the unstable and highly flammable cellulose nitrate film.
There are three methods to identify negatives: by inspecting edge printing and notch codes for dating information, by looking for acetate film-based deterioration and by testing the materials.
The edge printing on this negative is obvious, as the word "safety KODAK" is labeled on the emulsion side of the upper right edge of the negative border. There are also two V- and two U-shaped notch codes, which correspond to Kodak 14B safety negative sheet film.
As for dating the material, cellulose diacetate sheet film was used from 1925-1950 and cellulose acetate propionate was in production from 1930-1945. We also know the provenance of this negative of Sir Winston Churchill, which was taken by Yousuf Karsh on December 1941.
Therefore, we can accurately say that this negative is made with cellulose acetate.

Colour transparency on plastic film
Dates of major use: 1935-present
Inventors: Lumière Brothers (1930s); Rudolph Fisher (1909); and from Eastman Kodak, Leopold Mannes and Leopold Godowsky (1935)
A colour transparency is a positive on a plastic film support. This chromogenic process uses either a cellulose acetate or polyester as a support. It incorporates three layers of gelatin (cyan, magenta or yellow) in each layer as well as a silver halide (chloride, bromide or iodide) to make it photosensitive.
They come in standard formats such as 135 mm, 120 cm, 220 cm rolled film and also sheet film. For long-term preservation, keep in cold storage.

Credit: Bob Brooks
Chromogenic print
Dates of major use: 1960s-1990s
Inventor: Rudolph Fisher (1935)
The chromogenic print, introduced in the 1940s, is a silver-based photograph. The colours are formed by chemical synthesis during development. Traditionally a chromogenic print was produced using film in an enlarger. The enlarger projects light through the negative onto the photographic paper. With the advent of digital photography, a chromogenic print could be produced from a digital file using lasers or LED lights that project the image onto the photographic paper. The processes are similar as the photographic paper is processed using traditional chemicals.

Credit: Rodolphe Hammadi
Dye destruction print
Dates of major use: 1963-present
Inventor: Bela Gaspar (1930), CIBA (1963)
Marketed starting in the early 1960s under the name Cilchrome®, then Cibachrome® and finally Ilfochrome®, dye destruction print is a silver process colour print obtained using a positive transparency. An image is formed from the selected bleaching of dyes (subtractive three-colour synthesis). The print support is composed of gelatin layers of yellow, magenta and cyan on laminated paper or pigmented plastic film. The dyes are destroyed according to the amount of light received when exposed during development. The technique gradually disappeared after the 2013 bankruptcy of Ilford.

Credit: Loretta Lux
Dye diffusion print
Dates of major use:1948-2008
Inventor: Edwin H. Land (1937), Impossible Project 2008- currently
Dye diffusion prints were introduced in 1948 with the invention of the first Polaroid® device by Edwin H. Land. An integral film consisting of a receiving layer and a negative is loaded into the device. Immediately after the shot is taken, the film is manually removed or ejected from the device, a pod breaks open between two rollers releasing the reagent, which spreads between the negative and the receiving layer. The image is formed using a transfer/diffusion process. For black-and-white films, unexposed photosensitive salts are transferred from the negative to the positive. For colour Polaroid® films, introduced in 1963, dyes are transferred from one layer to another. A final chemical reaction fixes the image.
Link to Atelier de Restauration et de Conservation des Photographies de la Ville de Paris website

Credit: Anne Cartier-Bresson


