Trends in the global drug development pipeline, 2021–2025
Presented at ISPOR 2025, May 13–16, 2025 and at CAHSPR 2025, May 26–29, 2025
Brian O’Shea
Objective
The pharmaceutical industry invests heavily in new drug development to bring innovative therapies to markets around the world. Studying the research and development pipeline, especially in the later stages, provides insight into the novel therapies that will shape the pharmaceutical landscape in the near future.
This study presents a snapshot of the global pipeline and a breakdown of the various therapies undergoing clinical evaluation, as well as a look at the most notable drug development trends from 2021–2025.
Approach and data
A full list of pipeline medicines was retrieved from GlobalData’s Healthcare database in March 2025. To analyze historical trends, this extract is compared with historical extracts made in April 2024, September 2022, and September 2021. These snapshots of the pipeline over a specific time period are taken to be representative of the composition of medicines over the entire year. However, the pipeline is fairly dynamic and the share of medicines in any particular therapeutic area can vary.
New medicinal ingredients are identified in the GlobalData Healthcare database as those without prior market authorization from Canada, the United States, the United Kingdom, France, Germany, Italy, Spain, Brazil, Russia, India, China, South Africa, Japan, South Korea, and nine other countries. Medicines are cross-referenced with recorded sales data current to Q4 2024 from the IQVIA MIDAS® Database (all rights reserved) to confirm their novelty.
Orphan drugs are defined as medicines that received an orphan designation in either the US, EU, UK, or Japan. Therapeutic areas are assigned based on the indication under evaluation reported by GlobalData. A single new medicine may be undergoing multiple clinical studies for separate indications and could appear more than once in the therapeutic class distribution of a development stage.
Results
1. Trends in new medicines under development
- Despite a decline from the level observed in 2024, the number of new medicines under development in all phases of the pipeline shows an increase over the 2021–2022 totals (Figure 1).
- The 10,134 new medicines under development in March 2025 represent a 20% expansion compared to the pipeline as it was in September 2021.
- Orphan medicines, as designated by regulatory authorities in the US, UK, EU, and Japan, make up a growing share of the later stages of the pipeline (Figure 2).
- While the proportion of orphan medicines in pre-registration was lower in 2024–2025 than in 2021–2022, in absolute terms there have been 50–51 in each of the last three pipeline extracts.
Figure 1. Number of new medicines by highest phase of clinical evaluation, 2021–2025
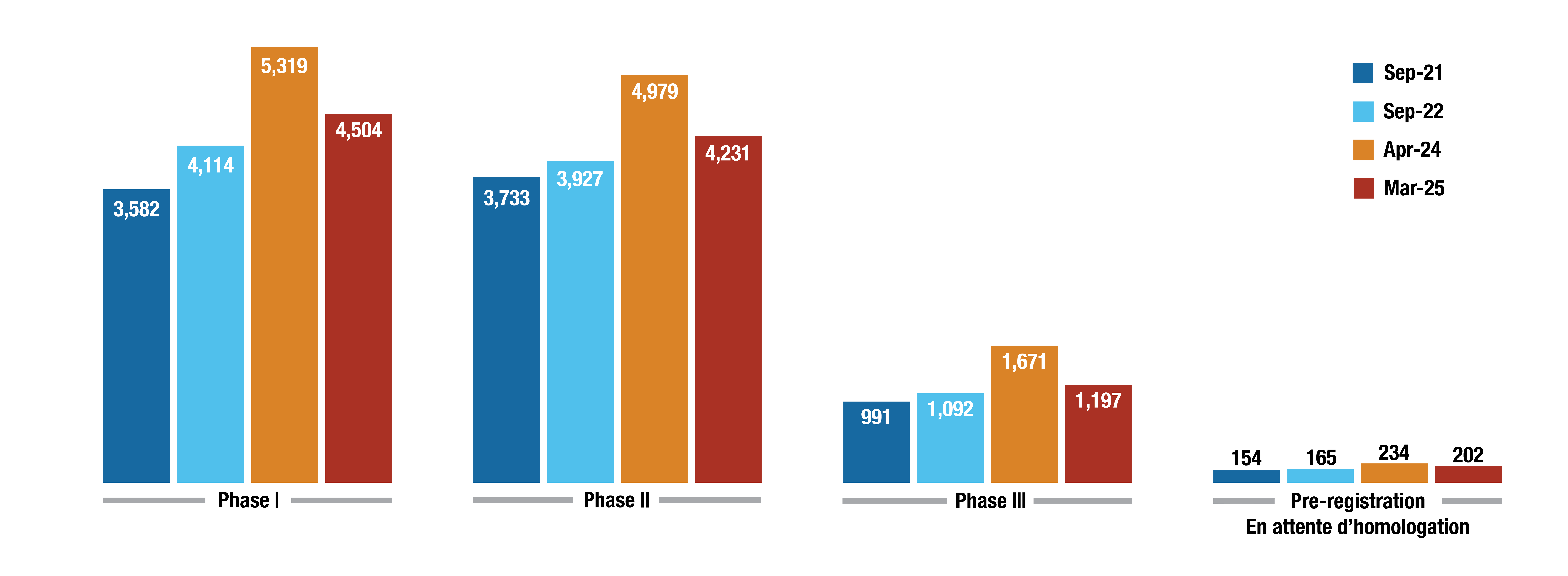
Figure - Text version
| September 2021 | September 2022 | April 2024 | March 2025 | |
|---|---|---|---|---|
Phase I |
3,582 |
4,114 |
5,319 |
4,504 |
Phase II |
3,733 |
3,927 |
4,979 |
4,231 |
Phase III |
991 |
1,092 |
1,671 |
1,197 |
Pre-registration |
154 |
165 |
234 |
202 |
Figure 2. Share of orphan medicines in the pipeline by highest phase of clinical evaluation, 2021–2025
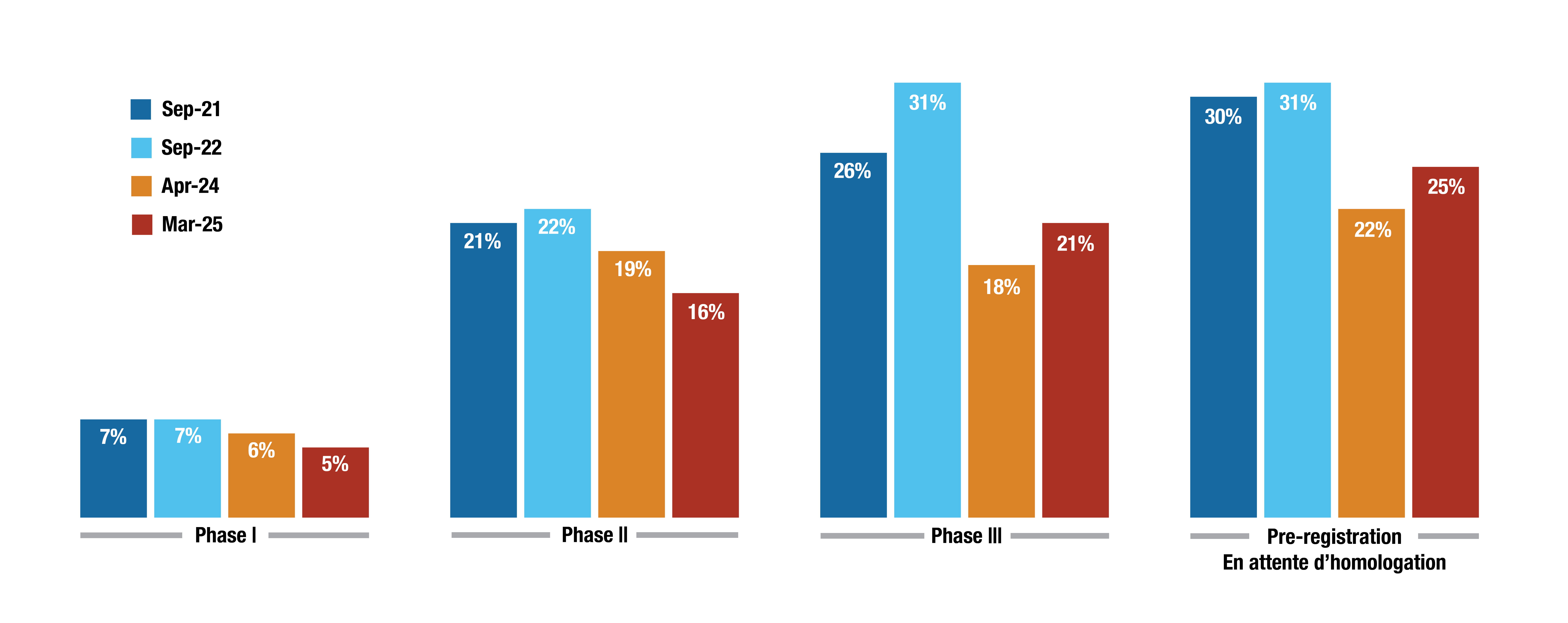
Figure - Text version
| September 2021 | September 2022 | April 2024 | March 2025 | |
|---|---|---|---|---|
Phase I |
7% |
7% |
6% |
5% |
Phase II |
21% |
22% |
19% |
16% |
Phase III |
26% |
31% |
18% |
21% |
Pre-registration |
30% |
31% |
22% |
25% |
2. Therapeutic classes and indications of new pipeline medicines
- In 2025, oncology medicines are a plurality at all stages of the pipeline, representing 38% of new medicines in development overall, and 25% at pre-registration (Figure 3).
- Metabolic disorders (representing only 6% of the entire pipeline) make up 15% of medicines in pre-registration, with multiple drugs for type 2 diabetes and hyperlipidemia in this late phase of development.
- Within the top 10 therapeutic areas for new medicines in pre-registration, Table 1 highlights common indications observed in the 202 medicines expected to enter the market in the near term.
- In addition to the 10 candidates in pre-registration for type 2 diabetes, and the 9 for HER2-negative breast cancer, there are also medicines for hemophilia B (3), Alzheimer’s disease (2), hemophilia A (2), and ovarian cancer (2), among other indications.
Figure 3. Therapeutic class distribution of new pipeline medicines by phase of clinical evaluation, 2025
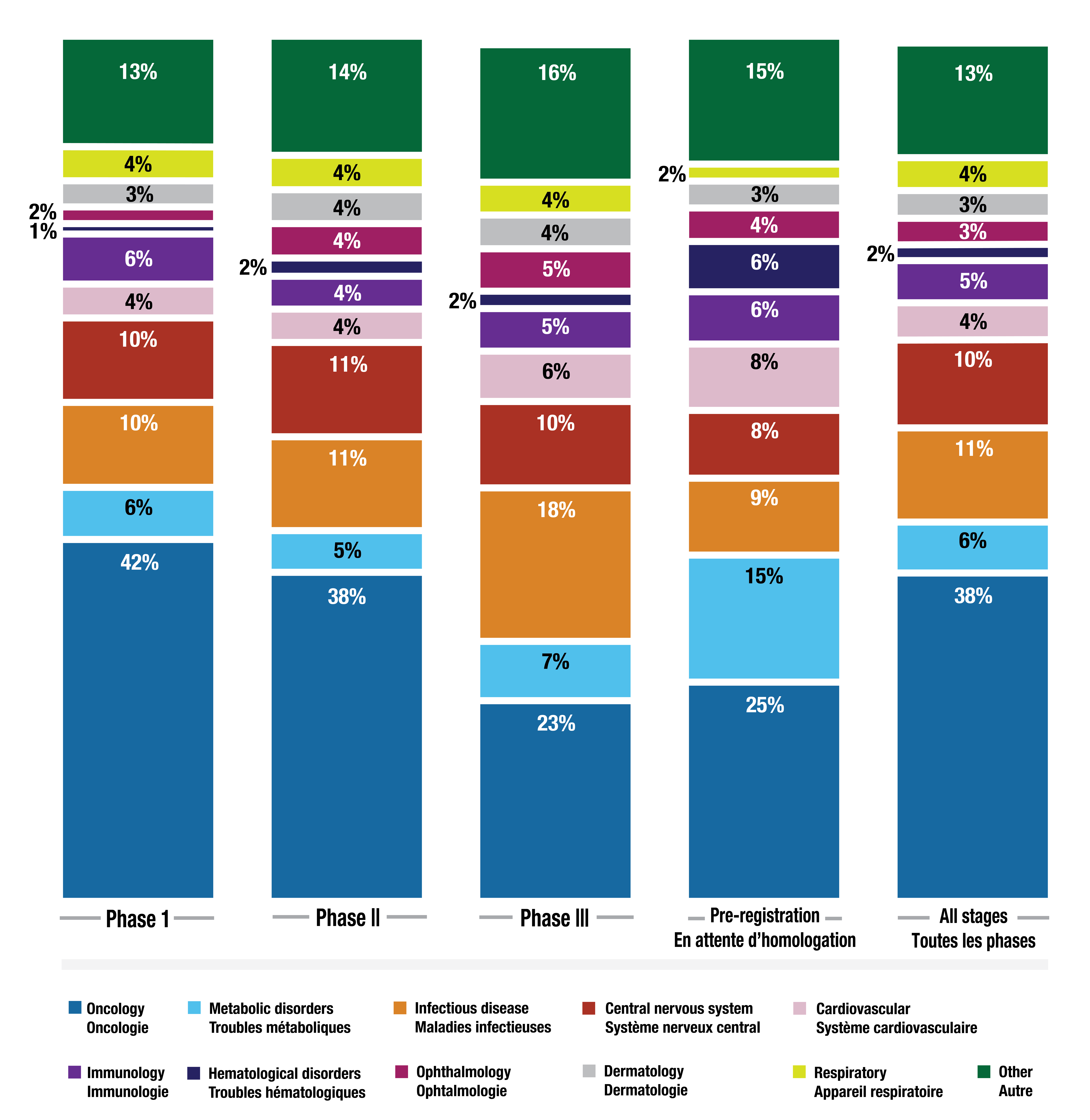
Figure - Text version
| Therapeutic Area | Phase I | Phase II | Phase III | Pre-registration | All Phases |
|---|---|---|---|---|---|
Oncology |
42% |
38% |
23% |
25% |
38% |
Metabolic disorders |
6% |
5% |
7% |
15% |
6% |
Infectious disease |
10% |
11% |
18% |
9% |
11% |
Central nervous system |
10% |
11% |
10% |
8% |
10.3% |
Cardiovascular |
4% |
4% |
6% |
8% |
4.4% |
Immunology |
6% |
4% |
5% |
6% |
5.0% |
Hematological disorders |
1% |
2% |
2% |
6% |
1.8% |
Ophthalmology |
2% |
4% |
5% |
4% |
3.2% |
Dermatology |
3% |
4% |
4% |
3% |
3.3% |
Respiratory |
4% |
4% |
4% |
2% |
3.8% |
Other |
13% |
14% |
16% |
15% |
13.4% |
Table 1. Top indications for major therapeutic areas in pre-registration, 2025
Oncology |
25% |
HER2-negative breast cancer (9) |
Non-small-cell lung cancer (7) |
||
Diffuse large B-cell lymphoma (3) |
||
Primary myelofibrosis (3) |
||
Relapsed/refractory multiple myeloma (3) |
||
Ovarian cancer (2) |
||
Metabolic disorders |
15% |
Type 2 diabetes (10) |
Hyperlipidemia (5) |
||
Other diabetes (4) |
||
Obesity (3) |
||
Infectious disease |
9% |
Influenza virus infections (6) |
COVID-19 (2) |
||
Respiratory syncytial virus (RSV) (2) |
||
Central nervous system |
8% |
Post-operative pain (3) |
Alzheimer's disease (2) |
||
Insomnia (2) |
||
Cardiovascular |
8% |
Hypertension (5) |
Ischemic stroke (3) |
||
Hypertrophic cardiomyopathy (2) |
||
Critical limb ichemia (2) |
||
Supraventricular tachycardia (2) |
||
Immunology |
6% |
Plaque psoriasis (5) |
Hereditary angiodema (3) |
||
Rheumatoid arthritis (2) |
||
Myasthenia gravis (2) |
||
Hematological disorders |
6% |
Immune thrombocytopenic purpura (3) |
Hemophilia B (3) |
||
Hemophilia A (2) |
||
Renal anemia (2) |
||
Ophthalmology |
4% |
Dry eye (4) |
Dermatology |
3% |
Atopic eczema (2) |
Alopecia (2) |
||
Respiratory |
2% |
Streptococcal pneumonia (2) |
Conclusion
The international drug development pipeline remains robust in the years following the COVID-19 pandemic. The global pipeline in 2025 contains over 10,000 new medicines in clinical development—a decrease from 2024, but still showing growth over 2021 and 2022 levels.
There are more and more new medicines for rare diseases toward the end of the pipeline. Orphan medicines are found in each of the top 10 most common therapeutic areas in pre-registration. This shows a continued focus on improving treatment options for underserved patient populations.
The pharmaceutical industry continues to prioritize the development of new cancer therapies, which make up one quarter of the pre-registration phase. The next most common therapeutic area in pre-registration is metabolic disorders, which encompasses treatments for type 2 diabetes and hyperlipidemia. In line with past observations of the pipeline, oncology is the largest therapeutic class across all stages of development, followed by infectious disease and central nervous system medicines.
Given the increasing globalization of drug development, horizon-scanning efforts provide the insights needed to prepare for the treatment landscape of tomorrow.
Disclaimer
Although based in part on data under license by IQVIA™, the statements, findings, conclusions, views and opinions expressed in this report are exclusively those of the PMPRB.