ARCHIVED - Lessons Learned Review: Public Health Agency of Canada and Health Canada Response to the 2009 H1N1 Pandemic
2. Background and context
This section provides a description of the nature of the H1N1 2009 influenza virus, its evolution and the experience in Canada in 2009-10. It also provides a broad overview of the roles and responsibilities for public health in Canada and internationally. In terms of the domestic response to pandemics (and H1N1 in particular), this section outlines roles and responsibilities across jurisdictions and mandates: municipal and provincial/territorial governments, non-governmental organizations, federal/provincial/territorial collaboration and the federal government. It also delineates the key committees, networks, plans and strategies.
H1N1 – The pandemic
Pandemic influenza
People are exposed to different strains of influenza viruses many times during their lives, allowing them to build immunity over time. Three to four times each century, for unknown reasons, a radical change takes place in the influenza A virus, causing a new strain to emerge. When this happens, people’s immune systems may not provide protection against the new strain causing them to be more vulnerable to becoming ill from the virus. An influenza pandemic occurs with the appearance of a new influenza virus against which none of us has any immunity, resulting in several, simultaneous epidemics worldwide.Footnote 1
The H1N1 2009 virus
The influenza A H1N1 2009 virus was determined to be a unique combination of influenza A virus genes, never before identified in either animals or people. Initial reports referred to the virus as “swine flu” because the virus genes appeared to be a combination of genes most closely related to North American and Eurasian H1N1 swine influenza viruses. Further investigation determined that the H1N1 virus contained genetic elements from North American swine influenza, North American avian influenza, human influenza and a Eurasian swine influenza.
Because H1N1 2009 was a new strain of influenza, a large population of humans had little to no natural immunity to the virus. While similar in some ways to seasonal influenza, H1N1 2009 led to patterns of death and illness not normally seen in seasonal influenza infections. Figure 2.1 highlights the differences between the H1N1 influenza and seasonal influenza.
Although the illness caused generally mild symptoms for most people, it proved to be severe in a small minority of cases. The World Health Organization indicated that, globally, some groups of people appeared to be at higher risk of more complicated or severe illness including:
- pregnant women
- infants and children under age five (especially those younger than two years old)
- people of any age with certain chronic health conditions (including asthma or chronic lung disease, liver disease, heart disease, diabetes, severe obesity, blood disorders, kidney disease or some neurological conditions)
- people with severely compromised immune systems (for example, people taking cancer drugs or people with HIV/AIDS).Footnote 2
Figure 2.1 Comparison between seasonal influenza and H1N1Footnote 3
| Seasonal influenza |
Influenza A pandemic H1N1 2009 |
|
|---|---|---|
| Origins of the virus |
Influenza A or B strains similar to previous years with minor variation. Many people have some immune protection from previous exposure. |
A new virus that:
Exposure to seasonal influenza does not protect most people from infection. |
| Epidemiological patterns |
In countries with a temperate climate, seasonal epidemics typically taper off in the spring and end before the summer. |
Epidemiological patterns differed from seasonal epidemics of influenza. A widespread, high level of infection with the new virus occurred during the summer in the northern hemisphere in multiple countries, and was followed by even higher levels during the fall and winter months. |
| Symptoms |
Seasonal influenza symptoms:
Nausea, vomiting and diarrhea may occur and is more common in children. |
Same as seasonal influenza. Nausea, vomiting and diarrhea may occur. |
| Pattern of illness and death |
Majority (more than 90 percent) of deaths occur in frail elderly people, who often suffer one or more chronic medical conditions. Most cases of pneumonia are caused by secondary bacterial infections, which usually respond well to antibiotics. |
People age 65 or older are the least likely to be infected with the virus, but those who do get sick are also at high risk of developing serious complications, just as they are from seasonal influenza. Unlike seasonal influenza, younger age groups, including those who were otherwise healthy, were most affected in all categories:
A frequent cause of death was viral pneumonia, caused directly by the virus and difficult to treat. While many of those who died had underlying medical conditions associated with a higher risk, many others who died were previously in good health. |
Evolution of H1N1 2009
Determined to be the cause of severe respiratory illness outbreaks in Mexico in March 2009, the H1N1 2009 virus spread to the United States and Canada within weeks (see Figure 2.2). On April 25, 2009, the Director General of the World Health Organization declared the 2009 H1N1 outbreak a “public health emergency of international concern” in accordance with the International Health Regulations. The World Health Organization warned of the “pandemic potential of the new swine flu virus which can be transmitted from human to human” and recommended that “all countries intensify surveillance for unusual outbreaks of influenza-like illness and severe pneumonia.”Footnote 4
Over the course of the next six weeks, the virus spread rapidly worldwide. When the World Health Organization raised the level of pandemic influenza alert to phase 6 on June 11, 2009, signalling the first pandemic of the 21st century, 74 countries and territories had reported laboratory-confirmed cases.Footnote 5 By July 18, 2010, the World Health Organization reported that worldwide more than 214 countries and overseas territories or communities had reported laboratory-confirmed cases of H1N1, including over 18,000 deaths.Footnote 6
Figure 2.2 Timeline: The H1N1 pandemic in Canada
MARCH
18 Detection of severe respiratory infections in Mexico
APRIL
20 Public Health Agency notifies provincial/
territorial health authorities of two confirmed cases of new H1N1 influenza in California
22 Activation of the Health Portfolio Emergency Operations Centre to level 2 (partial activation)
23 National Microbiology Laboratory confirms specimens from Mexico are positive for H1N1 influenza
23 Activation of the Health Portfolio Emergency Operations Centre to level 3 (partial activation)
26 Public Health Agency reports first case of H1N1 influenza in Canada
26 Activation of the Health Portfolio Emergency Operations Centre to level 4 (full 24/7 activation)
29 World Health Organization raises level of pandemic influenza alert to phase 5
29 Public Health Agency purchases 5.7 million doses of antivirals to supplement National Antiviral Stockpile
MAY
1 Chief Public Health Officer announces change in terminology from “swine flu” to H1N1 influenza A
1 Public Health Agency recommends antiviral treatment of moderately ill people at high risk for influenza
3 Public Health Agency launches pandemic planning page on website
6 International vaccine regulatory and public health information-sharing teleconference is initiated
JUNE
11 World Health Organization declares a global pandemic:
phase 6
JULY
2 Minister of Health and Chief Public Health Officer participate in international meeting on global response to H1N1 flu virus in Cancun, Mexico
28 Provinces and territories report surveillance data through FluWatch
AUGUST
6 Minister of Health announces Canada’s order of 50.4 million doses of H1N1 vaccine
29 First wave of H1N1 pandemic officially ends in Canada
30 Second wave of H1N1 pandemic officially begins in Canada
SEPTEMBER
16 Chief Public Health Officer issues guidance on H1N1 2009 vaccine sequencing
OCTOBER
19-25 Manufacturer begins shipment of H1N1 2009 vaccine to provinces, territories and federal agencies for pre-positioning
21 Health Canada authorizes the sale of adjuvanted H1N1 2009 vaccine
NOVEMBER
12 Health Canada authorizes the sale of unadjuvanted H1N1 2009 vaccine
DECEMBER
4 All FluWatch influenza indicators continue to decline
JANUARY
1 Public Health Agency and Health Canada begin to de-escalate H1N1 pandemic response
6 Minister of Health announces five million doses of the H1N1 2009 vaccine to be sent to Mexico by the manufacturer
27 Public Health Agency announces end of the second wave of the H1N1 pandemic
FEBRUARY
10 Health Portfolio Emergency Operations Centre demobilizes H1N1 response
H1N1 – The Canadian experience
The first Canadian cases of H1N1 were confirmed by the Public Health Agency of Canada on April 26, 2009.Footnote 7 Canada experienced two distinct waves of H1N1 (see Figure 2.2). The first wave occurred in the spring between April 12, 2009 and August 29, 2009. During this period, influenza activity reached its highest level during the first three weeks of June. The first wave was followed by a second wave in the fall between August 30, 2009, and January 27, 2010. Influenza activity during the second wave reached its highest level in early November 2009.Footnote 8
At the end of the second wave, Canada reported over 40,000 laboratory-confirmed cases of H1N1.Footnote 9 Footnote 10 This figure is a significant underestimation of the actual number of Canadian cases for several reasons. First, a large proportion of those affected did not seek medical attention because they had mild symptoms and so remained undetected. Second, the type of influenza was not determined for a large number of laboratory samples tested during the pandemic time period. Finally, beginning in early June 2009, given that treatment approaches for influenza symptoms were regularized and there were limited laboratory resources for testing, only hospitalized cases were tested in a number of provinces and territories. As a result, the number of reported laboratory-confirmed H1N1 cases cannot be compared between the first and second waves.
However, hospitalization rates, intensive care unit admissions and deaths can be compared. As seen in Figure 2.3, most of these hospitalizations, intensive care unit admissions and deaths occurred during the second wave of increased influenza activity. The second wave was substantially larger than the first and resulted in four to five times more hospitalizations and deaths compared with the first wave. All provinces and territories showed higher levels of transmission during the second wave, except Manitoba and Nunavut, which reported higher hospitalization rates in the first wave.Footnote 11
Figure 2.3 Cumulative numbers of hospitalized cases, intensive care unit admissions and deaths among pandemic H1N1 confirmed cases, Canada, April 12, 2009, to April 24, 2010Footnote 12
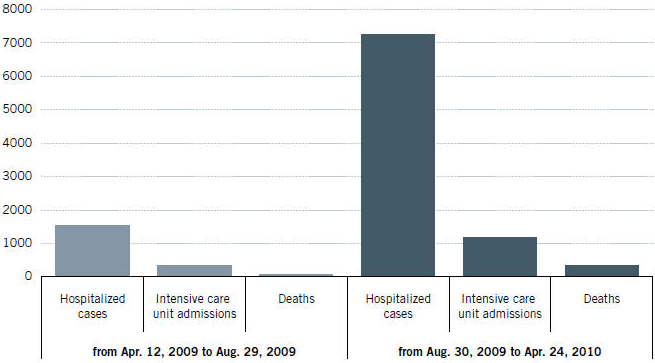
Figure 2.3 - Text Equivalent
Canadian population was exposed to two successive waves of pandemic influenza A/H1N1, which peaked respectively in June and November 2009. At the national level, the number of people affected by the pandemic was significantly larger in the second wave than it was in first wave. According to epidemiological data reported by provinces and territories, the number of hospitalizations, admissions to the intensive care units and deaths was 4 to 5 times increased in the second wave compared to the first wave. Aboriginal population, pregnant women and persons with underlying medical conditions were more likely to need hospitalization or special treatment in an intensive care unit. The same groups were also associated with an increase in mortality rate by H1N1 influenza virus compared to the general population.
Increased rate of hospitalization, intensive care unit admission and mortality were found to be highest among Aboriginal peoples, pregnant women and individuals with at least one underlying medical condition, although the risk for Aboriginal people and pregnant women decreased considerably in the second wave.
The virus is still having an impact. Some patients who spent time in the intensive care unit with the virus report lingering effects.
Roles and responsibilities for public health
Public health events such as the H1N1 pandemic involve international health authorities, as well as municipal, provincial/territorial health authorities, non-governmental organizations, the Public Health Agency of Canada, Health Canada and federal emergency response partners.
The H1N1 pandemic occurred against a backdrop of pandemic response planning at all levels of government including years of developing, refining and exercising response plans at the international, federal, provincial/territorial, regional and community levels in anticipation of an avian influenza A (H5N1) outbreak. Despite differences in planning scenarios and the actual H1N1 pandemic, many of the response plans and systems established through pandemic planning, and the roles and responsibilities defined in these plans, came into play in the response to H1N1.
International
In responding to a public health event, Canada works with its international partners through organizations such as the World Health Organization and mechanisms such as the Global Health Security Initiative. Canada also works closely with its North American partners, the United States and Mexico.
World Health Organization
The World Health Organization provides leadership on global health matters and has the lead in the international public health response to pandemic influenza.
The responsibilities of the World Health Organization in a disease outbreak include:
- coordinating the international response
- declaring the level of global pandemic alert (phases 1 to 6)
- selecting the pandemic vaccine strain and making recommendations for when to begin production of a pandemic vaccine instead of a seasonal influenza vaccine
- coordinating rapid containment operations
- providing early assessments of pandemic severity on health.Footnote 13
As a result of its experience with the Severe Acute Respiratory Syndrome (SARS) outbreak, the World Health Organization recognized the need to strengthen disease detection and reporting capabilities. It introduced the International Health Regulations (2005), an international agreement that came into force in June 2007 and to which Canada is a party. The Regulations provide a framework for detecting, reporting and managing international outbreaks, and for strengthening international public health security.Footnote 14 Each member state is required to designate or establish a national International Health Regulations focal point, responsible within its respective jurisdiction for the implementation of health measures under these Regulations.
Also in 2005, the World Health Organization began working with its international partners to plan for the possibility of an avian influenza outbreak. Through the Pandemic Influenza Preparedness and Response: A WHO Guidance Document, it sought to improve international coordination, transparency and management of risk in responding to such threats.Footnote 15
The World Health Organization’s Special Advisory Group of Experts was established in 1999 to provide technical guidance and support on immunization and vaccines. During the H1N1 pandemic, the Special Advisory Group of Experts activated an Ad Hoc Policy Advisory Working Group on influenza A (H1N1) vaccines. The Special Advisory Group of Experts reviewed the feasibility of influenza A (H1N1) vaccines and provided recommendations to the Director General of the World Health Organization on international guidelines and procedures on use and distribution of H1N1 vaccines.Footnote 16
The World Health Organization also maintains an electronic network, the Global Outbreak Alert and Response Network, to manage and regularly communicate critical epidemiological and operational information about outbreaks to its member countries, key international health professionals, laboratories and other members of the Network.
Global Health Security Initiative
Canada is a member of the Global Health Security Initiative, an international partnership among jurisdictions such as France, Germany, Italy, Japan, Mexico, the United Kingdom and the United States, intended to strengthen preparedness and response globally to public health threats. During the H1N1 pandemic, participation in this partnership helped Canada acquire critical information relevant to vaccine development and authorization, target groups, vaccination strategies, the use of antiviral drugs, and risk communications strategies and approaches.
In 2001, the Global Health Security Action Group of senior officials was established by health ministers of these countries to develop and implement concrete actions to improve global health security. It would also serve as a network of rapid communication/reaction in a crisis. The Global Health Security Action Group has established a number of technical working groups, among them three groups that had an important role in the global H1N1 pandemic response: the Global Health Security Laboratory Network, the Pandemic Influenza Working Group, and the Risk Management and Communications Working Group.
Health Canada acts as the international secretariat for the Global Health Security Initiative, ensuring coordination among member countries and convening senior officials to address collaborative efforts to respond to global risks to health.
North American collaboration
Canada works closely with its North American neighbours, the United States and Mexico. Among the priorities of this relationship is the protection of people from disease.
Guided by the North American Plan for Avian and Pandemic Influenza, Canada, Mexico and the United States work together to prepare for and manage an outbreak of avian influenza or an influenza pandemic in North America. Recognizing that the social and economic health of the three countries is closely intertwined, this Plan outlines a collaborative and coordinated North American approach to controlling the spread of avian influenza or a novel strain of human influenza.
The North American Plan for Avian and Pandemic Influenza describes joint activities to be carried out through six lines of action: health promotion and risk communications, coordination, epidemiological surveillance and laboratory practices, health care provision, strategic stockpile, and research and development, with the aim to:
- detect, contain and control an avian influenza outbreak and prevent transmission to humans
- prevent or slow the entry of a novel strain of human influenza to North America
- minimize illness and deaths
- sustain infrastructure and mitigate the impact to the economy and the functioning of society.Footnote 17
Canadian public health system
Public health is a shared responsibility in Canada. All levels of the public health system (municipal, provincial/territorial and federal) collect information to detect and monitor emerging disease threats and to detect changes in disease trends. Information collected locally is shared with the province or territory and with the Public Health Agency of Canada as appropriate, and if identified in existing information-sharing agreements.
Municipal and provincial/territorial roles
In general, a domestic infectious disease outbreak or emergency involving the health of the population is managed first at the local level by front-line public health responders and/or emergency management systems working with local health care providers. The role of local public health authorities includes monitoring and detection of health events, conducting outbreak investigations to identify the source (including laboratory testing, isolation and treatment), and following up with contacts of those affected.
Municipalities have a significant role to play in planning for and responding to a pandemic. Municipal governments are generally responsible for the first response to an emergency (e.g. police, ambulance and firefighter services). As highlighted by the Federation of Canadian Municipalities on its website,Footnote 18 business continuity and response services provided by other orders of government are dependent on core municipal infrastructure, including police, firefighters, water and wastewater treatment, public transit and municipal public health services.
Provincial or territorial health authorities may become engaged in a disease outbreak or an emergency if local public health authorities request assistance or resources. If an outbreak spreads beyond local borders, has serious human health implications or exceeds local capacity, the province or territory will assume leadership in coordinating the management of the response.Footnote 19 Provinces and territories have their own public health legislation, emergency management plans, standards and guidelines for responding to outbreaks, and laboratory services. The provinces and territories deliver health care services to the population as well as deliver vaccines through local health authorities.
Non-governmental organizations
Public health practice relies heavily on collaboration among government and non-governmental organizations, such as professional associations. These groups may be health-focused or may have primary interests in other related areas.Footnote 20
Non-governmental organizations play essential roles in responding to a pandemic and actively contribute in a manner consistent with their mandate. For example, various organizations may be involved in the development and dissemination of guidance documents, share information and provide advice through participation on various committees, work to ensure business continuity planning is in place, and deliver services in the community.
Federal/provincial/territorial collaboration
Conference of Deputy Ministers of Health
The federal/provincial/territorial Deputy Ministers of Health report to the Ministers of Health. During the H1N1 response, in addition to their regular face-to-face conferences, bilateral and multilateral calls were held weekly, and sometimes daily, to make decisions on key policy and other issues related to the response.
Pan-Canadian Public Health Network
The Pan-Canadian Public Health Network was established by Canada’s federal, provincial and territorial Health Ministers in 2005 (see Figure 2.4). Led by a 17-member Pan-Canadian Public Health Network Council, with representatives from each province and territory and the federal government, the Network enables different levels of government and experts to work together to improve public health in Canada. The Pan-Canadian Public Health Network takes a collaborative approach to public health that is critical at all times, but is especially important for coordination and collaboration during public health emergencies. The Public Health Agency of Canada acts as the Secretariat for the Pan-Canadian Public Health Network Council and its groups and committees.
The mandate of the Network is multifaceted, ranging from facilitating the sharing of information among all jurisdictions in Canada, to working with and providing policy and technical advice to federal/provincial/territorial Deputy Ministers of Health on public health matters, to supporting the public health challenges jurisdictions may face during emergencies and crises. The Network’s link with government decision makers and other key players in the public health arena supports strong and integrated public health policy development and implementation.
The Council of Chief Medical Officers of Health is also integrated into the Network’s structure: it reports through the Public Health Network Council to federal/provincial/territorial Deputy Ministers of Health. From the unique perspective of their roles and responsibilities, the Chief Medical Officers of Health contribute technical, strategic and policy advice.
Figure 2.4 Pan-Canadian Public Health Network composition as of March 31, 2010Footnote 21
Figure 2.4 - Text Equivalent
The Network is led by the Public Health Network Council, composed of federal, provincial and territorial representatives, which in turn is responsible to the Conference of Federal, Provincial, Territorial Deputy Ministers of Health. In addition, the Council of Chief Medical Officers of Health reports through the Public Health Network Council to the Conference of Deputy Ministers of Health.
The Network itself is composed of six Expert Groups, four Liaison Committees plus several time-limited task groups.
There are Expert Groups in the areas of:
- Communicable Disease Control,
- Emergency Preparedness and Response,
- Canadian Public Health Laboratories,
- Surveillance and Information,
- Chronic Disease and Injury Prevention and Control, and
- Population Health Promotion.
Expert Groups are supported, as needed, by Issue Groups.
Liaison Committees have been established on: Health and the Environment, Substance Use and Abuse, Tobacco Control and HIV/AIDS.
There are three time-limited task groups focused on: Public Health Human Resources, Antivirals for Prophylaxis, and Roles and Responsibilities in Pandemic Preparedness and Response.
Canadian Pandemic Influenza Plan for the Health Sector
In 2004, through the Pan-Canadian Public Health Network, the federal and provincial/territorial governments established the Canadian Pandemic Influenza Plan for the Health Sector. The Plan is the product of extensive dialogue and collaboration with representatives from all provinces and territories; Chief Medical Officers of Health; epidemiologists; virologists; communicable disease specialists; clinical, public health and laboratory specialists; and a wide group of stakeholders including non-governmental organizations, local governments, emergency planners and bioethicists.
Updated in 2006, the Plan is intended to provide a broad frame for Canada’s collaborative response to pandemic influenza, and guides the roles and responsibilities of the Public Health Agency of Canada, Health Canada, the provinces and territories. The goal of the Canadian Pandemic Influenza Plan for the Health Sector is to help minimize serious illness, death and societal disruption during and after a pandemic by assisting and facilitating a coordinated planning and response effort.
Federal government role
The federal government coordinates the nationwide pandemic influenza response, including surveillance, international liaison and coordination of the vaccine.Footnote 22
While the Public Health Agency of Canada and Health Canada play leading roles, other federal departments and agencies play specific roles in the response to H1N1, including the following:
- Central agencies, such as the Privy Council Office, coordinate responses to issues facing the Government and the country. As Head of the Public Service of Canada, the Clerk of the Privy Council serves as the principal link between the Prime Minister and the public service.
- Public Safety Canada is responsible for exercising leadership relating to emergency management in Canada by coordinating, among government institutions and in cooperation with the provinces and other entities, emergency management activities.
- The Canadian Food Inspection Agency has the lead role in responding to animal health emergencies. It works with the provinces and territories, the swine industry and private sector veterinarians to enhance monitoring of swine herds for signs of illness and to maintain enhanced biosecurity measures on farms across the country.
- The Department of Foreign Affairs and International Trade coordinates Canada’s international response.
- The Public Health Agency of Canada and Health Canada also work with the Royal Canadian Mounted Police, the Canada Border Services Agency and Citizenship and Immigration Canada to manage screening of travellers and events at the international border and points of entry.
Federal Emergency Response Plan
The Federal Emergency Response Plan outlines the processes and mechanisms to facilitate an integrated Government of Canada response to an emergency and to eliminate the need for departments to coordinate a wider Government of Canada response. The aim of the Federal Emergency Response Plan is to harmonize emergency response efforts by the federal and provincial/territorial governments, non-governmental organizations, and the private sector. In the Plan, Public Safety is identified as the federal coordinating department with responsibility for engaging relevant federal departments.
Assistant Deputy Ministers Emergency Management Committee
The Assistant Deputy Ministers Emergency Management Committee coordinates the federal response to an emergency and serves as a forum for the coordinated exchange of information and advice at the senior level before, during and after an emergency. During the H1N1 pandemic, the Committee put in place a subcommittee on H1N1 and, consistent with the Federal Emergency Response Plan, provided direction to the Government Operations Centre (managed by Public Safety Canada), as well as coordinated and recommended response options for senior decision makers.
Federal Healthcare Partnership – Pandemic Planning Working Group
The ad hoc Federal Healthcare Partnership – Pandemic Planning Working Group was created on May 11, 2009, to provide coordination between partners and federal organizations currently providing health care to federal populations. Federal partners include: Citizenship and Immigration Canada, Correctional Service Canada, the Department of National Defence, Health Canada, the Public Health Agency of Canada, the Royal Canadian Mounted Police and Veterans Affairs Canada. The Federal Healthcare Partnership Working Group was designed to answer questions posed by partners and to provide strategic guidance on issues related to H1N1, vaccination and access to the National Emergency Stockpile System and the National Antiviral Stockpile.
Health Portfolio
Under the Department of Health Act, the Minister of Health has a broad mandate to protect the people of Canada against risks to health and the spread of diseases. The Minister’s duties, functions and powers in relation to health include the investigation of and research into public health matters, including the monitoring of diseases. Under the Act, both Health Canada and the Public Health Agency of Canada exercise various duties, functions and powers on behalf of the Minister.
The Minister of Health is supported by the Health Portfolio, which comprises Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission, the Patented Medicine Prices Review Board and Assisted Human Reproduction Canada.
Within the Health Portfolio, the response to H1N1 was managed primarily by the Public Health Agency of Canada and Health Canada with research support from the Canadian Institutes of Health Research.
Health Portfolio Executive Group
The Health Portfolio Executive Group is the decision-making body that only convenes during an emergency or public health event, such as H1N1. The core Health Portfolio Executive Group consists of senior decision-makers from across the Health Portfolio. As needed, this group is expanded to include Directors General from the Health Portfolio to address specific event issues.
Health Portfolio Emergency Operations Centre
The Health Portfolio Emergency Operations Centre is the central command and coordination platform for emergency response for the Public Health Agency of Canada and Health Canada. It operates under an Incident Management System during emergencies. The Incident Management System is intended to be a flexible, scalable structure that can be expanded or contracted to meet the needs of specific events or emergencies. During the H1N1 pandemic, the activation level ranged from level 2 (partial activation) to level 4 (full 24/7 activation). It is based on the core functions of management, planning, logistics, finance/administration and operations, with the operations group typically customized to the event. The Incident Management System is designed to provide day-to-day operational leadership while the Health Portfolio Executive Group provides strategic leadership.
Health Portfolio Regional Offices
Regional offices within the Public Health Agency of Canada and Health Canada lead Health Portfolio emergency response activities in the regions. Activities include regional emergency preparedness committees, regional emergency coordination centres and bilateral arrangements among regional offices and various other government departments to ensure a strong interdepartmental and intergovernmental presence during public health events, such as H1N1.
Each province and territory has a Regional Federal Council at which the Regional Director for each department sits. Federal Councils play an interdepartmental communication and coordination role in each region and have done so in pandemic preparedness and response.
Health Portfolio Emergency Response Plan
The Health Portfolio Emergency Response Plan is structured as an “all hazards” plan for emergency response, including responding to a public health event like H1N1. It defines the scope, framework, roles and responsibilities within which the Public Health Agency of Canada and Health Canada operate to ensure an appropriate response to a range of emergencies that could affect the health and well-being of Canadians.Footnote 23
Avian and Pandemic Influenza Preparedness Program
The need for a coordinated and comprehensive plan specifically to address both avian and pandemic influenza was identified by the federal government. The 2006 Avian and Pandemic Influenza Preparedness framework comprises a suite of avian and pandemic preparedness initiatives being undertaken by the Public Health Agency of Canada, Health Canada, the Canadian Institutes of Health Research and the Canadian Food Inspection Agency. The suite of initiatives encompasses:
- vaccines and antivirals
- prevention and early warning
- emergency preparedness
- critical science and regulation
- surge capacity
- risk communications
- federal/provincial/territorial and international collaboration.
Health Canada’s Role
Health Canada is responsible for the regulatory regime governing the safety of products including: food, drugs, medical devices, natural health products, consumer products, chemicals, radiation-emitting devices, cosmetics and pesticides. To carry out its legislative mandate, the department is involved in:
- scientific research, evaluation, standard setting, regulation development, policy development, data collection, surveillance, testing for safety and efficacy, and education and outreach
- health information analysis and evaluation, to enhance Canada’s ability to prevent and respond to health crises
- social and natural scientific research and development of new laboratory methods and techniques to support policy research and analysis to enable decision making
- health surveillance, monitoring and exposure assessment to identify emerging issues and monitor existing ones
- measurement of Canada’s regulatory effectiveness and post-market monitoring of health products and pesticides
- strengthening capacity through internal and external research and research-related activities to accurately define health risks, trends and emerging issues
- supporting effective design and delivery of health programs and services.
During a public health event like the H1N1 pandemic, Health Canada engages and coordinates efforts among domestic and international health partners in the following areas:
- developing a regulatory framework for the review, approval and release for sale of pandemic vaccines and other health products used to prevent or treat the H1N1 influenza virus
- ensuring new influenza vaccines meet the highest standards of safety, quality and efficacy
- undertaking surveillance and risk management of post-market safety issues related to use of health products used to treat or prevent H1N1 influenza, including: antivirals, masks, hand sanitizers and disinfectants
- protecting public health on conveyances (e.g. aircraft, ships) and related infrastructure (e.g. airports, seaports) to reduce the spread of the pandemic into, across and out of Canada.
In recognition of the unique status and needs of on-reserve First Nations people in Canada, Health Canada collaborates with on-reserve First Nation communities to address health barriers and disease threats, and to attain health levels comparable to other Canadians living in similar locations. There is a high degree of variability in the way routine health services are provided in First Nation communities in Canada, based on factors such as location, level of integration with provincial health services and degree to which the community manages its own services.
In preparing for and responding to the threat of an influenza pandemic, such as H1N1, in on-reserve First Nation communities, Health Canada is responsible for the following:
- ensuring that health services are available and accessible to on-reserve First Nation communities
- representing and raising awareness of the pandemic planning needs of on-reserve First Nation communities among national and regional government and non-government stakeholders
- working closely with communities to advise on and support the development, testing and periodic revision of their influenza pandemic plans, which should be incorporated into existing emergency response plans in the community
- providing on-reserve First Nation communities with the resources to plan for an influenza pandemic, which include educational materials and training opportunities
- participating, in collaboration with the provinces, in the distribution, administration, and reporting (of adverse reactions) of vaccines using existing arrangements for on-reserve First Nation communities
- participating, in collaboration with the provinces, in the management, distribution, administration and reporting (of adverse reactions) of antiviral drugs using existing arrangements in on-reserve First Nation communities
- maintaining a personal protective equipment stockpile for health care workers and support staff assisting in the delivery of health care services
- providing information and guidance, based on guidelines developed by the Public Health Agency of Canada and/or the provinces, to health care workers providing services in on-reserve First Nation communities.
Public Health Agency of Canada’s role
The Public Health Agency of Canada is the lead federal agency responsible for addressing pandemic influenza preparedness and response. On behalf of the Health Portfolio, it is mandated to manage public health emergencies and the regional coordination of federal health emergency activities.
The Public Health Agency of Canada Act came into force in December 2006. The creation of the Public Health Agency of Canada was the culmination of numerous calls over the years for federal leadership in coordinating federal/provincial/territorial approaches to public health issues. Reports on how the 2003 Severe Acute Respiratory Syndrome (SARS) outbreak was handled, including Learning from SARS: Renewal of Public Health in Canada (Naylor Report), and the 2003 Standing Senate Committee on Social Affairs, Science and Technology report titled Reforming Health Protection and Promotion in Canada: Time to Act,Footnote 24 highlighted inadequacies in the public health response and the need to improve and strengthen coordination in the area of public health.
As noted in the Canadian Pandemic Influenza Plan for the Health Sector:
The Public Health Agency of Canada was created in response to growing concerns about the capacity of Canada’s public health system to anticipate and respond quickly and effectively to public health threats. The Agency will provide a clear focal point for federal leadership and accountability in managing public health emergencies and improved collaboration within and among jurisdictions.Footnote 25
In preparation for emergencies, and for responding to and recovering from the public health implications of pandemic influenza, the Public Health Agency of Canada plays a coordinating function in emergency planning, training and activities that engage all levels of government, as well as the voluntary and private sectors. It works with international partners, provinces and territories and other federal partners to monitor the international and domestic influenza situation and to mobilize a pan-Canadian response to disease outbreaks of national or international concern.
The Public Health Agency of Canada leads and/or undertakes the following activities:
- conducts scientific research to better identify, understand and track the influenza virus
- obtains surveillance (or tracking) information from its federal, provincial, territorial and local partners, and non-governmental organizations. Each week, this information is analyzed and interpreted, then shared with the broader public health community through FluWatch, the Public Health Agency of Canada’s online information-sharing vehicle. Influenza surveillance helps to determine: when, where and which influenza viruses are circulating; their intensity, spread and impact; and whether specific population groups are at higher risk for illness
- orders sufficient vaccine for the Canadian population in collaboration with the provinces and territories and monitors vaccine safety
- actively monitors adverse events, together with Health Canada, following immunization with the H1N1 influenza vaccine in Canada (with the collaboration of the provinces and territories, the Canadian Paediatric Society and a network of researchers)
- provides access to materials from federally controlled stockpiles (e.g. antiviral medication and general emergency supplies) to assist the provinces and territories with surge capacity, as well as facilitates the procurement of additional emergency supplies to complement provincial and territorial stockpiles
- provides public health advice for the general public and certain groups such as vulnerable populations through the coordination of pan-Canadian communications
- links with national and international experts, during the management of an outbreak, to develop advice and guidance for health professionals and other stakeholders on areas such as public health measures, laboratory testing and clinical management
- supports targeted communications to national Aboriginal organizations and communities through teleconference briefings and email notifications; Health Canada has the primary role for the federal response in on-reserve First Nation communities during a pandemic
- provides regional coordination of federal health emergency activities
- manages international aspects of pandemic preparedness and response, including liaising with the World Health Organization and acting as the focal point for coordinating the implementation of the International Health Regulations
- develops and supports the process required to update and maintain the Canadian Pandemic Influenza Plan for the Health Sector, in cooperation with Health Portfolio and provincial/territorial representatives.
Page details
- Date modified:
