Alignment between the estimated therapeutic value of medicines and their Canadian prices
Presented at CAPT 2022, October 17-18, 2022
Naghmeh Foroutan, PhD, and Jared Berger
Introduction
New medicines are introduced each year in therapeutic areas with limited or no treatment options or as treatment alternatives for patients. Increasingly, they come with high price tags, and many do not meet cost-effectiveness norms. Evidence from health technology assessment in Canada suggests that these medicines often require significant price reductions to meet established cost-effectiveness standards. This analysis provides insight into the level of alignment between the list prices in Canada and the therapeutic value of high-cost drugs and Expensive Medicines for Rare Diseases (EDRDs).
Approach
The research analyzed drugs approved by Health Canada from 2015 to 2021 with available pharmacoeconomic information published by the Canadian Agency for Drugs and Technologies in Health (CADTH). The analysis focused on (i) drugs with annual treatment costs between $30,000 and $100,000 (“high-cost”), and (ii) EDRDs, which were defined as medicines with at least one orphan designated indication through either the FDA or EMA, and with estimated treatment costs exceeding $100,000 per year for non-oncology medicines or $7,500 per 28 days for oncology medicines. Using these definitions, 55 high-cost drugs and 44 EDRDs were selected for analysis.
Health technology assessments often report on the Incremental Cost-Utility Ratio (“ICUR”), which estimates the benefits of new technologies relative to their costs. An ICUR is the incremental cost ($) for an additional unit of health benefits, expressed as a Quality-Adjusted Life Year (QALY). This helps payers and policymakers make informed decisions on how to allocate resources and prioritize spending within their healthcare systems.
This analysis provides a range of distribution, frequency and extent of medicines that would require a list price reduction to be cost-effective at various ICUR thresholds.
Results
High-cost drugs and EDRDs account for a significant share of the new drug landscape in Canada.
Drugs with a treatment cost of over $30,000 per year represent the vast majority of new medicine approvals (72% in 2020), accounting for an average of 67% from 2016 to 2020. Two-thirds of new medicines (66%) approved in 2020 had an annual treatment cost exceeding $100,000 per year.
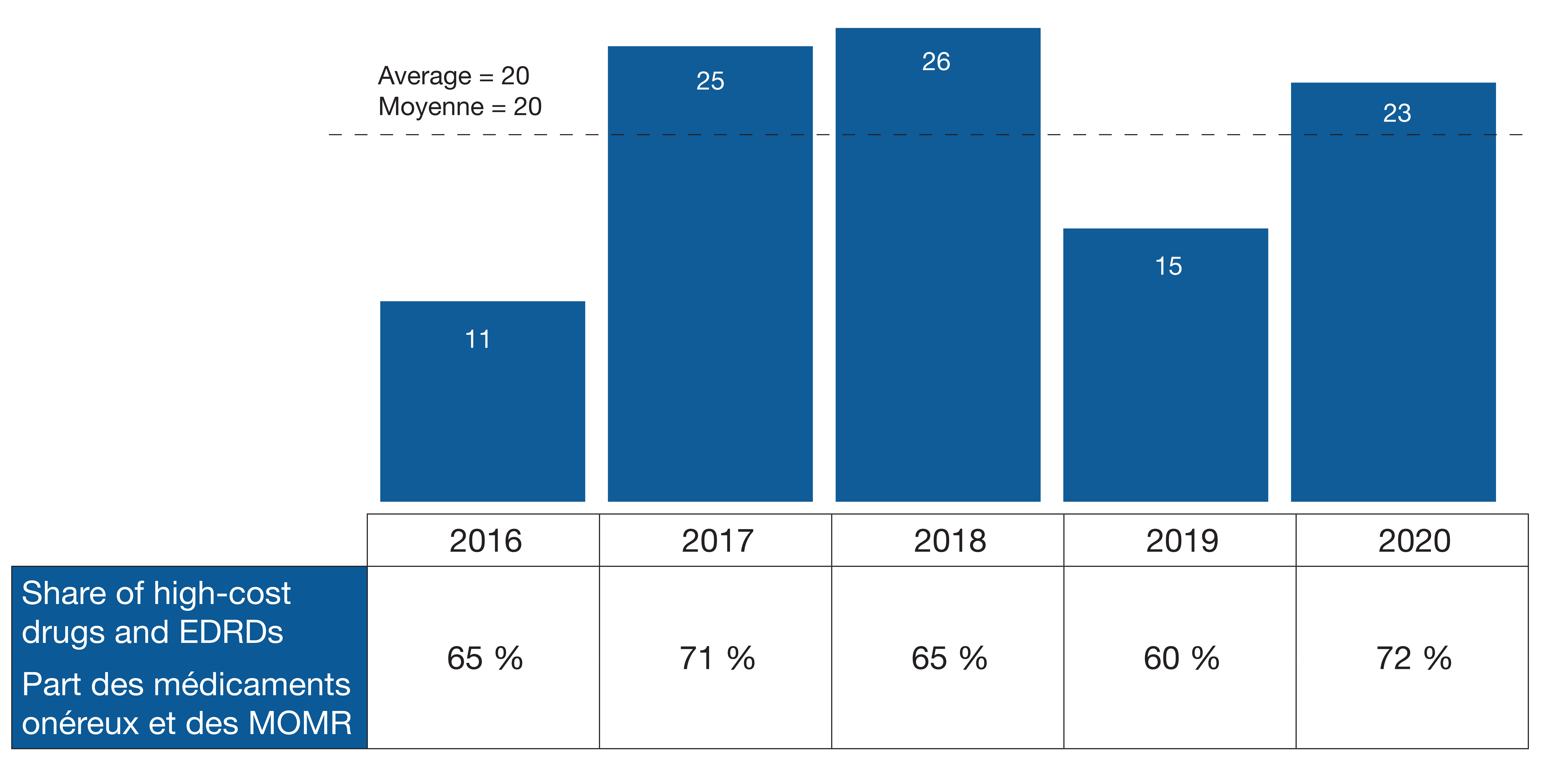
Figure description
This two-part figure is made up of a stacked bar graph and a pie chart illustrating the number and share of high-cost drugs and Expensive Drugs for Rare Diseases (EDRDs) approved in Canada between 2016 and 2020, and the share of new medicines by treatment cost in 2020.
A bar graph illustrates the number of new high-cost drugs and Expensive Drugs for Rare Diseases (EDRDs) approved in Canada in each year between 2016 and 2020, with an average number of 20.
| 2016 | 2017 | 2018 | 2019 | 2020 | Average |
|---|---|---|---|---|---|
11 |
25 |
26 |
15 |
23 |
20 |
A table under the bar graph gives the share of high-cost drugs and EDRDs as a percentage for each year between 2016 and 2020.
| Share of high-cost drugs and EDRDs | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
65% |
71% |
65% |
60% |
72% |
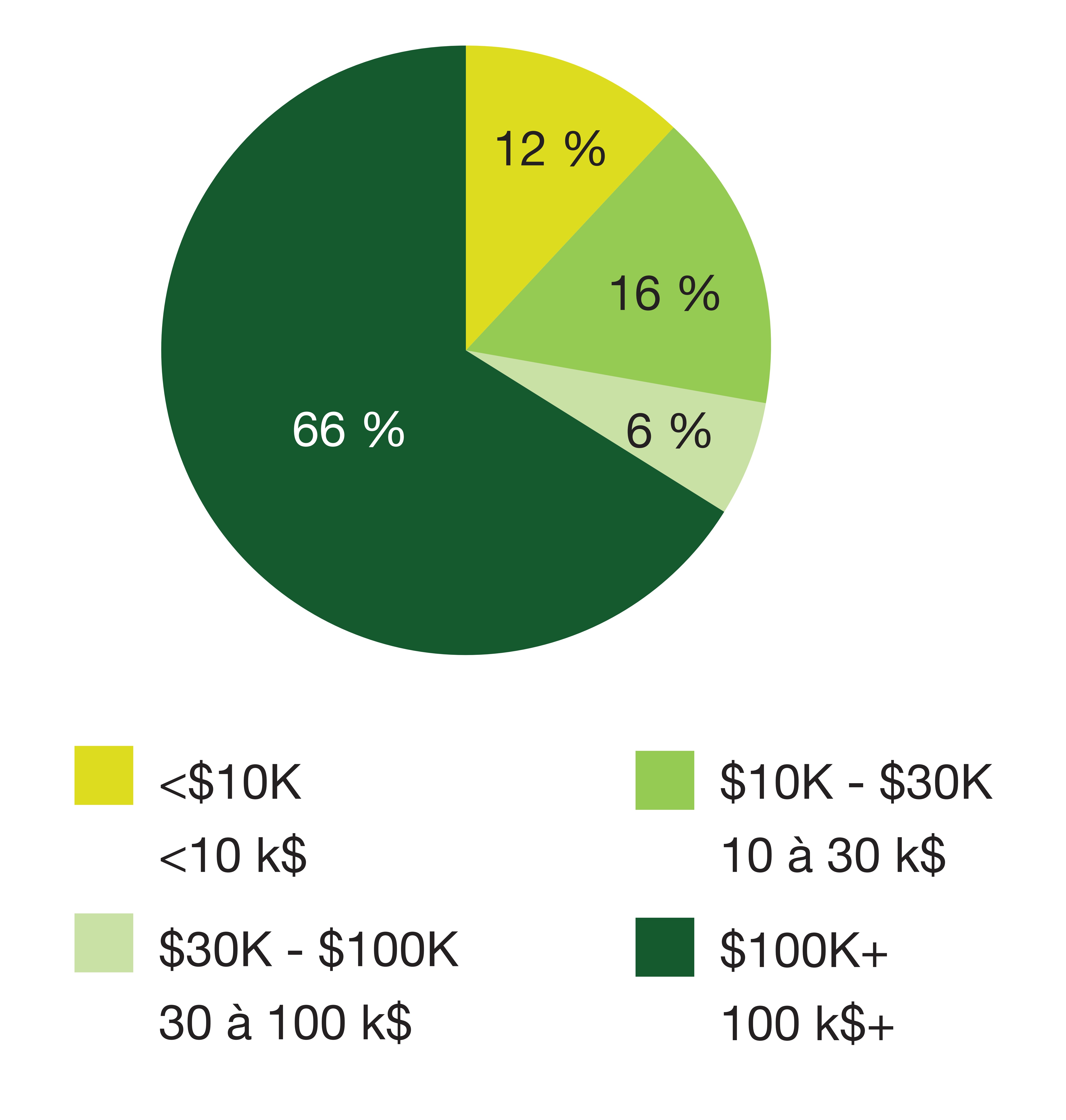
Figure description
A pie chart illustrates the share of new medicines by treatment cost for 32 new medicines in 2020. The percent share is given for four bands of treatment cost: less than $10,000; $10,000 to $30,000; $30,000 to $100,000; and $100,000 and over.
| Treatment cost | Share of New Medicines |
|---|---|
<$10K |
12% |
$10K-$30K |
16% |
$30K-$100K |
6% |
$100K+ |
66% |
The pace of EDRD approvals has increased over the last decade.
The number of EDRDs approved for use in Canada now totals 104, with sales accounting for over 12% of the Canadian pharmaceutical market in 2021. Most of the EDRDs reviewed by CADTH have received a recommendation to reimburse, largely on the condition that their cost-effectiveness is improved.
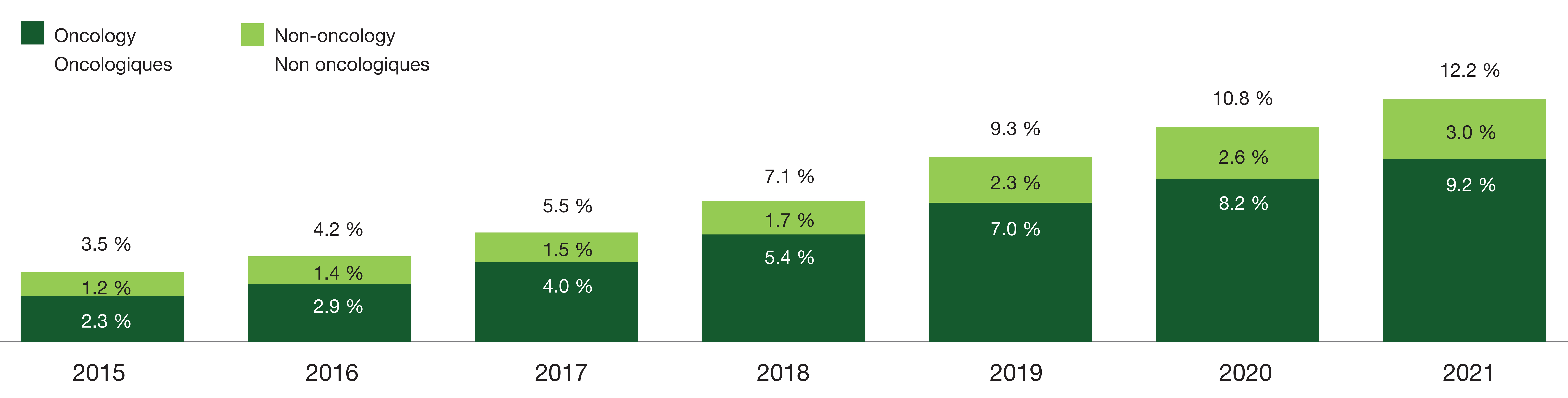
Figure description
A stacked bar graph illustrates the share of the pharmaceutical market in Canada captured by Expensive Drugs for Rare Diseases (EDRDs), for both oncology drugs and non-oncology drugs, as a percentage for each year between 2015 and 2021.
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | |
|---|---|---|---|---|---|---|---|
Oncology |
2.3% |
2.9% |
4.0% |
5.4% |
7.0% |
8.2% |
9.2% |
Non-oncology |
1.2% |
1.4% |
1.5% |
1.7% |
2.3% |
2.6% |
3.0% |
Total |
3.5% |
4.2% |
5.5% |
7.1% |
9.3% |
10.8% |
12.2% |
Data source: GlobalData (accessed September 2022).
The vast majority of high-cost drugs and EDRDs are not cost-effective at thresholds of $50K/QALY and $100K/QALY.
About three-quarters (76%) of high-cost drugs had an ICUR above $100K/QALY, with all EDRDs (100%) having an ICUR above $100K/QALY. Additionally, over half (56%) of high-cost drugs had an ICUR above $150K/QALY and a third (27%) were not cost-effective at a $200K/QALY threshold.
Most EDRDs had received a reimbursement recommendation from CADTH conditional on price reduction, and only about half were the subject of a successful price negotiation for an orphan indication with the pan-Canadian Pharmaceutical Alliance (pCPA).
While all EDRDs analyzed had an ICUR above $100K/QALY, over 80% of EDRDs had an ICUR above $200K/QALY and almost half (48%) were not cost-effective at a $400K/QALY threshold. Notably, almost a third (32%) of EDRDs had an average ICUR above $1M/QALY.

Figure description
An area graph depicts the trend in the number of Expensive Drugs for Rare Diseases (EDRDs) and the number of high-cost drugs that are above different Incremental Cost-Utility Ratio (ICUR) thresholds. The y-axis shows 0%, 50% and 100%. The trend is shown in the form of percentage labels along 7 bands, from left to right, on each curve for EDRDs and high-cost drugs.
| EDRDs | High-cost | |
|---|---|---|
Band 1 |
100% |
89% |
Band 2 |
100% |
76% |
Band 3 |
91% |
56% |
Band 4 |
84% |
27% |
Band 5 |
59% |
20% |
Band 6 |
48% |
13% |
Band 7 |
36% |
5% |
A table below the area graph gives the number of EDRDs and high-cost drugs for each ICUR threshold.
| ICUR threshold | 0 | $50K | $100K | $150K | $200K | $300K | $400K | $500K | $600K | $700K | $800K | $900K | $1M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Number of EDRDs |
44 |
44 |
44 |
40 |
37 |
26 |
21 |
16 |
15 |
14 |
14 |
14 |
14 |
Number of high-cost drugs |
55 |
49 |
42 |
31 |
15 |
11 |
7 |
3 |
3 |
3 |
2 |
2 |
2 |
Greater price reductions would be required for EDRDs to become cost-effective at various thresholds.
The average price reductions required to meet an ICUR threshold of $50K/QALY are 60% for high-cost drugs and 81% for EDRDs. Lower price reductions would be required for higher ICUR thresholds, as reported in the figure below. On average, EDRDs require a 27% greater price reduction than high-cost drugs across different ICUR thresholds ($50K-$200K).
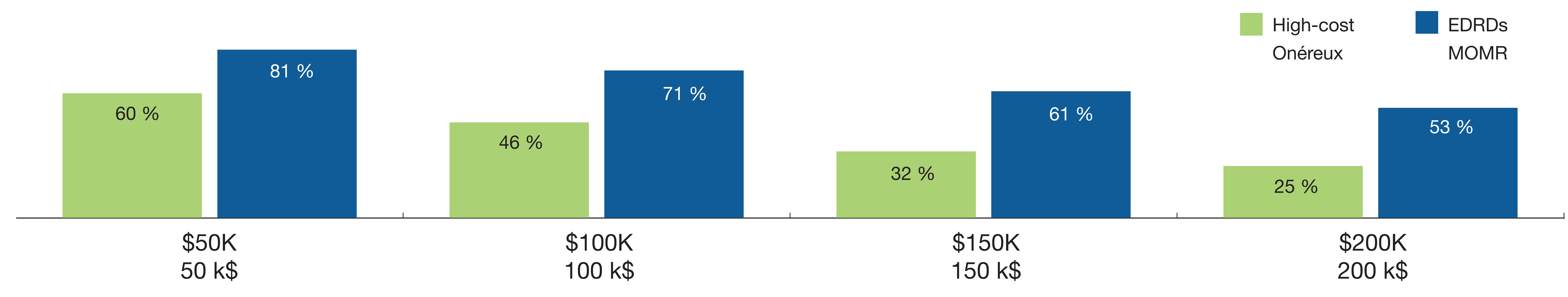
Figure description
A grouped bar graph depicts the average price reduction for two categories of drugs, high-cost drugs and EDRDs, for four Incremental Cost-Utility Ratio (ICUR) thresholds: $50K; $100K; $150K; and $200K. For each ICUR threshold, two bars are shown in different colours, representing high-cost drugs and EDRDs.
| ICUR threshold | Average price reduction (%) | |
|---|---|---|
| High-cost | EDRDs | |
$50K |
60% |
81% |
$100K |
46% |
71% |
$150K |
32% |
61% |
$200K |
25% |
53% |
EDRDs that were found by the PMPRB to bring a substantial improvement in therapeutic effects relative to other drug products sold in Canada require the greatest price reduction to meet various ICUR thresholds (avg. 81%; range: 66% –97%).
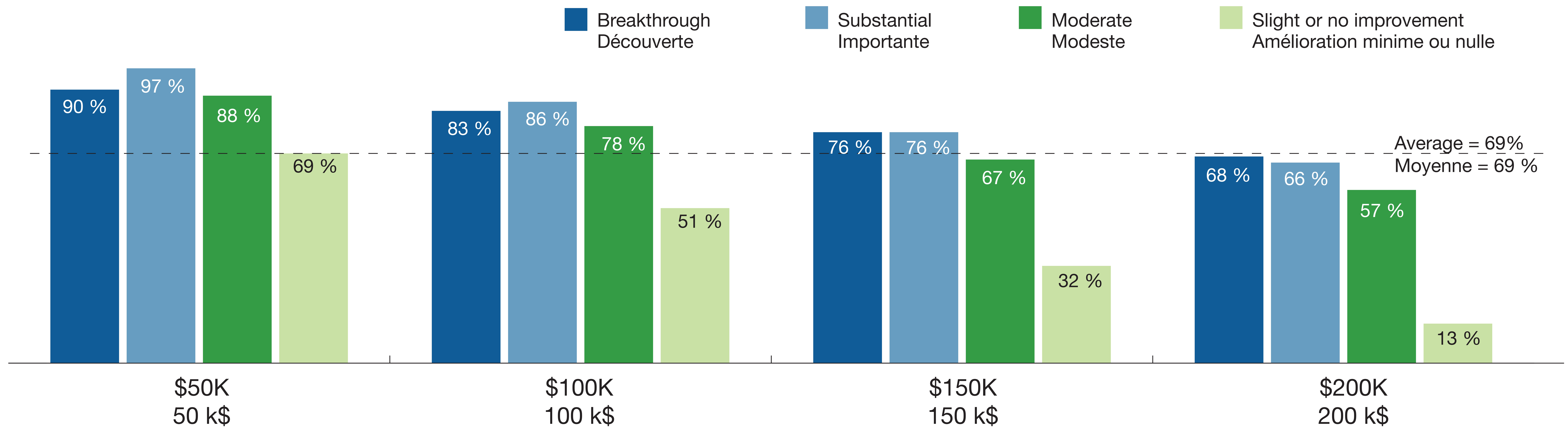
Figure description
A grouped bar graph depicts the price reduction for Expensive Drugs for Rare Diseases (EDRDs) based on four levels of therapeutic improvement: Breakthrough; Substantial; Moderate; or Slight or no improvement. The percent price reduction is given for each level of therapeutic improvement in four Incremental Cost-Utility Ratio (ICUR) thresholds: $50K; $100K; $150K; and $200K. The average is shown as 69%.
| ICUR threshold | Price reduction (%) by level of therapeutic improvement | |||
|---|---|---|---|---|
| Breakthrough | Substantial | Moderate | Slight or no improvement | |
$50K |
90% |
97% |
88% |
69% |
$100K |
83% |
86% |
78% |
51% |
$150K |
76% |
76% |
67% |
32% |
$200K |
68% |
66% |
57% |
13% |
EDRDs with a breakthrough, substantial or moderate level of therapeutic improvement tend to be associated with higher health gains. However, these drugs are also associated with substantially higher increased costs, resulting in ICURs averaging above $1M/QALY.
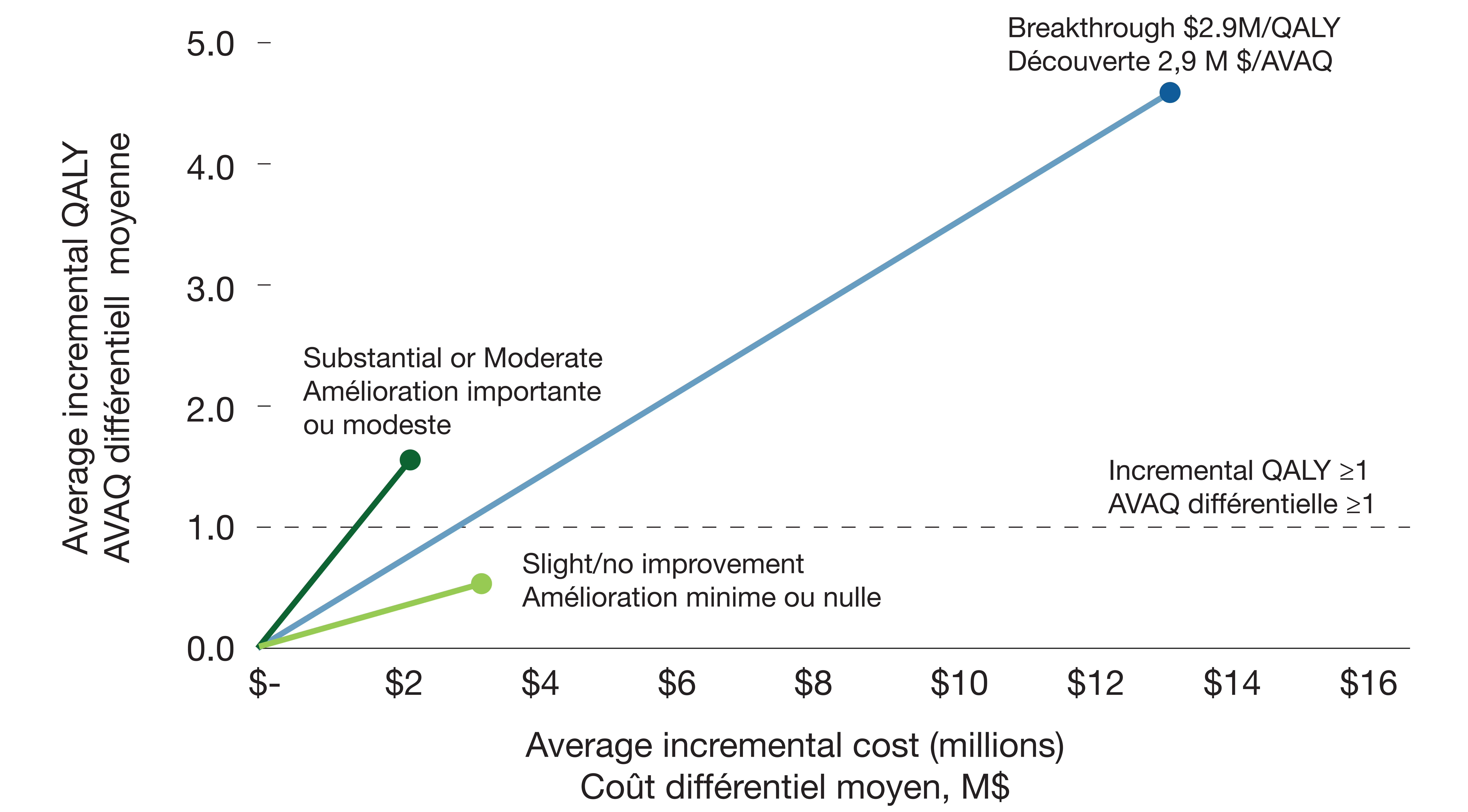
Figure description
This two-part figure is made up of a line graph and a horizontal bar chart.
A multiple line graph shows the average incremental cost (in millions) versus health benefits (expressed as an average incremental Quality-Adjusted Life Year (QALY)) for Expensive Drugs for Rare Diseases (EDRDs) based on three levels of therapeutic improvement: Breakthrough; Substantial or Moderate; Slight or no improvement. A dotted horizontal line delineates the average incremental QALY at 1.0. Above the line is the label “Incremental QALY≥1”. On the figure under Breakthrough, there is the label “$2.9M/QALY”. The y-axis (“Average incremental QALY”) has six labels: 0.0; 1.0; 2.0; 3.0; 4.0; 5.0. The x-axis (“Average incremental cost (millions)”) has nine labels: $-; $2; $4; $6; $8; $10; $12: $14; $16.
| Average incremental cost | Average incremental QALY | |
|---|---|---|
Breakthrough |
$13 million |
4.6 |
Substantial or Moderate |
$2 million |
1.6 |
Slight/no improvement |
$3 million |
0.6 |
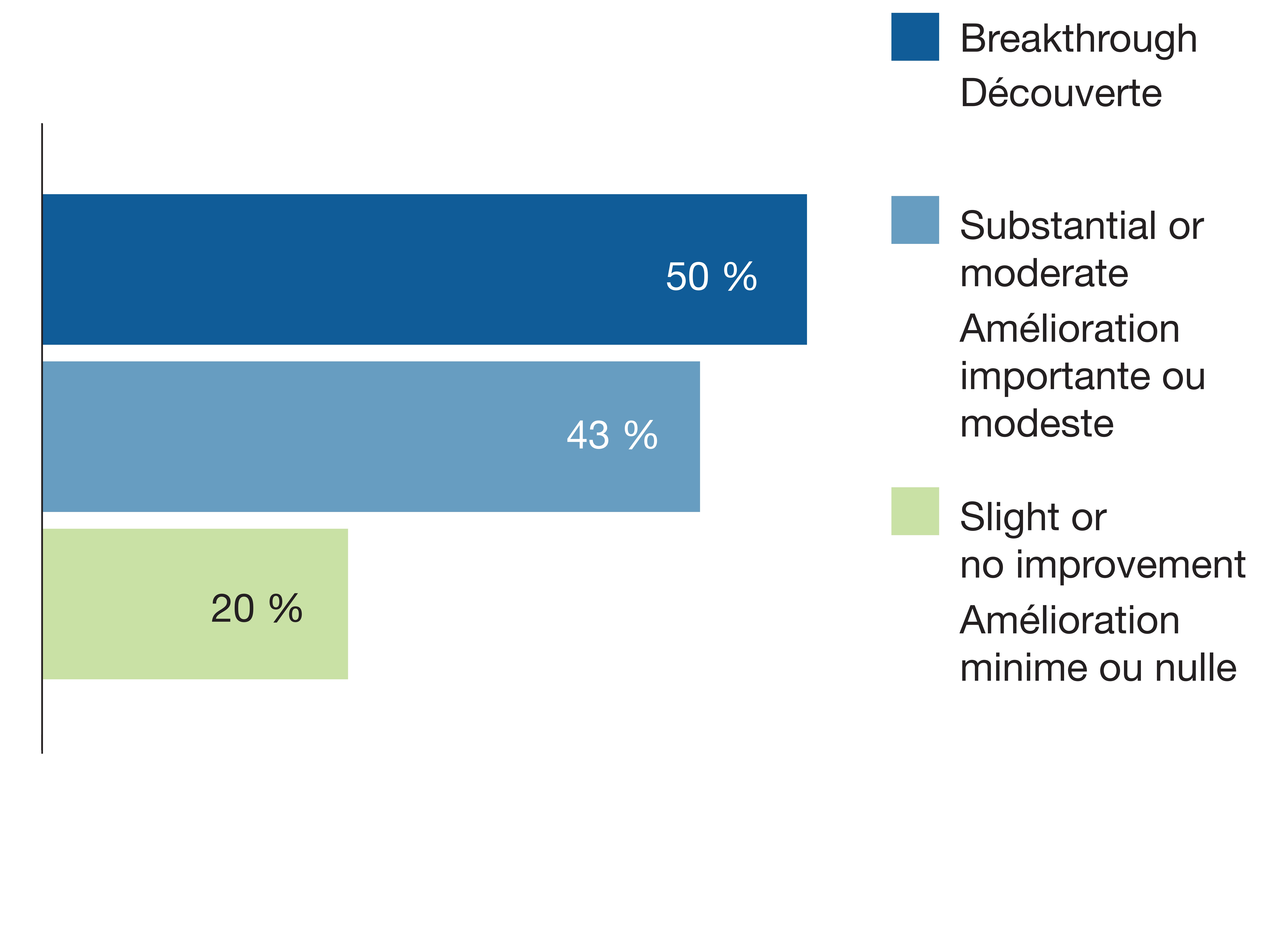
Figure description
A horizontal bar graph shows the percent share of medicines with an incremental Quality-Adjusted Life Year (QALY) over 1 in each level of therapeutic improvement: Breakthrough (50%); Substantial or moderate (43%); Slight or no improvement (20%).