Annual Report 2018

ISSN: 1495-0561
Cat. no.: H78E-PDF
PDF version (1.2 MB)
Table of Contents
- Statistical Highlights 2018
- Letter to the Minister
- Chairperson's Message
- About the Patented Medicine Prices Review Board: Acting in the Interest of Canadians
- Regulating Prices of Patented Medicines: Continued Vigilance Necessary
- Key Pharmaceutical Trends: More Expensive Medicines Continue to Influence Sales
- The National Prescription Drug Utilization Information System: Supporting Health Care Decision Making in Canada
- Analysis of Research and Development Expenditures: R&D Investment Falling Short of Target
- Appendix 1: Glossary
- Appendix 2: Patented Medicines First Reported to the PMPRB in 2018
- Appendix 3: Pharmaceutical Trends - Sales
- Appendix 4: Research and Development
Statistical Highlights 2018
Regulatory Mandate
- 1,403 patented medicines for human use were reported to the PMPRB, including 108 new medicines.
- 7 Voluntary Compliance Undertakings were accepted as at December 31, 2018.
- $315 thousand in excess revenues offset by way of payments to the Government of Canada, in addition to price reductions.
Reporting Mandate
Sales Trends:
- $16.7 billion in sales of patented medicines in Canada in 2018, decreasing slightly by 0.6% from the previous year.
- Patented medicines accounted for 59.0% of the total medicine sales in Canada, a decrease from 61.5% in 2017.
Price Trends:
- Prices of existing patented medicines were stable, while the Consumer Price Index rose by 2.3%.
- Canadian prices were fourth highest among the seven PMPRB comparator countries, lower than prices in Switzerland, Germany and the US.
Research and Development:
R&D-to-sales ratios decreased in 2018:
- 4.0% for all patentees, a slight decrease from 4.1% in 2017.
- 4.3% for Innovative Medicines Canada members, a decrease from 4.6% in 2017.
R&D expenditures:
- $892.6 million in total R&D expenditures reported by patentees, an increase of 2.4% over 2017.
- $723.0 million in R&D expenditures reported by Innovative Medicines Canada members, a decrease of 4.3% over 2017.
Letter to the Minister
February 28, 2020
The Honourable Patty Hajdu, P.C., M.P.
Minister of Health
House of Commons
Ottawa, Ontario
K1A 0A6
Dear Minister:
I have the pleasure to present to you, in accordance with sections 89 and 100 of the Patent Act, the Annual Report of the Patented Medicine Prices Review Board for the year ended December 31, 2018.
Yours very truly,
Dr. Mitchell Levine
Chairperson
Chairperson’s Message
The Patented Medicine Prices Review Board (PMPRB) is an independent quasi-judicial body established by Parliament in 1987 under the Patent Act (the Act). The PMPRB’s mandate is to protect and inform Canadians by ensuring that the prices of patented medicines sold in Canada are not excessive and by reporting on pharmaceutical trends.
In 2018, the PMPRB’s focus was on completing the final steps to modernize its regulations and guidelines. To that end, the PMPRB struck a steering committee composed of key stakeholders to provide it with high level feedback on a proposed new regulatory framework for protecting consumers from excessively priced patented medicines. To assist the committee, the PMPRB also established an expert working group to examine and advise on some of the more technically challenging aspects of the proposed new framework. The outcome of the deliberations of both the steering committee and the technical working group were presented to the Board for its consideration prior to the release of new draft guidelines for broader public consultation in the fall of 2019. Until such time as new regulations and guidelines are finalized and in force, the PMPRB will continue to administer its regulatory mandate under the existing rules to achieve the best possible results for Canadians.
In addition to progress on the PMPRB’s policy development objectives for new regulations and guidelines, 2018 also saw the legislative underpinnings to those two instruments further buttressed by the Supreme Court of Canada decision to deny Alexion’s leave to appeal application from the Federal Court of Appeal’s judgement in the Soliris matter. In doing so, the Supreme Court sent a strong signal that any doubts about the constitutional validity of the PMPRB’s legislative scheme should be firmly put to rest.
In terms of pharmaceutical trends, the most striking takeaway from this year’s Annual Report is the unprecedented 0.6% decrease in total spending on patented medicines. This can be explained by the fact that some key top-selling medicines stopped reporting to the PMPRB in 2018, including Remicade the highest selling prescription medicine in Canada. In 2018, medicines that previously reported to the PMPRB accounted for estimated sales of $3.3 billion, or 11.6% of all sales. This is a marked increase over the previous year and considerably more than a decade ago when medicines that formerly reported to the PMPRB accounted for $0.7 billion in sales, or 3.2% of all sales. Historically patented medicines have experienced a substantial erosion in market share upon loss of patent protection, however, recently that same effect has not been observed in a number of very high cost, biologic medicines that have come off patent. Given the significance of this phenomenon, the PMPRB has added new content to its Annual Report to track the impact of this trend as the market for these medicines matures.
Dr. Mitchell Levine
Chairperson
About the Patented Medicine Prices Review Board: Acting in the Interest of Canadians
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi-judicial body established by Parliament in 1987 under the Patent Act (Act).
The PMPRB is a consumer protection agency with a dual regulatory and reporting mandate. Through its regulatory mandate, it ensures that the prices of patented medicines sold in Canada are not excessive. The PMPRB also reports on trends in pharmaceutical sales and pricing for all medicines and on R&D spending by patentees. Its reporting mandate provides pharmaceutical payers and policy makers with information to make rational, evidence-based reimbursement and pricing decisions.
The PMPRB is part of the Health Portfolio, which includes Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research and the Canadian Food Inspection Agency. The Health Portfolio supports the Minister of Health in maintaining and improving the health of Canadians.
Our Mission:
The PMPRB is a respected public agency that makes a unique and valued contribution to sustainable spending on pharmaceuticals in Canada by:
- Providing stakeholders with price, cost, and utilization information to help them make timely and knowledgeable pricing, purchasing, and reimbursement decisions; and
- Acting as an effective check on the prices of patented medicines through the responsible and efficient use of its consumer protection powers.
Protecting Consumers in a Complex Marketplace
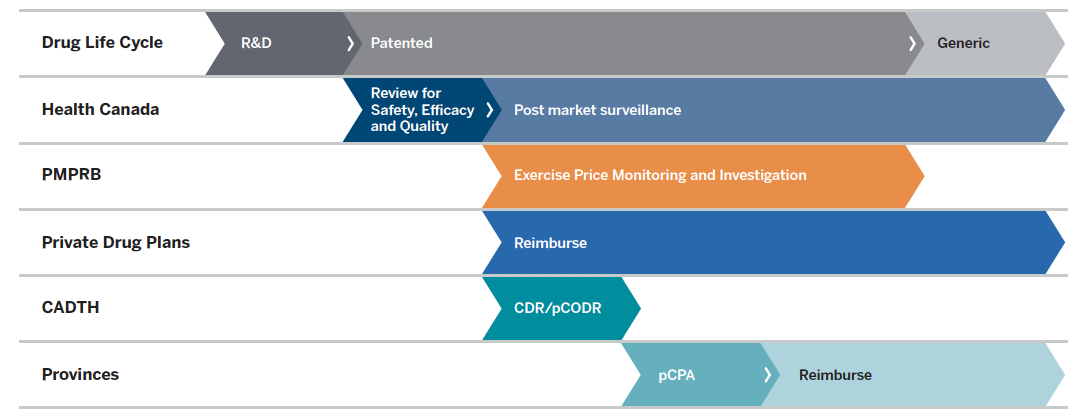
Figure description
This flowchart illustrates the role of Canadian regulators during the life cycle of a drug through research and development, the patent period, and post-patent generic period. Health Canada reviews for safety, efficacy, and quality at the start of the patent period and provides subsequent post-market surveillance. The PMPRB exercises price monitoring and investigation during the patent period, after Health Canada’s review. Private drug plans reimburse during and after the patented period, following Health Canada’s review. CADTH conducts the Common Drug Review and pan-Canadian Oncology Drug Review processes after Health Canada’s review. For provinces and territories, the pan-Canadian Pharmaceutical Alliance negotiations begin after CADTH reviews, and reimbursement is subsequent to these negotiations.
Although part of the Health Portfolio, because of its quasi-judicial responsibilities, the PMPRB carries out its mandate at arm’s length from the Minister of Health, who is responsible for the sections of the Act pertaining to the PMPRB. The PMPRB also operates independently of other healthcare related bodies such as:
- Health Canada, which approves medicines for marketing in Canada based on their safety, efficacy and quality;
- federal, provincial and territorial (F/P/T) public drug plans, working collectively as the pCPA, which approve the listing of medicines on their respective formularies for reimbursement purposes; and
- the Common Drug Review and pan-Canadian Oncology Drug Review, administered by the CADTH, which recommends which new medicines should qualify for reimbursement by the pCPA.
The PMPRB is composed of public servants (Staff) who are responsible for carrying out the organization’s day-to-day work, and Board Members, Governorin-Council appointees who serve as hearing panel members in the event of a dispute between Staff and a patentee over the price of a patented medicine.
Jurisdiction
Regulatory
The PMPRB regulates the maximum price at which patentees (companies) may sell their products to wholesalers, hospitals, pharmacies and other large distributors. This price is sometimes also known as the “factory gate” (ex-factory) price. The PMPRB does not regulate the prices of non-patented medicines.
The PMPRB’s jurisdiction is not limited to medicines for which the patent is for the active ingredient or for the specific formulation(s) or uses the patentee sells the medicine for in Canada. Rather, its jurisdiction also covers medicines for which a patent “pertains” including patents for manufacturing processes, delivery systems or dosage forms, indications/use and any formulations.
The Act requires patentees (which include any parties who benefit from patents regardless of whether they are owners or licensees under those patents and regardless of whether they operate in the “brand” or “generic” sector of the market) to inform the PMPRB of their intention to sell a new patented medicine. Upon the sale of a patented medicine, patentees are required to file price and sales information at introduction and, thereafter, until all patents pertaining have expired.
Our Vision:
A sustainable pharmaceutical system where payers have the information they need to make smart reimbursement choices and Canadians can afford the patented medicines they need to live healthy and productive lives.
Patentees are not required to obtain approval of the price to be able to market their products. However, the Act requires the PMPRB to ensure that the prices of patented medicines sold in Canada are not excessive.
Staff reviews the prices that patentees charge for each individual strength and form of a patented medicine. If the price of a patented medicine appears to be potentially excessive, Staff will first try to reach a voluntary resolution by the patentee. If this fails, the Chairperson can decide that the matter should go to a hearing. At the hearing, a panel composed of Board members acts as a neutral arbiter between Staff and the patentee. If a panel finds that the price of a patented medicine is excessive, it can order the price be reduced to a non-excessive level. It can also order a patentee to make a monetary payment to the Government of Canada to offset the excess revenues earned and, in cases where the panel determines there has been a policy of excessive pricing, it can double the amount of the monetary payment.
Reporting
As required by the Act, the PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends of all prescription medicines, and on the R&D expenditures reported by pharmaceutical patentees.
In addition, pursuant to an agreement by the F/P/T Ministers of Health in 2001, and at the request of the Minister of Health pursuant to section 90 of the Act, the PMPRB conducts critical analyses of price, utilization and cost trends for patented and non-patented prescription medicines under the National Prescription Drug Utilization Information System (NPDUIS). The PMPRB publishes the results of NPDUIS analyses in the form of research papers, posters, presentations and briefs. This program provides F/P/T governments and other interested stakeholders with a centralized, objective and credible source of information on pharmaceutical trends.
Among other initiatives under its reporting mandate, the PMPRB also hosts various forums, such as webinars, research forums and information sessions, with academics and policy experts to discuss and disseminate research on emerging areas for study on pharmaceutical trends in Canada and internationally.
1,403 Patented Medicines
1,403 Patented Medicines were reported to the PMPRB in 2018.
Communications and Outreach
The PMPRB takes a proactive and plain-language approach to its external communication activities. This includes targeted social media campaigns and more conventional (e.g., email and telephone) engagement with domestic, international and specialized news media. The PMPRB is actively pursuing additional opportunities to leverage new and emerging media to communicate with Canadians and its stakeholders.
The PMPRB recognizes the importance of openness and transparency as we continue to work on modernizing the way we carry out our mandate. We communicate regularly, through various channels, about our progress, including projected timelines, and key milestones. Engagement with stakeholders will remain a central part of our multi-faceted communications approach. Reporting on our progress helps ensure we remain focused on delivering results.
Governance
The Board consists of not more than five members who serve on a part-time basis. Board members, including a Chairperson and a Vice-Chairperson, are appointed by the Governor-in-Council. The Chairperson, designated under the Act as the Chief Executive Officer of the PMPRB, has the authority and responsibility to supervise and direct its work. By law, the Vice-Chairperson exercises all the powers and functions of the Chairperson when the Chairperson is absent or incapacitated, or when the office of the Chairperson is vacant.
The members of the Board, including the Chairperson, are collectively responsible for implementing the applicable provisions of the Act. Together, they establish the guidelines, rules, by-laws and other policies of the PMPRB provided for by the Act (section 96) and consult, as necessary, with stakeholders including provincial and territorial Ministers of Health, representatives of consumer groups, the pharmaceutical industry and others.
Members of the Board
Chairperson
Mitchell Levine,
BSc, MSc, MD, FRCPC, FISPE, FACP

Dr. Mitchell Levine was appointed Chairperson of the Board on February 13, 2018.
Dr. Levine is a professor in both the Department of Health Research Methods, Evidence and Impact and in the Department of Medicine, Division of Clinical Pharmacology & Toxicology at McMaster University in Hamilton, Ontario. He is also an Assistant Dean in the Faculty of Health Sciences and a faculty member of the Centre for Health Economics and Policy Analysis at McMaster.
Dr. Levine received his medical degree from the University of Calgary in 1979, followed by postgraduate medical training in Internal Medicine (FRCPC) and in Clinical Pharmacology at the University of Toronto (1981–1987). He received an MSc degree in Clinical Epidemiology from McMaster University in 1988.
Prior to his appointment to the Board, Dr. Levine was a member of the PMPRB’s Human Drug Advisory Panel. He currently acts on an ad hoc basis as a clinical pharmacology consultant to the Ontario Ministry of Health and Ministry of Long-Term Care. In addition, he is a Deputy Editor of the ACP Journal Club: Evidence-Based Medicine.
This is Dr. Levine’s second term as a Board member. He was initially appointed as its Vice-Chairperson in 2011.
Vice-Chairperson
Position vacant
Members
Carolyn Kobernick,
B.C.L., LL.B.

Carolyn Kobernick was appointed Member of the Board on June 13, 2014.
Ms. Kobernick is a lawyer and former public servant. Prior to her retirement in 2013, Ms. Kobernick was Assistant Deputy Minister of Public Law for the Department of Justice. As principal counsel to the Minister of Justice and Attorney General of Canada, Ms. Kobernick was instrumental in the development and delivery of policy for the Public Law sector. In addition to identifying key strategic, legal and operational matters, she tackled cross-cutting national issues as the liaison between the Department of Justice and other government organizations.
Ms. Kobernick joined the Department of Justice in 1980, where she practiced litigation and tax law at the Toronto Regional office. In 1991, she was appointed Senior General Counsel, Deputy Head, Business and Regulatory Law Portfolio, after working for over a decade in the legal services unit of the Correctional Service of Canada. In her role as Senior General Counsel, Ms. Kobernick was involved in complex federal policy and operational issues, including the Alaska Pipeline and Mackenzie Valley Pipeline files and the Sponsorship file.
During her career with the public service, Ms. Kobernick actively participated in many high-profile initiatives. She was Chair of the National Legal Advisory Committee and Departmental Champion for Aboriginal People and Gender Equity. She also served as the Senior Department of Justice official at the Domestic Affairs Cabinet Committee, and was appointed Senior Legal Advisor to the Government of Canada for the 2004 Gomery Inquiry.
Ms. Kobernick holds a B.C.L. and LL.B. from McGill University and is a member of the bar of Ontario. In 2012 she obtained a Certificate in Adjudication for Administrative Agencies, Boards and Tribunals from the Osgoode Hall Law School and the Society of Ontario Adjudicators and Regulators.
Dr. Ingrid Sketris,
BSc (Pharm), PharmD, MPA(HSA), Clinical Toxicology Residency

Dr. Ingrid Sketris was appointed Member of the Board on June 29, 2018.
Dr. Sketris is a licensed pharmacist and a professor at the College of Pharmacy, Dalhousie University, with cross appointments to Medicine and Health Administration.
Dr. Sketris received her Doctor of Pharmacy in 1979 from the University of Minnesota, followed by her residency in Clinical Toxicology at the University of Tennessee Centre for the Health Sciences. She also received a Master of Public Administration/Health Services Administration from Dalhousie University.
She is a leader in pharmacy, and has served as President of the Association of Faculties of Pharmacy of Canada and as a board member of the Canadian Council for Accreditation of Pharmacy Programs.
Dr. Sketris is a Fellow of the Canadian Society of Hospital Pharmacists, the American College of Clinical Pharmacy and the Canadian Academy of Health Sciences. She was previously elected to the US National Academies of Practice.
Matthew Herder,
B.Sc. (hons), LL.B., LL.M., J.S.M.

Matthew Herder was appointed Member of the Board on June 29, 2018.
Mr. Herder is the Director of the Health Law Institute at Dalhousie University as well as an Associate Professor in the Department of Pharmacology in the Faculty of Medicine, with a cross-appointment to the Schulich School of Law.
Mr. Herder’s research focuses on biomedical innovation policy, with a particular emphasis on intellectual property rights and the regulation of biopharmaceutical interventions. His work is often interdisciplinary and policy-oriented, and he has received grants from the Canadian Institutes of Health Research and the Royal Society of Canada, in addition to appearing as an expert witness before several Parliamentary committees on pharmaceutical regulation and policy.
Prior to arriving at Dalhousie, Mr. Herder was the Ewing Marion Kauffman Foundation Legal Research Fellow at New York University’s School of Law. He was a Law Clerk at the Federal Court of Canada and was admitted to the Law Society of Upper Canada. Mr. Herder holds a Master of the Science of Law degree from Stanford Law School as well as two law degrees from Dalhousie University.
Organizational Structure and Staff
PMPRB Organizational Chart
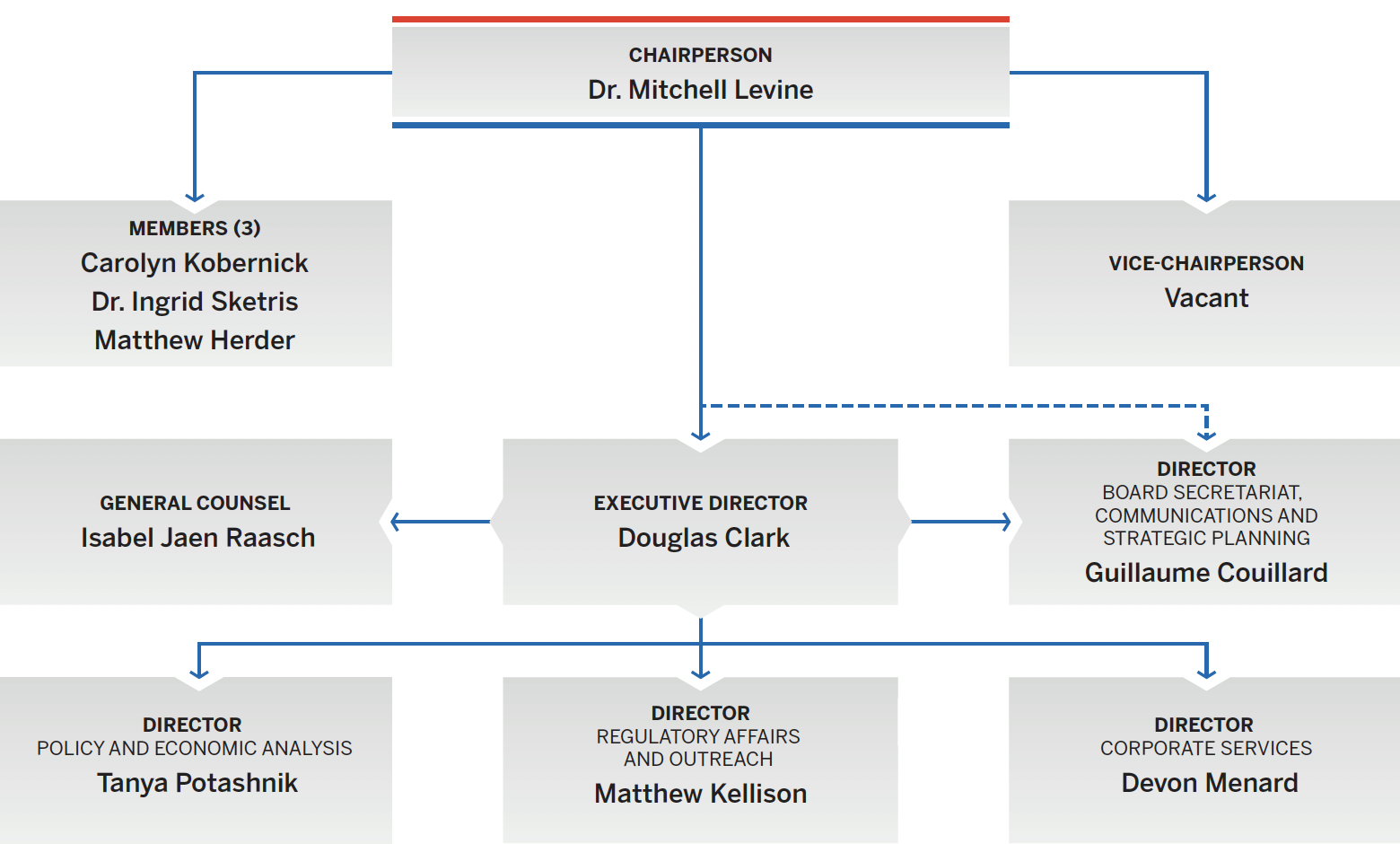
Figure description
This organizational chart illustrates the high-level reporting structure within the PMPRB, and lists the current Board and Senior Staff members. Board: Chairperson— Dr. Mitchell Levine; Vice-Chairperson— Mélanie Bourassa Forcier; Members— Carolyn Kobernick, Dr. Ingrid Sketris, Matthew Herder. Senior Staff: Executive Director—Douglas Clark; General Counsel—Isabel Jaen Raasch; Director Board Secretariat, Communications and Strategic Planning—Guillaume Couillard; Director Policy and Economic Analysis—Tanya Potashnik; Director Regulatory Affairs and Outreach— Matthew Kellison; Director Corporate Services—Devon Menard.
Executive Director
The Executive Director is responsible for advising the Board and for the leadership and management of Staff.
Regulatory Affairs and Outreach
The Regulatory Affairs and Outreach Branch reviews the prices of patented medicines sold in Canada to ensure they are not excessive; ensures that patentees are fulfilling their filing obligations; encourages patentees to comply voluntarily with the PMPRB’s Guidelines; implements related compliance policies; and investigates complaints into the prices of patented medicines.
Policy and Economic Analysis
The Policy and Economic Analysis Branch develops policy and strategic advice; leads stakeholder consultations and makes recommendations on possible amendments to the PMPRB’s Guidelines; conducts research and analysis on the prices of medicines, pharmaceutical market developments and R&D trends; and publishes studies aimed at providing F/P/T governments and other interested stakeholders with centralized, objective, and credible information in support of evidence based policy.
Corporate Services
The Corporate Services Branch provides advice and services in relation to human resources management; facilities; procurement; health, safety and security; information technology; and information management. It is also responsible for financial planning and reporting, accounting operations, audit and evaluation, and liaising with federal central agencies on these topics.
Board Secretariat, Communications and Strategic Planning
The Board Secretariat, Communications and Strategic Planning Branch develops and manages the PMPRB’s communications, media relations, and public enquiries; manages the Board’s meeting and hearing processes, including the official record of proceedings; and coordinates activities pursuant to the Access to Information Act and the Privacy Act. It is also responsible for strategic planning and reporting.
General Counsel
The General Counsel advises the PMPRB on legal matters and leads the legal team representing Staff in proceedings before the Board.
Budget
In 2018–19, the PMPRB had a budget of $14.872 million and an approved staff level of 72 full-time equivalent employees.
| 2017–18 | 2018–19 | 2019–20 | |
|---|---|---|---|
| Budget* | 10,866,321 | 14,871,872 | 16,612,511 |
| Salaries and employee benefits | 6,896,051 | 8,373,171 | 9,636,550 |
| Operating | 1,532,270 | 3,079,220 | 2,699,395 |
| Special Purpose Allotment** | 2,438,000 | 3,419,481 | 4,276,566 |
| Full Time Employees (FTEs) | 66 | 72 | 82 |
* The amounts are based on the Main Estimates.
** The Special Purpose Allotment is reserved strictly for external costs of public hearings (legal counsel, expert witnesses, etc.). Unspent funds are returned to the Consolidated Revenue Fund.
Regulating Prices of Patented Medicines: Continued Vigilance Necessary
Medical advancements have introduced many innovative new medicines to the Canadian marketplace to improve existing treatments and to treat conditions that previously had no pharmaceutical therapy. However, many of these new medicines come at a very high cost. Since 1987, pharmaceutical costs in Canada have grown at an average annual rate of 7.2%Footnote 1, outpacing all other health care costs and growing at well over 3 times the pace of inflation. At 15.7% of total health care spending, pharmaceuticals now rank ahead of spending on physicians.Footnote 2 About 1 in 5 Canadians reports having no prescription medicine coverage and many more are under-insured or face high deductibles or co-pays. Almost 1 in 10 Canadians have had to forego filling a prescription medicine in the past year for reasons related to cost.Footnote 3
The PMPRB protects the interests of Canadian consumers by ensuring that the prices of patented medicines sold in Canada are not excessive. It does this by reviewing the prices that patentees charge for each individual patented medicine and by ensuring that patentees reduce their prices and pay back excess revenues where appropriate.
Reporting Requirements
By law, patentees must file information about the sale of their medicines in Canada. The Act, along with the Patented Medicines Regulations (Regulations) set out the information required and Staff reviews pricing information on an ongoing basis until all relevant patents have expired.
There are several factors used for determining whether the price of a medicine is excessive, as outlined in section 85 of the Act.
The Compendium of Policies, Guidelines and Procedures (Guidelines) details the price tests used by Staff when it reviews the prices of patented medicines. The Guidelines are not binding and were developed in consultation with stakeholders, including the provincial and territorial Ministers of Health, consumer groups, and the pharmaceutical industry. When an investigation determines that the price of a patented medicine may be excessive, the patentee is offered the opportunity to voluntarily lower its price and/or refund its excess revenues through a Voluntary Compliance Undertaking (VCU). If the patentee disagrees with the findings of the investigation and chooses not to submit a VCU, the Chairperson may issue a Notice of Hearing. After hearing the evidence, if the Board finds that a price is excessive, it can issue an order requiring a patentee to reduce that price and/or refund excess revenues. Copies of the Act, the Regulations, the Guidelines, and the Patentee’s Guide to Reporting are available on the PMPRB’s website.
Failure to Report
The PMPRB relies on patentees’ full and timely disclosure of any and all patented medicines being sold in Canada to which a patent pertains. In 2018, 5 medicines were reported to the PMPRB for the first time despite being patented and sold prior to 2018. (See Table 2, Failure to Report the Sale of Patented Medicines)
Failure to File Price and Sales Data (Form 2)
Failure to file refers to the complete or partial failure of a patentee to file the information required by the Act and the Regulations to the PMPRB. There were no Board Orders issued for failure to file in 2018.
| Patentee | Brand Name | Medicinal Ingredient | Year Medicine Reported to the PMPRB as Under PMPRB’s Jurisdiction |
Year Medicine Reported to the PMPRB witith Subsequent Patent |
|---|---|---|---|---|
| Alkermes Inc. | Vivitrol | naltrexone | 2016 | blank |
| Avir Pharma Inc. | Cresemba | isavuconazole | 2016 | blank |
| Shire Pharma Canada Inc. | Xiidra | lifitegrast | 2018 | blank |
| Cipher Pharmaceuticals Inc. | Xydalba | Dalbavancin | 2018 | blank |
| Taiho Oncology Inc. | Lonsurf (2 DINs) | trifluridine/tipiracil | 2017 | blank |
Data source: PMPRB
Scientific Review
Human Drug Advisory Panel
A scientific evaluation is done on all new patented medicines as part of the price review process. The PMPRB established the Human Drug Advisory Panel (HDAP) to provide independent expertise and advice to Staff. HDAP conducts an evaluation when a patentee claims the new medicine provides a therapeutic improvement. HDAP members review and evaluate the appropriate scientific information available, including any submission by a patentee about the proposed level of therapeutic improvement, the selection of comparator medicines, and comparable dosage regimens.
HDAP evaluates the therapeutic benefit of new patented medicines according to the following definitions:
- Breakthrough: A medicine that is the first one sold in Canada to effectively treat a particular illness or effectively address a particular indication.
- Substantial Improvement: A medicine that, relative to other medicines sold in Canada provides substantial improvement in therapeutic effects.
- Moderate Improvement: A medicine that, relative to other medicines sold in Canada provides moderate improvement in therapeutic effects.
- Slight or No Improvement: A medicine that, relative to other medicines sold in Canada, provides slight or no improvement in therapeutic effects.
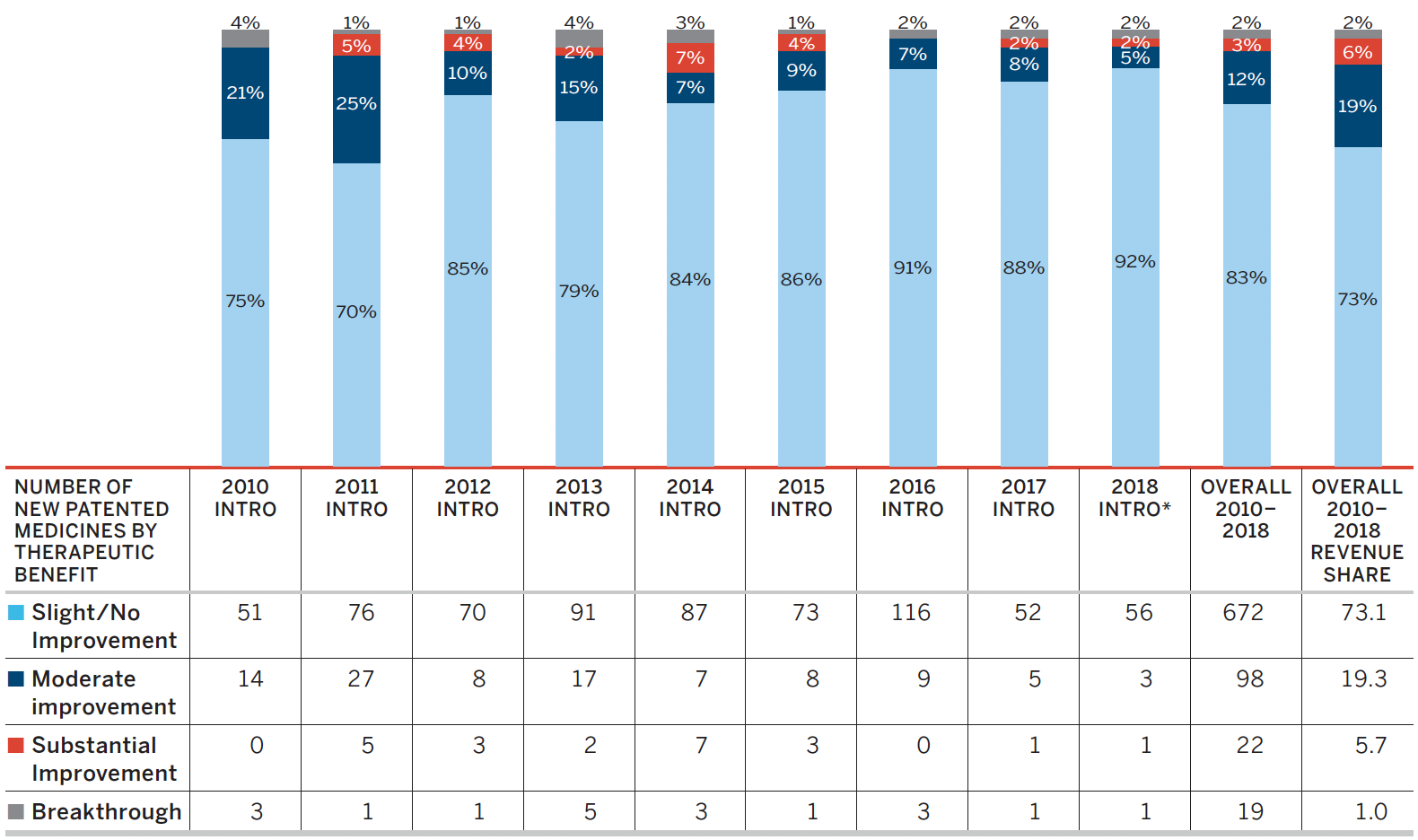
* Assessment as of March 31, 2019
Data source: PMPRB
Figure description
This bar graph depicts the breakdown in percentages of new patented medicines by therapeutic benefit over existing medicines in the year of introduction for the period 2010 to 2018. In 2010: 75% new patented medicines were slight or no improvement, 21% were moderate improvement, 0% were substantial improvement, and 4% were breakthrough; 2011: 70% new patented medicines were slight or no improvement, 25% were moderate improvement, 5% were substantial improvement, and 1% was breakthrough; 2012: 85% new patented medicines were slight or no improvement, 10% were moderate improvement, 4% were substantial improvement, and 1% was breakthrough; 2013: 79% new patented medicines were slight or no improvement, 15% were moderate improvement, 2% were substantial improvement, and 4% were breakthrough; 2014: 84% new patented medicines were slight or no improvement, 7% were moderate improvement, 7% were substantial improvement, and 3% were breakthrough; 2015: 86% new patented medicines were slight or no improvement, 9% were moderate improvement, 4% were substantial improvement, and 1% breakthrough; 2016: 91% new patented medicines were slight or no improvement, 7% were moderate improvement, 0% were substantial improvement, and 2% were breakthrough; 2017: 88% new patented medicines were slight or no improvement, 8% were moderate improvement, 2% was substantial improvement, and 2% was breakthrough; 2018: 92% new patented medicines were slight or no improvement, 5% were moderate improvement, 2% was substantial improvement, and 2% was breakthrough. The therapeutic benefit of all new patented medicines introduced from 2010 to 2018 is depicted by the Overall 2010-2018 bar. From 2010-2018; 83% new patented medicines were slight or no improvement, 12% were moderate improvement, 3% were substantial improvement, and 2% were breakthrough. The Overall 2010-2018 Revenue Share depicts the percentage of sales related to each level of therapeutic benefit: 73% of sales were medicines of little or no improvement, 19% were medicines of moderate improvement, 6% were medicines of substantial improvement, and 2% were breakthroughs.
Figure 1 illustrates the breakdown of new patented medicines in the year of introduction by therapeutic benefit for 2010 to 2018. The largest percentage of patented medicines (82.9%) introduced since 2010 were categorized as “Slight or No Improvement” in therapeutic benefit over existing therapies.Footnote 4
The “Overall 2010–2018” bar represents the therapeutic benefit breakdown for all new patented medicines introduced from 2010 to 2018. The “Overall 2010–2018 Revenue Share” bar illustrates the revenue share by therapeutic benefit for all new patented medicines introduced from 2010 to 2018.
Our motto

Protect, Empower, Adapt.
Price Review
The PMPRB reviews the average price of each strength of an individual dosage form of each patented medicine. In most cases, this unit is consistent with the Drug Identification Number(s) (DIN), (DINs) assigned by Health Canada at the time the medicine is approved for sale in Canada.
New Patented Medicines Reported to the PMPRB in 2018
For the purpose of this report, a new patented medicine in 2018 is defined as any patented medicine first sold in Canada, or previously sold but first patented, between December 1, 2017, and November 30, 2018.
There were 108 new patented medicines for human use reported as sold in 2018. Some are one or more strengths of a new active substance and others are new presentations of existing medicines. Of these 108 new patented medicines, 12 (11.1%) were being sold in Canada prior to the issuance of the Canadian patent that brought them under the PMPRB’s jurisdiction. Table 3 shows the year of first sale for these medicines.
| Year First Sold | Number of Medicines |
|---|---|
| 2014 | 2 |
| 2015 | 2 |
| 2016 | 7 |
| 2017 | 1 |
| 2018 | 96 |
| Total | 108 |
Data source: PMPRB
The list of New Patented Medicines Reported to PMPRB is available on the PMPRB’s website under “Regulating Prices”. This list includes information on the status of the review (i.e., whether the medicine is under review, within the Guidelines, under investigation, or subject to a VCU or Notice of Hearing). Figure 2 illustrates the number of new patented medicines for human use reported to the PMPRB from 1989 to 2018.
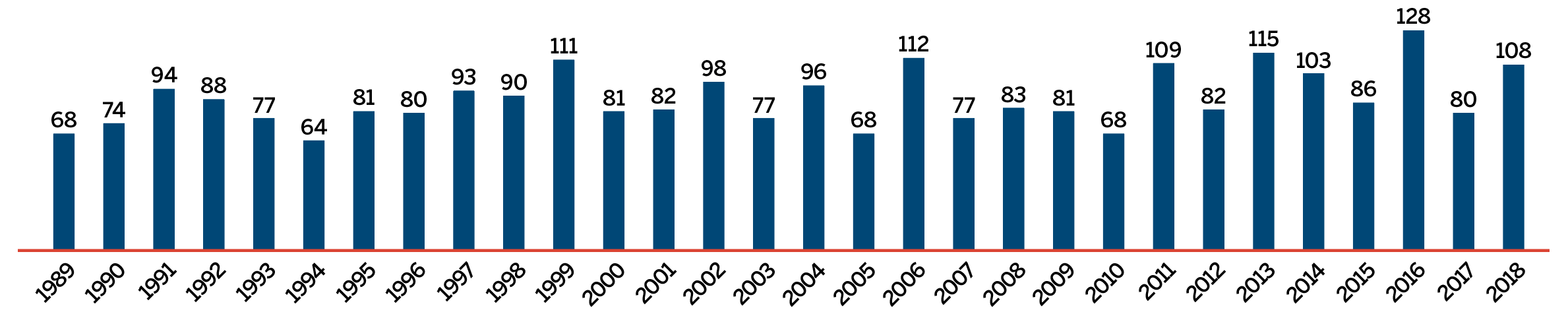
Data source: PMPRB
Figure description
This bar graph depicts the number of new patented medicines for human use reported to the Patented Medicine Prices Review Board by year. In 1989, 68 patented medicines for human use were reported to the PMPRB.
In 1990: 74; 1991: 94; 1992: 88; 1993: 77; 1994: 64; 1995: 81; 1996: 80; 1997: 93; 1998: 90; 1999: 111; 2000: 81; 2001: 82; 2002: 98; 2003: 77; 2004: 96; 2005: 68; 2006: 112; 2007: 77; 2008: 83; 2009: 81; 2010: 68; 2011: 109; 2012: 82; 2013: 115; 2014: 103; 2015: 86; 2016: 128; 2017: 80; 2018: 108.
Of the 108 new patented medicines, the prices of 61 had been reviewed as of March 31, 2019:
- 39 were found to be within the thresholds set out in the Guidelines;
- 6 were at a level that appeared to exceed the thresholds set out in the Guidelines by an amount that did not trigger the investigation criteria; and
- 16 were at levels that appeared to exceed the thresholds set out in the Guidelines and resulted in investigations being commenced.
- 8 of the 16 investigations were resolved by VCUs.
For a complete list of the 108 new patented medicines and their price review status, see Appendix 2.
Price Review of Existing Patented Drug Products for Human Use in 2018
For the purpose of this report, existing patented medicines include all patented medicines first sold and reported to the PMPRB prior to December 1, 2017.
At the time of this report, there were 1,295 existing patented medicines:
- 929 were priced within the thresholds set out in the Guidelines;
- 226 had prices that appeared to exceed the thresholds set out in the Guidelines by an amount that did not trigger the investigation criteria;
- 120 were the subject of investigations;
- 4 were under review;
- 13 were the subject of a Voluntary Compliance Undertaking;
- 2 are the subject of a hearing; and
- 1 is subject to a price reduction and excess revenue payment order (currently partially stayed).
Table 4 provides a summary of the status of the price review of the new and existing patented medicines for human use in 2018.
| New Medicines Introduced in 2018 | Existing Medicines | Total | |
|---|---|---|---|
| Total | 108 | 1295 | 1403 |
| Within Guidelines Thresholds | 39 | 929 | 968 |
| Under Review | 47 | 4 | 51 |
| Does Not Trigger Investigation | 6 | 226 | 232 |
| Under Investigation | 8 | 120 | 128 |
| Subject to Voluntary Compliance Undertaking | 8 | 13 | 211 |
| Price Hearing | 0 | 2 | 2 |
| Subject to Price Reduction Order (Stayed) | 0 | 1 | 1 |
1The terms and conditions of previous years VCUs that have carried over into 2018 are not captured in this count.
Data source: PMPRB
Update From the 2017 Annual Report
- Reviews of all but one medicine for human use that were reported as Under Review in the 2017 Annual Report have been completed.
- 98 of the 122 investigations reported in the 2017 Annual Report resulted in one of the following:
- the closure of the investigation where it was concluded the price was within the thresholds set out in the Guidelines;
- a VCU by the patentee to reduce the price and offset excess revenues through a payment and/or a reduction in the price of another patented medicine (see Voluntary Compliance Undertakings); or
- a public hearing to determine whether the price was excessive, including any remedial Order determined by the Board (see Hearings).
Patented Over-the-Counter Medicines and Patented Medicines For Veterinary Use
Staff reviews the prices of patented over-the-counter medicines, patented generic medicines and patented veterinary medicines only when they receive a complaint. No such complaints were received in 2018.
Voluntary Compliance Undertakings and Hearings
Voluntary Compliance Undertakings
A VCU is a written undertaking by a patentee to adjust its price to conform to the Board’s Guidelines. The Guidelines set out procedures for patentees to submit a VCU when Staff concludes, following an investigation, the price of a patented medicine sold in Canada appears to have exceeded the thresholds set out in the Guidelines. A VCU represents a promise by a patentee geared towards a satisfactory resolution of an investigation initiated by Staff as per the Guidelines. A VCU takes into account the specific facts and underlying context of a particular case. As such, VCUs are not intended to have precedential value.
In 2018, seven VCUs were finalized. In addition to price reductions for certain medicines, excess revenues totaling $315,070.73 were offset by way of payments to the Government of Canada.
In 2019, as of May 31, 2019, the Chairperson approved the consideration of five more VCUs totalling $2.2 million bringing the total payments to the Government of Canada for 2018 up to May 31, 2019 to $2.5 million.
| Patented Medicine Brand Name | Therapeutic Use | Patentee | Date of Approval | Offset of Excessive Revenues | |
|---|---|---|---|---|---|
| Price Reduction | Payment to the Government | ||||
| VCUs IN 2018 | |||||
| Travoprost and timolol ophthalmic solution (sold under trade name DuoTrav®PQ) (1 DIN) | Reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension who are insufficiently responsive to beta-blockers, prostaglandins, or other IOP lowering agents and when the use of DuoTrav® PQ (the fixed combination drug) is considered appropriate. | Novartis Pharmaceuticals Canada Inc. | January | ✓ | $275,000.00 |
| Methotrexate (sold under trade name Metoject® Subcutaneous) (4 DINs) | A Disease Modifying Antirheumatic Drug (“DMARD”) in the following diseases where standard therapeutic interventions fail:
|
Medexus Inc. | January | ✓ | |
| Brimonidine Gel (sold under trade name Onreltea) (1 DIN) | Topical treatment of facial erythema of rosacea in adults 18 years of age or older. | Galderma Canada Inc. | February | ✓ | |
| Panitumumab (sold under trade name Vectibix) (1 DIN) | Treatment of previously untreated patients with non-mutated (wild-type) RAS metastatic colorectal carcinoma in combination with FOLFOX (infusional 5-fluorouracil, leucovorin, and oxaliplatin). Also, as monotherapy for the treatment of patients with non-mutated (wild-type) TAS mCRC after failure of fluoropyrimidine-, oxaliplatim-, and irinotecan-containing chemotherapy regimens. | Amgen Canada Inc. | February | ✓ | |
| Evolocumab (sold under trade name Repatha) (1 DIN) | An adjunct to diet and maximally tolerated statin therapy in adult patients with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (CVD) who require additional lowering of low density lipoprotein cholesterol (LDL-C). | Amgen Canada Inc. | May | ✓ | $40,070.73 |
| Levofloxacin (sold under trade name Quinsair) (1 DIN) | Management of cystic fibrosis (CF) in patients aged 18 years or older with chronic pulmonary Pseudomonas aeruginosa (P. aeruginosa) infections. | HZNP Canada Limited | June | ✓ | |
| Olaparib (sold under trade name Lynparza) (3 DINs) | Monotherapy for maintenance treatment of adult patients with platinum-sensitive relapsed (PSR) BRCA-mutated (germline or somatic) high grade serous epithelial ovarian, fallopian tube or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. Monotherapy for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated human epidermal growth factor receptor 2 negative metastatic breast cancer who have previously been treated with chemotherapy in the neoadjuvant, adjuvant or metastatic setting. | AstraZeneca Canada Inc. | November | ✓ | |
| Total as of December 31, 2018 | $315,070.73 | ||||
| Triptorelin (sold under trade name Trelstar) (1 DIN) | Palliative treatment of hormone dependent advanced carcinoma of the prostate gland (stage D2) | Paladin Labs Inc. | January 2019 | ✓ | $157,159.70 |
| Belimumab (sold under trade name Benlysta) (2 DINs) | An adjunct to standard therapy for reducing disease activity in adult patients with active, autoantibody-positive, systemic lupus erythematosus (SLE) | GlaxoSmithKline Inc. | March 2019 | ✓ | |
| Dupilumab (sold under trade name Dupixent) (1 DIN) | Treatment of adult patients with moderateto-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. | Sanofi-aventis Canada Inc. | April 2019 | ✓ | $1,654,520.73 |
| Alirocumab (sold under trade name Praluent) (4 DINs) | An adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia (HeFH) or clinical atherosclerotic cardiovascular disease (CVD), who require additional lowering of low density lipoprotein cholesterol (LDL-C). | Sanofi-aventis Canada Inc. | April 2019 | ✕ | $426,955.62 |
| Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (sold under trade name Symtuza (1 DIN) | An antiretroviral agent indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 infection in adults and adolescents (aged 12 years and older with a body weight at least 40 kilograms) and with no known mutations associated with resistance to the individual components of Symtuza. | Janssen Inc. | April 2019 | ✕ | $4,590.73 |
| Total as of May 31, 2019 | $2,558,297.51 | ||||
Hearings
The PMPRB holds hearings into two types of matters:
- excessive pricing; and
- failure to file–jurisdiction.
Excessive Pricing
In the event that the price of a patented medicine appears to be excessive, the Chairperson can commence a public hearing. If the Hearing Panel finds the price is excessive, it can issue an order to reduce the price of the patented medicine in question (or of another patented medicine of the patentee) and/or to offset revenues received as a result of the excessive price. Judicial review of Board decisions can be sought in the Federal Court of Canada.
In January 2019, the PMPRB announced it would hold a public hearing in the matter of the price of the patented medicine cysteamine bitartrate sold under the trade name Procysbi by Horizon Therapeutics Canada. The purpose of this hearing is to determine whether the medicine has been or is being sold in any market in Canada at a price that, in the Board’s opinion, is or was excessive: and, if so, what order, if any, should be made to remedy the excessive pricing. The matter is ongoing.
Failure to File–Jurisdiction
When Staff believes a patentee has failed or refused to provide the PMPRB the pricing and sales information required by law, Staff will recommend that the Chairperson call a public hearing to determine whether the patentee has, in fact, breached the reporting requirements of the Act and Regulations. If the Hearing Panel finds, as the result of a public hearing, that the patentee has failed to report the required information, the Hearing Panel can order the patentee to file the required pricing and sales information.
There were no failure to file hearings as of March 31, 2019.
Summary
Excess revenues totaling $6,803,627.11 were offset by payments to the Government of Canada through VCUs and Board Orders in 2018 and up to May 31, 2019.
Since 1993, 145 VCUs have been approved and 30 public hearings initiated. These measures resulted in price reductions and the offset of excess revenues by additional price reductions and/or payments to the Government of Canada. Over $209 million has been collected through VCUs, settlements and Board Orders through payments to the Government of Canada and/or to customers such as hospitals and clinics.
Matters Before the Federal Court, Federal Court of Appeal and Supreme Court of Canada
On October 20, 2017, Alexion Pharmaceuticals Inc. filed an application for judicial review of the Board’s decision dated September 20, 2017 in respect of its finding that the patented medicine eculizumab sold under the trade name Soliris was being sold at an excessive price in Canada and ordering Alexion to lower its price (currently stayed) and make an excess revenue payment of $4,245,329.60. The Board’s decision was found to be reasonable by the Federal Court via a decision dated May 23, 2019. Alexion has appealed the Federal Court’s decision in the Federal Court of Appeal.
On January 18, 2017, Galderma Canada Inc. filed an application for judicial review of the Board’s decision dated December 19, 2016. In that decision the Board found that Canadian Patent No. 2,478,237 pertains to the patented medicine eculizumab sold under the trade name Differin and ordered Galderma to file the required information for the period between January 1, 2010 and March 14, 2016. The Federal Court granted Galderma’s judicial review application on November 9, 2017 and quashed the Board’s decision. On November 21, 2017, the Attorney General appealed the Federal Court’s grant of the judicial review application. On June 28, 2019, the Federal Court of Appeal granted the appeal and issued its decision sending the matter back to the Board for redetermination. The Board’s decision on redetermination is pending.
There are no matter before the Supreme Court of Canada.
| Medicine | Indication/Use | Patentee | Issuance of Notice of Hearing | Status |
|---|---|---|---|---|
| Eculizumab (sold under trade name Soliris) | Paroxysmal nocturnal hemoglobinuria Atypical hemolytic uremic syndrome |
Alexion Pharmaceuticals Inc. | January 20, 2015 | Board Order: September 27, 2017 Found the price of Soliris was and is excessive under Sections 83 & 85 of the Act Payment of excess revenues: $4,245,329.60 |
| Cysteamine bitartrate (sold under trade name Procysbi) | Nephropathic cystinosis | Horizon Thearpeutics Canada | January 14, 2019 | Ongoing |
| Medicine | Indication/Use | Patentee | Issuance of Notice of Hearing | Status |
|---|---|---|---|---|
| NIL |
| Medicine | Indication/Use | Patentee | Issuance of Notice of Hearing | Status |
|---|---|---|---|---|
| Eculizumab (sold under trade name Soliris) | Paroxysmal nocturnal hemoglobinuria Atypical hemolytic uremic syndrome |
Alexion Pharmaceuticals Inc. | Allegations of excessive pricing | Notice of Appeal (Federal Court of Appeal) filed on June 21, 2019. Court File A-237-19, Matter pending Notice of Application for Judicial Review – October 20, 2017 Court File T-1596-17 (Re. Board Panel’s decision of September 20, 2017): Hearing held November 15–16, 2018 Decision issued May 23, 2019. |
| Adapalene (sold under trade names Differin and Differin XP) | Acne | Galderma Canada Inc. | Failure to file (jurisdiction) | Notice of Appeal – November 21, 2017 Court File A-385-17: Hearing held January 17, 2019. Decision issued on June 28, 2019. Matter sent for redetermination by the Board. Redetermination decision pending. Court File T-83-17 Re. Board Panel’s decision of December 19, 2016): Decision rendered November 9, 2017 quashing in part Board Panel’s decision (appealed – see above) |
Key Pharmaceutical Trends: More Expensive Medicines Continue to Influence Sales
Overall spending on pharmaceuticals is influenced by many factors, including price, utilization, the entry of newer, more expensive medicines, and the loss of market exclusivity of older patented medicines. In 2018, there was a sizable increase in the volume of patented medicines sold, as well as a rise in the sales of more expensive medicines. At the same time, some key, top-selling medicines stopped reporting sales to the PMPRB, and as a result, the total spending on patented medicines decreased slightly by 0.6%. Canadian list prices of patented medicines remained among the highest in the Organisation for Economic Co-operation and Development (OECD) countries, ranking fourth, well behind the US and marginally less than Germany and Switzerland.
The PMPRB is responsible for reporting on trends in pharmaceutical sales and pricing for all medicines and for reporting research and development spending by patentees.
Under the Regulations, patentees are required to submit detailed information on their sales of patented medicines, including quantities sold, gross and net prices, and net revenues. The PMPRB uses this information to analyze trends in the sales, prices,Footnote 5 and use of patented medicines.Footnote 6 This section provides key trends, including analyses of Canadian national, public, and private payer markets for all medicines. Note that any reference to sales in this section should be interpreted as sales revenues unless otherwise noted.
$16.7 Billion Sales in Patented Medicines in 2018
Sales of patented medicines have grown by an average of 4.9% per year over the last five years.
Disclaimers
- Although select statistics reported in the KEY PHARMACEUTICAL TRENDS section are based in part on data obtained under license from the IQVIA MIDAS® database and the IQVIA Private Pay Direct Drug Plan Database, the statements, findings, conclusions, views, and opinions expressed in this Annual Report are exclusively those of the PMPRB and are not attributable to IQVIA.
- To provide a broader perspective on pharmaceutical trends in Canada, summaries of the results of NPDUIS analyses have been included as additional “Brief Insights” throughout the Pharmaceutical Trends section of the Annual Report. A variety of public and licensed data sources are used for NPDUIS analytical studies. Many of these sources do not differentiate between patented and non-patented generic medicines; in these instances, the general term “generic” is used to include both. NPDUIS is a research initiative that operates independently of the regulatory activities of the PMPRB.
Trends in Sales of Patented Medicines
Canadians spend much more on patented medicines today than they did a decade ago. Over the last five years, sales of these medicines grew by an average of 4.9% per year, reaching $16.7 billion in 2018. This section looks at the most important factors driving the change in sales revenues from 2017 to 2018 and compares them to trends from previous years.
Trends in Sales Revenues
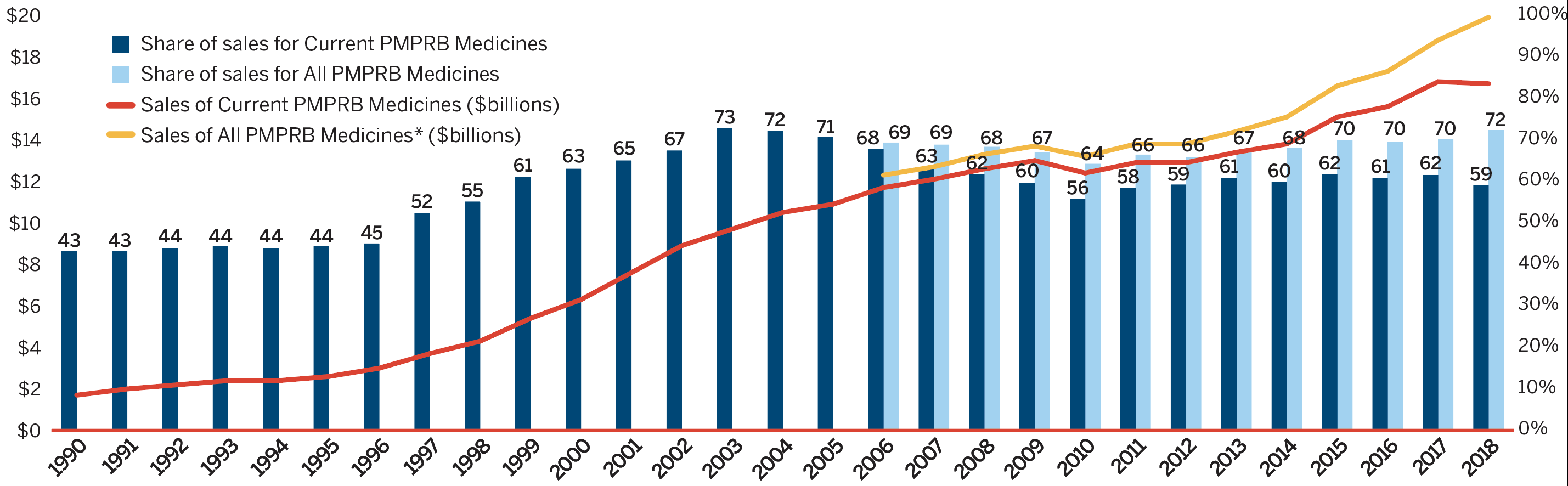
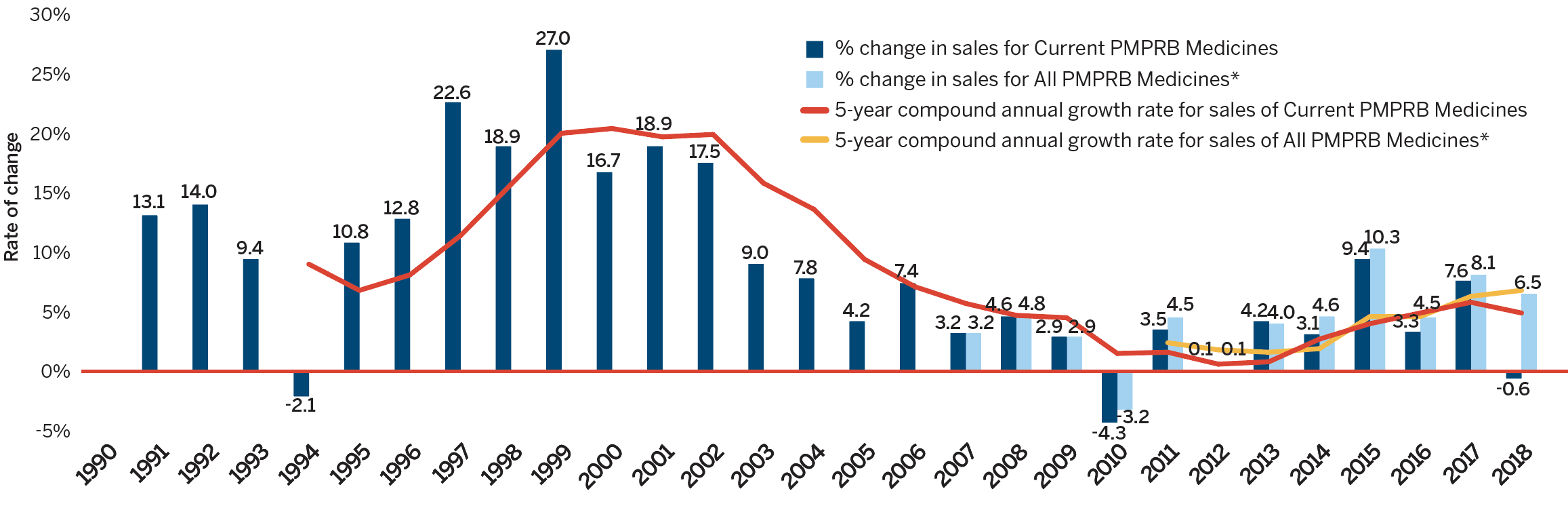
In 2018, the sales of patented medicines remained relatively unchanged, decreasing slightly by 0.6% from the previous year. Figure 3 reports on trends in the sales of patented medicines from 1990 to 2018. While annual sales have increased approximately 10-fold since 1990, the year-over-year rate of change within that period has varied. This trend is highlighted by the five-year compound annual growth rate given in Figure 3(b).
Figure 3(a) gives the sales of patented medicines as a share of overall medicine sales. This share, which reached a peak of 72.7% in 2003, declined from 2004 to 2010. Since then, patented medicines have accounted for approximately 60% of the sales of all medicines in Canada.
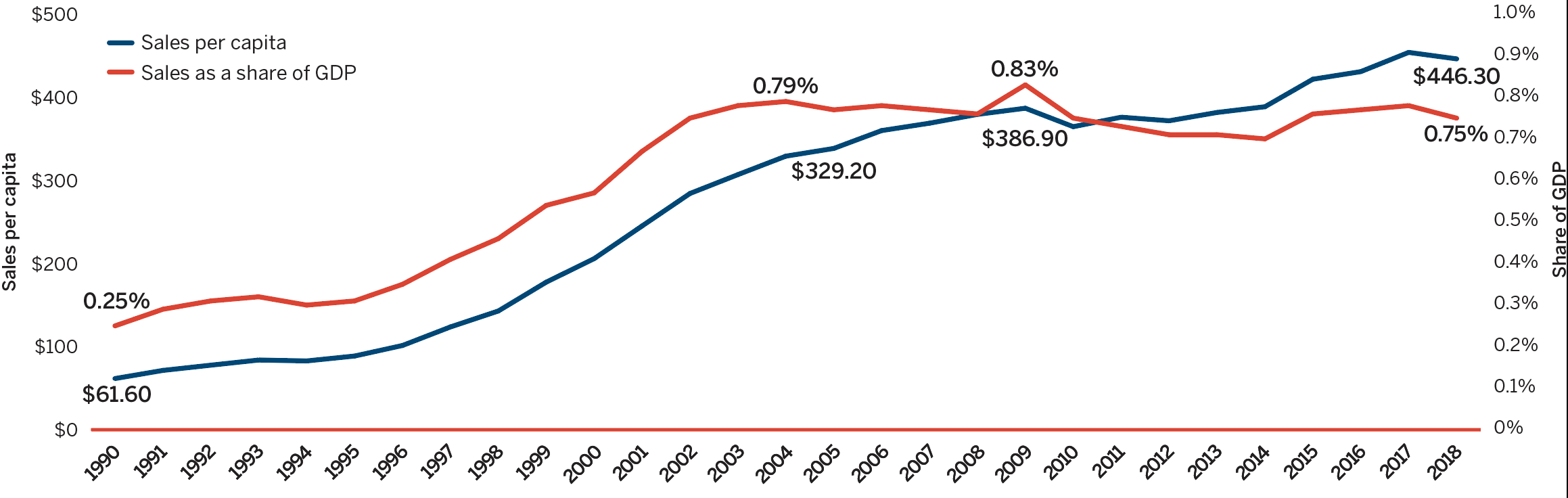
The trend in sales per capita and sales as a percentage of the gross domestic product (GDP) show the increasing importance of patented medicines in the Canadian economy. Overall, patented medicine per capita sales rose from $61.60 in 1990 to $446.30 in 2018, while sales as a percentage of GDP rose from 0.25% in 1990 to 0.75% in 2018 [Figure 3(c)].
To highlight the continuing impact of patented medicines, Figures 3(a) and 3(b) also provide results for “All PMPRB Medicines”. This broader category includes all medicines, current and historic, that ever reported sales to the PMPRB (since its creation).
In 2018, medicines that previously reported to the PMPRB accounted for estimated sales of $3.3 billion, or 11.6% of all sales. This is a marked increase over the previous year and considerably more than a decade ago when medicines that formerly reported to the PMPRB accounted for $0.7 billion in sales, or 3.2% of all sales
It is noted that the sales for All PMPRB Medicines rose by 6.5% in 2018, as compared to the decline of 0.6% observed among Current Patented Medicines. This is due to a handful of medicines that stopped reporting to the PMPRB in 2018, including Remicade, the highest-selling prescription medicine in Canada. Historically, patented medicines have experienced a substantial erosion in market share upon loss of patent protection; however, recently that same effect has not been observed in a number of very high-cost, biologic medicines that have come off patent.
A complete table of the data presented in Figure 3 for patented medicines currently reporting to the PMPRB is given in Appendix 3.
Figure 3. Trends in Patented Medicine Sales, 1990 to 2018
* Includes sales of currently patented medicines and medicines that once reported to the PMPRB but are no longer reporting a patent.
Data source: PMPRB; MIDAS® database, 1990–2018, IQVIA (all rights reserved)
Figure description
This line and bar graphic depicts the annual sales of patented medicines currently reporting to the PMPRB and patented medicines that once reported to the PMPRB but are no longer reporting a patent and the patented medicine share of sales for current PMPRB medicines and sales for all PMPRB medicines, for the period from 1990 to 2018. In 1990, the sales of current PMPRB patented medicines was $1.7 billion and the current PMPRB patented medicine share of all medicine sales was 43.2%. In 1991: $2.0 billion, 43.2%; 1992: $2.2 billion, 43.8%; 1993: $2.4 billion, 44.4%; 1994: $2.4 billion, 43.9%; 1995: $2.6 billion, 44.4%; 1996: $3.0 billion, 45.0%; 1997: $3.7 billion, 52.3%; 1998: $4.3 billion, 55.1%; 1999: $5.4 billion, 61.0%; 2000: $6.3 billion, 63.0%; 2001: $7.6 billion, 65.0%; 2002: $8.9 billion, 67.4%; 2003: $9.7 billion, 72.7%; 2004: $10.5 billion, 72.2%; 2005: $10.9 billion, 70.6%;
2006: the sales of current PMPRB medicines was $11.7 billion, current PMPRB medicines share of all medicine sales was 67.8%, the sales of all PMPRB medicines was $12.3 billion and the share of sales for all PMPRB medicines was 69.3%;
2007: $12.1 billion, 63.2%, $12.7 billion, 68.8%;
2008: $12.6 billion, 61.7%, $13.3 billion, 68.3%;
2009: $13.0 billion, 59.6%, $13.7 billion, 67.0%;
2010: $12.4 billion, 55.8%, $13.2 billion, 64.2%;
2011: $12.9 billion, 58.3%, $13.8 billion, 66.4%;
2012: $12.9 billion, 59.2%, $13.8 billion 65.8%;
2013: $13.4 billion, 60.7% $14.4 billion, 67.3%;
2014: $13.8 billion, 59.9%, $15.1 billion, 68.1%;
2015: $15.1 billion, 61.6%, $16.6 billion, 69.9%;
2016: $15.6 billion, 60.8%, $17.3 billion, 69.5%;
2017: $16.8 billion, 61.5%, $18.8 billion, 70.1%;
2018: $16.7 billion, 59.0%, $19.9 billion, 72.3%.
* Includes sales of currently patented medicines and medicines that once reported to the PMPRB but are no longer reporting a patent.
Data source: PMPRB; MIDAS® database, 1990–2018, IQVIA (all rights reserved)
Figure description
This line and bar graphic depicts the annual rate of change in patented medicine sales and the
5-year compound annual growth rate from 1990 to 2018 for patented medicines currently reporting to the PMPRB and patented medicines that once reported to the PMPRB but are no longer reporting a patent. In 1991, the rate of change in sales for Current PMPRB medicines was 13.1%. In 1992: 14.0%; 1993: 9.4%.
In 1994, the rate of change in sales for Current PMPB medicines was -2.1% and the 5-year compound annual growth rate for sales of Current PMPRB medicines was 9.0%.
1995: 10.8%, 6.8%;
1996: 12.8%, 8.1%;
1997: 22.6%, 11.4%;
1998: 18.9%, 15.7%;
1999: 27.0%, 20.0%;
2000: 16.7%, 20.4%;
2001: 18.9%, 19.7%;
2002: 17.5%, 19.9%;
2003: 9.0%, 15.8%;
2004: 7.8%, 13.6%;
2005: 4.2%, 9.4%;
2006: 7.4%, 7.1%;
In 2007: the rate of change in sales for Current PMPRB medicines was 3.2%, the 5-year compound annual growth rate for sales of Current PMPRB medicines was 5.7% and the rate of change in sales for All PMPRB medicines was 3.2%.
2008: 4.6%, 4.7%, 4.8%;
2009: 2.9%, 4.5%, 2.9%;
2010: -4.3%, 1.5%, -3.2%;
In 2011, the rate of change in sales for Current PMPRB medicines was 3.5%, the 5-year compound annual growth rate for sales of Current PMPRB medicines was 1.6%, the rate of change in sales for All PMPRB medicines was 4.5%, and the 5-year compound annual growth rate for sales of All PMPRB medicines was 2.4%;
2012: 0.1%, 0.6%, 0.1%, 1.8%;
2013: 4.2%, 0.8%, 4.0%, 1.6%;
2014: 3.1%, 2.7%, 4.6%, 1.9%;
2015: 9.4%, 4.0%, 10.3%, 4.6%;
2016: 3.3%, 4.9%, 4.5%, 4.6%;
2017: 7.6%, 5.8%, 8.1%, 6.3%;
2018: -0.6%, 4.9%, 6.5%, 6.8%.
Data source: PMPRB; Statistics Canada; OECD
Figure description
This line graphic depicts Current PMPRB medicine sales per capita and as a share of GDP from 1990 to 2018. In 1990, Current PMPRB medicine sales per capita was $61.60 and as a share of GDP 0.25. In 1991: $71.40, 0.29; 1992: $77.70, 0.31; 1993: $83.90, 0.32; 1994: $82.80, 0.30; 1995: $88.70, 0.31;
1996: $101.40, 0.35; 1997: $123.70, 0.41; 1998: $142.90, 0.46; 1999: $177.60, 0.54;
2000: $205.90, 0.57; 2001: $245.20, 0.67; 2002: $284.30, 0.75; 2003: $307.00, 0.78;
2004: $329.20, 0.79; 2005: $338.50, 0.77; 2006: $360.00, 0.78; 2007: $368.90, 0.77;
2008: $379.50, 0.76; 2009: $386.90, 0.83; 2010: $364.70, 0.75; 2011: $376.10, 0.73;
2012: $371.80, 0.71; 2013: $381.80, 0.71; 2014: $388.70, 0.70; 2015: $421.80, 0.76;
2016: $430.94, 0.77; 2017: $454.09, 0.78; 2018: $446.30, 0.75.
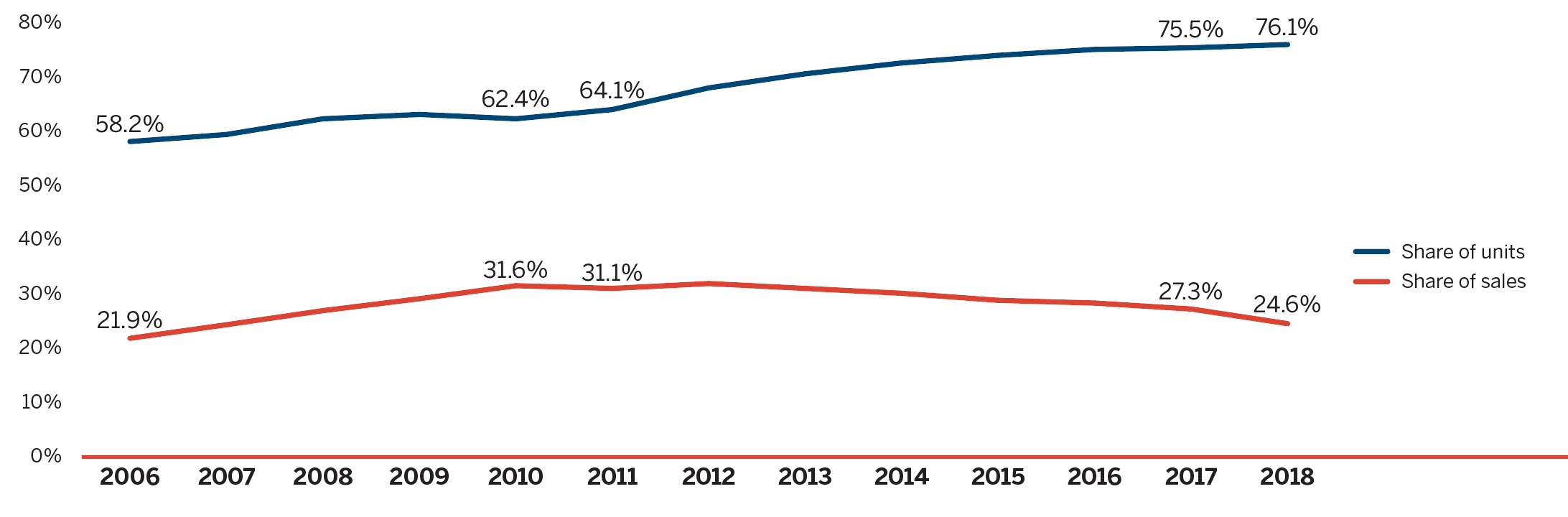
Brief Insights: Trends in the Sales of Generic Medicines
While the sales of patented medicines decreased slightly by 0.6% in 2018, sales of generic medicines dropped by a much greater rate of 7%. Generic sales have had low or negative rates of change since 2010, due in large part to the introduction of price-setting policies initiated by individual provincial governments and through the pan-Canadian Pharmaceutical Alliance (pCPA).
In 2018, the introduction of a five-year joint agreement between the pCPA and the Canadian Generic Pharmaceutical Association (CGPA) reduced the prices of 67 generic medicines to 10% or 18% of their brand reference price, driving expenditures down to virtually the same level as in 2010, even while generic use continued to increase.
Note: The results reflect prescription sales in the national retail market based on manufacturer ex-factory list prices.
Data source: MIDAS® database, IQVIA (all rights reserved)
[NPDUIS Report: Generics360, 2018]
Figure description
This line graph compares generic medicine share of the Canadian pharmaceutical retail market by percentage of units and percentage of sales from 2006 to 2018.
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Share of units |
58.2% |
59.5% |
62.4% |
63.2% |
62.4% |
64.1% |
68.1% |
70.7% |
72.7% |
74.1% |
75.2% |
75.5% |
76.1% |
Share of sales |
21.9% |
24.4% |
27.0% |
29.2% |
31.6% |
31.1% |
32.0% |
31.1% |
30.2% |
28.9% |
28.4% |
27.3% |
24.6% |
Drivers of the Growth in Sales Revenues
The growth in the sales revenue of patented medicines is influenced by changes in several key factors:
- Volume effect: changes in the quantity or amount of patented medicines sold. This effect focuses on established medicines that were on the market for the whole period. Increases in the population, changes in demographic composition (e.g., shifts in the age distribution), increases in the incidence of disease, and changes in prescribing practices are among the factors that may contribute to this effect.
- Mix effect: shifts in use between lower- and higher-cost patented medicines. This effect applies to both new medicines and those that were already on the market. The switch to new higher-priced medicines, the use of new medicines that treat conditions for which no effective treatment previously existed, and changes in physician prescribing practices are among the factors that may contribute to this change.
- Exiting effect: previously patented medicines that have stopped reporting sales revenue to the PMPRB or are no longer sold in Canada.
- Loss-of-exclusivity effect: medicines that have lost market exclusivity and are open to some level of generic competition, but still patented.
- Price effect: changes in the prices of existing patented medicines. This effect applies to both increases and decreases in the prices of patented medicines over the time period analyzed.
Some factors, such as the mix effect, will generally put an upward pressure on sales, while others, such as the loss-of-exclusivity effect, have the opposite effect.
The net result of all of the combined effects is the annual change in sales.
Occasionally the emergence of a new “blockbuster” medicine will have a significant influence on sales and will be monitored as a separate effect. For example, direct-acting antiviral (DAA) treatments for hepatitis C have had a notable impact on patented medicine sales over the past few years. Their greatest contribution was in 2015 when they pushed overall sales upward by almost 5%. Although their contribution to sales growth was more modest in 2018 they may continue to have an impact in the coming years, as new DAA medicines enter the market and as the extent of coverage offered by insurers expands.
Figure 5 focuses on the major factors that drove the year-by-year growth in patented medicine salesFootnote 7 between 2014 and 2018 (a) in absolute dollar amounts, and (b) as proportions of the overall annual change in sales.
Changes in the prices of patented medicines played a very minor role in the growth in patented medicine sales over the last five years, suggesting that, on average, the prices of existing patented medicines are fairly stable. However, this does not reflect the overall increases in treatment costs due to the entry of newer, higher-priced patented medicines (captured by the mix effect).
The results show that strong sales for new patented medicines and existing higher-cost patented medicines were the major contributors to sales growth over the five-year period. In 2018, the overall increase in the use of patented medicines, as well as higher-cost patented medicines, put a greater upward pressure on sales than in previous years. These factors will be analyzed in more detail in the upcoming sections.
Figure 5. Key Drivers of Change in the Sales of Patented Medicines, 2014 to 2018
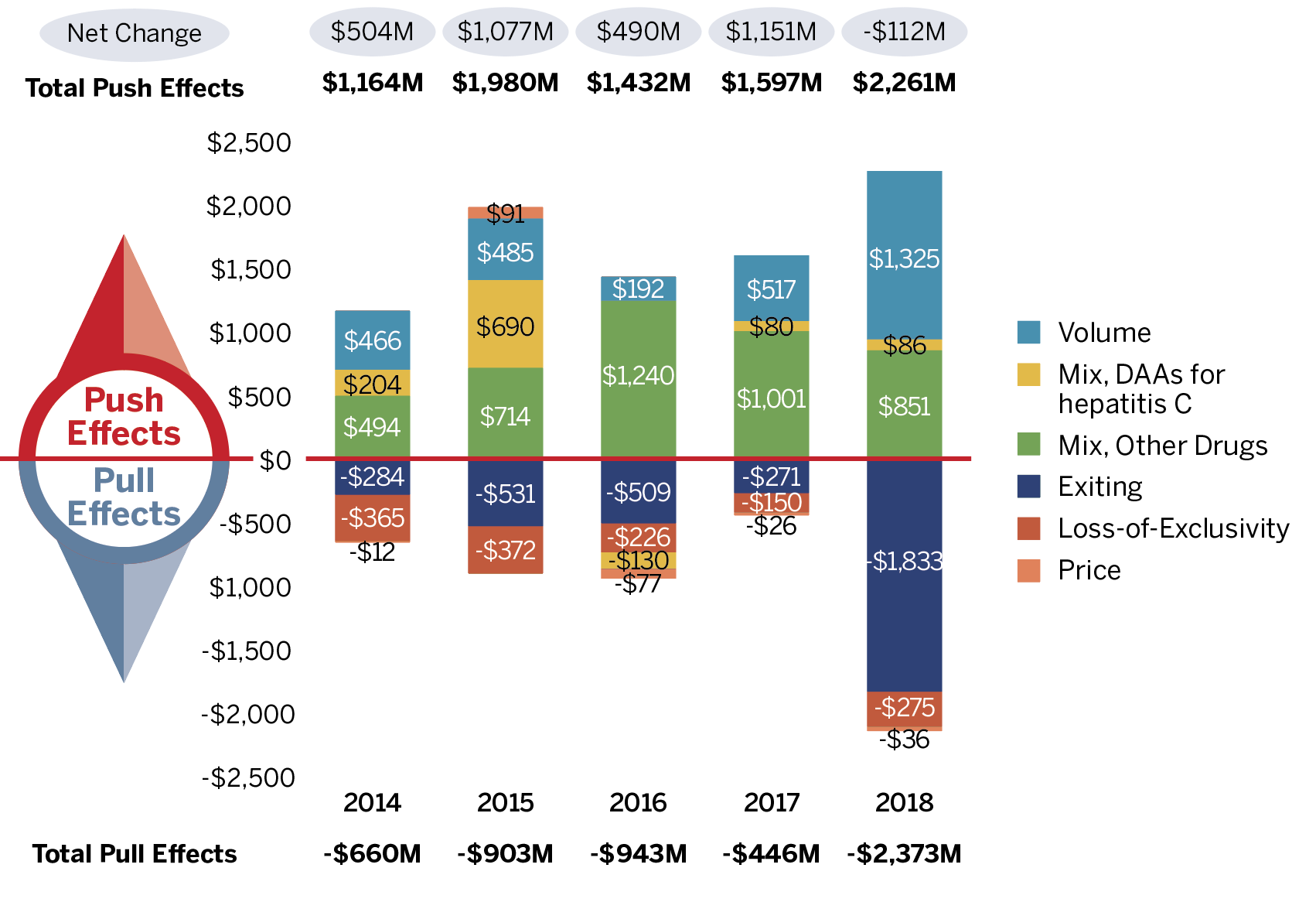
Note: When multiple factors change simultaneously, they create a residual or cross effect, which is not reported separately in this analysis,
but is accounted for in the total cost change.
Values may not add to the net change due to rounding and the cross effect.
Data source: PMPRB
Figure description
These two bar graphs describe the factors that impacted the annual rates of change in the sales of patented medicines from 2014 to 2018. The first graph gives the rate of change in absolute dollar amounts and the second gives the corresponding percent rate of growth for each contributing factor along with the total push up (positive) and pull down (negative) effects. Direct-acting antiviral (DAA) medicines for hepatitis C are presented separately from the rest of the drug-mix effect because of their high impact.
(a) Absolute change in millions of dollars
| Exiting | Loss-of-Exclusivity | Mix, Other Drugs | Mix, DAAs for hepatitis C | Volume | Price | |
|---|---|---|---|---|---|---|
2014 |
-$284,000,000 |
-$365,000,000 |
$494,000,000 |
$204,000,000 |
$466,000,000 |
-$12,000,000 |
2015 |
-$531,000.000 |
-$372,000,000 |
$714,000,000 |
$690,000,000 |
$485,000,000 |
$91,000,000 |
2016 |
-$509,000,000 |
-$226,000,000 |
$1,240,000,000 |
-$130,000,000 |
$192,000,000 |
-$77,000,000 |
2017 |
-$271,000,000 |
-$150,000,000 |
$1,001,000,000 |
$80,000,000 |
$517,000,000 |
-$26,000,000 |
2018 |
-$1,833,240,954 |
$275,094,724 |
$851,146,902 |
$ 85,642,876 |
$ 1,324,601,290 |
-$ 35,545,747 |
| Absolute change (millions of dollars) | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
Total Push Effects |
$1,164 |
$1,980 |
$1,432 |
$1,597 |
$2,261 |
Total Pull Effects |
-$660 |
-$903 |
-$943 |
-$446 |
-$2,373 |
Net Change |
$504 |
$1,077 |
$490 |
$1,151 |
$112 |
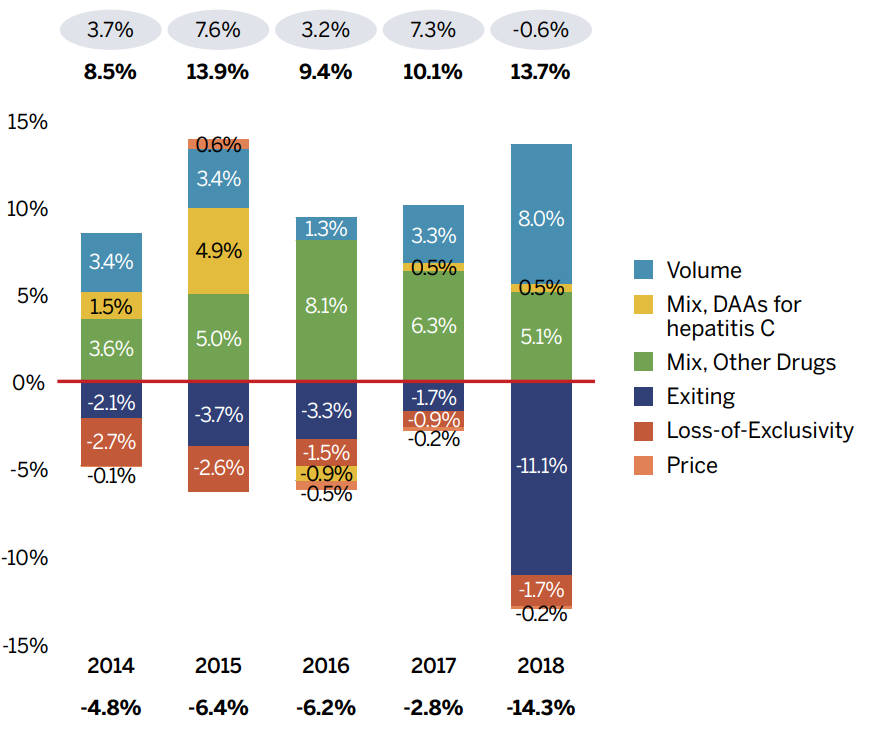
Note: When multiple factors change simultaneously, they create a residual or cross effect, which is not reported separately in this analysis,
but is accounted for in the total cost change.
Values may not add to the net change due to rounding and the cross effect.
Data source: PMPRB
Figure description
(b) Relative change in percent
| Exiting | Loss-of-Exclusivity | Mix, Other Drugs | Mix, DAAs for hepatitis C | Volume | Price | |
|---|---|---|---|---|---|---|
2014 |
-2.1% |
-2.7% |
3.6% |
1.5% |
3.4% |
-1.0% |
2015 |
-3.7% |
-2.6% |
5.0% |
4.9% |
3.4% |
0.6% |
2016 |
-3.3% |
-1.5% |
8.1% |
-0.9% |
1.3% |
-0.5% |
2017 |
-1.7% |
-0.9% |
6.3% |
0.5% |
3.3% |
-0.2% |
2018 |
-11.1% |
-1.7% |
5.1% |
0.5% |
8.0% |
-0.2% |
| Absolute change (millions of dollars) | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
Total Pull Effects |
-4.8% |
-6.4% |
-6.2% |
-2.8% |
-14.3% |
Total Push Effects |
8.5% |
13.9% |
9.4% |
10.1% |
13.7% |
Net Change |
3.7% |
7.6% |
3.2 |
7.3% |
-0.6% |
Counterbalancing this upward pressure, there was a significant market segment shift in 2018, as a number of high-selling medicines no longer reported their sales to the PMPRB. These included high-cost medicines and/or medicines with high utilization. In total, medicines accounting for $1.8 billion in expenditures exited the patented sales space in 2018, with Remicade alone accounting for over half of the total amount.
The exiting effect was significantly higher in 2018 than in previous years, offsetting increases in reported sales and resulting in a slight decrease in overall sales growth. Figure 6 illustrates the change in the impact of the exiting effect over the last five years and identifies the top-selling medicines that stopped reporting to the PMPRB in 2018.
Figure 6. Loss in Patented Medicine Sales from the Exiting Effect, 2014 to 2018
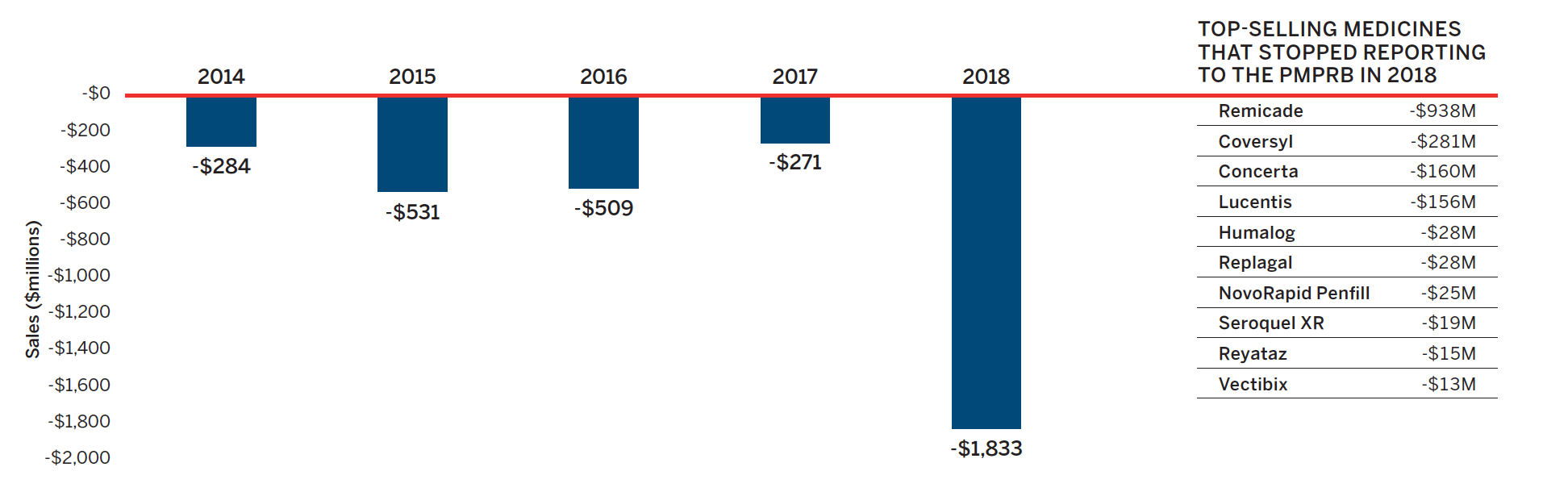
Data source: PMPRB
Figure description
This bar graph describes the change in patented medicine sales caused by patented medicines no longer reporting patents from 2014 to 2018. An accompanying table gives the change in sales for the top-selling medicines that stopped reporting patents in 2018.
| Year | Change in sales (millions of dollars) |
|---|---|
2014 |
-$284 |
2015 |
-$531 |
2016 |
-$509 |
2017 |
-$271 |
2018 |
-$1,833 |
| Top-selling medicines that stopped reporting to the PMPRB in 2018 | Change in sales (millions of dollars) |
|---|---|
Remicade |
-$938 |
Coversyl |
-$281 |
Concerta |
-$160 |
Lucentis |
-$156 |
Humalog |
-$28 |
Replagal |
-$28 |
NovoRapid Penfill |
-$25 |
Seroquel XR |
-$19 |
Reyataz |
-$15 |
Vectibix |
-$13 |
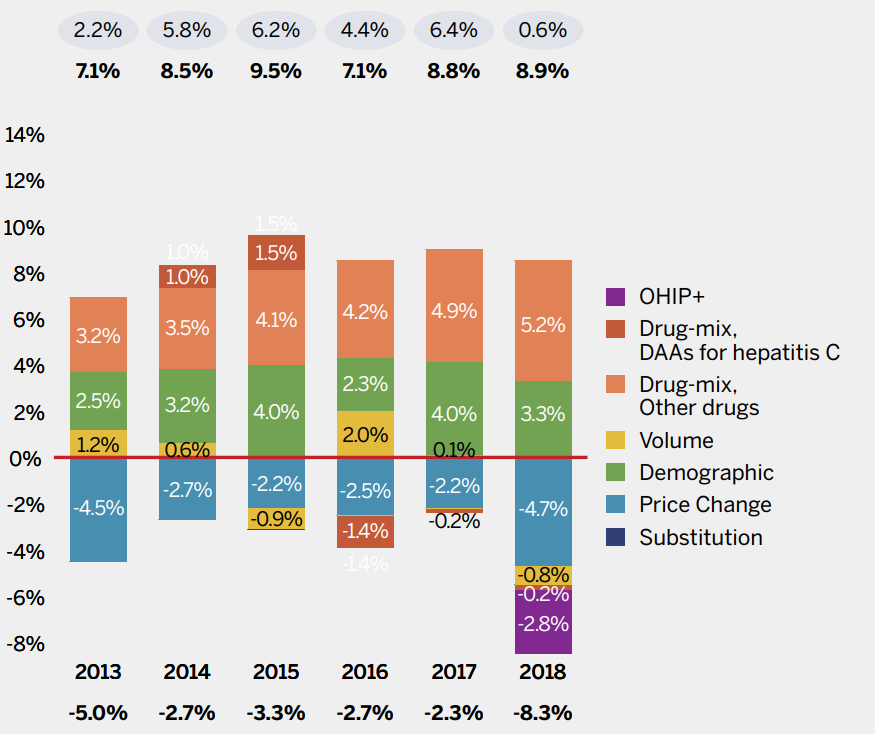
Brief Insights: Cost Drivers of Public and Private Drug Plans
Higher-cost medicines (other than DAAs for hepatitis C) are the primary cost driver in Canadian public and private drug plan expenditures. Over the past six years, they have exerted a consistent, upward pressure of 4% to 5% on drug costs. Conversely, savings from generic substitution and price reductions have been decreasing over the same period. A new generic pricing policy introduced in 2018 reduced cost growth for the year. However, the overall cost impact from policies such as this are not expected to be sustained over multiple years as the interventions serve as a one-time reduction in expenditures and do not suppress the underlying annual growth.
In addition to the use of higher-cost medicines, plan design changes can contribute significantly to growth, as was the case in 2018 with the introduction of OHIP+ in Ontario, which provided pharmaceutical benefits for Ontario residents 24 and under.
(A) NPDUIS PUBLIC DRUG PLANS*, 2012–13 TO 2017–18

Note: Public plans report on a fiscal year basis and private plans report on the calendar year. This has an impact on the magnitude of the effect of policies
such as the OHIP+ program or generic pricing initiative introduced in 2018, e.g., for public plans, most of the impact will be felt in the next fiscal year.
When multiple factors change simultaneously, they create a residual or cross effect, which is not reported separately in this analysis, but is
accounted for in the total cost change.
Values may not add to the net change due to rounding and the cross effect.
* British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador,
Yukon, and the Non-Insured Health Benefits Program
Data source: NPDUIS database, CIHI; IQVIA Private Pay Direct Drug Plan Database [NPDUIS Report: CompassRx 2017/18; NPDUIS Poster: Pressures behind the Rising Costs in Canadian Private Drug Plans, 2018]
Figure description
These two bar graphs describe the factors that impacted the annual rates of change in medicine costs for public and private plans. The first graph depicts the total for the combined NPDUIS public drug plans from fiscal year 2012-13 to 2017-18 and the second depicts the Canadian private drug plans from calendar year 2013 to 2018. The total positive or push effects, negative or pull effects, and net change is given above and below the bars for each year. Direct-acting antiviral (DAA) medicines for hepatitis C are presented separately from the rest of the drug-mix effect because of their high impact. The effects of the introduction of the OHIP+ program in Ontario are also shown separately for 2017-18 in the public plan results and for 2018 in the private plan results.
NPDUIS public drug plans, 2012-13 to 2017-18
|
2012-13 | 2013-14 | 2014-15 | 2015-16 | 2016-17 | 2017-18 |
|---|---|---|---|---|---|---|
OHIP+ |
– |
– |
– |
– |
– |
1.5% |
Drug-Mix, DAAs for Hepatitis C |
0.0% |
0.0% |
0.0% |
8.0% |
-2.3% |
2.4% |
Drug-Mix, Others |
4.1% |
5.4% |
4.9% |
4.1% |
4.4% |
4.7% |
Volume |
1.7% |
2.2% |
0.3% |
1.3% |
1.0% |
1.0% |
Demographic |
2.7% |
2.1% |
2.7% |
3.0% |
1.8% |
1.4% |
Price Change |
-2.0% |
-6.0% |
-3.0% |
-1.8% |
-1.0% |
-1.1% |
Generic Substitution |
-7.2% |
-1.5% |
-3.2% |
-2.3% |
-1.8% |
-1.3% |
Total pull effects |
-9.2% |
-7.5% |
-6.2% |
-4.1% |
-5.1% |
-2.3% |
Total push effects |
8.5% |
9.7% |
7.9% |
16.2% |
7.2% |
11.0% |
Net change |
-0.8% |
2.0% |
2.5% |
12.0% |
2.0% |
8.3% |
Note: Public plans report on a fiscal year basis and private plans report on the calendar year. This has an impact on the magnitude of the effect of policies
such as the OHIP+ program or generic pricing initiative introduced in 2018, e.g., for public plans, most of the impact will be felt in the next fiscal year.
When multiple factors change simultaneously, they create a residual or cross effect, which is not reported separately in this analysis, but is
accounted for in the total cost change.
Values may not add to the net change due to rounding and the cross effect.
* British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador,
Yukon, and the Non-Insured Health Benefits Program
Data source: NPDUIS database, CIHI; IQVIA Private Pay Direct Drug Plan Database [NPDUIS Report: CompassRx 2017/18; NPDUIS Poster: Pressures behind the Rising Costs in Canadian Private Drug Plans, 2018]
Figure description
(b) Private drug plans, 2013 to 2018
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|
OHIP+ |
– |
– |
– |
– |
– |
-2.8% |
Drug-Mix, DAAs for Hepatitis C |
0.0% |
1.0% |
1.5% |
-1.4% |
-0.2% |
-0.2% |
Drug-Mix, Others |
3.5% |
3.2% |
4.1% |
4.2% |
4.9% |
5.2% |
Volume |
1.2% |
0.6% |
-0.9% |
2.0% |
0.1% |
-0.8% |
Demographic |
2.5% |
3.2% |
4.0% |
2.3% |
4.0% |
3.3% |
Price Change |
-4.5% |
-2.7% |
-2.2% |
-2.5% |
-2.2% |
-4.7% |
Generic Substitution |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
0.0% |
Total pull effects |
-5.0% |
-2.7% |
-3.3% |
-2.7% |
-2.3% |
-8.3% |
Total push effects |
7.1% |
8.5% |
9.5% |
7.1% |
8.8% |
8.9% |
Net change |
2.2% |
5.8% |
6.2% |
4.4% |
6.4% |
0.6% |
New Medicines Driving Sales Revenues
Figure 8 breaks down 2018 sales of patented medicines according to the year in which the medicine was first issued a Notice of Compliance (NOC) by Health Canada and approved for market in Canada. Throughout the latter part of the 1990s and early 2000s, sales growth was largely driven by a succession of new “blockbuster” medicines that ultimately achieved very high sales volumes. As the patents for these medicines expired, their share of sales gradually decreased. Recently introduced higher-cost medicines such as biologics, oncology medicines, and treatments for hepatitis C are increasingly accounting for a growing share of sales.
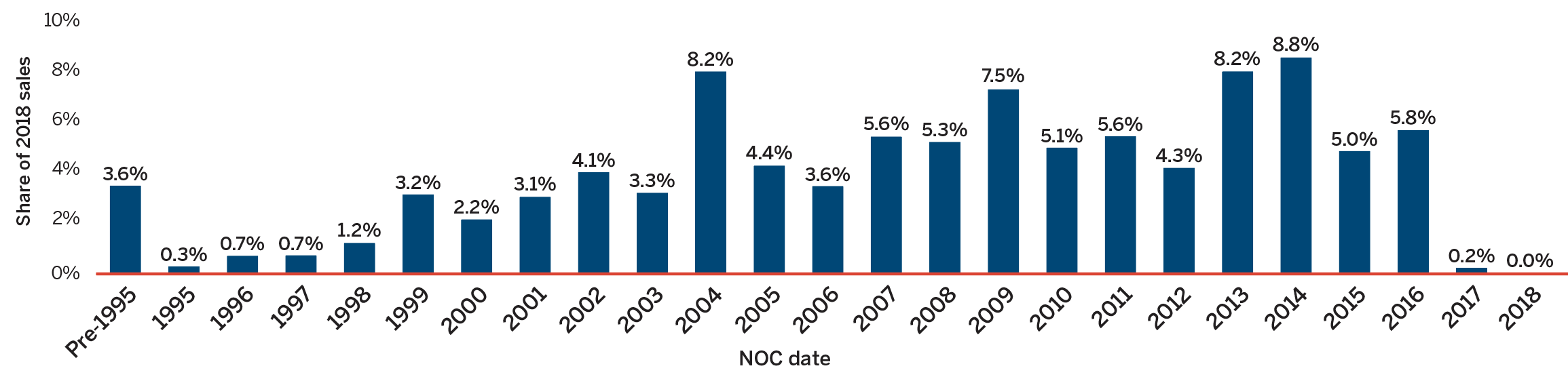
Data source: PMPRB
Figure description
This bar graph depicts the share of sales of patented medicines by the year in which the medicine was first issued a NOC by Health Canada and approved for sale in Canada. Medicines introduced before 1995 comprised 3.6% of the 2018 sales. In 1995: 0.3%; 1996: 0.7%; 1997: 0.7%;
1998: 1.2%; 1999: 3.2%; 2000: 2.2%; 2001: 3.1%; 2002: 4.1%; 2003: 3.3%; 2004: 8.2%; 2005: 4.4%;
2006: 3.6%; 2007: 5.6%; 2008: 5.3%; 2009: 7.5%; 2010: 5.1%; 2011: 5.6%; 2012: 4.3%; 2013: 8.2%;
2014: 8.8%; 2015: 5.0%; 2016: 5.8%; 2017: 0.2%; 2018: 0.0%.
Brief Insights: New Medicines Entering Canadian and International Markets
New medicines launched between 2009 and 2017 accounted for one third of the total brand-name pharmaceutical market in Canada in the fourth quarter of 2018. Approximately 50 new medicines received market approval through the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and/or Health Canada in both 2017 and 2018, the majority of which were high-cost speciality therapies. This represents a steep rise from the annual average of 36 approvals between 2009 and 2016.
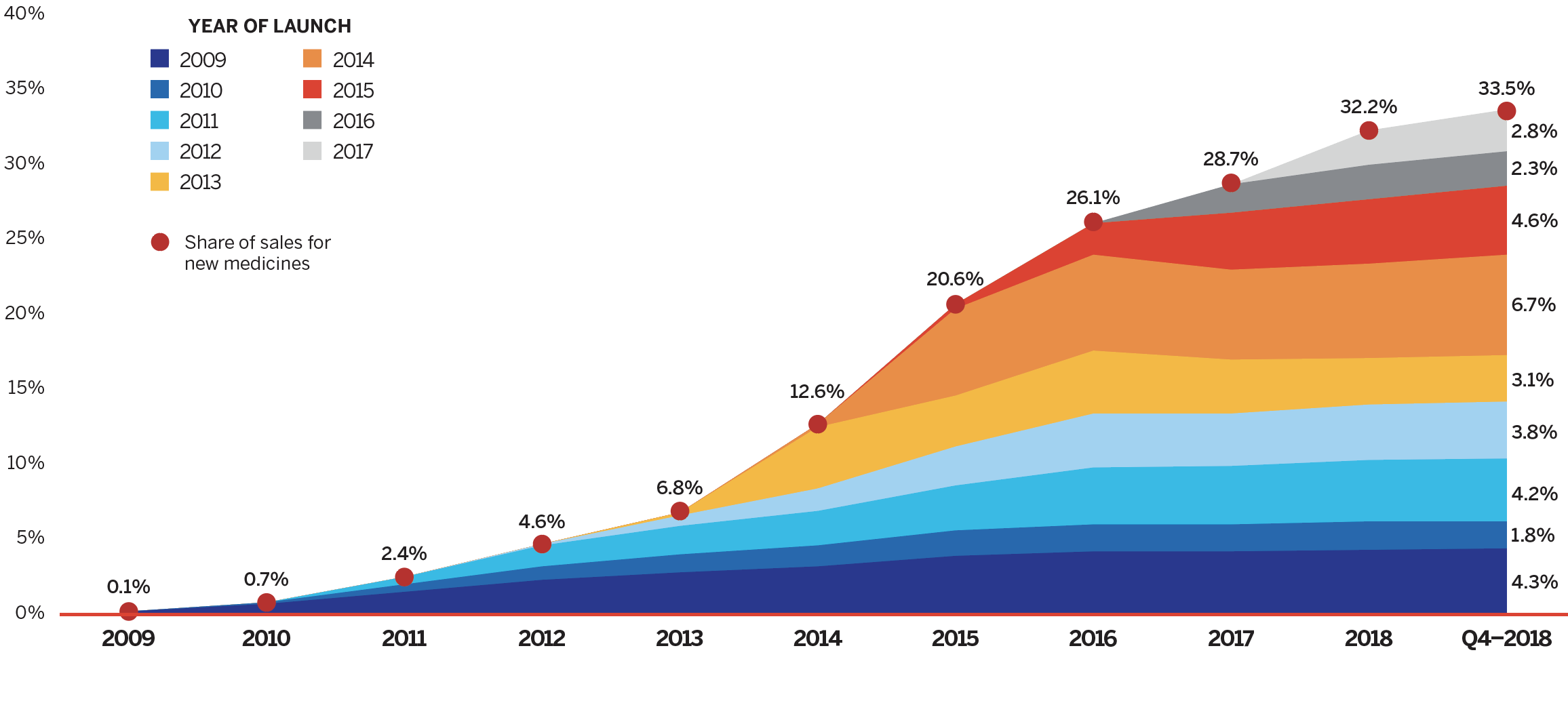
Data source: MIDAS® database, 2009–2018, IQVIA (all rights reserved)
[NPDUIS Poster: Early Insight into New Medicine Launches in Canadian and International Markets]
Figure description
Higher-Cost Medicines Driving Sales Revenues
Over the last decade, there has been a significant shift in pharmaceutical development toward more specialized medicines, with an increasing number of higher-cost medicines entering the market and gaining a significant market share.
In 2018, the shift to new or higher-cost medicines put an upward pressure on overall patented medicine sales. The top 10 contributors increased the sales of patented medicines by over $900 million between 2017 and 2018 (Table 7). Most of these medicines had an average annual treatment cost greater than $10,000.
| Medical Ingredient (Trade Name) | ATC | Sales ($Millions) 2017 | Sales ($Millions) 2018 | Absolute Change in Sales ($Millions) 2017–2018 | AVG. Annual Treatment Cost ($) 2018 |
|---|---|---|---|---|---|
| Pembrolizumab (Keytruda) | L01 | 74.8 | 206.3 | 131.4 | 37,637 |
| Empagliflozin (Jardiance) | A10 | 67.9 | 194.9 | 127.0 | 663 |
| Sofosbuvir/velpatasvir (Epclusa) | J05 | 518.2 | 645.2 | 126.9 | 43,563 |
| Aflibercept (Eylea) | S01 | 397.1 | 506.9 | 109.7 | 9,207 |
| Adalimumab (Humira) | L04 | 701.9 | 791.0 | 89.1 | 17,092 |
| Palbociclib (Ibrance) | L01 | 44.6 | 118.4 | 73.8 | 40,265 |
| Ibrutinib (Imbruvica) | L01 | 133.5 | 205.7 | 72.2 | 53,790 |
| Lenalidomide (Revlimid) | L04 | 338.5 | 408.4 | 69.9 | 60,312 |
| Apixaban (Eliquis) | B01 | 195.0 | 256.6 | 61.6 | 720 |
| Ustekinumab (Stelara) | L04 | 206.0 | 263.4 | 57.3 | 21,179 |
| Total top 10 medicines* | 2,677.5 | 3,596.8 | 918.9 |
* Values may not add to totals due to rounding.
Data source: PMPRB, IQVIA Private Pay Direct Drug Plan Database, 2018
While Table 7 reports the top 10 medicines contributing to the increase in the sales of patented medicines, Table 8 compares the 10 top-selling patented medicines in 2006 and 2018, along with their treatment costs. In 2006, Remicade was the only biologic medicine to make the top 10 list, with an average annual treatment cost of $17,759. This was much higher than the rest of the medicines on the list, none of which exceeded $1,000 annually. By 2018, however, half of the top 10 medicines were biologics, with annual treatment costs ranging from $9,207 to $37,637. Of the remaining 10 top sellers in 2018, only three had annual treatment costs of less than $1,000, and the highest treatment cost exceeded $60,000. With collective annual sales of approximately $3.8 billion, these 10 medicines accounted for close to one quarter of the total sales for all patented medicines in 2018.
| 2006 | 2018 | ||||||
|---|---|---|---|---|---|---|---|
| MEDICINAL INGREDIENT (TRADE NAME) | ATC | AVG. ANNUAL TREATMENT COST | MEDICINAL INGREDIENT (TRADE NAME) | ATC | AVG. ANNUAL TREATMENT COST | SALES ($MILLIONS) | SHARE OF PATENTED SALES (%) |
| 1. Atorvastatin calcium (Lipitor) | C10A | $511 | 1. Adalimumab (Humira) | L04A | $17,092 | $791.0 | 4.8 |
| 2. Amlodipine besylate (Norvasc) | C08C | $417 | 2. Sofosbuvir / velpatasvir (Epclusa) | J05A | $43,563 | $645.2 | 3.9 |
| 3. Ramipril (Altace) | C09A | $271 | 3. Aflibercept (Eylea) | S01L | $9,207 | $506.9 | 3.0 |
| 4. Venlafaxine hydrochloride (Effexor) | N06A | $446 | 4. Lenalidomide (Revlimid) | L04A | $60,312 | $408.4 | 2.5 |
| 5. Pantoprazole sodium (Pantoloc) | A02B | $330 | 5. Etanercept (Enbrel) | L04A | $13,654 | $292.3 | 1.8 |
| 6. Clopidogrel bisulfate (Plavix) | B01A | $607 | 6. Ustekinumab (Stelara) | L04A | $21,179 | $263.4 | 1.6 |
| 7. Rosuvastatin calcium (Crestor) | C10A | $341 | 7. Apixaban (Eliquis) | B01A | $720 | $256.6 | 1.5 |
| 8. Olanzapine (Zyprexa) | N05A | $977 | 8. Rivaroxaban (Xarelto) | B01A | $586 | $241.6 | 1.5 |
| 9. Salmeterol xinafoate/fluticasone propionate (Advair) | R03A | $343 | 9. Budesonide / formoterol fumarate (Symbicort) | R03A | $291 | $209.1 | 1.3 |
| 10. Infliximab (Remicade) | L04A | $17,759 | 10. Pembrolizumab (Keytruda) | L01X | $37,637 | $206.3 | 1.2 |
| Total top 10 medicines* | $3,820.6 | 23.0 | |||||
| Total patented medicines | $16,653.1 | ||||||
Note: Biologic medicines are highlighted.
* Values may not add to totals due to rounding.
Data source: PMPRB, IQVIA Private Pay Direct Drug Plan Database, 2018.Figure 10 details the trend in treatment costs for patented medicines over the last decade. For many years, the majority of the 20 top-selling patented medicines had annual treatment costs under $1,000; however, 2015 marked a turning point, and now most of the top sellers cost in the thousands or tens of thousands of dollars per year. This shift is reflected in the exceptional 10-fold growth in the median annual treatment cost between 2006 and 2015, which was $5,163 in 2018 after reaching a high of $8,584 in 2016. In addition to their higher cost, these medicines have had a strong uptake in use, resulting in a weighted average annual treatment cost of $18,414 for the 20 top-selling patented medicines in 2018. This is only slightly less than the maximum average annual treatment cost a decade before.
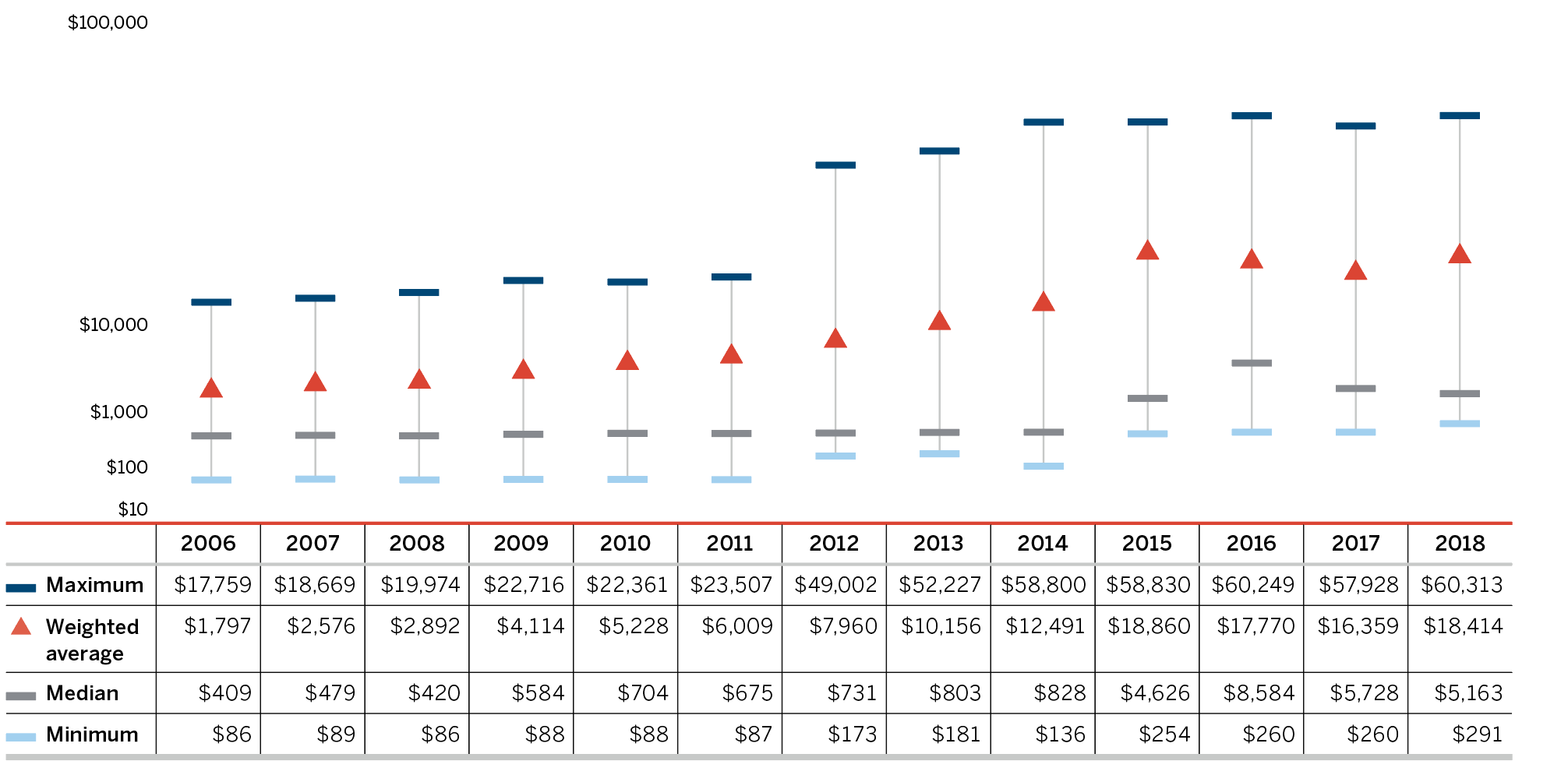
Data source: PMPRB; IQVIA Private Pay Direct Drug Plan Database, 2006–2018
Figure description
This graph depicts the minimum, maximum, median, and weighted average annual treatment costs for the 20 top-selling patented medicines sold in Canada from 2006 to 2018.
| Treatment cost | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Maximum |
$17,759 |
$18,669 |
$19,974 |
$22,716 |
$22,361 |
$23,507 |
$49,022 |
$52,227 |
$58,800 |
$58,830 |
$60,249 |
$57,928 |
$60,313 |
Weighted average |
$1,797 |
$2,576 |
$2,892 |
$4,114 |
$5,228 |
$6,009 |
$7,960 |
$10,156 |
$12,491 |
$18,860 |
$17,770 |
$16,359 |
$18,414 |
Median |
$409 |
$479 |
$420 |
$584 |
$704 |
$675 |
$731 |
$803 |
$828 |
$4,626 |
$8,584 |
$5,728 |
$5,163 |
Minimum |
$86 |
$89 |
$86 |
$88 |
$88 |
$87 |
$173 |
$181 |
$136 |
$254 |
$260 |
$260 |
$291 |
Figure 11 shows that high-cost medicines represent an increasingly significant share of the total sales of patented medicines, rising steeply from 5.0% in 2006 to 42.1% in 2018. This sustained growth was evident in all cost bands ($10 to $20 thousand; $20 to $50 thousand; and $50+ thousand), with a steep increase in the higher-cost medicines. Despite the sharp increase in the share of costs, less than 1% of the population use these medicines.
Between 2006 and 2018 the number of patented medicines in Canada with an annual average treatment cost of at least
$10,000 more than tripled and now high-cost medicines account for over 40% of patented medicine sales as compared to 5% in 2006.
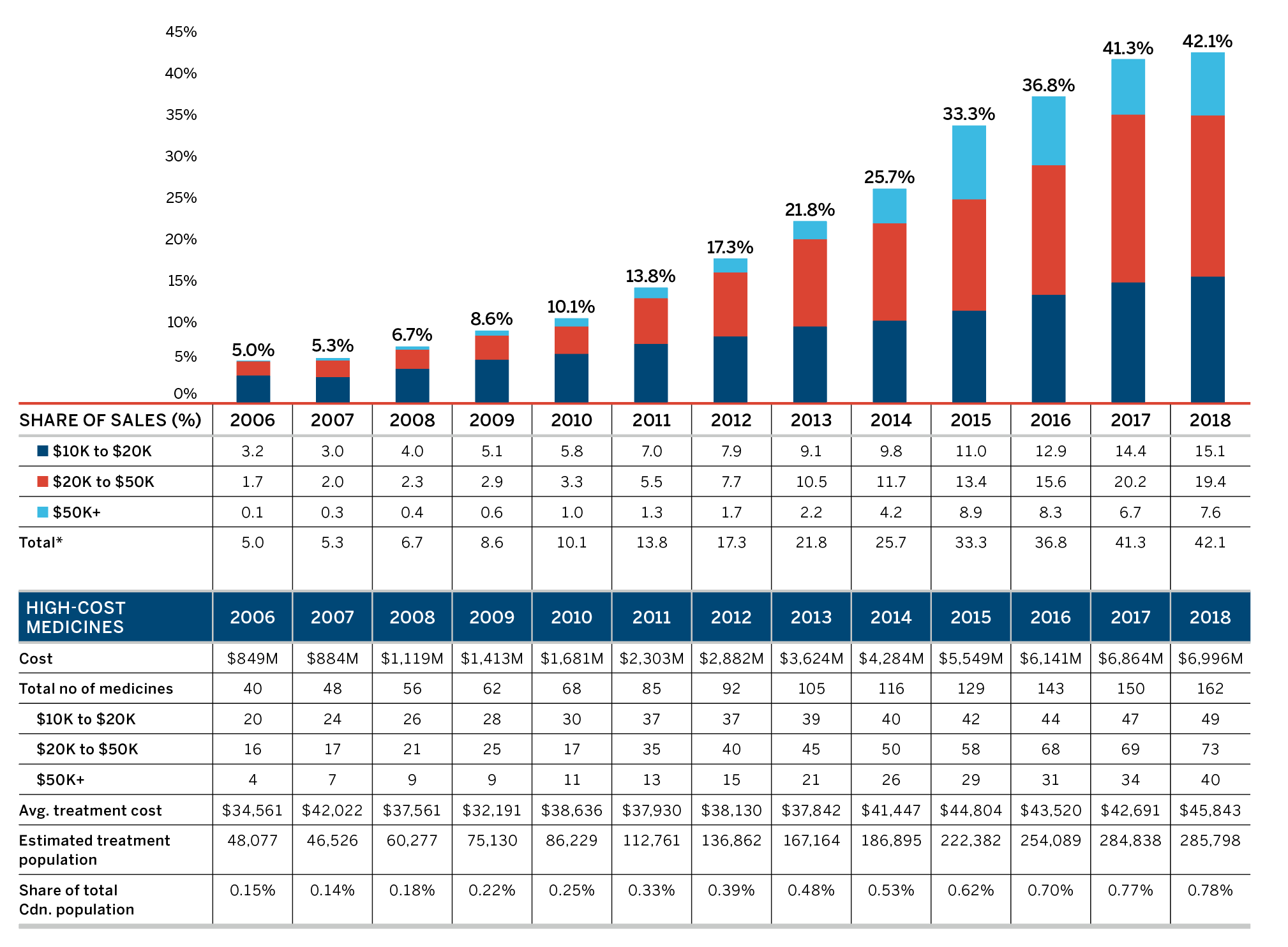
Note: The methodology for this analysis has been revised, and as such, historical results may not match those reported in previous editions.
* Values may not add to totals due to rounding.
Data source: PMPRB; IQVIA Private Pay Direct Drug Plan Database, 2006–2018
Figure description
This bar graph depicts the high-cost medicine share of total patented medicine sales per year by annual treatment cost from 2006 to 2018. The bars are subdivided into three bands based on average annual treatment cost: $10 to $20 thousand; $20 to $50 thousand; and greater than $50 thousand.
| Year | Share of sales for medicines costing $10 to $20 thousand | Share of sales for medicines costing $20 to $50 thousand | Share of sales for medicines costing greater than $50 thousand | Total share of sales of high-cost medicines |
|---|---|---|---|---|
2006 |
3.2% |
1.7% |
0.1% |
5.0% |
2007 |
3.0% |
2.0% |
0.3% |
5.3% |
2008 |
4.0% |
2.3% |
0.4% |
6.7% |
2009 |
5.1% |
2.9% |
0.6% |
8.6% |
2010 |
5.8% |
3.3% |
1.0% |
10.1% |
2011 |
7.0% |
5.5% |
1.3% |
13.8% |
2012 |
7.9% |
7.7% |
1.7% |
17.3% |
2013 |
9.1% |
10.5% |
2.2% |
21.8% |
2014 |
9.8% |
11.7% |
4.2% |
25.7% |
2015 |
11.0 |
13.4% |
8.9% |
33.3% |
2016 |
12.9% |
15.6% |
8.3% |
36.8% |
2017 |
14.4% |
20.2% |
6.7% |
41.3% |
2018 |
15.1% |
19.4% |
7.6% |
42.1% |
The table below the graph gives additional information including the medicine cost, the number of medicines, the average annual treatment cost, and the estimated treatment population and corresponding share of total Canadian population.
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Medicine cost (millions of dollars) |
$849 |
$884 |
$1,119 |
$1,413 |
$1,681 |
$2,303 |
$2,882 |
$3,624 |
$4,284 |
$5,549 |
$6,141 |
$6,864 |
$6,996 |
Total number of medicines |
40 |
48 |
56 |
62 |
68 |
85 |
92 |
105 |
116 |
129 |
143 |
150 |
162 |
Number of medicines costing $10,000 to $20,000 |
20 |
24 |
26 |
28 |
30 |
37 |
37 |
39 |
40 |
42 |
44 |
47 |
49 |
Number of medicines costing $20,000 to $50,000 |
16 |
17 |
21 |
25 |
17 |
35 |
40 |
45 |
50 |
58 |
68 |
69 |
73 |
Number of medicines costing more than $50,000 |
4 |
7 |
9 |
9 |
11 |
13 |
15 |
21 |
26 |
29 |
31 |
34 |
40 |
Average annual treatment cost |
$34,561 |
$42,022 |
$37,561 |
$32,191 |
$38,636 |
$37,930 |
$38,130 |
$37,842 |
$41,447 |
$44,804 |
$43,520 |
$42,691 |
$45,843 |
Estimated treatment population |
48,077 |
46,526 |
60,277 |
75,130 |
86,229 |
112,761 |
136,862 |
167,164 |
186,895 |
222,382 |
254,089 |
284,838 |
285,798 |
Share total Canadian population |
0.15% |
0.14% |
0.18% |
0.22% |
0.25% |
0.33% |
0.39% |
0.48% |
0.53% |
0.62% |
0.70% |
077% |
0.78% |
The shift toward higher-cost treatments is especially evident in oncology medicines. Figure 12 shows the share of total sales for patented oncology medicines by treatment cost based on a standard 28-day treatment regimen.
From 2006 to 2018, the average treatment cost for oncology medicines more than doubled, from $3,555 to $7,152. Many treatment regimens use multiple medicines resulting in even higher treatment costs per beneficiary. There may be some overlap in the medicines reported in Figures 11 and 12, as the oncology medicines that exceeded $10,000 in annual treatment costs are reported in both graphs.
The dual pressures of increasing average treatment costs and growing utilization mean that this therapeutic area is likely to continue to grow as a proportion of patented medicine sales.
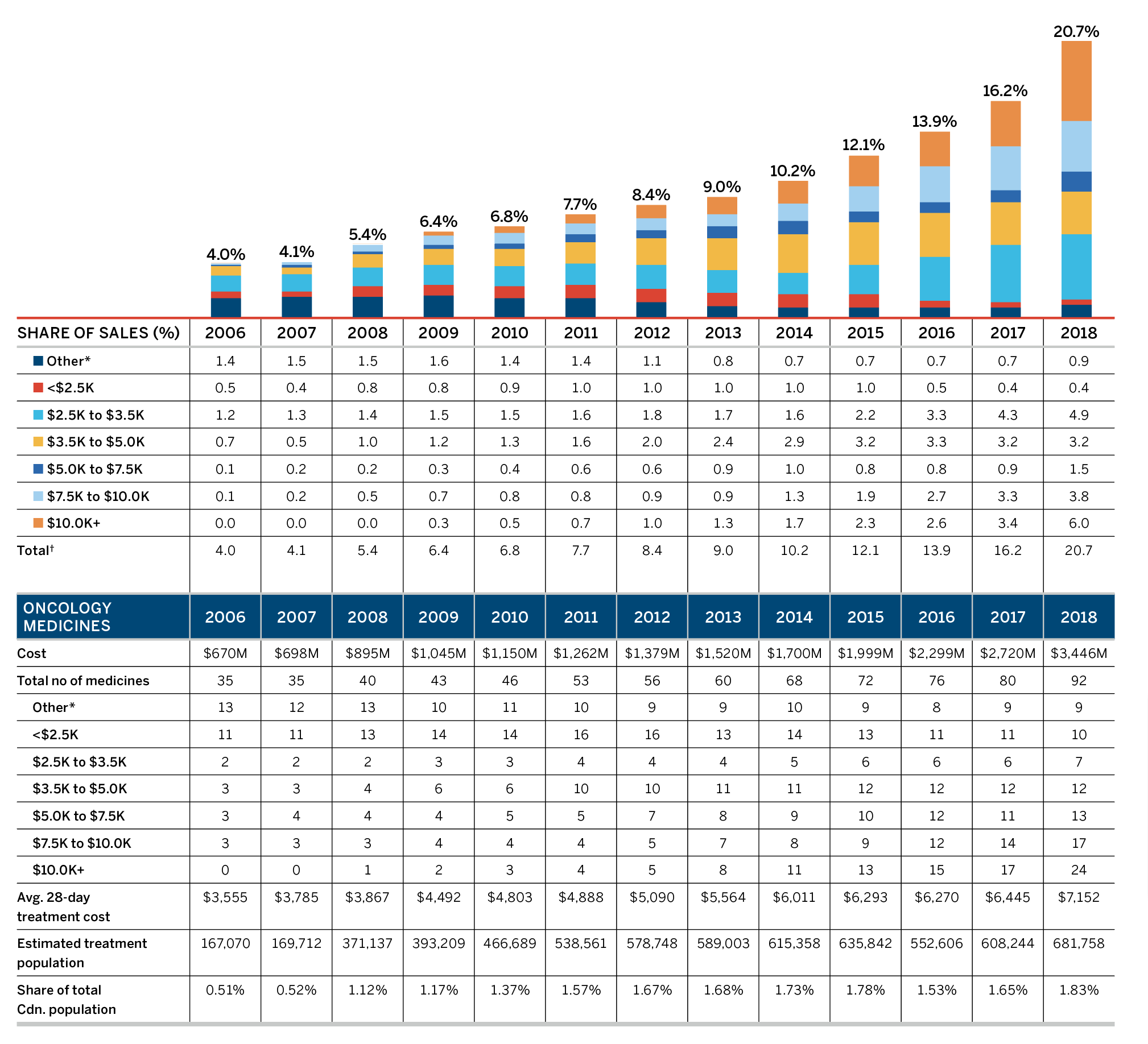
Note: These results reflect the total sales for patented medicines used in the treatment of cancer. While some of these medicines may also be used to treat other conditions, the data used for this analysis does not distinguish between indications, and thus, the reported sales may reflect some non-cancer use.
* Treatment costs not available for these medicines.
† Values may not add to totals due to rounding.
Data source: PMPRB; CADTH pCODR
Figure description
This bar graph depicts the high-cost patented oncology medicine share of total patented medicine sales from 2006 to 2018. The bars are subdivided into seven bands based on the average 28-day treatment cost: medicines whose costs are not available; medicines costing less than $2.5 thousand; $2.5 to $3.5 thousand; $3.5 to $5.0 thousand; $5.0 to $7.5 thousand; $7.5 to $10.0 thousand; and more than $10 thousand.
| Year | Share of sales for patented oncology medicines for which treatment cost is unavailable | Share of sales for patented oncology medicines for which treatment cost is less than $2,500 | Share of sales for patented oncology medicines for which treatment cost is $2,500 to $3,500 | Share of sales for patented oncology medicines for which treatment cost is $3,500 to $5,000 | Share of sales for patented oncology medicines for which treatment cost is $5,000 to $7,500 | Share of sales for patented oncology medicines for which treatment cost is $7,500 to $10,000 | Share of sales for patented oncology medicines for which treatment cost is more than $10,000 | Total share of sales for patented oncology medicines |
|---|---|---|---|---|---|---|---|---|
2006 |
1.4% |
0.5% |
1.2% |
0.7% |
0.1% |
0.1% |
0.0% |
4.0% |
2007 |
1.5% |
0.4% |
1.3% |
0.5% |
0.2% |
0.2% |
0.0% |
4.1% |
2008 |
1.5% |
0.8% |
1.4% |
1.0% |
0.2% |
0.5% |
0.0% |
5.4% |
2009 |
1.6% |
0.8% |
1.5% |
1.2% |
0.3% |
0.7% |
0.3% |
6.4% |
2010 |
1.4% |
0.9% |
1.5% |
1.3% |
0.4% |
0.8% |
0.5% |
6.8% |
2011 |
1.4% |
1.0% |
1.6% |
1.6% |
0.6% |
0.8% |
0.7% |
7.7% |
2012 |
1.1% |
1.0% |
1.8% |
2.0% |
0.6% |
0.9% |
1.0% |
8.4% |
2013 |
0.8% |
1.0% |
1.7% |
2.4% |
0.9% |
0.9% |
1.3% |
9.0% |
2014 |
0.7% |
1.0% |
1.6% |
2.9% |
1.0% |
1.3% |
1.7% |
10.2% |
2015 |
0.7% |
1.0% |
2.2% |
3.2% |
0.8% |
1.9% |
2.3% |
12.1% |
2016 |
0.7% |
0.5% |
3.3% |
3.3% |
0.8% |
2.7% |
2.6% |
13.9% |
2017 |
0.7% |
0.4% |
4.3% |
3.2% |
0.9% |
3.3% |
3.4% |
16.2% |
2018 |
0.9% |
0.4% |
4.9% |
3.2% |
1.5% |
3.8% |
6.0% |
20.7% |
The table below the graph gives additional information including the medicine cost, the number of medicines, the average 28-day treatment cost, and the estimated treatment population and corresponding share of total Canadian population.
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Medicine cost |
$670M |
$698M |
$895M |
$1,045M |
$1,150M |
$1,262M |
$1.379M |
$1,520M |
$1,700M |
$1,999M |
$1,2,299M |
$2,720M |
$3,446M |
Total number of medicines |
35 |
35 |
40 |
43 |
46 |
53 |
56 |
60 |
68 |
72 |
76 |
80 |
92 |
Number of medicines for which cost is unavailable |
13 |
12 |
13 |
10 |
11 |
10 |
9 |
9 |
10 |
9 |
8 |
9 |
9 |
Number of medicines costing less than $2,500 |
11 |
11 |
13 |
14 |
14 |
16 |
16 |
13 |
14 |
13 |
11 |
11 |
10 |
Number of medicines costing $2,500 to $3.,500 |
2 |
2 |
2 |
3 |
3 |
4 |
4 |
4 |
5 |
6 |
6 |
6 |
7 |
Number of medicines costing $3,500 to $5,000 |
3 |
3 |
4 |
6 |
6 |
10 |
10 |
11 |
11 |
12 |
12 |
12 |
12 |
Number of medicines costing $5,000to $7,500 |
3 |
4 |
4 |
4 |
5 |
5 |
7 |
8 |
9 |
10 |
12 |
11 |
13 |
Number of medicines costing $7,500 to $10,000 |
3 |
3 |
3 |
4 |
4 |
4 |
5 |
7 |
8 |
9 |
12 |
14 |
17 |
Number of medicines costing more than $10,000 |
0 |
0 |
1 |
2 |
3 |
4 |
5 |
8 |
11 |
13 |
15 |
17 |
24 |
Average 28-day treatment cost |
$3,555 |
$3,785 |
$3,867 |
$4,492 |
$4,803 |
$4,888 |
$5,090 |
$5,564 |
$6,011 |
$6,293 |
$6,270 |
$6,445 |
$7,152 |
Estimated treatment population |
167,070 |
169,712 |
371,137 |
393,209 |
466,689 |
538,561 |
578,748 |
589,003 |
615,358 |
635,842 |
552,606 |
608,244 |
681,758 |
Share total Canadian population |
0.51% |
0.52% |
1.12% |
1.17% |
1.37% |
1.57% |
1.67% |
1.68% |
1.73% |
1.78% |
1.53% |
1.65% |
1.83% |
Brief Insights: High-Cost Medicines in Public and Private Drug Plans
High-cost medicines account for approximately 30% of all public and private drug plan expenditures. This is lower than the share for patented medicines reported in Figure 11, since public and private plan drug costs also include non-patented generic and non-patented single-source medicines.
In 2018, private drug plans covered 189 high-cost medicines, while public plans covered 99 high-cost medicines in fiscal year 2017–18. Note that the number of oncology medicines and other high-cost medicines covered by public plans may be underestimated, as some are reimbursed through specialized programs, such as cancer care, that are not captured in the data.
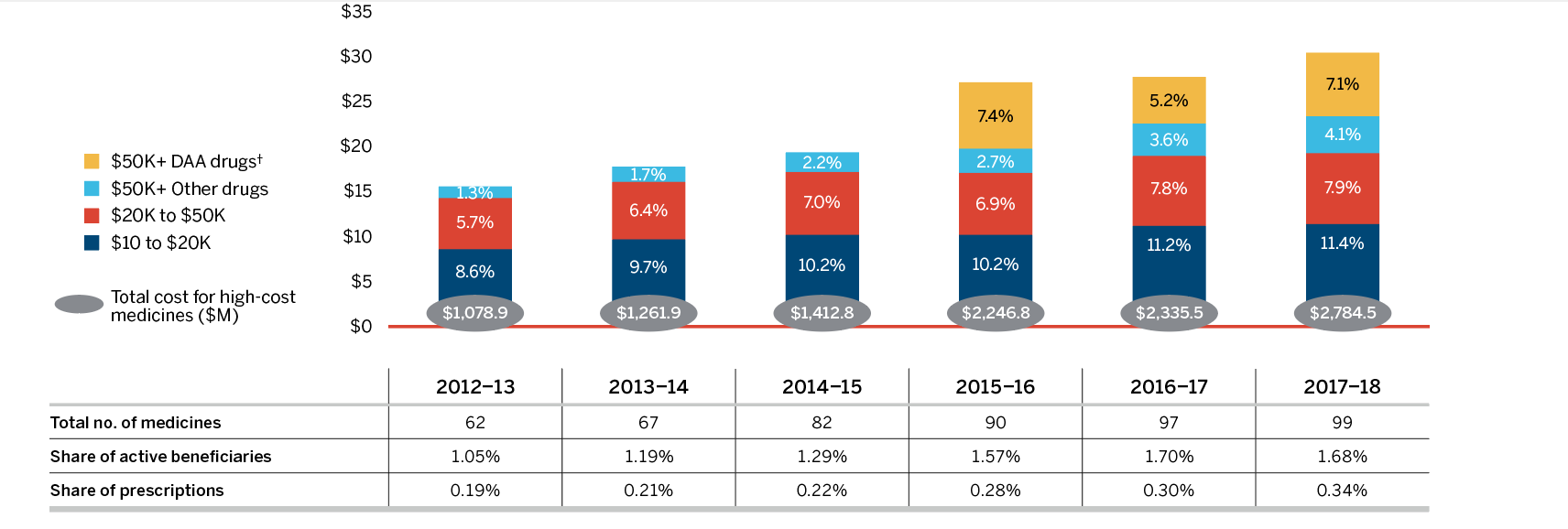
Note: Values may not add to totals due to rounding.
*British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, Yukon and the Non-Insured Health Benefits Program.
† DAA: Direct-acting antivirals for the treatment for hepatitis C, which were launched in 2014 and 2015
Data source: NPDUIS database, CIHI (fiscal year data) [NPDUIS Report: CompassRx 2017/18]
Figure description
This stacked bar graph depicts the high-cost medicine share of total medicine costs for the NPDUIS public drug plans from fiscal year 2012-13 to 2017-18. The bars are subdivided into bands based on average annual costs per active beneficiary: $10 to $20 thousand; $20 to $50 thousand; and more than $50 thousand. The share of new direct-acting antiviral (DAA) medicines for hepatitis C is reported separately. An accompanying table gives the total number of high-cost medicines, the share of beneficiaries using these medicines, and the share of prescriptions they represent.
High-cost medicines share of total medicine costs
| 2012-13 | 2013-14 | 2014-15 | 2015-16 | 2016-17 | 2017-18 | |
|---|---|---|---|---|---|---|
Medicine cost in millions of dollars |
$1,078.9 |
$1,261.9 |
$1,412.8 |
$2,246.8 |
$2,335.5 |
$2,784.5 |
Total number of medicines |
62 |
67 |
82 |
90 |
97 |
99 |
Average cost per active beneficiary of $10,000‒$20,000: share of total medicine cost |
8.6% |
9.7% |
10.2% |
10.2% |
11.2% |
11.4% |
Average cost per active beneficiary of $20,000‒$50,000: share of total medicine cost |
5.7% |
6.4% |
7.0% |
6.9% |
7.8% |
7.9% |
Average cost per active beneficiary of greater than $50,000: share of total medicine cost for medicines other than DAAs for hepatitis C |
1.3% |
1.7% |
2.25 |
2.7% |
3.6% |
4.1% |
Average cost per active beneficiary of greater than $50,000: share of total medicine cost for DAAs |
0% |
0% |
0% |
7.4% |
5.2% |
7.1% |
High-cost medicines - share of active beneficiaries |
1.05% |
1.19% |
1.29% |
1.57% |
1.70% |
1.68% |
High-cost medicines - share of total prescriptions |
0.19% |
0.21% |
0.22% |
0.28% |
0.30% |
0.34% |
Top Therapeutic Classes Driving Sales Revenues
“Antineoplastics and immunomodulating agents,” “general antiinfectives for systemic use and antiparasitic products,” and “alimentary tract and metabolism” were the three top-selling therapeutic classes in 2018, accounting for 60% of all patented medicine sales. After experiencing double-digit growth in 2017, sales for the top-selling “antineoplastics and immunomodulating agents” class decreased in 2018, as some important medicines stopped reporting sales to the PMPRB.
In 2018, 6 of the 10 top-selling medicines
had annual treatment costs exceeding $10K
Figure 14 breaks out the sales of patented medicines in Canada by therapeutic class using level 1 of the World Health Organization’s (WHO) Anatomical Therapeutic Chemical (ATC) classification systemFootnote 8. Two donut graphs compare the share of total sales for each therapeutic class in 2018 to the share in 2008. The associated table gives the 2018 sales for each class and the sales growth from 2017 to 2018.
The “antineoplastics and immunomodulating agents” class accounted for a much larger share of sales in 2018 (33.7%) than in 2008 (15.6%), as more high-cost medicines entered the market over the past decade. By contrast, the share of sales of cardiovascular system medicines decreased dramatically from 24.5% to 3.8%.
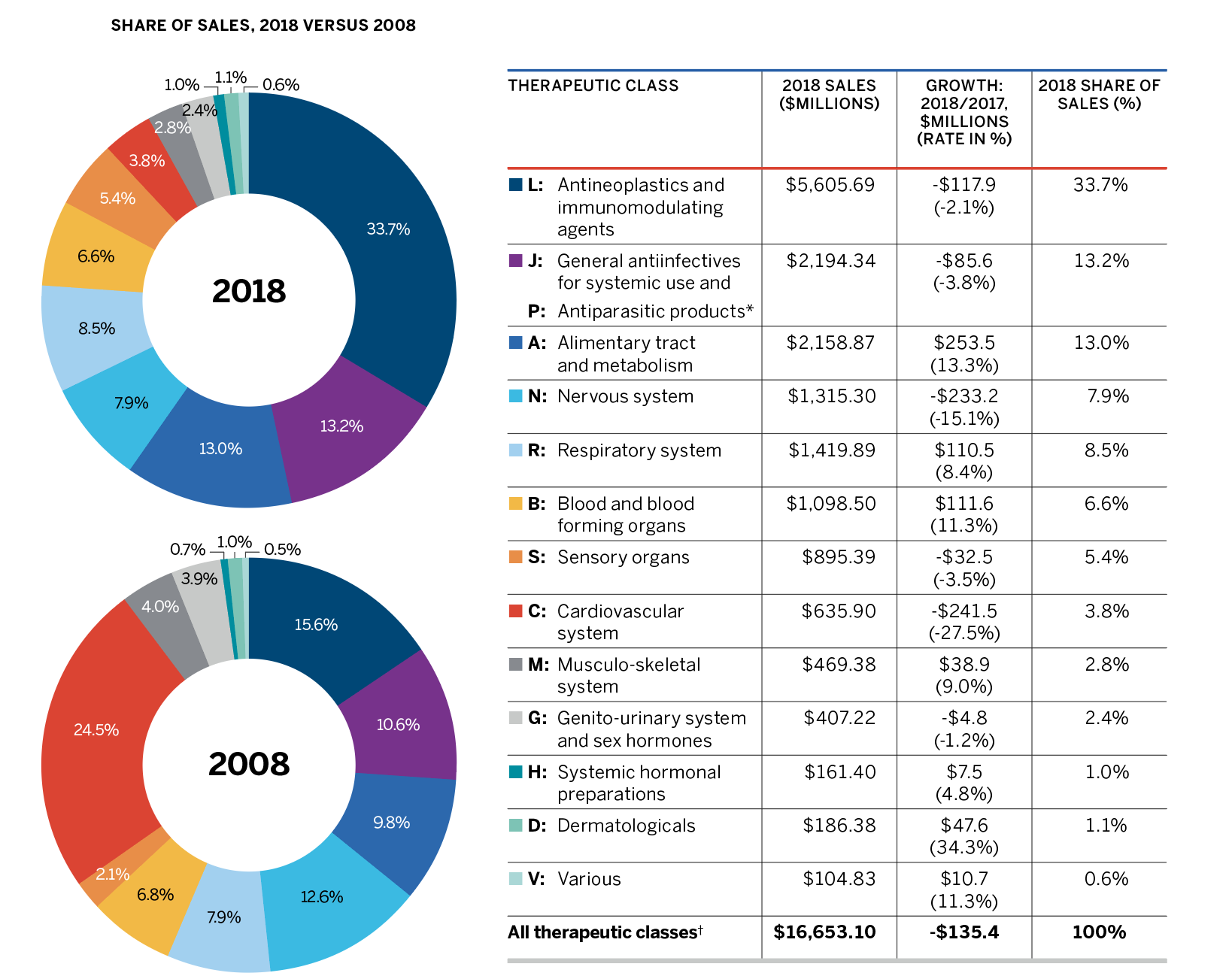
* These groups have been combined for reasons of confidentiality.
† Values may not add to totals due to rounding.
Data source: PMPRB
Figure description
These two pie charts depict the sales of patented medicines as a percentage of all medicine sales by therapeutic class in 2018 and 2008.
| Therapeutic Class | 2018 Share of Sales (%) | 2008 Share of Sales (%) |
|---|---|---|
L: Antineoplastics and immunomodulating agents |
33.7 |
15.6 |
J: General antiinfectives for systemic use and P: Antiparasitic products |
13.2 |
10.6 |
A: Alimentary tract and metabolism |
13.0 |
9.8 |
N: Nervous system |
7.9 |
12.6 |
R: Respiratory system |
8.5 |
7.9 |
B: Blood and blood forming organs |
6.6 |
6.8 |
S: Sensory organs |
5.4 |
2.1 |
C: Cardiovascular system |
3.8 |
24.5 |
M: Musculo-skeletal system |
2.8 |
4.0 |
G: Genito-urinary system and sex hormones |
2.4 |
3.9 |
H: Systemic hormonal preparations |
1.0 |
0.7 |
D: Dermatologicals |
1.1 |
1.0 |
V: Various |
0.6 |
0.5 |
The accompanying table gives the 2018 total sales by therapeutic class, the growth in sales (millions of dollars) and the growth rate (percent) from 2017 to 2018, and the 2018 share of sales.
| Therapeutic Class | 2018 Sales (millions of dollars) |
Growth: 2018/2017, $M (rate in %) | 2018 share of sales (%) |
|---|---|---|---|
L: Antineoplastics and immunomodulating agents |
$5,605.69 |
-$117.9 |
33.7% |
J: General antiinfectives for systemic use and P: Antiparasitic products |
$2,194.34 |
-$85.6 |
13.2% |
A: Alimentary tract and metabolism |
$2,158.87 |
$253.5 |
13.0% |
N: Nervous system |
$1,315.30 |
-$233.2 |
7.9% |
R: Respiratory system |
$1,419.89 |
$110.5 |
8.5% |
B: Blood and blood forming organs |
$1,098.50 |
$111.6 |
6.6% |
S: Sensory organs |
$895.39 |
-$32.5 |
5.4% |
C: Cardiovascular system |
$635.90 |
-$241.5 |
3.8% |
M: Musculo-skeletal system |
$469.38 |
$38.9 |
2.8% |
G: Genito-urinary system and sex hormones |
$407.22 |
-$4.8 |
2.4% |
H: Systemic hormonal preparations |
$161.40 |
$7.5 |
1.0% |
D: Dermatologicals |
$186.38 |
$47.6 |
1.1% |
V: Various |
$104.83 |
$10.7 |
0.6% |
All therapeutic classes |
$16,653.10 |
-$134.4 |
100% |
Biologic medicines
Biologic medicines, the majority of which are in high-cost categories, have been capturing an increasing share of the Canadian market, from 16% of patented medicine sales in 2008 to 39% in 2018. In 2018, Humira, Eylea, and Enbrel were the top-selling biologics, collectively accounting for almost 10% of all patented medicine sales. Figure 15 breaks down the annual growth in biologic patented medicine sales by major therapeutic class.
Although the share of biologic medicine sales has increased in many therapeutic classes, immunosuppressants had the highest uptake over the last decade, growing from 4% of total patented medicine sales in 2008 to 17% in 2017, primarily driven by the sales of three medicines: Remicade, Humira, and Enbrel.
However, in 2018, the immunosuppressant share of patented medicine sales decreased from 17% to 13%, as top-selling medicine Remicade stopped reporting to the PMPRB. Although the sales of these medicines continued to have a noteworthy impact on the overall Canadian pharmaceutical market, they were no longer captured in PMPRB data. In 2018, the overall biologics share of patented medicine sales dropped from 42% to 39% due to the exiting effect of these important medicines. The share of all other major classes of biologics remained the same as in 2017, except for oncology medicines, which represent a steadily growing share of the market.
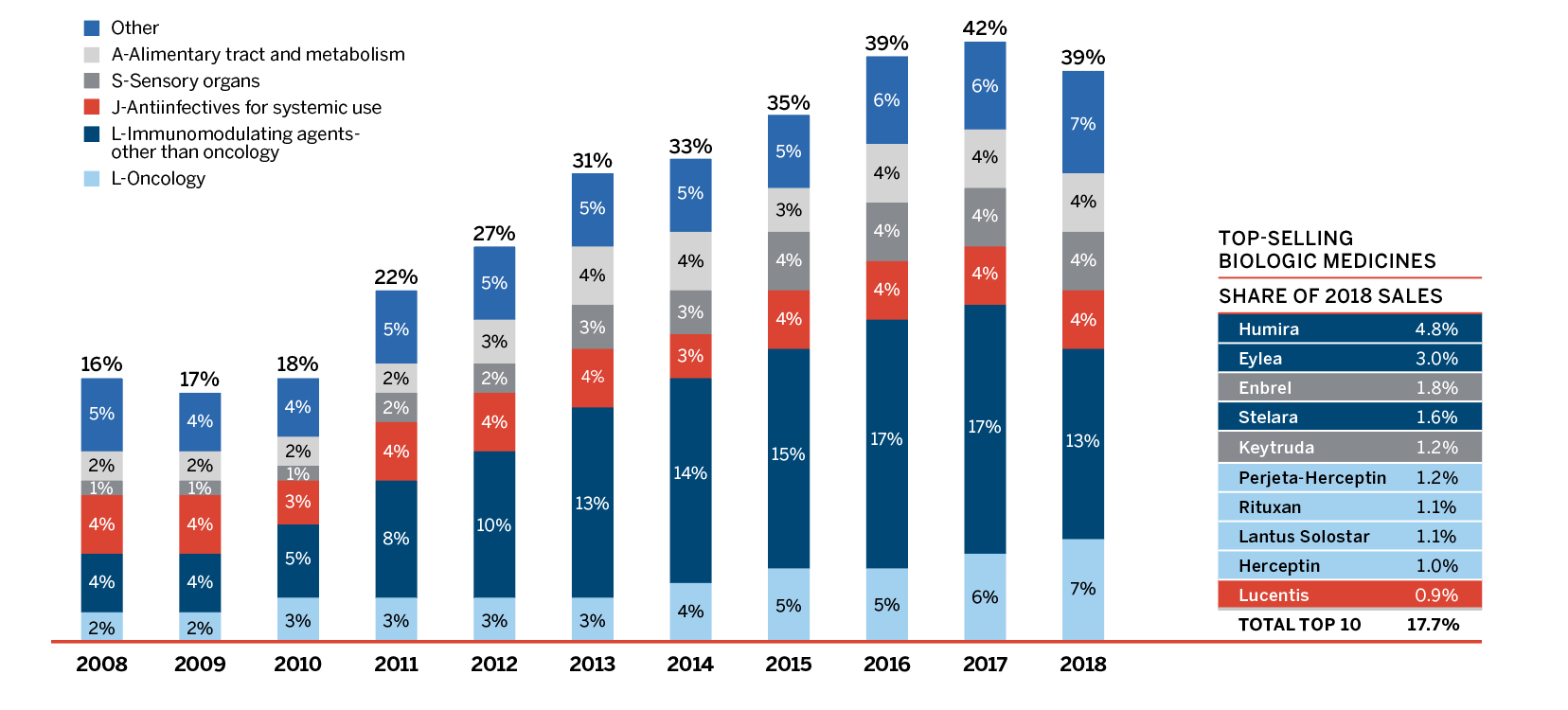
Data source: PMPRB
Figure description
This bar graph depicts the biologic medicine share of total patented medicine sales by therapeutic class from 2008 to 2018. Each bar is subdivided into the therapeutic class bands: Other; Alimentary tract and metabolism; Sensory organs; Antiinfectives for systemic use; Immunomodulating agents other than oncology; and Oncology. The total sales in billions of dollars is given for each year.
| Therapeutic Class | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|---|---|
Other |
5% |
4% |
4% |
5% |
5% |
5% |
5% |
5% |
6% |
6% |
7% |
A: Alimentary tract and metabolism |
2% |
2% |
2% |
2% |
3% |
4% |
4% |
3% |
4% |
4% |
4% |
S: Sensory organs |
1% |
1% |
1% |
2% |
2% |
3% |
3% |
4% |
4% |
4% |
4% |
J: Antiinfectives for systemic use |
4% |
4% |
3% |
4% |
4% |
4% |
3% |
4% |
4% |
4% |
4% |
L: Immunomodulating agents – other than Oncology |
4% |
4% |
5% |
8% |
10% |
13% |
14% |
15% |
17% |
17% |
13% |
L: Oncology |
2% |
2% |
3% |
3% |
3% |
3% |
4% |
5% |
5% |
6% |
7% |
Sales (billions of dollars) |
$2.6 |
$2.8 |
$2.9 |
$3.7 |
$4.4 |
$5.1 |
$5.5 |
$5.9 |
$6.5 |
$7.0 |
$6.5 |
The accompanying table gives the share of 2018 sales for the 10 top- selling biologics and their therapeutic class.
| Medicine | Therapeutic Class | Share of 2018 Sales |
|---|---|---|
Humira |
L: Immunomodulating agents other than oncology |
4.8% |
Eylea |
S – sensory organs |
3.0% |
Enbrel |
L – immunomodulating agents-other than oncology |
1.8% |
Stelara |
L: Immunomodulating agents other than oncology |
1.6% |
Keytruda |
L - oncology |
1.2% |
Perjeta-Herceptin |
L: Oncology |
1.2% |
Rituxan |
L: Oncology |
1.1% |
Lantus Solostar |
A – alimentary tract and metabolism |
1.1% |
Herceptin |
L: Oncology |
1.0% |
Lucentis |
S – sensory organs |
0.9% |
Total Top 10 Biologics |
17.7% |
|
Brief Insights: Biosimilar Uptake
Given the high use and cost of biologics in Canada, biosimilars offer an opportunity for significant cost savings. However, unlike generics, biosimilars are not identical to their originator medicines, but are rather highly similar versions. Health Canada’s authorization of a biosimilar is not a declaration of equivalence to the originator biologic, which is one of the requirements for provincial/territorial authorities to determine interchangeability. This makes it more difficult to switch one medicine for another, and new patients and prescribers may be more cautious about using a biosimilar version. Thus, a loss of exclusivity does not necessarily equate to a loss of sales for originator biologic medicines.
The prices of biosimilars are generally higher in Canada than in other countries, and biosimilar uptake is lower. In addition, when biosimilars do become available, new patients may be more likely to start on other originator biologics rather than the biosimilar alternative.
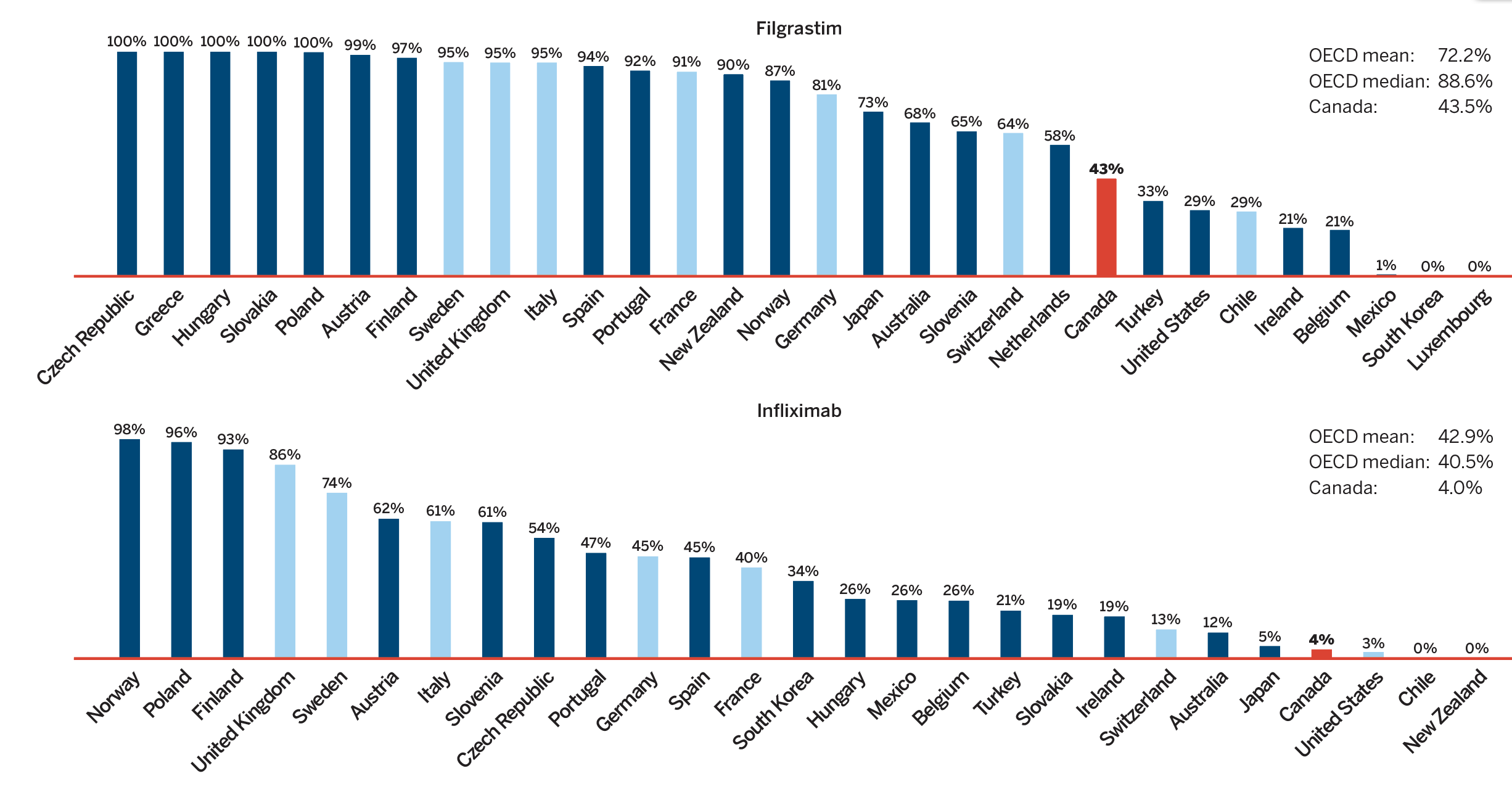
Note: Countries without recorded biosimilar sales in MIDAS® are excluded from the calculation of OECD averages.
Data source: International Policies on the Appropriate Use of Biosimilar Drugs (Canadian Agency for Drugs and Technologies in Health, 2018);
MIDAS® database, 2017, IQVIA (all rights reserved)
[NPDUIS Report: Meds Entry Watch, 2017]
Figure description
These two bar graphs illustrate the uptake of two biologic medicines: filgrastim and infliximab. The share of units sold is given for each of the Organisation for Economic Co-operation and Development (OECD) countries in the last quarter of 2017. The OECD mean and median are also indicated.
| OECD country | Filgrastim share of units sold |
|---|---|
Estonia |
|
Czech Republic |
100% |
Greece |
100% |
Hungary |
100% |
Slovak Republic |
100% |
Poland |
100% |
Austria |
99% |
Finland |
97% |
Sweden |
95% |
United Kingdom |
95% |
Italy |
95% |
Spain |
94% |
Portugal |
92% |
France |
91% |
New Zealand |
90% |
Norway |
87% |
Germany |
81% |
Japan |
73% |
Australia |
69% |
Slovenia |
65% |
Switzerland |
64% |
Netherlands |
59% |
Canada |
44% |
Turkey |
34% |
United States |
29% |
Chile |
29% |
Ireland |
22% |
Belgium |
21% |
Mexico |
1% |
Korea |
0% |
Luxembourg |
0% |
OECD mean |
72.2% |
OECD median |
88.6% |
| OECD country | Inflixmab share of units sold |
|---|---|
Estonia |
|
Greece |
N/A |
Norway |
98% |
Poland |
96 % |
Finland |
93% |
United Kingdom |
86% |
Sweden |
74% |
Austria |
62% |
Italy |
61% |
Slovenia |
61% |
Czech Republic |
54% |
Portugal |
47% |
Germany |
45% |
Spain |
45% |
France |
41% |
Korea |
35% |
Hungary |
27% |
Mexico |
26% |
Belgium |
26% |
Turkey |
21% |
Slovak Republic |
19% |
Ireland |
19% |
Switzerland |
13% |
Australia |
12% |
Japan |
6% |
Canada |
4% |
United States |
3% |
Chile |
0% |
New Zealand |
0% |
OECD mean |
42.9% |
OECD median |
40.5% |
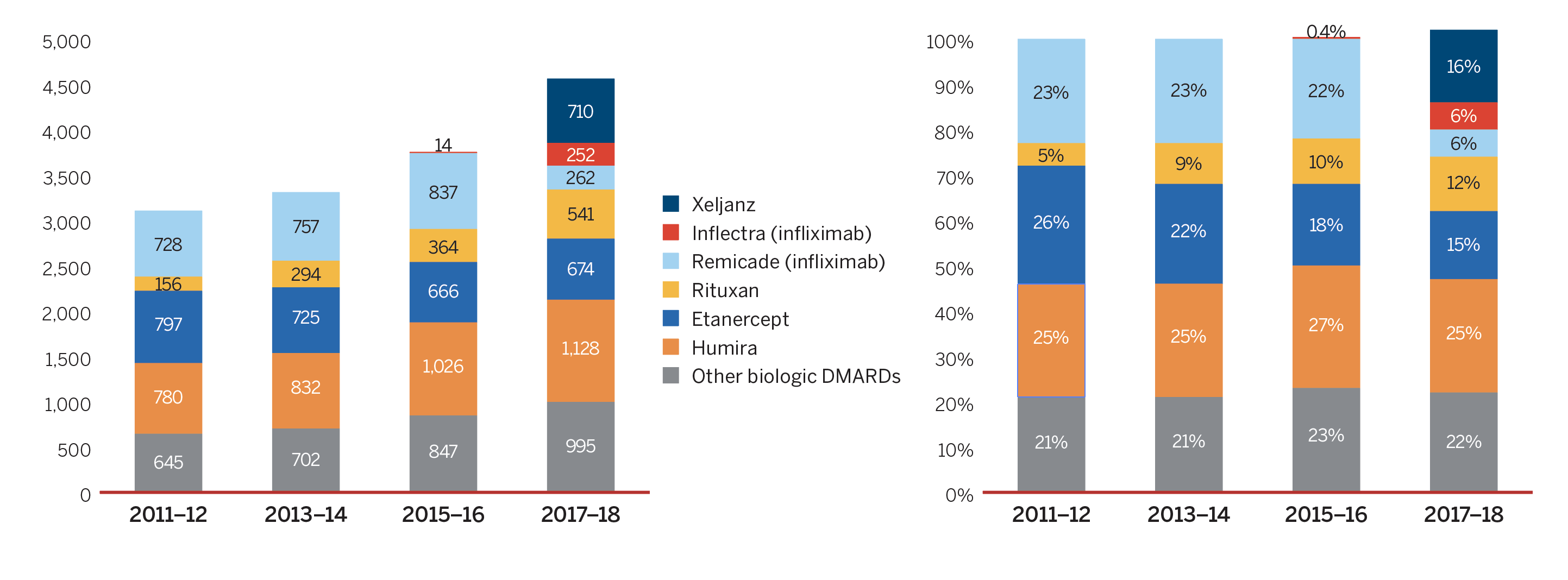
Note:Other biologic DMARDs include Simponi, Orencia, Actemra, and Cimzia. Results do not distinguish between use for rheumatoid arthritis and for other indications.
Data source: NPDUIS database, CIHI
[NPDUIS Report: CompassRx, 2017/18]
Figure description
These two stacjed bar graph break down the distribution of new patients started on select disease-modifying antirheumatics by drug every second year from 2011-12 to 2017-18. The first graph gives the number of new patients started on each drug, while the second gives the distribution of new patients as a share of all select disease-modifying antirheumatics.
| Other Biologic DMARDs | Humira | Etanercept | Rituxan | Remicade (infliximab) | Inflectra (infliximab) | Xeljanz | |
|---|---|---|---|---|---|---|---|
2011-12 |
645 |
780 |
797 |
156 |
728 |
– |
– |
2013-14 |
702 |
832 |
725 |
294 |
757 |
– |
– |
2015-16 |
847 |
1,026 |
666 |
364 |
837 |
14 |
– |
2017-18 |
995 |
1,128 |
674 |
541 |
262 |
252 |
710 |
| Other Biologic DMARDs | Humira | Etanercept | Rituxan | Remicade (infliximab) | Inflectra (infliximab) | Xeljanz | |
|---|---|---|---|---|---|---|---|
2011-12 |
21% |
25% |
26% |
5% |
23% |
– |
– |
2013-14 |
21% |
25% |
22% |
9% |
23% |
– |
– |
2015-16 |
23% |
27% |
18% |
10% |
22% |
0.4% |
– |
2017-18 |
22% |
25% |
15% |
12% |
6% |
6% |
16% |
Oncology medicines
Figure 18 illustrates the growth in the sales of all oncology medicines (biologic and non-biologic) over the last decade. Oncology medicines now account for 20.7% of total patented medicine sales, a substantial increase from 5.4% in 2008. Oral forms of cancer treatment are a noteworthy emerging segment, increasing their share of the patented medicine market from 1.7% to 9.2% over the same period. Revlimid was the top-selling oncology medicine, accounting for 2.5% of all patented medicine sales.Footnote 9
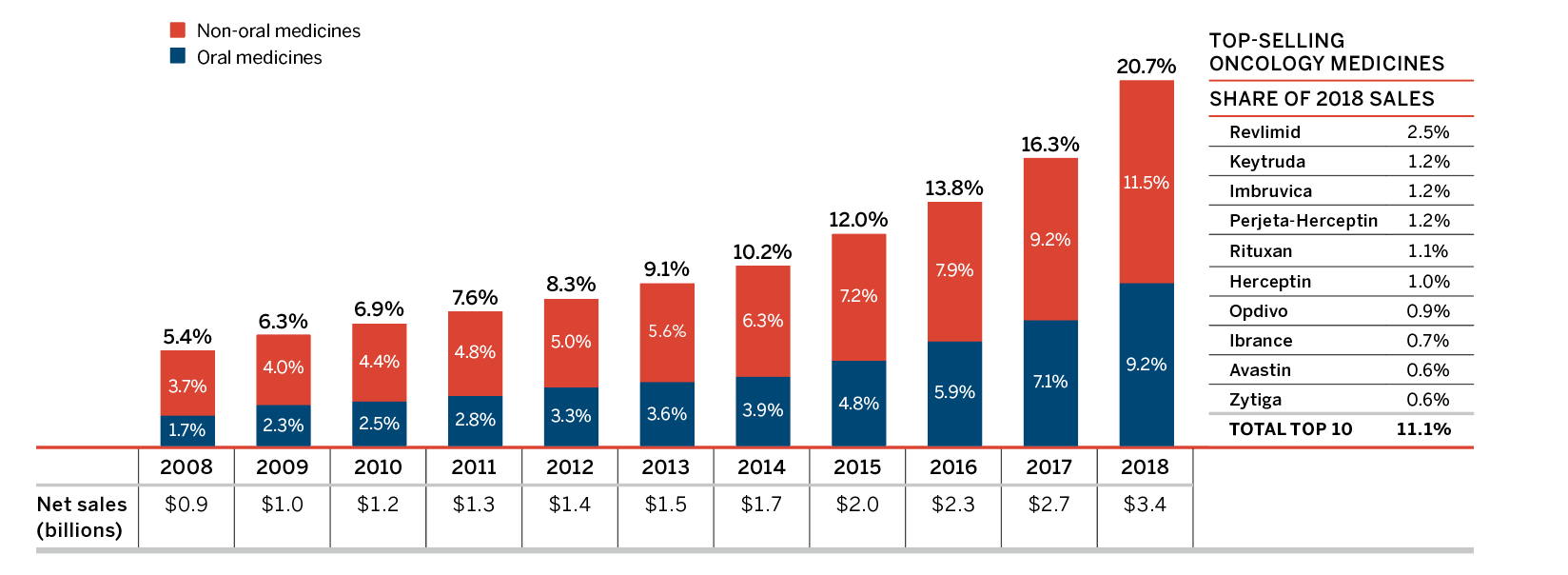
Note: These results reflect the total sales for patented medicines used in the treatment of cancer. While some of these medicines may also be used to treat other conditions, the data used for this analysis does not distinguish between indications, and thus, the reported sales may reflect some non-cancer use.
Data source: PMPRB
Figure description
This stacked bar graph depicts the oncology medicine share of patented medicine sales by formulation from 2008 to 2018. Each bar is subdivided into non-oral medicine and oral medicine formulations. The combined total oncology share of patented medicine sales as well as the net sales in billions of dollars are also indicated.
Formulation |
2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|---|---|
Non-oral medicines |
3.7% |
4.0% |
4.4% |
4.8% |
5.0% |
5.6% |
6.3% |
7.2% |
7.9% |
9.2% |
11.5% |
Oral medicines |
1.7% |
2.3% |
2.5% |
2.8% |
3.3% |
3.6% |
3.9% |
4.8% |
5.9% |
7.1% |
9.2% |
Total oncology share |
5.4% |
6.3% |
6.9% |
7.6% |
8.3% |
9.1% |
10.2% |
12.0% |
13.8% |
16.3% |
20.7% |
Sales (billions of dollars) |
$0.9 |
$1.0 |
$1.2 |
$1.3 |
$1.4 |
$1.5 |
$1.7 |
$2.0 |
$2.3 |
$2.7 |
$3.4 |
The accompanying table gives the share of 2018 sales for the 10 top-selling oncology medicines.
| Oncology medicine | Share of 2018 sales |
|---|---|
Revlimid |
2.5% |
Keytruda |
1.2% |
Imbruvica |
1.2% |
Perjeta-Herceptin |
1.2% |
Rituxan |
1.1% |
Herceptin |
1.0% |
Opdivo |
0.9% |
Ibrance |
0.7% |
Avastin |
0.6% |
Zytiga |
0.6% |
Total top 10 |
11.1% |
Price Trends
The PMPRB uses the Patented Medicines Price Index (PMPI) to monitor trends in the prices of patented medicines. The PMPI measures the average yearover- year change in the ex-factory prices of patented medicines sold in Canada using a sales-weighted average of price changes at the level of individual medicines.Footnote 10 This is similar to the approach Statistics Canada uses to construct the Consumer Price Index (CPI). The PMPI is based on an average transaction price and sales information submitted by patentees for a six-month period.
The PMPI only measures the sales growth attributable to changes in the prices of patented medicines. It does not measure changes in the use of patented medicines; this is measured by the quantity index or PMQI (see the Utilization of Patented Medicines section). Nor does it measure the cost impact of changes in prescribing patterns or the introduction of new medicines.
The Patent Act requires the PMPRB to consider changes in the CPI, among other factors, in determining whether the price of a patented medicine is excessive. Figure 19 compares year-over-year changes in the PMPI to corresponding changes in the CPI from 2003 to 2018. The PMPI is reported based on two measures: the national average transaction price, a “net” price which includes rebates and discounts; and the national list price, a “gross”price. Both measures are reported to the PMPRB by patentees. General price inflation, as measured by the CPI, has exceeded the average increase in the prices of patented medicines almost every year since 2003. In 2018, the CPI rose by 2.3%, while the PMPI increased by 0.1%.
It is not surprising that the PMPI has seldom kept pace with the CPI. The PMPRB’s Guidelines envisage that the price of a patented medicine should not rise by more than the CPI over any three-year period.Footnote 11 (The Guidelines also contemplate a cap on year-over-year price increases equal to one and one-half times the current year rate of CPI inflation.) This effectively establishes CPI inflation as an upper bound on the amount by which individual prices could rise over any three-year period. Increases in the PMPI normally do not reach this upper bound because many patentees do not raise their prices by the full amount envisaged under the Guidelines.
Patented medicine prices increased less than CPI
In 2018, the increase in patented medicine prices was, on average, less than the rate of inflation, as measured by the Consumer Price Index (CPI), and therefore, did not contribute to sales growth.
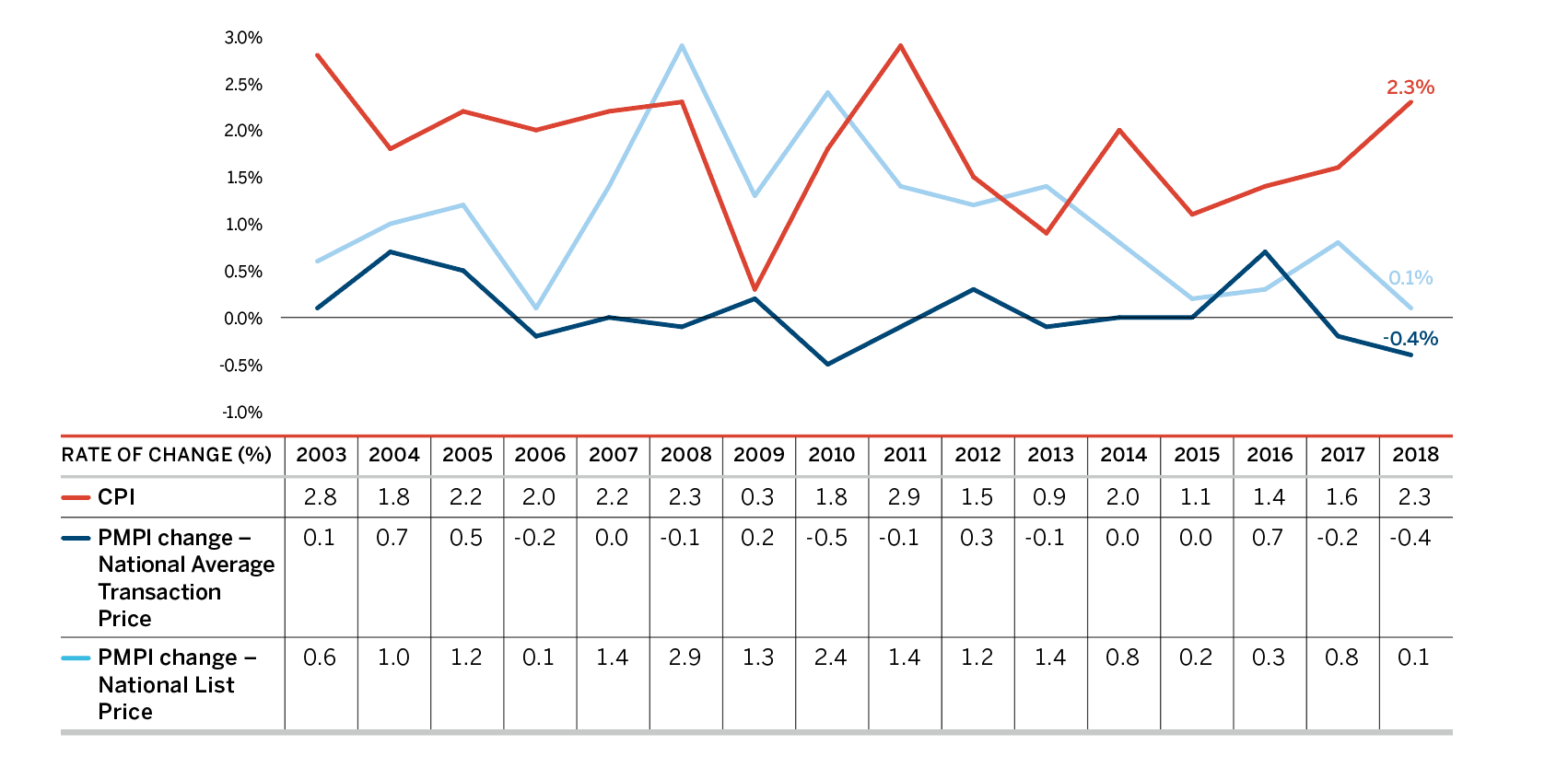
Data source: PMPRB; Statistics Canada
Figure description
This line graph depicts the year-over-year percent changes in the PMPI and CPI for the years 2003 to 2018. The PMPI is represented by two lines – one based on the national average transaction price and one based on the national list price.
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
PMPI change (National Average Transaction Price) |
0.1 |
0.7 |
0.5 |
-0.2 |
0.0 |
-0.1 |
0.2 |
-0.5 |
-0.1 |
0.3 |
-0.1 |
0.0 |
0.0 |
0.7 |
-0.2 |
-0.4 |
PMPI change (National List Price) |
0.6 |
1.0 |
1.2 |
0.1 |
1.4 |
2.9 |
1.3 |
2.4 |
1.4 |
1.2 |
1.4 |
0.8 |
0.2 |
0.3 |
0.8 |
0.1 |
CPI change |
2.8 |
1.8 |
2.2 |
2.0 |
2.2 |
2.3 |
0.3 |
1.8 |
2.9 |
1.5 |
0.9 |
2.0 |
1.1 |
1.4 |
1.6 |
2.3 |
Price Behaviour After Introduction
Does the price of a typical patented medicine change much in the years after it enters the Canadian market? To answer this question, Figure 20 provides the average ratio of the 2018 price to introductory price (the price at which the medicine was sold in its first year on the Canadian market).
The results in Figure 20 suggest a consistent trend: prices remain stable early in their life cycle, and then gradually rise by a small amount, year-over-year, afterwards. This is consistent with the effect of the PMPRB’s CPI methodology.Footnote 12 For example; average prices of medicines introduced a decade ago are still at the same level in 2018.
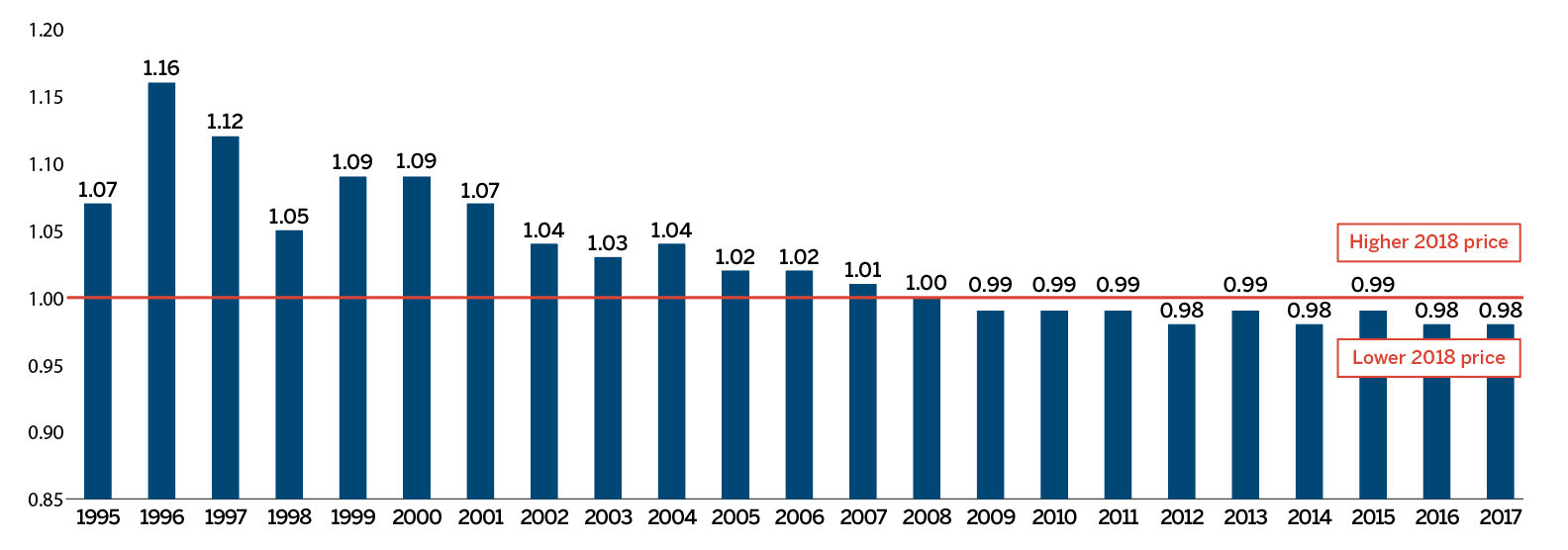
Data source: PMPRB
Figure description
This bar graph depicts the average ratio of the 2018 price of a typical patented medicine to the introductory price (the price at which the medicine was sold in its first year on the Canadian market) for medicines introduced between 1995 and 2017. A line at 1.00 indicates the 2018 price.
For medicines introduced in 1995, the average ratio of the 2018 price to the introductory price is 1.07. In 1996, 1.16; 1997, 1.12; 1998, 1.05; 1999, 1.09; 2000, 1.09; 2001, 1.07; 2002, 1.04; 2003, 1.03; 2004, 1.04; 2005, 1.02; 2006, 1.02; 2007, 1.01; 2008, 1.00; 2009, 0.99; 2010, 0.99; 2011, 0.99; 2012, 0.98;
2013, 0.99; 2014, 0.98; 2015, 0.99; 2016, 0.98; 2017, 0.98.
Price Change by Country
In accordance with the Act and the Regulations, patentees must report publicly available prices of patented medicines for seven foreign comparator countries (PMPRB7): France, Germany, Italy, Sweden, Switzerland, the United Kingdom (UK), and the United States (US).
The PMPRB uses this information:
- to conduct international price comparison tests; and
- to compare the Canadian prices of patented medicines to those prevailing in other countries.
Figure 21 gives the average annual rates of price change for Canada and each of the PMPRB7 countries. These results were obtained by applying the PMPI methodology (with weights based on Canadian sales patterns) to the international price data that patentees submitted to the PMPRB. Note that prices from the US Federal Supply Schedule (FSS)Footnote 13 are incorporated into the US results.
In 2018, US prices rose by an average of 5.9%, while prices in all other countries declined. Germany saw the greatest decrease, at -10.5%. These results are consistent with a long-term tendency for patented medicine prices to slowly fall over time in most comparable countries (with the exception of the US).
The foreign market results are based on publicly available gross prices, namely ex-factory price information (generally for the retail customer class) submitted by patentees to the PMPRB. The Canadian rate of change, however, is based on net prices, namely actual average transaction prices net of rebates and discounts provided by manufacturers to their direct customers.
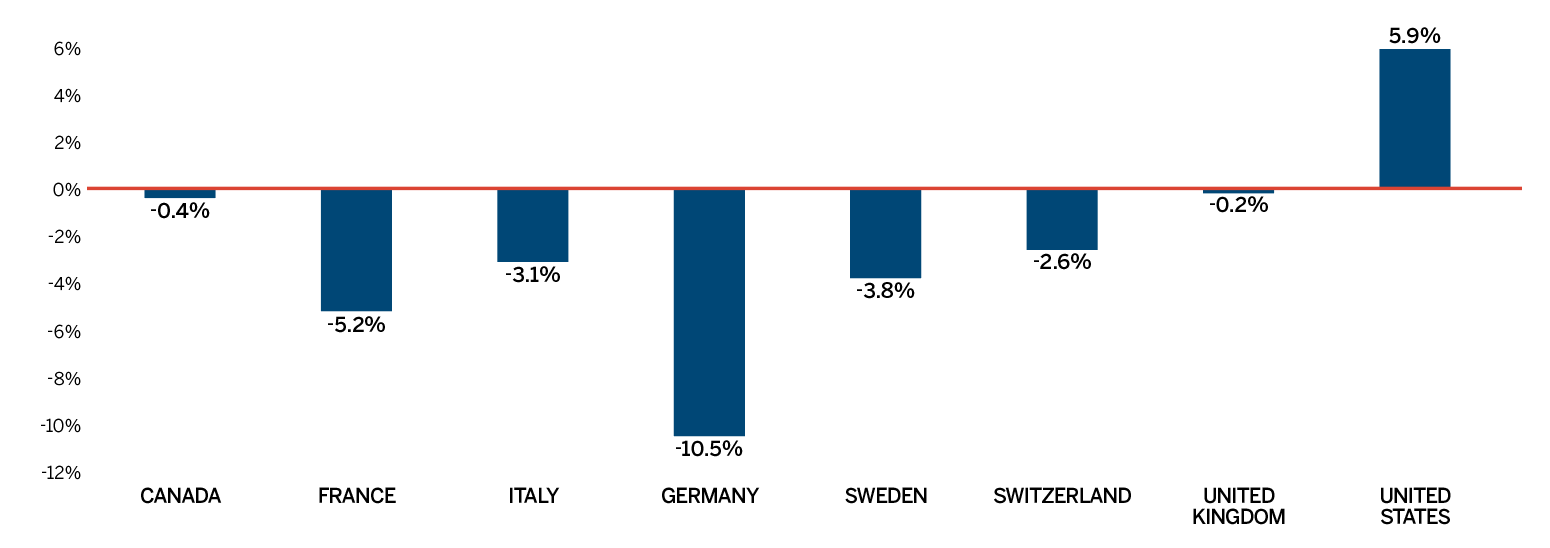
Data source: PMPRB
Figure description
This bar graph depicts the average annual rates of price change for Canada and each of the PMPRB7 comparator countries for 2018. In Canada, the average annual rate of price change was -0.4%.
France, -5.2%; Italy, -3.1%; Germany, -10.5%; Sweden, -3.8%; Switzerland, -2.6%; UK, -0.2%; US, 5.9%.
Comparison of Canadian Prices to Foreign Prices
Tables 9 and 10 provide detailed statistics comparing the foreign prices of patented medicines to their Canadian prices. Each table provides two sets of average price ratios. These are differentiated according to the method by which foreign prices were converted to their Canadian dollar equivalents. The tables also give the numbers of strengths and dosage forms of medicines (DINs) and the volume of sales encompassed by each reported price ratio.Footnote 14
The average price ratios given in Tables 9 and 10 are sales-weighted arithmetic means of price ratios obtained for individual DINs, with weights based on Canadian sales patterns. Average price ratios constructed in this way provide answers to questions such as:
How much more/less would Canadians have paid for the patented medicines they purchased in 2018 had they paid Country X prices rather than Canadian prices?
For example, Table 9 states that the 2018 average France-to-Canada price ratio was 0.74. This means Canadians would have paid 26% less for the patented medicines they purchased in 2018 if they had paid French prices.
For many years, the PMPRB has reported average foreign-to-Canadian price ratios with foreign prices converted to their Canadian dollar equivalents by means of market exchange rates. (More exactly, the 36-month moving averages of market rates the PMPRB normally uses in applying its Guidelines.) Tables 9 and 10 also report foreign-to-Canadian price ratios with currency conversion at purchasing power parity (PPP). The PPP between any two countries measures their relative costs of living expressed in units of their own currencies. In practice, cost of living is determined by pricing out a standard “basket” of goods and services at the prices prevailing in each country.
Because PPPs are designed to represent relative costs of living, they offer a simple way to account for differences in overall national price levels when comparing individual prices, incomes, and other monetary values across countries. When applied to the calculation of average foreign-to-Canadian price ratios they produce statistics answering questions such as:
How much more/less consumption of other goods and services would Canadians have sacrificed for the patented medicines they purchased in 2018 had they lived in Country X?
Questions of this type cannot be answered by simply comparing the prices of medicines. Rather, one must first calculate what each price represents in terms of goods and services foregone. PPPs are designed for such purposes.
Bilateral Price Comparisons
Table 9 provides bilateral comparisons of prices in each of the PMPRB7 countries to corresponding Canadian prices. Focusing on the results with currency conversion at market exchange rates, it appears that, as in previous years, Canadian prices were typically within the range of prices observed among the comparator countries. Prices in France were appreciably lower than Canadian prices, followed by Sweden and Italy. Prices in the UK were equivalent to those in Canada, while prices in Germany and Switzerland were higher. As in previous years, prices reported for the United States were much higher than prices in Canada or any other comparator country.
Table 9. Average Foreign-to-Canadian Price Ratios, Bilateral Comparisons, 2017
| Canada | France | Italy | Germany | Sweden | Switzerland | United Kingdom | United States | |
|---|---|---|---|---|---|---|---|---|
| Average price ratio 2018 | 1.00 | 0.74 | 0.97 | 1.09 | 0.94 | 1.08 | 1.00 | 3.59 |
| Average price ratio 2017 | 1.00 | 0.75 | 0.95 | 1.12 | 0.93 | 1.12 | 0.94 | 3.36 |
| Canada | France | Italy | Germany | Sweden | Switzerland | United Kingdom | United States | |
|---|---|---|---|---|---|---|---|---|
| Average price ratio 2018 | 1.00 | 0.76 | 1.09 | 1.13 | 0.82 | 0.82 | 0.99 | 3.07 |
| Average price ratio 2017 | 1.00 | 0.79 | 1.12 | 1.20 | 0.83 | 0.88 | 0.98 | 3.25 |
| Number of patented medicines 2018 | 1,392 | 662 | 812 | 1,015 | 820 | 863 | 976 | 1,074 |
| Sales ($millions) | 16,325.7 | 10,767.8 | 12,700.9 | 14,200.9 | 12,362.0 | 13,868.6 | 13,213.86 | 14,758.5 |
Data source: PMPRB
It is important to note that it is not always possible to find a matching foreign price for each and every strength and dosage form of a patented medicine sold in Canada. Table 9 displays how often an international price comparison was available for each of the comparator countries. For example, out of 1,392 DINs for patented medicines reported as under the PMPRB’s jurisdiction in 2018, a publicly available ex-factory price for France was available 48% of the time, whereas for the US the number was 77%. Given the integrated nature of the Canadian and US supply chain, it is not uncommon for the US to be the only comparator country with an available price for a strength and dosage form of a medicine sold in Canada. In this case, it is considered to constitute the international median price, as per the PMPRB’s methodology.
Average price ratios obtained with currency conversion at PPPs tell the same story. When international differences in the cost of living are considered, it appears that Canadians incurred a larger consumption cost for the patented medicines they purchased in 2018 than did residents of France, Sweden, Switzerland, and the UK.
Figure 22 puts these results in historical perspective. In 2008, Canadian prices were, on average, slightly higher than prices in Italy, France, Sweden, and the UK, and approximately the same as prices in Switzerland. By 2018, the gap between Canadian prices and prices in France and Sweden had grown slightly greater as the relative prices in these countries dropped, while the prices in Italy and the UK in 2018 were more in line with Canadian levels. Price levels in Switzerland, Germany, and the US all exceeded those in Canada in 2018.
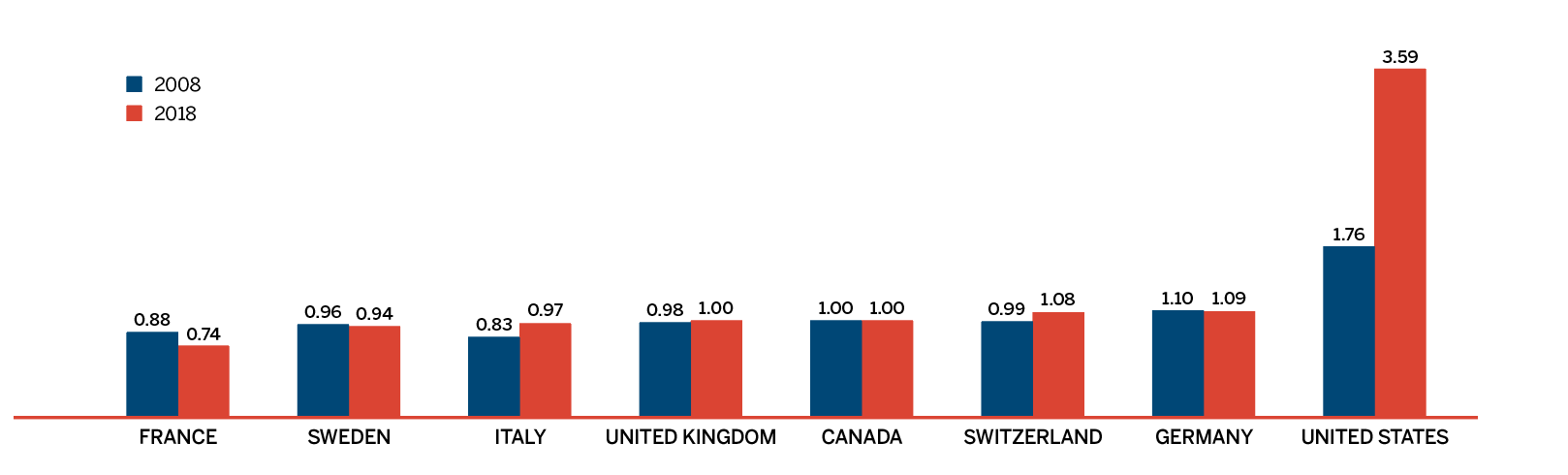
Data source: PMPRB
Figure description
This bar graph depicts the average foreign-to-Canadian price ratios in 2008 and 2018 for the PMPRB7 comparator countries.
- France — 2008; 0.88, 2018: 0.74
- Sweden — 2008; 0.96, 2018: 0.94
- Italy — 2008; 0.83, 2018: 0.97
- UK — 2008; 0.98, 2018: 1.00
- Canada — 2008: 1.00, 2018: 1.00
- Switzerland — 2008; 0.99, 2018: 1.08
- Germany — 2008; 1.10, 2018: 1.09
- US — 2008; 1.76, 2018: 3.59
If the patented medicine is being sold in one or more of the PMPRB7 countries, the patentee must report the publicly available ex-factory prices to the PMPRB for each class of customer.Footnote 15 In order to assess how Canada compares to a basket of countries beyond the PMPRB7, Figure 23 uses Canadian and international prices reported in the IQVIA MIDAS® database at the ex-factory manufacturer level, reflecting all sales to the pharmacy and hospital sectors.
The international price comparisons reported in Figure 23 provide a bilateral price comparison using all countries in the OECD with available MIDAS® data. The average foreign-to-Canadian price ratios are calculated using the same approach employed to produce the ratios presented in Figure 22. These are Canadian sales-weighted arithmetic averages of the corresponding foreign-to-Canadian price ratios for individual medicines.Footnote 16 As shown in Figure 23, median OECD prices are, on average, approximately 17% below price levels in Canada, which are the fourth highest among the 31 countries. Notably, the top three highest-priced countries are the US, Germany, and Switzerland.
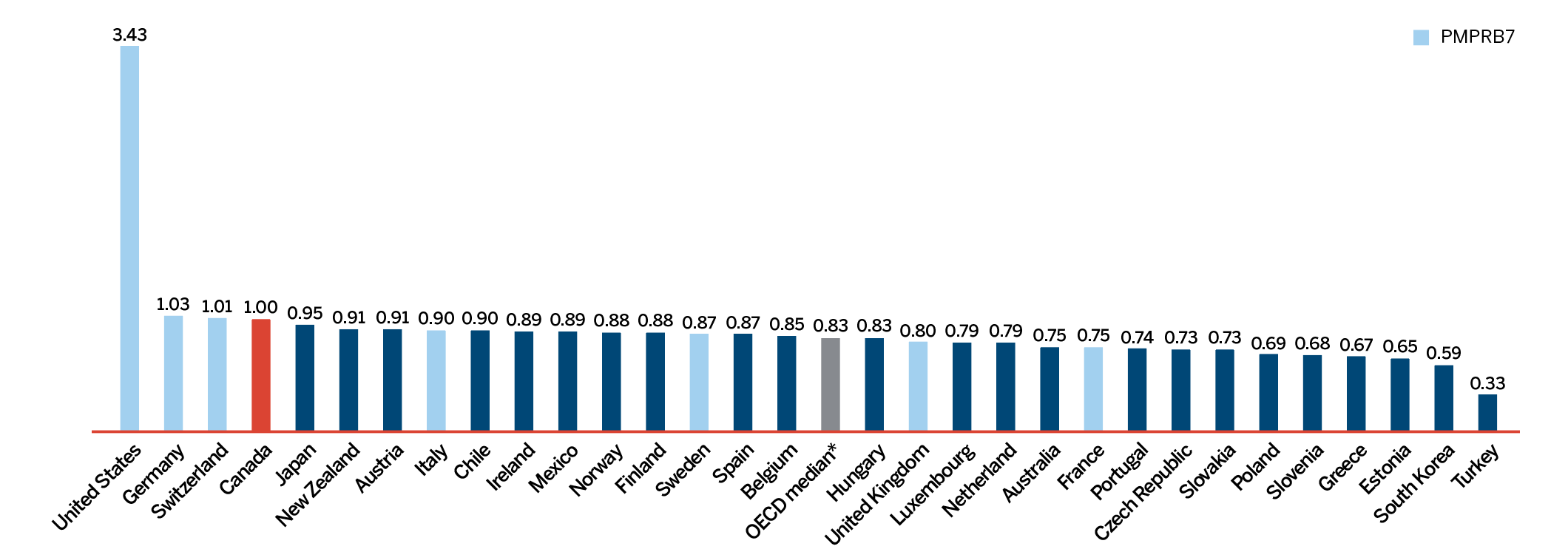
* Calculated at the medicine level for medicines with prices available in at least three foreign markets.
Data source: MIDAS® database, 2018, IQVIA (all rights reserved)
Figure description
This bar graph depicts the average foreign-to-Canadian price ratios for patented medicines in 2018 for OECD countries using Canadian and international prices reported in the IQVIA MIDAS® database. The given OECD median value is calculated at the medicine level for medicines with prices available in at least three foreign markets.
US: 3.43; Germany: 1.03; Switzerland: 1.01; Canada: 1.00; Japan: 0.95; New Zealand: 0.91; Austria: 0.91; Italy: 0.90; Chile: 0.90; Ireland: 0.89; Mexico: 0.89; Norway: 0.88; Finland: 0.88; Sweden: 0.87;
Spain: 0.87; Belgium: 0.85; OECD Median*: 0.83; Hungary: 0.83; UK: 0.83; Luxembourg: 0.79; Netherlands: 0.79; Australia: 0.75; France: 0.75; Portugal: 0.74; Czech Republic: 0.73; Slovakia: 0.73;
Poland: 0.69; Slovenia: 0.68; Greece: 0.67; Estonia: 0.65; South Korea: 0.59; Turkey: 0.33.
Brief Insights: Trends in the Price of Generic Medicines
The average price of generic medicines in Canada has dropped substantially, by 59% relative to price levels a decade ago (Figure 24). This exceeded the overall rates of price reductions in all PMPRB7 markets, as generic price decreases, coupled with a weakening Canadian dollar, gradually reduced the sizable historic gap between Canadian and foreign generic price levels. The latest Canadian generic pricing policy, implemented in 2018, brought Canadian generic prices in line with average prices in Italy, the US, and France. However, price levels are still lower in Germany, and significantly lower in the UK and Sweden, with the average prices for all OECD countries 15% lower than prices in Canada (Figure 25).
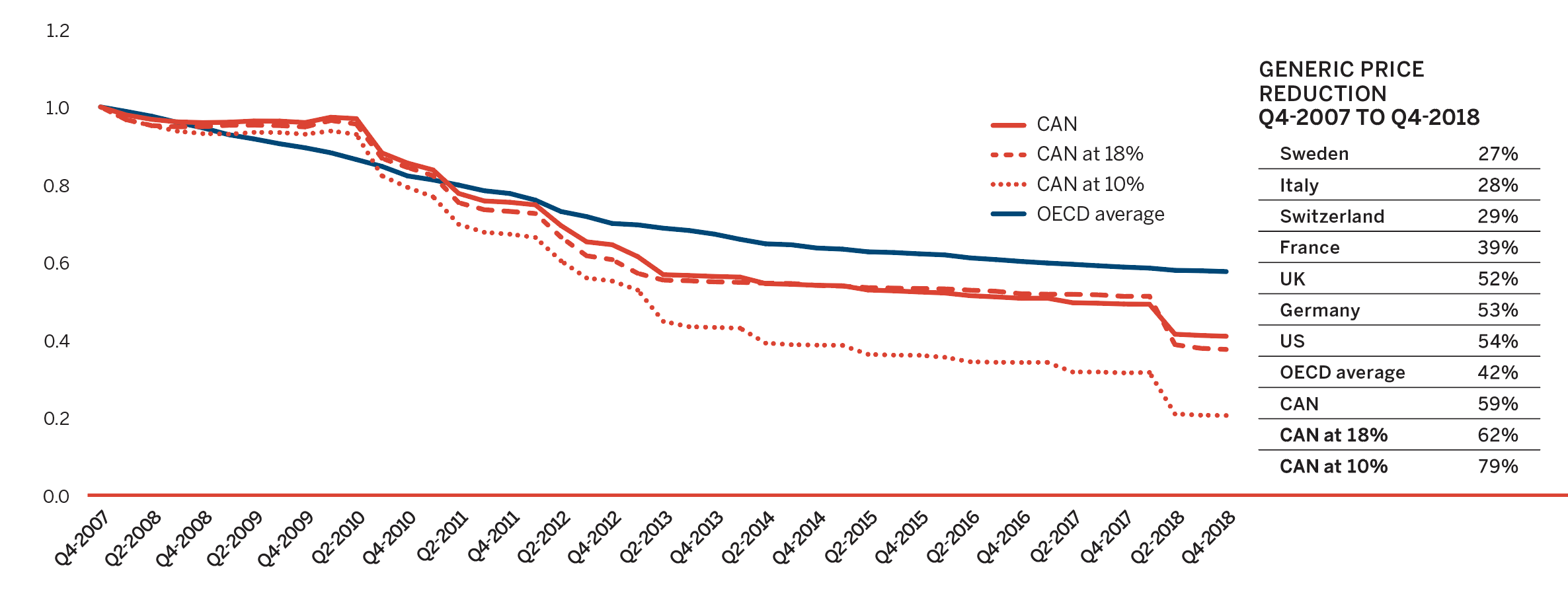
Note: The term “generic” used in this analysis includes both patented and non-patented generic medicines. Results are based on manufacturer ex-factory list prices in the national retail markets. The analysis was restricted to oral solid generic medicines that had been on the market for at least one year. CAN at 18% and 10% refer to the 67 generic medicines reduced to 18% and 10% of their brand reference prices through the generic pricing policy introduced in April 2018.
Data source: MIDAS® database, October–December 2007 to October–December 2018, IQVIA (all rights reserved)
[NPDUIS Report: Generics360, 2018]
Figure description
This line graph and accompanying table focus on the price reductions for generic medicines from the fourth quarter of 2007 to the fourth quarter of 2018. The graph gives the price indices for all generic medicines in Canada, as well as those at 18% and 10% of their brand-reference prices, and compares these with the average for the Organisation for Economic Co-operation and Development (OECD) countries over the same period. Note that the term “generic” used in this analysis includes both patented and non-patented generic medicines.
| Quarter year | Canada | Canada medicines at 18% | Canada medicines at 10% | OECD average |
|---|---|---|---|---|
2007: fourth quarter |
1.00 |
1.00 |
1.00 |
1.00 |
2008: first quarter |
0.98 |
0.97 |
0.97 |
0.99 |
2008: second quarter |
0.97 |
0.95 |
0.95 |
0.98 |
2008: third quarter |
0.96 |
0.95 |
0.94 |
0.96 |
2008: fourth quarter |
0.96 |
0.95 |
0.93 |
0.95 |
2009: first quarter |
0.96 |
0.95 |
0.93 |
0.93 |
2009: second quarter |
0.96 |
0.95 |
0.93 |
0.92 |
2009: third quarter |
0.96 |
0.95 |
0.93 |
0.91 |
2009: fourth quarter |
0.96 |
0.95 |
0.93 |
0.89 |
2010: first quarter |
0.97 |
0.96 |
0.94 |
0.88 |
2010: second quarter |
0.97 |
0.96 |
0.93 |
0.86 |
2010: third quarter |
0.88 |
0.87 |
0.82 |
0.85 |
2010: fourth quarter |
0.86 |
0.84 |
0.79 |
0.82 |
2011: first quarter |
0.84 |
0.82 |
0.77 |
0.81 |
2011: second quarter |
0.78 |
0.75 |
0.70 |
0.80 |
2011: third quarter |
0.76 |
0.74 |
0.68 |
0.78 |
2011: fourth quarter |
0.75 |
0.73 |
0.67 |
0.78 |
2012: first quarter |
0.75 |
0.73 |
0.66 |
0.76 |
2012: second quarter |
0.69 |
0.66 |
0.60 |
0.73 |
2012: third quarter |
0.65 |
0.62 |
0.56 |
0.72 |
2012: fourth quarter |
0.65 |
0.61 |
0.55 |
0.70 |
2013: first quarter |
0.61 |
0.57 |
0.53 |
0.70 |
2013: second quarter |
0.57 |
0.55 |
0.45 |
0.69 |
2013: third quarter |
0.57 |
0.55 |
0.43 |
0.68 |
2013: fourth quarter |
0.56 |
0.55 |
0.43 |
0.67 |
2014: first quarter |
0.56 |
055 |
0.43 |
0.66 |
2014: second quarter |
0.55 |
0.55 |
0.39 |
0.65 |
2014: third quarter |
0.54 |
0.55 |
0.39 |
0.65 |
2014: fourth quarter |
0.54 |
0.54 |
0.39 |
0.64 |
2015: first quarter |
0.54 |
0.54 |
0.39 |
0.63 |
2015: second quarter |
0.53 |
0.53 |
0.36 |
0.63 |
2015: third quarter |
0.53 |
0.53 |
0.36 |
0.63 |
2015: fourth quarter |
0.52 |
0.53 |
0.36 |
0.62 |
2016: first quarter |
0.52 |
0.53 |
0.36 |
0.62 |
2016: second quarter |
0.51 |
0.53 |
0.34 |
0.61 |
2016: third quarter |
0.51 |
0.53 |
0.34 |
0.61 |
2016: fourth quarter |
0.51 |
0.53 |
0.34 |
0.61 |
2017: first quarter |
0.51 |
0.52 |
0.34 |
0.60 |
2017: second quarter |
0.50 |
0.52 |
0.32 |
0.59 |
2017: third quarter |
0.50 |
0.52 |
0.32 |
0.59 |
2017: fourth quarter |
0.49 |
0.51 |
0.32 |
0.59 |
2018: first quarter |
0.49 |
0.51 |
0.32 |
0.58 |
2018: second quarter |
0.41 |
0.39 |
0.21 |
0.58 |
2018: third quarter |
0.41 |
0.38 |
0.21 |
0.58 |
2018: fourth quarter |
0.41 |
0.38 |
0.21 |
0.58 |
The accompanying table gives the associated generic price reductions from the fourth quarter of 2007 to the fourth quarter of 2018 for the Canadian markets and OECD average, as well as for each of the PMPRB7 countries.
| Country | Generic price reduction |
|---|---|
Sweden |
27% |
Italy |
28% |
Switzerland |
29% |
France |
39% |
UK |
52% |
Germany |
53% |
US |
54% |
OECD average |
42% |
Canada: all generics |
59% |
Canada: generics at 18% |
62% |
Canada: generics at 10% |
79% |
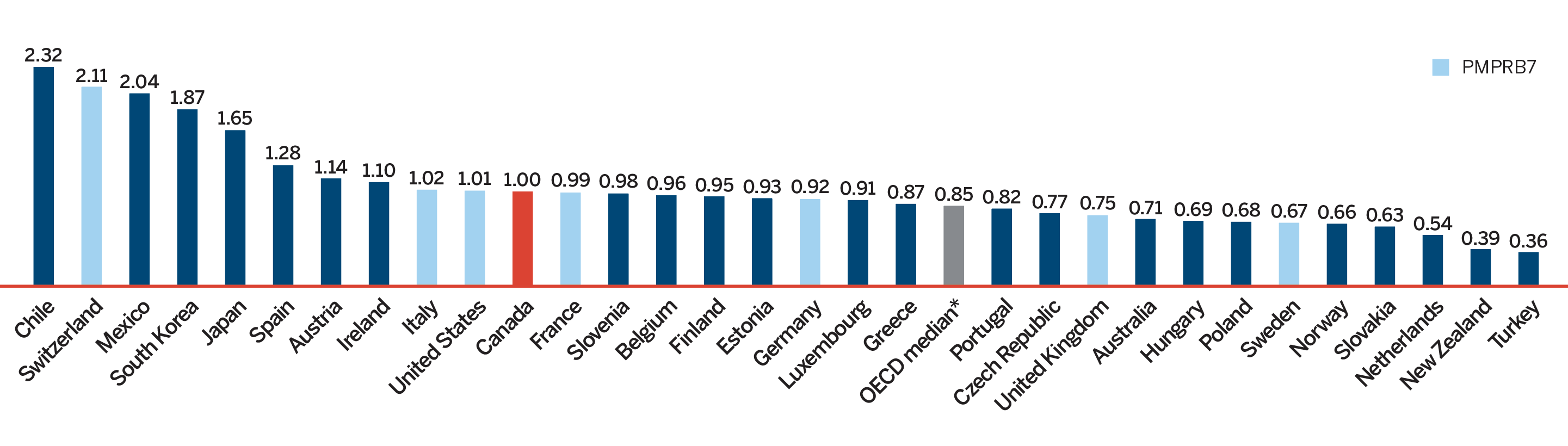
Note: The term “generic” used in this analysis includes both patented and non-patented generic medicines. Results are based on manufacturer ex-factory list prices in the national retail markets. The analysis was restricted to oral solid generic medicines that had been on the market for at least one year. Results were calculated at the medicine level for medicines with prices available in at least three foreign markets.
Data source: MIDAS® database, October–December 2018, IQVIA (all rights reserved)
[NPDUIS Report: Generics360, 2018]
Figure description
This bar graph compares the bilateral foreign-to-Canadian generic medicine price ratios for OECD countries for the fourth quarter of 2018. A value for the OECD median is also provided, calculated at medicine level for medicines with prices available in at least three foreign markets.
Note that the term “generic” used in this analysis includes both patented and non-patented generic medicines. Results are based on manufacturer ex-factory list prices in the national retail markets. The analysis was restricted to oral solid generic medicines that had been on the market for at least one year.
| Country | Foreign-to-Canadian price ratio |
|---|---|
Chile |
2.32 |
Switzerland |
2.11 |
Mexico |
2.04 |
South Korea |
1.87 |
Japan |
1.65 |
Spain |
1.28 |
Austria |
1.14 |
Ireland |
1.10 |
Italy |
1.02 |
US |
1.01 |
Canada |
1.00 |
France |
0.99 |
Slovenia |
0.98 |
Belgium |
0.96 |
Finland |
0.95 |
Estonia |
0.93 |
Germany |
0.92 |
Luxembourg |
0.91 |
Greece |
0.87 |
OECD median |
0.85 |
Portugal |
0.82 |
Czech Republic |
0.77 |
UK |
0.75 |
Australia |
0.71 |
Hungary |
0.69 |
Poland |
0.68 |
Sweden |
0.67 |
Norway |
0.66 |
Slovakia |
0.63 |
Netherlands |
0.54 |
New Zealand |
0.39 |
Turkey |
0.36 |
Multilateral Price Comparisons
Table 10 provides average foreign-to-Canadian price ratios using several multilateral measures of foreign prices. The median international price (MIP) is the median of prices observed among the PMPRB7. Other multilateral price ratios compare the minimum, maximum, and simple mean of foreign prices to their Canadian counterparts.
Focusing again on the results based on market exchange rates, the average MIP-to-Canadian price ratio was 1.20 in 2018, lower than the 1.26 ratio in 2017 (Figure 26). Note that mean foreign prices produce higher foreign-to-Canadian price ratios than do MIPs. This is due to the influence of US prices, which are typically much higher than prices elsewhere. Although US prices nearly always figure importantly in determining the mean foreign price, they have less impact on median international prices. Nevertheless, the US does exercise a significant influence over the average ratio of median international prices relative to Canadian prices, as the US is sometimes the only country with an available ex-factory price for a patented medicine sold in Canada.
| Median | Minimum | Maximum | Mean | |
|---|---|---|---|---|
| Average price ratio at market exchange rates | 1.20 | 0.91 | 3.50 | 1.53 |
| Average price ratio at purchasing power parities | 1.24 | 0.94 | 3.51 | 1.59 |
| Number of patented medicines | 1,287 | 1,287 | 1,287 | 1,287 |
| Sales ($millions) | 15,843.19 | 15,843.19 | 15,843.19 | 15,843.19 |
Data source: PMPRB
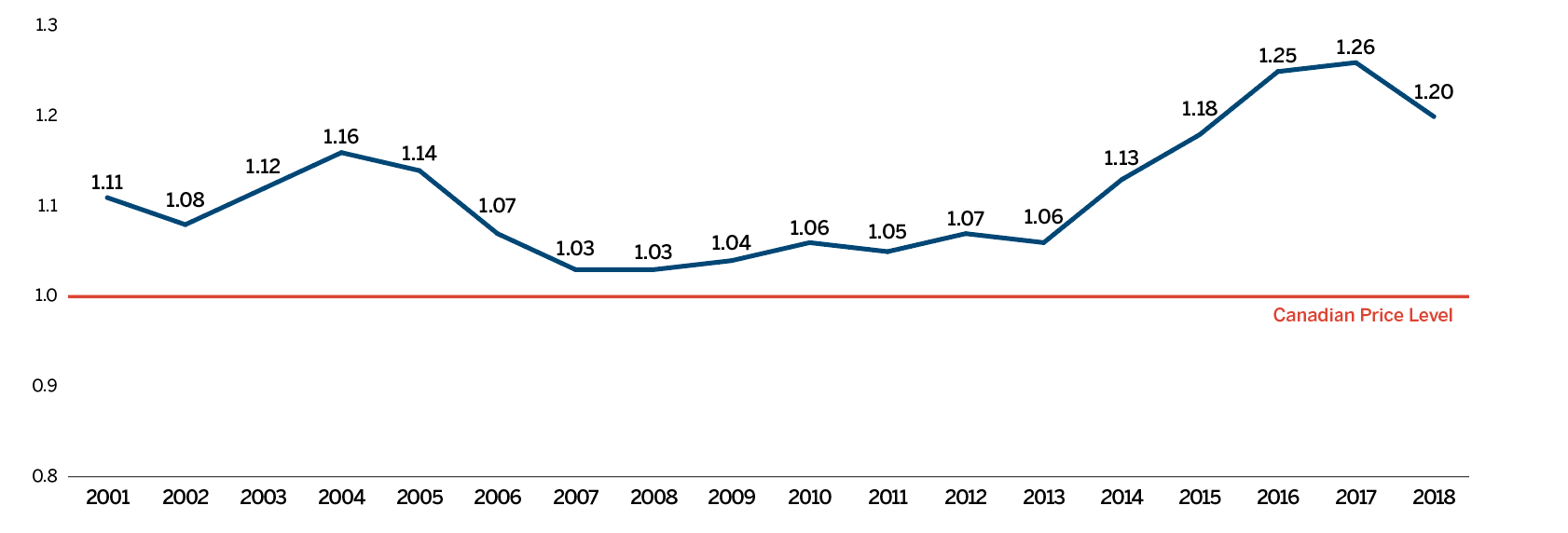
Data source: PMPRB
Figure description
This line graph depicts the trend in the average MIP-to-Canadian price ratios from 2001 to 2018 using the average transaction price (ATP) in Canada. In 2001, the average ratio of Median International Price (MIP) to Canadian price, at market exchange rates, was 1.11. In 2002: 1.08; 2003: 1.12; 2004: 1.16; 2005: 1.14; 2006: 1.07; 2007: 1.03; 2008: 1.03; 2009: 1.04; 2010: 1.06; 2011: 1.05; 2012: 1.07;
2013: 1.06; 2014: 1.13; 2015: 1.18; 2016: 1.25; 2017: 1.26; 2018: 1.20.
Figure 27 provides alternate results for the average MIP-to-Canadian price ratio at market exchange rates in 2018. To address the point that Canadian prices are national average transaction prices whereas foreign prices are list prices, a list price to list price ratio is also calculated. Using this method, the average ratio decreases from 1.20 to 1.07. It is important to keep in mind that confidential rebates provided to payers are currently not captured in this data.
To account for the large impact of US prices in determining the median foreign price, a ratio excluding the US and a ratio including at least five countries in the calculation of the median are also provided in Figure 27.
With these restrictions, the average MIP-to-Canadian price ratios drop to 0.87 and 0.91, respectively, suggesting that median foreign list prices are, on average, 13% to 9% lower than Canadian list prices. In many of the comparator countries, discounts off list prices are available to all payers, both public and private. By contrast, a large portion of the Canadian market pays list prices, or close to list prices. Furthermore, it should be noted that these are average ratios—some patentees charge Canadian consumers less than median international prices, while others charge more. For patentee level median-to-Canadian price ratios, please refer to Table 21 in Appendix 4 of this report.
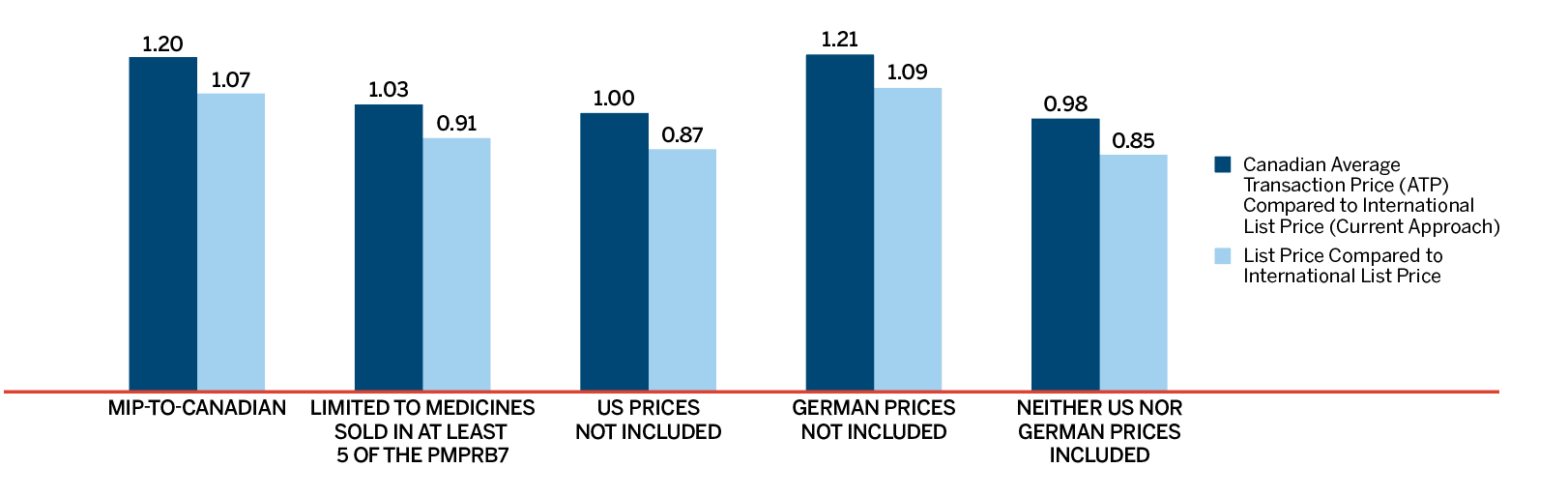
Data source: PMPRB
Figure description
This bar graph depicts the average MIP-to-Canadian price ratios at market exchange rates for 2018 using (1) the average transaction price in Canada (ATP) compared to the PMPRB7 international list price, and (2) the Canadian list price compared to the international list price.
| Ratio of the Average Transaction Price (ATP) to International List Price | Ratio of the Canadian List Price to International List Price | |
|---|---|---|
MIP to Canadian |
1.20 |
1.07 |
Median limited to medicines sold in at least 5 of the PMPRB7 |
1.03 |
0.91 |
US prices not included |
1.00 |
0.87 |
German prices not included |
1.21 |
1.09 |
Neither US nor German prices included |
0.98 |
0.85 |
Figure 28 offers more detail on the medicine-level MIP-to-Canadian ratios underlying the averages reported in Table 10. This figure distributes the 2018 sales of each patented medicine according to the value of its MIP-to-Canadian price ratio (more exactly, according to the range into which the ratio fell).Footnote 17 These results show substantial dispersion in medicine-level price ratios: while patented medicines with MIP-to-Canadian price ratios between 0.90 and 1.10 accounted for 32.5% of sales, those with ratios less than 0.90 accounted for 37.1% of sales, and medicines with ratios exceeding 1.10 accounted for 30.4%.
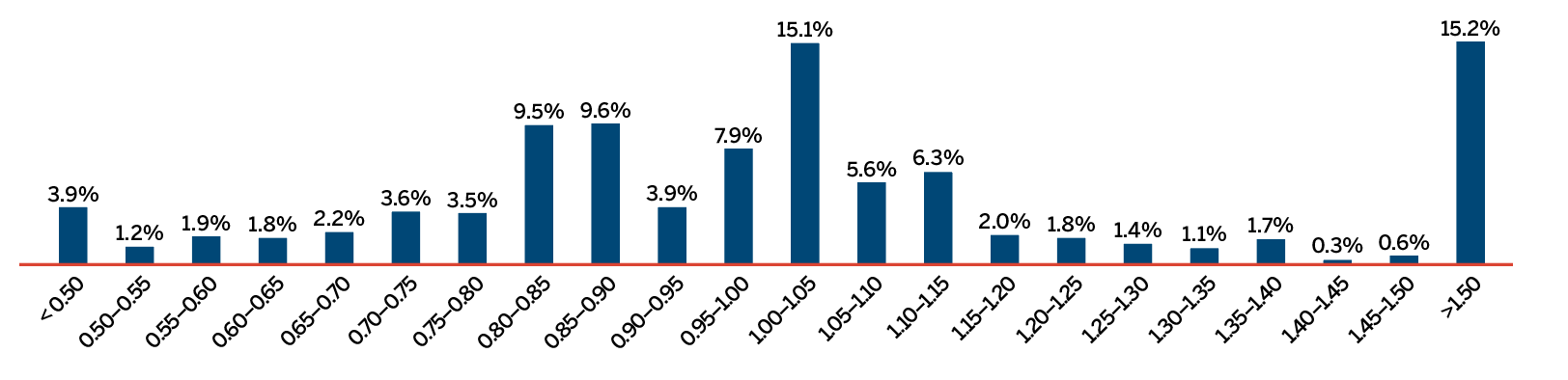
Data source: PMPRB
Figure description
This bar graph depicts the distribution of 2018 sales of patented medicines by their range of MIP-to-Canadian price ratio. Ratios between less than 0.50 and greater than 1.50 are given in increments of 0.05.
Patented medicines with price ratios less than 0.50 accounted for 3.2% of sales. For 0.50 to 0.55: 0.5%; 0.55 to 0.60: 1.8%; 0.60 to 0.65: 4.4%; 0.65 to 0.70: 1.9%; 0.70 to 0.75: 3.3%; 0.75 to 0.80: 3.9%; 0.80 to 0.85: 3.0%; 0.85 to 0.90: 8.5%; 0.90 to 0.95: 7.0%; 0.95 to 1.00: 10.7%; 1.00 to 1.05: 8.2%; 1.05 to 1.10: 8.8%; 1.10 to 1.15: 8.7%; 1.15 to 1.20: 3.7%; 1.20 to 1.25: 1.0%; 1.25 to 1.30: 0.5%; 1.30 to 1.35: 1.3%; 1.35 to 1.40: 3.3%; 1.40 to 1.45: 1.2%; 1.45 to 1.50: 2.2%; >1.50: 12.8%.
In 2018, approximately 50% of Canadian patented medicines were priced above the median international level.Footnote 18 Table 11 shows which therapeutic categories in particular are priced above the median international levels in Canada. Medicines that share the fourth level ATC (“ATC4”)Footnote 19 are grouped to identify distinct chemical/ pharmacological/therapeutic subgroups, allowing for a calculation of the average MIP-to-Canadian price ratios among medicines that may be used to treat the same conditions. Table 11 identifies the top 10 ATC4s in 2018 in which the difference between Canadian and median prices had the largest effect on Canadian patented medicine spending. For example, had Canadian prices been in line with the international median for these classes of medicines in 2018, sales in Canada would have been reduced by over $1 billion (an average reduction of 22% for these ATC4s). Of the 149 DINs classified into these 10 ATC4s, 63% were priced above the median international price.
| Description | ATC4 | NO. of Companies | NO. of Chemicals in ATC4 (NO. Currently Under Patent)Footnote 20 | Total Patented Dins | Patented Dins Greater Than Median Price | 2018 Net Revenue For Patented Dins ($millions) | Patented Dins ATC4 Share Of 2018 Revenues | Mip-to-canadian Ratio (Min. 5) Of Patented Dins | Impact Of Difference On Patented Medicines In 2018 |
|---|---|---|---|---|---|---|---|---|---|
| Antiinfectives for systemic use | J05AX | 6 | 14 (14) | 20 | 9 | $982.3 | 5.90% | 91% | $273.5 |
| Adrenergics in combination with corticosteroids or other medicines excluding anticholinergics | R03AK | 3 | 4 (4) | 11 | 9 | $568.0 | 3.41% | 59% | $231.8 |
| DPP-4 inhibitors | A10BH | 4 | 4 (4) | 9 | 9 | $349.0 | 2.10% | 75% | $89.7 |
| Glucocorticoids | R03BA | 7 | 9 (6) | 17 | 11 | $211.1 | 1.27% | 65% | $81.6 |
| Combinations of oral blood glucose lowering medicines | A10BD | 7 | 12 (12) | 36 | 22 | $356.6 | 2.14% | 69% | $78.9 |
| Antineovascularisation agents | S01LA | 2 | 2 (1) | 1 | 1 | $506.8 | 3.04% | 80% | $77.1 |
| Other blood glucose lowering drugs, excl. insulins | A10BX | 6 | 8 (7) | 13 | 11 | $547.7 | 3.29% | 87% | $73.6 |
| Insulins and analogues for injection, long-acting | A10AE | 3 | 5 (3) | 5 | 4 | $282.3 | 1.70% | 80% | $55.5 |
| Proton pump inhibitors | A02BC | 5 | 8 (7) | 13 | 10 | $186.3 | 1.12% | 45% | $54.3 |
| Other antineoplastic agents | L01XC | 11 | 19 (17) | 24 | 8 | $967.9 | 5.81% | 99% | $53.2 |
Data source: PMPRB
Canada is a Top 10 Global Market
Canada is an important market for pharmaceuticals representing 2.1% of worldwide sales. Canada spends approximately the same amount as the UK on pharmaceuticals despite having only half its population.
Utilization of Patented Medicines
The price and sales data used to calculate the PMPI also allow the PMPRB to examine trends in the quantities of patented medicines sold in Canada. The PMPRB maintains the Patented Medicines Quantity Index (PMQI) for this purpose. Figure 29 provides average rates of utilization growth, as measured by the PMQI, from 1988 through 2018. These results confirm that in recent years, growth in the utilization of patented medicines has been a primary source of rising sales.
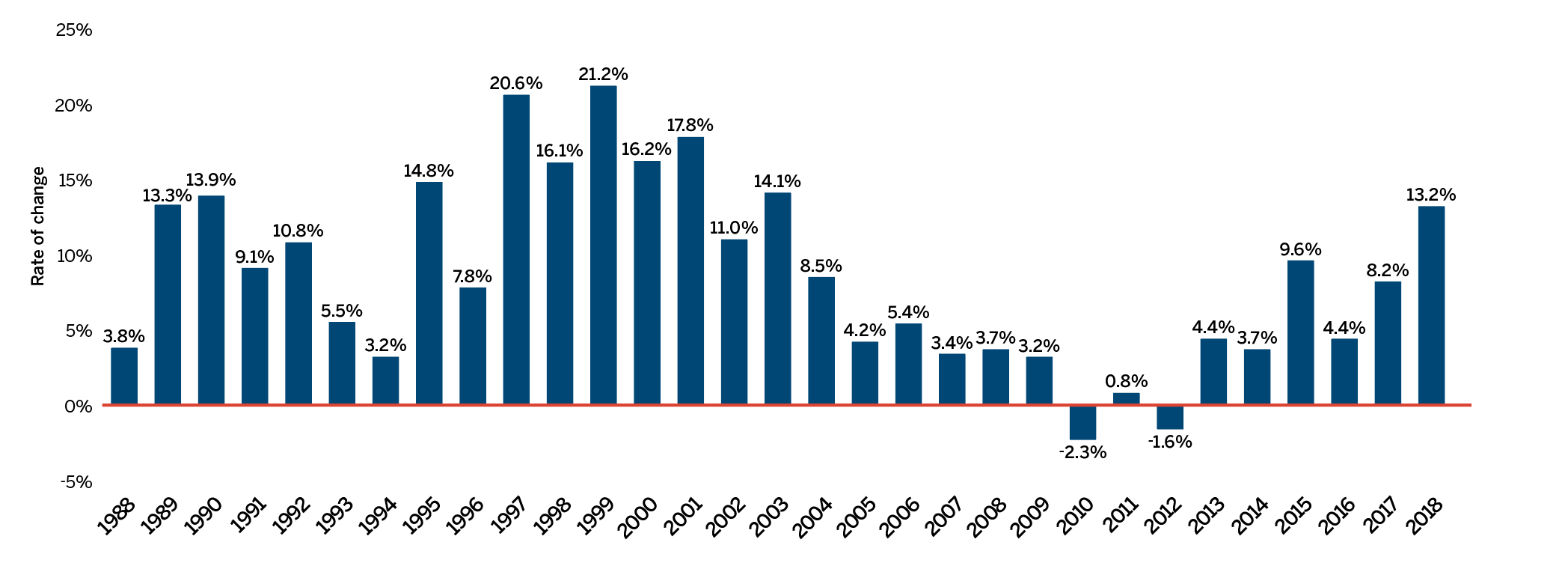
Data source: PMPRB
Figure description
This bar graph depicts the average annual rates of growth in utilization, as measured by the Patented Medicines Quantity Index (PMQI), from 1988 to 2018.
The rate of change in 1988 was 3.8%; 1989: 13.3%; 1990: 13.9%; 1991: 9.1%; 1992: 10.8%; 1993: 5.5%; 1994: 3.2%; 1995: 14.8%; 1996: 7.8%; 1997: 20.6%; 1998: 16.1%; 1999: 21.2%; 2000: 16.2%; 2001: 17.8%; 2002: 11.0%; 2003: 14.1%; 2004: 8.5%; 2005: 4.2%; 2006: 5.4%; 2007: 3.4%; 2008: 3.7%; 2009: 3.2%; 2010: -2.3%; 2011: 0.8%; 2012: -1.6%; 2013: 4.4%; 2014 : 3.7%; 2015: 9.6%; 2016: 4.4%; 2017: 8.2%; 2018: 13.2%.
Canadian Medicine Expenditures in the Global Context
IQVIAFootnote 21 regularly reports on medicine sales across a large number of countries. Based on sales data from this source, Figure 30 provides shares of global sales for Canada and other major national markets including the PMPRB7 countries.Footnote 22 The Canadian market accounted for 2.1% of the global market in 2018.
Data source: MIDAS® database, 2018, IQVIA (all rights reserved)
Figure description
This pie chart depicts the distribution of medicine sales among major global markets in 2018.
The US market accounted for 44.5% of the global market in 2017; Japan: 7.1%; Germany: 4.4%;
France: 3.4%; Italy: 3.1%; UK: 2.4%; Spain 2.2%; Canada: 2.1%; Switzerland: 0.6%; Sweden: 0.4%; the rest of the world: 29.8%.
Figure 31 provides Canada’s share of global sales for 2005 to 2018. The Canadian share has remained between 1.9% and 2.7% throughout this period. Although 2.1% is at the low end for Canada’s average share of global sales in recent years, the US share grew from 40.4% in 2014 to 44.5% in 2018, resulting in declining shares for all other major countries.
1.8% Medicine expenditures in Canada
In 2016, Canadians spent 1.8% of gross domestic product on medicines. This is the 2nd highest share in the PMPRB7, behind only the United States.
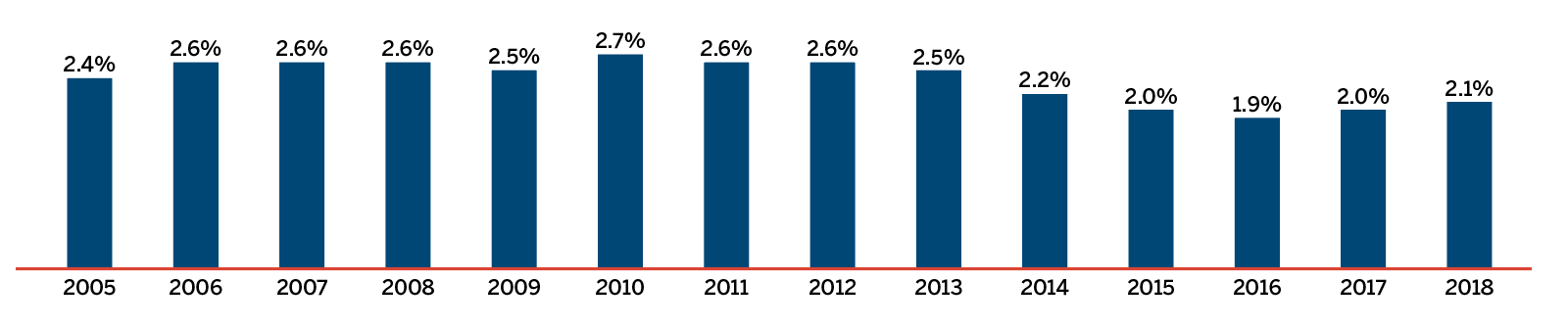
Data source: MIDAS® database, 2005–2018, IQVIA (all rights reserved)
Figure description
This bar graph depicts Canada’s share of global medicine sales from 2005 to 2018.
In 2005, Canada made up 2.4% of global sales; 2006: 2.6%; 2007: 2.6%; 2008: 2.6%; 2009: 2.5%; 2010: 2.7%; 2011: 2.6%; 2012: 2.6%; 2013: 2.5%; 2014: 2.2%; 2015: 2.0%; 2016: 1.9%; 2017: 2.0%; 2018: 2.1%.
Figure 32 gives the average annual rate of growth in total medicine sales for Canada and the PMPRB7, individually and collectively. From 2005 to 2018, medicine sales in Canada rose at an average annual rate of approximately 4.6%. This is less than the average rate of growth in medicine sales among the PMPRB7 countries over the same period, though it is clear from the figure that this growth rate is heavily skewed by the influence of US sales.
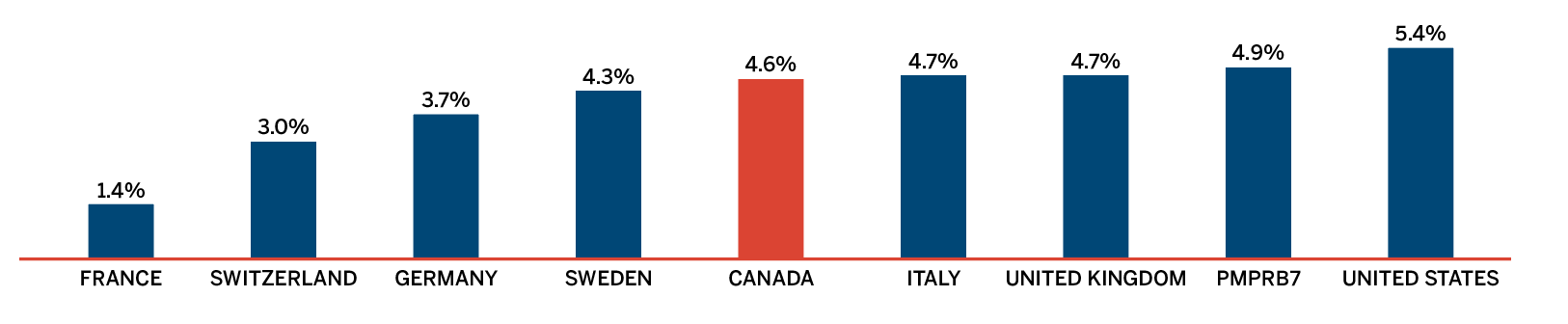
Data source: MIDAS® database, 2005–2018, IQVIA (all rights reserved)
Figure description
This bar graph depicts the average annual rate of growth in total medicine sales for Canada and the PMPRB7 comparator countries from 2005 to 2018.
In France, the average rate of growth was 1.4%; Switzerland: 3.0%; Germany: 3.7%; Sweden: 4.3%; Canada: 4.6%; Italy: 4.7%; UK: 4.7%; all of the PMPRB7: 4.9%; US: 5.4%.
Figure 33 compares rates of year-over-year growth in medicine sales for the entire pharmaceutical market in Canada and the PMPRB7 countries combined. In 2018, sales grew at a slightly faster rate in Canada than in the other PMPRB7 countries. This growth includes medicines that are no longer reporting to the PMPRB, such as Remicade.
Data source: MIDAS® database, 2005–2018, IQVIA (all rights reserved)
Figure description
This line graph depicts the average annual rate of change in medicine sales, using constant 2018 market exchange rates, for Canada, the average of the PMPRB7 countries, and the PMPRB7 median from 2006 to 2018.
| Year | Canada | PMPRB7 average | PMPRB7 median |
|---|---|---|---|
2006 |
8.0 |
7.3 |
4.3 |
2007 |
6.9 |
4.6 |
5.9 |
2008 |
8.6 |
2.5 |
5.3 |
2009 |
6.6 |
4.7 |
4.3 |
2010 |
2.0 |
2.7 |
2.6 |
2011 |
-1.0 |
3.0 |
1.3 |
2012 |
-1.8 |
0.3 |
-0.3 |
2013 |
1.6 |
2.3 |
3.5 |
2014 |
4.7 |
10.8 |
5.1 |
2015 |
6.6 |
10.7 |
7.5 |
2016 |
4.0 |
6.0 |
4.6 |
2017 |
6.9 |
1.9 |
3.7 |
2018 |
4.6 |
4.4 |
4.3 |
The proportion of national income allocated to the purchase of medicines provides another way to compare medicine costs across countries.Footnote 23 Figure 34 gives medicine expenditures as a share of gross domestic product (GDP) for Canada and the PMPRB7 countries based on data for 2016. Medicine expenditures absorbed between 1.1% and 2.1% of the GDP in the PMPRB7. The Canadian value of 1.8% was second only to the US.
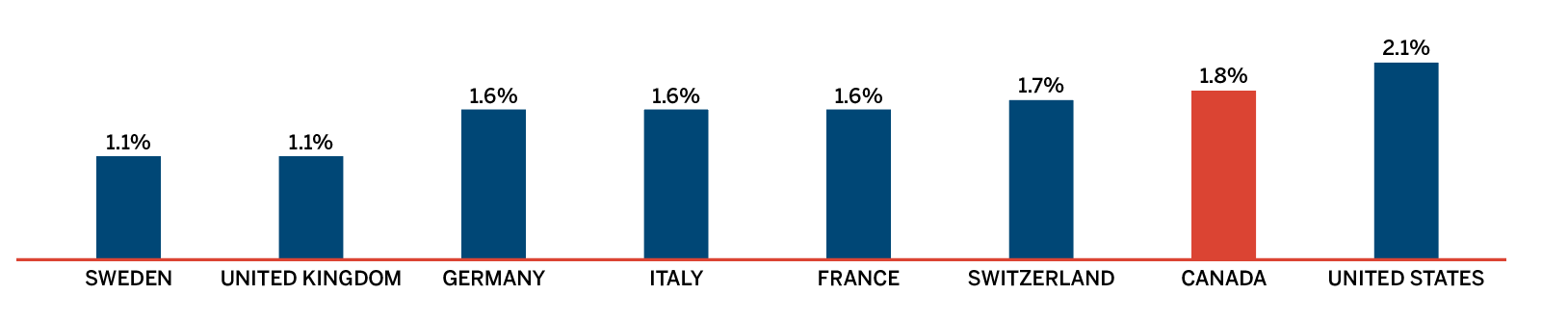
Data source: OECD
Figure description
This bar graph depicts the expenditures for medicines as a share of the gross domestic product (GDP) for Canada and the PMPRB7 comparator countries based on data for 2016.
In Sweden, medicine expenditure was 1.1% of the GDP; UK: 1.1%; Germany: 1.6%; Italy: 1.6%; France: 1.6%; Switzerland 1.7%; Canada: 1.8%; US: 2.1%.
Table 12 provides a historical perspective on the expenditures-to-GDP ratio.Footnote 24 In 2005, Canada’s ratio was fourth highest of the PMPRB7. Since that time, Canada’s ratio has risen, while the ratios of three other countries (France, Italy, and Sweden) have declined. In 2016, Canada had the third highest spending per capita on medicines compared to the PMPRB7, 26% higher than the median of these countries.
| Share: Medicine Expenditures/GDP 2016 (%) | Share: Medicine Expenditures/GDP 2005 (%) | Growth: GDP 2005–2016 (%)) | Medicine Spending Per Capita 2005 ($US PPP) | Medicine Spending Per Capita 2016 ($US PPP) | |
|---|---|---|---|---|---|
| Canada | 1.84 | 1.64 | 39.8 | 593 | 833 |
| France | 1.60 | 1.79 | 46.1 | 545 | 663 |
| Germany | 1.59 | 1.58 | 54.9 | 509 | 777 |
| Italy | 1.59 | 1.70 | 37.7 | 505 | 607 |
| Sweden | 1.07 | 1.15 | 57.1 | 396 | 524 |
| Switzerland | 1.68 | 1.09 | 84.8 | 427 | 1,080 |
| United Kingdom | 1.12 | 1.00 | 34.8 | NA | 476 |
| United States | 2.10 | 1.88 | 42.0 | 832 | 1,208 |
Data source: OECD
Table 13 gives the composition of patentees’ sales by therapeutic class for Canada and PMPRB7, individually by country and as an aggregate.Footnote 25 The results suggest a remarkable degree of similarity across countries.
| Therapeutic Class | Canada | PMPRB7 | France | Italy | Germany | Sweden | Switzerland | United Kingdom | United States |
|---|---|---|---|---|---|---|---|---|---|
| A: Alimentary tract and metabolism | 13.1 | 15.2 | 9.5 | 10.0 | 10.7 | 10.1 | 10.4 | 10.8 | 16.8 |
| B: Blood and blood-forming organs | 4.6 | 6.1 | 8.4 | 8.9 | 8.1 | 9.1 | 6.3 | 6.3 | 5.5 |
| C: Cardiovascular system | 7.4 | 4.7 | 7.1 | 8.5 | 6.9 | 4.2 | 8.7 | 6.0 | 3.9 |
| D: Dermatologicals | 2.9 | 2.4 | 2.1 | 1.7 | 2.6 | 2.2 | 2.9 | 2.1 | 2.5 |
| G: Genito-urinary system and sex hormones | 4.3 | 3.6 | 2.7 | 2.9 | 2.6 | 3.5 | 3.8 | 3.3 | 3.9 |
| H: Systemic hormonal preparations | 1.3 | 2.6 | 2.2 | 1.8 | 2.0 | 2.1 | 1.3 | 2.0 | 2.9 |
| J: General antiinfectives for systemic use | 10.0 | 11.5 | 12.4 | 19.0 | 9.1 | 12.9 | 10.7 | 12.2 | 11.1 |
| L: Antineoplastics and immunomodulating agents | 21.6 | 22.4 | 23.9 | 20.7 | 24.3 | 24.4 | 23.4 | 23.9 | 22.1 |
| M: Musculo-skeletal system | 3.0 | 3.3 | 2.7 | 3.0 | 4.0 | 3.7 | 5.0 | 2.6 | 3.3 |
| N: Nervous system | 16.2 | 15.6 | 13.9 | 12.1 | 15.2 | 15.8 | 16.5 | 14.9 | 16.0 |
| P: Antiparasitic products | 0.2 | 0.2 | 0.2 | 0.0 | 0.2 | 0.1 | 0.1 | 0.1 | 0.2 |
| R: Respiratory system | 7.2 | 7.0 | 5.9 | 5.2 | 6.6 | 6.7 | 5.8 | 7.7 | 7.2 |
| S: Sensory organs | 4.7 | 2.6 | 3.7 | 2.1 | 2.9 | 2.8 | 4.4 | 4.7 | 2.4 |
| V: Various | 3.4 | 2.8 | 5.3 | 4.1 | 4.8 | 2.3 | 0.6 | 3.3 | 2.3 |
| All therapeutic classes* | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
* Values may not add to 100 due to rounding.
Data source: MIDAS® database 2018, IQVIA (all rights reserved)
National Prescription Drug Utilization Information System: Supporting Health Care Decision Making in Canada
How medications are used—where, by whom, and for what—has an impact on the amount that we spend on medicines. The PMPRB contributes to Canada’s understanding of medicine usage through the National Prescription Drug Utilization Information System (NPDUIS) initiative, generating comprehensive, accurate information to help guide decision making and support continued sustainability of our pharmaceutical system.
Background
NPDUIS is a research initiative established by federal, provincial, and territorial Ministers of Health in September 2001. It is a partnership between the PMPRB and the Canadian Institute for Health Information (CIHI).
At the request of the Minister of Health pursuant to section 90 of the Patent Act, the PMPRB has the mandate to conduct analysis that provides decision makers with critical information and intelligence on price, utilization, and cost trends of patented and non-patented prescription medicines. This ensures that Canada’s healthcare system has more comprehensive and accurate information on how medicines are being used and on sources of cost pressures.
The specific research priorities and methodologies for NPDUIS are established with the guidance of the NPDUIS Advisory Committee and reflect the priorities of the participating jurisdictions. The Advisory Committee is composed of representatives from public drug plans in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, Yukon, the Non-Insured Health Benefits (NIHB) Program, and Health Canada. It also includes observers from CIHI, 54 PATENTED MEDICINE PRICES REVIEW BOARD the Canadian Agency for Drugs and Technologies in Health (CADTH), the Ministère de la Santé et des Services sociaux du Québec (MSSS), and the pan-Canadian Pharmaceutical Alliance (pCPA) Office.
NPDUIS operates independently of the regulatory activities of the PMPRB. NPDUIS reports do not contain information that is confidential or privileged under sections 87 and 88 of the Patent Act.
Highlights
Since the release of the last Annual Report, the PMPRB has published eight analytical reports, one Chartbook, and six posters under the NPDUIS banner.
Published Reports:
- Generics360: Generic Drugs in Canada, 2016 (February 2018)
- Meds Entry Watch, 2016 (June 2018)
- CompassRx, 4th Edition, 2016/17 (September 2018)
- Market Intelligence Report: Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Drugs for Retinal Conditions, 2017 (December 2018)
- Meds Entry Watch, 2017 (February 2019)
- Meds Pipeline Monitor, 2018 (May 2019) [formerly the New Drug Pipeline Monitor]
- Generics360 : Generic Drugs in Canada, 2018 (August 2019)
- CompassRx, 5th Edition, 2017/18 (September 2019)
Chartbook
- The market for prescription oral solid opioids, 2010 to 2017 (January 2019)
Poster Presentations:
- Generic Drug Pricing in Canada: Closing the Gap
- Uncovering the Forces Driving Costs in Canada’s Public Drug Plans, 2017/18
- The Oncology Drug Market: a High-Growth, High-Price Therapeutic Area
- Combination Asthma Inhalers in Canada: Locked on High Prices
- Alignment of Oncology Drug Coverage Across Canada
- Pressures Behind the Rising Costs in Canadian Private Drug Plans, 2018
In addition, the NPDUIS conducted a number of ad hoc studies at the request of the NPDUIS participating jurisdictions.
The PMPRB continued to support and strengthen its NPDUIS engagement activities by regularly consulting with the NPDUIS Advisory Committee, participating in conferences and stakeholder committees, and organizing information sessions with interested stakeholders to share the results of the analytical studies.
Research Agenda
In 2018, the PMPRB introduced a series of short, graphic-based analyses called Chartbooks. The first, released in January 2019, focused on the market for prescription oral solid opioids in Canada and the United States. Topics for upcoming analyses include an overview of Canadian and international markets for biosimilars, expensive drugs for rare diseases (EDRDs), and oncology medicines.
The NPDUIS research agenda for the upcoming fiscal year also includes plans to publish the following analytical studies:
- Meds Entry Watch, 2018
- Meds Pipeline Monitor, 2019
- Market Intelligence Reports on (1) Combination Inhalers for Asthma, 2018; and (2) New Oral Antidiabetic Drugs, 2018
- Alignment among Public Formularies in Canada, Part 2: Oncology Drugs Assessed Through the pan-Canadian Oncology Drug Review (pCODR) Process.
- Private Drug Plans in Canada Part 2: Major Factors Driving Drug Costs and Dispensing Fees in Private Plans
Additional research topics may be pursued based on consultation with the NPDUIS Advisory Committee.
Analysis of Research and Development Expenditures: R&D Investment Falling Short of Target
Innovation is vital to advancing health care. In part, the provisions of Canada’s Patent Act are intended to foster an investment climate favorable to pharmaceutical research and development (R&D) in Canada. However, the ratio of R&D expenditures to sales revenues for pharmaceutical patentees in Canada has been falling since the late 1990’s and has been under the agreed-upon target of 10% since 2003. In 2018, it was at 4.0% for all patentees and 4.3% for members of Innovative Medicines Canada.
Analysis of Research and Development Expenditures
The Act mandates the PMPRB to monitor and report on pharmaceutical R&D spending. This chapter provides key statistics on the current state of pharmaceutical R&D investment in Canada.
4.0% R&D-to-Sales Ratio
The R&D-to-sales ratio for all patentees was 4.0% in 2018. This represents a 66% decrease from a peak of 11.7% in 1995.
Data Sources
The statistical results in this report were entirely derived from data submitted to the PMPRB by patentees.
The Act requires each patentee to report its total gross revenues from sales of all medicines for human or veterinary use (including revenues from sales of non-patented medicines and from licensing agreements) and R&D expenditures in Canada related to medicines (both patented and non-patented for human or veterinary use). Patentees transmit this information to the PMPRB by means of its Form 3 (Revenues and Research and Development Expenditures Provided Pursuant to subsection 88(1) of the Patent Act).
The Patented Medicines Regulations (Regulations) require that each submitted Form 3 be accompanied by a certificate stating the information it contains is “true and correct”. The Board does not audit Form 3 submissions, but it does review submitted data for anomalies and inconsistencies, seeking corrections or clarifications from patentees where necessary. To confirm that PMPRB staff has correctly interpreted the data submitted, each patentee is given the opportunity to review and confirm the accuracy of its own R&D-to-sales ratio before that ratio is published.
Failure to File (Form 3)
It is a patentee’s responsibility to ensure a complete and accurate Form 3 is filed within the time frame set out in the Regulations. If a patentee fails to meet these filing requirements, the Board may issue an Order demanding compliance. No such Board Orders were issued for the 2018 reporting period.
Coverage
Note that companies without sales of patented medicines do not need to report their R&D expenditures to the PMPRB. This has two implications:
First, the statistical results reported here should not be taken to cover all pharmaceutical research conducted in Canada. For example, a company may sell only non-patented medicines but may still perform considerable research in Canada. Similarly, a company may conduct research and have no medicine sales at all.Footnote 26 The results presented below will not reflect the R&D expenditures of firms in either situation.
Second, as new patented medicines come onto the Canadian market and existing relevant patents expire, the number and identity of companies required to file R&D data may change from year to year. In 2018, 93 companies reported on their R&D activity. Of these, 32 were members of Innovative Medicines Canada.
Definition of Sales Revenues
For reporting purposes, sales revenues are defined as total gross revenues from sales in Canada of all medicines and from licensing agreements (e.g., royalties and fees accruing to the patentee related to sales in Canada by licensees).
Definition of R&D Expenditures
Pursuant to section 6 of the Regulations, patentees are required to report R&D expenditures that would have qualified for an investment tax credit in respect to scientific research and experimental development (SR&ED) under the provisions of the Income Tax Act that came into effect on December 1, 1987.Footnote 27 By this definition, R&D expenditures may include current expenditures, capital equipment costs and allowable depreciation expenses. Market research, sales promotions, quality control or routine testing of materials, devices or products and routine data collection are not eligible for an investment tax credit and, therefore, are not to be included in the R&D expenditures reported by patentees.
Total Sales Revenues and R&D Expenditures
Table 14 provides an overview of reported sales revenues and R&D expenditures over the period 1988 through 2018.
Patentees reported total 2018 sales revenues of $22.7 billion, an increase of 7.2% from 2017. Sales revenues reported by Innovative Medicines Canada members were $16.8 billion, accounting for 74% of the total. (Less than 1% of reported sales revenues were generated by licensing agreements.)
Patentees reported R&D expenditures of $892.6 million in 2018, an increase of 2.4% over 2017. Innovative Medicines Canada members reported R&D expenditures of $723.0 million in 2018, a decrease of 4.3% over the previous year. Innovative Medicines Canada members accounted for 81% of all reported R&D expenditures in 2018.
R&D-to-Sales Ratios
Table 14 and Figure 35 also provide ratios of R&D expenditures to sales revenues. It should be noted that, with the adoption of the 1987 amendments to the Act, Innovative Medicines Canada made a public commitment to increase its members’ annual R&D expenditures to 10% of sales revenues by 1996.Footnote 28 This level of R&D expenditure was reached by 1993, with the ratio exceeding 10% in some years.
The ratio of R&D expenditures to sales revenues among all patentees was 4.0% in 2018, a slight decrease from 4.1% in 2017. The overall R&D-to-sales ratio has been less than 10% for the past 18 consecutive years.
The corresponding R&D-to-sales ratio for members of Innovative Medicines Canada was 4.3% in 2018, a decrease from 4.6% in 2017.Footnote 29 The Innovative Medicines Canada ratio has been less than 10% for the past 16 consecutive years.
Table 20 in Appendix 4 provides details on the range of 2018 R&D-to-sales ratios. Of the 93 companies reporting in 2018, 82.8% had R&D-to-sales ratios below 10%.
| Year | All Patentees | Innovative Medicines Canada Patentees | R&D-To-sales Ratio: All Patentees (%) | R&D-To-sales Ratio: Innovative Medicines Canada Patentees (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number Of Companies Reporting | R&D Expenditures By All Patentees ($millions) | Change From Previous Year (%) | Sales Revenues ($millions) | Change From Previous Year (%) | R&D Expenditures By Innovative Medicines Canada Patentees ($millions) | Change From Previous Year (%) | Sales Revenues ($millions) | Change From Previous Year (%) | |||
| 2018 | 93 | 892.6 | 2.4 | 22,663.4 | 7.2 | 723.0 | -4.3 | 16,789.7 | 2.7 | 4.0 | 4.3 |
| 2017 | 85 | 871.4 | -5.1 | 21,147.2 | 1.4 | 755.8 | -1.8 | 16,349.8 | 4.8 | 4.1 | 4.6 |
| 2016 | 78 | 918.2 | 5.7 | 20,855.7 | 5.9 | 769.9 | 0.3 | 15,599.9 | 0.2 | 4.4 | 4.9 |
| 2015 | 77 | 869.1 | 9.7 | 19,693.3 | 6.7 | 767.4 | 7.8 | 15,565.1 | 4.7 | 4.4 | 4.9 |
| 2014 | 75 | 792.2 | -0.8 | 18,455.1 | 1.0 | 711.7 | 2.0 | 14,861.1 | 9.2 | 4.3 | 4.8 |
| 2013 | 81 | 798.3 | -14.7 | 18,268.1 | 1.4 | 697.5 | -15.4 | 13,614.8 | 3.4 | 4.4 | 5.1 |
| 2012 | 85 | 936.1 | -5.6 | 18,021.1 | 1.3 | 824.1 | -8.6 | 13,162.8 | -2.1 | 5.2 | 6.3 |
| 2011 | 79 | 991.7 | -15.8 | 17,798.8 | 4.7 | 901.2 | -9.9 | 13,446.1 | 10.7 | 5.6 | 6.7 |
| 2010 | 82 | 1,178.2 | -7.4 | 17,000.0 | -0.3 | 1,000.2 | -11.7 | 12,149.0 | -11.8 | 6.9 | 8.2 |
| 2009 | 81 | 1,272.0 | -2.9 | 17,051.9 | 4.5 | 1,132.9 | -3.4 | 13,780.0 | 4.6 | 7.5 | 8.2 |
| 2008 | 82 | 1,310.7 | -1.1 | 16,316.7 | 2.0 | 1,172.2 | -1.0 | 13,178.2 | -1.4 | 8.1 | 8.9 |
| 2007 | 82 | 1,325.0 | 9.5 | 15,991.0 | 7.3 | 1,184.4 | 24.8 | 13,359.8 | 20.0 | 8.3 | 8.9 |
| 2006 | 72 | 1,210.0 | -1.9 | 14,902.0 | 4.7 | 949.0 | -8.8 | 11,131.2 | -5.8 | 8.1 | 8.5 |
| 2005 | 80 | 1,234.3 | 5.5 | 14,231.3 | 0.5 | 1,040.1 | 3.9 | 11,821.4 | 0.0 | 8.7 | 8.8 |
| 2004 | 84 | 1,170.0 | -2.0 | 14,168.3 | 4.0 | 1,000.8 | 0.8 | 11,819.0 | 8.8 | 8.3 | 8.5 |
| 2003 | 83 | 1,194.3 | -0.4 | 13,631.1 | 12.8 | 992.9 | -3.6 | 10,865.7 | 5.2 | 8.8 | 9.1 |
| 2002 | 79 | 1,198.7 | 13.0 | 12,081.2 | 12.5 | 1,029.6 | 10.1 | 10,323.8 | 16.8 | 9.9 | 10.0 |
| 2001 | 74 | 1,060.1 | 12.6 | 10,732.1 | 15.3 | 935.2 | 14.7 | 8,835.4 | 14.3 | 9.9 | 10.6 |
| 2000 | 79 | 941.8 | 5.3 | 9,309.6 | 12.0 | 815.5 | 4.0 | 7,728.8 | 11.6 | 10.1 | 10.6 |
| 1999 | 78 | 894.6 | 12.0 | 8,315.5 | 19.2 | 784.3 | 9.9 | 6,923.4 | 22.8 | 10.8 | 11.3 |
| 1998 | 74 | 798.9 | 10.2 | 6,975.2 | 10.9 | 713.7 | 8.6 | 5,640.2 | 10.6 | 11.5 | 12.7 |
| 1997 | 75 | 725.1 | 9.0 | 6,288.4 | 7.4 | 657.4 | 10.3 | 5,098.2 | 4.9 | 11.5 | 12.9 |
| 1996 | 72 | 665.3 | 6.4 | 5,857.4 | 9.9 | 595.8 | 6.5 | 4,859.5 | 8.7 | 11.4 | 12.3 |
| 1995 | 71 | 625.5 | 11.5 | 5,330.2 | 7.5 | 559.5 | 9.8 | 4,468.8 | 1.4 | 11.7 | 12.5 |
| 1994 | 73 | 561.1 | 11.4 | 4,957.4 | 4.4 | 509.5 | 10.4 | 4,407.2 | 2.0 | 11.3 | 11.6 |
| 1993 | 70 | 503.5 | 22.1 | 4,747.6 | 14.0 | 461.4 | 24.0 | 4,321.4 | 14.4 | 10.6 | 10.7 |
| 1992 | 71 | 412.4 | 9.6 | 4,164.4 | 6.9 | 372.1 | 9.0 | 3,778.4 | 6.5 | 9.9 | 9.8 |
| 1991 | 65 | 376.4 | 23.2 | 3,894.8 | 18.1 | 341.4 | 24.7 | 3,546.9 | 19.5 | 9.7 | 9.6 |
| 1990 | 65 | 305.5 | 24.8 | 3,298.8 | 11.0 | 273.8 | 25.8 | 2,967.9 | 10.5 | 9.3 | 9.2 |
| 1989 | 66 | 244.8 | 47.4 | 2,973.0 | 9.4 | 217.6 | 34.7 | 2,685.5 | 7.3 | 8.2 | 8.1 |
| 1988 | 66 | 165.7 | — | 2,718.0 | — | 161.5 | — | 2,502.3 | — | 6.1 | 6.5 |
Data source: PMPRB
Data source: PMPRB
Figure description
This line graph depicts the annual the R&D-to-sales ratio for all patentees and members of Innovative Medicines Canada (IMC) from 1988 to 2018.
IMC Patentees: 1988, 6.5%; 1989, 8.1%; 1990, 9.2%; 1991, 9.8%; 1992, 9.8%; 1993, 10.7%; 1994, 11.6%; 1995, 12.5%; 1996, 12.3%; 1997, 12.9%; 1998, 12.7%; 1999, 11.3%; 2000, 10.6%; 2001, 10.6%; 2002, 10.0%; 2003, 9.1%; 2004, 8.5%; 2005, 8.8%; 2006, 8.5%; 2007, 8.9%; 2008, 8.9%; 2009, 8.2%; 2010, 8.2%; 2011, 6.7%; 2012, 6.3%; 2013, 5.7%; 2014, 4.8%; 2015, 4.9%; 2016, 4.9%; 2017, 4.6%; 2018, 4.3%.
All Patentees: 1988, 6.1%; 1989, 8.2%; 1990, 9.3%; 1991, 9.7%; 1992, 9.9%; 1993, 10.6%; 1994, 11.3%; 1995, 11.7%; 1996, 11.4%; 1997, 11.5%; 1998, 11.5%; 1999, 10.8%; 2000, 10.1%; 2001, 9.9%; 2002, 9.9%; 2003, 8.8%; 2004, 8.3%; 2005, 8.7%; 2006, 8.1%; 2007, 8.2%; 2008, 8.1%; 2009, 7.5%; 2010, 6.9%; 2011, 5.6%; 2012, 5.2%; 2013, 4.4%; 2014, 4.3%; 2015, 4.4%; 2016, 4.4%; 2017, 4.1%; 2018, 4.0%.
Current R&D Expenditures by Type of Research
Table 15 and Figure 36 (as well as Figure 38 in Appendix 4) provide information on the allocation of 2018 current R&D expendituresFootnote 30 among basic and applied research and other qualifying R&D.Footnote 31 Patentees reported spending $106.9 million on basic research in 2018, representing 12.3% of current R&D expenditures, a decrease of 2.0% over the previous year. Patentees reported spending $517.1 million on applied research, representing 59.2% of current R&D expenditures. Clinical trials accounted for 77.3% of applied research expenditures.
| Type Of Research | Expenditures: 2018 ($millions) | Share: 2018 (%) | Expenditures: 2017 ($millions) | Share: 2017 (%) | Annual Change In Expenditures (%) |
|---|---|---|---|---|---|
| Basic | 106.9 | 12.3 | 109.0 | 13.1 | -2.0 |
| Chemical | 69.5 | 8.0 | 61.4 | 7.4 | 13.2 |
| Biological | 37.4 | 4.3 | 47.6 | 5.7 | -21.4 |
| Applied | 517.1 | 59.2 | 501.9 | 60.3 | 3.0 |
| Manufacturing process | 71.0 | 8.1 | 72.9 | 8.8 | -2.6 |
| Pre-clinical Trial I | 19.8 | 2.3 | 31.6 | 3.8 | -37.3 |
| Pre-clinical Trial II | 26.8 | 3.1 | 34.6 | 4.2 | -29.8 |
| Clinical Trial Phase I | 50.5 | 5.8 | 41.2 | 4.9 | 22.6 |
| Clinical Trial Phase II | 83.1 | 9.5 | 58.7 | 7.0 | 41.6 |
| Clinical Trial Phase III | 265.9 | 30.4 | 262.9 | 31.6 | 1.1 |
| Other qualifying R&D | 250.2 | 28.6 | 222.2 | 26.7 | 12.6 |
| Total* | 874.1 | 100 | 833.1 | 100 | 4.9 |
* Values may not add to totals due to rounding.
Data source: PMPRB
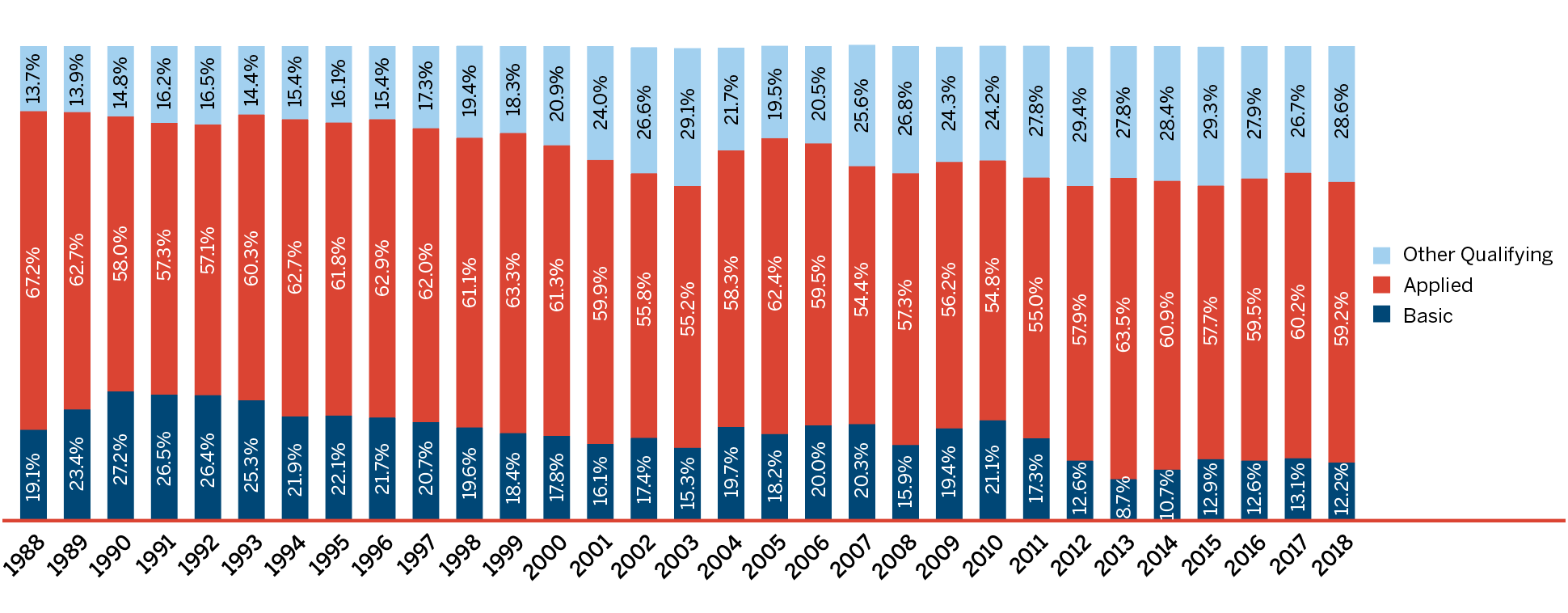
Data source: PMPRB
Figure description
This stacked bar graph depicts the share of current R&D expenditures from 1988 to 2018 by type of research: basic, applied, and other qualifying.
| Year | Basic research (%) | Applied research (%) | Other qualifying research (%) |
|---|---|---|---|
1988 |
19.1 |
67.2 |
13.7 |
1989 |
23.4 |
62.7 |
13.9 |
1990 |
27.2 |
58.0 |
14.8 |
1991 |
26.5 |
57.3 |
16.2 |
1992 |
26.4 |
57.1 |
16.5 |
1993 |
25.3 |
60.3 |
14.4 |
1994 |
21.9 |
62.7 |
15.4 |
1995 |
22.1 |
61.8 |
16.1 |
1996 |
21.7 |
62.9 |
15.4 |
1997 |
20.7 |
62.0 |
17.3 |
1998 |
19.6 |
61.1 |
19.4 |
1999 |
18.4 |
63.3 |
18.3 |
2000 |
17.8 |
61.3 |
20.9 |
2001 |
16.1 |
59.9 |
24.0 |
2002 |
17.4 |
55.8 |
26.6 |
2003 |
15.3 |
55.2 |
29.1 |
2004 |
19.7 |
58.3 |
21.7 |
2005 |
18.2 |
62.4 |
19.5 |
2006 |
20.0 |
59.5 |
20.5 |
2007 |
20.3 |
54.4 |
25.6 |
2008 |
15.9 |
57.3 |
26.8 |
2009 |
19.4 |
56.2 |
24.3 |
2010 |
21.1 |
54.8 |
24.2 |
2011 |
17.3 |
55.0 |
27.8 |
2012 |
12.6 |
57.9 |
29.4 |
2013 |
8.7 |
63.5 |
27.8 |
2014 |
10.7 |
60.9 |
28.4 |
2015 |
12.9 |
57.7 |
29.3 |
2016 |
12.6 |
59.5 |
27.9 |
2017 |
13.1 |
60.2 |
26.7 |
2018 |
12.2 |
59.2 |
28.6 |
Current R&D Expenditures by Performer
Patentees report expenditures on research they conduct themselves (intramural) and research performed by other establishments, such as universities, hospitals, and other manufacturers (extramural). Table 16 shows that 49.2% of 2018 current research expenditures were intramural. Research performed by other companies on behalf of patentees made up 23.7% of current expenditures, while research conducted in universities and hospitals accounted for 18.5%.
| R&D Performer | Expenditures: 2018 ($millions) | Share: 2018 (%) | Expenditures: 2017 ($millions) | Share: 2017 (%) | Annual change In Expenditures (%) |
|---|---|---|---|---|---|
| INTRAMURAL | |||||
| Patentees | 429.7 | 49.2 | 375.3 | 45.1 | 14.5 |
| EXTRAMURAL | |||||
| Universities and hospitals | 162.4 | 18.5 | 148.7 | 17.9 | 9.2 |
| Other companies | 207.0 | 23.7 | 222.6 | 26.7 | -7.0 |
| Others | 75.1 | 8.6 | 86.5 | 10.4 | -13.2 |
| Total* | 874.1 | 100.0 | 833.1 | 100.0 | 4.9 |
* Values may not add to totals due to rounding.
Source: PMPRB
The PMPRB7 Average R&D Ratio is 5 times Greater than in Canada
The R&D-to-sales ratio obtained by aggregating R&D spending and sales across all seven comparator countries was 23.5%, more than five times Canada’s.
Current R&D Expenditures by Region
Table 17 (as well as Table 22 and Table 23 in Appendix 4) show current R&D expenditures by region. As in previous years, current expenditures were heavily concentrated in Ontario and Quebec in 2018, with these provinces accounting for 80.1% of total expenditures. However, while current R&D expenditures increased at a modest year-over-year rate of 1.2% in Ontario and 1.0% in Quebec, they rose by 23.0% in Western Canada and 26.6% in the Atlantic Provinces.
| Region | Expenditures: 2018 ($millions) | Share: 2018 (%) | Expenditures: 2017 ($millions) | Share: 2017 (%) | Annual Change In Expenditures (%) |
|---|---|---|---|---|---|
| Atlantic provinces | 19.8 | 2.3 | 15.7 | 1.9 | 26.6 |
| Quebec | 285.8 | 32.7 | 283.1 | 34.0 | 1.0 |
| Ontario | 414.5 | 47.4 | 409.5 | 49.1 | 1.2 |
| Western provinces | 153.6 | 17.6 | 124.9 | 15.0 | 23.0 |
| Territories | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total* | 874.1 | 100.0 | 833.1 | 100.0 | 4.9 |
* Values may not add to totals due to rounding.
Data source: PMPRB
Total R&D Expenditures by Source of Funds
Table 18 provides information on the sources of funds used by patentees to finance their R&D activity. Internal company funds remained by far the single largest source of funding in 2018, accounting for 91.0% of total expenditures. Funds received from government amounted to 0.5% of total expenditures.
| Source Of Funds | Expenditures: 2018 ($millions) | Share: 2018 (%) | Expenditures: 2017 ($millions) | Share: 2017 (%) | Annual Change In Expenditures (%) |
|---|---|---|---|---|---|
| Company funds | 812.1 | 91.0 | 791.1 | 90.8 | 2.7 |
| Federal/provincial governments | 4.5 | 0.5 | 6.0 | 0.7 | -25.0 |
| Others | 75.9 | 8.5 | 74.3 | 8.5 | 2.2 |
| Total* | 892.6 | 100.0 | 871.4 | 100.0 | 2.4 |
* Values in this line may not add due to rounding
Data source: PMPRB
The Global Context
Figure 37 compares Canadian pharmaceutical R&D-to-sales ratios for 2000 and 2016 to those in the PMPRB7.Footnote 32 In 2000, Canada had an R&D-to-sales ratio of 10.1%, lower than all other PMPRB7 countries except for Italy, at 6.2%. Switzerland had the highest ratio at 102.5%.
In 2016, Canada’s R&D-to-sales ratio was the lowest among the comparator countries at 4.4%. Italy had a slightly higher ratio of 5.7%, while all other PMPRB7 countries remained well above Canada. The ratio obtained by aggregating R&D spending and sales across all PMPRB7 countries was 23.5%, more than five times Canada’s.
The R&D-to-sales ratios represented in Figure 37 may be compared to the average bilateral price ratios reported in Table 9 (see the section on Comparison of Canadian Prices to Foreign Prices). A number of comparator countries with patented medicine prices that are, on average, lower than prices in Canada, have achieved much higher R&D-to-sales ratios.
As noted in previous annual reports, there are a multitude of factors that drive the location of pharmaceutical R&D. These include where companies can find the best science base at reasonable cost and ready access to a quality clinical trials infrastructure. Although price levels and intellectual property protection are often cited as an important policy lever for attracting R&D, the data has not supported this link domestically or internationally.
Data source: PMPRB; European Federation of Pharmaceutical Industries and Associations (EFPIA): The Pharmaceutical Industry in Figures 2017; PhRMA 2017 profile
Figure description
This bar graph depicts R&D-to-sales ratios for Canada and the PMPRB7 comparator countries for 2000 and 2016.
2000: Canada, 10.1%; PMPRB7, 20.4%; France, 16.8%; Germany, 17.3%; Italy, 6.2%;
Sweden, 44.4%; Switzerland, 102.5%; UK, 35.1%; US, 18.4%.
2016: Canada, 4.4%; PMPRB7, 23.5%; France, 15.7%; Germany, 20.2%; Italy, 5.7%;
Sweden, 36.6%; Switzerland, 125.3%; UK, 27.3%; US, 24.1%.
Appendix 1: Glossary
These definitions are provided for general assistance only; they have no legal force and should be read in conjunction with the applicable legislation.
Active Ingredient or Medicinal Ingredient: Chemical or biological substance responsible for the claimed pharmacologic effect of a medicine.
ATC: Anatomical Therapeutic Chemical (ATC) classification system, developed and maintained by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology, divides medicines into different groups according to their site of action and therapeutic and chemical characteristics. This system is used by the PMPRB as a guide for selecting comparable medicines for purposes of price review under the Guidelines.
Drug Identification Number (DIN): A registration number (drug identification number) that the Health Products and Food Branch of Health Canada assigns to each prescription and non-prescription drug product marketed under the Food and Drugs Regulations. A DIN uniquely identifies the following product characteristics: manufacturer; product name; active ingredient(s); strength(s) of active ingredient(s); pharmaceutical dosage form; route of administration.
Drug Product: A particular presentation of a medicine characterized by its pharmaceutical dosage form and the strength of the active ingredient(s) (see “medicine” below).
Failure to File: The complete or partial failure of a patentee to comply with regulatory filing requirements pursuant to the Patent Act and the Patented Medicines Regulations.
Failure to Report: The complete failure of a patentee to have reported a patented medicine being sold in accordance with regulatory filing requirements pursuant to the Patent Act and the Patented Medicines Regulations.
License, Voluntary: A contractual agreement between a patent holder and a licensee under which the licensee is entitled to enjoy the benefit of the patent or to exercise any rights in relation to the patent for some consideration (e.g., royalties in the form of a share of the licensee's sales).
Medicine: A medicinal ingredient and/or a substance or a mixture of substances manufactured, sold or represented for use in the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms, in human beings or animals; or restoring, correcting or modifying organic functions in human beings or animals.
Notice of Compliance (NOC): Means a notice issued under section C.08.004 or C.08.004.01 of the Food and Drug Regulations. The issuance of an NOC indicates that a drug product meets the required Health Canada standards for use in humans or animals and that the product is approved for sale in Canada.
Patent: An instrument issued by the Commissioner of Patents in the form of letters patent for an invention.
Patented Medicine Price Index (PMPI): The PMPI was developed by the PMPRB as a measure of average year-over-year change in the transaction prices of patented medicines sold in Canada, based on the price and sales information reported by patentees.
Patentee: As defined by subsection 79(1) of the ?Patent Act, “the person for the time being entitled to the benefit of the patent for that invention and includes, where any other person is entitled to exercise any rights in relation to that patent other than under a license continued by subsection 11(1) of the Patent Act Amendment Act, 1992, that other person in respect of those rights;”
PMPRB7: France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States.
Research and Development (R&D): Basic or applied research for the purpose of creating new, or improving existing, materials, devices, products or processes (e.g., manufacturing processes).
Research and Development—Basic Research: R&D directed toward a specific practical application, comprising research intended to improve manufacturing processes, pre-clinical trials and clinical trials.
Research and Development—Other Qualifying: Includes eligible research and development expenditures that cannot be classified into any of the preceding categories of “type of research and development”. It includes regulatory submissions, bioavailability studies and Phase IV clinical trials.
Research and Development Expenditures: For the purposes of the Patented Medicines Regulations, in particular Sections 5 and 6, research and development includes activities for which expenditures would have qualified for the investment tax credit for scientific research and experimental development under the Income Tax Act as it read on December 1, 1987.
Research and Development Expenditures–Current: Consist of the following non-capital expenses that are directly related to research work: (a) wages and salaries, (b) direct material, (c) contractors and subcontractors, (d) other direct costs such as factory overhead, (e) payments to designated institutions, (f) payments to granting councils, and (g) payments to other organizations. These elements are described in greater detail in the Patentees' Guide to Reporting—Form 3, available from the PMPRB Website under Regulatory Filings.
Special Access Programme (SAP): A program operated by Health Canada to give practitioners access to medicines that are not approved or otherwise available in Canada.
Voluntary Compliance Undertaking (VCU): A written undertaking by a patentee to adjust its price to conform to the Board's Guidelines. A VCU represents a compromise between the PMPRB and the patentee as a result of negotiations between the parties geared towards a satisfactory resolution of an investigation initiated by Board Staff as per the Guidelines. A VCU takes into account the specific facts and underlying context of a particular case. As such, VCUs are not intended to have precedential value.
Appendix 2: Patented Medicines First Reported to the PMPRB in 2018
| Brand Name | Company | DIN | Status (Full Year 2018) | Level Of Therapeutic Improvement/category* | |
|---|---|---|---|---|---|
| 1 | Addyi – 100 mg/tablet | Sprout Pharmaceuticals, Inc. | 2473550 | Under Review | Under Review |
| 2 | Alecensaro –150 mg/capsule | Hoffmann-La Roche Limited, Canada | 2458136 | Within Guidelines | SN |
| 3 | Alunbrig – 30 mg/tablet | Takeda Canada Inc. | 2479206 | Under Review | Under Review |
| 4 | Alunbrig – 90 mg/tablet | Takeda Canada Inc. | 2479214 | Under Review | Under Review |
| 5 | Alunbrig – 180 mg/tablet | Takeda Canada Inc. | 2479222 | Under Review | Under Review |
| 6 | Alunbrig 90/180 – 270 mg pack | Takeda Canada Inc. | 2479230 | Under Review | Under Review |
| 7 | ARZERRA – 100 mg/mL | Novartis Pharmaceuticals Canada Inc. | 2381559 | Under Review | Under Review |
| 8 | ARZERRA – 1000 mg/mL | Novartis Pharmaceuticals Canada Inc. | 2381567 | Under Review | Under Review |
| 9 | Bavencio – 20 mg/mL | EMD Serono Canada Inc | 2469723 | Within Guidelines | SN |
| 10 | Belbuca – 75 mcg film | Purdue Pharma | 2465221 | Within Guidelines | SN |
| 11 | Belbuca – 150 mcg film | Purdue Pharma | 2465248 | Within Guidelines | SN |
| 12 | Belbuca – 300 mcg film | Purdue Pharma | 2465256 | Within Guidelines | SN |
| 13 | Belbuca – 450 mcg film | Purdue Pharma | 2465264 | Within Guidelines | SN |
| 14 | Beleodaq – 500 mg/vial | Servier Canada Inc. | -374 | Under Review | Under Review |
| 15 | Benlysta – 200 mg /mL | GlaxoSmithKline Inc. | 2470489 | Under Review | Under Review |
| 16 | Besponsa – 0.9 mg/vial | Pfizer Canada Inc. | 2473909 | Within Guidelines | MI-S |
| 17 | Biktarvy 25/50/200 – 275 mg/tablet | Gilead Sciences, Inc. | 2478579 | Under Review | Under Review |
| 18 | Brinavess – 20 mg/mL | Cipher Pharmaceuticals Inc. | 2462400 | Does Not Trigger Investigation | SN |
| 19 | Cabometyx – 20 mg/tablet | Ipsen Biopharmaceuticals Canada Inc. | 2480824 | Under Review | Under Review |
| 20 | Cabometyx – 40 mg/tablet | Ipsen Biopharmaceuticals Canada Inc. | 2480832 | Under Review | Under Review |
| 21 | Cabometyx – 60 mg/tablet | Ipsen Biopharmaceuticals Canada Inc. | 2480840 | Under Review | Under Review |
| 22 | Constella – 72 mcg/capsule | Allergan Inc | 2469510 | Within Guidelines | SN |
| 23 | Contrave 8/90 – 98 mg/tablet | Bausch Health, Canada Inc. | 2472945 | Within Guidelines | SN |
| 24 | Cresemba – 200 mg/vial | Avir Pharma Inc. | 2483998 | Under Review | Under Review |
| 25 | Cubicin RF – 500 mg/vial | Sunovion Pharmaceuticals Inc. | 2465493 | Does Not Trigger Investigation | SN |
| 26 | Darazalex – 100 mg/vial | Janssen Inc. | 2455951 | Under Review | Under Review |
| 27 | Darazalex – 400 mg/vial | Janssen Inc. | 2455978 | Under Review | Under Review |
| 28 | Dupixent – 150mg/mL | Sanofi-aventis Canada Inc. | 2470365 | VCU | SN |
| 29 | Erleada – 60 mg/tablet | Janssen Inc. | 2478374 | Under Review | Under Review |
| 30 | Eucrisa – 20 mg/gram | Pfizer Canada Inc. | 2476991 | Under Review | Under Review |
| 31 | Fasenra – 30 mg/dose | AstraZeneca Canada Inc. | 2473232 | Within Guidelines | SN |
| 32 | Folotyn – 20 mg/mL | Servier Canada Inc. | 2481820 | Under Review | Under Review |
| 33 | Foquest – 25 mg/capsule | Purdue Pharma | 2470292 | Within Guidelines | SN |
| 34 | Foquest – 35 mg/capsule | Purdue Pharma | 2470306 | Within Guidelines | SN |
| 35 | Foquest – 45 mg/capsule | Purdue Pharma | 2470314 | Within Guidelines | SN |
| 36 | Foquest –55 mg/capsule | Purdue Pharma | 2470322 | Within Guidelines | SN |
| 37 | Foquest – 70 mg/capsule | Purdue Pharma | 2470330 | Within Guidelines | SN |
| 38 | Foquest – 85 mg/capsule | Purdue Pharma | 2470349 | Within Guidelines | SN |
| 39 | Foquest – 100 mg/capsule | Purdue Pharma | 2470357 | Within Guidelines | SN |
| 40 | Galafold – 123 mg/capsule | Amicus Therapeutics | 2468042 | Subject to Investigation | MI-S |
| 41 | Herceptin SC – 600 mg/vial | Hoffmann-La Roche Limited, Canada | 2480697 | Under Review | Under Review |
| 42 | Imfinzi – 50 mg/mL | AstraZeneca Canada Inc. | 2468816 | Within Guidelines | SN |
| 43 | Juluca 50/25 – 75 mg/tablet | ViiV HealthCare ULC | 2475774 | Under Review | Under Review |
| 44 | Kadcyla – 160 mg/vial | Hoffmann-La Roche Limited, Canada | 2473224 | Under Review | Under Review |
| 45 | Kanuma – 2 mg/mL | Alexion Pharmaceuticals, Inc. | 2469596 | Within Guidelines | B |
| 46 | Kevzara – 150 mg/pen | Sanofi-aventis Canada Inc. | 2472961 | Under Review | Under Review |
| 47 | Kevzara – 200 mg/pen | Sanofi-aventis Canada Inc. | 2472988 | Under Review | Under Review |
| 48 | Kisqali – 200 mg/tablet | Novartis Pharmaceuticals Canada Inc. | 2473569 | Does Not Trigger Investigation | SN |
| 49 | Lartruvo 500 mg/vial | Eli Lilly Canada Inc. | 2469227 | Subject to Investigation | MI-P |
| 50 | Lenvima – 8 mg/day | Eisai Limited | 2468220 | Within Guidelines | SN |
| 51 | Lenvima – 18 mg/day | Eisai Limited | 2468239 | Within Guidelines | SN |
| 52 | Lynparza – 100 mg/tablet | AstraZeneca Canada Inc. | 2475200 | VCU | SN |
| 53 | Lynparza – 150 mg/tablet | AstraZeneca Canada Inc. | 2475219 | VCU | SN |
| 54 | Metoject Subcutaneous – 15 mg/syringe | Medexus Inc. | 2454858 | Under Review | Under Review |
| 55 | Monoprost – 50 mcg/mL | Labtician Théa | 2456230 | Does Not Trigger Investigation | SN |
| 56 | Nuwiq – 3000 IU/vial | Octapharma Canada Inc. | 2474050 | Under Review | Under Review |
| 57 | Nuwiq – 4000 IU/vial | Otsuka Canada Pharmaceutical Inc. | 2474069 | Under Review | Under Review |
| 58 | Ofirmev – 10 mg/mL | Mallinckrodt Canada Inc. | 2408066 | Under Review | Under Review |
| 59 | Olumiant – 2 mg/tablet | Eli Lilly Canada Inc. | 2480018 | Under Review | Under Review |
| 60 | Opdivo – 40 mg/vial | Bristol-Myers Squibb Canada Co. | 2446626 | Under Review | Under Review |
| 61 | Opdivo – 100 mg/vial | Bristol-Myers Squibb Canada Co. | 2446634 | Under Review | Under Review |
| 62 | Orilissa – 150 mg/tablet | Abbvie | 2481332 | Under Review | Under Review |
| 63 | Ozanex – 10 mg/gram | Cipher Pharmaceuticals Inc. | 2463504 | Within Guidelines | SN |
| 64 | Ozempic – 1 mg/dose | Novo Nordisk Canada Inc. | 2471469 | Within Guidelines | SN |
| 65 | Ozempic – 1 N.A./dose | Novo Nordisk Canada Inc. | 2471477 | Within Guidelines | SN |
| 66 | Pazeo – 7 mg/mL | Novartis Pharmaceuticals Canada Inc. | 2458551 | Within Guidelines | SN |
| 67 | Pifeltro – 100 mg/tablet | Merck Canada Inc. | 2481545 | Under Review | Under Review |
| 68 | Praluent – 75 mg/mL | Sanofi-aventis Canada Inc. | 2453754 | VCU | SN |
| 69 | Praluent – 150 mg/mL | Sanofi-aventis Canada Inc. | 2453762 | VCU | SN |
| 70 | Praluent – 75 mg/mL | Sanofi-aventis Canada Inc. | 2453819 | VCU | SN |
| 71 | Praluent – 150 mg/mL | Sanofi-aventis Canada Inc. | 2453835 | VCU | SN |
| 72 | Prevymis – 20 mg/mL | Merck Canada Inc. | 2469367 | Subject to Investigation | SN |
| 73 | Prevymis – 240 mg/tablet | Merck Canada Inc. | 2469375 | Subject to Investigation | SN |
| 74 | Prevymis – 480 mg/tablet | Merck Canada Inc. | 2469383 | Subject to Investigation | SN |
| 75 | Prevymis – 20 mg/mL | Merck Canada Inc. | 2469405 | Subject to Investigation | SN |
| 76 | Probuphine – 80 mg/implant | Knight Therapeutics Inc. | 2474921 | Under Review | Under Review |
| 77 | Rebinyn – 500 | Novo Nordisk Canada Inc. | 2470187 | Within Guidelines | SN |
| 78 | Rebinyn – 1000 | Novo Nordisk Canada Inc. | 2470268 | Within Guidelines | SN |
| 79 | Rebinyn – 2000 | Novo Nordisk Canada Inc. | 2470276 | Within Guidelines | SN |
| 80 | Restasis MultiDose – 0.5 mg/mL | Allergan Inc | 2476835 | Under Review | Under Review |
| 80 | Rhopressa – 0.2 mg/mL | Aerie Pharmaceuticals, Inc | Under Review | Under Review | |
| 80 | Rituxan SC – 120 mg/mL | Hoffmann-La Roche Limited, Canada | 2473976 | Within Guidelines | SN |
| 80 | Rixubis – 3000 unit/vial | Baxalta Canada Corporation | 2431963 | Subject to Investigation | SN |
| 80 | Segluromet 2.5/1000 – 1002.5 mg/tablet | Merck Canada Inc. | 2476223 | Under Review | Under Review |
| 80 | Segluromet 2.5/500 – 502.5 mg/tablet | Merck Canada Inc. | 2476215 | Under Review | Under Review |
| 80 | Segluromet 7.5/1000 – 1007.5 mg/tablet | Merck Canada Inc. | 2476258 | Under Review | Under Review |
| 80 | Segluromet 7.5/1000 – 507.5 mg/tablet | Merck Canada Inc. | 2476231 | Under Review | Under Review |
| 80 | Shingrix – 50 mcg/dose | GlaxoSmithKline Inc. | 2468425 | Subject to Investigation | SI |
| 80 | Siliq – 210 mg/syringe | Bausch Health, Canada Inc. | 2473623 | Under Review | Under Review |
| 80 | Soliqua – 3 mL/pen | Sanofi-aventis Canada Inc. | 2478293 | Under Review | Under Review |
| 80 | Steglatro – 5 mg/tablet | Merck Canada Inc. | 2475510 | Within Guidelines | SN |
| 80 | Steglatro – 15 mg/tablet | Merck Canada Inc. | 2475529 | Within Guidelines | SN |
| 80 | Steglujan 5/100 – 105 mg/tablet | Merck Canada Inc. | 2475928 | Under Review | Under Review |
| 80 | Steglujan 15/100 – 115 mg/tablet | Merck Canada Inc. | 2475901 | Under Review | Under Review |
| 80 | Strensiq – 40 mg/mL | Alexion Pharmaceuticals, Inc. | 2444631 | Does Not Trigger Investigation | SN |
| 80 | Symdeko 100/150/150 – 400 mg/tablet | Vertex Pharmaceuticals Canada Inc. | 2478080 | Under Review | Under Review |
| 80 | Symtuza 800/150/200/10 – 1160 mg/tablet | Janssen Inc. | 2473720 | VCU | SN |
| 80 | Takhzyro – 150 mg/mL | Shire Pharma Canada Inc. | 2480948 | Under Review | Under Review |
| 80 | Trelegy Ellipta 100/62.5/25 – 187 mcg/dose | GlaxoSmithKline Inc. | 2474522 | Within Guidelines | SN |
| 80 | Trumenba – 1 N.A./dose | Pfizer Canada Inc. | 2468751 | Within Guidelines | SN |
| 80 | Xarelto – 2.5 mg/tablet | Bayer Inc. | 2480808 | Under Review | Under Review |
| 80 | Xeljanz XR – 11 mg/tablet | Pfizer Canada Inc. | 2470608 | Does Not Trigger Investigation | SN |
| 80 | Zevtera – 500 mg/vial | Avir Pharma Inc. | 2446685 | Within Guidelines | SN |
| 80 | Zonovate – 250 | Novo Nordisk Canada Inc. | 2435187 | Within Guidelines | SN |
| 80 | Zonovate – 500 | Novo Nordisk Canada Inc. | 2435195 | Within Guidelines | SN |
| 80 | Zonovate – 1000 | Novo Nordisk Canada Inc. | 2435209 | Within Guidelines | SN |
| 80 | Zonovate – 2000 | Novo Nordisk Canada Inc. | 2435225 | Within Guidelines | SN |
| 80 | Zonovate – 3000 | Novo Nordisk Canada Inc. | 2435233 | Within Guidelines | SN |
* Compliance status as of the end of the January to December 2017 reporting period. Medicines shown as under review are as of March 31, 2019.
- SN Slight or No Improvement
- MI-S Moderate Improvement – Secondary
- MI-P Moderate Improvement – Primary
- SI Substantial Improvement
- B Breakthrough
Appendix 3: Pharmaceutical Trends - Sales
| Year | Patented medicine | 5-year compound annual growth rate | Sales of patented medicines as a share of all medicine sales (%)* |
Patented medicine sales per capita | Change (%) | Patented medicine sales per GDP (%) | |
|---|---|---|---|---|---|---|---|
| Sales ($billions) | Change (%) | ||||||
| 2018 | 16.7 | -0.6 | 4.9 | 59.0 | $446.30 | -1.7 | 0.751 |
| 2017 | 16.8 | 7.6 | 5.8 | 61.5 | $454.1 | 5.4 | 0.783 |
| 2016 | 15.6 | 3.3 | 4.9 | 60.8 | $430.9 | 2.2 | 0.770 |
| 2015 | 15.1 | 9.4 | 4.0 | 61.6 | $421.8 | 8.5 | 0.760 |
| 2014 | 13.8 | 3.1 | 2.7 | 59.9 | $388.7 | 1.8 | 0.696 |
| 2013 | 13.4 | 4.2 | 0.8 | 60.7 | $381.8 | 2.7 | 0.706 |
| 2012 | 12.9 | 0.1 | 0.6 | 59.2 | $371.8 | -1.2 | 0.708 |
| 2011 | 12.9 | 3.5 | 1.6 | 58.3 | $376.1 | 3.1 | 0.729 |
| 2010 | 12.4 | -4.3 | 1.5 | 55.8 | $364.7 | -5.7 | 0.746 |
| 2009 | 13.0 | 2.9 | 4.5 | 59.6 | $386.9 | 1.9 | 0.829 |
| 2008 | 12.6 | 4.6 | 4.7 | 61.7 | $379.5 | 2.9 | 0.762 |
| 2007 | 12.1 | 3.2 | 5.7 | 63.2 | $368.9 | 2.5 | 0.769 |
| 2006 | 11.7 | 7.4 | 7.1 | 67.8 | $360.0 | 6.3 | 0.784 |
| 2005 | 10.9 | 4.2 | 9.4 | 70.6 | $338.5 | 2.8 | 0.769 |
| 2004 | 10.5 | 7.8 | 13.6 | 72.2 | $329.2 | 7.2 | 0.789 |
| 2003 | 9.7 | 9.0 | 15.8 | 72.7 | $307.0 | 8.0 | 0.776 |
| 2002 | 8.9 | 17.5 | 19.9 | 67.4 | $284.3 | 16.0 | 0.748 |
| 2001 | 7.6 | 18.9 | 19.7 | 65.0 | $245.2 | 19.1 | 0.666 |
| 2000 | 6.3 | 16.7 | 20.4 | 63.0 | $205.9 | 15.9 | 0.571 |
| 1999 | 5.4 | 27.0 | 20.0 | 61.0 | $177.6 | 24.3 | 0.538 |
| 1998 | 4.3 | 18.9 | 15.7 | 55.1 | $142.9 | 15.4 | 0.459 |
| 1997 | 3.7 | 22.6 | 11.4 | 52.3 | $123.7 | 22.1 | 0.409 |
| 1996 | 3.0 | 12.8 | 8.1 | 45.0 | $101.4 | 14.2 | 0.350 |
| 1995 | 2.6 | 10.8 | 6.8 | 43.9 | $88.7 | 7.2 | 0.314 |
| 1994 | 2.4 | -2.1 | 9.0 | 40.7 | $82.8 | -1.4 | 0.304 |
| 1993 | 2.4 | 9.4 | — | 44.4 | $83.9 | 7.9 | 0.322 |
| 1992 | 2.2 | 14.0 | — | 43.8 | $77.7 | 8.8 | 0.307 |
| 1991 | 2.0 | 13.1 | — | 43.2 | $71.4 | 16.0 | 0.286 |
| 1990 | 1.7 | — | — | 43.2 | $61.6 | — | 0.245 |
* The denominator in this ratio comprises sales of patented and non-patented brand medicines and patented and non-patented generic medicines. Starting with the estimate for 2005, this value is derived from data contained in IQVIA’s MIDAS® database. In previous years, IQVIA data was used to calculate sales of generic medicines only, while sales of non-patented brand products were estimated from data submitted by patentees. This approach was abandoned because of anomalies related to year-to-year changes in the set of companies reporting to the PMPRB. Ratios reported for years before 2005 likely overstate the patented share, but by only a small amount. This small bias in no way invalidates the strong upward trend evinced by the results for the years 1990 through 2003. Ratios since 2009 have also been revised slightly as a result of data updates from IQVIA—none of these adjustments resulted in a change greater than 0.4%.
Source: PMPRB; MIDAS™ database, 2005−2017, IQVIA. All rights reserved.
Appendix 4: Research and Development
| Range: R&D-to-sales ratio |
Number of reporting companies: 2018 |
Sales revenues: 2018 ($millions) |
Share: 2018(%) | Number of reporting companies: 2017 |
Sales revenues: 2017 ($millions) |
Share: 2017(%) |
|---|---|---|---|---|---|---|
| 0% | 38 | 2,764.4 | 12.2 | 33 | 1,662.1 | 7.8 |
| ≤10% | 39 | 17,682.7 | 78.0 | 41 | 17,566.3 | 83.1 |
| > 10% | 16 | 2,216.3 | 9.8 | 11 | 1,918.8 | 9.1 |
| Total* | 93 | 22,663.4 | 100.0 | 85 | 21,147.2 | 100.0 |
* Values may not add to totals due to rounding.
Data source: PMPRB
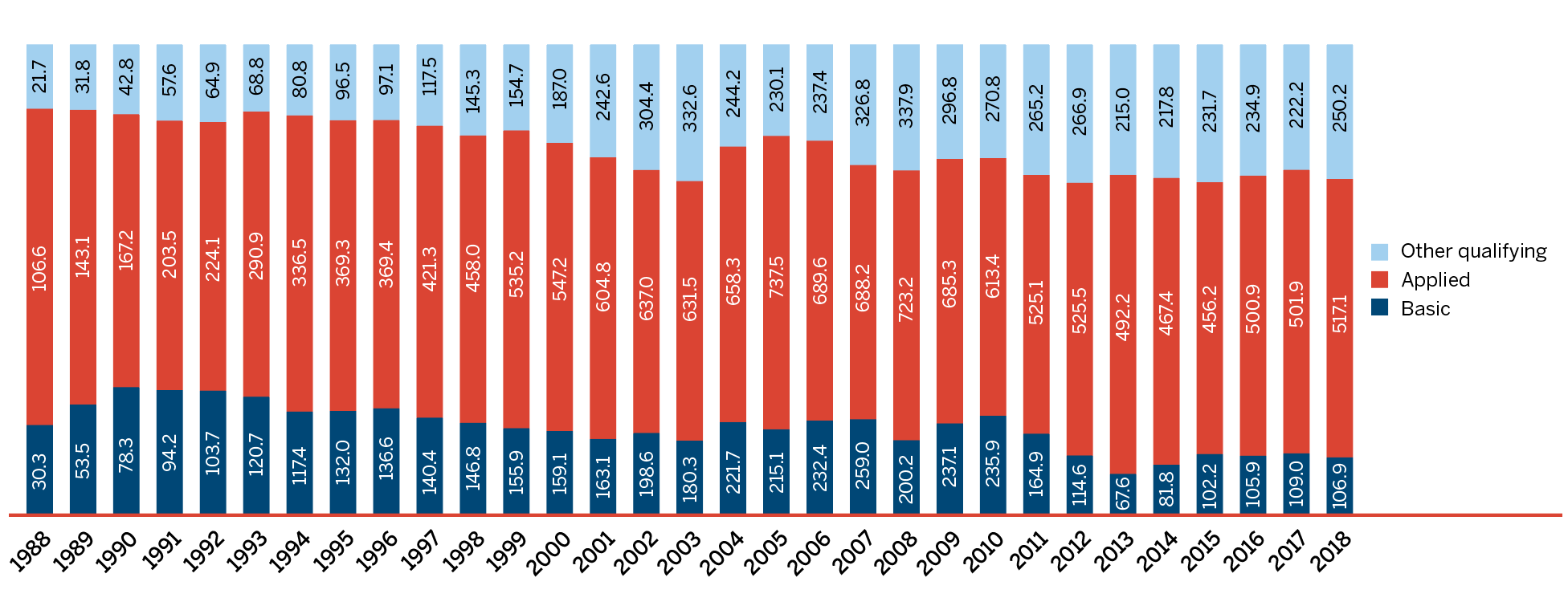
Source: PMPRB
Figure description
This stacked bar graph depicts current R&D expenditures in millions of dollars from 1988 to 2018 by type of research: basic, applied, and other qualifying.
| Year | Basic research (millions of dollars) | Applied research (millions of dollars) | Other qualifying research (millions of dollars) |
|---|---|---|---|
1988 |
30.3 |
106.6 |
21.7 |
1989 |
53.5 |
143.1 |
31.8 |
1990 |
78.3 |
167.2 |
42.8 |
1991 |
94.2 |
203.5 |
57.6 |
1992 |
103.7 |
224.1 |
64.9 |
1993 |
120.7 |
290.9 |
68.8 |
1994 |
117.4 |
336.5 |
80.8 |
1995 |
132.0 |
369.3 |
96.5 |
1996 |
136.6 |
369.4 |
97.1 |
1997 |
140.4 |
421.3 |
117.5 |
1998 |
146.8 |
458.0 |
145.3 |
1999 |
155.9 |
535.2 |
154.7 |
2000 |
159.1 |
547.2 |
187.0 |
2001 |
163.1 |
604.8 |
242.6 |
2002 |
198.6 |
637.0 |
304.4 |
2003 |
180.3 |
631.5 |
332.6 |
2004 |
221.7 |
658.3 |
244.2 |
2005 |
215.1 |
737.5 |
230.1 |
2006 |
232.4 |
689.6 |
237.4 |
2007 |
259.0 |
688.2 |
326.8 |
2008 |
200.2 |
723.2 |
337.9 |
2009 |
237.1 |
685.3 |
296.8 |
2010 |
235.9 |
613.4 |
270.8 |
2011 |
164.9 |
525.1 |
265.2 |
2012 |
114.6 |
525.5 |
266.9 |
2013 |
67.6 |
492.2 |
215.0 |
2014 |
81.8 |
467.4 |
217.8 |
2015 |
102.2 |
456.2 |
231.7 |
2016 |
105.9 |
500.9 |
234.9 |
2017 |
109.0 |
501.9 |
222.2 |
2018 |
106.9 |
517.1 |
250.2 |
| Company | R&D-to-sales ratio (%) 2017 | R&D-to-sales ratio (%) 2016 | MIP-to-Cdn Price Ratio (%) – 5 country limit | Canadian share of sales to PMPRB7 (2017) | Canadian share of sales to OECD (2017) |
|---|---|---|---|---|---|
| AbbVie Corporation2,3 | 2.7 | 2.2 | – | 2.6 | 2.2 |
| Acerus Pharmaceuticals | 0.0 | 0.0 | – | – | – |
| Actelion Pharmaceuticals Canada Inc.2 | 2.9 | 4.2 | 103 | 4.0 | 2.5 |
| Aerie Pharmaceuticals Inc.4 | 0.0 | – | – | – | – |
| Alexion Pharmaceuticals Inc.3 | 0.0 | 0.0 | 97 | – | – |
| ALK-Abelló A/S | 0.3 | 0.0 | 119 | 3.3 | 2.6 |
| Alkermes Inc.4 | 10,732.8 | – | – | – | – |
| Allergan Inc. | 1.4 | 1.1 | 79 | 1.0 | 1.0 |
| Altius Healthcare Inc. | 17.8 | 0.0 | – | – | – |
| Amgen Canada Inc.2,3 | 3.6 | 3.7 | 154 | 2.5 | 2.2 |
| Amicus Therapeutics UK Ltd4 | 0.0 | – | 242 | – | – |
| Aralez Pharmaceuticals Inc.4 | 0.0 | – | 101 | 8.1 | 7.5 |
| Aspen Pharmacare Canada Inc. | 0.0 | 0.0 | 96 | 3.7 | 1.1 |
| Astellas Pharma Canada Inc.2 | 1.1 | 1.8 | 148 | 3.2 | 2.0 |
| AstraZeneca Canada Inc.2,3 | 7.7 | 7.5 | 81 | 4.9 | 3.9 |
| Avir Pharma Inc.3,4 | 0.0 | – | 102 | – | – |
| Bausch Health Canada Inc.4 | 0.6 | – | 66 | 23.0 | 8.3 |
| Baxalta Canada Corp. | 0.0 | 0.0 | 299 | – | – |
| Baxter Corporation | 0.0 | 0.02 | 136 | 0.5 | 0.3 |
| Bayer Inc.2,3 | 5.4 | 6.8 | 102 | 13.9 | 6.2 |
| BGP Pharma ULC | 0.0 | 0.0 | 52 | 910.4 | 55.5 |
| Biogen Idec Canada Inc.3 | 16.5 | 11.9 | 109 | 1.7 | 1.4 |
| BioMarin Canada Inc.3 | 7.6 | 11.2 | 100 | – | – |
| BioSyent Pharma Inc. | 0.0 | 0.0 | – | – | – |
| Bioverativ Canada Inc.3 | 1.7 | 0.7 | 173 | – | – |
| Boehringer Ingelheim (Canada) Ltd.2 | 2.2 | 3.8 | 101 | 3.5 | 2.8 |
| Bracco Diagnostics Canada Inc. | 0.0 | 0.0 | – | – | – |
| Bristol-Myers Squibb Canada2 | 10.4 | 10.3 | 106 | 32.8 | 24.7 |
| BTG International Ltd.5 | 0.0 | 0.0 | – | – | – |
| Celgene Inc.3 | 1.9 | 1.1 | 104 | 0.5 | 0.4 |
| Cheplapharm Arzneimittel GmbH. | 0.0 | 0.0 | 72 | – | – |
| Cipher Pharmaceuticals Inc. | 1.3 | 2.1 | – | – | – |
| CSL Behring Canada Inc. | 0.2 | 0.5 | 526 | – | – |
| Duchesnay Inc. | 0.7 | 0.7 | – | 10.8 | 9.8 |
| Eisai Limited3 | 3.2 | 7.4 | 97 | 1.3 | 0.6 |
| Eli Lilly Canada Inc. (includes Provel Animal Health Division)2,3 | 7.3 | 9.5 | 91 | 1.4 | 1.3 |
| EMD Serono Canada Inc.2 | 0.0 | 0.0 | 78 | 3.5 | 3.4 |
| Ferring Pharmaceuticals Inc.3 | 0.0 | 0.0 | 87 | 3.8 | 2.6 |
| Galderma Canada Inc. | 0.0 | 0.0 | 50 | 5.4 | 4.5 |
| GE Healthcare Inc.4 | 0.0 | – | – | – | – |
| Gilead Sciences Canada, Inc.2 | 10.3 | 11.1 | 95 | 3.5 | 2.8 |
| GlaxoSmithKline Inc.2 | 5.4 | 5.9 | 63 | 66.9 | 14.4 |
| Grifols Canada Ltd. (Talecris Biotherapeutics Ltd.)3 | 0.0 | 0.0 | – | – | – |
| Hoffmann-La Roche Ltd. Canada2,3 | 6.8 | 5.9 | 96 | 15.1 | 7.1 |
| Horizon Pharma PLC. 2,3 | 0.0 | 0.0 | 110 | – | – |
| Intercept Pharmaceuticals Inc. | 13.4 | 27.4 | – | 0.2 | 0.2 |
| Ipsen Biopharmaceuticals Inc.2,3 | 0.2 | 0.3 | 105 | 0.7 | 0.5 |
| Janssen Inc.2,3 | 2.4 | 2.6 | 112 | 7.4 | 5.8 |
| Jazz Pharmaceuticals3 | 9.3 | 11.9 | – | 0.4 | 0.4 |
| Johnson & Johnson Medical Products | 0.9 | 0.4 | – | 2.4 | 1.4 |
| Knight Therapeutics Inc.2 | 18.2 | 24.8 | 58 | – | – |
| Labtician Théa.4 | 37.1 | – | 84 | – | – |
| Lantheus MI Canada Inc. | 0.0 | 0.0 | – | – | – |
| LEO Pharma Inc.2 | 0.1 | 0.04 | 66 | 10.2 | 6.9 |
| Lundbeck Canada Inc.2 | 0.5 | 1.1 | 75 | 5.1 | 3.9 |
| Lupin Pharma Canada Limited | 0.0 | 0.0 | 101 | 0.2 | 0.2 |
| Medexus Inc. | 0.0 | 0.0 | 76 | – | – |
| Merck Canada Inc.2,3 | 4.3 | 3.8 | 97 | 5.2 | 4.0 |
| Merus Labs | 0.0 | 0.0 | 98 | 24.1 | 13.8 |
| Merz Pharma Canada Ltd. | 0.0 | 1.9 | 97 | 1.9 | 1.3 |
| Novartis Pharmaceuticals Canada Inc.2,3 | 3.4 | 3.9 | 89 | 5.6 | 3.8 |
| Novo Nordisk Canada Inc.2,3 | 1.6 | 1.6 | 95 | 1.8 | 1.6 |
| Octapharma Canada Inc. | 0.5 | 20.6 | 500 | – | – |
| Otsuka Canada Pharmaceutical Inc. (OCPI)2 | 0.2 | 1.0 | 93 | 6.0 | 3.0 |
| Paladin Labs Inc.2 | 0.2 | 0.3 | 90 | – | – |
| Partner Therapeutics Inc.4 | 519.6 | – | – | – | – |
| Pediapharm Inc. | 0.0 | 0.0 | – | – | – |
| Pfizer Canada Inc.2,3 | 0.3 | 0.6 | 110 | 3.6 | 3.0 |
| Pharmascience Inc. | 12.4 | 9.2 | – | – | – |
| Pierre Fabre Dermo-CosmétiquIe Canada Inc. | 0.0 | 0.0 | 103 | – | – |
| Purdue Pharma2 | 2.1 | 3.6 | 140 | 13.6 | 12.0 |
| PTC Therapeutics International Ltd. | 637.0 | 149.6 | – | – | – |
| Sandoz Canada Inc.4 | 0.0 | – | – | 10.9 | 7.2 |
| Sanofi Canada Inc.2,3 | 1.5 | 1.7 | 84 | 28.5 | 11.6 |
| Sanofi Pasteur Ltd.2,3 | 51.7 | 72.1 | – | – | – |
| Seattle Genetics Inc. | 16.6 | 5.7 | 108 | – | – |
| Seqirus Canada Inc.3 | 870.2 | 20.8 | 165 | – | – |
| Servier Canada Inc.2,3 | 4.4 | 1.8 | 114 | 61.9 | 11.0 |
| Shire Canada Inc.3 | 0.0 | 0.0 | 113 | 3.8 | 3.2 |
| Shire Rare Disease Business Unit3 | 0.0 | 0.0 | 134 | – | – |
| Sprout Pharmaceuticals Inc.4 | 0.0 | – | – | – | – |
| Sunovion Pharmaceuticals Canada Inc.2 | 0.0 | 0.0 | 99 | 1.0 | 0.9 |
| Swedish Orphan Biovitrum AB (Sobi)3 | 0.0 | 0.0 | 90 | 0.03 | 0.01 |
| Taiho Oncology Inc.3,4 | 0.0 | – | 61 | 2.4 | 0.4 |
| Takeda Canada Inc.2,3 | 0.2 | 0.9 | 68 | 3.3 | 1.8 |
| Theratechnologies Inc. | 0.0 | 0.0 | – | – | – |
| Teva Canada Innovation3 | 0.1 | 0.1 | 100 | 4.8 | 3.8 |
| ThromboGenics N.V.4 | 359.7 | – | 102 | – | – |
| UCB Canada Inc.3 | 41.5 | 9.9 | 95 | 1.6 | 1.2 |
| Valneva Austria GmbH.3 | 0.0 | 0.0 | – | 92.4 | 43.1 |
| Vertex Pharma Canada Inc.3 | 0.0 | 5.5 | 106 | – | – |
| VIIV Healthcare ULC2 | 0.0 | 0.0 | 118 | 2.8 | 2.3 |
1 To avoid double counting sales revenues, revenues from royalties are included in calculating each company’s ratio but not included in calculating industry-wide ratios. Federal and provincial government grants are subtracted from the R&D expenditure in calculating individual R&D-to-sales ratios but are included in calculating industry-wide ratios. Differences between the list of companies filing data on prices and those filing R&D data are due to differences in the reporting practices of patentees and their affiliates or licensees. Note as well that some veterinary patentees (i.e., those without revenue from sales of products for human use) are required to file information on R&D expenditures but not price and sales information.
2 Member of Innovative Medicines Canada.
3 Member of BIOTECanada.
4 Not a patentee in 2017.
Data source: PMPRB
| Province | Expenditures: All patentees ($thousands) | Regional share (%) | Expenditures: Innovative Medicines Canada ($thousands) | Regional share (%) |
|---|---|---|---|---|
| Newfoundland | 2,039.74 | 0.234 | 1,507.73 | 0.213 |
| Prince Edward Island | 4,435.29 | 0.507 | 0.00 | 0.000 |
| Nova Scotia | 9,770.95 | 1.118 | 7,166.03 | 1.015 |
| New Brunswick | 3,574.56 | 0.409 | 2,590.46 | 0.367 |
| Quebec | 285,832.53 | 32.699 | 196,068.21 | 27.762 |
| Ontario | 414,466.33 | 47.415 | 369,334.50 | 52.296 |
| Manitoba | 12,438.87 | 1.423 | 10,272.08 | 1.454 |
| Saskatchewan | 3,022.70 | 0.346 | 1,136.07 | 0.161 |
| Alberta | 97,189.93 | 11.119 | 89,641.24 | 12.693 |
| British Columbia | 40,991.48 | 4.689 | 28,280.09 | 4.004 |
| Territories | 365.69 | 0.042 | 246.25 | 0.035 |
| Canada* | 874,128.06 | 100.0 | 706,242.66 | 100.0 |
* Provincial/territorial values may not add to totals for Canada due to rounding.
Data source: PMPRB
| Province | blank | Patentees | Other Companies | Universities | Hospitals | Others |
|---|---|---|---|---|---|---|
| Newfoundland | $000 | 1,019.39 | 356.59 | 131.4 | 143.35 | 388.97 |
| % | 49.9 | 17.5 | 6.4 | 7.0 | 19.1 | |
| Prince Edward Island | $000 | 443.48 | 3,991.81 | 0.00 | 0.00 | 0.00 |
| % | 10.0 | 90.0 | 0.0 | 0.0 | 0.0 | |
| Nova Scotia | $000 | 1,575.44 | 3,266.45 | 1,212.53 | 643.83 | 3,072.70 |
| % | 16.1 | 33.4 | 12.4 | 6.6 | 31.4 | |
| New Brunswick | $000 | 1,160.96 | 645.33 | 859.20 | 293.63 | 615.44 |
| % | 32.5 | 18.1 | 24.0 | 8.2 | 17.2 | |
| Quebec | $000 | 103,984.05 | 102,585.91 | 16,665.01 | 26,109.23 | 36,488.33 |
| % | 36.4 | 35.9 | 5.8 | 9.1 | 12.8 | |
| Ontario | $000 | 217,892.16 | 72,693.18 | 40,878.55 | 57,033.53 | 25,966.91 |
| % | 52.6 | 17.5 | 9.9 | 13.8 | 6.2 | |
| Manitoba | $000 | 9,838.65 | 968.01 | 239.18 | 440.33 | 952.70 |
| % | 79.1 | 7.8 | 1.9 | 3.5 | 7.7 | |
| Saskatchewan | $000 | 1,142.95 | 643.59 | 1,081.51 | 0.00 | 154.65 |
| % | 37.8 | 21.3 | 35.8 | 0.0 | 5.1 | |
| Alberta | $000 | 74,003.39 | 9,147.54 | 5,714.28 | 4,246.96 | 4,077.75 |
| % | 76.1 | 9.4 | 5.8 | 4.3 | 4.2 | |
| British Columbia | $000 | 18,476.99 | 12,695.15 | 4,756.68 | 1,710.52 | 3,352.13 |
| % | 45.1 | 30.9 | 11.6 | 4.1 | 8.2 | |
| Territories | $000 | 119.44 | 11.00 | 235.25 | 0.00 | 0.00 |
| % | 32.7 | 3.0 | 64.3 | 0.0 | 0.0 | |
| Canada | $000 | 429,656.89 | 207,004.56 | 71,773.65 | 90,623.38 | 75,069.58 |
| % | 49.1 | 23.7 | 8.2 | 10.4 | 8.6 |
Notes:
- The percentage under each R&D category gives the percentage of all money spent in that category in that province.
- Expenditures as a percentage of total means percentage of R&D expenditures in that province compared to total R&D in Canada.
- Rows and columns may not equal totals due to rounding.
- Current expenditures plus capital expenditures (equipment + depreciation) = total R&D expenditures.
Data source: PMPRB