Canadian Integrated Program for Antimicrobial Resistance Surveillance: A 20-year tour
Download in PDF format
(460 KB, 1 pages)
Organization: Public Health Agency of Canada
Date published: 2022-11-21
Cat.: HP40-325/2-2022E-PDF
ISBN: 978-0-660-45982-0
Pub.: 220509
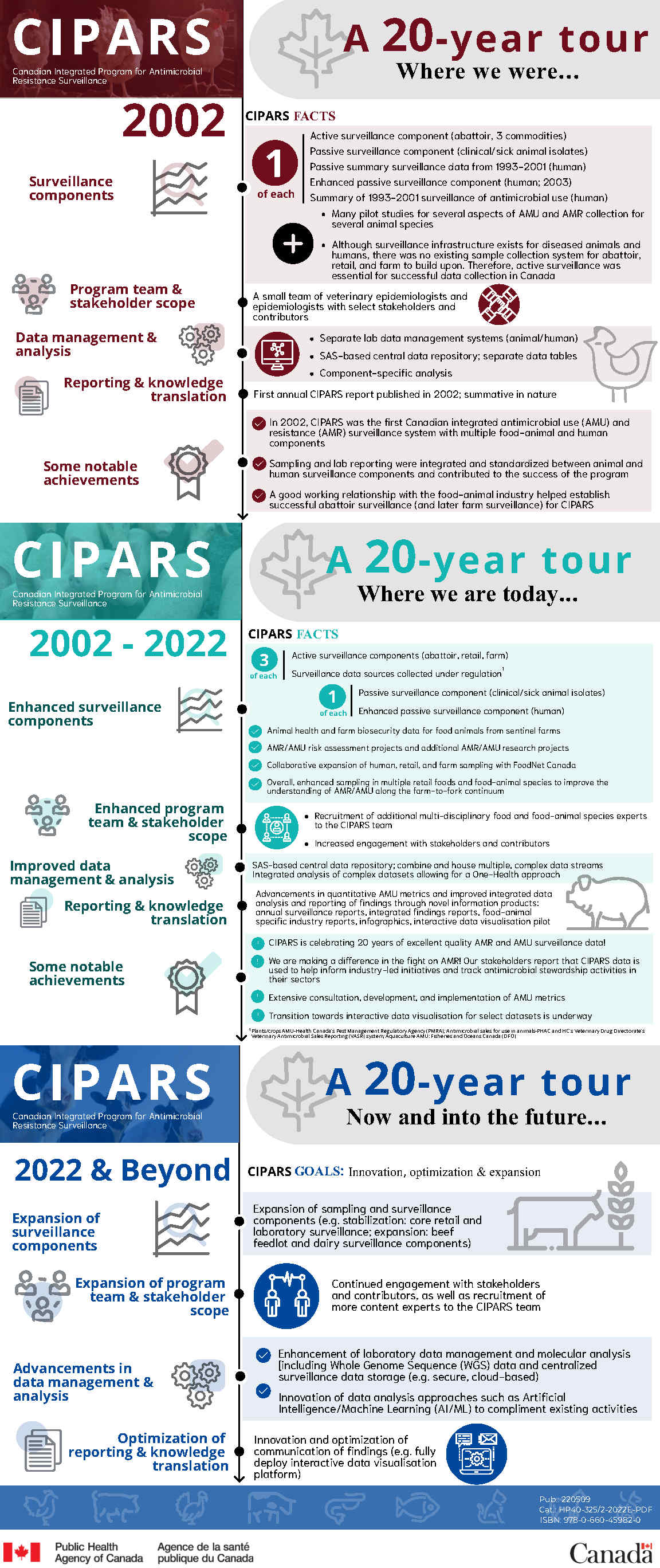
Where we were
2002: CIPARS facts
Surveillance component
One of each:
- Active surveillance component (abattoir, 3 commodities)
- Passive surveillance component (clinical/sick animal isolates)
- Passive summary surveillance data from 1993 to 2001 (human)
- Enhanced passive surveillance component (human; 2003)
- Summary of 1993 to 2001 surveillance of antimicrobial use (human)
Additionally:
- Many pilot studies for several aspects of AMU and AMR collection for several animal species
- Although surveillance infrastructure exists for diseased animals and humans, there was no existing sample collection system for abattoir, retail, and farm to build upon. Therefore, active surveillance was essential for successful data collection in Canada
Program team and stakeholder scope:
- A small team of veterinary epidemiologists and epidemiologists with select stakeholders and contributors
Data management and analysis:
- Separate lab data management systems (animal/human)
- SAS-based central data repository; separate data tables
- Component-specific analysis
Reporting and knowledge translation:
- First annual CIPARS report published in 2002; summative in nature
Some notable achievements:
- In 2002, CIPARS was the first Canadian integrated antimicrobial use (AMU) and resistance (AMR) surveillance system with multiple food-animal and human components
- Sampling and lab reporting were integrated and standardized between animal and human surveillance components and contributed to the success of the program
- A good working relationship with the food-animal industry helped establish successful abattoir surveillance (and later farm surveillance) for CIPARS
Where we are today
2002 to 2022: CIPARS facts
Enhanced surveillance components:
Three of each:
- Active surveillance components (abattoir, retail, farm)
- Surveillance data sources collected under regulationFootnote 1
One of each:
- Passive surveillance component (clinical/sick animal isolates)
- Enhanced passive surveillance component (human)
And:
- Animal health and farm biosecurity data for food animals from sentinel farms
- AMR/AMU risk assessment projects and additional AMR/AMU research projects
- Collaborative expansion of human, retail, and farm sampling with FoodNet Canada
- Overall, enhanced sampling in multiple retail foods and food-animal species to improve the understanding of AMR/AMU along the farm-to-fork continuum
Enhanced program team and stakeholder scope:
- Recruitment of additional multi-disciplinary food and food-animal species experts to the CIPARS team
- Increased engagement with stakeholders and contributors
Improved data management and analysis:
- SAS-based central data repository; combine and house multiple, complex data streams
- Integrated analysis of complex datasets allowing for a One-Health approach
Reporting and knowledge translation:
- Advancements in quantitative AMU metrics and improved integrated data analysis and reporting of findings through novel information products: annual surveillance reports, integrated findings reports, food-animal specific industry reports, infographics, interactive data visualisation pilot
Some notable achievements:
- CIPARS is celebrating 20 years of excellent quality AMR and AMU surveillance data!
- We are making a difference in the fight on AMR! Our stakeholders report that CIPARS data is used to help inform industry-led initiatives and track antimicrobial stewardship activities in their sectors
- Extensive consultation, development, and implementation of AMU metrics
- Transition towards interactive data visualisation for select datasets is underway
Now and into the future
2022 and beyond, CIPARS goals: innovation, optimization and expansion
Enhanced surveillance components:
- Expansion of sampling and surveillance components (example, stabilization: core retail and laboratory surveillance; expansion: beef feedlot and dairy surveillance components)
Expansion of program team and stakeholder scope:
- Continued engagement with stakeholders and contributors, as well as recruitment of more content experts to the CIPARS team
Advancements in data management and analysis:
- Enhancement of laboratory data management and molecular analysis including Whole Genome Sequence (WGS) data and centralized surveillance data storage (example, secure, cloud-based)
- Innovation of data analysis approaches such as Artificial Intelligence/Machine Learning (AI/ML) to complement existing activities
Optimization of reporting and knowledge translation:
- Innovation and optimization of communication of findings (example, fully deploy interactive data visualisation platform)