Information sessions on the Guidelines for PMPRB Staff
Introduction
- Welcoming remarks by Guillaume Couillard, Director General, Patented Medicine Prices Review Board
- Word from Anie Perrault, Acting Chair, Patented Medicine Prices Review Board
Outline
- Filing
- Review Process
- Initial Review
- Annual Review
- Additional Information
- In-Depth Review
- Communications and Timelines
- Questions and Answers
Filing
Filing – Online Filing Tool
- The Online Filing Tool (OFT) is an online portal which facilitates Rights Holders’ regulatory filing obligations based on the PMPRB’s guiding legislation.
- Mainly:
- Form 1 – Identity of medicines, Rights Holders and patents (from the outset and following any changes)
- Form 2 – Price and Sales Data (from the outset and semi-annually onwards) through Block 4 (Canadian sales) and Block 5 (publicly available ex-factory prices in Canada and PMPRB11 countries)
- Form 3 – Revenues and R&D Expenditures
- It is the responsibility of each Rights Holder to ensure all required information is submitted by the set deadlines and is accurate.
- Detailed information on when and how to submit can be found on our Website.
Filing Requirements Pertaining to Price Reviews
Form 1: Rights Holder and Medicine Information
| Information | Timing | Patent Act | Regulations | Form |
|---|---|---|---|---|
| Identity of medicine, rights holder and patent(s) | Earliest of: Seven (7) days after the date the first Notice of Compliance issued Seven (7) days after the date the medicine is first offered for sale in Canada |
80(1)(a) 80(2)(a) |
3(1) 3(2) 3(3) |
1 |
| Updating information on identity of medicine/rights holder | Within thirty (30) days after any modification of information | 3(4) | 1 |
Form 2: Price and Sales Data
| Information | Timing | Patent Act | Regulations | Form |
|---|---|---|---|---|
Price and sales data for the medicine sold to province/territory in Canada Publicly available ex-factory price sold in Australia, Belgium, France, Germany, Italy, Japan, Netherlands, Norway, Spain, Sweden and United Kingdom |
When a drug is first offered for sale in Canada, no later than thirty (30) days after the first day of sales On or before July 30 (January 1 to June 30 reporting period) On or before January 30 (July 1 to December 31 reporting period) |
80(1)(b) 80(2)(b) |
4(1) 4(2) |
2 |
Form 3: Revenue and R&D Expenditures
| Information | Timing | Patent Act | Regulations | Form |
|---|---|---|---|---|
| Revenues from sales and expenditures on R&D | On or before March 1 of each year | 88(1) 88(2) | 5, 6 | 3 |
Filing – Form 2, Block 4 and 5 Information
- Approximately 6 weeks before a semi-annual filing is due, Staff sends all Rights Holders information on the upcoming filing, including the list of medicines currently filed with the PMPRB.
- You should flag any anomalies and ensure all required information is included for the filing.
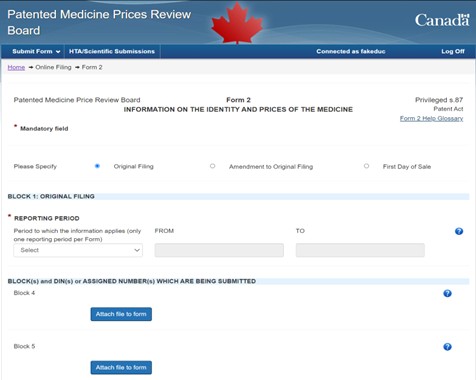
Figure description
This image is a screen capture of the «Form 2 - Information on the Identity and Prices of the Medicine» submission page on the Online Filing Tool. This form includes fields to fill out information on Block 1 (reporting period), as well as a section to attach files to submit Block 4 and Block 5 information
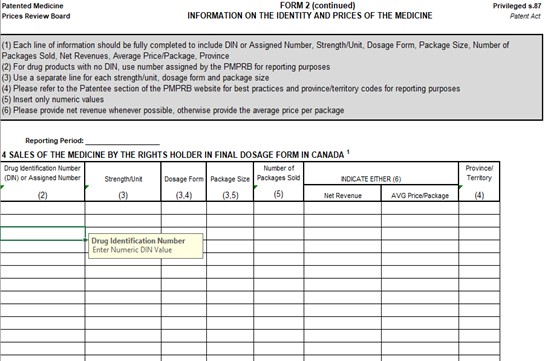
Figure description
This image is an example of an Excel document that is provided by the PMPRB to rights holders to file Block 4 information. It is a table to includes columns to note sales information for Canada including DIN number, Strenght/Unit, Dosage form, Package size, Number of packages sold, if its either net revenues of average price per package, and province/Territory.
- The PMPRB uses information provided by Rights Holders to review prices. Under the new framework, information submitted in Block 5 is especially important for the initial/annual review.
- Staff may follow-up if information seems to be missing, or if there appears to be a clerical error.
- Examples could be, if the Canadian list price is missing, if the reporting unit has changed (i.e. pack-size), or if a list price has changed in a way that seems to indicate an error (e.g. goes from $1.35 to $135).
- The objective is to avoid situations where an in-depth review is opened where it was not necessary. It is therefore important to communicate back with PMPRB staff as soon as possible after an inquiry has been made, or if the Rights Holder realizes that there was a filing error.
- Reminder that the following information should be filed for each reporting period, for each medicine for which the regulations apply to:
- Canadian sales information
- Canadian list price
- List price in other PMPRB11 countries where the medicine is sold
- Should there be a situation where there is no Canadian ex-factory price publicly available, Rights Holders should provide a rationale to allow PMPRB staff to maintain the appropriate records.
Review Process
Review Process – Overview
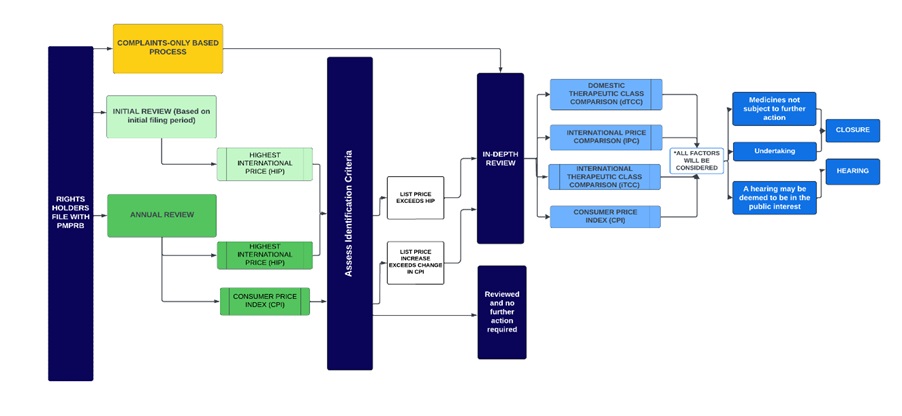
Figure description
This diagram sets out PMPRB's general review processes for patented medicines contemplated under these Guidelines. It is divided into several sections to highlight the various steps in the process.
The framework is described below:
- The first step is an Initial Review (light green) or Annual Review (dark green) and applies to all patented medicines sold in Canada, with certain limited exceptions (set out below under “Special Provisions on Complaints”).
- Complaints (yellow) serve as a separate process by which medicines can be identified for an In-Depth Review.
- Initial Review (light green box):
- Staff uses a medicine’s first semi-annual price filing to conduct an Initial Review against the highest international price among the Schedule Countries filed by the Rights Holder (“HIP”).
- Patented medicines whose prices are above the HIP threshold are subject to an In-Depth Review.
- Annual Review (dark green box):
- Staff conducts an Annual Review of list prices for each patented medicine under the PMPRB’s jurisdiction.
- The Annual Review applies the same IPC identification criteria (the HIP) used during the Initial Review.
- Staff also compares the price change of each patented medicine against changes in the Consumer Price Index (CPI) as an identification criterion to prioritize medicines that warrant an In-Depth Review.
- The result of the Initial Review or Annual Review may either be (a) no further review; or (b) referral for In-Depth Review.
- The In-Depth Review (light blue) is the process by which Staff analyses and balances all the information related to the section 85 factors to prepare a recommendation to the Chairperson on whether a matter should be brought to a hearing. The result of an In-Depth Review is either (a) recommendation for closure of the In-Depth Review; or (b) recommendation for a hearing.
Initial Review
- The Initial Review period for a medicine spans from its date of first sale to the end of that reporting period, either June 30 or December 31.
- Using the methodology described previously for exchange rates, Staff will compare the highest Canadian list price of the medicine reported for the Initial Review period to the Highest International Price (HIP) of the PMPRB11, as filed by the Rights Holder.
- This is known as the International Price Comparison (IPC)
- If the highest Canadian list price is above the HIP, the medicine will be subject to In-Depth Review.
- The In-Depth Review will include the DIN that triggered the In-Depth Review, as well as all Associated DINs (as filed by rights holders).
- If the highest Canadian list price is below the HIP, the medicine will be considered Reviewed.
- If the IPC cannot be conducted because no prices are filed for the medicine in any of the PMPRB11 comparator countries during the Initial Review period, the medicine will also be considered Reviewed.
Initial Review – Spring 2026
- The new Guidelines define a New Medicine as any patented medicine first sold on or after July 1, 2022.
- The Initial Reviews completed in Spring 2026 will include all New Medicines first sold between July 1, 2022, and December 31, 2025. These Initial Reviews will be based on:
- The July to December 2025 Form 2, Block 5 filings reported by Rights Holders; and
- The 36-month average exchange rates ending February 2025.
- The results of Initial Reviews will be communicated to Rights Holders within 60 days following the semi-annual filing deadline.
- After this first exercise, Initial Reviews will be conducted after each semi-annual filing, for each medicine that is newly reported.
Additional Information – Exchange Rates
- The PMPRB has adjusted the 36-month average exchange rates used during the Initial and Annual Reviews to be more consistent, and to enhance predictability and transparency for Rights Holders.
- During the Initial Review, the exchange rates used will be the 36-month average ending in the second month of the reporting period prior to the Initial Review period. Examples:
- Any medicine with an Initial Review period of January to June 2040 will use the 36-month average exchange rates ending in August 2039.
- Any medicine with an Initial Review period of July to December 2040 will use the 36-month average exchange rates ending in February 2040.
- During the Annual Review, the exchange rates used will be the 36-month average ending in the second month of the reporting period under review. Example:
- During the Annual Review of the July to December 2035 prices, the 36-month average exchange rates ending August 2035 will be used.
- Rights Holders will know the exchange rates that will be used by PMPRB Staff at least five months before the filing deadlines.
Summary of criteria – Initial Review
Examples of initial reviews based on date of first sale:
| Date of First Sale | Initial Review | Reporting Period Reviewed | Exchange Rate Used** | HIP Applies? | CPI Applies? | First Annual Review | Reporting Period Used |
|---|---|---|---|---|---|---|---|
July 1, 2022* |
Jan/Feb 2026 |
2025-2 |
03/22 - 02/25 |
Yes |
No |
Jan/Feb 2027 |
2026-2 |
March 5, 2024 |
Jan/Feb 2026 |
2025-2 |
03/22 - 02/25 |
Yes |
No |
Jan/Feb 2027 |
2026-2 |
Sept. 9, 2025 |
Jan/Feb 2026 |
2025-2 |
03/22 - 02/25 |
Yes |
No |
Jan/Feb 2027 |
2026-2 |
May 12, 2027 |
July/Aug 2027 |
2027-1 |
09/23 - 08/26 |
Yes |
No |
Jan/Feb 2028 |
2027-2 |
Oct. 26, 2028 |
Jan/Feb 2029 |
2028-2 |
03/25 - 02/28 |
Yes |
No |
Jan/Feb 2030 |
2029-2 |
*Earliest date of first sale to be considered a new medicine. Medicines first sold earlier are considered existing medicines under the new guidelines.
** Simple average of the thirty-six (36) monthly average noon spot exchange rates for each country as published by the Bank of Canada as explained earlier.
Annual Review
Annual Review – Process
- Staff will conduct an Annual Review of the list prices of all patented medicines under the PMPRB’s jurisdiction beginning in January of each year.
- Two identification criteria will be applied during the Annual Review:
- The International Price Comparison (IPC)
- Consumer Price Index (CPI)
- International Price Comparison:
- During the Annual Review, Staff will compare the highest Canadian list price of the medicine to the HIP of the PMPRB11.
- Staff will use the Form 2, Block 5 data filed by the Rights Holder for the July to December reporting period.
- If the highest Canadian list price is above the HIP, the medicine (including all Associated DINs as filed by rights holders) will be subject to In-Depth Review.
Annual Review – CPI
- Consumer Price Index:
- The second identification criteria compares changes to the list price of a medicine to the change in CPI.
- Staff will use one-year lagged CPI factors in this evaluation.
- Example: for the Annual Review of patented medicines in February 2028, Staff will compare list price increases taken in 2027 against the 2026 CPI factors, published by Statistics Canada in January 2027.
- Rights Holders will know the CPI factors that will be used by Staff in advance of the Annual Review.
- The source Staff uses to calculate the CPI is the “Consumer Price Index, monthly, not seasonally adjusted” as published by Statistics Canada.
- If a Rights Holder increases the list price of a patented medicine by an amount greater than the change in the CPI in any given year, Staff will open an In-Depth Review unless:
- The Rights Holder did not take a list price increase in the previous year, and
- The increase in the second year is lower than or equal to the total change in CPI over those two years.
Annual Review – CPI Schematic

Figure description
This image represents the decision matrix when determining how to apply the Consumer Price Index criterion against a list price increase.
The first question is «Was the list price increase greater than one-year CPI? If the answer is no, then the medicine is considered reviewed.
If the answer is yes, then the next question is «Was the list price increased in the previous year? If the answer is yes, then the medicine is subject to in-depth review.
If the answer is no, the next question is «Was the list price increase greater than two-year CPI? If the answer is no, then the medicine is considered reviewed.
If the answer is yes, then the medicine is subject to in-depth review.
Note: this schematic only represents the CPI criterion and assumes the list price is below the HIP.
Annual Review – CPI Examples
- Example 1: (Date of first sale is February 2, 2029)
| Regulatory Period | Highest List Price | List Price Change | 1-Year CPI |
|---|---|---|---|
Jan29 – Dec29 |
$11.00 |
-- |
-- |
Jan30 – Dec30 |
$11.20 |
1.8% |
2.3% |
- In 2030, the highest Canadian list price increased by 1.8%.
- As this increase is less than one-year CPI (2.3%), the list price increase does not trigger an In-Depth Review.
- Example 2: (Date of first sale is February 2, 2029)
| Regulatory Period | Highest List Price | List Price Change | 1-Year CPI |
|---|---|---|---|
Jan29 – Dec29 |
$11.00 |
-- |
-- |
Jan30 – Dec30 |
$11.20 |
1.8% |
2.3% |
- In 2030, the highest Canadian list price increased by 3.2%.
- Two-year CPI cannot be considered as the medicine is only in its second year of sales in Canada.
- As this increase is greater than one-year CPI (2.3%), the list price increase does trigger an In-Depth Review.
- Example 3: (Date of first sale is February 2, 2029)
| Regulatory Period | Highest List Price | List Price Change | 1-Year CPI | 2-year CPI |
|---|---|---|---|---|
Jan29 – Dec29 |
$11.00 |
-- |
-- |
-- |
Jan30 – Dec30 |
$11.00 |
0.0% |
2.3% |
-- |
Jan31 – Dec31 |
$11.35 |
3.2% |
2.1% |
4.4% |
- In 2031, the highest Canadian list price increased by 3.2%. This increase is greater than one-year CPI (2.1%).
- No list price increase was taken in 2030; therefore, Staff compare the increase to two-year CPI.
- As this increase is less than two-year CPI (4.4%), the list price increase does not trigger an In-Depth Review.
- Example 4: (Date of first sale is February 2, 2029)
| Regulatory Period | Highest List Price | List Price Change | 1-Year CPI | 2-year CPI |
|---|---|---|---|---|
Jan29 – Dec29 |
$11.00 |
-- |
-- |
-- |
Jan30 – Dec30 |
$11.00 |
0.0% |
2.3% |
-- |
Jan31 – Dec31 |
$11.35 |
3.2% |
2.1% |
4.4% |
- In 2030, the highest Canadian list price increased by 2.0%. This increase was less than one-year CPI (2.3%).
- In 2031, the highest Canadian list price increased by 2.3%. This increase is greater than one-year CPI (2.1%).
- While the two-year increase (4.36%) is less than two-year CPI (4.4%), two-year CPI cannot be considered as the medicine took a list price increase in the previous year.
- As the 2031 increase is greater than one-year CPI, the list price increase does trigger an In-Depth Review.
Annual Review
- If the list price of a medicine triggers either of the identification criteria, an In-Depth Review will be initiated.
- A medicine will only be considered Reviewed if neither identification criteria are triggered.
- The results of Annual Reviews will be communicated to Rights Holders by letter within 60 days following the semi-annual filing deadline in January.
Additional Information
Transition period – New versus Existing
- To account for the interim period between the previous framework and the 2025 Guidelines, as well as the new basket of comparator countries established on July 1, 2022, transition measures were put in place. These mostly apply to existing medicines.
- New versus Existing:
- New Medicine – patented medicine first sold on or after July 1, 2022
- Existing Medicine – patented medicine first sold prior to July 1, 2022.
- First reporting period for review:
- Guidelines for Staff come into effect in January 2026, therefore the 2025-2 reporting period will be the first one reviewed under the first step of the new framework.
- This would not preclude the PMPRB from using previous data in the case of an in-depth review or a hearing.
Transition period – Existing Medicines
- Existing Medicines have a transition period of two years from the date the Guidelines go into effect before Staff will conduct the review of their prices for the first time.
- January 2026 – No review of the 2025-2 data
- January 2027 – No review of the 2026-2 data
- January 2028 – Annual Review of the 2027-2 data
- This first review (January 2028) will not be an Initial Review, but an “abbreviated” Annual Review, in which only the IPC identification criterion will be applied (comparison with international prices).
- The first “standard” Annual Review for Existing Medicines, applying both identification criteria, will be conducted by Staff in 2029.
Summary of criteria – Annual Review
Examples of Annual Reviews considering transition measures:
| Date | Existing Medicines (first sold before July 1, 2022) | New Medicines (first sold between July 1, 2022, and December 31, 2025) | Onwards Example: Medicine first sold between January 1, 2026, and June 30, 2026 | Onwards Example: Medicine first sold between July 1, 2026, and December 31, 2026 |
|---|---|---|---|---|
March 2026 |
Information Report |
Initial Review |
N/A |
N/A |
Sept. 2026 |
Information Report |
N/A |
Initial Review |
N/A |
March 2027 |
Information Report |
Annual Review |
Annual Review |
Initial Review |
Sept. 2027 |
Information Report |
N/A |
N/A |
N/A |
March 2028 |
Annual Review (no CPI) |
Annual Review |
Annual Review |
Annual Review |
Sept. 2028 |
N/A |
N/A |
N/A |
N/A |
March 2029 |
Annual Review |
Annual Review |
Annual Review |
Annual Review |
*Simple average of the thirty-six (36) monthly average noon spot exchange rates for each country as published by the Bank of Canada as explained earlier.
Existing Medicines – Information Reports
- To avoid a gap in communications with Rights Holders on the prices of their Existing Medicines, Staff will send Information Reports following the receipt of each semi-annual filing leading up to their first Annual Review.
- Data included on the Information Reports is based on the Form 2, Block 5 filings, and summarizes each medicine’s highest Canadian list price, HIP, and the country setting the HIP.
- These Information Reports will be like those sent to Rights Holders this fall, but will be limited to Existing Medicines, as New Medicines will be subject to Initial and Annual Reviews, as described in the New Guidelines.
- An Existing Medicine will only become subject to In-Depth Review during this two-year transition period if:
- It is the subject of a complaint
- It is the Associated DIN of a medicine that is subject to In-Depth Review

Figure description
This image shows an example of information reports that are sent to rights holders. The title is «Internationale/List Price Information Report (month/month year)Reporting Period.
The top portion provides information on the name of the Rights Holders. Then we see a table with 5 columns:
- Column 1 - Brand Name
- Column 2 - DIN
- Column 3 - Highest Canadian List Price
- Column 4 - Highest International Price (HIP)
- Column 5 - Comparator Country Setting HIP.
Communications Following Initial/Annual Reviews
Expected communications with Rights Holders:
| Date | Existing Medicines (first sold before July 1, 2022) | New Medicines (first sold between July 1, 2022, and December 31, 2025) | Onwards Example: Medicine first sold between January 1, 2026, and June 30, 2026 | Onwards Example: Medicine first sold between July 1, 2026, and December 31, 2026 |
|---|---|---|---|---|
March 2026 |
Information Report |
Initial Review |
N/A |
N/A |
Sept. 2026 |
Information Report |
N/A |
Initial Review |
N/A |
March 2027 |
Information Report |
Annual Review |
Annual Review |
Initial Review |
Sept. 2027 |
Information Report |
N/A |
N/A |
N/A |
March 2028 |
Annual Review (no CPI) |
Annual Review |
Annual Review |
Annual Review |
Sept. 2028 |
N/A |
N/A |
N/A |
N/A |
March 2029 |
Annual Review |
Annual Review |
Annual Review |
Annual Review |
Special Consideration – Complaints
- In addition to the HIP and CPI identification criteria, an In-Depth Review may be triggered at any time following the receipt of a complaint by an approved individual (or organization).
- The Federal Minister of Health or any of their Provincial/Territorial counterparts.
- Senior officials who are authorized to represent Canadian publicly-funded drug programs.
- Basic information will need to be confirmed before initiating at In-Depth Review. After receiving the complaint, Staff will:
- Confirm that the complainant is one of the above approved individuals
- Confirm that the subject of the complaint is a patented medicine under the PMPRB’s jurisdiction
- All complaints will be acknowledged by the PMPRB. However, the complainant is not informed of the commencement of an In-Depth Review, nor of its outcome.
- The scientific and price review processes for an In-Depth Review opened due to a complaint are the same as those opened through the Initial or Annual Reviews.
In-Depth Review
In-Depth Review – Initiation
- In-Depth Reviews will be initiated based on the result of an Initial or Annual Review, or following a complaint.
- Under the Guidelines:
- Staff does not calculate a ceiling price for each medicine each year.
- Instead, Staff use triggers to identify medicines in need of a more thorough review.
- These triggers should not be confused with ceilings, nor are they the arbiter of whether a price is excessive or non-excessive: only the Board can make that determination during a price hearing.
- Accordingly, Staff does not calculate potential excess revenues.
- This allows for increased transparency: the triggers are based on the Rights Holders’ filings of their Canadian list price and HIP, as well as exchange rates and CPI factors that are available in advance. A Rights Holder will know at the time of their filing whether or not their medicine(s) will be subject to In-Depth Review.
In-Depth Review – Scientific Review
- The Scientific Review starts at the very beginning of the In-Depth Review process.
- This review aims at identifying comparators, comparable dosage regimens and assigning a level of similarity with the medicine under review. This will inform the Therapeutic Class Comparison (TCC) analysis.
- The Staff scientific team is responsible for the Scientific Review. The team is comprised of health care professionals with significant education, background and expertise in various areas of clinical practice, drug evaluation and drug utilization.
- This exercise is done using various sources such as available medical literature, clinical evaluations from health technology organizations, research from a drug information centre, Rights Holder submission, etc.
- A list of potential sources can be found in section 91 of the Guidelines.
- The Human Drug Advisory Panel (HDAP) may be consulted to assist scientific evaluations, as needed. More information on HDAP can be found on our Website.
- The Scientific Review will identify comparators for each approved indication or use for the medicine at the time of the review.
- Each comparator will be assigned a level of similarity and a comparable dosage regimen. These will be used to provide context for the TCC analysis, which will inform the price review.

Figure description
This table illustrates the spectrum of comparability, ranging from more comparable (top-left) to less comparable (bottom-right). The horizontal axis represents the qualitative class (A, B, C, D), while the vertical axis indicates groupings (1, 2, 3, 4). The figure effectively shows how comparability decreases progressively across qualitative classes (left to right) and across groupings (top to bottom).
Comparability is visually distinguished by color:
- Green: High comparability (e.g., A1, A2 and B1)
- Blue: Medium comparability (e.g., A3, A4, B2, B3, C1, C2, D1)
- Yellow/Orange/Pink: Low comparability (e.g., B4, C3, C4, D2, D3 and D4)
- Please note:
- Level of therapeutic improvement is no longer a factor (e.g. breakthrough)
- At no point in the scientific review will prices be considered
- Rights Holders may choose to provide written input relating to the Scientific Review.
- Materials should be submitted using the PMPRB Online Filing Tool, as per past practice.
- After receiving notification of an In-Depth Review, Rights Holders are asked to inform Staff if they intend to make a scientific submission. If so, this submission should be made as soon as possible to ensure it is captured in the scientific review.
- The PMPRB Website provides guidance for the suggested format and content of scientific submissions.
- The content should align with submissions made previously. For each approved indication:
- A summary of the Right’s Holders perspective on the medicine’s place in therapy
- Comparators
- Comparable dosage regimens
- Supporting scientific literature
In-Depth Review – Scientific Review Outputs
- The scientific review will generally be completed within eight months of the commencement of the In-Depth Review.
- Potentially longer if the Human Drug Advisory Panel (HDAP) is consulted.
- Upon the completion of the scientific review, Rights Holders will receive a summary document listing:
- The identified comparators
- The comparable dosage regimens of the medicine under review and all identified comparators
- The comparability score of each comparator
- This summary document will also include:
- An outline of the rationale used in the assessment of each comparator.
- A list of references evaluated during the science review process.
In-Depth Review – Price Review
- At the same time as the scientific review is initiated, Staff will conduct the preliminary price review.
- The first appendix of the Guidelines provides insight into the type of analysis that will be conducted. At this time, without the scientific review, the focus will be on 85(1)(a), 85(1)(c), and 85(1)(d), such as:
- Examination of current and historic list prices
- Current and historic list price changes compared to CPI
- The number of reported PMPRB11 countries, current and historic
- Distribution of the price of the medicine across the PMPRB11
- Changes in local currency prices and exchange rates across the PMPRB11
- Once the scientific review is complete, the price review continues with the domestic Therapeutic Class Comparison (dTCC) and international Therapeutic Class Comparison (iTCC) analyses.
- Covering 85(1)(b) and 85(1)(c), respectively.
- The dTCC and iTCC analyses are not “tests”. They do not produce a singular result with a pass/fail outcome. Staff consider the treatment costs of all comparators, determining the weight to assign the two TCC analyses based on the comparability scores from the scientific review.
- The image illustrates how the TCC can be used to weigh treatment costs during the pricing review component of the In-Depth Review.
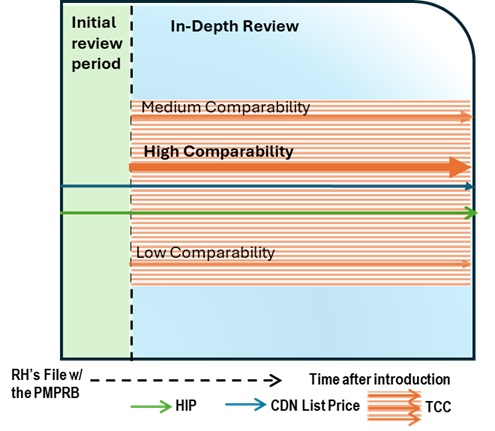
Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is above the HIP, resulting in an In-Depth Review. The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will also evaluate the comparators for their level of comparability to the medicine under review. The chart is divided into two sections: the green section for the Initial Review period and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, the green line represents the HIP, and the orange lines represent the TCC comparators. A high comparability comparator priced above both the Canadian list price and the HIP was identified.
In-Depth Review – Recommendation
- At the conclusion of the price review, Staff will weigh the analysis conducted under each individual 85(1) factor to determine a recommendation for the outcome of the In-Depth Review.
- It is estimated that the full process, including scientific review, could take between 12 and 28 months, depending on complexity.
- The outcome of an In-Depth Review will either be to recommend to the Chairperson:
- the issuance of a Notice of Hearing; or
- closure of the file (with or without the acceptance of an Undertaking).
- The appendix in the Guidelines describes situations which could lead to a recommendation for a Hearing versus closure.
- If the Chairperson decides to close an In-Depth Review, the Guidelines allow for a grace period of at least two filing periods during which the medicine will not be subject to another In-Depth Review.
Communications and Timelines
- Input on pricing-related factors
- Written submission within three months of the commencement of the In-Depth Review, sent via email.
- Assigned Senior Regulatory Officer throughout the In-Depth Review.
- Input on science-related factors
- As soon as possible following the commencement of the In-Depth Review.
- Submitted through the Online Filing Tool, with an email to the Filing inbox.
- Second opportunity to respond following receipt of the results of the scientific review.
- Undertakings
- A Rights Holder may submit an Undertaking at any point during the In-Depth Review, up to two months after the date on which the Rights Holders is notified by Staff that they have recommended to the Chairperson that a Notice of Hearing be issued.
- All Undertakings received will be analyzed by Staff and presented to the Chairperson for consideration.
- If Staff intend to recommend a Hearing, the Rights Holder will be notified when that recommendation is sent to the Chairperson. The Chairperson’s final decision will come three months after they are presented with the recommendation from Staff.
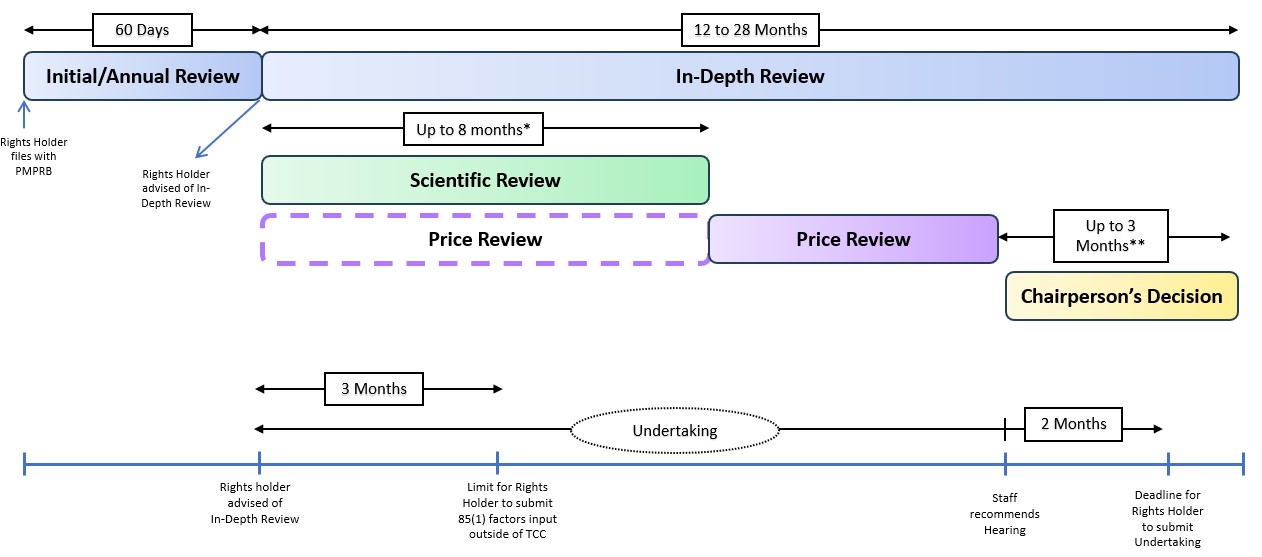
Figure description
This image presents two timelines illustrating deadlines and target dates considered under the Draft Guidelines.
The first timeline describes the PMPRB internal process from the information filed by the Rights Holder to the Chairperson’s decision.
The process is described below:
- The first step (first blue box) is an Initial Review or Annual Review, which would be completed within 60 days. At the end of this period, the Rights Holder would be advised of an In-Depth Review.
- The second step (second blue box) is the In-Depth Review, which can take between 12 to 28 months. This Review is divided in three sections:
- The first section (green box) is the Scientific Review, which can take up to eight months, and even longer if the HDAP is consulted.
- The second section (purple box) is the Price Review. It begins at the same time as the Scientific Review but lasts up to three months before the end of the process.
- The third section (yellow box) is the Chairperson’s decision, which can take up to three months, and at least one month after the submission of an Undertaking.
The second timeline (blue timeline) describes the interactions between Rights Holders and the PMPRB throughout the process.
The interactions are described below:
- First, after the Initial or Annual Review, the Rights Holder is notified that an In-Depth Review is initiated.
- From that moment, the Rights Holder has three months to submit information related to the factors outlined in subsection 85(1) other than the TCC.
- From the moment the Rights Holder is notified that an In-Depth Review is initiated, it is also possible to submit an Undertaking for the Chairperson to consider. The deadline for a Rights Holder to submit an Undertaking is two months after the PMPRB Staff recommends to the Chairperson that a hearing be held.
Questions and Answers
- Technical question period
- Questions will be taken live
- Participants who wish to ask a question should raise their hand virtually
- Only technical questions on implementation of the guidelines will be addressed today
- As this session is mean to increase understanding of the general application of the new framework, questions should not pertain to specific cases. Company-specific issues can be discussed after the session by contacting the PMPRB Regulatory Affairs and Outreach group.
- Questions remaining?
- Contact the Regulatory Affairs and Outreach group:
Stéphanie Plouffe, Senior Director Regulatory Affairs and Outreach
stephanie.plouffe@pmprb-cepmb.gc.ca