Meds Pipeline Monitor 2021
 April 2022
April 2022
ISSN 2562-3834
Cat. No.: H79-5E-PDF
Contact Information
Patented Medicine Prices Review Board
Standard Life Centre
Box L40
333 Laurier Avenue West
Suite 1400
Ottawa, ON K1P 1C1
Tel.: 1-877-861-2350
TTY 613-288-9654
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Acknowledgements
This report was prepared by the Patented Medicine Prices Review Board (PMPRB) as part of the National Prescription Drug Utilization Information System (NPDUIS) initiative.
The PMPRB wishes to acknowledge the members of the NPDUIS Advisory Committee for their expert oversight and guidance in the preparation of this report. Please note that the statements and findings for this report do not necessarily reflect those of the members or their organizations.
We gratefully acknowledge Patricia Carruthers-Czyzewski, BScPhm, MSc, Sintera Inc. for providing pharmaceutical expertise and for her contribution to the scientific analysis.
Appreciation goes to Allison Carey for leading this project, and Tanya Potashnik and Brian O’Shea for their oversight in the development of the report. The PMPRB also wishes to acknowledge the contribution of the editorial staff Sarah Parker and Laura Fortune.
Disclaimer
NPDUIS operates independently of the regulatory activities of the Board of the PMPRB. The research priorities, data, statements, and opinions expressed or reflected in NPDUIS reports do not represent the position of the PMPRB with respect to any regulatory matter. NPDUIS reports do not contain information that is confidential or privileged under sections 87 and 88 of the Patent Act, and the mention of a medicine in an NPDUIS report is not and should not be understood as an admission or denial that the medicine is subject to filings under sections 80, 81, or 82 of the Patent Act or that its price is or is not excessive under section 85 of the Patent Act.
Although this information is based in part on data obtained under license from GlobalData and the MIDAS® Database proprietary to IQVIA Solutions Canada Inc. and/or its affiliates (“IQVIA”), the statements, findings, conclusions, views, and opinions expressed in this report are exclusively those of the PMPRB and are not attributable to either GlobalData or IQVIA.
Executive Summary
Meds Pipeline Monitor (MPM) is a horizon scanning report that features a selection of new medicines in the late stages of clinical evaluation that may have a significant impact on future clinical practice and drug spending in Canada.
Medicines in Phase III clinical trials or pre-registration are considered as candidates if they have the potential to address an unmet therapeutic need, offer a novel mechanism of action or therapeutic benefit over existing therapies, or treat a serious condition. The final selection features medicines that treat a broad range of therapeutic areas. In addition to identifying new medicines for inclusion in the list, medicines featured in the previous edition of the MPM are also reviewed to report on changes to their status in the pipeline. A section focused on Canada highlights potentially significant medicines currently under review by Health Canada.
This edition of the report also includes a section on COVID-19, which provides an overview of medicines undergoing Phase I, II, and III clinical trials or in pre-registration for the treatment and prevention of the novel coronavirus disease.
The report collects data from two main sources: GlobalData’s Healthcare database is used to identify medicines currently undergoing clinical evaluation, while Health Canada’s Drug and Health Product Submissions Under Review list provides information on new medicines under review in Canada.
Together with its companion publication Meds Entry Watch, this report series monitors the continuum of new and emerging medicines in Canada and internationally, providing key information to decision makers, researchers, patients, and clinicians, among other stakeholders.
Highlights of the Meds Pipeline 2021
- In 2021, the pipeline contained nearly 8,500 new medicines in various stages of evaluation, compared to just under 7,000 the year before. The higher-than-average number of new medicines in the pipeline may be attributable to the ongoing COVID-19 pandemic, which has delayed clinical trials for other therapeutic areas.
- Consistent with previous years, the 1,145 new medicines undergoing Phase III clinical trials and pre-registration in 2021 represented a wide range of therapeutic areas and accounted for 13% of the total pipeline.
- Oncology continued to dominate the therapeutic mix in 2021, with cancer treatments representing one third (35%) of medicines in all phases of clinical trials. Treatments for infectious diseases held the second largest share of the pipeline, at 14%, due to the rapid response to the COVID-19 pandemic.
- One third (33%) of medicines in Phase III clinical trials or pre-registration had an early orphan designation approved through the US FDA or the EMA, which is consistent with the increasing trend in the prevalence of orphan-designated medicines entering the pharmaceutical market.
- Thirty-one late-stage new medicines were selected for addition to the 2021 MPM based on their potential impact on the Canadian healthcare system. Some of these medicines may offer breakthroughs in treating previously unmet needs or may have the potential to treat large patient populations.
- Five of the new medicines added to the MPM in 2021 have forecasted annual global revenues of over US $1 billion by 2027.
- Of the 27 new medicines featured in the 2020 edition of the MPM, nine received market authorization, 10 were retained on the list as they continued to satisfy the selection criteria, and eight were removed as their clinical trials were discontinued or they no longer meet the selection criteria.
- As of September 2021, 663 vaccines and therapies were undergoing clinical evaluation globally for the prevention and treatment of COVID-19.
- As of February 2022, Health Canada is reviewing the safety and efficacy of 18 new and supplemental drug submissions for the prevention and treatment of COVID-19.
List of Terms
For the purpose of this report, the following terms and associated definitions apply.
- Clinical efficacy
- The maximum response achievable from a medicine in research settings and the capacity for sufficient therapeutic effect in clinical settings.Footnote i
- Gene therapy
- A technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene, as defined by Health Canada.Footnote ii
- Market authorization
- The process of approval for a medicine to be marketed in a given country. In Canada, market approval is granted following a substantive scientific evaluation of a product's safety, efficacy, and quality, as required by the Food and Drugs Act and Regulations.Footnote iii
- Medicinal ingredient
- A chemical or biological substance responsible for the claimed pharmacologic effect of a drug product. Sometimes referred to as a molecule, active substance, or active ingredient.Footnote iv
- Medicine
- A broad term encompassing both the final drug product and medicinal ingredient(s); this encompasses chemically manufactured active substances and biologics, including gene therapies. Medicines are reported at the medicinal ingredient level and can refer to a single ingredient or a unique combination of ingredients.
- Medicine pipeline
- A set of new medicine candidates under active research and development by biotechnology and pharmaceutical companies.
- New medicine
- A medicinal ingredient that has not previously received market authorization by a regulator.Footnote iv
- Orphan medicine
- A medicine used to treat a rare disease. For the purposes of this study, orphan medicines are defined as having an orphan designation granted by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for the relevant indication.
- Phase I
- These trials test an experimental medicine on a small group of people for the first time. The purpose is to look at the medicine's safety, determine a safe dosage range, and monitor if there are any side effects.
- Phase II
- In this phase, the medicine is given to a larger group of people (usually 100 or more) to gather data on how well the medicine works to treat a disease or condition, check its safety on a wider range of people, and determine the best dose.Footnote v
- Phase III
- These controlled or uncontrolled trials are conducted after preliminary evidence suggesting efficacy of the medicine has been demonstrated. They are intended to gather additional and confirmatory information about the clinical efficacy and safety of the medicine under the proposed conditions of use.Footnote ii Phase III trials are usually randomized with double-blind testing in several hundred to several thousand patients.
- Pre-registration
- A medicine is in the pre-registration phase once all the necessary clinical trials have been completed and it is waiting for registration or approval for use by a governing body.Footnote vi
Phases of clinical trials
Introduction
This 11th edition of the Meds Pipeline Monitor (MPM) features a selection of medicines in Phase III clinical trials or pre-registration in 2021 that have the potential to significantly impact clinical practice and drug spending in Canada.
The methodology, which is detailed in the next section, uses a specific set of criteria to identify a list of pipeline candidates from the GlobalData Healthcare database, as well as a list of candidates currently under review in Canada from Health Canada’s Drug and Health Product Submissions Under Review (SUR) lists. Medicines reported in the previous edition are also reviewed in this report, including those that continue to qualify for the list of candidates as well as those that have since received market authorization. Likewise, the new medicines featured in this report will be monitored in future editions of the MPM to identify candidates that successfully enter the market.
To provide context for the selection of medicines, the MPM includes a snapshot of the entire pipeline, with an emphasis on the therapeutic breakdown of each phase of clinical evaluation. This edition of the report also highlights select vaccines and other medicines undergoing evaluation for the treatment and prevention of COVID-19, in global markets as well as in Canada. The medicines assessed for this portion of the analysis include new therapies as well as previously marketed treatments that have been repurposed.
Meds Pipeline Monitor is a companion publication to Meds Entry Watch, which analyzes the market dynamics of newly approved medicines in Canada and internationally. Together, these two PMPRB reports monitor the market continuum of late-stage pipeline medicines and new approvals, providing decision makers, researchers, patients, clinicians, and other stakeholders with information on the emerging medicines and evolving cost pressures.
Methodology
Snapshot of the Pipeline
The snapshot of the pipeline captures the composition of medicines in various phases of clinical evaluation at a single point in time. For the purpose of this analysis, a full list of pipeline medicines was retrieved from GlobalData’s Healthcare database in September 2021.
New medicinal ingredients are identified as those with no prior approvals through the US Food and Administration (FDA), the European Medicines Agency (EMA), or Health Canada. The distribution of new medicines by therapeutic area corresponds to the indication under evaluation, as reported by GlobalData. Note that a single new medicine may be undergoing multiple clinical studies for separate indications.
The list of medicines used for the analysis of orphan medicines in the pipeline was retrieved in September 2021. For the purposes of this analysis, orphan medicines were defined as new medicines that had been granted an orphan designation by the US FDA or the EMA.
Meds Pipeline Monitor
The MPM focuses on new medicines in Phase III clinical trials or pre-registration in Canada, the United States, and Europe. Pipeline medicines are selected for inclusion using a two-stage process (Figure 1). The initial screening stage selects medicines in the late phases of clinical evaluation, while the analytic review stage involves a more rigorous appraisal of each potential candidate to identify medicines that may have a significant clinical and budgetary impact. The second stage considers a specific set of criteria, in addition to the results of a thorough review of clinical evidence and scientific literature.
This methodology is reviewed annually and refined as required.
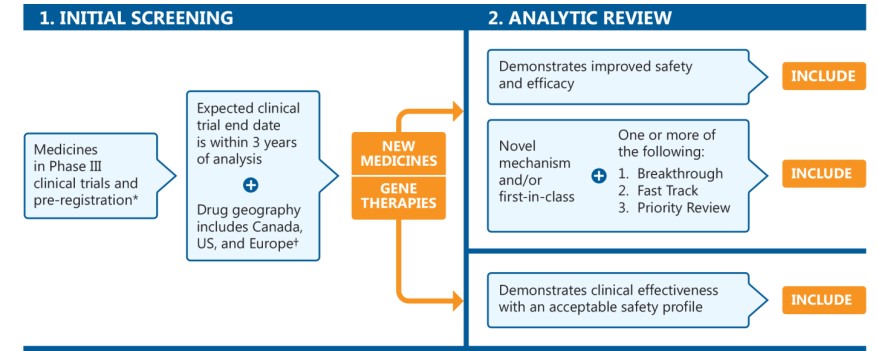
* In pre-registration with the US Food and Drug Administration (FDA).
† Has Phase III clinical trials in Canada, the United States, or geographic Europe (excluding Russia and Turkey).
Figure description
This is a flowchart describing the process used to select the listed medicines. The chart consists of two steps:
1. Initial Screening
This step begins with all medicines in Phase III clinical trials or pre-registration with the US Food and Drug Administration. Of these medicines, the next step includes only those with expected clinical trial end dates within three years of the analysis and drug geography including Canada, the US, and Europe. To qualify for the drug geography, a medicine must have Phase III clinical trials in Canada, the US, and/or geographic Europe (excluding Russia and Turkey).
2. Analytic Review
The analytic review step of the process is divided into two parts: one path for new medicines and the other for gene therapies.
New medicines must meet at least one of the following requirements to be included in the list:
- Demonstrates improved safety and efficacy
- Novel mechanism and/or first-in-class, with the addition of one or more of Breakthrough, Fast Track, and Priority Review designations
Gene therapies must demonstrate clinical effectiveness with an acceptable safety profile to be included in the list.
Stage 1. Initial screening
GlobalData’s Healthcare database is used to identify a list of medicines undergoing Phase III clinical trials or in pre-registration. These medicines serve as the basis for the initial screening stage.
The drug geography, defined as the geographical region or country in which the medicine is either marketed or in pipeline development, is restricted to Canada and other countries with similar regulatory and approval processes: the US and geographic Europe (excluding Russia and Turkey). Only new medicinal ingredients that have adequate data that supports increased efficacy and safety from clinical trials are considered as candidates for inclusion.
Medicines approved or sold in Canada, the US, or Europe for any other indication or in any other strength or formulation are excluded during the selection process, as are medicines whose clinical trials are inactive, suspended, withdrawn, or terminated.
The selection process groups pipeline candidates into two categories: (a) new medicines and (b) new gene therapies. As illustrated in Figure 1, the initial screening process for both groups is the same, but the analytic review stage is slightly different, as the available data for gene therapies is limited.
Stage 2: Analytic screening
Selection criteria
Following the initial screening, the second stage of the process considers a number of selection criteria to determine the final list of pipeline candidates. These criteria are detailed in Table 1.
Gene therapies are selected using a broader approach, as the clinical evidence available for this group is relatively limited. A gene therapy is retained on the list if the preliminary (or completed) results from Phase III trials suggest that there is evidence of clinical effectiveness with an acceptable safety profile.
Table 1. Selection criteria for the Meds Pipeline Monitor
| Selection criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
Novel mechanism / First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class In addition, the medicine must fall into one or more of the three following FDA designations for expedited development and review:
|
Additional descriptive information
A profile of each successful pipeline candidate is provided, including a brief outline of the indication and mechanism of action, as well as a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified. Table 2 provides a detailed description of these key attributes.
Table 2. Key attributes of new medicines selected for the Meds Pipeline Monitor
| Attribute | Relevance | Data sources |
|---|---|---|
| Phase III clinical trials in Canada | Medicines tested in Canada are likely to be of interest to Canadians |
GlobalData Healthcare; Health Canada Clinical Trials Database; Health Canada Drug and Health Product Submissions Under Review; National Institutes of Health (NIH) Clinical Trial Registry |
| Rare or orphan designation | Medicines used to treat rare diseases or conditions that generally have high treatment costs and may result in substantial spending | GlobalData Healthcare |
| Biologic medicine | These complex molecules produced by living organisms are expected to have high costs, resulting in substantial spending | |
| Add-on therapy | Medicines designed to be used in conjunction with existing medicines may increase the treatment cost and contribute to higher spending |
The profile also provides details of potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
The indications and therapeutic areas of the featured medicines correspond to their Phase III clinical trial or pre-registration stage. A single clinical trial may assess multiple indications within the same therapeutic area. These medicines may also have additional indications at various phases of clinical evaluation that are not mentioned in this report. The scientific description and key attributes provided are focused on the specified indication(s) for the selected medicines.
Medicines reported for a given year are reassessed for each following edition of the MPM. They may be retained on the MPM list if they continue to meet the selection criteria, or they may be removed if they have been granted market authorization through the US FDA, the EMA, or Health Canada. Medicines for which clinical trials have been discontinued or for which the selection criteria is no longer met are not reported in subsequent editions.
Spotlight on Canada
Health Canada’s Drug and Health Product Submissions Under Review (SUR) are assessed using a modified approach to the selection criteria to establish a list of medicines that may have the potential to significantly affect Canadian drug spending.
Medicines listed in the SUR include new drug submissions containing medicinal ingredients that have not been approved in Canada for any indication, in any strength or form. Unlike the selection of medicines identified in the pipeline lists, these medicines may have previously received market authorization through the US FDA or the EMA.
Selection Criteria
Following this initial screening, the medicine must demonstrate at least one of three selection criteria to qualify for inclusion in the report. These criteria are listed in Table 3.
Table 3. Selection criteria for the list of medicines currently under review by Health Canada
| Selection Criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
| Novel mechanism / First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class |
| Gene therapy: a technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene |
Additional descriptive information
The profile of each medicine under review includes the key attributes listed in Table 2, as well as a brief outline of the indication and mechanism of action, and a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified, as well as potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
Although FDA designations for expedited development or review are not a selection criteria for this list, relevant Breakthrough, Fast Track, and Priority Review designations are indicated where available. For a description of these designations, see Table 1.
Indications and therapeutic areas correspond to the information provided by GlobalData. The scientific description and key attributes provided are focused on the specified indication(s) for the selected medicine. For medicines under review for multiple indications, the primary indication is used.
Emerging COVID-19 Therapies
Vaccines and medicines under development worldwide with an indication for COVID-19 were extracted for this section of the report, based on a development stage of Phase I, II, and III clinical trials or pre-registration. All such medicines were assessed for this analysis, both new and existing. New medicines were identified as those that have not yet been marketed for any indication, while existing medicines include previously marketed therapies undergoing evaluation for new indications related to the treatment of COVID-19.
This section also highlights the COVID-19 medicines that have been approved in Canada as well as the medicines that are currently undergoing an expedited review process.
Data Sources
The GlobalData Healthcare database is the primary data source for the identification of pipeline medicines and their corresponding clinical information, including the clinical trial end date. GlobalData Healthcare tracks medicines from pre-clinical discovery, through clinical trials, to market launch and subsequent sales. The database is a comprehensive resource of medicines under various stages of clinical development. Search capabilities allow for controlled selection of specific attributes, including but not limited to the following: phase of clinical development, therapeutic area, molecule type, indication, drug geography, mechanism of action, and regulatory designations.
The Health Canada Drug and Health Product Submissions Under Review (SUR) lists are used to determine the featured selection of new medicines currently undergoing review by Health Canada. The SUR is a publicly available set of lists that identify pharmaceutical and biologic drug submissions containing new medicinal ingredients not previously approved in Canada that have been accepted for review. This applies to submissions accepted on or after April 1, 2015.
As this selection is restricted to new medicines, additional sources of information are cross-referenced to confirm that the candidates have not previously been approved or sold. These include recorded sales data from the IQVIA MIDAS® Database (all rights reserved); regulatory approval records from the National Institutes of Health (NIH), US FDA, the EMA, and Health Canada; and information in Health Canada’s Clinical Trials database and ClinicalTrials.org.
Limitations
This analysis captures a snapshot of the pipeline over a specific time period. Although it is assumed to be representative of the composition of medicines over the entire year, the pipeline is fairly dynamic and the share of medicines in any particular therapeutic area will vary.
This assessment is restricted to medicines under development for market in Canada and other countries with similar regulatory and approval processes: the US and Europe (excluding Russia and Turkey). Medicines that have not yet received market authorization in these countries were considered as potential pipeline candidates, even if they have been approved elsewhere in the world.
Some of the selected medicines may be undergoing clinical trials for additional indications; this analysis only reports on indications in the late stages of development—that is, in Phase III clinical trials or pre-registration with the US FDA—that satisfy the selection criteria set out in the methodology.
For each selected pipeline medicine, the primary manufacturer(s) and trade name, if available, are given along with the indication. In some cases, additional manufacturers, including subsidiaries, may also be involved in the development of the medicine with the primary companies, or other manufacturers may be developing the same medicine for other indications.
Although this report attempts to identify the most important pipeline medicines, the selection is not exhaustive and some medicines that are not included in this selection may have a significant impact on future clinical practice and drug spending in Canada.
Unless otherwise specified, the featured lists capture the composition of the pipeline as of September 2021 and are validated as of March 2022. Due to the unpredictability and fast-moving nature of pipeline medicines entering the market, some of the medicines listed in this edition may have been approved or marketed in Canada, the US, or Europe following this date. Pipeline medicines that have not been included in this report due to the timing of the selection may presently meet the selection criteria; these, along with the rest of the drug pipeline, will be considered for the next edition of the report.
Snapshot of the 2021 Pipeline
Pharmaceutical innovation is transforming the development and application of medical treatments worldwide. Nearly 8,500 new medicines were in clinical evaluation or pre-registration in 2021.
Figure 2 provides a snapshot of the pipeline in 2021, including the number of new medicinal ingredients in each phase of clinical evaluation. Of the 8,460 new medicines, 1,145 (13%) were in Phase III clinical trials or pre-registration.
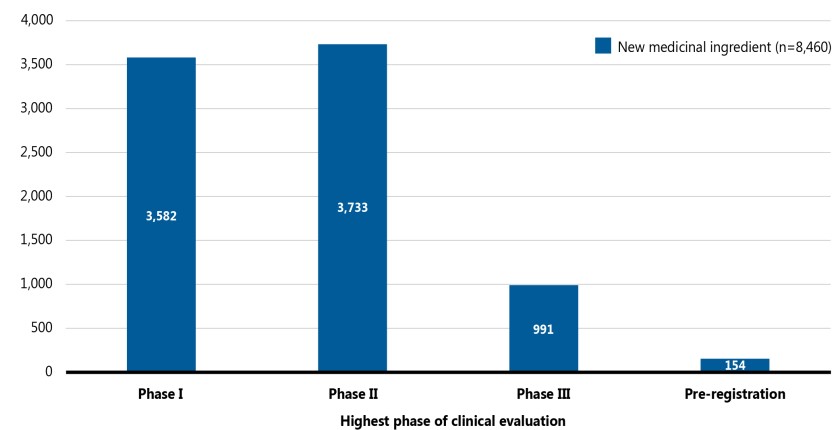
Figure description
This stacked bar graph illustrates the number of new medicinal ingredients in the pipeline by their highest phase of clinical evaluation in September 2021. There were 8,460 new medicines across the pipeline, of which 3,582 were in Phase I clinical trials, 3,733 were in Phase II, 991 were in Phase III, and 154 were in pre-registration.
Data source: GlobalData Healthcare database (accessed September 2021); IQVIA MIDAS© Database.
Figure 3 illustrates the distribution of new medicines by therapeutic area from Phase I through pre-registration. Although the findings show that pipeline medicines represented a wide range of therapeutic areas in 2021, cancer treatments dominated the therapeutic mix across the pipeline, accounting for over one third (35%) of medicines in all phases of clinical evaluation. Other important pipeline therapies include those for infectious diseases (such as COVID-19) and central nervous system therapies.
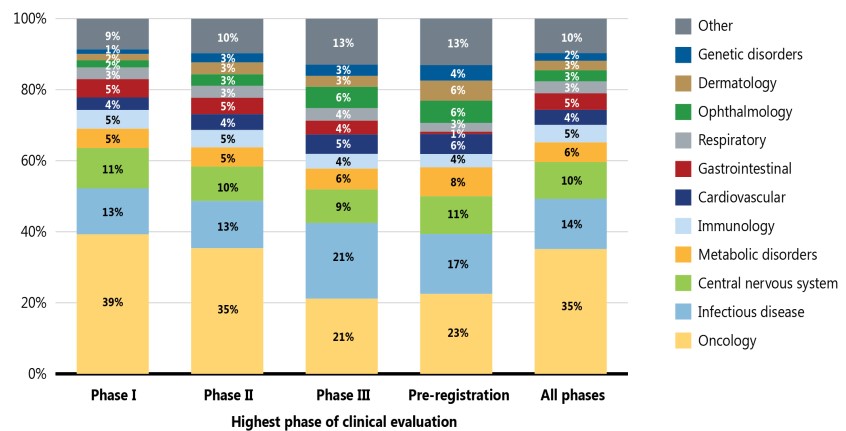
Figure description
A stacked bar graph shows the distribution of new medicines in the pipeline by their therapeutic area. The distribution is given as a percentage of all new medicines in each phase of development, as well as a total share for all phases.
| Therapeutic area | Phase I | Phase II | Phase III | Pre-registration | All phases |
|---|---|---|---|---|---|
Oncology |
39% |
35% |
21% |
23% |
35% |
Infectious Disease |
13% |
13% |
21% |
17% |
14% |
Central Nervous System |
11% |
10% |
9% |
11% |
10% |
Metabolic Disorders |
5% |
5% |
6% |
8% |
6% |
Immunology |
5% |
5% |
4% |
4% |
5% |
Cardiovascular |
4% |
4% |
5% |
6% |
4% |
Gastrointestinal |
5% |
5% |
4% |
1% |
5% |
Respiratory |
3% |
3% |
4% |
3% |
3% |
Ophthalmology |
2% |
3% |
6% |
6% |
3% |
Dermatology |
2% |
3% |
3% |
6% |
3% |
Genetic Disorders |
1% |
3% |
3% |
4% |
2% |
Other |
9% |
10% |
13% |
13% |
10% |
Data source: GlobalData Healthcare database (accessed September 2021).
Orphan medicines, as designated by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA), accounted for a notable proportion of the total medicine pipeline in 2021. Figure 4 provides the shares of orphan and other medicines in the pipeline from Phase I to pre-registration. Orphan medicines made up a greater share of medicines in the later stages of clinical evaluation, accounting for 8% of pipeline medicines in Phase I clinical trials and 30% of those in pre-registration.
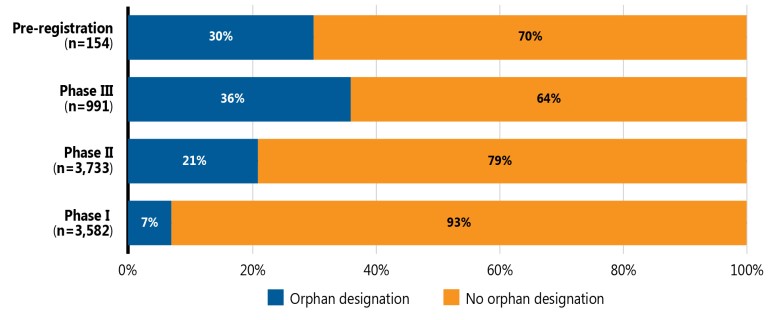
Figure description
A stacked bar graph gives the share of orphan medicines in each phase of development (Phase I, Phase II, Phase III, and pre-registration) as of September 2021. Orphan medicines were defined as pipeline medicines that have been granted an orphan designation by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
Orphan |
7% |
21% |
36% |
30% |
Non-orphan |
93% |
79% |
64% |
70% |
Total number of medicines |
3,582 |
3,733 |
991 |
154 |
Note: Includes all pipeline medicines with a highest development stage of Phase I to pre-registration that are being developed for market in Canada, the United States, or geographic Europe (excluding Russia and Turkey). Orphan medicines were defined as pipeline medicines that have been granted an orphan designation by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
Data source: GlobalData Healthcare database (accessed September 2021).
Meds Pipeline Monitor 2021
The following tables list the selection of new pipeline medicines in 2021, those retained from earlier editions of the Meds Pipeline Monitor, as well as medicines featured in previous editions that have since gained market authorization. These pipeline medicines will continue to be monitored in future editions of this report.
Applying the screening criteria described in the Methodology section, 31 of the 1,145 pipeline medicines in late stages of clinical evaluation were selected for inclusion in the 2021 new medicines list (Table 4).
Of the new medicines featured in the 2020 report, 10 were retained as recent evidence continues to support promising clinical benefit and satisfies the selection criteria (Table 5). Nine of the 2020 pipeline medicines had received market authorization in the US, Europe, or Canada as of December 2021 (Table 6), while eight were removed from the list as their clinical trials were discontinued or they no longer fulfill the selection criteria.
Table 4. Selected new medicines for 2021
Cardiovascular
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Apabetalone Resverlogix Corp.
|
Coronary artery disease (CAD) |
|
CSL-112 CSL Ltd
|
Acute coronary syndrome (ACS) |
|
Central Nervous System
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
AL-001 Alector Inc.
|
Dementia |
|
Valiltramiprosate Alzheon Inc.
|
Alzheimer's disease (AD) |
|
Ampion Ampio Pharmaceuticals Inc.
|
Pain; Osteoarthritis |
|
Lecanemab Eisai Co. Ltd
|
Alzheimer's disease (AD) |
|
ND-0612 Mitsubishi Tanabe Pharma Corp.
|
Parkinson's disease (PD) |
|
Midomafetamine [MDMA] Multidisciplinary Association for Psychedelic Studies
|
Post-traumatic stress disorder (PTSD) |
|
Gastrointestinal Disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Brazikumab AstraZeneca PLC
|
Crohn's disease |
|
Lirentelimab Allakos Inc.
|
Eosinophilic esophagitis (EoE); Gastritis; Gastroenteritis |
|
RBX-2660 Rebiotix Inc.
|
Clostridioides difficile infections (C. difficile associated disease) |
|
Genito Urinary System and Sex Hormones
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Bardoxolone methyl Reata Pharmaceuticals Inc.
|
Chronic kidney disease (chronic renal failure) caused by Alport syndrome and various forms of chronic kidney disease (CKD) |
|
Gepotidacin mesylate GlaxoSmithKline PLC
|
Cystitis; Urinary tract infections (UTI) |
|
Hematological Disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Bentracimab PhaseBio Pharmaceuticals Inc.
|
Bleeding and clotting disorders |
|
Danicopan Alexion Pharmaceuticals Inc.
|
Paroxysmal nocturnal hemoglobinuria (PNH) |
|
Hormonal Disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
ACP-014 Ascendis Pharma AS
|
Hypoparathyroidism |
|
Infectious Diseases
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Oteseconazole Mycovia Pharmaceuticals Inc.
|
Recurrent vulvovaginal candidiasis (RVVC) |
|
Ridinilazole Summit Therapeutics Ltd
|
Clostridioides difficile infections (C. difficile associated disease) |
|
V-7 Immunitor Inc.
|
Tuberculosis (TB) |
|
Men's Health
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Fexapotide triflutate Nymox Pharmaceutical Corp.
|
Benign prostatic hyperplasia (BPH) |
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Birtamimab Prothena Corp PLC
|
Primary systemic amyloidosis |
|
Donislecel CellTrans Inc.
|
Type 1 diabetes (T1D; juvenile diabetes) |
|
KSI-301 Kodiak Sciences Inc.
|
Diabetic macular edema; Diabetic retinopathy |
|
Oncology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Arfolitixorin Isofol Medical AB
|
Metastatic colorectal cancer |
|
Elacestrant A. Menarini Industrie Farmaceutiche Riunite SRL
|
Human epidermal growth factor receptor 2 negative breast cancer (HER2- breast cancer); Metastatic breast cancer |
|
SGX-301 Soligenix Inc.
|
Cutaneous T-cell lymphoma (CTCL) |
|
Motixafortide BioLineRx Ltd
|
Multiple myeloma (Kahler’s disease) |
|
Ophthalmology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Avacincaptad pegol sodium Iveric Bio Inc.
|
Geographic atrophy (GA) |
|
NCX-470 NicOx SA
|
Ocular hypertension; Open-angle glaucoma |
|
Toxicology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Avasopasem manganese Galera Therapeutics Inc.
|
Chemotherapy-induced oral mucositis |
|
Women’s Health
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Fezolinetant Astellas Pharma Inc.
|
Vasomotor symptoms of menopause (hot flashes) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2021, and are given in US dollars.
Data source: GlobalData Healthcare database.
Table 5. Pipeline medicines retained from the 2020 Meds Pipeline Monitor
Central Nervous System
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Gantenerumab Hoffmann-La Roche Ltd
|
Alzheimer’s disease |
|
Genetic Disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Pegunigalsidase alfa Chiesi Farmaceutici SpA
|
Fabry disease (FD) |
|
Hematological
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Etranacogene dezaparvovec CSL Ltd
|
Hemophilia B (factor IX deficiency) |
|
Fidanacogene elaparvovec Pfizer Inc.
|
Hemophilia B (factor IX deficiency) |
|
Fitusiran Sanofi
|
Hemophilia A; Hemophilia B |
|
Vadadustat Otsuka Holdings Co., Ltd
|
Anemia in chronic kidney disease (CKD; renal anemia) |
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Teplizumab Provention Bio Inc.
|
Type 1 diabetes (T1D; juvenile diabetes) |
|
Oncology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Ipatasertib Genentech, Inc.
|
Metastatic hormone refractory (castration-resistant, androgen-independent) prostate cancer |
|
Ofranergene obadenovec Vascular Biogenics Ltd
|
Epithelial ovarian cancer
|
|
Ublituximab TG Therapeutics, Inc.; LFB S.A.
|
Chronic lymphocytic leukemia (CLL); Relapsed chronic lymphocytic leukemia (CLL); Relapsing multiple sclerosis (RMS) |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2021, and are given in US dollars.
Data source: GlobalData Healthcare database.
Table 6. Pipeline medicines from the 2020 Meds Pipeline Monitor that have gained market authorization
Cardiovascular
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Inclisiran Novartis Pharmaceuticals Corp.
|
Atherosclerosis |
|
Central Nervous System
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Aducanumab Biogen Inc.
|
Alzheimer’s disease |
|
Immunology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Anifrolumab AstraZeneca PLC
|
Systemic lupus erythematosus |
|
Infectious Disease
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Ibrexafungerp Scynexis Inc.
|
Vulvovaginal candidiasis |
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Dasiglucagon Zealand Pharma US Inc.
|
Hypoglycemia |
|
Oncology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Idecabtagene vicleucel Celgene Inc.
|
Refractory multiple myeloma; Relapsed multiple myeloma
|
|
Lisocabtagene maraleucel Juno Therapeutics Inc.
|
Diffuse large B-cell lymphoma; Follicular lymphoma; Primary mediastinal large B-cell lymphoma |
|
Umbralisib tosylate TG Therapeutics Inc.
|
Marginal zone B-cell lymphoma (mucosa-associated lymphoid tissue or MALT-lymphoma); Follicular lymphoma (FL) |
|
Respiratory
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Tezepelumab Amgen Inc.
|
Asthma |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2021, and are given in US dollars.
Data source: GlobalData Healthcare database.
Spotlight on Canada
This section includes a list of select medicines currently under review by Health Canada that may have a significant impact on future clinical practice and drug spending. Medicines included on this list are new to Canada but may have been approved in other jurisdictions.
Table 7 highlights four new medicines currently on Health Canada’s Drug and Health Product Submissions Under Review (SUR) list that have a novel mechanism of action or have demonstrated improved safety and efficacy in clinical trials. Of the six medicines reported in the 2020 edition, five have since received market authorization from Health Canada while one application was cancelled by the sponsor.
The SUR is a publicly available source that identifies pharmaceutical and biologic drug submissions with new medicinal ingredients that have been accepted for review in Canada.
Table 7. Selected new medicines currently under review by Health Canada, 2021
Central Nervous System
| Medicine (Trade name) Company | Indication(S)* | Description and Key Attributes |
|---|---|---|
Sodium phenylbutyrate, ursodoxicoltaurine Amylyx Pharmaceuticals Inc.
|
Amyotrophic lateral sclerosis (ALS) |
|
Genetic Disorders
| Medicine (Trade name) Company | Indication(S)* | Description and Key Attributes |
|---|---|---|
Selumetinib AstraZeneca Canada Inc.
|
Neurofibromatoses type 1 |
|
Oncology
| Medicine (Trade name) Company | Indication(S)* | Description and Key Attributes |
|---|---|---|
Asciminib hydrochloride Novartis Pharmaceuticals Canada Inc.
|
Chronic myeloid leukemia (CML) |
|
Mogamulizumab Kyowa Kirin Inc.
|
Mycosis fungoides |
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2021, and are given in US dollars.
Data source: GlobalData Healthcare database.
Emerging COVID-19 Therapies
This section of the Meds Pipeline Monitor includes an overview of new and existing pipeline medicines that are under evaluation for indications related to the prevention and treatment of COVID-19. An analysis of global markets provides information on COVID-19 medicines in all phases of clinical trials and pre-registration.
Global markets
There have been significant strides in the COVID-19 drug pipeline worldwide. However, published information to confirm safety and efficacy of the various treatments for COVID-19 is continuously evolving.
In addition to the wide variety of vaccines under development, many novel and repurposed medicines are currently being evaluated in clinical trials for their potential benefits in the treatment of COVID-19. These include antivirals, monoclonal antibodies, mesenchymal stem cells, convalescent plasma, and cytokine adsorbers.202
A breakdown of COVID-19 pipeline vaccines and treatments by phase of clinical evaluation is given in Figure 5. For this snapshot, data was extracted for medicines indicated for the treatment of COVID-19 with a development stage of Phase I, II, III, or pre-registration. These medicines are presented in three categories: vaccines, which are used to prevent infection of the novel coronavirus; COVID-19 treatments (new), which are new medicines used for the prevention or reduction of some of the complications associated with COVID-19 (e.g., pneumonia or respiratory complications and hyperinflammation); and COVID-19 treatments (existing), which are previously marketed medicines that have been repurposed to treat COVID-19 or its symptoms.
Brief Insights
The pipeline for COVID-19 medicines is growing rapidly, with clinical investigations of novel and existing drugs:
- Current approaches to COVID-19 therapies generally fall into two categories: antivirals, which prevent the virus from multiplying; and immune modulators, which help the immune system to fight the virus.
- In 2020, there were 53 trials for COVID-19 categorized as pivotal/registration studies, whereas in 2021, this decreased to 41 trials. A pivotal or registration clinical trial is a trial seeking to prove the efficacy of new therapeutics and vaccines.
- As new strains of COVID-19, such as the Omicron variant, continue to raise the number of cases around the world, focus is placed on the efficacy of booster vaccinations.
- The 2020 pipeline had more ongoing and completed clinical trials while 2021 had a greater number of planned clinical trials.
- Following the approvals of the four most common vaccines used in Canada and the US—Pfizer/BioNTech’s Comirnaty, Moderna’s Spikevax, AstraZeneca’s Vaxzevria, and the Janssen vaccine—sponsors continued to focus testing on different age groups in 2021 (e.g., authorization for Pfizer/BioNTech’s Comirnaty vaccine for children aged 5 to 11 years).
- Vaccine manufactures have estimated that by the end of 2021, 12 billion COVID-19 vaccine doses will have been produced globally.
Source: Pharma COVID-19 Bulletin, GlobalData (December 30, 2021); Health Canada (December 2021).
Figure 5 illustrates the breakdown of vaccines and new or existing treatments for COVID-19 by highest development stage. The majority of treatments undergoing clinical evaluation are new medicines with an increasing proportion of existing medicines in the later stages of clinical development.
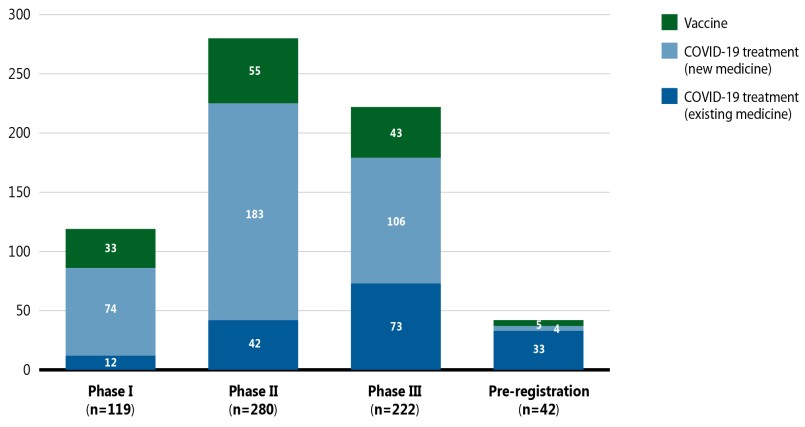
Figure description
This stacked bar graph illustrates the number of COVID-19 treatments and vaccine in the pipeline by their highest phase of clinical evaluation in September 2021. Totals are given for vaccines and COVID-19 treatments, with separate totals for new and existing medicines.
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
Vaccine |
33 |
55 |
43 |
5 |
COVID-19 treatment, new medicine |
74 |
183 |
106 |
4 |
COVID-19 treatment, existing medicine |
12 |
42 |
73 |
33 |
Total |
119 |
280 |
222 |
42 |
Figure 6 breaks down the COVID-19 vaccines by mechanism of action and highest development phase.Footnote vii Vaccines are categorized into various vaccine types based on their mechanism of action; for example, while live attenuated vaccines target the whole virus, subunit and recombinant vaccines target one specific part of the virus.
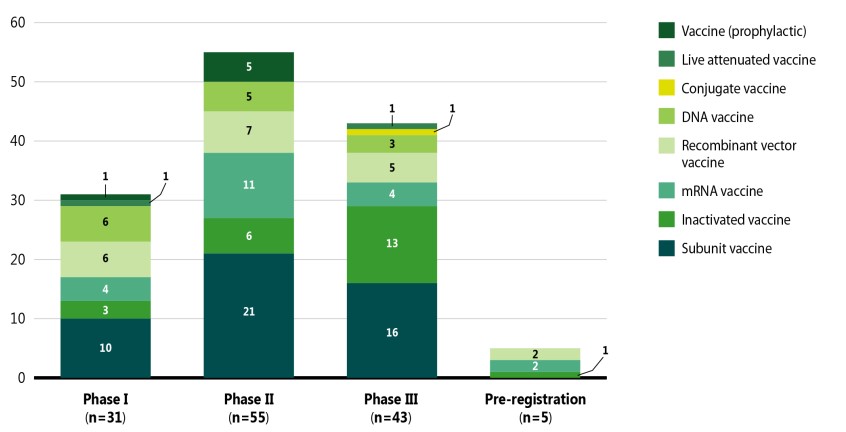
Data source: GlobalData (accessed September 2021).
Figure description
A stacked bar graph gives the distribution of COVID-19 vaccines in each phase of clinical evaluation by vaccine type, as of September 2021.
| Subunit vaccine | Inactivated vaccine | mRNA vaccine | Recombinant vector vaccine | DNA vaccine | Conjugate vaccine | Live attenuated vaccine | Vaccine (prophylactic) | Total | |
|---|---|---|---|---|---|---|---|---|---|
Phase I |
10 |
3 |
4 |
6 |
6 |
– |
1 |
1 |
31 |
Phase II |
21 |
6 |
11 |
7 |
5 |
– |
– |
5 |
55 |
Phase III |
16 |
13 |
4 |
5 |
3 |
1 |
1 |
– |
43 |
Pre-registration |
– |
1 |
2 |
2 |
– |
– |
– |
– |
5 |
Canada
As COVID-19 disease activity continues to accelerate, Canada’s health authorities have committed to the immunization response plan published in December 2020. Key elements of Canada’s immunization plan include securing sufficient supply; regulatory authorization for safety and efficacy; managing allocation and distribution of vaccines efficiently and securely; administering vaccines rapidly and equitably; and continuing to monitor vaccine safety, effectiveness and coverage. As of December 2021, the eligibility regulations for booster doses have permitted individuals 18 years and older residing in many Canadian provinces to receive a third shot.
Table 8 gives the number of medicines approved by Health Canada for the prevention and treatment of COVID-19, while Table 9 gives the number of COVID-19 medicines currently under review. As the COVID-19 pandemic remains a top priority in Canada, the current submissions undergoing Health Canada’s review process for COVID-19 are being reviewed under expedited approval timelines. This effort has been supported by the use of interim orders intended to put temporary regulations into place in order to make drugs available to address large-scale public health emergencies. For example, the Interim Order Respecting the Importation, Sale and Adversiting of Drugs for Use in Relation to COVID-19, approved on May 23, 2020, introduced an alternate pathway to facilitate clinical trials for potential COVID-19 drugs and medicinal devices, while upholding strong patient safety requirements and validity of trial data.
Table 8. COVID-19 treatment and vaccines approved by Health Canada, 2021
| Therapeutic area | Applicant | Medicinal ingredient(s) | Outcome of application | Date of decision/outcome |
|---|---|---|---|---|
Antivirals for systemic use |
Veklury |
Remdesivir (solution for injection) |
Approved under: |
27-Jul-20 |
Antivirals for systemic use |
Paxlovid |
Nirmatrelvir and ritonavir (tablets for oral administration) |
Approved under: |
17-Jan-22 |
Immune sera and immunoglobulins |
Casirivimab and imdevimab |
Casirivimab and imdevimab (solution for injection) |
Approved under: |
09-Jun-21 |
Immune sera and immunoglobulins |
Bamlanivimab |
Bamlanivimab (solution for injection) |
Approved under: |
20-Nov-20 |
Immune sera and immunoglobulins |
Sotrovimab |
Sotrovimab (solution for injection) |
Approved under: |
30-Jul-21 |
Vaccines |
Covifenz |
Virus-like particles of SARS-CoV-2 spike protein |
Approved under: |
24-Feb-22 |
Vaccines |
Nuvaxovid |
SARS-CoV-2 recombinant spike protein |
Approved under: |
17-Feb-22 |
Vaccines |
Vaxzevria |
ChAdOx1-S [recombinant] |
Approved under: |
19-Nov-21 |
| Interim order |
26-Feb-21 |
|||
Vaccines |
Comirnaty |
Tozinameran [mRNA vaccine, BNT162b2] (suspension for injection) |
Approved under: |
19-Nov-21 |
Food and Drug Regulations; booster dose | 09-Nov-21 | |||
Food and Drug Regulations; authorized with terms and conditions | 16-Sept-21 | |||
Interim order; pediatric indication (ages 12-15) | 05-May-21 | |||
Interim order | 09-Dec-20 | |||
Vaccines |
Spikevax |
Elasomeran |
Approved under: |
17-Mar-22 |
Food and Drug Regulations; | 12-Nov-21 | |||
Food and Drug Regulations; authorized with terms and conditions | 16-Sept-21 | |||
Interim order; pediatric indication (ages 12-17) | 27-Aug-21 | |||
Interim order | 23-Dec-20 | |||
Vaccines |
Janssen Inc. |
AD26.COV2.S [recombinant] |
Approved under: |
23-Nov-21 |
Interim order | 05-Mar-21 | |||
Vaccines |
Covishield |
ChAdOx1-S (recombinant) |
Approved under: |
26-Feb-21 (expired |
* The Interim Order Respecting the Importation, Sale and Adversiting of Drugs for Use in Relation to COVID-19, approved on May 23, 2020, introduced an alternate pathway to facilitate clinical trials for potential COVID-19 drugs and medicinal devices, while upholding strong patient safety requirements and validity of trial data.
Data source: Drug and vaccine authorizations for COVID-19: List of applications received, Health Canada (accessed February 2022):
https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/applications.html
Table 9. COVID-19 treatment and vaccines under review by Health Canada, 2021
| Therapeutic area | Applicant | Medicinal ingredient(s) | Date submission accepted |
|---|---|---|---|
Vaccine |
Pfizer Canada ULC/ BioNTech SE |
Tozinameran |
Feb-22 |
Vaccines - Booster dose |
AstraZeneca Canada Inc. |
ChAdOx1-S; [recombinant] |
Dec-21 |
Vaccines - Booster dose |
Janssen Inc. |
Ad26.COV2.S |
Dec-21 |
Vaccines |
Novavax Inc. |
NVX-CoV2373 |
Aug-21 |
Vaccines |
Sanofi Pasteur Ltd |
SARS-CoV-2 prefusion spike delta TM protein [recombinant] |
Jul-21 |
Vaccines |
Vaccigen Ltd |
Whole virion inactivated coronavirus |
Jul-21 |
Immune sera and immunoglobulins |
AstraZeneca Canada Inc. |
Cilgavimab, tixagevimab |
Nov-21 |
Immune sera and immunoglobulins |
Celltrion HealthCare Co. Ltd |
Regdanvimab |
May-21 |
Immune sera and immunoglobulins |
Eli Lilly Canada Inc. |
Bamlanivimab* |
Jun-21 |
Immune sera and immunoglobulins |
Hoffmann-La Roche Ltd |
Casirivimab, imdevimab* |
Sept-21 |
Immune sera and immunoglobulins |
GlaxoSmithKline Inc. |
Sotrovimab* |
Oct-21 |
Immune sera and immunoglobulins |
Eli Lilly Canada Inc. |
Etesevimab |
Sept-21 |
Immunosuppressants |
Eli Lilly Canada Inc. |
Baricitinib |
Sept-21 |
Antivirals for systemic use |
Gilead Sciences Canada Inc. |
Remdesivir |
Apr-21 |
Antivirals for systemic use |
Merck Canada Inc. |
Molnupiravir |
Aug-21 |
| Ceased Reviews | |||
Immune sera and immunoglobulins |
CytoDyn Inc. |
Leronlimab |
Mar-21 |
Antigout preparations |
Pendopharm Division of Pharmascience Inc |
Colchicine |
Jan-21 |
Other nervous system drugs |
Sanotize Research & Development Corp. |
Nitric oxide |
Jun-21 |
* The applicant has filed a new drug submission under the Food and Drug Regulations to transition this product from the interim order. The product continues to be approved for sale in Canada during this transition period.
Data source: Drug and vaccine authorizations for COVID-19: List of applications received, Health Canada (accessed February 2022): https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/applications.html
References
- Ray KK, Nicholls SJ, Buhr KA, et al.; BETonMACE Investigators and Committees. 2020. Effect of Apabetalone Added to Standard Therapy on Major Adverse Cardiovascular Events in Patients With Recent Acute Coronary Syndrome and Type 2 Diabetes: A Randomized Clinical Trial. JAMA. 323(16):1565-73. doi: 10.1001/jama.2020.3308.
- A Phase III Multi-Center, Double-Blind, Randomized, Parallel Group, Placebo-Controlled Clinical Trial in High-Risk Type 2 Diabetes Mellitus (T2DM) Subjects With Coronary Artery Disease (CAD) to Determine Whether Bromodomain Extraterminal Domain (BET) Inhibition Treatment With RVX000222 Increases the Time to Major Adverse Cardiovascular Events (MACE). ClinicalTrials.gov: NCT02586155 (completed). https://clinicaltrials.gov/ct2/show/NCT02586155?term=NCT02586155&draw=2&rank=1
- Brandts J, Ray KK. 2020. Apabetalone — BET protein inhibition in cardiovascular disease and Type 2 diabetes. Future Cardiol. 16(5):385-95. doi: 10.2217/fca-2020-0017.
- Nicholls SJ, Schwartz GG, Buhr KA, et al.; BETonMACE Investigators. 2021. Apabetalone and hospitalization for heart failure in patients following an acute coronary syndrome: a prespecified analysis of the BETonMACE study. Cardiovasc Diabetol. 20(1):13. doi: 10.1186/s12933-020-01199-x.
- An Open-Label Study to Assess the Safety and Effect on Clinical Course and Key Biomarkers of Oral Apabetalone in Hospitalized Subjects With Covid-19 Infection in Addition to Standard of Care (SOC). ClinicalTrials.gov: NCT04894266 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04894266?term=NCT04894266&draw=2&rank=1
- Gille A, Duffy D, Tortorici MA, Wright SD, Deckelbaum LI, D'Andrea DM. 2019. Moderate Renal Impairment Does Not Impact the Ability of CSL112 (Apolipoprotein A-I [Human]) to Enhance Cholesterol Efflux Capacity. J Clin Pharmacol. 59(3):427-36. doi: 10.1002/jcph.1337.
- Hatch P. 2019. Heart-stopper: CSL's $800m heart attack trial. 30 Dec 2019. The Sydney Morning Herald. Available: https://www.smh.com.au/business/companies/heart-stopper-csl-s-800m-heart-attack-trial-20191210-p53iog.html
- Hatch P. 2019. Heart-stopper: CSL's $800m heart attack trial. 30 Dec 2019. The Sydney Morning Herald. Available: https://www.smh.com.au/business/companies/heart-stopper-csl-s-800m-heart-attack-trial-20191210-p53iog.html
- A Phase 3, Multicenter, Double-blind, Randomized, Placebo-controlled, Parallel-group Study to Investigate the Efficacy and Safety of CSL112 in Subjects With Acute Coronary Syndrome. ClinicalTrials.gov: NCT03473223 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03473223?term=NCT03473223&draw=2&rank=1
- Alector, Inc. 2021. Press Release: Alector Presents 12-Month Results from the INFRONT-2 Phase 2 Open-label Clinical Study of AL001 for the Treatment of Symptomatic Frontotemporal Dementia Patients with a Progranulin Mutation. 29 July 2021. Available: https://www.globenewswire.com/en/news-release/2021/07/29/2271495/0/en/Alector-Presents-12-Month-Results-from-the-INFRONT-2-Phase-2-Open-label-Clinical-Study-of-AL001-for-the-Treatment-of-Symptomatic-Frontotemporal-Dementia-Patients-with-a-Progranulin.html
- Alector, Inc. 2021. Press Release: Alector Presents 12-Month Results from the INFRONT-2 Phase 2 Open-label Clinical Study of AL001 for the Treatment of Symptomatic Frontotemporal Dementia Patients with a Progranulin Mutation. 29 July 2021. Available: https://www.globenewswire.com/en/news-release/2021/07/29/2271495/0/en/Alector-Presents-12-Month-Results-from-the-INFRONT-2-Phase-2-Open-label-Clinical-Study-of-AL001-for-the-Treatment-of-Symptomatic-Frontotemporal-Dementia-Patients-with-a-Progranulin.html
- A Phase 3, Multicenter, Randomized, Double Blind, Placebo Controlled Study to Evaluate the Efficacy and Safety of AL001 in Individuals at Risk for or With Frontotemporal Dementia Due to Heterozygous Mutations in the Progranulin Gene. ClinicalTrials.gov: NCT04374136 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04374136?term=NCT04374136&draw=2&rank=1
- Alector, Inc. 2021. Press Release: Alector Presents 12-Month Results from the INFRONT-2 Phase 2 Open-label Clinical Study of AL001 for the Treatment of Symptomatic Frontotemporal Dementia Patients with a Progranulin Mutation. 29 July 2021. Available: https://www.globenewswire.com/en/news-release/2021/07/29/2271495/0/en/Alector-Presents-12-Month-Results-from-the-INFRONT-2-Phase-2-Open-label-Clinical-Study-of-AL001-for-the-Treatment-of-Symptomatic-Frontotemporal-Dementia-Patients-with-a-Progranulin.html
- Alzheon. 2021. Press Release: Alzheon CEO Presents Overview of Oral Anti-Amyloid ALZ-801 Phase 3 Program at 9th Neurodegenerative Drug Development Summit. 24 Feb 2021. Available: https://alzheon.com/alzheon-ceo-presents-overview-of-oral-anti-amyloid-alz-801-phase-3-program-at-9th-neurodegenerative-drug-development-summit/
- Tolar M, Abushakra S, Hey JA, Porsteinsson A, Sabbagh M. 2020. Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval. Alzheimers Res Ther. 12(1):95. doi: 10.1186/s13195-020-00663-w.
- Alzheon. Alzheon ALZ-801 potentially the first oral disease-modifying treatment for Alzheimer’s disease. Available: https://alzheon.com/pipeline/alzheon-alz-801/
- Tolar M, Abushakra S, Sabbagh M. 2020. The path forward in Alzheimer's disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 16(11):1553-60. doi: 10.1016/j.jalz.2019.09.075.
- Tolar M, Hey J, Power A, Abushakra S. 2021. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer's Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int J Mol Sci. 22(12):6355. doi: 10.3390/ijms22126355.
- A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study of the Efficacy, Safety and Biomarker Effects of ALZ-801 in Subjects With Early Alzheimer's Disease and APOE4/4 Genotype. ClinicalTrials.gov: NCT04770220 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04770220?term=NCT04770220&draw=2&rank=1
- Alzheon. 2021. Press Release: Alzheon CEO Presents Overview of Oral Anti-Amyloid ALZ-801 Phase 3 Program at 9th Neurodegenerative Drug Development Summit. Business Wire. 24 Feb 2021. Available: https://www.businesswire.com/news/home/20210224005082/en/Alzheon-CEO-Presents-Overview-of-Oral-Anti-Amyloid-ALZ-801-Phase-3-Program-at-9th-Neurodegenerative-Drug-Development-Summit
- Alzheon. Alzheon ALZ-801 potentially the first oral disease-modifying treatment for Alzheimer’s disease. Available: https://alzheon.com/pipeline/alzheon-alz-801/
- Ampio Pharmaceuticals, Inc. ABOUT AMPION. Available: https://ampiopharma.com/pipeline/ampion/
- A Phase 3 Randomized Study to Confirm the Efficacy of an Intra-Articular Injection of Ampion™ in Adults With Pain Due to Severe Osteoarthritis of the Knee. ClinicalTrials.gov: NCT03182686 (completed). https://clinicaltrials.gov/ct2/show/NCT03182686?term=NCT03182686&draw=2&rank=1
- A Randomized, Placebo-Controlled, Double-Blind Study to Evaluated the Efficacy and Safety of an Intra-Articular Injection of AMPION™ in Adults With Pain Due to Osteoarthritis of the Knee. ClinicalTrials.gov: NCT02556710 (completed). https://clinicaltrials.gov/ct2/show/NCT02556710?term=NCT02556710&draw=2&rank=1
- A Randomized, Placebo-Controlled, Double-Blind Study to Evaluate the Safety and Efficacy of Three Intra-articular Injections of Ampion™ (4 mL) Administered Two Weeks Apart in Adults With Pain Due to Osteoarthritis of the Knee. ClinicalTrials.gov: NCT02242435 (completed). https://clinicaltrials.gov/ct2/show/NCT02242435?term=NCT02242435&draw=2&rank=1
- Ampio Pharmaceuticals, Inc. 2021. Press Release: Ampio Provides Update on Osteoarthritis of the Knee (OAK) Program, Reiterates Compelling Data in Earlier Phase III Trials of Ampion in Severe OAK. BioSpace. 17 June 2021. Available: https://www.biospace.com/article/releases/ampio-provides-update-on-osteoarthritis-of-the-knee-oak-program-reiterates-compelling-data-in-earlier-phase-iii-trials-of-ampion-in-severe-oak/
- An Open Label Extension Study to Assess the Safety of Long-Term Treatment With a 4 mL Intra-Articular Injection of Ampion in Adults With Pain Due to Severe Osteoarthritis of the Knee. ClinicalTrials.gov: NCT03349645 (study terminated by Sponsor before completion due to absence of significant safety findings). https://clinicaltrials.gov/ct2/show/NCT03349645?term=NCT03349645&draw=2&rank=1
- A Randomized, Controlled, Double-Blind Study to Evaluate the Efficacy and Safety of an Intra-Articular Injection of Ampion in Adults With Pain Due to Severe Osteoarthritis of the Knee. ClinicalTrials.gov: NCT03988023 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03988023?term=NCT03988023&draw=2&rank=1
- Swanson CJ, Zhang Y, Dhadda S, et al. 2021. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 13(1):80. doi: 10.1186/s13195-021-00813-8.
- AlzForum. 2021. Lecanemab Follows Aduhelm’s Path to Accelerated Approval. AlzForum. 20 Oct 2021. Available: https://www.alzforum.org/news/research-news/lecanemab-follows-aduhelms-path-accelerated-approval.
- AHEAD 3-45 Study: A Placebo-Controlled, Double-Blind, Parallel-Treatment Arm, 216 Week Study to Evaluate Efficacy and Safety of Treatment With BAN2401 in Subjects With Preclinical Alzheimer's Disease and Elevated Amyloid (A45 Trial) and in Subjects With Early Preclinical Alzheimer's Disease and Intermediate Amyloid (A3 Trial). ClinicalTrials.gov: NCT04468659 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04468659?term=NCT04468659&draw=2&rank=1
- A Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study With an Open-Label Extension Phase to Confirm Safety and Efficacy of BAN2401 in Subjects With Early Alzheimer's Disease. ClinicalTrials.gov: NCT03887455 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03887455?term=NCT03887455&draw=2&rank=1
- Meglio M. 2021. ND0612 Subcutaneous Levodopa Delivery System Demonstrates Efficacy in Phase 2 Setting. Proceedings of International Parkinson and Movement Disorder Society (MDS) Virtual Congress 2021; 17 Sept 2021. NeurologyLive. Available: https://www.neurologylive.com/view/nd0612-subcutaneous-levodopa-delivery-system-demonstrates-efficacy-phase-2
- The Michael J. Fox Foundation. Funded Studies: Continuous Subcutaneous Administration of Levodopa/Carbidopa (ND0612) for the Treatment of Parkinson's Disease. Available: https://www.michaeljfox.org/grant/continuous-subcutaneous-administration-levodopacarbidopa-nd0612-treatment-parkinsons-disease
- A Multicenter, Randomized, Active-controlled, Double-blind, Double-dummy, Parallel Group Clinical Trial, Investigating the Efficacy, Safety, and Tolerability of Continuous Subcutaneous ND0612 Infusion in Comparison to Oral IR-LD/CD in Subjects With Parkinson's Disease Experiencing Motor Fluctuations (BouNDless). ClinicalTrials.gov: NCT04006210 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04006210?term=NCT04006210&draw=2&rank=1
- Jerome L, Feduccia AA, Wang JB, et al. 2020. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacology (Berl). 237(8):2485-97. doi: 10.1007/s00213-020-05548-2.
- Jerome L, Feduccia AA, Wang JB, et al. 2020. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacology (Berl). 237(8):2485-97. doi: 10.1007/s00213-020-05548-2.
- Richard Staines. 2020. Evidence builds for use of MDMA as PTSD therapy. Pharmaphorum. 16 June 2020. Available: https://pharmaphorum.com/news/evidence-builds-for-use-of-mdma-as-ptsd-therapy/
- Marseille E, Kahn JG, Yazar-Klosinski B, Doblin R. 2020. The cost-effectiveness of MDMA-assisted psychotherapy for the treatment of chronic, treatment-resistant PTSD. PLoS One. 15(10):e0239997. doi: 10.1371/journal.pone.0239997.
- A Randomized, Double-Blind, Placebo-Controlled, Multi-Site Phase 3 Study of the Efficacy and Safety of Manualized MDMA-Assisted Psychotherapy for the Treatment of Severe Posttraumatic Stress Disorder. ClinicalTrials.gov: NCT03537014 (completed). https://clinicaltrials.gov/ct2/show/NCT03537014?term=NCT03537014&draw=2&rank=1
- A Multi-Site Open-Label Safety Extension Study of Manualized MDMA-Assisted Psychotherapy for the Treatment of Participants With Posttraumatic Stress Disorder. ClinicalTrials.gov: NCT04714359 (enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT04714359?term=NCT04714359&draw=2&rank=1
- A Randomized, Double-Blind, Placebo-Controlled, Multi-Site Phase 3 Study of the Efficacy and Safety of Manualized MDMA-Assisted Psychotherapy for the Treatment of Posttraumatic Stress Disorder of Moderate or Greater Severity. ClinicalTrials.gov: NCT04077437 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04077437?term=NCT04077437&draw=2&rank=1
- Gottlieb ZS, Sands BE. 2021. Personalized Medicine with IL-23 Blockers: Myth or Reality? J Crohns Colitis. doi: 10.1093/ecco-jcc/jjab190. [Online ahead of print.]
- Ma C, Panaccione R, Khanna R, Feagan BG, Jairath V. 2019. IL12/23 or selective IL23 inhibition for the management of moderate-to-severe Crohn's disease? Best Pract Res Clin Gastroenterol. 38-39:101604. doi: 10.1016/j.bpg.2019.02.006.
- Wong U, Cross RK. 2019. Expert opinion on interleukin-12/23 and interleukin-23 antagonists as potential therapeutic options for the treatment of inflammatory bowel disease. Expert Opin Investig Drugs. 28(5):473-9. doi: 10.1080/13543784.2019.1597053.
- An Open-label, Long-term Extension Study of Brazikumab in Participants With Moderately to Severely Active Crohn's Disease (INTREPID OLE). ClinicalTrials.gov: NCT03961815 (enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT03961815?term=NCT03961815&draw=2&rank=1
- A 52-Week, Multicenter, Randomized, Double-blind, Placebo and Active-Controlled, Operationally Seamless Phase 2b/3, Parallel-group Study to Assess the Efficacy and Safety of Brazikumab in Participants With Moderately to Severely Active Crohn's Disease (INTREPID Lead-In). ClinicalTrials.gov: NCT03759288 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03759288?term=NCT03759288&draw=2&rank=1
- Allakos. 2019. Press Release: Allakos Announces AK002 Met All Prespecified Primary and Secondary Endpoints in Phase 2 Randomized, Double-Blind, Placebo-Controlled Study in Patients with Eosinophilic Gastritis (EG) and/or Eosinophilic Gastroenteritis (EGE). 5 Aug 2019. Available: https://investor.allakos.com/news-releases/news-release-details/allakos-announces-ak002-met-all-prespecified-primary-and
- Bruba K. 2021. Lirentelimab safe, effective in long-term gastritis, duodenitis treatment. Healio. 12 Oct 2021. Available: https://www.healio.com/news/gastroenterology/20211012/lirentelimab-safe-effective-in-longterm-gastritis-duodenitis-treatment.
- Apfed. 2020. Lirentelimab Shows Promise as a Future Therapy for EG/EGE/EoD. American Partnership for Eosinophilic Disorders. 8 Nov 2020. Available: https://apfed.org/lirentelimab-shows-promise-as-a-future-therapy-for-eg-ege-eod/
- A Phase 3, Multicenter, Open-Label, Extension Study to Evaluate the Efficacy and Safety of AK002 in Patients That Were Previously Enrolled in AK002-016 or AK002-012 Studies and Have Eosinophilic Gastritis and/or Eosinophilic Duodenitis (Formerly Referred to as Eosinophilic Gastroenteritis). ClinicalTrials.gov: NCT04620811 (enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT04620811?term=NCT04620811&draw=2&rank=1
- A Phase 2/3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Lirentelimab (AK002) in Adult and Adolescent Patients With Active Eosinophilic Esophagitis. ClinicalTrials.gov: NCT04322708 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04322708?term=NCT04322708&draw=2&rank=1
- A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of AK002 in Patients With Moderately to Severely Active Eosinophilic Duodenitis Who Have an Inadequate Response With, Lost Response to, or Were Intolerant to Standard Therapies. ClinicalTrials.gov: NCT04856891 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04856891?term=NCT04856891&draw=2&rank=1
- A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Efficacy and Safety of AK002 in Patients With Moderately to Severely Active Eosinophilic Gastritis and/or Eosinophilic Duodenitis (Formerly Referred to as Eosinophilic Gastroenteritis). ClinicalTrials.gov: NCT04322604 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04322604?term=NCT04322604&draw=2&rank=1
- A Phase 3 Prospective, Randomized, Double-blinded, Placebo-controlled Clinical Study to Evaluate the Efficacy and Safety of RBX2660 (Microbiota Suspension) for the Prevention of Clostridium Difficile Infection. ClinicalTrials.gov: NCT03244644 (completed). https://clinicaltrials.gov/ct2/show/NCT03244644?term=NCT03244644&draw=2&rank=1
- Walter K. 2021. RBX2660 Shows Promise as a C Difficile Treatment. HCPLive. 22 May 2021. Available: https://www.hcplive.com/view/rbx2660-c-difficile-treatment
- Walter K. 2021. RBX2660 Shows Promise as a C Difficile Treatment. HCPLive. 22 May 2021. Available: https://www.hcplive.com/view/rbx2660-c-difficile-treatment
- Ferring Pharmaceuticals. 2021. Ferring and Rebiotix Present Landmark Phase 3 Data Demonstrating Superior Efficacy of Investigational RBX2660 Versus Placebo to Reduce Recurrence of C. difficile Infection. Business Wire. 21 May 2021. Available: https://www.businesswire.com/news/home/20210521005335/en/Ferring-and-Rebiotix-Present-Landmark-Phase-3-Data-Demonstrating-Superior-Efficacy-of-Investigational-RBX2660-Versus-Placebo-to-Reduce-Recurrence-of-C.-difficile-Infection
- A Phase 3 Open-Label Clinical Study to Evaluate the Safety and Tolerability of Rebiotix RBX2660 (Microbiota Suspension) in Subjects With Recurrent Clostridium Difficile Infection. ClinicalTrials.gov: NCT03931941 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03931941?term=NCT03931941&draw=2&rank=1
- Nangaku M, Kanda H, Takama H, Ichikawa T, Hase H, Akizawa T. 2020. Randomized Clinical Trial on the Effect of Bardoxolone Methyl on GFR in Diabetic Kidney Disease Patients (TSUBAKI Study). Kidney Int Rep. 5(6):879-90. doi: 10.1016/j.ekir.2020.03.030.
- Chertow GM, Appel GB, Andreoli S, et al. 2021. Study Design and Baseline Characteristics of the CARDINAL Trial: A Phase 3 Study of Bardoxolone Methyl in Patients with Alport Syndrome. Am J Nephrol. 52(3):180-189. doi: 10.1159/000513777.
- Healio. 2019. Phase 3 trials: Bardoxolone methyl slowed progression of kidney disease in Alport patients. Healio. 27 Nov 2019. Available: https://www.healio.com/news/nephrology/20191127/phase-3-trials-bardoxolone-methyl-slowed-progression-of-kidney-disease-in-alport-patients
- GlaxoSmithKline. 2019. Press Release: GSK starts a phase III clinical programme for a potential first-in-class antibiotic, gepotidacin. 28 Oct 2019. Available: https://www.gsk.com/en-gb/media/press-releases/gsk-starts-a-phase-iii-clinical-programme-for-a-potential-first-in-class-antibiotic-gepotidacin/
- GlaxoSmithKline. 2019. Press Release: GSK starts a phase III clinical programme for a potential first-in-class antibiotic, gepotidacin. 28 Oct 2019. Available: https://www.gsk.com/en-gb/media/press-releases/gsk-starts-a-phase-iii-clinical-programme-for-a-potential-first-in-class-antibiotic-gepotidacin/
- GlaxoSmithKline. 2019. Press Release: GSK starts a phase III clinical programme for a potential first-in-class antibiotic, gepotidacin. 28 Oct 2019. Available: https://www.gsk.com/en-gb/media/press-releases/gsk-starts-a-phase-iii-clinical-programme-for-a-potential-first-in-class-antibiotic-gepotidacin/
- A Phase III, Randomized, Multicenter, Parallel-Group, Double-Blind, Double-Dummy Study in Adolescent and Adult Female Participants Comparing the Efficacy and Safety of Gepotidacin to Nitrofurantoin in the Treatment of Uncomplicated Urinary Tract Infection (Acute Cystitis). ClinicalTrials.gov: NCT04020341 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04020341?term=NCT04020341&draw=2&rank=1
- A Phase III, Randomized, Multicenter, Parallel-Group, Double-Blind, Double-Dummy Study in Adolescent and Adult Female Participants Comparing the Efficacy and Safety of Gepotidacin to Nitrofurantoin in the Treatment of Uncomplicated Urinary Tract Infection (Acute Cystitis). ClinicalTrials.gov: NCT04187144 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04187144?term=NCT04187144&draw=2&rank=1
- PhaseBio. 2021. Press Release: PhaseBio Announces Topline Results From Phase 2b Trial for Bentracimab. Business Wire. 3 Nov 2021. Available: https://www.businesswire.com/news/home/20211103005318/en/PhaseBio-Announces-Topline-Results-From-Phase-2b-Trial-for-Bentracimab
- Makhdoum A, Dhingra NK, Kirubaharan A, et al. 2021. Ticagrelor use and practice patterns among Canadian cardiac surgeons. J Card Surg. 36(8):2793-801. doi: 10.1111/jocs.15636.
- A Phase 3, Multicenter, Open-Label, Single-Arm Study of Bentracimab (PB2452) in Ticagrelor-Treated Patients With Uncontrolled Major or Life-Threatening Bleeding or Requiring Urgent Surgery or Invasive Procedure (REVERSE-IT Trial). ClinicalTrials.gov: NCT04286438 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04286438?term=NCT04286438&draw=2&rank=1.
- Risitano AM, Kulasekararaj AG, Lee JW, et al. 2020. Danicopan: an oral complement factor D inhibitor for paroxysmal nocturnal hemoglobinuria. Haematologica. doi: 10.3324/haematol.2020.261826. [Online ahead of print.]
- Alexion Pharamceuticals, Inc. 2019. Press Release: Achillion Reports Third Quarter 2019 Financial Results and Provides Corporate Update. 25 Sept 2019. GlobeNewswire. Available: https://www.globenewswire.com/news-release/2019/09/25/1920498/0/en/Achillion-Receives-Breakthrough-Therapy-Designation-from-FDA-for-Danicopan-for-Treatment-of-Paroxysmal-Nocturnal-Hemoglobinuria.html
- Kulesekararaj A, Risitano A, Maciejewski JP, et al. 2021. Phase 2 Study of Danicopan in Paroxysmal Nocturnal Hemoglobinuria Patients with an Inadequate Response to Eculizumab. Blood. doi: 10.1182/blood.2021011388. [Online ahead of print.]
- Kulasekararaj AG, Risitano AM, Lee JW, et al. 2020. 756 Phase 3 Study of Danicopan, an Oral Complement Factor D Inhibitor, As Add-on Therapy to a C5 Inhibitor in Patients with Paroxysmal Nocturnal Hemoglobinuria with Clinically Evident Extravascular Hemolysis [abstract]. Presented at the 62nd American Society of Hematology Annual Meeting and Exposition; 5 Dec 2020; Washington, DC. Available: https://ash.confex.com/ash/2020/webprogram/Paper134388.html
- Kulasekararaj AG, Risitano A, Lee JW, et al. 2021. Danicopan Improves Hemoglobin, Reduces Transfusion Requirements in Eculizumab-Treated Patients With PNH. 1 Sept 2021. ASH Clinical News. Available: https://www.ashclinicalnews.org/news/from-the-blood-journals/written-in-blood/danicopan-improves-hemoglobin-reduces-transfusion-requirements-eculizumab-treated-patients-pnh/
- A Phase 3 Study of Danicopan (ALXN2040) as Add-on Therapy to a C5 Inhibitor (Eculizumab or Ravulizumab) in Patients With Paroxysmal Nocturnal Hemoglobinuria Who Have Clinically Evident Extravascular Hemolysis (EVH). ClinicalTrials.gov: NCT04469465 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04469465?term=NCT04469465&draw=2&rank=1
- Khan AA, Rejnmark L, Rubin M, et al. 2021. PaTH Forward: A randomized, double-blind, placebo-controlled phase 2 trial of TransCon PTH in adult hypoparathyroidism. J Clin Endocrinol Metab. doi: 10.1210/clinem/dgab577. [Online ahead of print.]
- Acendis Pharma. TRANSCON PTH Designed to replace parathyroid hormone to physiologic levels in patients with oypoparathyroidism. Available: https://ascendispharma.com/pipeline/endocrinology/transcon-pth/
- Khan AA, Rejnmark L, Rubin M, et al. 2021. PaTH Forward: A randomized, double-blind, placebo-controlled phase 2 trial of TransCon PTH in adult hypoparathyroidism. J Clin Endocrinol Metab. doi: 10.1210/clinem/dgab577. [Online ahead of print.]
- PaTHway TRIAL: A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Trial, With an Open-Label Extension, Investigating the Safety, Tolerability and Efficacy of TransCon PTH Administered Subcutaneously Daily in Adults With Hypoparathyroidism. ClinicalTrials.gov: NCT04701203 (Phase 3, active not recruiting). https://clinicaltrials.gov/ct2/show/NCT04701203?term=NCT04701203&draw=2&rank=1
- Gawel R. 2021. Oteseconazole proves safe, effective against recurrent vulvovaginal candidiasis. 1 Oct 2021. Healio. Available: https://www.healio.com/news/womens-health-ob-gyn/20211001/oteseconazole-proves-safe-effective-against-recurrent-vulvovaginal-candidiasis
- Mycovia Pharmaceuticals, Inc. 2021. Press Release: Mycovia Pharmaceuticals Announces U.S. FDA Acceptance and Priority Review of New Drug Application for Oteseconazole for the Treatment of Recurrent Vulvovaginal Candidiasis. 28 July 2021. Drugs.com. Available: https://www.drugs.com/nda/oteseconazole_210728.html
- Carlson TJ, Endres BT, Bassères E, Gonzales-Luna AJ, Garey KW. 2019. Ridinilazole for the treatment of Clostridioides difficile infection. Expert Opin Investig Drugs. 28(4):303-10. doi: 10.1080/13543784.2019.1582640.
- Qian X, Yanagi K, Kane AV, et al. 2020. Ridinilazole, a narrow spectrum antibiotic for treatment of Clostridioides difficile infection, enhances preservation of microbiota-dependent bile acids. Am J Physiol Gastrointest Liver Physiol. 319(2):G227-G237. doi: 10.1152/ajpgi.00046.2020.
- A Randomized, Double Blind, Active Controlled Study to Evaluate the Safety and Tolerability of Ridinilazole Compared With Vancomycin and to Assess the Pharmacokinetics of Ridinilazole in Adolescent Subjects (Aged 12 to <18 Years) With Clostridioides Difficile Infection. ClinicalTrials.gov: NCT04802837 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04802837?term=NCT04802837&draw=2&rank=1
- A Phase 3, Randomized, Double-blind, Active Controlled Study to Compare the Efficacy and Safety of Ridinilazole (200 mg, Bid) for 10 Days With Vancomycin (125 mg, Qid) for 10 Days in the Treatment of Clostridium Difficile Infection (CDI). ClinicalTrials.gov: NCT03595566 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03595566?term=NCT03595566&draw=2&rank=1
- A Phase 3, Randomized, Double-blind, Active Controlled Study to Compare the Efficacy and Safety of Ridinilazole (200 mg, Bid) for 10 Days With Vancomycin (125 mg, Qid) for 10 Days in the Treatment of Clostridium Difficile Infection (CDI). ClinicalTrials.gov: NCT03595553 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03595553?term=NCT03595553&draw=2&rank=1
- Immunitor, Inc. 2020. Press Release: Immunitor unveils breakthrough immunotherapy to treat TB in as short as one month. 1 Feb 2020. TB Online. Available: https://www.tbonline.info/posts/2020/2/1/immunitor-unveils-breakthrough-immunotherapy-treat/
- Phase III and Dose Ranging Trial of Heat-killed M. Vaccae (V7). ClinicalTrials.gov: NCT01977768 (completed). https://clinicaltrials.gov/ct2/show/NCT01977768?term=NCT01977768&draw=2&rank=1
- Bourinbaiar AS, Batbold U, Efremenko Y, et al. 2019. Phase III, placebo-controlled, randomized, double-blind trial of tableted, therapeutic TB vaccine (V7) containing heat-killed M. vaccae administered daily for one month. J Clin Tuberc Other Mycobact Dis. 18:100141. doi: 10.1016/j.jctube.2019.100141.
- Shore N, Tutrone R, Roehrborn CG. 2019. Efficacy and safety of fexapotide triflutate in outpatient medical treatment of male lower urinary tract symptoms associated with benign prostatic hyperplasia. Ther Adv Urol. 11:1756287218820807. doi: 10.1177/1756287218820807.
- Nymox. Fexapotide for BPH. Available: https://nymox.com/science/fexapotide-for-bph
- Phase III Multicenter Prospective Randomized Parallel-Group Placebo-Controlled Double Blind Clinical Evaluation of NX-1207 for the Treatment of BPH NX02-0018. ClinicalTrials.gov: NCT00945490 (completed). https://clinicaltrials.gov/ct2/show/NCT00945490?term=NCT00945490&draw=2&rank=1
- Phase III Multicenter Prospective Randomized Parallel-Group Placebo-Controlled Double Blind Clinical Evaluation of NX-1207 for the Treatment of BPH NX02-0017. ClinicalTrials.gov: NCT00918983 (completed). https://clinicaltrials.gov/ct2/show/NCT00918983?term=NCT00918983&draw=2&rank=1
- Phase 3 Multicenter Prospective Open Label Clinical Safety Evaluation of Re-Injection of NX-1207 for the Treatment of BPH: Two Doses 1-7 Years Apart. ClinicalTrials.gov: NCT01438775 (completed). https://clinicaltrials.gov/ct2/show/NCT01438775?term=NCT01438775&draw=2&rank=1
- Phase 3 Multicenter Prospective Open Label Clinical Safety Evaluation of Re-Injection of NX-1207 for the Treatment of BPH: Two Doses 1-8 Years Apart. ClinicalTrials.gov: NCT01846793 (completed). https://clinicaltrials.gov/ct2/show/NCT01846793?term=NCT01846793&draw=2&rank=1
- Efficacy and Safety of a Single Transrectal Ultrasound(TRUS)-Guided Intraprostatic Injection of NX-1207 in Patients With Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia: A Phase III European Study. ClinicalTrials.gov: NCT02003742. https://clinicaltrials.gov/ct2/show/NCT02003742?term=NCT02003742&draw=2&rank=1.
- Popkova T, Hajek R, Jelinek T. 2020. Monoclonal antibodies in the treatment of AL amyloidosis: co-targetting the plasma cell clone and amyloid deposits. Br J Haematol. 189(2):228-238. doi: 10.1111/bjh.16436.
- Gertz MA, Cohen AD, Comenzo RL, et al. 2019. Results of the Phase 3 VITAL Study of NEOD001 (Birtamimab) Plus Standard of Care in Patients with Light Chain (AL) Amyloidosis Suggest Survival Benefit for Mayo Stage IV Patients. Blood. 134(Supplement 1):3166. doi.org/10.1182/blood-2019-124482.
- Prothena Corporation PLC. Al Amyloidosis. Available: https://www.prothena.com/pipeline/birtamimab/
- A Phase 3, Randomized, Multicenter, Double-Blind, Placebo-Controlled, Efficacy and Safety Study of Birtamimab Plus Standard of Care vs. Placebo Plus Standard of Care in Mayo Stage IV Subjects With Light Chain (AL) Amyloidosis. ClinicalTrials.gov: NCT04973137 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04973137?term=NCT04973137&draw=2&rank=1
- FDA. 2021. Proceedings of the Cellular, Tissue, and Gene Therapies Advisory Committee Meeting. 15 Apr 2021. Available: https://www.fda.gov/media/147529/download
- Expanded Access to Donislecel for Treatment Use. ClinicalTrials.gov: NCT03791567. https://clinicaltrials.gov/ct2/show/NCT03791567?term=NCT03791567&draw=2&rank=1
- Chandrasekaran PR, Madanagopalan VG. 2021. KSI-301: antibody biopolymer conjugate in retinal disorders. Ther Adv Ophthalmol. 13:25158414211027708. doi:10.1177/25158414211027708.
- Guttman Krader C. 2020. KSI-301 for exudative retinal disease showing safety, efficacy, and durability. 1 Nov 2020. Modern Retina. Available: https://www.modernretina.com/view/ksi-301-for-exudative-retinal-disease-showing-safety-efficacy-and-durability
- Kodiak Sciences Inc. 2021. Press Release: Kodiak Sciences Announces 1-Year Durability, Efficacy and Safety Data from Ongoing Phase 1b Study of KSI-301 in Patients with Wet Age-Related Macular Degeneration, Diabetic Macular Edema and Retinal Vein Occlusion at the Angiogenesis, Exudation and Degeneration 2021 Annual Meeting. 13 Feb 2021. Cision PR Newswire. Available: https://www.prnewswire.com/news-releases/kodiak-sciences-announces-1-year-durability-efficacy-and-safety-data-from-ongoing-phase-1b-study-of-ksi-301-in-patients-with-wet-age-related-macular-degeneration-diabetic-macular-edema-and-retinal-vein-occlusion-at-the-angiogene-301227910.html
- A Prospective, Randomized, Double-masked, Active Comparator-controlled, Multi-center, Two-arm, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 Compared With Intravitreal Aflibercept in Participants With Visual Impairment Due to Treatment-naïve Macular Edema Secondary to Retinal Vein Occlusion (RVO). ClinicalTrials.gov: NCT04592419. https://clinicaltrials.gov/ct2/show/NCT04592419?term=NCT04592419&draw=2&rank=1
- A Prospective, Randomized, Double-masked, Active Comparator-controlled, Multi-center, Two-arm, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 Compared With Intravitreal Aflibercept in Participants With Visual Impairment Secondary to Treatment-naïve Diabetic Macular Edema (DME). ClinicalTrials.gov: NCT04603937. https://clinicaltrials.gov/ct2/show/NCT04603937?term=NCT04603937&draw=2&rank=1
- A Prospective, Randomized, Double-masked, Active Comparator-controlled, Multi-center, Two-arm, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 Compared With Intravitreal Aflibercept in Participants With Visual Impairment Secondary to Treatment-naïve Diabetic Macular Edema (DME). ClinicalTrials.gov: NCT04611152. https://clinicaltrials.gov/ct2/show/NCT04611152?term=NCT04611152&draw=2&rank=1
- A Prospective, Randomized, Double-masked, Sham-controlled, Multicenter, Two-arm, Phase 3 Study to Evaluate the Efficacy and Safety of Intravitreal KSI-301 in Participants With Moderately Severe to Severe Non-proliferative Diabetic Retinopathy (NPDR). ClinicalTrials.gov: NCT05066230. https://clinicaltrials.gov/ct2/show/NCT05066230?term=NCT05066230&draw=2&rank=1
- Tell R, Di Cara A, Odin E, et al. 2021. Folate pathway gene expression in metastatic colorectal cancer patients treated with arfolitixorin/5-FU-based chemotherapy. 22 Jan 2021. 39(3):99. J Clin Oncol. doi: 10.1200/JCO.2021.39.3_suppl.99
- Isofol Medical AB. 2021. Press Release: Isofol receives notice that Clinical Use Patent in Europe for Drug Candidate arfolitixorin will be approved. Cision PR Newswire. 22 Mar 2021. Available: https://www.prnewswire.com/news-releases/isofol-receives-notice-that-clinical-use-patent-in-europe-for-drug-candidate-arfolitixorin-will-be-approved-301252744.html
- Isofol Medical AB. 2021. Arfolitixorin- A new drug candidate for colorectal cancer. Isofol Medical AB Biotech Centre. Available: https://isofolmedical.com/arfolitixorin/
- A Study to Compare the Efficacy of Arfolitixorin Versus Leucovorin in Combination With 5 Fluorouracil, Oxaliplatin, and Bevacizumab in Patients With Advanced Colorectal Cancer (AGENT). ClinicalTrials.gov: NCT03750786. https://clinicaltrials.gov/ct2/show/NCT03750786?term=NCT03750786&draw=2&rank=1
- Virgil H. 2021. First Oral SERD Elacestrant Achieves Positive Findings in EMERALD Trial for ER+/HER2– Advanced or Metastatic Breast Cancer. 21 Oct 2021. Cancer Network. Available: https://www.cancernetwork.com/view/first-oral-serd-elacestrant-achieves-positive-findings-in-emerald-trial-for-er-her2-advanced-or-metastatic-breast-cancer
- Chen YC, Yu J, Metcalfe C, et al. 2021. Latest generation estrogen receptor degraders for the treatment of hormone receptor-positive breast cancer. Expert Opin Investig Drugs. doi: 10.1080/13543784.2021.1983542. [Online ahead of print.]
- Elacestrant Monotherapy vs. Standard of Care for the Treatment of Patients With ER+/HER2- Advanced Breast Cancer Following CDK4/6 Inhibitor Therapy: A Phase 3 Randomized, Open-label, Active-controlled, Multicenter Trial. ClinicalTrials.gov: NCT03778931 (active not recruiting). https://clinicaltrials.gov/ct2/show/NCT03778931?term=NCT03778931&draw=2&rank=1
- Stocum L. 2021. Study: SGX301 Demonstrates Efficacy for Treatment of Cutaneous T-cell Lymphoma. 6 May 2021. DermatologyTimes. Available: https://www.dermatologytimes.com/view/study-sgx301-demonstrates-efficacy-for-treatment-of-cutaneous-t-cell-lymphoma
- PCG Digital Holdings LLC. 2021. Press Release: Soligenix Poised to Announce US Commercialization Plans of SGX301 in the Treatment of Cutaneous T-Cell Lymphoma. 25 Jan 2021. BioSpace. Available: https://www.biospace.com/article/soligenix-poised-to-announce-us-commercialization-plans-of-sgx301-in-the-treatment-of-cutaneous-t-cell-lymphoma/
- PCG Digital Holdings LLC. 2021. Press Release: Soligenix Poised to Announce US Commercialization Plans of SGX301 in the Treatment of Cutaneous T-Cell Lymphoma. 25 Jan 2021. BioSpace. Available: https://www.biospace.com/article/soligenix-poised-to-announce-us-commercialization-plans-of-sgx301-in-the-treatment-of-cutaneous-t-cell-lymphoma/
- A Phase 3 Multicenter, Randomized, Double-Blind, Placebo Controlled Study to Determine the Efficacy of Topical SGX301 (Synthetic Hypericin) and Fluorescent Bulb-Light Irradiation for the Treatment of Cutaneous T-Cell Lymphoma. ClinicalTrials.gov: NCT02448381 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT02448381?term=NCT02448381&draw=2&rank=1
- Martin M, Mayer IA, Walenkamp AME, Lapa C, Andreeff M, Bobirca A. 2021. At the Bedside: Profiling and treating patients with CXCR4-expressing cancers. J Leukoc Biol. 109(5):953-67. doi: 10.1002/JLB.5BT1219-714R.
- Sternberg A. 2021. Motixafortide Reaches Primary End Point of Improved Stem Cell Mobilization in Multiple Myeloma Trial. 5 May 2021. CancerNetwork. Available: https://www.cancernetwork.com/view/motixafortide-reaches-primary-end-point-of-improved-stem-cell-mobilization-in-multiple-myeloma-trial
- BioLineRx Ltd. 2021. Press Release: BioLineRx Announces Positive Results from Pharmacoeconomic Study Positioning Motixafortide as Potential Standard of Care in Stem Cell Mobilization. 13 Oct 2021. Cision PR Newswire. Available: https://www.prnewswire.com/news-releases/biolinerx-announces-positive-results-from-pharmacoeconomic-study-positioning-motixafortide-as-potential-standard-of-care-in-stem-cell-mobilization-301399211.html
- Jaffe GJ, Westby K, Csaky KG, et al. 2021. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology. 128(4):576-86. doi: 10.1016/j.ophtha.2020.08.027.
- Linnehan R. 2020. Avacincaptad pegol reduces geographic atrophy growth rate at 18 months. 17 Nov 2020. Healio. Available: https://www.healio.com/news/ophthalmology/20201117/avacincaptad-pegol-reduces-geographic-atrophy-growth-rate-at-18-months
- A Phase 2/3 Randomized, Double-Masked, Controlled Trial to Assess the Safety and Efficacy of Intravitreous Administration of Zimura™ (Anti-C5 Aptamer) in Subjects With Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration. ClinicalTrials.gov: NCT02686658 (completed). https://clinicaltrials.gov/ct2/show/NCT02686658?term=NCT02686658&draw=2&rank=1.
- A Phase 3 Multicenter, Randomized, Double Masked, Sham- Controlled Clinical Trial to Assess the Safety and Efficacy of Intravitreal Administration of Zimura (Complement C5 Inhibitor) in Patients With Geographic Atrophy Secondary to Age-Related Macular Degeneration. ClinicalTrials.gov: NCT04435366 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04435366?term=NCT04435366&draw=2&rank=1.
- Nicox. 2021. Press Release: Nicox’s NCX 470 Demonstrates Significant Intraocular Pressure Lowering in Dolomites Phase 2 Glaucoma Trial. 1 July 2021. GlobeNewswire. Available: https://www.globenewswire.com/en/news-release/2021/07/01/2256215/0/en/Nicox-s-NCX-470-Demonstrates-Significant-Intraocular-Pressure-Lowering-in-Dolomites-Phase-2-Glaucoma-Trial.html
- Edison. 2021. NCX-4251 setback sharpens focus on NCX-470. 24 Sept 2021. Edison Investment Research Ltd. Available: https://www.edisongroup.com/publication/ncx-4251-setback-sharpens-focus-on-ncx-470/29966
- A Phase 3, Randomized, Multi-Regional, Double-Masked, Parallel-Group Trial Evaluating the Safety and Efficacy of NCX 470 0.1% vs. Latanoprost 0.005% in Subjects With Open-Angle Glaucoma or Ocular Hypertension (Denali). ClinicalTrials.gov: NCT04630808 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04630808?term=NCT04630808&draw=2&rank=1
- Phase 3, Randomized, Adaptive Dose-Selection, Multi-regional, Double-Masked, Parallel-Group, 3-Month Trial Evaluating the Safety and Efficacy of NCX 470 vs. Latanoprost 0.005% in Subjects With Open-Angle Glaucoma or Ocular Hypertension (Mont Blanc). ClinicalTrials.gov: NCT04445519 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04445519?term=NCT04445519&draw=2&rank=1
- Sonis ST. 2021. Superoxide Dismutase as an Intervention for Radiation Therapy-Associated Toxicities: Review and Profile of Avasopasem Manganese as a Treatment Option for Radiation-Induced Mucositis. Drug Des Devel Ther. 15:1021-9. doi: 10.2147/DDDT.S267400.
- UTSouthwestern Medical Center. 2021. Press Release: Experimental drug makes radiation therapy more effective, less damaging. 20 May 2021. Available: https://www.utsouthwestern.edu/newsroom/articles/year-2021/avasopasem-manganese.html
- ROMAN: Reduction in Oral Mucositis With Avasopasem Manganese (GC4419) - Phase 3 Trial in Patients Receiving Chemoradiotherapy for Locally-Advanced, Non-Metastatic Head and Neck Cancer. ClinicalTrials.gov: NCT03689712 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03689712?term=NCT03689712&draw=2&rank=1
- Santoro N, Waldbaum A, Lederman S, et al. 2020. Effect of the neurokinin 3 receptor antagonist fezolinetant on patient-reported outcomes in postmenopausal women with vasomotor symptoms: results of a randomized, placebo-controlled, double-blind, dose-ranging study (VESTA). Menopause. 27(12):1350-6. doi: 10.1097/GME.0000000000001621.
- Depypere H, Lademacher C, Siddiqui E, Fraser GL. 2021. Fezolinetant in the treatment of vasomotor symptoms associated with menopause. Expert Opin Investig Drugs. 30(7):681-694. doi: 10.1080/13543784.2021.1893305.
- A Phase 3, Randomized, Placebo-controlled, 12-week Double-blind Study, Followed by a Non-Controlled Extension Treatment Period, to Assess the Efficacy and Safety of Fezolinetant in Women Suffering From Moderate to Severe Vasomotor Symptoms (Hot Flashes) Associated With Menopause. ClinicalTrials.gov: NCT04003142 (completed). https://clinicaltrials.gov/ct2/show/NCT04003142?term=NCT04003142&draw=2&rank=1
- A Phase 3, Randomized, Placebo-controlled, 12-week Double-blind Study, Followed by a Non-Controlled Extension Treatment Period, to Assess the Efficacy and Safety of Fezolinetant in Women Suffering From Moderate to Severe Vasomotor Symptoms (Hot Flashes) Associated With Menopause. ClinicalTrials.gov: NCT04003155 (completed). https://clinicaltrials.gov/ct2/show/NCT04003155?term=NCT04003155&draw=2&rank=1
- A Randomized, Placebo-Controlled, Double-Blind Phase 3 Clinical Study to Investigate the Long-Term Safety of Fezolinetant in Women Suffering From Vasomotor Symptoms (Hot Flashes) Associated With Menopause. ClinicalTrials.gov: NCT04003389 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04003389?term=NCT04003389&draw=2&rank=1
- A Phase 3, Randomized, Placebo-controlled, 12-week Double-blind Study, Followed by a Non-Controlled Extension Treatment Period, to Assess the Efficacy and Safety of Fezolinetant in Women in Asia Suffering From Moderate to Severe Vasomotor Symptoms (Hot Flashes) Associated With Menopause. ClinicalTrials.gov: NCT04234204 (active, not yet recruiting). https://clinicaltrials.gov/ct2/show/NCT04234204?term=NCT04234204&draw=2&rank=1
- A Single-Arm Phase 3 Clinical Study to Investigate the Long-Term Safety of Fezolinetant in Women in China Suffering From Vasomotor Symptoms (Hot Flashes) Associated With Menopause. ClinicalTrials.gov: NCT04451226 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04451226?term=NCT04451226&draw=2&rank=1
- A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study of Gantenerumab in Patients With Early (Prodromal to Mild) Alzheimer's Disease. ClinicalTrials.gov: NCT03443973 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03443973?term=gantenerumab&phase=2&draw=2&rank=1
- An Open-Label, Multicenter, Rollover Study to Evaluate the Safety and Tolerability of Long-Term Administration of Gantenerumab in Participants With Alzheimer's Disease. ClinicalTrials.gov: NCT04339413 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04339413?term=gantenerumab&phase=2&draw=2&rank=2
- A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study of Gantenerumab in Patients With Early (Prodromal to Mild) Alzheimer's Disease. ClinicalTrials.gov: NCT03444870 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03444870?term=gantenerumab&phase=2&draw=2&rank=3
- An Open-Label, Multicenter, Rollover Study to Evaluate the Safety, Tolerability, and Efficacy of Long-Term Gantenerumab Administration in Participants With Alzheimer's Disease. ClinicalTrials.gov: NCT04374253 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04374253?term=gantenerumab&phase=2&draw=2&rank=4
- Safety and Efficacy Study of Gantenerumab in Participants With Early Alzheimer's Disease (AD). ClinicalTrials.gov: NCT03443973. https://www.clinicaltrials.gov/ct2/show/NCT03443973?term=Gantenerumab&rank=2
- Efficacy and Safety Study of Gantenerumab in Participants With Early Alzheimer's Disease (AD). ClinicalTrial.gov: NCT03444870. https://www.clinicaltrials.gov/ct2/show/NCT03444870?term=Gantenerumab&rank=3
- A Study of Gantenerumab in Participants With Mild Alzheimer Disease. ClinicalTrial.gov: NCT02051608. https://www.clinicaltrials.gov/ct2/show/NCT02051608?term=Gantenerumab&rank=6
- A Study of Gantenerumab in Participants With Prodromal Alzheimer's Disease. ClinicalTrial.gov: NCT01224106. https://www.clinicaltrials.gov/ct2/show/NCT01224106?term=Gantenerumab&rank=8
- Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia. A Study of Potential Disease Modifying Treatments in Individuals at Risk for or With a Type of Early Onset Alzheimer's Disease Caused by a Genetic Mutation. Master Protocol DIAN-TU001 (DIAN-TU). ClinicalTrial.gov: NCT01760005. https://www.clinicaltrials.gov/ct2/show/NCT01760005?term=Gantenerumab&rank=12
- Fabry Disease News. 2021. PRX-102 (Pegunigalsidase Alfa). 20 Sept 2021. Fabry Disease News. Available: https://fabrydiseasenews.com/prx-102-pegunigalsidase-alfa/
- Schiffmann R, Goker-Alpan O, Holida M, et al. 2019. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. J Inherit Metab Dis. 42(3):534-44. doi: 10.1002/jimd.12080.
- Protalix BioTherapeutics, Inc. 2020. Press Release: Protalix BioTherapeutics Presents Clinical Data of Pegunigalsidase Alfa for the Treatment of Fabry Disease at the 16th Annual WORLDSymposium 2020. 10 Feb 2020. Cision PR Newswire. Available: https://www.prnewswire.com/news-releases/protalix-biotherapeutics-presents-key-clinical-data-of-pegunigalsidase-alfa-for-the-treatment-of-fabry-disease-at-the-16th-annual-worldsymposium-2020-301002142.html
- Schiffmann R, Goker-Alpan O, Holida M, et al. 2019. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. J Inherit Metab Dis. 42(3):534-44. doi: 10.1002/jimd.12080.
- UniQure Inc. 2021. Press Release: uniQure Announces Positive 52-Week Clinical Data from HOPE-B Pivotal Trial of Etranacogene Dezaparvovec Gene Therapy in Patients with Hemophilia B and Provides Regulatory Update. 22 June 2021. GlobeNewswire. Available: https://www.globenewswire.com/en/news-release/2021/06/22/2251227/0/en/uniQure-Announces-Positive-52-Week-Clinical-Data-from-HOPE-B-Pivotal-Trial-of-Etranacogene-Dezaparvovec-Gene-Therapy-in-Patients-with-Hemophilia-B-and-Provides-Regulatory-Update.html
- CSL Behring. 2021. Press Release: CSL Behring Announces Closing of Global Commercialization and License Agreement with uniQure for etranacogene dezaparvovec. 6 May 2021. Available: https://www.cslbehring.com/newsroom/2021/cslbehring-to-commercialize-amt-061
- Von Drygalski A, Giermasz A, Castaman G, et al. 2019. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3(21):3241-7. doi: 10.1182/bloodadvances.2019000811.
- Von Drygalski A, Giermasz A, Castaman G, et al. 2019. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3(21):3241-7. doi: 10.1182/bloodadvances.2019000811.
- Von Drygalski A, Giermasz A, Castaman G, et al. 2019. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 3(21):3241-7. doi: 10.1182/bloodadvances.2019000811.
- George LA, Sullivan SK, Rasko JEJ, et al. 2019. Efficacy and Safety in 15 Hemophilia B Patients Treated with the AAV Gene Therapy Vector Fidanacogene Elaparvovec and Followed for at Least 1 Year. Am Soc Hematol. 134(Supplement 1):3347. doi: 10.1182/blood-2019-124091.
- George LA, Sullivan SK, Rasko JEJ, et al. 2019. Efficacy and Safety in 15 Hemophilia B Patients Treated with the AAV Gene Therapy Vector Fidanacogene Elaparvovec and Followed for at Least 1 Year. Am Soc Hematol. 134(Supplement 1):3347. doi: 10.1182/blood-2019-124091.
- Spark Therapeutics. 2018. Press Release: Pfizer Initiates Pivotal Phase 3 Program for Investigational Hemophilia B Gene Therapy. 16 July 2018. Available:https://sparktx.com/press_releases/pfizer-initiates-pivotal-phase-3-program-for-investigational-hemophilia-b-gene-therapy/
- Talyor P. 2021. Sanofi eyes 2022 filing for haemophilia drug fitusiran, despite safety issue. 28 April 2021. Pharmaphorum. Available: https://pharmaphorum.com/news/sanofi-eyes-2022-filing-for-haemophilia-drug-fitusiran-despite-safety-issue/
- Pasi KJ, Rangarajan S, Georgiev P, et al. 2017. Targeting of Antithrombin in Hemophilia A or B with RNAi Therapy. N Engl J Med. 377(9):819-828. doi: 10.1056/NEJMoa1616569.
- Martin ER, Smith MT, Maroni BJ, Zuraw QC, deGoma EM. 2017. Clinical Trial of Vadadustat in Patients With Anemia Secondary to Stage 3 or 4 Chronic Kidney Disease. Am J Nephrol. 45(5):380-388. doi: 10.1159/000464476.
- Haase VH, Chertow GM, Block GA, et al. 2018. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. doi: 10.1093/ndt/gfy055.
- Healio. 2021. FDA accepts filing of new drug application for vadadustat. 2 June 2021. Healio. Available: https://www.healio.com/news/nephrology/20210602/fda-accepts-filing-of-new-drug-application-for-vadadustat
- Pharmaceutical Technology. 2021. FDA declines to approve Provention Bio’s teplizumab for diabetes. 7 July 2021. Pharmaceutical Technology. Available: https://www.pharmaceutical-technology.com/news/fda-declines-teplizumab-approval/
- Herold KC, Bundy BN, Long A, et al. 2019. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 381(7):603-13. doi: 10.1056/NEJMoa1902226.
- Hippensteele A. 2020. Study: Teplizumab Delays Progression to Type 1 Diabetes by 3 Years in High-risk Patients. 22 June 2020. Pharmacy Times. Available: https://www.pharmacytimes.com/news/study-teplizumab-delays-progression-to-type-1-diabetes-by-3-years-in-high-risk-patients
- Mistry S. 2021. Ipatasertib Plus Abiraterone: A New Potential Treatment for Metastatic Castration-Resistant Prostate Cancer With PTEN Loss. 26 July 2021. Cancer Therapy Advisor. Available: https://www.cancertherapyadvisor.com/home/cancer-topics/prostate-cancer/prostate-cancer-ipatasertib-abiraterone-treatment-risk-potential/
- Sweeney C, Bracarda S, Sternberg CN, et al. 2021. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 398(10295):131-142. doi: 10.1016/S0140-6736(21)00580-8.
- de Bono JS, De Giorgi U, Nava Rodrigues D, et al. 2018. Randomized Phase II Study of Akt Blockade With or Without Ipatasertib in Abiraterone-Treated Patients With Metastatic Prostate Cancer With and Without PTEN Loss. Clin Cancer Res. doi:10.1158/1078-0432.CCR-18-0981.
- Broderick JM. 2020. VB-111 Hits Milestone in Phase III Platinum-Resistant Ovarian Cancer Trial. 26 Mar 2020. OncLive. Available: https://www.onclive.com/view/vb111-hits-milestone-in-phase-iii-platinumresistant-ovarian-cancer-trial
- Arend RC, Monk BJ, Herzog TJ, et al. 2021. Utilizing an interim futility analysis of the OVAL study (VB-111-701/GOG 3018) for potential reduction of risk: A phase III, double blind, randomized controlled trial of ofranergene obadenovec (VB-111) and weekly paclitaxel in patients with platinum resistant ovarian cancer. Gynecol Oncol. 161(2):496-501. doi: 10.1016/j.ygyno.2021.02.014.
- Arend RC, Beer HM, Cohen YC, et al. 2020. Ofranergene obadenovec (VB-111) in platinum-resistant ovarian cancer; favorable response rates in a phase I/II study are associated with an immunotherapeutic effect. Gynecol Oncol. 157(3):578-84. doi: 10.1016/j.ygyno.2020.02.034.
- Arend RC, Beer HM, Cohen YC, et al. 2020. Ofranergene obadenovec (VB-111) in platinum-resistant ovarian cancer; favorable response rates in a phase I/II study are associated with an immunotherapeutic effect. Gynecol Oncol. 157(3):578-84. doi: 10.1016/j.ygyno.2020.02.034.
- TG Therapeutics. 2021. Press Release: TG Therapeutics Announces Positive Results from the ULTIMATE I & II Phase 3 Trials of Ublituximab in Multiple Sclerosis to be Presented at American Academy of Neurology 73rd Annual Meeting. 16 Apr 2021. GlobeNewswire. Available: https://www.globenewswire.com/news-release/2021/04/16/2211550/8790/en/TG-Therapeutics-Announces-Positive-Results-from-the-ULTIMATE-I-II-Phase-3-Trials-of-Ublituximab-in-Multiple-Sclerosis-to-be-Presented-at-American-Academy-of-Neurology-73rd-Annual-M.html
- Babiker HM, Glode AE, Cooke LS, Mahadevan D. 2018. Ublituximab for the treatment of CD20 positive B-cell malignancies. Expert Opin Investig Drugs. 27(4):407-12. doi:10.1080/13543784.2018.1459560.
- Pithadia DJ, Reynolds KA, Lee EB, et al. 2019. Tildrakizumab in the treatment of psoriasis: latest evidence and place in therapy. Ther Adv Chronic Dis. 10:2040622319865658. doi:10.1177/2040622319865658.
- Sharman JP, Farber CM, Mahadevan D, et al. 2017. Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial. Br J Haematol. 176(3):412-20. doi: 10.1111/bjh.14447.
- Paganoni S, Macklin EA, Hendrix S, et al. 2020. Trial of Sodium Phenylbutyrate-Taurursodiol for Amyotrophic Lateral Sclerosis. N Engl J Med. 383(10):919-30. doi: 10.1056/NEJMoa1916945.
- Paganoni S, Hendrix S, Dickson SP, et al. 2021. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve. 63(1):31-39. doi: 10.1002/mus.27091.
- Amylyx Pharamceuticals, Inc. 2021. Press Release: Amylyx Pharmaceuticals Submits New Drug Application (NDA) for AMX0035 for the Treatment of ALS. 2 Nov 2021. BusinessWire. Available: https://www.businesswire.com/news/home/20211102005320/en/Amylyx-Pharmaceuticals-Submits-New-Drug-Application-NDA-for-AMX0035-for-the-Treatment-of-ALS
- Amylyx Pharamceuticals, Inc. 2021. Press Release: Amylyx Pharmaceuticals Submits New Drug Application (NDA) for AMX0035 for the Treatment of ALS. 2 Nov 2021. BusinessWire. Available: https://www.businesswire.com/news/home/20211102005320/en/Amylyx-Pharmaceuticals-Submits-New-Drug-Application-NDA-for-AMX0035-for-the-Treatment-of-ALS
- AstraZeneca Pharmaceuticals LP. 2020. OSELUGO (selumetinib) capsules, for oral use [FDA label]. US Food and Drug Administration. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf
- Gross AM, Wolters PL, Dombi E, et al. 2020. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N Engl J Med. 382(15):1430-42. doi: 10.1056/NEJMoa1912735.
- Gross AM, Wolters PL, Dombi E, et al. 2020. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N Engl J Med. 382(15):1430-42. doi: 10.1056/NEJMoa1912735.
- Gross AM, Dombi E, Widemann BC. 2020. Current status of MEK inhibitors in the treatment of plexiform neurofibromas. Childs Nerv Syst. 36(10):2443-52. doi: 10.1007/s00381-020-04731-2.
- AstraZenenca. 2020. Press Release: Koselugo (selumetinib) approved in US for paediatric patients with neurofibromatosis type 1 plexiform neurofibromas. 13 Apr 2020. Available: https://www.astrazeneca.com/media-centre/press-releases/2020/koselugo-selumetinib-approved-in-us-for-paediatric-patients-with-neurofibromatosis-type-1-plexiform-neurofibromas.html
- Manley PW, Barys L, Cowan-Jacob SW. 2020. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk Res. 98:106458. doi: 10.1016/j.leukres.2020.106458.
- Novartis. 2021. Press Release: FDA approves Novartis Scemblix® (asciminib), with novel mechanism of action for the treatment of chronic myeloid leukemia. 29 Oct 2021. Available: https://www.novartis.com/news/media-releases/fda-approves-novartis-scemblix-asciminib-novel-mechanism-action-treatment-chronic-myeloid-leukemia.
- Réa D, Mauro MJ, Boquimpani C, et al. 2021. A Phase 3, Open-Label, Randomized Study of Asciminib, a STAMP Inhibitor, vs Bosutinib in CML After≥ 2 Prior TKIs. Blood. 138(21):2031-2041. doi: 10.1182/blood.2020009984.
- FDA Approves Asciminib, Drug With Novel Action in CML. 2 Nov 2021. https://www.medscape.com/viewarticle/962073
- Novartis. 2021. Press Release: FDA approves Novartis Scemblix® (asciminib), with novel mechanism of action for the treatment of chronic myeloid leukemia. 29 Oct 2021. Available: https://www.novartis.com/news/media-releases/fda-approves-novartis-scemblix-asciminib-novel-mechanism-action-treatment-chronic-myeloid-leukemia
- Chustecka Z. 2021. FDA Approves Asciminib, Drug With Novel Action in CML. 2 Nov 2021. Medscape. Available: https://www.medscape.com/viewarticle/962073
- FDA. 2021. FDA approves asciminib for Philadelphia chromosome-positive chronic myeloid leukemia. 29 Oct 2021. US Food and Drug Administration. Available: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-asciminib-philadelphia-chromosome-positive-chronic-myeloid-leukemia
- Kyowa Kirin, Inc. 2018. POTELIGEO® (mogamulizumab-kpkc) injection, for intravenous use [FDA label]. US Food and Drug Administration. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761051s000lbl.pdf
- Blackmon AL, Pinter-Brown L. 2020. Spotlight on Mogamulizumab-Kpkc for Use in Adults with Relapsed or Refractory Mycosis Fungoides or Sézary Syndrome: Efficacy, Safety, and Patient Selection. Drug Des Devel Ther. 14:3747-54. doi: 10.2147/DDDT.S185896.
- Moore DC, Elmes JB, Shibu PA, Larck C, Park SI. 2020. Mogamulizumab: An Anti-CC Chemokine Receptor 4 Antibody for T-Cell Lymphomas. Ann Pharmacother. 54(4):371-9. doi: 10.1177/1060028019884863.
- Samaddar A, Grover M, Nag VL. 2020. Pathophysiology and Potential Therapeutic Candidates for COVID-19: A Poorly Understood Arena. Front Pharmacol. 11:585888. doi: 10.3389/fphar.2020.585888.