The COVID-19 Pipeline: Vaccines and Treatments on the Horizon
Presented at CAPT 2022, October 17-18, 2022
Allison Carey
Introduction
The COVID-19 pandemic has prompted scientists, drugmakers and governments to move with unprecedented speed over the past 2 and a half years to develop and manufacture vaccines and therapeutics for the prevention and treatment of the novel coronavirus. Next-generation sequencing strategies have been successful in understanding the evolution of infectious diseases as well as facilitating the development of molecular diagnostics and treatments.
This poster provides a snapshot of the global COVID-19 pipeline and a breakdown of the various vaccines and treatments undergoing clinical evaluation, as well as a spotlight on Canada, showing the number of COVID-19 medicines that have been approved or are under review by Health Canada.
Approach
Using GlobalData’s drug product database, medicines indicated for COVID-19 undergoing clinical trials were extracted based on a development stage of Phase I, II, and III or pre-registration. All such medicines were assessed for this analysis, both new and repurposed. New medicines were identified as those that have not yet been marketed for any indication, while repurposed medicines include previously marketed therapies undergoing evaluation for new indications related to the treatment of COVID-19.
The spotlight on Canada section highlights the COVID-19 medicines that have been approved in Canada as well as the medicines that are currently undergoing an expedited review process with Health Canada.
Results
The global pipeline for COVID-19 medicines is growing rapidly, with clinical trials examining novel and existing drugs for the treatment and prevention of COVID-19.
As of September 2022, 550 new and repurposed vaccines and therapies were undergoing phase I, II, III clinical development or pre-registration globally for the prevention and treatment of COVID-19.
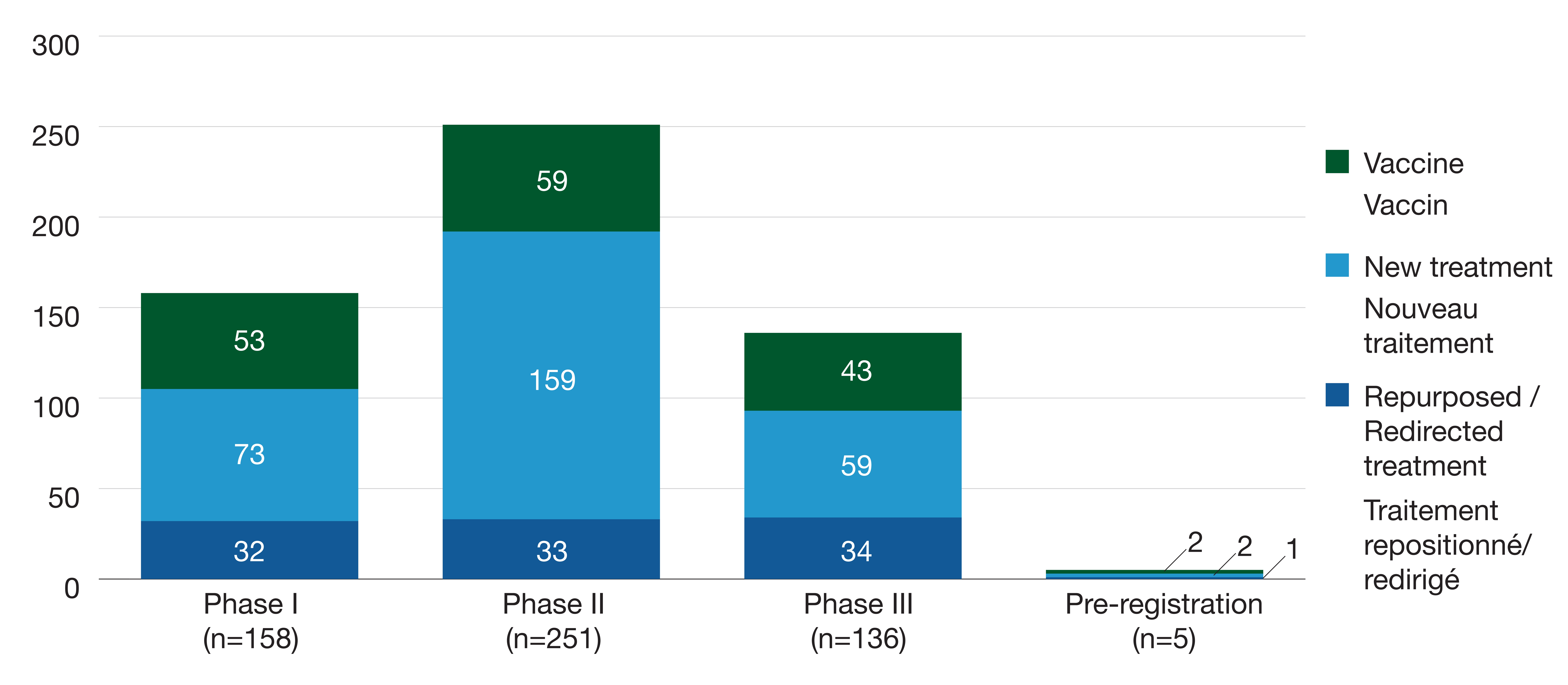
Figure description
This stacked bar graph illustrates the number of COVID-19 treatments and vaccines in the pipeline by their highest phase of clinical evaluation in September 2022. Totals are given for vaccines and COVID-19 treatments, with separate totals for new treatments and repurposed or redirected treatments.
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
Vaccine |
53 |
59 |
43 |
2 |
New treatment |
73 |
159 |
59 |
1 |
Repurposed / Redirected treatment |
32 |
33 |
34 |
2 |
Total |
158 |
251 |
136 |
5 |
The majority of vaccines in the pipeline (97%) are new medicines intended to prevent the COVID-19 infection. Antivirals are the most common therapy used to treat COVID-19 symptoms, with 74% new medicines in the pipeline. Other treatment options include monoclonal antibodies, cell therapies and synthetic peptides, that have a larger percentage of redirected and repurposed medicines in the pipeline.
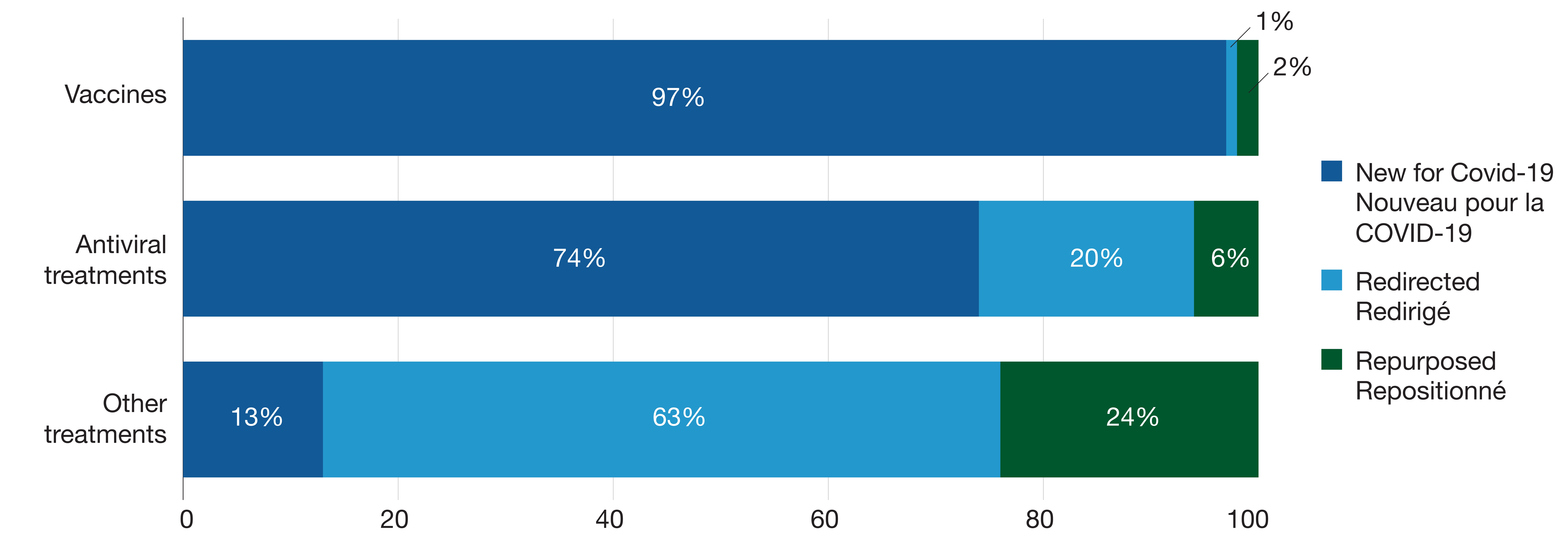
Figure description
A horizontal bar graph gives the new and repurposed treatment status for COVID-19 pipeline medicines by therapy type. The share of vaccines, antiviral treatments and other treatments that are either new for Covid-19, redirected for Covid-19 or repurposed for Covid-19 is given as a percentage.
| New for Covid-19 | Redirected | Repurposed | |
|---|---|---|---|
Vaccines |
97% |
1% |
2% |
Antiviral treatments |
74% |
20% |
6% |
Other treatments |
13% |
63% |
24% |
Data source: GlobalData (accessed September 2022).
Figure 3 displays the various COVID-19 vaccines categorized by mechanism of action and highest development phase.Footnote 1 Vaccines are categorized into different types based on their mechanism of action; for example, while live attenuated vaccines target the whole virus, protein subunit and recombinant vaccines target specific parts of the virus.
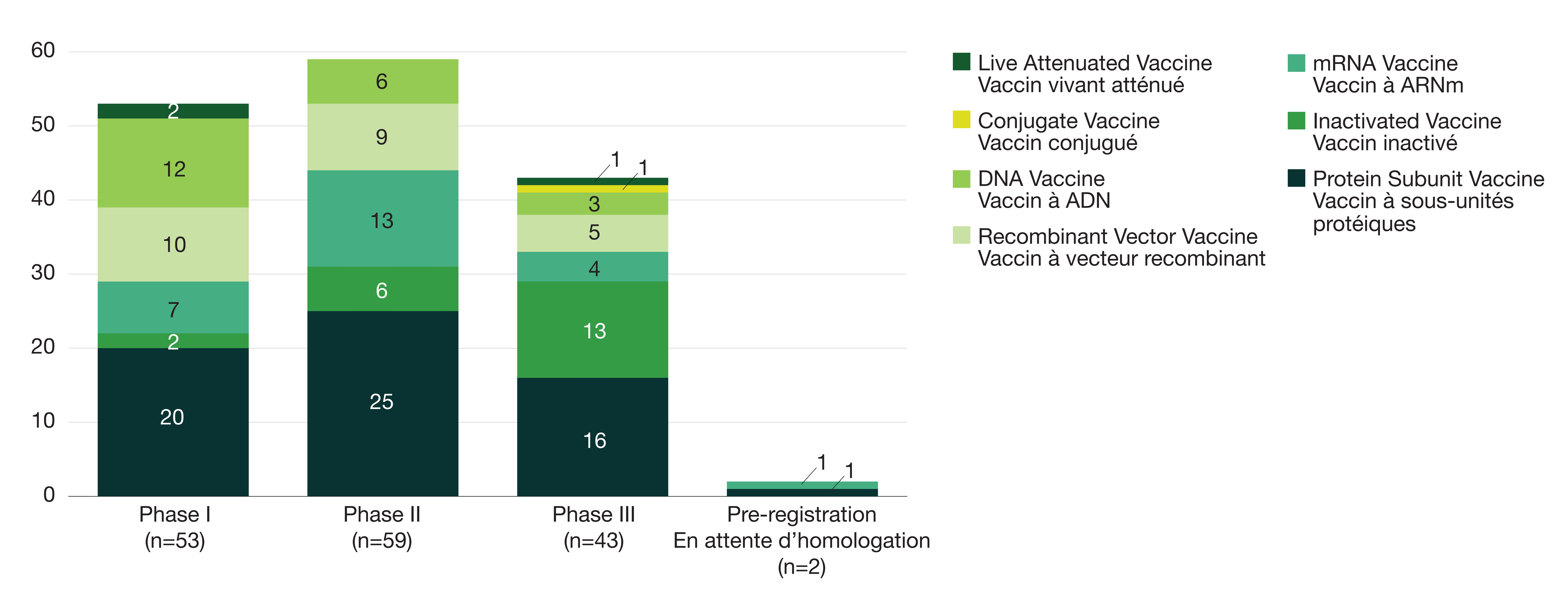
Figure description
A stacked bar graph gives the distribution of coronavirus vaccines in each phase of clinical evaluation by vaccine type, as of September 2022.
| Protein Subunit Vaccine | Inactivated Vaccine | mRNA Vaccine | Recombinant Vector Vaccine | DNA Vaccine | Conjugate Vaccine | Live Attenuated Vaccine | Total | |
|---|---|---|---|---|---|---|---|---|
Phase I |
20 |
2 |
7 |
10 |
12 |
0 |
2 |
53 |
Phase II |
25 |
6 |
13 |
9 |
6 |
0 |
0 |
59 |
Phase III |
16 |
13 |
4 |
5 |
3 |
1 |
1 |
43 |
Pre-registration |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
2 |
Treatments constitute 78% of marketed medicines indicated for COVID-19. Marketed treatments include new and repurposed medicines that are used to decrease the severity of the disease in symptomatic patients.
- As of September 2022, there were 140 marketed COVID-19 medicines, 109 treatments and 31 vaccines available globally for the prevention and treatment of COVID-19.
- As new strains of COVID-19, such as Omicron variants BA.2, BA.4, and BA.5 continue to raise the number of cases around the world, many clinical trials are being expedited to get approvals for Omicron bivalent boosters.
- Current approaches to COVID-19 treatments generally fall into two categories: antivirals (e.g. Veklury and Paxlovid, which prevent the virus from multiplying); and monoclonal antibodies (e.g. casirivimab and imdevimab, which help the immune system to fight the virus).
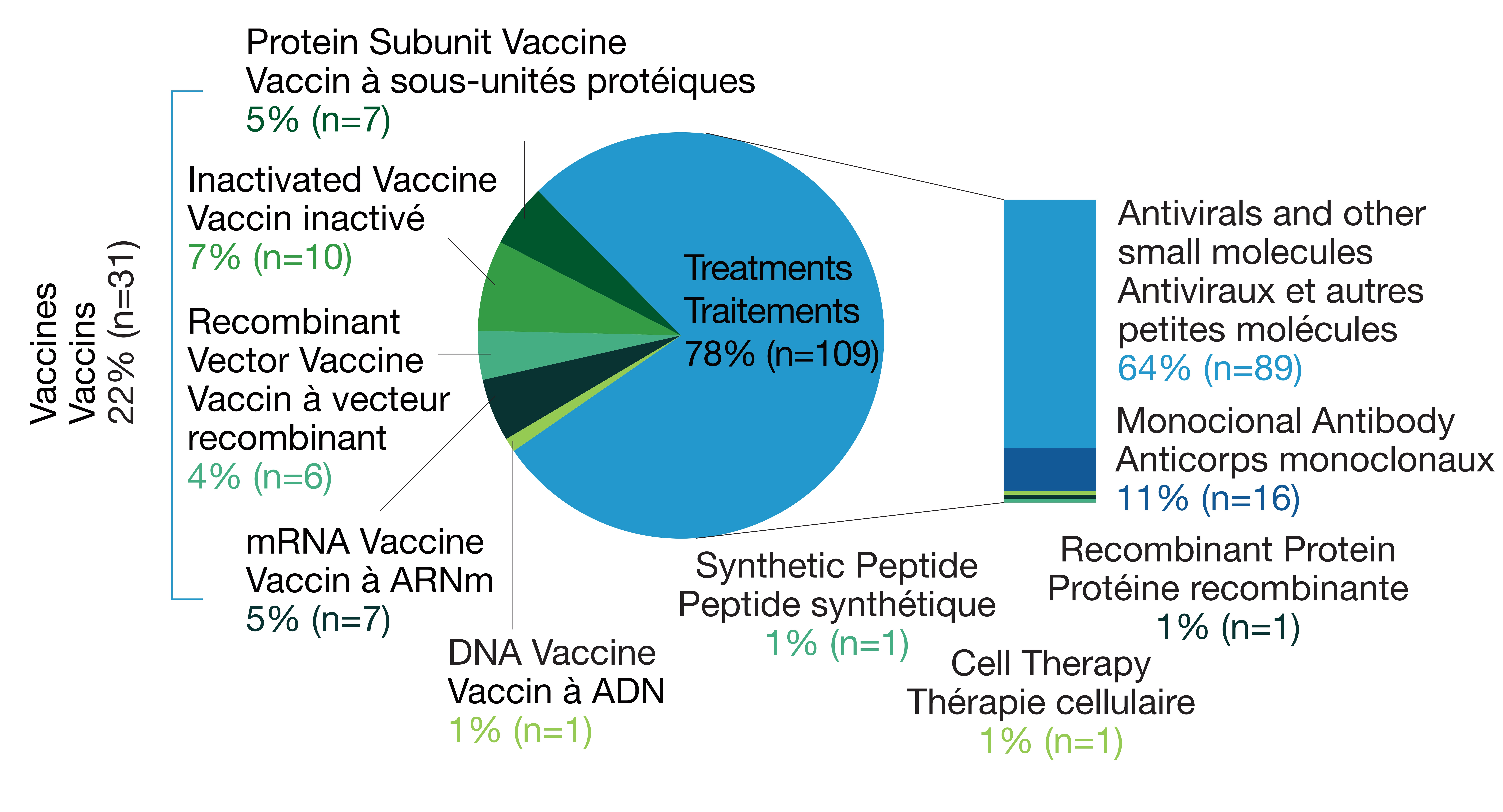
Figure description
This two-part figure examines the total marketed medicines for COVID-19. The first section of the figure is a pie chart that displays the percentage of total marketed vaccines and total marketed treatments. The total treatments make up 78% of the marketed medicines. The second part of this figure is a bar graphs showing the various treatment types for COVID-19.
| Total globally marketed vaccines and treatments for COVID-19 | ||
|---|---|---|
| Type of marketed medicine | Percentage of market | Number of medicines marketed |
DNA Vaccine |
1% |
n=1 |
mRNA Vaccine |
5% |
n=7 |
Recombinant Vector Vaccine |
4% |
n=6 |
Inactivated Vaccine |
7% |
n=10 |
Protein Subunit Vaccine |
5% |
n=7 |
Vaccines |
22% |
n=31 |
Treatments |
78% |
n=109 |
| Types of treatments for COVID-19 | ||
|---|---|---|
| Type of marketed treatment | Percentage of market | Number of medicines marketed |
Small Molecule |
64% |
n=89 |
Monoclonal Antibody |
11% |
n=16 |
Recombinant Protein |
1% |
n=2 |
Synthetic Peptide |
1% |
n=1 |
Cell Therapy |
1% |
n=1 |
Spotlight on Canada
As COVID-19 disease activity continues to accelerate, Canada’s health authorities have committed to the immunization response plan published in December 2020. Key elements of Canada’s immunization plan include securing sufficient supply; regulatory authorization for safety and efficacy; managing allocation and distribution of vaccines efficiently and securely; administering vaccines rapidly and equitably; continuing to monitor vaccine safety, effectiveness and coverage.
As the COVID-19 pandemic remains a top priority in Canada, the current submissions undergoing Health Canada’s review process for COVID-19 are being reviewed under expedited approval timelines. This effort has been supported by the use of interim orders intended to put temporary regulations into place in order to make drugs available to address large-scale public health emergencies.
As of September 2022, six vaccines and six therapies indicated for COVID-19 have been approved in Canada. In addition to the approved medicines, there are six vaccines and six therapy options currently under review by Health Canada for the prevention and treatment of COVID-19.
Table: COVID-19 treatment and vaccines approved and under review by Health Canada, 2022
| Treatments | |||
|---|---|---|---|
| Review Status | Vaccines | Antivirals for systemic use | Immune sera, immunoglobulins and immunosuppressants |
| Approved | Covifenz (Medicago’s plant-based vaccine) | Veklury (Remdesivir) | Casirivimab and/et imdevimab |
| Nuvaxovid (Novavax’s protein-subunit vaccine) | Paxlovid (Nirmatrelvir and ritonavir) | Bamlanivimab | |
| Vaxzevria (AstraZeneca’s viral vector-based vaccine) | Sotrovimab | ||
| Comirnaty (Pfizer- BioNTech’s mRNA vaccine) | Evusheld (Cilgavimab, tixagevimab) | ||
| Spikevax (Moderna’s mRNA vaccine) | |||
| Jcovden (Janssen’s viral vector-based vaccine) | |||
| Under Review | SARS-CoV-2 recombinant spike protein* (Novavax Inc. first booster and adolescent dose) | Remdesivir** | Regdanvimab |
| Whole virion inactivated corona virus (Vaccigen Ltd) | Molnupiravir | Baricitinib | |
| Elasomeran* Omicron BA.4/BA.5 (Moderna’s Bivalent booster)* | Baricitinib** | Tocilizumab** | |
| SARS-CoV-2 prefusion spike delta TM protein recombinant (Sanofi Pasteur Limited) | |||
| Tozinameran* (Pfizer-BioNTech Bivalent booster) | |||
| Omicron BA.4/BA.5, tozinameran (Pfizer-BioNTech) | |||
* The applicant has filed a new drug submission under the Food and Drug Regulations to transition this product from the interim order. The product continues to be approved for sale in Canada during this transition period.
The Interim Order Respecting the Importation, Sale and Adversiting of Drugs for Use in Relation to COVID-19, approved on May 23, 2020, introduced an alternate pathway to facilitate clinical trials for potential COVID-19 drugs and medicinal devices, while upholding strong patient safety requirements and vaility of trial data.
** New applications and doses are being reviewed for the treatment of COVID-19 by Health Canada. This product is approved in Canada for other uses.
‡ Covisheild was approved in Canada under the interim order on Feb 26, 2021 and expired on September 16, 2021.
Data source: Drug and vaccine authorizations for COVID-19: List of applications received, Health Canada (accessed April 2022): https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/applications.html