Organizational Structure: Patented Medicine Prices Review Board
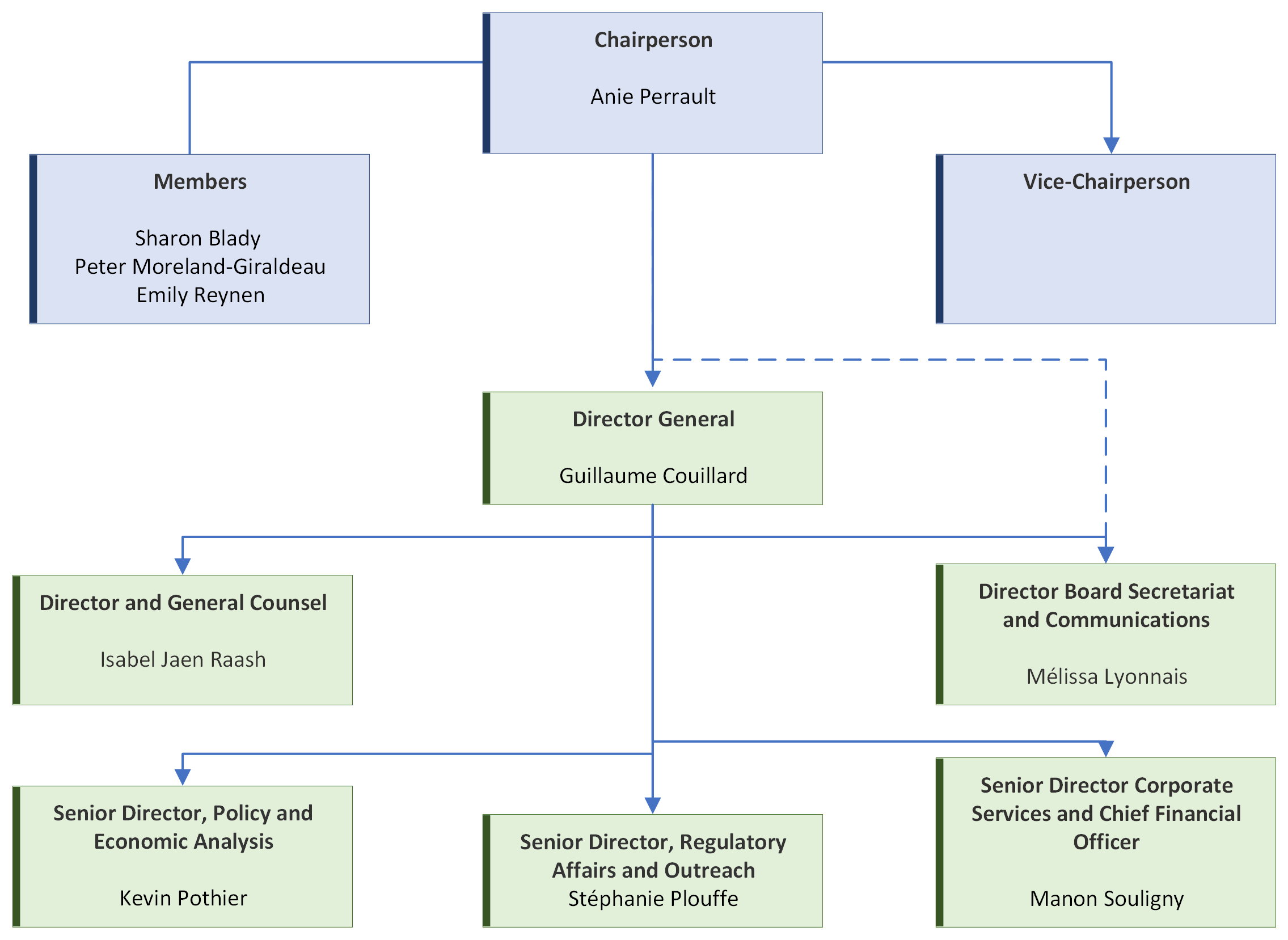
Board Members
The Board consists of not more than five members who serve on a part-time basis, appointed by the Governor-in-Council, including a Chairperson and a Vice-chairperson. The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB with the authority and responsibility to supervise and direct its work. By operation of law, the Vice-chairperson exercises all the powers and functions of the Chairperson should the Chairperson be absent or incapacitated, or if the office of the Chairperson is vacant.
Find out more about the roles and responsibilities of Board Members.
Chairperson
Anie Perrault

Ms. Anie Perrault is a lawyer by training (University of Ottawa – 1992; Barreau 1993) with more than 30 years of professional experience in the public and private sectors. Her career has focused on communications and public affairs related to genomic research and biotechnology and she has held several strategic positions at a national level in this field. She was Director General of BIOQuébec from 2013 to 2022 and Vice President, Communications of Genome Canada from 2001 to 2006.
Named Sun Life Leadership – Exceptional Woman finalist in the prestigious Les Mercuriades 2021 competition, Ms. Perrault is also an accredited mediator from the Institute of Mediation and Arbitration of Quebec. Ms. Perrault was a Member (administrative judge) of the Canadian Human Rights Tribunal from 2015 to 2021, and continues to act as a mediator there today.
In 2013, Ms. Perrault received the title of Certified Corporate Administrator from the College of Corporate Administrators at Laval University. She is president of the board of directors of Génome Québec. She has also served on the board of directors of ACCESSA since 2020. She was a member of the boards of the Jeanne-Mance Foundation from 2016 to 2022, Loto-Québec from 2011 to 2021 and the University of Sherbrooke from 2016 to 2019.
Vice-Chairperson
Vacant
Members
Sharon Blady

Sharon Blady (PhD) is a senior executive with more than 15 years of healthcare, government, and public policy expertise. She is the former Health Minister of Manitoba and Founder-CEO of Speak Up: Mental Health + Neurodiversity. Sharon has served on numerous healthcare-related boards, and taught in Indigenous studies, gender studies, nursing, social work, and psychology.
Sharon has written two pieces of first-in-Canada legislation supporting interpersonal violence survivors, persons with disabilities, and service animal users. As Health Minister, she led system-wide transformation addressing systemic racism identified in the Brian Sinclair Inquest Report, while strengthening vaccination, cancer, and mental health protocols.
Sharon has provided expertise in healthcare policy and government relations to various groups, ranging from local NGOs to international media. She created two mental health and neurodivergence programs drawing from her research, healthcare, and lived experience to foster engagement, understanding, and respect.
Peter Moreland-Giraldeau

Peter Moreland-Giraldeau is an administrative law lawyer currently working as Legal Counsel for the Appeals Commission for Alberta Workers’ Compensation. Peter obtained his LLB from the University of Leicester and his LLM from the University of British Columbia.
Peter has significant experience in administrative law, particularly in the context of quasi-judicial tribunals. He has worked for various government organizations, including the Government of Ontario, the UK Federal Civil Service, the Scottish Government, and the Government of Alberta.
Dr. Emily Reynen

Dr. Emily Reynen completed both undergraduate and doctorate degrees in Pharmacy at the University of Toronto Leslie Dan Faculty of Pharmacy. Dr. Reynen obtained her medical degree from the McGill University Faculty of Medicine. She completed internal medicine and critical care subspecialty training at Queen’s University. Dr. Reynen holds certification in Internal Medicine and Critical Care Medicine from the Royal College of Physicians and Surgeons of Canada and the American Board of Internal Medicine. Dr. Reynen currently practices as a staff intensivist at Quinte Health Care Belleville General Hospital site and is an Adjunct Assistant Professor at Queen’s University.
Directorates
Regulatory Affairs and Outreach
The Regulatory Affairs and Outreach Branch reviews the prices of patented drug products sold in Canada to ensure that they are not excessive; encourages patentees to comply voluntarily with the Board’s Guidelines; implements related compliance policies; and investigates complaints into the prices of patented medicines. This branch also informs and educates patentees on the Board’s Guidelines and filing requirements.
Policy and Economic Analysis
The Policy and Economic Analysis Branch develops policy and strategic advice; makes recommendations on possible amendments to the Board’s Guidelines; conducts research and analysis on the prices of drugs, pharmaceutical market developments and R&D trends; and publishes studies aimed at providing F/P/T governments and other interested stakeholders with centralized, credible information in support of evidence based policy.
Corporate Services
The Corporate Services Branch provides advice and services in relation to human resources management; facilities; procurement; health, safety and security; information technology; and information management. It is also responsible for financial planning and reporting, accounting operations, audit and evaluation, and liaising with federal central agencies on these topics.
Board Secretariat
The Board Secretariat manages the Board’s meeting and hearing processes, including the official record of proceedings.
General Counsel
The General Counsel advises the PMPRB on legal matters and leads the legal team representing Board Staff in proceedings before the Board.