Factors delaying the public listing of drugs in Canada
Presented at ISPOR 2025, May 13–16, 2025 and at CAHSPR 2025, May 26–29, 2025
Étienne Gaudette, Shirin Rizzardo, Kevin R. Pothier, and Mina Tadrous
Objective
The time to public listing for new drugs in Canada has been criticized, citing decentralized public payer structure and institutions leading to a prolonged listing process. We aim to identify and analyze key factors contributing to delays in the time-to-listing.
Approach and data
We use a cohort study approach to examine the 160 prescription drugs with a first Canadian public listing between 2018 and 2023. We document time-to-listing components, timeline exceptions, reimbursement recommendations, and negotiation results using the Canadian Institute for Health Information’s (CIHI) Formulary Coverage tool, Health Canada’s Notice of Compliance (NOC) database, Canada’s Drug Agency (CDA-AMC) Reimbursement Review reports, and the pan-Canadian Pharmaceutical Alliance’s (pCPA) Brand Name Drug Negotiation Status website. Time-to-listing is defined as the time from the NOC, which grants market authorization for a new drug, to the earliest date at which the drug is added to a public drug plan formulary.
Results
1. How long does it take for new drugs to be publicly reimbursed in Canada?
- On average, new drugs are added to a Canadian public formulary 906 days (29.8 months, SD 640 days) after receiving the NOC (Figure 1).
- For most drugs, the process entails completing five key steps, with durations ranging from 1.5 months to 7.9 months.
Figure 1. Average time in months to reach key steps of the listing process
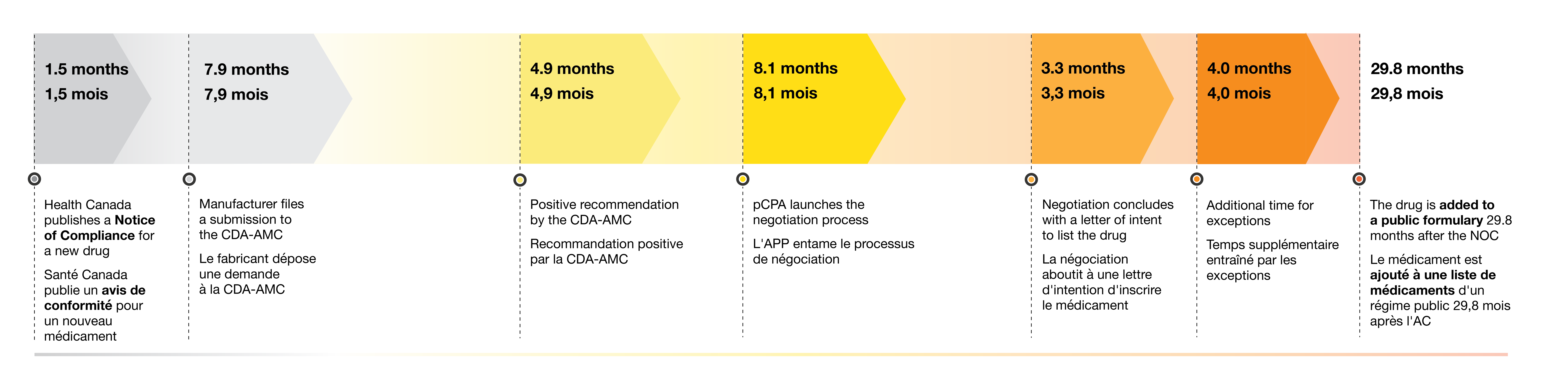
Figure - Text version
| Key step of the listing process | Average time to reach the next step in the process |
|---|---|
1. Health Canada publishes a Notice of Compliance for a new drug |
1.5 months |
2. Manufacturer files a submission to Canada’s Drug Agency |
7.9 months |
3. Positive recommendation by Canada’s Drug Agency |
4.9 months |
4. The pan-Canadian Pharmaceutical Alliance launches the negotiation process |
8.1 months |
5. Negotiation concludes with a letter of intent to list the drug |
3.3 months |
6. Additional time for exceptions |
4.0 months |
7. The drug is added to a public formulary 29.8 months after the Notice of Compliance |
|
Data source: CDA-AMC, CIHI, Health Canada, pCPA.
Note: NOC = Health Canada Notice of Compliance; CDA-AMC = Canada’s Drug Agency; pCPA = pan-Canadian Pharmaceutical Alliance. The additional time for exceptions accounts for divergences from the standard process (e.g., drugs requiring multiple negotiations).
2. What are the main factors delaying the listing process?
- Five common factors are identified, associated with delays ranging from 68 days to 3 years (Table 1).
- Of these factors, late manufacturer submissions for CDA-AMC review—despite pre-NOC submissions being permitted since 2012Footnote 1—are the most common and account for 49% of overall delays.
- Drugs not impacted by delaying factors (streamlined process) are listed on average 17 months (518 days, SD 214 days) after the NOC, while drugs impacted are listed on average 39 months (1,179 days, SD 697 days) after the NOC (Figure 2).
Table 1. Factors causing delays during the listing process
| Step | Streamlined process average duration | Factors causing delays (% contribution to overall delay) |
|---|---|---|
Manufacturer submission to CDA-AMC |
127 days before the NOC |
- Late submission: 44% of drugs are submitted after the NOC, with post-NOC submissions occurring 368 days later on average (49% of overall delay). |
CDA-AMC review |
236 days |
- Queued review: 5% of drugs are placed in a queue due to capacity constraints, adding an average 68 days to the review process (1% of overall delay). |
pCPA negotiation engagement |
177 days |
|
Negotiation |
215 days |
- Unsuccessful negotiation: 6% of drugs with a positive recommendation undergo a first negotiation that concludes without an agreement, or negotiations are not pursued, adding 490 days on average from engagement to public listing (7% of overall delay). |
Public drug plan listing |
107 days |
|
Overall |
518 days |
The average delay is 661 days for drugs impacted by one or more factors. |
Data source: CDA-AMC, CIHI, Health Canada, pCPA.
Note: NOC = Health Canada Notice of Compliance; CDA-AMC = Canada’s Drug Agency; pCPA = pan-Canadian Pharmaceutical Alliance. The average time-to-listing of drugs not impacted by delays is lower than the sum of individual components due to 3% of drugs being listed prior to completing all steps.
Figure 2. Distribution of time-to-listing by drug group
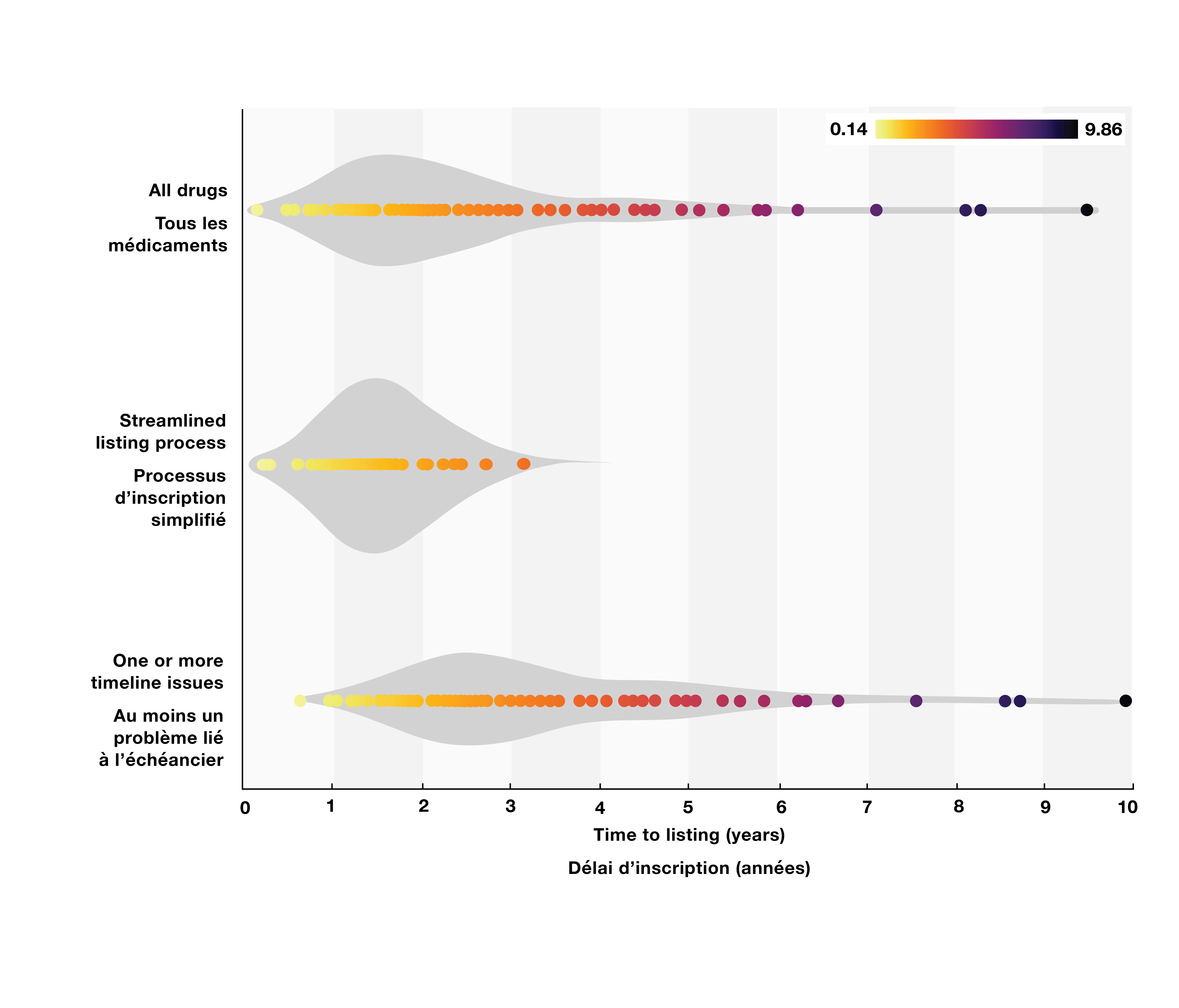
Figure - Text version
| Drug group | Distribution of time-to-listing (in years) |
|---|---|
All drugs |
0.14 to 9.86 |
Streamlined listing process |
0.14 to 3.22 |
One or more timeline issues |
0.64 to 9.86 |
Data source: CDA-AMC, CIHI, Health Canada, pCPA.
Note: The “One or more timeline issues” group includes 94 drugs with at least one of the delaying factor described in Table 1, while the “Streamlined listing process” group includes the remaining 66 drugs.
Conclusions
Adding new drugs to public formularies takes over 2 years on average in Canada. Although Canada’s Drug Agency (CDA-AMC) review processes and pan-Canadian Pharmaceutical Alliance (pCPA) negotiation times have been criticized for contributing to slow access, the average time-to-listing masks important variations and is skewed by identifiable factors that cannot be exclusively ascribed to the CDA-AMC or pCPA.
These delaying factors include manufacturer decisions on whether and when to submit to CDA-AMC, negative listing recommendations that appropriately safeguard public resources until cost-effectiveness is demonstrated, and unsuccessful price negotiations, which reflect a shared responsibility between manufacturers and public payers represented by the pCPA.
Limitations
The methods are observational, and unobserved factors may contribute to differences in time-to-listing between groups. The time-to-listing measure considers the earliest date at which a province added the drug to its public drug plan formulary; subsequent listing in other provinces may take several additional months. Reviews conducted by the Institut national d’excellence en santé et en services sociaux, which serves a similar role to the CDA-AMC for Québec, are excluded from the analysis. The reasons for unsuccessful price negotiations are not publicly available.