Draft Guidelines for PMPRB Staff
Administrative Process for Excessive Price Hearing Recommendation
Version for consultation, December, 2024
Draft Guidelines
Table of Contents
The Role of These Guidelines
- The Patented Medicine Prices Review Board (“PMPRB”) was established via amendments to the Patent Act (the “Act”) in 1987 as the response to a major set of reforms to the Act which strengthened Canada’s patent protection for medicines. The PMPRB is an independent quasi-judicial statutory body with a mandate to monitor the prices of patented medicines sold in Canada to ensure that they are not excessive. The PMPRB fulfills its mandate by holding public hearings to determine whether the prices of specific patented medicines are excessive. The PMPRB is also empowered to issue non-binding Guidelines on matters within its jurisdiction.
- In 2019, the Government proposed to amend the Patented Medicines Regulations (“Regulations”) to introduce new price review factors (pharmacoeconomic value, market size and gross domestic product) for the PMPRB to consider when monitoring for excessive pricing, changed the schedule of countries for which Rights Holders are to report price information to the PMPRB and also required Rights Holders to file information net of all price adjustments, including rebates provided through product listing agreements.
- The proposed amendments were challenged in both provincial and federal courts, resulting in new judicial guidance in two court proceedings – Merck Canada Inc. c. Procureur general du Canada and Innovative Medicines Canada v. Canada (Attorney General)Footnote 1– as to the scope of the PMPRB’s mandate and authority. Subsequently the Governor in Council decided not to proceed with the amendments related to the new price review factors and the net price information filing. Instead, the Government moved forward only with the implementation of the updated schedule of eleven countries (“PMPRB11”) and reduced reporting requirements for medicines believed to be at the lowest risk of excessive pricing: changes which were upheld as both constitutional and vires the Act in the Court proceedings. These two regulatory changes came into force on July 1, 2022.
- These non-binding Guidelines have been drafted to address the amendments to the Regulations, and to reflect the guidance provided by the case law. These Guidelines are directed to PMPRB Staff (“Staff”) and are intended to provide transparency to interested parties regarding the process the Staff uses to identify potential candidates to recommend that the Chairperson consider for hearings.
- These Guidelines are designed to ensure procedural fairness and consistency in that all similarly placed Rights Holders are subject to the same process and process timelines. The process described in these Guidelines consists of two “screening” steps designed to prioritize the cases that are advanced for recommendation for a hearing. The goal of the process is to allow the PMPRB to focus its limited hearings-related resources most efficiently. The first screening step – Initial Review and Annual Review – prioritizes cases for In-Depth Review. The second screening step – In-Depth Review – prioritizes cases for recommendation for hearing. Neither step determines whether a price is excessive. The steps only determine which cases Staff recommends for hearing. In all cases, the discretion to determine whether to issue a Notice of Hearing rests with the Chairperson and not with the Staff. At all times, the discretion to determine whether a given price is excessive remains with the panel of Board members assigned to an excessive price hearing (“Hearing Panel”).
- These Guidelines are not directed to Rights Holders, are not intended to provide certainty on ultimate outcomes for particular cases, are not intended to suggest or set prices in Canada and are not intended to encourage “compliance” with any tests or price ceilings. These Guidelines do not calculate “price ceilings”, “non-excessive prices”, or “potential excess revenues” either at introduction or at any subsequent point nor do they deem or presume any prices or price thresholds to be excessive or not excessive.
- The PMPRB can neither define the term “excessive” nor decide whether the price of any given patented medicine is or is not excessive through general Guidelines. The Board can only decide that prices are excessive and order price reductions to a maximum ceiling and/or order excess revenue payments on a case-by-case basis, based on the factors enumerated in the Act, in the context of a public hearing. During hearings, the Board cannot be fettered in its discretion by the Guidelines.
- The Board recognizes that the approach presented in these Guidelines is a departure from its historical practice of issuing Guidelines directed to Rights Holders to encourage “voluntary compliance” with specific price ceilings identified through the Guidelines. The Board has chosen this new approach based on the recent litigation and jurisprudence regarding its mandate and powers, and the input of interested parties during consultation processes the Board has undertaken with respect to the Guidelines over the past several years.
- As per the Act, unless they are subject to an order of the Board after a hearing into the price of a particular patented medicine, Rights Holders may price their patented medicines in Canada as they see fit at any time. Rights Holders do not require any “approval” from the PMPRB, or “compliance” with the Guidelines, to sell their patented medicines in Canada. The PMPRB cannot and does not authorize or approve prices.
- Certain aspects of these Guidelines may be revisited by the PMPRB as circumstances change. If any changes to the Guidelines are contemplated because of an internal review, interested parties will be consulted by the PMPRB as per subsection 96(5) of the Act.
Overview of the PMPRB and its Legislation
Mandate
- The PMPRB’s mandate and jurisdiction are set out in sections 79-103 of the Act. The PMPRB’s principal mandate is to ensure that the prices of patented medicines sold in Canada are not excessive. In this regard, the PMPRB monitors and reviews the prices of patented medicines and conducts price hearings which may result in orders to reduce prices to a non-excessive level.
- The Act does not define what an “excessive” price is. Instead, subsection 85(1) of the Act sets out factors that the Board must take into consideration when it is holding a hearing to determine whether a patented medicine is being sold or has been sold at an excessive price “in any market” in Canada by a Rights Holder or former Rights Holder. These factors are:
- The prices at which the medicine has been sold in the relevant market;
- The prices at which other medicines in the same therapeutic class have been sold in the relevant market;
- The prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada;
- Changes in the Consumer Price Index; and
- Such other factors as may be specified in any regulations made for the purposes of this subsection.Footnote 2
- If after considering the above factors, the Board is unable to determine if a price is excessive, subsection 85(2) of the Act stipulates that it may consider the costs of making and marketing the medicine, as well as other factors which can be specified by regulationsFootnote 3 or that the Board considers relevant in the circumstances.
- In addition to ensuring that the prices of patented medicines sold in Canada are not excessive, the PMPRB is mandated to provide information on pricing trends in the pharmaceutical industry via its Annual Reports. Moreover, further to a request from the Minister of Health under section 90 of the Act, the PMPRB also prepares reports to the Minister on a variety of subject matter related to pharmaceutical pricing and usage. These reports, also known as the National Prescription Drug Utilization Information System (NPDUIS) reports, are available on the PMPRB’s website. Information filed by Rights Holders with the PMPRB is not used for these reports to the Minister.
Structure and Administrative Operation
- The PMPRB is composed of up to five Board members, appointed by the Governor–in-Council pursuant to subsection 91(1) of the Act. One of the Board members is also designated as the Chairperson of the Board and, in that capacity, acts as the Chief Executive Officer (“CEO”) of the PMPRB. In addition to their role as a Board member, the Chairperson is responsible for administrative supervision over and direction of the work of the Board, including issuing Notices of Hearing, appointing members of the Board to serve as panel members on hearings, and the management of the PMPRB’s internal affairs and the duties of its staff. Another Board member is designated as a Vice-Chairperson and, in that capacity, is empowered to assume the powers and functions of the Chairperson during their absence, incapacity or in case of a vacancy.
- Collectively, the PMPRB Board members issue the Guidelines and PMPRB reports. In addition, Board members assigned to a Hearing Panel constitute the Board for the purpose of the hearing. Only Board members assigned to a Hearing Panel have the discretion to exercise the PMPRB’s powers to conduct hearings and issue binding orders, including making decisions on whether a price is excessive under section 85 of the Act and ordering appropriate remedies under the Act. Outside the issuance of Guidelines and by-laws, Board members other than the Chairperson do not direct or instruct Staff on its day-to-day work.
- Orders issued by the PMPRB’s Hearing Panels are enforceable in the same manner as orders of the Federal Court or any superior court in Canada and may be enforced by the PMPRB or by the Federal Court. Decisions embodied in orders issued by the PMPRB may be subject to judicial review by the Federal Court in accordance with administrative law principles and the Federal Courts Act.
- The PMPRB’s Staff consists of civil servants appointed pursuant to subsection 94(1) of the Act. The role of Staff is to assist with the proper conduct of the work of the PMPRB. Staff is headed by a Director General, who reports to the Chairperson of the PMPRB. Staff has no discretionary power to issue Guidelines, commence hearings, make decisions regarding whether a patented medicine is priced excessively, issue binding orders or directions, or to compel Rights Holders to comply with legislation. All such discretion resides with the Board as a whole, the Chairperson, or Board members sitting on a Hearing Panel as per the applicable provisions in the Act.
- Because the PMPRB is established under the Act as an independent, quasi-judicial body, it maintains an arm’s length relationship from the rest of the Federal Government, including the Minister of Health (who is responsible for the sections of the Act pertaining to the PMPRB), and the Minister of Innovation, Science and Economic Development (who is responsible for the Act as a whole) with regard to the exercise of its mandate. Under the Patent Act, the PMPRB has no specific authority or obligation to align itself with any other policy of government relating to pharmaceuticals or the life-sciences ecosystem in Canada. Equally, no express or implicit power is provided under the Act to Health Canada or any other government entity to direct the PMPRB in the exercise of its price monitoring function. That said, the PMPRB is part of the Federal Government, and the Minister of Health is the designated Minister for the PMPRB for the purposes of the Financial Administration Act, in addition to having specific limited duties and authorities vis-a-vis the PMPRB as set out in the Patent Act.
- Internally, the PMPRB is structured in a manner that separates the work and functions of Staff, the Chairperson and Board Members. Adjudication functions are reserved for Board Members sitting in a Hearing Panel. Internal administrative functions, including reviews of information filed by Rights Holders, are performed by Staff under the direction of the Chairperson as the CEO. The Board Secretariat operates separately and independently from other Staff with respect to hearings and other confidential matters related to the operation of the Board. It is responsible for managing the activities of the Board, including Board meetings, consultations, hearings and other related processes.
- To preserve the impartiality of Board members, until a matter is brought before a Hearing Panel at a public hearing, no Board member other than the Chairperson is informed of details of internal reviews of information filed by Rights Holders or of any recommendations made to the Chairperson. Once a hearing has commenced, Staff represents the public interest as the party opposing the Rights Holder in the hearing. As such, Staff does not engage in any ex-parte communication with the Hearing Panel members about the hearing, and only interacts with the Hearing Panel members in the manner set out in the PMPRB Rules of Practice and ProcedureFootnote 4 for hearings. Similarly, Board members not on the Hearing Panel do not discuss the hearing with the Hearing Panel members.
General Role of Guidelines
- Under subsection 96(4) of the Act, the PMPRB may, but is not obligated to, issue non-binding guidelines (“Guidelines”) on matters within its jurisdiction.
- The Board does not have the administrative capacity or resources to conduct hearings for each patented medicine under its jurisdiction. As a result, the Board needs a mechanism to prioritize the cases that are advanced for recommendation for a hearing.
- Consequently, as described in the Introduction, the Board has drafted these Guidelines to guide Staff and to provide transparency to interested parties regarding the process the Staff uses to identify potential cases to recommend that the Chairperson consider for hearings. They are designed to ensure procedural fairness and consistency in that all similarly placed Rights Holders are subject to the same process and process timelines.
- Since the Act stipulates that the Board is to only consider the factors in subsection 85(2) if it is unable to determine whether the medicine is being sold or has been sold at an excessive price after considering the factors in subsection 85(1), these Guidelines are directed at only the subsection 85(1) factors and do not contemplate considerations which could only be raised pursuant to subsection 85(2).
- Once Staff has followed the processes described in these Guidelines, it informs the Chairperson whether it recommends that a hearing into the price of a patented medicine be held and provides the Chairperson with all the relevant information that relates to the recommendation. Subsequently, the Chairperson decides whether to issue a Notice of Hearing.
- When deciding whether to issue a Notice of Hearing, the Chairperson’s involvement takes place only in their management capacity as the CEO of the PMPRB, pursuant to subsection 93(2) of the Act, which is done solely for the purpose of determining whether a hearing is in the public interest, and not to determine whether the price of a patented medicine is excessive.
Jurisdiction of the PMPRB
- The Act gives the PMPRB jurisdiction to determine whether a Rights Holder or former Rights Holder of an invention pertaining to a medicine is selling or has sold the medicine at an excessive price in any market in Canada if the following criteria are satisfiedFootnote 5.
Rights Holder or Former Rights Holder
- Subsection 79(1) of the Act defines a “Rights Holder” as “a patentee and the person for the time being entitled to the benefit of a certificate of supplementary protection for that invention, and includes, if any other person is entitled to exercise rights in relation to the certificate, that other person in respect of those rights.”
- The PMPRB also has jurisdiction over a former Rights Holder of an invention, while it was a Rights Holder (see, e.g. subsections 80(2), 83(3)).
Invention of the Patent pertains to the medicine
- The case law to date has established the following principles regarding whether an invention pertains to the medicine:
- A patent pertains to a medicine if it is “intended or capable of being used for medicine or for the preparation or production of the medicine” ;
- On the face of the patent, there must be a rational connection or nexus between the invention described in the patent and the medicine and that connection can be the medicine itself. The threshold for finding a connection between a patent and a medicine is low and does not require the Board or Staff to engage in claims construction or otherwise go beyond the face of the patentFootnote 6;
- The Board may also consider clinical similarities when determining whether a patent pertains to a given medicine.Footnote 7
- The PMPRB’s jurisdiction over the price at which a patented medicine is sold in any market in Canada persists after the patent has been dedicated and until the cancellation or surrender of the patent pursuant to the express provisions of the Act or the expiry of the term of the patent. Patent dedication is not expressly recognized in the Act as a mechanism by which patent rights may be terminated before the normal expiry of the patent termFootnote 8.
Medicine
- Subsection 79(1) of the Act provides that the term “medicine” “includes a drug, as defined in section 104, and a medicinal ingredient.”
- Section 104 of the Act defines a “drug” as “a substance or a mixture of substances manufactured, sold or represented for use in (a) the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms, in human beings or animals; or (b) restoring, correcting or modifying organic functions in human beings or animals.”
- The PMPRB has historically interpreted these definitions to exclude medical devices per se (as opposed to active substances used in medical devices), in vitro diagnostic products and disinfectants that are not used in vivo.
Invention/Patent
- Subsection 79(2) of the Act provides that “an invention pertains to a medicine if the invention is intended or capable of being used for medicine or for the preparation or production of medicine.”
- Types of patented inventions that may pertain to a medicine include, but are not restricted or limited to:
- Patented inventions for active ingredients;
- Patented inventions for processes of manufacture;
- Patented inventions for a particular delivery system or dosage form that are integral to the delivery of the medicine;
- Patented inventions for indications/uses; and
- Patented inventions for formulations.
Sale in any market in Canada
- The Rights Holder or former Rights Holder must be selling or have sold the patented medicine “in any market” in Canada (see e.g. subsection 83(1)).
- The PMPRB reviews the prices of patented medicines sold at arm’s-length by Rights Holders. Sales in Canada may include, but are not limited to, patented medicines subject to a Notice of Compliance (NOC), Notice of Compliance with Conditions (NOC/c), the Special Access Programme (“SAP”), or the List of Drugs for an Urgent Public Health NeedFootnote 9. The PMPRB has no authority over prices charged by parties other than Rights Holders, such as prices charged by wholesalers or retailers, or over pharmacists’ professional fees.
Filing Requirements and Protection of Filed Information
- The Patented Medicines Regulations (“Regulations”) set out the information that Rights Holders are to report to the PMPRB and the timeframes in which that information is to be provided. This includes information such as the identity of the medicine and information related to the price and sales of the medicine.
- The Regulatory filing requirements are mandatory and neither the Board nor Staff have the authority to vary them, grant exceptions or extend deadlines set by the Regulations.
- Evidence of failure to file any of the information set out in the Regulations in the manner prescribed therein may be brought to the attention of the Chairperson who may recommend that a panel of the Board be appointed to hold a hearing into whether to issue an order requiring production of this information.
- In the alternative or in addition, the matter may be referred to the Attorney General of Canada to determine if summary conviction proceedings should be commenced under subsection 76.1(1) of the Act.
- Pursuant to subsection 87(1) of the Act, any information or document provided to the PMPRB under sections 80, 81 or 82 of the Act, or in any proceeding under section 83, is privileged, and cannot be disclosed without the authorization of the person who provided it, unless it has been disclosed at a public hearing under section 83. Additional protective measures set out in the Access to Information Act or the Privacy Act may apply.
- Receipt of information or documents filed by Rights Holders does not constitute acceptance, verification, or approval of their contents by Staff or Board Members.
Review Process
- The following diagram sets out the general review processes contemplated under these Guidelines and is separated into two steps or screens. The first step is an Initial Review or Annual Review and applies to all patented medicines sold in Canada, with certain limited exceptions (set out below under “Special Provisions on Complaints”). The result of the first step may either be (a) no further review; or (b) referral for In-Depth Review. The second step only applies to patented medicines who have gone through the first screen and been referred for In-Depth Review. The result of the second step is either (a) recommendation for closure of the In-Depth Review; or (b) recommendation for a hearing.
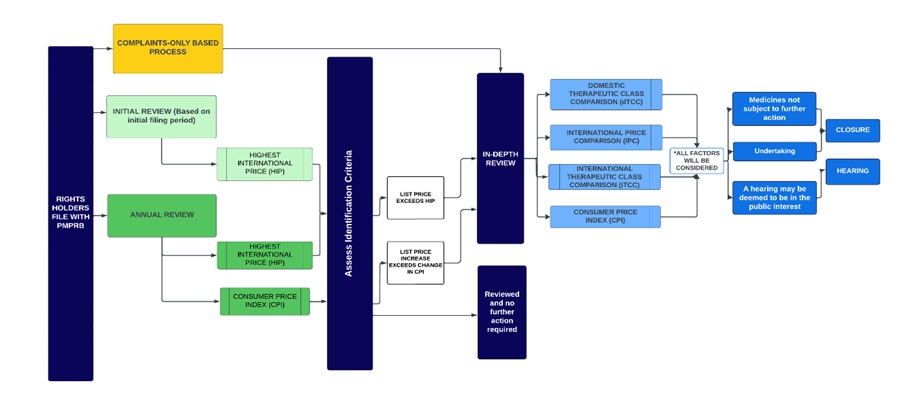
Figure description
This diagram sets out PMPRB's general review processes for patented medicines contemplated under these Guidelines. It is divided into several sections to highlight the various steps in the process.
The framework is described below:
- The first step is an Initial Review (light green) or Annual Review (dark green) and applies to all patented medicines sold in Canada, with certain limited exceptions (set out below under “Special Provisions on Complaints”).
- Complaints (yellow) serve as a separate process by which medicines can be identified for an In-Depth Review.
- Initial Review (light green box):
- Staff uses a medicine’s first semi-annual price filing to conduct an Initial Review against the highest international price among the Schedule Countries filed by the Rights Holder (“HIP”).
- Patented medicines whose prices are above the HIP threshold are subject to an In-Depth Review.
- Annual Review (dark green box):
- Staff conducts an Annual Review of list prices for each patented medicine under the PMPRB’s jurisdiction.
- The Annual Review applies the same IPC identification criteria (the HIP) used during the Initial Review.
- Staff also compares the price change of each patented medicine against changes in the Consumer Price Index (CPI) as an identification criterion to prioritize medicines that warrant an In-Depth Review.
- The result of the Initial Review or Annual Review may either be (a) no further review; or (b) referral for In-Depth Review.
- The In-Depth Review (light blue) is the process by which Staff analyses and balances all the information related to the section 85 factors to prepare a recommendation to the Chairperson on whether a matter should be brought to a hearing. The result of an In-Depth Review is either (a) recommendation for closure of the In-Depth Review; or (b) recommendation for a hearing.
- Patented Medicines are reviewed by Staff at the DIN level in the Initial and Annual Reviews. If one DIN meets the criteria for In-Depth Review, all Associated DINsFootnote 10 sold by the Rights Holder are considered as part of the In-Depth Review. If multiple list prices are reported either in Canada or in a Schedule Country for a patented medicine, Staff considers the highest list price in the relevant market so long as the prices filed by the Rights Holder meet the requirements of the Regulations. In cases where there is reason to believe that a price filed by the Rights Holder does not conform to the requirements of the Regulations (e.g., the reported price appears to be a public sale price as opposed to the ex-factory price required by the Regulations), Staff may contact the Rights Holder to raise the issue of potential correction of the data.
- When selecting the list price for any therapeutic comparators, Staff considers the highest publicly-available list price for each comparator. The information about the prices of comparators used by Staff comes from public sources, not Rights Holder filings. Provincial formularies are typically the starting point for Canadian prices, while official formularies for the Schedule Countries (where available) are typically used for international prices. In the event of a hearing, however, all prices for the patented medicine and its comparators – not just the highest – may be considered by a Hearing Panel.
- For prices filed by Rights Holders for the Schedule Countries, Staff converts local currency prices filed for the Schedule Countries into Canadian dollars using exchange rates calculated as the simple average of the thirty-six (36) monthly average noon spot exchange rates for each country as published by the Bank of Canada. During a patented medicine’s Initial Review, the thirty-six (36) months ending in the second month of the previous reporting period (i.e., February or August) is generally used. In Annual Reviews, the thirty-six (36) months ending in the second month of the reporting period under review is generally used to convert prices filed by the Rights Holders.
- No “margins” are provided to account for exchange rate or other temporary price fluctuations when determining whether a patented medicine meets the criteria for In-Depth Review, but issues around foreign exchange fluctuations may be considered by Staff as part of the In-Depth Review.
First Step
Initial Review
- Staff uses a patented medicine’s first semi-annual price filing to conduct an Initial Review against the highest international price among the Schedule Countries filed by the Rights Holder (“HIP”) based on paragraph 85(1)(c) of the Act. The review compares the list price(s) of a patented medicine in Canada with the information the Rights Holder files regarding the list price(s) of the medicine in the Schedule Countries.
- Patented medicines whose prices are above the HIP threshold are subject to an In-Depth Review. As noted above, while Staff reviews patented medicines at the DIN level, if one DIN meets the criteria to commence an In-Depth Review, Associated DINs are considered as part of the In-Depth Review.
- If an International Price Comparison (“IPC”) cannot be conducted during the Initial Review because no list prices are filed for the medicine in any of the Schedule Countries, the list price is considered reviewed for the purpose of the Initial Review and is not reviewed again until the Annual Review. For further clarity, an IPC is conducted if one or more list prices are filed for the medicine in any of the Schedule Countries.
- The Initial Review service standard for Staff is to advise Rights Holders whether their patented medicine(s) is subject to an In-Depth Review within 60 days of the filing deadline for their first semi-annual filing.
Annual Review
- Staff conducts an Annual Review of list price for each patented medicine under the PMPRB’s jurisdiction beginning in January of each year to see whether they should be subject to an In-Depth Review. “New medicines” and “Existing medicines” are subject to the same Annual Review process, although Existing medicines will be reviewed starting one (1) year from the date these Guidelines go into effect. New medicines will be reviewed immediately after these Guidelines come into effect.
- As in the Initial Review, the service standard for Staff is that Rights Holders are advised whether any of their patented medicines are subject to In-Depth Review within 60 days of the filing deadline for their semi-annual filing in January. The Annual Review applies the same IPC identification criteria (the HIP) and methodology (e.g. foreign exchange, selection of price when multiple prices are filed for a country, etc.) used during the Initial Review, but focuses on the most recently filed domestic and international pricing data.
- Note that since the Annual Review will compare the list price(s) of a patented medicine against the HIP based on the prevailing list prices in the Schedule Countries, changes in the HIP could render the applicable threshold lower or higher in subsequent years than it was at introduction, depending on how the international landscape evolves. For example, if a patented medicine which was previously listed in only some of the Schedule Countries is subsequently listed in another Schedule Country at a higher price point than the original countries, the price threshold for initiating an In-Depth Review could rise accordingly. Likewise, if the highest price for the medicine in the other Schedule Countries where it is available declines, this could mean that the threshold becomes lower over time. Any such shifts are considered in the context of the In-Depth Review.
- During an Annual Review, Staff also compares the price change of each patented medicine against changes in the Consumer Price Index (CPI) as an identification criterion to prioritize medicines that warrant an In-Depth Review.
- If an IPC cannot be conducted because no list prices are filed for the patented medicine in the Schedule Countries, an In-Depth Review is only initiated based on the change in the CPI, or in response to a complaint.
- If an In-Depth Review for a given patented medicine is still open at the beginning of a subsequent Annual Review period, the medicine does not trigger a new Annual Review at that time.
- A patented medicine that has been subject to an In-Depth Review which has been closed will not be subject to another In-Depth Review for at least the two subsequent filing periods.
Changes in the Consumer Price Index (CPI)
- List prices are reviewed against the change in the CPI each year using the one-year lagged CPI (e.g. for patented medicines reviewed in 2028, Staff will compare list price increases taken in 2027 against the 2026 CPI factors, published by Statistics Canada in January 2027). If a Rights Holder increases the list price of a patented medicine by an amount greater than the change in the CPI in any given year, Staff opens an In-Depth Review into the medicine unless the Rights Holder did not take a list price increase in the previous year and the increase in the second year is lower than or equal to the total change in CPI over those two years. Staff does not consider the total changes to the CPI for more than the most recent two years in determining whether to open an In-Depth Review, but the total change in the CPI for more than two years may be a relevant consideration during an In-Depth Review.
- The source Staff uses to calculate the CPI is the “Consumer Price Index, monthly, not seasonally adjusted” as published by Statistics Canada.Footnote 11 This is the same source Staff has historically consulted.
- An In-Depth Review commenced due to the list price increases above the CPI described above subsequently proceeds in the same manner as all other In-Depth Reviews.
Special Provisions on Complaints
- Patented generic medicines, over the counter medicines and veterinary medicines are only subject to In-Depth Review if a complaint is received.
- In addition, for all other patented medicines, the receipt of a complaint from an approved individual or organization (see para. 67) who believes that the price of a patented medicine may be excessive in any market in Canada will automatically lead to an In-Depth Review even if the Initial Review or Annual Review criteria have not otherwise been met.
- The approved individuals or organizations whose complaints lead to In-Depth Reviews are set out below:
- the Federal Minister of Health or any of their Provincial or Territorial counterparts;
- Publicly-funded drug programs; or,
- Canadian life and health insurance companies and their trade association(s).
- Complaints from other parties do not lead to In-Depth Reviews. Other parties who have concerns about the list prices of specific patented medicines are encouraged to raise their concerns with their relevant Minister(s) of Health, drug program, or insurance company, who may choose to make a complaint to the PMPRB on their behalf.
- Approved individuals and organizations may submit a complaint using the contact information available on the “How to Make a Complaint” page of the PMPRB’s website. Information about the complainant is not provided to the Rights Holder of the patented medicine subject to complaint, unless required by law. Neither the information about the complainant, nor the patented medicine subject to complaint and corresponding Rights Holder is made public by the PMPRB, unless the matter results in a Hearing or as otherwise required by law. The complainant is not required to provide any documents or evidence to the PMPRB and if they choose to do so, Staff only consider any information which is relevant to the subsection 85(1) factors.
- Due to limitations on disclosure set out in section 87 and section 88 of the Act and in the Access to Information Act, the complainant is only informed that the complaint has been received, and of the outcome of an In-Depth Review if the process results in an Undertaking or a Notice of Hearing. For clarity, due to these confidentiality obligations, if an In-Depth Review is concluded with no further action being taken (i.e., it is closed by the Chairperson), only the Rights Holder is advised and not the complainant.
- An In-Depth Review commenced due a complaint subsequently proceeds in the same manner as all other In-Depth Reviews.
Second Step
In-Depth Review
- In-Depth Review is the process by which Staff analyses and balances all the information related to the section 85 factors to prepare a recommendation to the Chairperson on whether a matter should be brought to a hearing. This Section as well as the following Sections (“Scientific Review: Therapeutic Class Comparison Selection and Analysis” and “Price Review”) provide further details on In-Depth Review.
- Note that the selection of the HIP as an identification criterion for Initial Review and Annual Review is based on administrative efficiency and resource prioritization and does not pre-suppose that prices above or below the HIP are excessive or not excessive. The Board recognizes that the determination of whether a price is excessive can only be made by Hearing Panels in hearings and must consider all the factors set out in subsection 85(1) of the Act. As such, it is possible that a price above or below the HIP could be found to be excessive or non-excessive, depending on how the factors and related information are weighted by a Hearing Panel in the circumstances of a specific case.Footnote 12 Staff keeps these principles in mind when conducting an In-Depth Review, and considers all available prices in the Schedule Countries as part of an In-Depth Review.
Deferral Letters
- In the event of resource constraints, Staff prioritizes patented medicines with list prices significantly above the HIP or whose list price increases are significantly above the CPI. Depending on the PMPRB’s internal resources, other patented medicines may receive deferral letters. It is possible that some patented medicines with list prices above the HIP may be deferred multiple times depending on the PMPRB’s internal resources. Deferral letters do not provide relief from any calculation of excess revenue by a Hearing Panel that may be accruing during the period of deferral.
Scientific Review: Therapeutic Class Comparison Selection and Analysis
- Once an In-Depth Review is initiated, the Staff scientific team identifies the comparators for the purpose of conducting a Therapeutic Class Comparison (“TCC”). Comparators are identified for each approved indication or use of the patented medicine under In-Depth Review at the time the In-Depth Review is initiated. To best prioritize the resources of the Staff scientific team, only the approved indications or uses of the patented medicine under review will be considered for the purpose of populating the TCC during In-Depth Reviews. If there is evidence to demonstrate that the patented medicine is used for other indications which have not been approved, those other indications could potentially be relevant in the case of a hearing.
- All products having the same indication or use as the medicine under review will be identified for the purposes of a TCC. TCC assessments are conducted from a population therapeutics perspective for each indication rather than from the perspective of the needs of individual patients and are not intended to address all possible unique patient circumstances or needs. TCC assessments are only intended for use in internal PMPRB price reviews under the Guidelines and are not intended for use in PMPRB hearings or in third-party clinical, medical, or drug evaluation settings. TCC assessments are confidential to the Rights Holder and will not be shared with third parties by the PMPRB, unless required by law.
- Each comparator is assigned a level of similarity to the patented medicine subject to In-Depth Review in accordance with the following two-step framework. Comparability is assigned for each comparator identified for a single indication. Comparable dosage forms are also established at this stage. The most appropriate strength of the drug product is chosen for a particular dosage regimen. Generally, a dosage regimen based on a course of treatment is applicable to acute indications, while a per-day regimen (based on a maintenance dose) is applicable for chronic indications.
- Rights Holders are generally notified regarding the selection of comparators for each approved indication or use within up to 8 months of the initiation of the In-Depth Review (potentially longer if Staff decides to consult the Human Drug Advisory Panel (HDAP)).
Step 1: Qualitative Assessment
| Qualitative Class | Example RationaleFootnote 13 |
|---|---|
A |
Comparable: comparable safety and efficacy demonstrated in high quality head-to-head trials or meta-analyses. Recommended or used in the same place in therapy as the patented medicine under review. |
B |
Less comparable: efficacy, safety or patient-assessed outcomes are meaningfully different (either better or worse) than the patented medicine under review based on published literature. Recommended or used in a similar place in therapy as the medicine under review. |
C |
Undetermined comparability: could be used alternatively or in place of the patented medicine under review in many circumstances but comparability is unclear based on available published literature. |
D |
No data: No quality published data available to assess comparability to the patented medicine under review. |
X |
No comparators. Do not proceed to step 2.* |
*Note: for the purposes of a TCC, a patented medicine that is identified as the only effective treatment sold in Canada for a particular illness or indication is designated as a Qualitative Class X. This medicine therefore has no comparators.
Step 2: Grouping Assessment
| Group | Description of the comparator |
|---|---|
1 |
Same 4th level ATC†, materially similar approved indication or use as the patented medicine under review as per Health Canada. |
2 |
Different 4th level ATC, materially similar approved indication as the patented medicine under review as per Health Canada. |
3 |
Same 4th level ATC, used in Canada for a materially similar indication or use as the patented medicine under review as per published literature but not indicated by Health Canada. |
4 |
Different 4th level ATC, used in Canada for a materially similar indication or use as the patented medicine under review as per published literature but not indicated by Health Canada. |
†Anatomic and Therapeutic Classification System as published by the World Health Organization |
|
- For example, if there are three comparators identified, one may be described as a B2 comparator, another could be an A1 comparator, and another as a D2 comparator.
- Note that the Scientific Review does not consider the pricing of either the patented medicine under In-Depth Review or the comparators as part of this assessment. The purpose of this part of the analysis is to determine how comparable medicines of the same therapeutic class are to the patented medicine under In-Depth Review in order to provide context for the comparators used in the TCC as a paragraph 85(1)(b) factor.
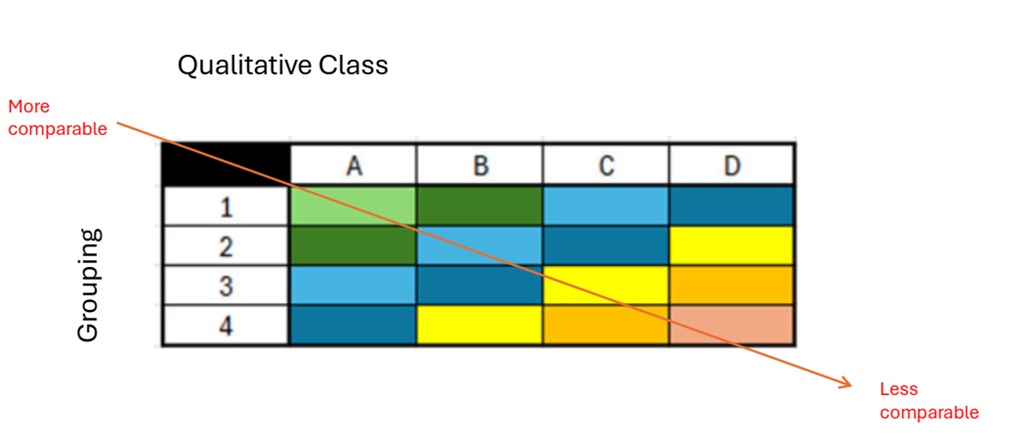
Figure description
This table illustrates the spectrum of comparability, ranging from more comparable (top-left) to less comparable (bottom-right). The horizontal axis represents the qualitative class (A, B, C, D), while the vertical axis indicates groupings (1, 2, 3, 4). The figure effectively shows how comparability decreases progressively across qualitative classes (left to right) and across groupings (top to bottom).
Comparability is visually distinguished by color:
- Green: High comparability (e.g., A1, A2 and B1)
- Blue: Medium comparability (e.g., A3, A4, B2, B3, C1, C2, D1)
- Yellow/Orange/Pink: Low comparability (e.g., B4, C3, C4, D2, D3 and D4)
| High comparability | Medium comparability | Low comparability |
|---|---|---|
|
|
|
Figure description
This table consists of three columns, each outlining examples of potential features for different comparability categories. The first column focuses on the high comparability, the second on medium comparability, and the third on the low comparability category.
Domestic TCC (dTCC)
- The Scientific Review of the dTCC involves a separate evaluation of each approved indication or use of the patented medicine subject to In-Depth Review at the time the In-Depth Review is conducted. If approved indications or uses in Canada change over time, this is reflected in any subsequent In-Depth Review. A separate dTCC assessment is prepared for each approved indication or use. Medicines used for the treatment of the indication under review may form part of the dTCC for a given patented medicine, regardless of their approved indication(s) or use(s), if there is appropriate evidence to support their inclusion. For clarity, comparators which are generic products or biosimilars (patented or otherwise) may be included in the dTCC if considered appropriate based on the available evidence.
International TCC (iTCC)
- In general, the Staff scientific team uses the comparators identified in the dTCC to populate the iTCC. This approach is the fastest and easiest to operationalize and therefore best prioritizes the Board’s use of resources, but it may not necessarily reflect the therapeutic comparators available in each of the Schedule Countries.
- In some cases, the Staff scientific team may also populate an iTCC which identifies lists of comparators for each relevant Schedule Country. The Staff scientific team is more likely to conduct individual reviews of the comparators in each Schedule Country in situations where the science is particularly complex; the therapeutic class could differ significantly across the Schedule Countries due to the indication(s) of the patented medicine under In-Depth Review; and/or if the analysis of the other subsection 85(1) factors suggests the case may be a candidate for a hearing and the more comprehensive analysis is worth the additional expenditure of resources.
Communications with Rights Holders on Comparability
- Once the assessment of the comparators has been completed by the Staff scientific team, Rights Holders are provided with the list of comparators with comparable dosage regimens (the dTCC and/or iTCC), the comparability score of each comparator, a list of references reviewed and a summary of the rationale used in the assessment of each comparator.
- Rights Holders may, but are not obligated to, provide input on Staff’s TCC assessment at the beginning of the Scientific Review, or after they receive the TCC assessment, or at both points at their discretion. Rights Holders are, however, encouraged to submit any relevant information they wish to be considered as soon as possible after being notified of an In-Depth Review. Rights Holders are not limited in the written input they may provide to the Scientific Review or in their response to the proposed TCC and may provide any information they deem appropriate. It is anticipated that input could include (but is not required to include) commentary and analysis of the patented medicine from relevant sources. Submissions which clearly explain the rationale behind the Rights Holder’s proposals for level of comparability, drug products for comparison purposes, and comparable dosage regimens are likely to be the most useful to the Staff scientific team. This may include evidence regarding the indication(s) of the patented medicine under review and its potential comparators, a brief description of the patented medicine and its place in therapy, and a summary of the available clinical evidence. High level clinical evidence may include phase III trials (or phase II trials in cases where phase III trials are not yet available), high quality meta-analyses or systematic reviews, and head-to-head comparisons with comparators used for the indication(s) under review.
Human Drug Advisory Panel (HDAP)
- The HDAP is an advisory body comprised of independent health professionals who are contracted by the PMPRB to assist with scientific evaluations in view of their broad general knowledge of drug therapy, drug evaluation, drug utilization and clinical research methodology. HDAP members are consulted to provide objective evaluations of available clinical evidence and as experts in drug evaluation and are not asked to review the materials as specific subject-matter clinical experts and thus may seek the input of clinical experts from time to time.
- Staff may consult the HDAP on an ad hoc basis when Staff identifies specific issues or questions necessitating additional advice. The HDAP is not an adjudicatory body, and Rights Holders cannot request a review of their patented medicine by the HDAP. If Staff decides to consult the HDAP and the Rights Holder has submitted input for the Scientific Review prior to the HDAP reviewing the patented medicine, that input is provided to the HDAP. Staff may also, but is not obliged to, seek input from additional experts or specialists in exceptional cases if they feel additional input is necessary.
- The HDAP’s role is not to adjudicate disputes between Staff and Rights Holders over the selection or relative weights of comparators and it is not anticipated that the HDAP will typically be consulted more than once about a patented medicine during a particular In-Depth Review. Rather, HDAP’s role is to provide recommendations on comparators and comparable dosage regimens to assist the PMPRB’s scientific staff when needed.
Sources
- When assembling and assigning a comparability rating to medicines in the TCC, the Staff scientific team consults the following sources:
- ATC classification;
- Approved indications or uses, or proposed indications, where applicable;
- Available medical literature;
- Clinical evaluations undertaken by recognized health technology assessment organizations (e.g. Canada’s Drug Agency, INESSS, WHO, NICE, etc.);
- Written input provided by the Rights Holder (if any);
- Research by a Drug Information Centre (DIC) – Staff may use the services of various drug information centres to obtain scientific information, such as clinical trial information, clinical practice guidelines, etc. The basis of the review by the DIC is the product monograph (or information like that contained in a product monograph if a NOC has not been granted). Product monographs (and like information) are filed by Rights Holders pursuant to the Regulations;
- Research by Staff – Staff may also update research and supplement data and evidence from the Rights Holder and DIC using other sources;
- PMPRB Human Drug Advisory Panel (HDAP) – On an ad hoc basis, Staff may consult with the HDAP to provide clinical context pertaining to the scientific information that is being considered by Staff; and
- Guidelines or consensus statements from recognized Canadian or foreign clinical groups pertaining to the treatment of the approved Health Canada indication of the patented medicine under review.
Price Review
- In parallel with the Scientific Review, Staff commences its Price Review of the patented medicine in anticipation of the results of the Scientific Review. If they choose to, Rights Holders may provide input to the pricing team on issues relevant to the subsection 85(1) factors other than the TCC within three months of being notified that their patented medicine is subject to In-Depth Review. As with the Scientific Review, Rights Holders are not limited in terms of the information they may choose to provide to Staff as context for Staff’s consideration of the subsection 85(1) factors. Information provided by Rights Holders which would only be relevant as part of a subsection 85(2) analysis or other material that is not relevant to the factors in subsection 85(1) of the Act may not be considered by Staff as part of the In-Depth Review. Any relevant additional information provided by Rights Holders may form part of Staff’s recommendation to the Chairperson.
- Once the Scientific Review has been concluded, Staff adds pricing information to the TCC analysis. The pricing for medicines in the TCC takes account of the treatment regimen for each medicine and indication as identified in the Scientific Review.
- Staff reviews and analyses all the information related to the patented medicine provided pursuant to section 3 of the Regulations and information related to the price of the patented medicine provided pursuant to section 4 of the Regulations or collected by Staff that relates to factors in section 85 of the Act, namely:
- information relating to the price of the medicine in Canada;
- information relating to the price of the medicine in the Schedule Countries;
- information relating to the TCC selection and analysis; and
- information relating to changes in the CPI over the relevant period.
- Staff considers all the information collected and prepares a recommendation to the Chairperson that the In-Depth Review either be closed (with or without an Undertaking) or that a hearing be held. The Appendix “Assessment of individual s. 85(1) factors, separately” sets out examples of how Staff begins its assessment of the information collected by first considering the individual subsection 85(1) factors separately. The Appendix “Weighting of individual factors during In-Depth Review” sets out examples of how Staff typically balances the subsection 85(1) factors in preparing its Recommendation. Note that the result of the analysis is not a price ceiling, recommended price, or a decision on whether a price is or is likely to be excessive; rather it is a recommendation that a hearing be held or not.
- In addition to the above information, the recommendation includes any input received from the Rights Holder, and any proposed Undertaking(s). Each recommendation is reviewed by the Senior Director of Regulatory Affairs & Outreach and the Director General of the PMPRB to ensure consistency in the analytical approach and resulting recommendation.
- Generally, an In-Depth Review could take between 12 and 28 months to complete, depending on its complexity (e.g. number of comparators during the Scientific Review, IPC calculations).
- Rights Holders whose patented medicines are identified for In-Depth Review cannot avoid or prematurely terminate In-Depth Review by unilaterally changing their list prices once the In-Depth review is initiated, although Rights Holders may propose Undertakings for the Chairperson’s consideration at any point between being advised that their patented medicine is subject to In-Depth Review and up to two months after being informed that Staff recommends a hearing. If a Rights Holder submits a proposed Undertaking early in the In-Depth Review, Staff may pause the review to manage resources while the Chairperson considers the Undertaking.
Recommendations
- If the result of the In-Depth Review is a recommendation to the Chairperson to issue a Notice of Hearing, Staff informs the Rights Holder of that recommendation when it is submitted to the Chairperson. Rights Holders are not provided with details of Staff’s analysis in the Pricing Review beyond what is provided in respect of the Scientific Review (see para. 84, above). The information provided about the Scientific Review, the change in the CPI (which is publicly available), and the prices for the patented medicine in the Schedule Countries (which are filed by the Rights Holder) should be sufficient to assist the Rights Holder in determining why its patented medicine is recommended for a hearing by Staff. Rights Holders have two months from the date they are informed of the recommendation to provide a proposed Undertaking theymay wish for the Chairperson to consider in making their decision. The Chairperson will render their decision within one month of receiving a proposed Undertaking or within no more than three months after receiving Staff’s recommendation.
- If the Chairperson decides that an In-Depth Review should be closed, the Rights Holder is advised by Board Staff of the Chairperson’s decision.
- If the Chairperson decides that it is in the public interest that a Notice of Hearing be issued, the Rights Holder is advised of the Chairperson’s decision. If a Rights Holder against whom a Notice of Hearing has been issued would like to resolve the matter before a hearing is concluded, the Rights Holder may seek a settlement with the Hearing Panel.
Undertakings and Settlement Proposals
- Undertakings are unilateral promises made by Rights Holders and are not binding agreements. As such, Undertakings and discussions related thereto are not covered by settlement privilege and are not treated on a “without prejudice” basis.
- Rights Holders are advised if an In-Depth Review is commenced. Rights Holders may submit an Undertaking at any point during the In-Depth Review and for two months after they are advised that Staff recommends a hearing to the Chairperson. Any Undertaking submitted is referred to the Chairperson.
- Because Undertakings are unilateral and these Guidelines are not designed as price compliance Guidelines, neither Staff nor the Chairperson provides Rights Holders with suggestions or guidance on the substance of any proposed Undertaking, nor negotiate the terms. The Chairperson considers all Undertakings received, and if they are of the view that the recommended hearing would not be in the public interest in view of the terms of the Undertaking, the Chairperson directs Staff to close the In-Depth Review, and the matter does not proceed to a Notice of Hearing. Undertakings accepted by the Chairperson are intended to resolve a specific In-Depth Review and are not intended to provide assurances about prices going forward. The prices of the patented medicine continue to be reviewed in accordance with the Guidelines for as long as it remains subject to the jurisdiction of the Board.
- For clarity, Staff do not calculate potential excess revenues as part of the In-Depth Review, and such calculation is not a factor in the recommendation to the Chairperson, which is based on list price only. Should an Undertaking be proposed by a Rights Holder which includes a repayment of potential excess revenues, Staff reviews the excess revenue calculation provided by the Rights Holder based on the average price per package and/or net revenue (ATP) information filed by the Rights Holder pursuant to the Regulations, and not on list prices.
- Once a Notice of Hearing is issued, Undertakings can no longer be accepted. After a Notice of Hearing has been issued, a Rights Holder may only seek to settle the matter through a formal settlement approved by the designated Hearing Panel. All proposed settlements must be presented to and approved by the Hearing Panel. This is usually done through a motion for discontinuance based on the proposed settlement. Negotiations and discussions with Staff regarding a settlement proposal after the issuance of a Notice of Hearing may be conducted on a without prejudice basis and are subject to settlement privilege (see detailed discussion of hearing processes in Section “Price Hearings”, below).
- The PMPRB reports publicly on all Undertakings accepted by the Chairperson and all settlements accepted by Hearing Panels. The information reported ordinarily includes the name of the patented medicine and/or the rights holder and such other information as is considered appropriate. This information is included in the PMPRB’s Annual Report and published on the PMPRB website. It may also be published in the NEWSletter or other publications.
Price Hearings
- PMPRB hearings are commenced by the issuance of a Notice of Hearing which is provided to the relevant parties under the Act.
- PMPRB hearings are public and are “de novo” proceedings, meaning that the Hearing Panel considers all the law and evidence from a blank slate. PMPRB hearings are not reviews of the application of the Guidelines or of Staff’s actions prior to the issuance of the Notice of Hearing. During a hearing, submissions and evidence from the parties are heard by a Hearing Panel consisting of at least two Board members. The Hearing Panel determines whether a patented medicine is being or has been sold at an excessive price in any market in Canada by taking into consideration the available information relating to the factors set out in section 85 of the Act. The Hearing Panel cannot be bound or fettered in its discretion by these Guidelines or their application to the patented medicine that is the subject of the hearingFootnote 14.
- For more information about hearings, please consult the PMPRB Rules of Practice and ProcedureFootnote 15, the published standard set of procedures to be followed by all participants in hearings before the PMPRB. The Rules set out the PMPRB’s procedures in accordance with the requirement under the Act to resolve matters as informally and expeditiously as the circumstances and considerations of fairness permit. Practice directions and further information about previous and ongoing hearings are also publicly available on the PMPRB’s website.
- As per section 83 of the Act, where, following a hearing, the Board finds that a Rights Holder is selling a patented medicine in any market in Canada at an excessive price, the Board may order the Rights Holder to reduce the maximum price at which the Rights Holder sells the medicine in that market.
- In addition, where, following a hearing, the Board finds that a Rights Holder or former Rights Holder, while a Rights Holder, has sold a patented medicine in any market in Canada at an excessive price, the Board may order the Rights Holder or former Rights Holder to offset the amount of excess revenues estimated by it to have been derived by the Rights Holder or former Rights Holder from the sale of the medicine at an excessive price. While these Guidelines require Staff to assess a patented medicine’s list price against certain criteria, historically, when the Board has issued an order requiring the repayment of excess revenues, that calculation has taken the medicine’s net or average transaction price into account.
- Where, following a hearing, the Board finds that the Rights Holder or former Rights Holder has engaged in a policy of selling the patented medicine at an excessive price “having regard to the extent and duration of the sales of the medicine at the excessive price” (see subsection 83(4) of the Act), the Board may order the Rights Holder or former Rights Holder to offset up to twice the amount of excess revenues estimated by it to have been derived by the Rights Holder or former Rights Holder from the sale of the medicine at an excessive price. As above, these calculations consider the net or average transaction price(s) of the patented medicine(s).
- To offset excess revenues, the Board may order a Rights Holder or former Rights Holder to:
- reduce the price at which the Rights Holder or former Rights Holder sells the medicine in any market in Canada;
- reduce the price at which the Rights Holder or former Rights Holder sells an other patented medicine in any market in Canada; and/or
- make a payment to His Majesty in right of Canada.
Glossary and list of Acronyms and Abbreviations
The following definitions are provided for general assistance only and are limited to terms that are not already defined in section 79 of the Act; they have no legal force and should be read in conjunction with the applicable legislation.
Existing Medicine: Patented medicines first sold before July 1, 2022.
New Medicine: Patented medicines first sold on or after July 1, 2022.
PMPRB11: Australia, Belgium, France, Germany, Italy, Japan, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
Schedule Countries: The countries currently set out in the Schedule to the Patented Medicines Regulations.
TCC: Therapeutic Class Comparison
iTCC: international Therapeutic Class Comparison
dTCC: domestic Therapeutic Class Comparison
HDAP: Human Drug Advisory Panel
IPC: International Price Comparison
CPI: Consumer Price Index
HIP: The highest price of the same strength and dosage form of the same patented medicine among the Schedule Countries.
ATC: Anatomic Therapeutic Classification as outlined by the World Health Organization.
List Price: the gross price (before any rebates or discounts) at the ex-factory level filed by the Rights Holder pursuant to paragraph 4(1)(f)(i) or (ii) of the Regulations.
Associated DINs: includes all other dosage strengths and may include alternate dosage forms and prolonged release products but does not include combination products.
DIN: drug identification number as assigned by Health Canada.
Appendices
Assessment of individual subsection 85(1) factors, separately
The purpose of Appendix “Assessment of individual s. 85(1) factors, separately” and Appendix “Weighting of individual factors during In-Depth Review” is to provide a degree of transparency and predictability on the process to ensure consistency across In-Depth Reviews. It is not intended to address the outcomes for specific In-Depth Reviews.
When initiating an In-Depth Review, Staff begins by considering the individual subsection 85(1) factors separately. Information considered by Staff during this phase of the review may include but is not limited to the following:
85(1)(a) – the prices at which the medicine has been sold in the relevant market:
- How many different list prices have been reported by the Rights Holder for the medicine?
- Is there differential pricing across markets? How big is the difference?
- How have the list prices changed over time? Increased? Decreased?
- Are there other strengths of this medicine available in Canada? Are they level priced? Proportionally priced? Linearly priced?
- How do the average price per package or net revenue reported by the Rights Holder compare to the list price(s)? Have benefits been reported?
- Is this medicine a tendered product? Has the Rights Holder reported contract sales?
- Has this medicine been genericized? Is the generic version “authorized” by the Rights Holder? How does its price compare to the generic version?
85(1)(b) – the prices at which other medicines in the same therapeutic class have been sold in the relevant market:
- Is this medicine a line extension? A combination product? A biosimilar? A patented generic?
- How many indications is this medicine approved for?
- How many comparators were identified during the Scientific Review?
- Do the comparators share the same indication as the medicine, or are used in the same way without a shared approved indication?
- How strong are the levels of similarity across the comparators?
- What is the range in treatment costs? Tight? Narrow?
- Are there any high or low outliers among the treatment costs?
- Have any of the comparators been genericized?
- If the medicine is a patented biologic, are any of the comparators biosimilars?
- Did the Rights Holder express disagreement with any aspect of the Scientific Review? If so, on what grounds?
85(1)(c) – the prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada:
- If the In-Depth Review was triggered by the list price being above the HIP, by how much?
- How many comparator countries have been reported for the medicine?
- What is the range in prices for the medicine across the Schedule Countries? Tight? Narrow?
- How does the price of the medicine in Canada compare with other prices in the Schedule Countries generally?
- Has the number of comparator countries changed over time? If so, how?
- Do we anticipate additional countries to be reported in the future? If so, why?
- Has a price reduction (in local currency) been reported for any of the comparator countries?
- Has a price increase (in local currency) been reported for any of the comparator countries?
- Has there been an impact of exchange rates on the HIP?
- Are the comparators identified during the Scientific Review available across the Schedule Countries?
- How do the treatment costs of this medicine compare to the treatment costs of its comparators across the basket of comparator countries?
- If the medicine is consistently higher or lower priced than its comparators internationally, how does that compare to the dTCC?
85(1)(d) – changes in the Consumer Price Index:
- If the In-Depth Review was triggered by the list price increase being greater than CPI, by how much?
- Is there a difference between the one-year lagged CPI value used as the trigger and the CPI from the year in which the list price increase was taken? If so, how does the list price increase compare to both CPI factors?
- How many list price increases have been taken for this medicine, and how do each of those list prices increases compare to CPI?
- How does the cumulative increase since introduction compare to cumulative CPI over the same period?
Other possible considerations:
- How does the change in CPI and corresponding list price increase compare to the changes in the list prices in the Schedule Countries?
- Has this medicine previously been subject to In-Depth Review? If so, what was the outcome of that In-Depth Review? Are circumstances different? The same?
- When is the last reported patent for this medicine set to expire?
- Does the Rights Holder have a Certificate of Supplementary Protection for this medicine?
- Has a complaint been received regarding the price of this medicine?
- Was there any additional information shared by the Rights Holder that is within the scope of the factors that the Chairperson should be aware of?
Weighting of individual factors during In-Depth Review
The purpose of this Appendix “Weighting of individual factors during In-Depth Review” is to provide general information about how Staff may balance the information related to the subsection 85(1) factors in an In-Depth Review.
All recommendations arising from In-Depth Reviews are vetted for consistency by the Senior Director of Regulatory Affairs & Outreach and the Director General before they are presented to the Chairperson for consideration.
Based on the results of the Staff's analysis of each case (see example questions in Appendix “Assessment of individual s. 85(1) factors, separately”), Staff makes a recommendation to the Chairperson on whether a Notice of Hearing should be issued or the In-Depth Review should be closed based on a balancing of the information related to the subsection 85(1) factors obtained during the In-Depth Review. The Staff’s recommendation to the Chairperson is merely advice, and the Chairperson retains the sole discretion on the ultimate decision as to whether to issue a Notice of Hearing or to close the In-Depth Review.
In-Depth Reviews are conducted on a case-by-case basis, consequently it is impossible to anticipate all possible scenarios Staff may encounter and some individual cases may be subject to deviations from the general approach.
See the “Case Studies” Appendix for illustratives case studies of the general approach for this Appendix.
Recommendation for Hearing
More particularly, scenarios that likely tilt the balance towards a recommendation for a hearing include:
- Cases where both IPC and TCC are available, and the Canadian list price(s) is/are above both.
- Cases that suggest a “policy of selling the medicine at an excessive price” as per the terms of subsection 83(4) of the Patent Act.
- Where both IPC and TCC are available, and the Canadian list price(s) is/are between them: significant differential between the price(s) of the most similar comparator(s) and the IPC, especially if the comparator(s) is/are very similar and priced below the IPC.
- Where TCC is not possible (no comparators) and the Canadian list price(s) is/are above the IPC: significant differential between the IPC price and the Canadian list price(s), especially when the differential does not appear to relate to exchange rate shifts.
- Where the IPC is not possible (no international prices) but TCC is possible (there are comparators) and the Canadian list price(s) is/are above the TCC: significant differential between the price of the most similar comparator(s) and the Canadian list price(s), especially if the comparator(s) is/are very similar.
Recommendation for Closure
Scenarios that likely tilt the balance towards a recommendation for closure include:
- Cases where both IPC and TCC are available, and the Canadian list price(s) is/are below both.
- Where both IPC and TCC are available, and the Canadian list price(s) is/are between them: de minimis differential between the price of the most similar comparator(s) and the IPC.
- Where TCC is not possible (no comparators) and the Canadian list price(s) is/are above the IPC: de minimis differential between the IPC price and the Canadian list price(s), especially when the differential appears to relate to exchange rate shifts.
- Where the IPC is not possible (no international prices) but TCC is possible (there are comparators) and the Canadian list price(s) is/are above the TCC: de minimis differential between the price(s) of the most similar comparator(s) and the Canadian list price(s).
- Where the IPC is not possible (no international prices) but TCC is possible (there are comparators): where the most similar comparator(s) is/are not very high on the comparability scale to the medicine under review.
- Cases that suggest that data filing errors or other unusual circumstances are causing a price fluctuation that is temporary and will likely be resolved within 1 filing period.
- Cases where the Rights Holder has submitted an Undertaking that would result in one of the above situations.
Case Studies

Figure description
This flowchart depicts the general review processes contemplated under these Guidelines, while highlighting the case studies intended to demonstrate how Staff may weigh the factors during the In-Depth Review process in order to arrive at a recommendation to the Chairperson. The case studies are distinguished into two parts: Part 1 represents scenarios where the identification criteria are not met and thus no In-Depth Reviews were commenced. Part 2 includes scenarios where an In-Depth Review is initiated.
No In-Depth Review:
- Case Study 1: List price below HIP
- Case Study 2: List price increase within CPI and list price below HIP
In-Depth Review:
- Case Study 3: List price above HIP, without TCC
- Case Study 4: List price above HIP, with TCC below HIP
- Case Study 5: List price above HIP, with TCC above HIP
- Case Study 6: List price increase above CPI, with TCC
- Case Study 7: Complaint, with TCC
To help interested parties understand how Staff may weigh the factors during the in-depth review process in order to arrive at a recommendation to the Chairperson, several case studies have been prepared for illustration purposes.
The case studies are distinguished into two parts: Part 1 represents scenarios where the identification criteria are not met and thus no In-Depth Reviews were commenced. Part 2 includes scenarios where an In-Depth Review is initiated. In these scenarios, the case studies illustrate the decision-making framework that may be applied by PMPRB Staff in their recommendation to the Chairperson based on how the available information about the relevant factors from subsection 85(1) may appear in a given situation.
These case studies are not exhaustive and are not intended to cover all possible permutations of scenarios that may appear in In-Depth Reviews. They are for illustration purposes only. In particular, the magnitude differential represented by the lines and the distance between them should be ignored, as the examples only seek to represent the relative positions of the lines, not the distance between them or specific weight.
| Part | Case Study |
|---|---|
|
1. No In-Depth Review |
Case Study 1: List price below HIP |
Case Study 2: List price increase within CPI and list price below HIP |
|
|
2. In-Depth Review |
Case Study 3: List price above HIP, without TCC |
Case Study 4: List price above HIP, with TCC below HIP |
|
Case Study 5: List price above HIP, with TCC above HIP |
|
Case Study 6: List price increase above CPI, with TCC |
|
Case Study 7: Complaint, with TCC |
Given that the TCC figures prominently in the case studies in Part 2, it is important to be familiar with the process described in the “Scientific Review: Therapeutic Class Comparison Selection and Analysis” section. In any given review, multiple comparators will be identified and classified by their level of comparability. The case studies presented represent simplified scenarios.
These examples are not intended to bind the Staff or the Board in any In-Depth Review or hearing and the analytical approach may be departed from based on the facts available during the review. All recommendations from the Staff and decisions from the Chairperson/Board will depend on the particular circumstances of the matter in question.
Part 1 (No In-Depth Reviews)
Case Study 1: List price below HIP

Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is below the HIP. During the Annual Review period, both the Canadian list price and the HIP decrease over time. The Canadian list price remains below the HIP, which does not lead to an In-Depth Review. The chart is divided into two sections, separated by a dashed line: the left represents the Initial Review period, and the right represents the Annual Review period. The blue line represents the Canadian list price, and the green line represents the HIP.
Issue/Facts:
- During the Initial Review, the Canadian list price is less than the HIP.
- The HIP trends downward with time, but the Canadian list price also decreases.
- With no list price increases occurring, changes in CPI are not a consideration.
- No complaint is received regarding this medicine.
Analysis:
- No additional analysis is required, as the medicine does not trigger an In-Depth Review.
Potential Recommendation:
- N/A – medicine not subject to In-Depth Review.
Case Study 2: List price increase within CPI and list price below HIP
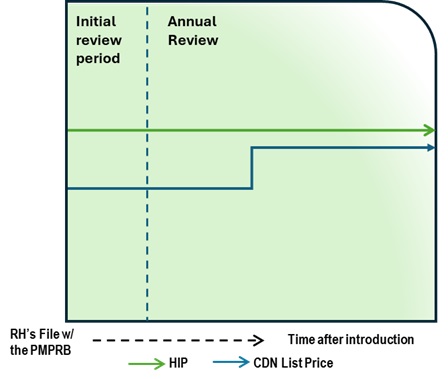
Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is below the HIP. During the Annual Review period, the Canadian list price increase is less than CPI and thus does not lead to an In-Depth Review. The chart is divided into two sections, separated by a dashed line: the left represents the Initial Review period, and the right represents Annual Review period. The blue line represents the Canadian list price, and the green line represents the HIP.
Issue/Facts:
- During the Initial Review, the Canadian list price is less than the HIP.
- The HIP is constant over time, but the Canadian list price increases.
- The list price increase is less than CPI.
- No complaint is received regarding this medicine.
Analysis:
- No additional analysis is required, as the medicine does not trigger an In-Depth Review.
Potential Recommendation:
- N/A – medicine not subject to In-Depth Review.
Part 2 (In-Depth Review)
Case Study 3: List price above HIP, without TCC
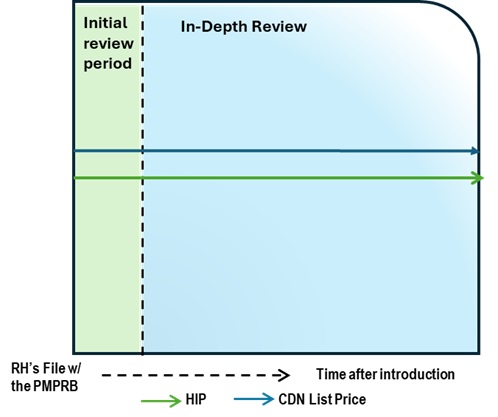
Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is above the HIP. This results in an In-Depth Review. The chart is divided into two sections: the green section for the Initial Review period and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, and the green line represents the HIP.
Issue/Facts:
- During the Initial Review, the Canadian list price is greater than the HIP. This results in an In-Depth Review.
Analysis:
- The commencement of an In-Depth Review prompts a Scientific Review; however, there are no therapeutic comparators and a TCC analysis cannot be conducted.
- During the In-Depth Review, it is observed that the Canadian list price continues to be greater than the HIP, but no list price increases are taken by the Rights Holder.
- With no TCC and no list price increase to measure against CPI, the only 85(1) factor available for consideration is the HIP. PMPRB Staff may consider the difference between the Canadian list price and the HIP, the number of countries with a reported price for the medicine, as well as the range in prices across the PMPRB11.
Potential Recommendation:
- This case could result in a recommendation for closure or Notice of Hearing, depending upon how the Canadian list price is positioned relative to the more comprehensive analysis of the international market.
Case Study 4: List price above HIP, with TCC below HIP
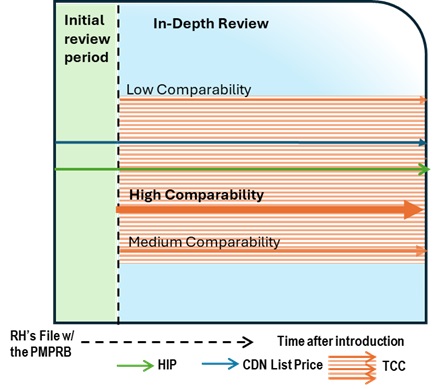
Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is above the HIP, resulting in an In-Depth Review. The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will also evaluate the comparators for their level of comparability to the medicine under review. The chart is divided into two sections: the green section for the Initial Review period and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, the green line represents the HIP, and the orange lines represent the TCC comparators. A high comparability comparator priced below both the Canadian list price and the HIP was identified.
Issue/Facts:
- During the Initial Review, the Canadian list price is greater than the HIP. This results in an In-depth Review.
Analysis:
- The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will evaluate the comparators for their level of comparability to the medicine under review.
- While no list price increase has been taken by the Rights Holder, the analysis suggests that the Canadian list price is above both the HIP and TCC.
- PMPRB Staff may consider the strength of the TCC, as well as the difference between the Canadian list price and the HIP, the number of countries with a reported price for the medicine, and the range in prices across the PMPRB11.
Potential Recommendation:
- With the Canadian list price above both the HIP and certain TCC comparators, the case-specific context would need to be supportive of the price of the medicine for PMPRB Staff to recommend closure of this In-Depth Review. More likely, given the existence of a high comparability comparator whose price is lower than both the Canadian list price and the HIP, this case would result in the recommendation for a Notice of Hearing.
Case Study 5: List price above HIP, with TCC above HIP

Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is above the HIP, resulting in an In-Depth Review. The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will also evaluate the comparators for their level of comparability to the medicine under review. The chart is divided into two sections: the green section for the Initial Review period and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, the green line represents the HIP, and the orange lines represent the TCC comparators. A high comparability comparator priced above both the Canadian list price and the HIP was identified.
Issue/Facts:
- During the Initial Review, the Canadian list price is greater than the HIP. This results in an In-Depth Review.
Analysis:
- The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will evaluate the comparators for their level of comparability to the medicine under review. A high comparability comparator priced above both the Canadian list price and the HIP was identified.
- No list price increase has been taken by the Rights Holder, as a result the CPI factor is not taken into consideration.
- PMPRB Staff may consider the strength of the TCC, as well as the difference between the Canadian list price and the HIP, the number of countries with a reported price for the medicine, and the range in prices across the PMPRB11.
Potential Recommendation:
With the Canadian list price above the HIP but below certain TCC comparators, the case-specific context would need to be supportive of the price of the medicine for PMPRB Staff to recommend closure of this In-Depth Review. Given the existence of a high comparability comparator whose price is higher than both the Canadian list price and the HIP, Staff will likely recommend a closure of the In-Depth Review.
Case Study 6: List price increase above CPI, with TCC below HIP

Figure description
This chart illustrates the case where, after a period of time on the market, the Canadian list price increases greater than CPI, resulting in an In-Depth Review. The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will also evaluate the comparators for their level of comparability to the medicine under review. The chart is divided into two sections: the green section for the Initial Review period and Annual Review period prior to the price increase and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, the green line represents the HIP, and the orange lines represent the TCC comparators. A high comparability comparator priced above the Canadian list price and below the HIP was identified.
Issue/Facts:
- After a period of time on the market, the list price increases greater than CPI.
Analysis:
- The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will evaluate the comparators for their level of comparability to the medicine under review. A high comparability comparator priced below both the Canadian list price and the HIP was identified.
- PMPRB Staff may consider the strength of the TCC, as well as the difference between the Canadian list price and the HIP, the number of countries with a reported price for the medicine, and the range in prices across the PMPRB11.
Potential Recommendation:
- The Canadian list price is below the HIP. Given the increase above CPI and the lower TCC the case-specific context would need to be considered, including the difference between the Canadian list price and the HIP, the extent of the price increase and the relative comparability of the TCC. Given the existence of a high comparability comparator whose price is lower than both the Canadian list price and the HIP, this case would likely result in the recommendation for a Notice of Hearing.
Case Study 7: Complaint, with TCC
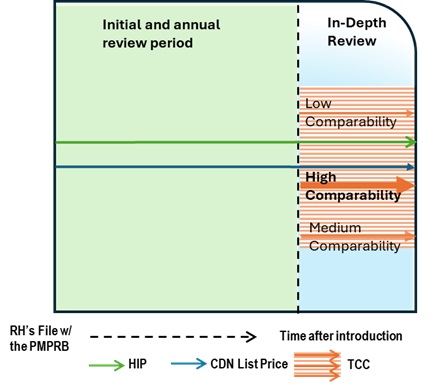
Figure description
This chart illustrates the case where the Canadian list price is below the HIP and remains unchanged. After a period of time on the market, a complaint is received, resulting in an In-Depth Review. The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will also evaluate the comparators for their level of comparability to the medicine under review. The chart is divided into two sections: the green section for the Initial review period and Annual Review period prior to the receipt of a complaint and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, the green line represents the HIP, and the orange lines represent the TCC comparators. A high comparability comparator priced below both the Canadian list price and the HIP was identified.
Issue/Facts
- Over time on the market, the Canadian list price and the HIP remain unchanged.
- A complaint is received. This will result in an In-Depth Review.
Analysis:
- The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will evaluate the comparators for their level of comparability to the medicine under review. A high comparability comparator priced below both the Canadian list price and the HIP was identified.
- Staff must take the TCC into account and weigh it against the HIP and the fact that the Canadian list price has not changed. Staff’s recommendation will depend heavily on the TCC context and price differential magnitude.
Potential Recommendation:
- In this case, a high comparability comparator whose price is lower than both the Canadian List and the HIP was identified. This will have to be weighed against the fact that the Canadian list price is lower than the HIP. In a situation where the HIP has considerably more weight than the TCC given the context, Staff may recommend that the In-Depth Review be closed. If the reverse is the case, Staff may recommend that a Notice of Hearing be issued.
Comparable Dosage Forms
This Appendix identifies comparable dosage forms for the purpose of the Scientific Review for patented medicines. Formulations within each group are considered comparable, but dosage forms in a different group are not.
The PMPRB reviews the list of comparable dosage forms periodically to ensure that it includes those currently used.
| Topical | Nasal (N)/Pulmonary (P) | Oral Solid (S) |
|---|---|---|
Aerosol |
Aerosol |
Bar (chewable) |
| Oral Liquid (L) | Vaginal (V) | Parenteral (J) |
Drops |
Cone |
Bolus |
| Otic (E)/Ophthalmic (Y) | Rectal (R) | Dental/Sublingual Buccal (M) |
Drops |
Cream |
Emulsion |
Simplified Overview
A Simplified Overview of the Draft Guidelines
Disclaimer
This document is for general informational purposes only. Pursuant to the Treasury Board Secretariat Directive on the Management of Communications, this overview of the Patented Medicine Prices Review Board (PMPRB) Draft Guidelines has been written in clear, straightforward language for the public to more easily understand the PMPRB Draft Guidelines.
As a result, this overview is not a legal document. It is not intended to have any legal impact or authority. This overview does not create, interpret, or affect any legal rights, responsibilities, or obligations. It does not constitute legal, technical, business, or other advice, and should not be relied upon as such.
The PMPRB is seeking feedback on the Draft Guidelines document, not this overview. If there are any perceived differences between this overview and the Draft Guidelines, the Draft Guidelines document is the definitive text.
The PMPRB Mandate
The main mandate of the PMPRB is to review the prices of patented drugs. If a price appears too high, and excessive, when compared to the price of the drug in other countries, the inflation rate or the price of similar treatments, it may hold a hearing which could result in the price being lowered.
Guidelines
The role of guidelines, when finalized, will be to provide a review process to be used by the PMPRB staff to recommend a price hearing for drugs that might be priced too high. The PMPRB staff cannot review in-depth the prices of each patented drug that is reported by Right Holders. Therefore, guidelines, when finalized, will provide an administrative process that help identify drugs that are more at risk to be potentially priced too high compared to its price in other countries or to similar treatments. It is also desired that guidelines should provide a transparent, predictable, and procedurally fair process around price review for those who sell drugs in Canada.
The Draft Guidelines Proposal
The Board is now consulting on a proposal for new guidelines, which it calls the Draft Guidelines. A plain language description of certain important aspects of the proposal is provided below.
The Comparison in the Review Process
The review process of the PMPRB is based on a comparison mechanism. To identify cases where a drug might be priced too high, the PMPRB staff compares its price to the highest price of the same drug sold in 11 other countries and considers the inflation rate, calculated using the Consumer Price Index. These 11 countries are called the PMPRB11:
Australia |
Belgium |
France |
Germany |
Italy |
Japan |
Netherlands |
Norway |
Spain |
Sweden |
United Kingdom |
|
The PMPRB staff also compare the drug under review to similar drugs or treatments based on therapeutic indications and their use for similar medical conditions.
Through price and therapeutic comparisons, the PMPRB staff can recommend either an excessive price hearing to the Chairperson to determine if the price is too high, or closure of the review process.
The Review Process
The Draft Guidelines propose a 2-step review process.
Step 1 – First Reviews
The first step serves as a screening process to identify drugs that might be priced too high. If the price of a patented drug is caught by one of the screens described in the Draft Guidelines, the PMPRB staff will do a more detailed review of the price.
Moments at which the PMPRB staff do the first review are:
- Initial Review – The PMPRB staff review the price of a patented drug when it is first sold in Canada
- Annual Review – The PMPRB staff review the price for all patented drugs sold in Canada each year
- Review Following Complaint – Certain people and groups can send formal complaints to the PMPRB.
For this first review, the PMPRB staff compare the drug price to see if the Canadian price is higher than its price in the PMPRB11 countries .
The PMPRB staff also use the consumer price index (CPI) to identify if a price increase is higher than the inflation rate.
If one or each comparison indicates that the price could be too high, the PMPRB staff then start a more in-depth review.
Step 2 - In-Depth Review
An In-Depth Review happens when the PMPRB staff compare the drug under review to similar drugs and treatments available for similar medical conditions. They examine how the drug is used and its treatment plan. Based on this information, the PMPRB staff then perform a price comparison between the drug under review and the similar drugs and treatments.
It is only at the end of this extended analysis that the PMPRB staff can recommend a hearing to the Chairperson, and only if the analysis indicates that the drug could be priced too high in Canada. If not, the PMPRB staff recommend to close the review, and will reanalyze the drug's price during the annual review using the first-step analysis.
Recommendations and next steps
If the PMPRB staff believe the price of a drug is too high after the In-Depth Review, they can recommend a hearing to the Chairperson. Then, the Chairperson of the PMPRB can decide to request a hearing or not.
The Chairperson decides if it is in the public interest for the price of the patented drug to be reviewed by a Hearing Panel. Only a Hearing Panel can decide if a price is too high, based on all the relevant legal factors.
Alternatively, and following the recommendation of the PMPRB staff, the Chairperson can also close the In-Depth Review.
Undertakings and Settlement Proposals
Rights holders are informed when the PMPRB staff begin an In-Depth Review. In some cases, rights holders may provide a written commitment to lower the price of a drug under review or to repay excess revenue earned to the Government of Canada. The Chairperson or the Hearing Panel (if a hearing has started) may decide to accept or reject this commitment.
Price Hearings
PMPRB hearings are public. During the hearing, submissions and evidence are heard by the Hearing Panel consisting of at least 2 Board Members. The Hearing Panel then determines if a patented drug is being or has been sold at a price that is considered too high in Canada. The hearing can result in the Board directing the price of the drug be lowered and/or a payment of excess revenue be made by the Rights Holder to the Government of Canada.
The Hearing Panel determines whether a price is too high, considering all relevant legal factors. The PMPRB staff and the rights holder selling the medicine each submit their own evidence and arguments to the Hearing Panel, which makes the decision during the hearing.
Submissions
Submissions
Webinar
PMPRB Draft Guidelines Webinar
February 18, 2025
Background
The PMPRB:
- is an independent, quasi-judicial body established by Parliament in 1987 under the Patent Act
- has a dual price review and pricing trends reporting mandate
- fulfills its price review mandate by holding public hearings to determine whether the prices of specific patented medicines are excessive
- operates within the boundaries of the Patent Act sections 79 to 103 and the Patented Medicines Regulations (SOR/94-688)
- Is empowered to issue non-binding Guidelines on matters within its jurisdiction
Amendments to the Patented Medicines Regulations Key changes in effect since July 1, 2022
1. An updated schedule of comparator countries (the new “PMPRB11”).
| PMPRB7 | ||
|---|---|---|
 |
X | United States |
 |
X | Switzerland |
 |
Italy* |
|
 |
Germany* |
|
 |
United Kingdom* |
|
 |
Sweden* |
|
 |
France* |
|
| Added countries | |
|---|---|
 |
Spain |
 |
Norway |
 |
Belgium |
 |
Japan |
 |
Netherlands |
 |
Australia |
2. Reduced reporting obligations for medicines with lowest risk of excessive pricing (i.e. veterinary, over-the-counter, and certain “generic” medicines
Additionally, the Board released Interim Guidance that came into force in August 2022 and decided to consult on a new set of guidelines.
Refreshing the Dialogue: Comprehensive Consultation
The PMPRB has made significant efforts to consult with as many stakeholders as possible on the direction and substance of new Draft Guidelines.
- Phase 1 (November 2023 to January 2024)
- Scoping Paper on November 10, 2023: outlined themes and questions to inform the development of final PMPRB Guidelines
- Policy Roundtables on December 5 and 6: two-day consultation where stakeholders from across the country had the opportunity to voice their opinions on any topic or themes relevant to the development of new PMPRB Guidelines.
- The What we Learned Report, commissioned by the PMPRB, was published in January 2024. It summarizes the feedback and insights gathered during the Policy Roundtable discussions.
- Phase 2 (January 2024 to October 2024)
- Discussion Guide on June 26, 2024: outlined the proposed structure for the price review process envisioned by the Board and highlighted key issues for stakeholder input.
- Webinar on the Discussion Guide on August 13, 2024: information webinar designed to clarify issues raised and questions received from stakeholders
- PMPRB Stakeholder Meetings from September to October 2024: Five virtual meetings providing stakeholders an opportunity to voice their opinions on any topic relevant to the development of the Draft Guidelines
- Phase 3 (December 2024 to March 2025)
- Draft Guidelines released December 19, 2024 - 90-day consultation period begins
- Webinar on the Draft Guidelines on February 18 and 19, 2025
Phase 3 Consultation
December 2024 – March 2025
On December 19, 2024 the PMPRB released new Draft Guidelines
A 90-day consultation period is underway, during which interested parties are invited to provide feedback through the PMPRB consultation portal
The deadline to submit feedback is March 19, 2025
Key features of the new Draft Guidelines
Review Process – An Overview of the Two-Step Framework
The draft Guidelines work as a mechanism to prioritize the cases that are advanced for recommendation for a hearing.
The draft Guidelines would provide transparency to interested parties regarding Staff’s procedures, thereby ensuring procedural fairness and consistency.
The review process, as contemplated under the draft Guidelines, is separated into two steps or screens:
- First Step: Initial or Annual Review
- Second Step: In-Depth Review
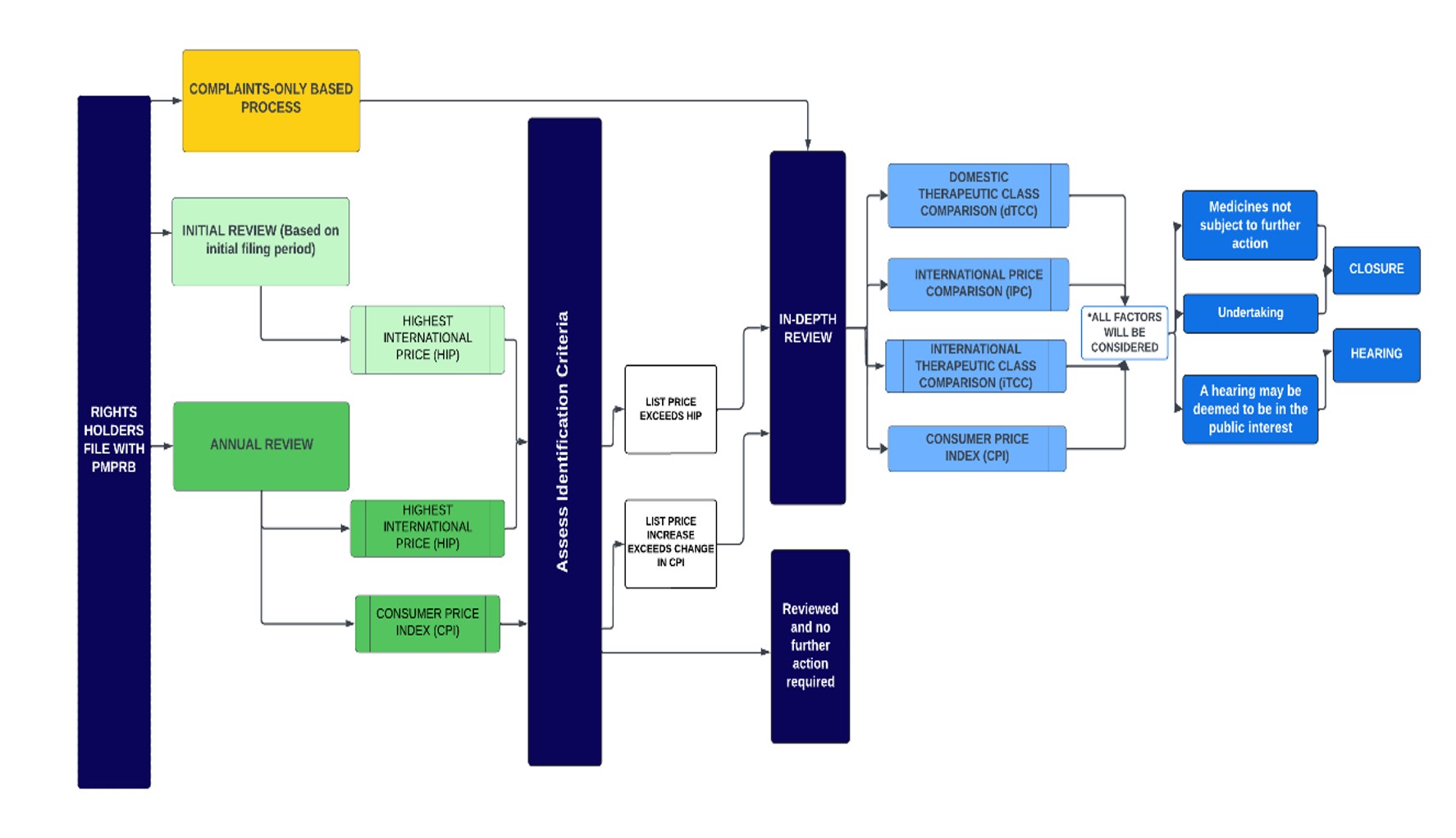
Figure description
This diagram sets out PMPRB's general review processes for patented medicines contemplated under these Guidelines. It is divided into several sections to highlight the various steps in the process.
The framework is described below:
- The first step is an Initial Review (light green) or Annual Review (dark green) and applies to all patented medicines sold in Canada, with certain limited exceptions (set out below under “Special Provisions on Complaints”).
- Complaints (yellow) serve as a separate process by which medicines can be identified for an In-Depth Review.
- Initial Review (light green box):
- Staff uses a medicine’s first semi-annual price filing to conduct an Initial Review against the highest international price among the Schedule Countries filed by the Rights Holder (“HIP”).
- Patented medicines whose prices are above the HIP threshold are subject to an In-Depth Review.
- Annual Review (dark green box):
- Staff conducts an Annual Review of list prices for each patented medicine under the PMPRB’s jurisdiction.
- The Annual Review applies the same IPC identification criteria (the HIP) used during the Initial Review.
- Staff also compares the price change of each patented medicine against changes in the Consumer Price Index (CPI) as an identification criterion to prioritize medicines that warrant an In-Depth Review.
- The result of the Initial Review or Annual Review may either be (a) no further review; or (b) referral for In-Depth Review.
- The In-Depth Review (light blue) is the process by which Staff analyses and balances all the information related to the section 85 factors to prepare a recommendation to the Chairperson on whether a matter should be brought to a hearing. The result of an In-Depth Review is either (a) recommendation for closure of the In-Depth Review; or (b) recommendation for a hearing.
First Step – Initial Review
PMPRB Staff would review a patented medicine’s highest Canadian list price against the highest international price (“HIP”) among the Schedule Countries (“PMPRB11”), as filed by the Rights Holder.
- Also known as “International Price Comparison” or “IPC”.
Staff would use the medicine’s first semi-annual price filing to conduct the Initial Review
Patented medicines whose prices are above the HIP threshold would be subject to an In-Depth Review.
- The Initial Review would be performed at the DIN level; however, if one DIN met the criteria for In-Depth Review, all associated DINs sold by the Rights Holder would be considered part of the In-Depth Review.
If the IPC cannot be conducted because no list prices are filed for the medicine in any of the PMPRB11 during the Initial Review, the list price would be considered reviewed and would not be reviewed again until the Annual Review.
Service Standard: 60 days of the filing deadline for the first semi-annual filing.
First Step – Annual Review
Staff would review a patented medicine’s highest Canadian list price against the highest international price (“HIP”) among the PMPRB11, as filed by the rights holder; and
- Also known as “International Price Comparison” or “IPC”.
Staff would compare the price change of each patented medicine against the Consumer Price Index (CPI).
Patented medicines whose prices are above the HIP and/or have increased beyond CPI would be subject to an In-Depth Review.
- The Annual Review would be performed at the DIN level; however, if one DIN met the criteria for In-Depth Review, all Associated DINs sold by the Rights Holder would be considered part of the In-Depth Review.
When the IPC cannot be conducted (i.e., no filed PMPRB11 prices), an In-Depth Review could still be initiated based on the change in CPI, or in response to a complaint.
Service Standard: 60 days of the filing deadline for the semi-annual filing in January.
Annual Review – Changes in the Consumer Price Index (CPI)
As part of the Annual Review, changes in list prices would be reviewed against the change in CPI using the one-year lagged CPI.
- e.g. List price increases taken in 2027 would be compared against the 2026 CPI factors, published by Statistics Canada in January 2027.
If a list price increase is greater than the change in CPI, the patented medicine would be subject to an In-Depth Review, unless:
- A list price increase was not taken in the previous year and the increase in the second year is lower than or equal to the total change in CPI over those two years.
Special Provisions on Complaints
The receipt of a complaint from an approved individual/organization would automatically lead to an In-Depth Review, whether the Initial or Annual Review criteria have been met or not.
- This would apply to all patented medicines, including patented generics, over the counter, and veterinary medicines.
Approved individuals or organizations:
- the Federal Minister of Health or any of their Provincial or Territorial counterparts;
- Publicly-funded drug programs; and,
- Canadian life and health insurance companies and their trade association(s).
Neither the information about the complainant, nor the patented medicine subject to complaint and corresponding Rights Holder is made public by the PMPRB, unless the matter results in a Hearing or as otherwise required by law.
Second Step – In-Depth Review
In-Depth reviews would be initiated for all medicines that have been identified either through initial review, annual review or based on a complaint.
- Potential exception: Medicines for which an In-Depth review is still ongoing and Medicines for which an In-Depth review was closed within the last two filing periods.
In-Depth Review is the process by which Staff would analyse and balance all the information related to the section 85(1) factors to prepare a recommendation to the Chairperson on whether a matter should be brought to a hearing.
The process would include both a Scientific Review (Therapeutic Class Comparison Selection and Analysis) and a Price Review.
Deferral Letters: In the event of resource constraints, Staff would prioritize medicines with list prices significantly above the HIP or whose list price increases are significantly above CPI. Other medicines subject to an In-Depth Review may receive deferral letters.
Scientific Review: Therapeutic Class Comparison Selection and Analysis
Upon initiation of an In-Depth Review, PMPRB Staff’s scientific team would identify and rate relevant comparators for the purposes of conducting a Therapeutic Class Comparison (“TCC”) for each Health Canada approved indication of the medication under review.
- Types of sources consulted by Staff are outlined in section 89 of the draft Guidelines
Each comparator would be assigned a level of similarity to the patented medicine subject to In-Depth Review in accordance with a two-step framework.
- Step 1: Qualitative Assessment
- Step 2: Grouping Assessment
The Scientific Team may, at their discretion, consult the Human Drug Advisory Panel (HDAP)(or a subset thereof), for further analysis of the comparability ratings of any or all identified comparators.
Rights Holders would generally be notified regarding the selection of comparators for each approved indication or use within 8 months of the initiation of the In-Depth Review (potentially longer if HDAP is consulted).
Step 1: Qualitative Assessment
| Qualitative Class | Example Rationale |
|---|---|
A |
Comparable: comparable safety and efficacy demonstrated in high quality head-to-head trials or meta-analyses. Recommended or used in the same place in therapy as the patented medicine under review. |
B |
Less comparable: efficacy, safety or patient-assessed outcomes are meaningfully different (either better or worse) than the patented medicine under review based on published literature. Recommended or used in a similar place in therapy as the medicine under review. |
C |
Undetermined comparability: could be used alternatively or in place of the patented medicine under review in many circumstances but comparability is unclear based on available published literature. |
D |
No data: No quality published data available to assess comparability to the patented medicine under review. |
X |
No comparators. Do not proceed to step 2.* |
*Note: for the purposes of a TCC, a patented medicine that is identified as the only effective treatment sold in Canada for a particular illness or indication is designated as a Qualitative Class X. This medicine therefore has no comparators.
Step 2: Grouping Assessment
| Group | Description of the Comparator |
|---|---|
1 |
Same 4th level ATC†, materially similar approved indication or use as the patented medicine under review as per Health Canada. |
2 |
Different 4th level ATC, materially similar approved indication as the patented medicine under review as per Health Canada. |
3 |
Same 4th level ATC, used in Canada for a materially similar indication or use as the patented medicine under review as per published literature but not indicated by Health Canada. |
4 |
Different 4th level ATC, used in Canada for a materially similar indication or use as the patented medicine under review as per published literature but not indicated by Health Canada. |
†Anatomic and Therapeutic Classification System as published by the World Health Organization |
|
Visual representation of comparability

Figure description
This table illustrates the spectrum of comparability, ranging from more comparable (top-left) to less comparable (bottom-right). The horizontal axis represents the qualitative class (A, B, C, D), while the vertical axis indicates groupings (1, 2, 3, 4). The figure effectively shows how comparability decreases progressively across qualitative classes (left to right) and across groupings (top to bottom).
Comparability is visually distinguished by color:
- Green: High comparability (e.g., A1, A2 and B1)
- Blue: Medium comparability (e.g., A3, A4, B2, B3, C1, C2, D1)
- Yellow/Orange/Pink: Low comparability (e.g., B4, C3, C4, D2, D3 and D4)
| High Comparability – Green | Medium Comparability – Blue | Low Comparability – Yellow/Orange/Pink |
|---|---|---|
|
|
|
As part of the scientific review, PMPRB Staff scientific team would generate:
- A domestic therapeutic class comparison (dTCC) for each reviewed indication, including comparable dosage regimens;
- An international therapeutic class comparison (iTCC) (if required), including comparable dosage regimens;
- In general, the Staff scientific team would use the comparators identified in the dTCC to populate the iTCC.
- A list of references used for establishing the comparability ratings; and
- A context summary of the rationale for the assigned ratings.
Rights Holders would be provided with the above information and would have the opportunity to make a submission prior to the Chairperson’s decision on a potential Hearing.
- Rights Holders are encouraged to submit any relevant information that they wish to be considered as soon as possible after being notified of an In-Depth Review. They may provide any information they deem appropriate.
Price Review
In parallel with the Scientific Review, Staff commences its Price Review of the patented medicine.
- Rights Holders could provide input to the pricing team on issues relevant to the subsection 85(1) factors other than the TCC within three months of being notified of the In-Depth Review.
Staff would add pricing information to the TCC analysis once the Scientific Review is complete.
As part of the review/analysis, all information provided by the Rights Holder pursuant to the Regulations and/or collected by Staff would be considered as it relates to the factors outlined in section 85(1) of the Act, namely:
- information relating to the price of the medicine in Canada;
- information relating to the price of the medicine in the Schedule Countries;
- information relating to the TCC selection and analysis; and
- information relating to changes in the CPI over the relevant period.
Recommendation
Staff would consider all the information collected and prepares a recommendation to the Chairperson that the In-Depth Review either be closed (with or without an Undertaking) or that a hearing be held.
- The recommendation would NOT include a price ceiling, recommended price, or decision on excessivity.
- Appendices “Assessment of individual s. 85(1) factors, separately” and “Weighting of individual factors during In-Depth Review” provide further details on/examples of how Staff would assess information and balances the subsection 85(1) factors in preparing its Recommendation.
If the result of the In-Depth Review is a recommendation to the Chairperson to issue a Notice of Hearing, Staff would inform the Rights Holder of that recommendation when it is submitted to the Chairperson.
The Chairperson would then decide whether the In-Depth Review should be closed or if it is in the public interest that a Notice of Hearing be issued.
- The Rights Holder would be advised of the Chairperson’s decision.
Undertakings and Settlement Proposals
Undertakings
- Unilateral promises;
- Not binding agreements and therefore not covered by settlement privilege nor treated on a “without prejudice” basis;
- Could be submitted from notification of In-Depth Review up until two months after notification that a recommendation of a Hearing has been made to the Chairperson;
- Any undertaking submitted would be referred to the Chairperson; Staff would not negotiate terms or provide guidance to Rights Holders on the substance of an Undertaking.
Settlement Proposals
- Could be submitted after issuance of a Notice of Hearing;
- May only be approved by the designated Hearing Panel (e.g. through motion to discontinue);
- Negotiations/discussions with Staff may be conducted on a without prejudice basis and are subject to settlement privilege
Timelines and Deadlines
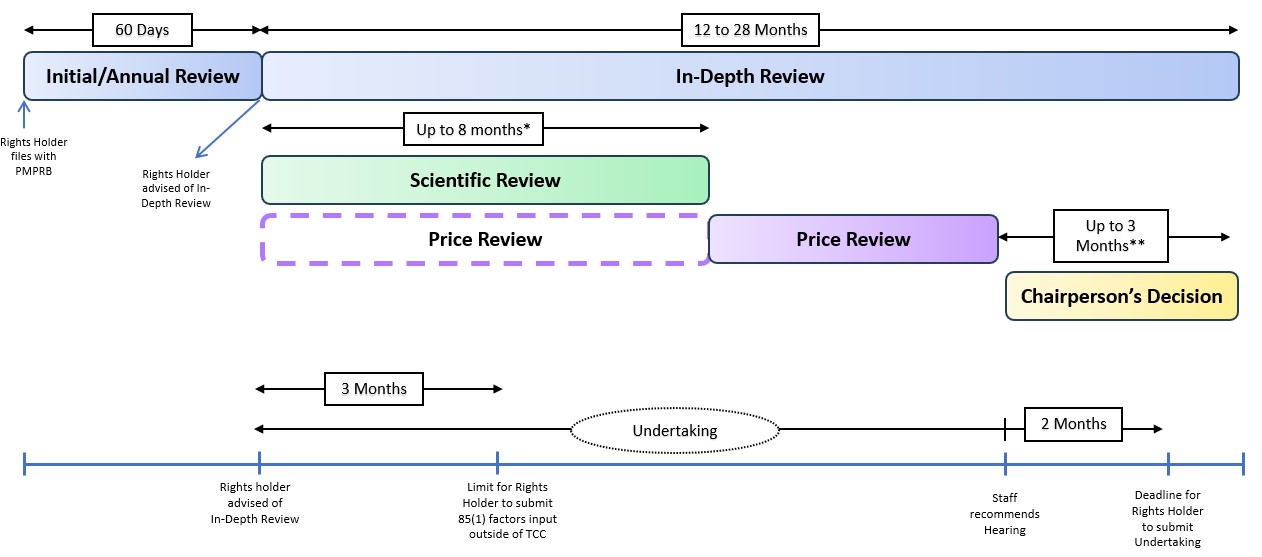
Description de la figure
This image presents two timelines illustrating deadlines and target dates considered under the Draft Guidelines.
The first timeline describes the PMPRB internal process from the information filed by the Rights Holder to the Chairperson’s decision.
The process is described below:
- The first step (first blue box) is an Initial Review or Annual Review, which would be completed within 60 days. At the end of this period, the Rights Holder would be advised of an In-Depth Review.
- The second step (second blue box) is the In-Depth Review, which can take between 12 to 28 months. This Review is divided in three sections:
- The first section (green box) is the Scientific Review, which can take up to eight months, and even longer if the HDAP is consulted.
- The second section (purple box) is the Price Review. It begins at the same time as the Scientific Review but lasts up to three months before the end of the process.
- The third section (yellow box) is the Chairperson’s decision, which can take up to three months, and at least one month after the submission of an Undertaking.
The second timeline (blue timeline) describes the interactions between Rights Holders and the PMPRB throughout the process.
The interactions are described below:
- First, after the Initial or Annual Review, the Rights Holder is notified that an In-Depth Review is initiated.
- From that moment, the Rights Holder has three months to submit information related to the factors outlined in subsection 85(1) other than the TCC.
- From the moment the Rights Holder is notified that an In-Depth Review is initiated, it is also possible to submit an Undertaking for the Chairperson to consider. The deadline for a Rights Holder to submit an Undertaking is two months after the PMPRB Staff recommends to the Chairperson that a hearing be held.
* Longer if HDAP is consulted; ** 1 month upon submission of Undertaking
Case Study 4: List Price above HIP, with TCC below HIP
Facts & Analysis:
- An In-Depth Review is commenced following the Initial Review which reveals that the Canadian list price (“CDN list price”) is greater than the HIP.
- The Scientific Review identifies a therapeutic class containing multiple comparators, with varying degrees of comparability.
- The prices of the comparators are seen to be both higher and lower than the CDN list price and the HIP. Of note, a high comparability comparator is priced below both the CDN list price and the HIP.
- Throughout the course of the In-Depth Review, the CDN list price remains steadily above the HIP and TCC.
- PMPRB Staff may consider: the strength of the TCC, the difference between the CDN list price and the HIP, the number of reported prices, and the range in prices across the PMPRB11.

Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is above the HIP, resulting in an In-Depth Review. The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will also evaluate the comparators for their level of comparability to the medicine under review. The chart is divided into two sections: the green section for the Initial Review period and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, the green line represents the HIP, and the orange lines represent the TCC comparators. A high comparability comparator priced below both the Canadian list price and the HIP was identified.
Potential Recommendation: With the CDN list price above both the HIP and the high comparability comparator(s), this case would likely result in the recommendation for a Notice of Hearing.
Case Study 5: List Price above HIP, with TCC above HIP
Facts & Analysis:
- An In-Depth Review is commenced following the Initial Review which reveals that the Canadian list price (“CDN list price”) is greater than the HIP.
- The Scientific Review identifies a therapeutic class containing multiple comparators, with varying degrees of comparability.
- The prices of the comparators are seen to be both higher and lower than the CDN list price and the HIP. Of note, a high comparability comparator is priced above both the CDN list price and the HIP.
- Throughout the course of the In-Depth Review, the CDN list price remains above the HIP.
- PMPRB Staff may consider: the strength of the TCC, the difference between the CDN list price and the HIP, the number of reported prices, and the range in prices across the PMPRB11.

Figure description
This chart illustrates the case where, during the Initial Review, the Canadian list price is above the HIP, resulting in an In-Depth Review. The commencement of the In-Depth Review prompts a Scientific Review. This review identifies a therapeutic class containing multiple comparators with prices both higher and lower than the Canadian list price and the HIP. The Scientific Review will also evaluate the comparators for their level of comparability to the medicine under review. The chart is divided into two sections: the green section for the Initial Review period and the blue section for the In-Depth Review period. The blue line represents the Canadian list price, the green line represents the HIP, and the orange lines represent the TCC comparators. A high comparability comparator priced above both the Canadian list price and the HIP was identified.
Potential Recommendation: The CDN list price is above the HIP; however, the existence of a high comparability comparator priced above both the CDN List price and the HIP would likely result in the recommendation to close the In-Depth Review.
Implementation approach considered – New Medicines
New Medicines - Patented medicines first sold on or after July 1, 2022.
| Immediately after the Guidelines come into effect |
|
|---|---|
| January of the second calendar year after publication of the Guidelines |
|
Implementation approach considered – Existing Medicines
Existing Medicines - Patented medicines first sold before July 1, 2022.
Immediately after the Guidelines are published |
|
|---|---|
One year after the Guidelines come into effect |
|
Consultation Period – Draft Guidelines
The deadline to provide feedback on the Draft Guidelines is March 19, 2025.