The COVID-19 Pipeline: Vaccines and Treatments on the Horizon
Presented at CAHSPR 2022, May 31-June 2, 2022
Introduction
The current COVID-19 outbreak has created a major health pandemic worldwide. Scientists, drugmakers and governments have moved with unprecedented speed over the past 24 months to develop and manufacture vaccines and therapeutics for the prevention and treatment of the novel coronavirus. Many new and repurposed medicines such as antivirals and monoclonal antibodies have been studied for the treatment of COVID-19. This poster provides a snapshot of the global COVID-19 pipeline and a breakdown of the various vaccines and treatments undergoing clinical evaluation, as well as a spotlight on Canada, showing the number of COVID-19 medicines that have been approved or under review by Health Canada.
Methodology
Using GlobalData’s drug product database, medicines indicated for COVID-19 undergoing clinical trials were extracted based on a development stage of Phase I, II, and III or pre-registration. All such medicines were assessed for this analysis, both new and existing. New medicines were identified as those that have not yet been marketed for any indication, while existing medicines include previously marketed therapies undergoing evaluation for new indications related to the treatment of COVID-19.
The spotlight on Canada section highlights the COVID-19 medicines that have been approved in Canada as well as the medicines that are currently undergoing an expedited review process with Health Canada.
Results
The global pipeline for COVID-19 medicines is growing rapidly, with clinical investigations examining novel and existing drugs for the treatment and prevention of COVID-19.
- As of September 2021, 663 vaccines and therapies were undergoing clinical evaluation globally for the prevention and treatment of COVID-19. Of the 663 vaccines and therapies in development for COVID-19, most were novel medicines in Phase II or III of their clinical evaluations.
- The majority of treatments undergoing clinical evaluation are new medicines with an increasing proportion of existing medicines in the later stages of clinical development.
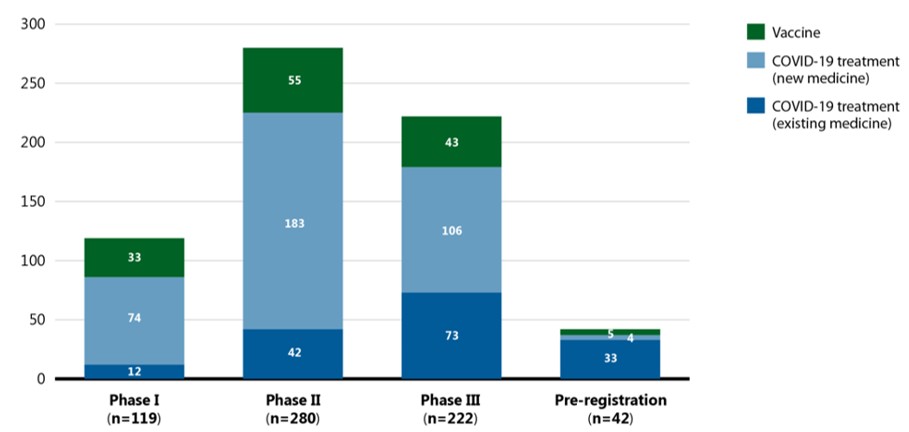
Data source: GlobalData (accessed September 2021).
Figure description
This stacked bar graph illustrates the number of COVID-19 treatments and vaccines in the pipeline by their highest phase of clinical evaluation in September 2021. Totals are given for vaccines and COVID-19 treatments, with separate totals for new and existing medicines.
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
Vaccine |
33 |
55 |
43 |
5 |
COVID-19 treatment, new medicine |
74 |
183 |
106 |
4 |
COVID-19 treatment, existing medicine |
12 |
42 |
73 |
33 |
Total |
119 |
280 |
222 |
42 |
Figure 2 displays the various COVID-19 vaccines categorized by mechanism of action and highest development phase.Footnote 1 Vaccines are categorized into different types based on their mechanism of action; for example, while live attenuated vaccines target the whole virus, subunit and recombinant vaccines target one specific part of the virus.
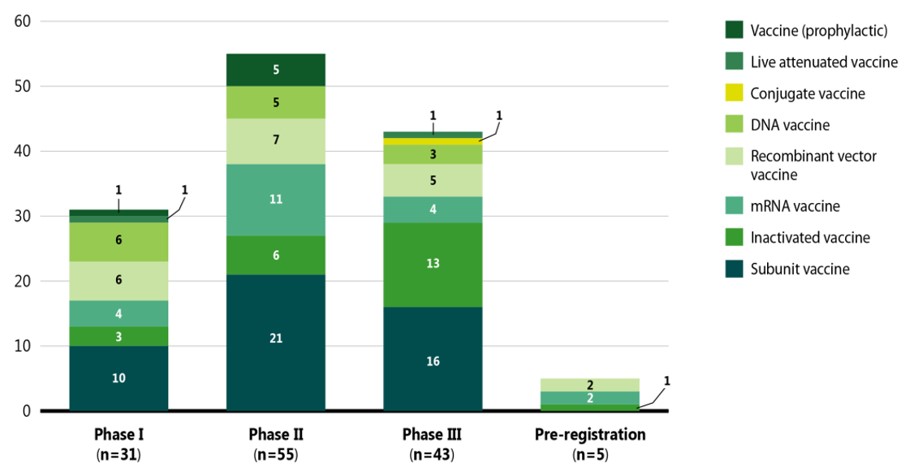
Figure description
A stacked bar graph gives the distribution of coronavirus vaccines in each phase of clinical evaluation by vaccine type, as of September 2021.
| Vaccine (prophylactic) | Live attenuated vaccine | Conjugate vaccine | DNA vaccine | Recombinant vector vaccine | mRNA vaccine | Inactivated vaccine | Subunit vaccine | Total | |
|---|---|---|---|---|---|---|---|---|---|
Phase I |
1 |
1 |
– |
6 |
6 |
4 |
3 |
10 |
31 |
Phase II |
5 |
– |
– |
5 |
7 |
11 |
6 |
21 |
55 |
Phase III |
– |
1 |
1 |
3 |
5 |
4 |
13 |
16 |
43 |
Pre-registration |
– |
– |
– |
– |
2 |
2 |
1 |
– |
5 |
Treatments constitute over 80% of marketed medicines indicated for COVID-19. Marketed treatments include new and repurposed medicines used to decrease the severity of the disease in symptomatic patients.
- As of April 2022, there were 118 marketed COVID-19 medicines available globally for the prevention and treatment of COVID-19.
- As new strains of COVID-19, such as the Omicron variant continue to raise the number of cases around the world, upcoming clinical trials focus on treatments over vaccines.
- Current approaches to COVID-19 treatments generally fall into two categories: antivirals, including Veklury and Paxlovid, which prevent the virus from multiplying; and monoclonal antibodies, including casirivimab and imdevimab, which help the immune system to fight the virus.
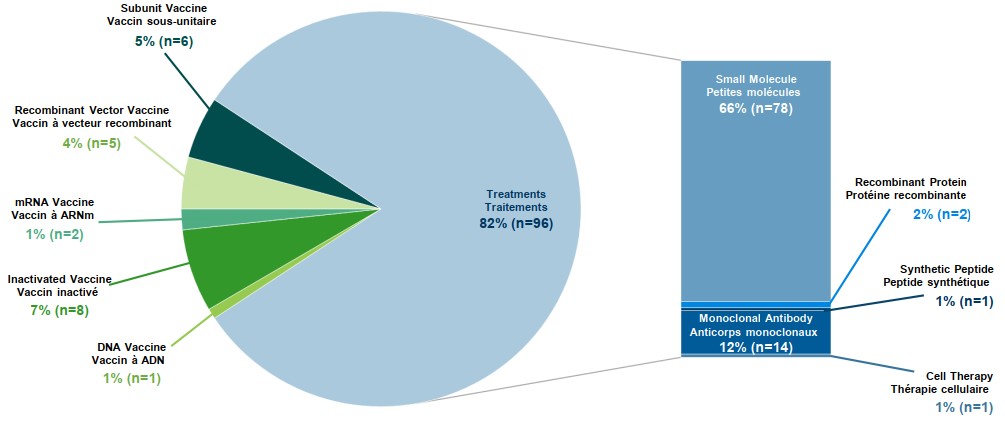
Figure description
This two-part figure examines the total marketed medicines for COVID-19. The first section of the figure is a pie chart that displays the percentage of total marketed vaccines and total marketed treatments. The total treatments make up 82% of the marketed medicines. The second part of this figure is a bar graphs showing the various treatment types for COVID-19.
| Total globally marketed vaccines and treatments for COVID-19 | ||
|---|---|---|
| Type of marketed medicine | Percentage of market | Number of medicines marketed |
Subunit vaccine |
5% |
n=6 |
Recombinant vector vaccine |
4% |
n=5 |
mRNA vaccine |
1% |
n=2 |
Inactivated vaccine |
7% |
n=8 |
DNA vaccine |
1% |
n=1 |
Treatments |
82% |
n=96 |
| Types of treatments for COVID-19 | ||
|---|---|---|
| Type of marketed treatment | Percentage of market | Number of medicines marketed |
Small molecule |
66% |
n=78 |
Recombinant protein |
2% |
n=2 |
Synthetic peptide |
1% |
n=1 |
Monoclonal antibody |
12% |
n=14 |
Cell therapy |
1% |
n=1 |
Spotlight on Canada
As COVID-19 disease activity continues to accelerate, Canada’s health authorities have committed to the immunization response plan published in December 2020. Key elements of Canada’s immunization plan include securing sufficient supply; regulatory authorization for safety and efficacy; managing allocation and distribution of vaccines efficiently and securely; administering vaccines rapidly and equitably; and continuing to monitor vaccine safety, effectiveness and coverage.
As the COVID-19 pandemic remains a top priority in Canada, the current submissions undergoing Health Canada’s review process for COVID-19 are being reviewed under expedited approval timelines. This effort has been supported by the use of interim orders intended to put temporary regulations into place in order to make drugs available to address large-scale public health emergencies.
Six vaccines and five therapies for COVID-19 have been approved in Canada, and six vaccines and nine therapy options are currently under review by Health Canada for the prevention and treatment of COVID-19.
Table. COVID-19 treatment and vaccines approved and under review by Health Canada
| Review Status | Vaccines | Treatments | |
|---|---|---|---|
| Antivirals for systemic use | Immune sera, immunoglobulins and immunosuppressants | ||
Approved
|
Covifenz |
Veklury |
Casirivimab and imdevimab |
Nuvaxovid |
Paxlovid |
Bamlanivimab |
|
Vaxzevria |
|
Sotrovimab |
|
Comirnaty |
|
|
|
Spikevax |
|
|
|
Janssen Inc. vaccine |
|
|
|
Covishield ‡ (expired) |
|
|
|
Under Review
|
Tozinameran |
Remdesivir** |
Cilgavimab, tixagevimab |
[ChAdOx1-S; [recombinant] |
Molnupiravir |
Regdanvimab |
|
Ad26.COV2.S |
|
Bamlanivimab* |
|
Elasomeran |
|
Casirivimab, imdevimab* |
|
SARS-CoV-2 prefusion spike delta TM protein |
|
Sotrovimab* |
|
Whole virion inactivated coronavirus |
|
Baricitinib |
|
|
|
Etesevimab |
|
* The applicant has filed a new drug submission under the Food and Drug Regulations to transition this product from the interim order. The product continues to be approved for sale in Canada during this transition period.
The Interim Order Respecting the Importation, Sale and Adversiting of Drugs for Use in Relation to COVID-19, approved on May 23, 2020, introduced an alternate pathway to facilitate clinical trials for potential COVID-19 drugs and medicinal devices, while upholding strong patient safety requirements and vaility of trial data.
** New applications and doses are being reviewed for the treatment of COVID-19 by Health Canada
‡ Covisheild was approved in Canada under the interim order on Feb 26, 2021 and expired on September 16, 2021.
Data source: Drug and vaccine authorizations for COVID-19: List of applications received, Health Canada (accessed April 2022):
https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/applications.html