ARCHIVED - Recommendations on a Human Papillomavirus Immunization Program
5. Cost-effectiveness of HPV immunization
While clinical studies are sufficient for vaccine licensure, they cannot provide information on the long-term epidemiologic and economic consequences of the vaccine. When data from long-term, follow-up clinical studies are not available, an alternative information source is mathematical models. Mathematical models have been developed to project the long-term benefits and costs of vaccination and to evaluate alternative HPV vaccination strategies. Two types of mathematical model have been used: Markov models (also referred to as cohort models) and transmission dynamic models. Markov models are probabilistic and linear: the progression of HPV disease is simulated for a single cohort over its expected lifetime, much as a cohort is tracked in a life-table analysis. Dynamic models are deterministic and nonlinear: individuals constantly enter the model as they are born and exit it as they die. A dynamic model does not track just a single cohort but, rather, the changing population over time. Dynamic modelling accounts for how HPV vaccination reduces the prevalence of infection in the population over time, assessing the impact of herd immunity(25). However, fitting parameters into dynamic models is often more challenging than in Markov models because of high computing demands.
At this time, the direct costs of the immunization program are vaccine costs (three doses of quadrivalent HPV 6/11/16/18 vaccine and a booster shot if immunity to primary vaccination is not shown to be lifelong; $134.95/dose) and the cost of administering each dose (approximately $10 to $13/dose if administered by a nurse through a school-based program). A school-based vaccination program would entail few indirect costs, whereas the indirect costs associated with a physician-based program would include patient and/or parent time taken for three (or four if a booster dose is required) office visits. For Canada, the total program cost would include cost of vaccine, distribution of the vaccine, cold chain maintenance, education, obtaining informed consent and administration of the vaccine by public health nurses through a school-based program. This has been estimated by the British Columbia (BC) Centre for Disease Control at $9 to $10 million per cohort of school-aged girls in BC (approximately 30,000 girls/cohort).
The clinical trials published to date have shown a decrease in the incidence of HPV 16 and 18 infections, CIN 1 and 2/3, vaginal and vulvar cancers, and genital warts following HPV vaccination(26-28). None of these studies examined the impact of vaccination on anal, penile, head and neck cancers or recurrent respiratory papillomatosis. The longest follow-up after vaccination in these clinical trials was 5.5 years(18). Since the interval from HPV infection to cervical cancer is long in most cases (usually decades), to date none of these trials has reported invasive cervical squamous cell carcinoma, adenocarcinoma or mortality outcomes.
Since long-term efficacy data of the HPV vaccine are still lacking, mathematical models are used to project the impact of a HPV vaccine on HPV prevalence, CIN and cervical cancer incidence. Each of the published studies to date included various assumptions on vaccine coverage, efficacy and duration of protection in their models (Appendix 2).
5.1 International modelling studies
The published dynamic models, assuming either a 10-year or lifelong duration of vaccine immunity, predict approximately 95% reduction in HPV infections, 62% to 93% reduction in cervical cancer cases (if vaccinating girls only) and 64% to 91% reduction in cervical cancer cases if vaccinating girls and boys(29-34). The studies using a Markov model found that the use of HPV vaccine in 12-year-old girls would reduce the incidence of HPV infections by approximately 13%, CIN 1 by 20% to 30% and CIN 2/3 by 46% to 66%. Kulasingam and Myers(35) and Sanders and Tairs(36) showed a reduction in cervical cancer cases of 15% and 20% respectively when the duration of protection is assumed to be 10 years, whereas other studies assuming lifelong duration of immunity show approximately 50% to 75% reduction(37-40).
Published cost-effectiveness studies have included direct medical costs, such as the cost of the vaccine, as well as the costs of managing and treating cancer precursors and cervical cancer (Appendix 2). None of these studies included the costs associated with some non-cervical cancers (vaginal, vulvar, anal and head/neck cancers). The dynamic models(30,32,34) showed a lower incremental cost-effectiveness ratio for the bivalent vaccine, which ranged from approximately $15,000 to $25,000 for a girls-only program. The use of a quadrivalent vaccine in girls only gives an incremental cost per quality adjusted life year (QALY ) varying from approximately $3000 to $37,000, depending on the model used, duration of immunity and other assumptions(34,40). A universal immunization program for girls aged 14 and under would cost an estimated $15,000 to $31,000 per QALY if the vaccine were effective for life and approximately $400 per person vaccinated. This threshold could be considered acceptable for a health intervention. Cost per QALY increases progressively after the age of 14, as does the proportion of girls infected with one of the types contained in the vaccine. The studies using a Markov model(35,36,38,40) produced similar results to the dynamic models, showing an incremental cost per life year gained ranging from approximately $32,000 to $93,000 when bivalent HPV vaccine was introduced for 12-year-old girls, as compared with the current screening programs; the cost per QALY in these studies ranged from $23,000 to $31,000.
Introduction of the HPV vaccine for girls and boys was estimated at an incremental cost of approximately $170,000(34) to $440,000(30) per QALY.
Published mathematical models have shown that the cost-effectiveness of HPV vaccination is highly sensitive to the duration of vaccine protection. However, varying vaccine coverage from 70% to 100% has little impact on the cost-effectiveness predictions.
5.2 Canadian modelling studies
Pourbohloul and Günther(42) have developed a transmission dynamic model and performed an extensive sensitivity analysis to predict HPV 16/18-associated disease prevalence and incidence in British Columbia. The transmission dynamic model assesses the epidemiologic consequences of alternative strategies for immunization with HPV vaccines in British Columbia, as well as in Canada.
Figure 1 shows the expected number of new cervical cancer cases using different vaccine parameters (10 years, 25 years or lifelong immunity) for vaccination of 14-year-old females, based on data from British Columbia.
Figure 1: Incidence of cervical cancer following immunization of 14-year-old females, assuming 10 years, 25 years or lifelong duration of vaccine immunity
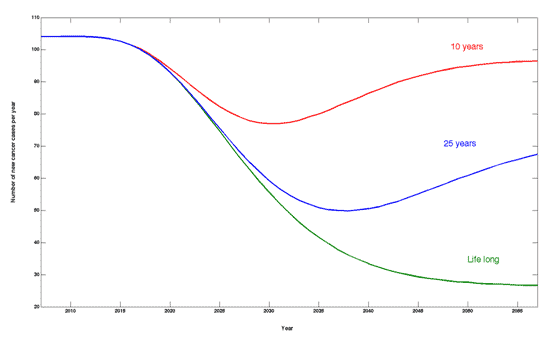
For each program strategy, the impact of vaccinating girls only versus boys and girls had a modest impact on the estimated number of new cervical cancer cases, especially when considering that twice as many vaccines would be needed for the latter strategy.
With the assumption of lifelong vaccine protection, all strategies caused the annual cancer incidence to drop significantly over 50 years after the initial delay (due to the long time lag between HPV infection and development of full-blown cervical cancer). It was further shown that the reduction in cancer incidence after 50 years was largest when the vaccine was administered at a young age and smallest when administered at an older age. With lifelong protection, vaccine immunity will not wane; therefore, vaccination of girls at an age as young as possible results in the best performance, especially when combined with a 3-year catch-up program for 14-year-old girls. On the other hand, the results for an assumed vaccine protection of 10 years were qualitatively different (Figure 1). Even though the annual cancer incidence dropped for all strategies after an initial delay, the reduction was significantly smaller compared with the assumption of lifelong vaccine protection, and a rebound was observed around the year 2030.
Figure 2 illustrates the projected number of new cervical cancer cases per year if vaccinating girls only at different ages (11, 14 or 17 years old) assuming lifelong vaccine immunity.
Figure 2: Projected cancer incidence after various HPV vaccination strategies (42)

The model* has been used to assess the cost-effectiveness ratio in terms of CDN$ per QALY of three school-based strategies: (1) a girls-only program at age 11 (F11); (2) a girls-only program at age 14 (F14); (3) a combined F11 and F14 program for girls only for 3 years followed by an F11 program. Compared with the screening program, all three strategies were similarly cost-effective, at $24,530/QALY with vaccination of 14-year-old girls, $24,945/QALY with vaccination of 11-year-old girls and $25,417/QALY with the combined program(34).
Similar results were obtained with the compartmental deterministic model developed by Brisson and collaborators(40,43). Among 12-year-old girls, these authors estimated that the number needing to be vaccinated to prevent an episode of genital warts would be 8 and to prevent a case of cervical cancer would be 324(43). For Canada, this model estimated that vaccination of 12-year-old girls would result in a decrease of 62% in cervical cancer cases at a cost of $20,512 to $31,060 per QALY(40). Similar estimates were obtained by the compartmental deterministic model and the transmission dynamic model as to the impact of the duration of vaccine protection on disease incidence.
*QALY takes into account both the quantity and the quality of life generated by health care interventions. It is the arithmetic product of life expectancy and a measure of the quality of the remaining life years. QALYs provide a common currency to assess the extent of the benefits gained from a variety of health interventions. When combined with the costs of providing the interventions, cost-utility ratios result; these indicate the additional costs required to generate a year of perfect health (one QALY)(41).
*Assumptions: 100% vaccine efficacy, 85% and 80% vaccine uptake for the 11 and 14-year-olds respectively, lifelong duration of immunity, vaccine cost of $135.95 and administration cost $12.66 per dose, and cost of Pap and cytology $74.
Page details
- Date modified: