Federal Framework on Lyme Disease - Consultation Summary

Download the entire report
(PDF format, 843 KB, 9 pages)
Organization: Public Health Agency of Canada
Type: Report
Date published: 2017-05-30
May 2017
Table of contents
Introduction
The Federal Framework on Lyme Disease Act requires the federal government to develop a Federal Framework on Lyme disease that would include the three pillars of surveillance; guidelines and best practices; and education and awareness.
The Framework is intended to guide a way forward in areas where the Government of Canada has a role. To inform development of the Framework, the Public Health Agency of Canada, on behalf of the Minister of Health:
- Conducted a 30-day online consultation in June 2015;
- Co-hosted, with the Canadian Institutes of Health Research, a Best Brains Exchange on Lyme disease diagnostics in June 2015; and
- Hosted a conference in May 2016, where participants included provincial and territorial health ministers and other stakeholders, including representatives of patient groups and the medical community.
Informed through these activities, a draft version of the Framework was posted for a public comment period, which closed on March 8, 2017.
For further information on this process, or to view the Federal Framework, please visit the Government of Canada's website (www.canada.ca).
Feedback overview
Stakeholder classification
In the spirit of openness and transparency, and to ensure that stakeholders' views were included to the greatest extent possible, comments received up to March 31, 2017, were included in the consultation results. In total, 409 Footnote 1 respondents provided feedback on the draft framework. Patients, patient families and patient groups comprised the majority of responses; followed by the general public, referred to as 'Other'; health professional organizations or experts, including veterinarians; local or provincial public health; academia; Members of Parliament; and industry. Feedback broken down by stakeholder classification is summarized in Figure 1.

Figure 1 text description
This figure shows stakeholder feedback broken down by stakeholder classification. This is a chart with vertical bars showing the number of responses received by stakeholder groups. There is one bar for each type of stakeholder. From left to right: 1 response from Industry, 6 responses from Public Health (Local and Provincial), 23 responses from Health organizations and Experts, 255 responses from Patients, Patient Families and Patient Groups, 6 responses from Academia, 2 responses from Members of Parliament (MP) and 116 responses from Other.
In addition to this feedback formally submitted through the consultation, there are two petitions that have called for the framework to be re-written. Footnote 2, Footnote 3
Stakeholder feedback
Patients, patient families and patient groups (62.3% of total feedbackFootnote 4)
Overall, patients, patient families and patient groups were not supportive of the draft framework. Only 2% of this specific stakeholder group supported its contents and felt it took steps to "address all the necessary basics". Many found that the draft did not address knowledge gaps related to the diagnosis and treatment of Lyme disease, including for Lyme disease patients who have persistent symptoms following treatment, and for patients who experience various symptoms consistent with Lyme disease or similar ailments.
Additionally, this stakeholder group expressed frustration over the lack of transparent communication in the development of this draft and what they deemed a "status quo" approach to a significant public health issue.
Many of the points raised by this group, such as financial assistance for medical treatment, are outside the scope of the federal role.
Echoing the sentiments of many in this group, this patient stated:
"Je suis très déçue. Cette ébauche de cadre fédéral n'est aucunement proactive. C'est du devoir de fond, supposément scientifique, supposément responsable, mais pas d'action concrète auprès des individus qui en souffre."
Other (28.4% of total feedbackFootnote 4)
Of those stakeholders classified as 'other', 9% supported its contents while noting that more could be done. Comments from this group included the need to conduct more research, and education and awareness activities; that patients should have access to better diagnosis and treatment; and expressing the need for increased funding to address the burden of Lyme disease.
Health organizations and experts (5.6% of total feedbackFootnote 4)
While most health organizations and experts generally supported the draft framework, a number of technical revisions were suggested. This included the need for stronger surveillance, education and awareness; the need for a balanced approach between clinical diagnosis and laboratory testing; and improving timely access to care by drawing on the expertise of nurse practitioners.
Local or provincial public health (1.5% of total feedbackFootnote 4)
There was mixed support from local or provincial public health groups. While some supported the framework with its evidence-based approach, others commented that the framework should include detailed scientific and clinical evidence. Suggestions included increasing public education and awareness, enhancing surveillance, defining roles and responsibilities across sectors, and increasing concrete actions to more comprehensively address Lyme disease.
Academia (1.5% of total feedbackFootnote 4)
Approximately 30% of this stakeholder group was supportive of the draft framework. Technical edits that were recommended related to surveillance and monitoring, research, and increasing education.
Industry (< 1% of total feedbackFootnote 4)
This stakeholder requested that reference to tick repellents be included in future guidelines and best practices for the prevention of Lyme disease.
Members of Parliament (<1% of total feedbackFootnote 4)
Members of Parliament that responded were not supportive of the framework, and requested the inclusion of action from both the federal and provincial/territorial governments to ensure all Lyme disease patients have access to timely treatment.
Feedback - Areas of focus
Those affected by Lyme disease, as well as those represented in the 'other' stakeholder group, wished to see diagnosis and treatment emphasized in the framework. Feedback to address diagnosis and treatment of Lyme disease included addressing the financial burden of seeking testing and treatment abroad, improving access in Canada to medical treatment, and addressing co-infections and chronic Lyme disease.
Select stakeholders in this group also suggested that the Public Health Agency of Canada should consider the "importance of other non-Lyme chronic diseases that are more prevalent" when moving forward on action.
The top five priorities as identified by data from patients, patient families and patient groups, and 'other' stakeholders are outlined in Figure 2.
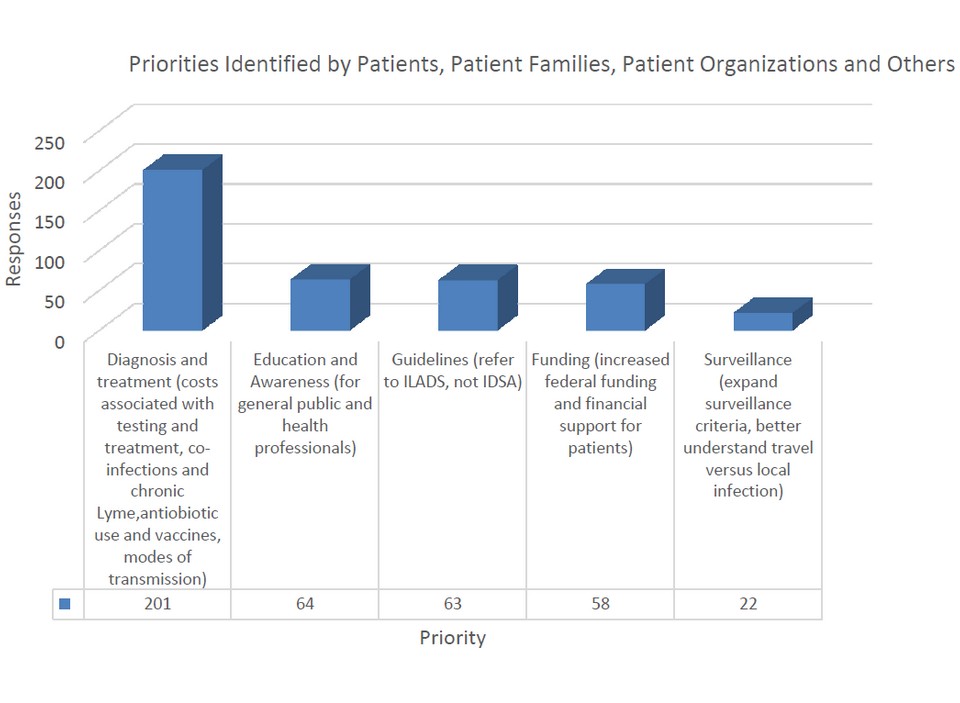
* IDSA: Infectious Diseases Society of America; ILADS: International Lyme and Associated Disease Society
Figure 2 text description
This figure shows the top five areas of focus, as identified by data from patients, patient families and patient groups, and "other" stakeholders. This is a chart with vertical bars showing the priorities identified by patients, patient families, patient organizations and others. There is one bar for each identified priority (5 in total). From left to right: 201 respondents identified as a priority: Diagnosis and treatment (costs associated with testing and treatment, co-infections and chronic Lyme, antibiotic use and vaccines, modes of transmission), 64 respondents identified as a priority: Education and Awareness (for general public and health professionals), 63 respondents identified as a priority: Guidelines (refer to ILADS, not IDSA), 58 respondents identified as a priority: Funding (increased federal funding and financial support for patients and 22 respondents identified as a priority: Surveillance (expand surveillance criteria, better understand travel versus local infection).
On the other hand, those in the health or academic fields expressed a clear desire for expanding the evidence base on Lyme disease while also balancing the need for better education and awareness at all levels. Further, it was acknowledged that evidence-based guidelines are necessary to guide the practice of medicine; however, opinions differed on whether the best course of action would be to follow the Infectious Diseases Society of America (IDSA) guidelines, or the International Lyme and Associated Disease Society (ILADS) guidelines. More than one respondent from this group suggested employing an external adjudication body to resolve stakeholder differences given the varying opinions.
The top five priorities as identified by data from health organizations and experts, local and provincial public health, and academia are outlined in Figure 3.

Figure 3 text description
This figure shows the top five areas of focus, as identified by health organizations and experts, local and provincial public health, and academia. This is a chart with vertical bars showing the priorities identified by health organizations and experts, local and provincial public health, and academia. There is one bar for each identified priority (5 in total). From left to right: 11 respondents identified as a priority: Surveillance (expand surveillance criteria, better understand travel versus local infection, morbidity database), 8 respondents identified as a priority: Education and Awareness (for general public and health professionals), 7 respondents identified as a priority: Research (explore co-infections, chronic Lyme), 7 respondents identified as a priority: Guidelines (need for development and dissemination) and 4 respondents identified as a priority: Diagnosis and treatment (interpretation, co-infection and chronic Lyme, antibiotic use).
Implications for framework revisions
In reflection of the comments received, the framework has been revised to more clearly acknowledge the perspective of patients by including the summary report of the conference that occurred in 2016.
The framework continues to encourage an evidence-based approach to addressing the three pillars of surveillance; guidelines and best practices; and education and awareness. Technical revisions have been incorporated following the expert feedback received.
In addition, the framework has been revised to include actions where the federal government has a role, including the establishment of a national research network on Lyme disease. While these actions will reflect those that can be undertaken by the Government of Canada, all interested parties are invited to identify actions that their respective organizations can take to contribute to progress for patients and the front-line health professionals that care for them.
Footnotes
- Footnote 1
-
Some individuals submitted multiple submissions; multiple submissions from the same individual were considered as a single submission, but all of the collective feedback was considered. A total of 387 submissions were received by March 8, 2017, and a total of 409 submissions were received by March 31, 2017.
- Footnote 2
-
Ticking Lyme Bomb in Canada: 39,361 signatures as of April 26, 2017 https://www.change.org/p/minister-philpott-ticking-lyme-bomb-in-canada-fix-canada-s-lyme-action-plan-now
- Footnote 3
-
E-petitions e-903 (Lyme disease), sponsored by Elizabeth May (closes June 28, 2017): 4,996 signatures as of April 26, 2017 https://petitions.parl.gc.ca/en/Petition/Details?Petition=e-903
- Footnote 4
-
Total does not equal 100% due to rounding.
Page details
- Date modified: