National Enteric Surveillance Program (NESP) annual summary 2022
Download in PDF format
(5,46 MB 43 pages)
Organization: Public Health Agency of Canada
Published: 2024-06-25
Prepared by:
The National Microbiology Laboratory (NML), the Centre for Foodborne, Environmental and Zoonotic Infectious Diseases (CFEZID), Public Health Agency of Canada, and the provincial public health laboratories
Acknowledgements
National Enteric Surveillance Program (NESP)
Coordination team:
Celine Nadon, Director, Division of Enteric Diseases, National Microbiology Laboratory (NML)
Sara Christianson, Chief, Diagnostic and Reference Services Section, Division of Enteric Diseases, NML
Lori Lozinski, Surveillance Clerk, Division of Enteric Diseases, NML
Brent Avery, A/Surveillance Manager, Foodborne Disease and AMR Surveillance Division (FDASD), Centre for Foodborne, Environmental and Zoonotic Infectious Diseases (CFEZID)
Lauren Sherk, Epidemiologist, FDASD, CFEZID
Provincial laboratory partners:
BC Centre for Disease Control Public Health Laboratory
Alberta Precision Laboratories
Roy Romanow Provincial Laboratory (Saskatchewan)
Cadham Provincial Microbiology Laboratory (Manitoba)
Public Health Ontario
Laboratoire de santé publique du Québec (LSPQ)
New Brunswick Public Health Laboratories
Nova Scotia Public Health Laboratories
Prince Edward Island Public Health Laboratories
Newfoundland and Labrador Public Health Laboratory
Provincial/territorial epidemiology partners:
British Columbia Centre for Disease Control
Alberta Health
Saskatchewan Ministry of Health
Manitoba Health
Public Health Ontario
Ministère de la santé et des services sociaux du Québec
New Brunswick Health
Nova Scotia Department of Health and Wellness
Prince Edward Island Department of Health and Wellness
Newfoundland and Labrador Department of Health and Community Services
Yukon Health and Social Services
Northwest Territories Department Health and Social Services
Nunavut Health and Social Services
Overview
The National Enteric Surveillance Program (NESP) is a collaboration between the Public Health Agency of Canada (PHAC) and the provincial public health laboratories. Through NESP, weekly analysis and reporting is conducted for 14 different organisms causing enteric illness, including 10 organisms that are nationally notifiable. The data and information derived from this surveillance system supports detection of multi-provincial clusters and outbreaks, guides public health interventions, and are designed to integrate with national and international efforts to limit the transmission of enteric diseases.
In 2022, a total of 12,523 isolate results were reported to NESP; a similar number as the average number of notifications received in the previous five years (12,677). However, this number remains lower than the 2015 to 2019 (pre-COVID-19 pandemic) five-year average number of notifications (15,313). Salmonella spp. continues to be the most common organism identified with 4,826 notifications provided in 2022, representing 39% of all isolates reported to NESP. As in previous years, Salmonella Enteritidis (1,840 isolates; 38%) and S. Typhimurium (319 isolates; 7%), represent the top two serotypes among all Salmonella reported in 2022. In 2022, S. ssp I 4,[5],12:i:- (265 isolates; 5%) was the third most commonly reported serotype. Collectively, these three serotypes represent 50% of all Salmonella serotypes identified.
The 2022 incidence rate of Shiga toxin-producing Escherichia coli (STEC) O157 is slightly higher than the 2021 rate of O157 STEC with 0.77 cases per 100,000 population reported, but remains lower than the relatively stable rate seen from 2010 to 2019 (between 0.95 to 1.40 cases per 100,000 population). A slight increase was observed in the incidence rate of non-O157 STEC isolates in 2022 (1.32 cases per 100,000 population) compared to 2021 (0.98 cases per 100,000 population). However, this remains lower than an all-time high of 1.58 cases per 100,000 population reported to NESP in 2019. This is the sixth consecutive year where more non-O157 STEC isolates were reported than O157 STEC isolates.
The incidence rate of invasive listeriosis in 2022 (0.47 per 100,000 population) is slightly higher than what was reported in 2021, but similar to 2019. The incidence rate of Hepatitis A increased in 2022 (0.80 cases per 100,000 population) compared to 2021 (0.69 cases per 100,000 population). However, this is still lower than the highest incidence reported in 2019 (1.30 cases per 100,000 population). In contrast to 2019 and years before where Shigella sonnei represented the majority of Shigella species reported, 2022 followed a similar trend to 2021 and 2020 where Shigella flexneri represented over 50% of all Shigella reported. In 2022, the rate of Shigella flexneri (1.45 per 100,000 population) was also higher than the rate of Shigella sonnei (0.96 per 100,000 population). Trends for all other Shigella species in 2022 were similar to 2021, remaining lower compared to previous years.
Table of contents
- Acknowledgements
- Overview
- About the National Enteric Surveillance Program (NESP)
- Laboratory-confirmed isolate counts and incidence rates
- Salmonella
- Escherichia coli
- Listeria monocytogenes
- Shigella
- Hepatitis A
Tables
- Table 1. Number of isolates reported to NESP by major organism group per province or territory, 2022
- Table 2. Annual national totals and rates (per 100,000 population) for enteric pathogens and organism groups reported to NESP, 2017 to 2022Footnote 1
- Table 3. Annual rates (per 100,000 population) of infection per province and territory for select groups of pathogens routinely reported to NESP, 2022Footnote 1
- Table 4. Number of isolates reported to NESP per province and territory for the ten most commonly reported Salmonella serotypes, 2022
- Table 5. National total counts (overall rank) for the ten most commonly reported Salmonella serotypes to NESP, 2017 to 2022
Figures
- Figure 1. Proportion of Salmonella serotypes causing human illness as reported to NESP, 2022 (n=4,826)
- Figure 2. Annual counts between 2013 to 2022 for the top five non-typhoidal Salmonella serotypes reported to NESP in 2022
- Figure 3. Relative incidence rates (per 100,000 population) of S. Enteritidis, S. Typhimurium, S. ssp I 4,[5],12:i:-, and other Salmonella serotypes reported to NESP by year, 2018 to 2022 compared to the 2013 to 2017 baseline periodFootnote 1
- Figure 4. Incidence rates (per 100,000 population) of O157 STEC, non-O157 STEC, and other non-typed E. coli reported to NESP, 1997 to 2022
- Figure 5. Distribution of non-O157 STEC serogroups reported to NESP in 2022 (n=513)
- Figure 6. Incidence rate (per 100,000 population) of the top five serotyped non-O157 STEC serogroups reported to NESP, 2013 to 2022
- Figure 7. Incidence rate (per 100,000 population) of invasive listeriosis reported to NESP by province, 2013 to 2022Footnote 1
- Figure 8. Incidence rate (per 100,000 population) of Shigella species reported to NESP, 1997 to 2022
- Figure 9. National and provincial incidence rate (per 100,000 population) of Hepatitis A reported to NESP, 2013 to 2022
Appendices
- Appendix A. Canadian Notifiable Disease Surveillance System (CNDSS) and the National Enteric Surveillance Program (NESP)
- Appendix B. Species and serotype data reported to NESP by province and territory, 2022
- Appendix C. NESP support for outbreak investigations
- Appendix D. Impacts of COVID-19: Comparison of NESP weekly isolate counts from 2022, 2021 and 2020 compared to the 2015 to 2019 historical average for select pathogens
- Figure 10. All Salmonella reported to NESP in 2022, 2021 and 2020 compared to the 2015 to 2019 historical average
- Figure 11. All Salmonella reported to NESP excluding Salmonella Enteritidis in 2022, 2021 and 2020 compared to the 2015 to 2019 historical average
- Figure 12. Salmonella Enteritidis reported to NESP in 2022, 2021 and 2020 compared to the 2015 to 2019 historical average
- Figure 13. O157 STEC reported to NESP in 2022, 2021 and 2020 compared to the 2015 to 2019 historical average
- Figure 14. Non-O157 STEC reported to NESP in 2022, 2021 and 2020 compared to the 2015 to 2019 historical average
- Figure 15. Listeria monocytogenes reported to NESP in 2022, 2021 and 2020 compared to the 2015 to 2019 historical average
- Figure 16. Shigella reported to NESP in 2022, 2021 and 2020 compared to the 2015 to 2019 historical average
About the National Enteric Surveillance Program (NESP)
In Canada, the national surveillance of human enteric diseases is conducted through NESP and the Canadian Notifiable Diseases Surveillance System (CNDSS)Footnote 1. NESP is jointly administered by PHAC's National Microbiology Laboratory (NML) and the Centre for Foodborne, Environmental and Zoonotic Infectious Diseases (CFEZID). Since 1997, weekly analysis and reporting on laboratory-confirmed cases of enteric illness by the provincial public health laboratories has been conducted through NESP.
NESP provides the most timely data (at a level of characterization that is primarily species and serotype) that are critical to, and integrated with other surveillance programs. Monitoring these aggregated data allows for the rapid evaluation and response to enteric illness outbreaks. In addition, these data allow for the description of trends in pathogen subtypes and in the incidence of nationally notifiable enteric pathogens. CNDSS receives data that are collected by local health units, which is forwarded to provincial/territorial health authorities and collated by PHAC's Centre for Communicable Diseases and Infection Control (CCDIC). These data may be more representative of total numbers of annual illnesses; however, CNDSS is not designed to provide timely information required for cluster or outbreak detection. These two surveillance systems (CNDSS and NESP) are complementary in providing both epidemiological and laboratory results; however, discrepancies between them do exist. Due to the reporting protocols and requirements, CNDSS is a more reliable source of information in terms of total number of illnesses, while NESP data are more current and responsive to trends. A comparison of national case counts and incidence rates for enteric diseases is included (Appendix 1).
NESP is also highly complementary to another laboratory-based surveillance system, PulseNet CanadaFootnote 2. Also administered by PHAC, PulseNet Canada collects high resolution (i.e., whole genome sequence) data in real-time on cases of enteric diseases for the purpose of outbreak detection and response. Due to the additional testing performed (genomic subtyping, whole genome sequencing), there are differences in turnaround time compared to weekly NESP data. Further, PulseNet Canada surveillance is conducted only for a subset of the organisms that are tracked by NESP.
Data collection
Isolates (or specimens) are submitted to provincial public health laboratories for testing and/or confirmation of the enteric pathogen. On a weekly basis, each provincial public health laboratory summarizes the number of enteric microorganisms isolated from human patients. The information details the genus, species, pathovar (where appropriate) and serotype (where appropriate). The 'report week' for NESP spans the period from Sunday to Saturday and is based on the date the laboratory test was completed, except for in Alberta, where it is based on the date received. Data are submitted to NML either directly (through email), or by entering the data via the web-based application (webNESP) hosted on the Canadian Network for Public Health Intelligence (CNPHI). The information is submitted as soon as possible and no later than the second day after a weekend or holiday. An exception to this reporting scheme occurs when the isolate must be sent to another laboratory for completion of the identification. In this case, the isolate is reported at the level of typing or identification attained (e.g. Salmonella spp.) for the week in which it was sent to the reference laboratory. The NESP record is then updated when the final identification is received from the reference laboratory (e.g. report in week 35 indicates that one "Salmonella spp." reported in week 33 has been confirmed as "S. Banana"). This updated information is submitted with the next weekly NESP report form.
All data submitted are aggregated by province and pathogen and do not contain any patient identifiers, locators, or other confidential information. NESP partners endeavor to include only the number of isolates from new cases identified at the laboratory that week, or updates to previously reported numbers. To avoid duplication, the provincial public health laboratories attempt to identify multiple, repeat, or follow-up specimens from the same individual. For example, when multiple isolates are collected from a single patient, the laboratories would consider this as a single case if all identical isolates are collected over a reasonable time period (typically three months).
Enhanced subtype data collected for surveillance purposes are primarily generated using whole genome sequencing (WGS) in silico predictions instead of by classical microbiological methods. This includes in silico predictions of species identification and serotype (where applicable). Use of WGS data for NESP analyses helps ensure this system will remain compatible with surveillance in the genomics era. Since 2018, the majority of the data collected and analyzed by NESP has been generated via WGS.
Data analysis and dissemination
Data analysis is conducted weekly by using an algorithm to determine if the current week case counts are significantly higher than the expected baseline. Statistical significance is based on the cumulative Poisson probability between the reported case count and the retrospective five-year median.
Results from the weekly analysis included in the "NESP Weekly Report" are disseminated to all provincial public health laboratories, at least one epidemiologist or Medical Officer of Health in each province/territory and multiple stakeholders at the federal level. Protocol allows sharing of the reports with other public health professionals who have an operational need to have this information, although, the weekly reports are not intended for public distribution. No response is required by public health professionals to the statistical elevations noted in the reports. The aim is to provide useful and timely information for those responsible for public health action.
In addition to NESP Weekly Reports, partners can perform real-time data analysis, examine trends and display their respective jurisdictions' data within the webNESP application. PulseNet Canada uses these data in conjunction with whole genome sequencing based subtyping data and other molecular/genomic data to detect disease clusters and outbreaks. The resulting data analyses are also shared on CNPHI with provincial public health laboratories, the Canadian Food Inspection Agency (CFIA), Health Canada (HC), PHAC and provincial/territorial epidemiologists. The coordinated assessment of laboratory evidence collected through these complementary laboratory surveillance networks allows for the interpretation of clinical microbiological evidence during multi-jurisdictional epidemiologic investigations, as described in the Food-borne Illness Outbreak Response Protocol (FIORP)Footnote 3.
For this annual summary, initial 2022 data validation activities were performed in collaboration with the provinces and territories. Once the final dataset was validated and closed, summary statistics using SAS softwareFootnote 4 were conducted for the 14 different enteric organisms causing enteric illness that are reported to NESP.
Limitations
There are some inherent limitations of these data. For some organisms, the number of isolates reported is a subset of laboratory isolations and may not reflect the incidence of disease at the provincial or national level. For example, Campylobacter isolates are not routinely forwarded to provincial public health or central reference laboratories for further testing beyond genus/species characterizations, and are therefore greatly under-represented in NESP. By contrast, Salmonella and O157 STEC isolates captured by NESP are more representative of the true incidence of disease in Canada, as the number of cases reported to CNDSS and isolates reported to NESP show a high degree of concurrence for both diseases. There may be over-reporting of organisms in NESP due to reporting of multiple specimens from a single patient, but efforts are made to minimize this occurrence. Information regarding extra-intestinal isolation sites and foreign travel are not consistently reported to NESP from all provincial public health laboratories and therefore any interpretation should be considered with caution.
In March of 2020, the COVID-19 pandemic was declaredFootnote 5 and global public health action was taken to address it. Across Canada and within specific provinces/territories and regions, various public health measures were put in place. These public health measures and the adaptations Canadians made to combat COVID-19 not only helped to reduce the transmission of COVID-19, but have also impacted other reported infectious diseases to varying degrees in various ways.
Similar to the 2020 and 2021 NESP Annual Summaries, interpretation of the data and findings in the 2022 NESP Annual Summary should be interpreted with caution, as the public health measures invoked to help limit the spread of COVID-19 likely impacted disease incidence spanning all pandemic-related years, as well as data collection and reporting to NESP (Appendix D). In addition, COVID-19 impacts on laboratory services (e.g., delays, break in service, reduced service, change in service) likely impacted the observed incidence of these enteric pathogens reported to NESP during the pandemic years.
Questions and correspondence may be forwarded via email to:
nesp-pnsme@phac-aspc.gc.ca
Laboratory-confirmed isolate counts and incidence rates
In 2022, provincial public health laboratories reported 12,523 cases of illness caused by enteric pathogens to NESP, similar to the average number of notifications reported in the previous five years (12,677). However, this number remains lower than the 2015 to 2019 (pre-COVID-19 pandemic) five-year average number of notifications (15,313). The most frequently reported enteric pathogen group was Salmonella, followed by enteric viruses (Norovirus, Hepatitis A, Rotavirus, Adenovirus, Astrovirus, Sapovirus and Enterovirus)and E. coli (Table 1). Organism isolate counts reported by province and territory in 2022 can be found in Appendix B.
| GroupFootnote 4 | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total | % of total isolates reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella | 781 | 598 | 146 | 111 | 1,977 | 949 | 121 | 80 | 7 | 46 | 0 | 8 | 2 | 4,826 | 38.54 |
| VirusesFootnote 1 | 258 | 811 | 223 | 236 | 569 | 57 | 151 | 67 | 30 | 383 | 0 | 1 | 3 | 2,789 | 22.27 |
| E. coliFootnote 2 | 133 | 325 | 58 | 156 | 183 | 154 | 17 | 1 | 5 | 296 | 0 | 0 | 0 | 1,328 | 10.60 |
| CampylobacterFootnote 1 | 6 | 164 | 119 | 135 | 69 | 186 | 187 | 84 | 32 | 105 | 1 | 0 | 0 | 1,088 | 8.69 |
| ParasitesFootnote 1 | 147 | 7 | 88 | 67 | 432 | NRFootnote 3 | 102 | 68 | 19 | 79 | 21 | 0 | 0 | 1,030 | 8.22 |
| Shigella | 304 | 275 | 3 | 5 | 231 | 146 | 4 | 1 | 0 | 7 | 1 | 1 | 0 | 978 | 7.81 |
| Yersinia | 65 | 33 | 4 | 4 | 95 | 30 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 233 | 1.86 |
| Listeria | 14 | 14 | 1 | 5 | 69 | 59 | 4 | 12 | 1 | 4 | 0 | 0 | 0 | 183 | 1.46 |
| Vibrio | 27 | 3 | 3 | 0 | 17 | 4 | 11 | 0 | 3 | 0 | 0 | 0 | 0 | 68 | 0.54 |
| Total | 1,735 | 2,230 | 645 | 719 | 3,642 | 1,585 | 598 | 313 | 97 | 921 | 23 | 10 | 5 | 12,523 | 100.00 |
|
|||||||||||||||
Annual national incidence rates for the groups of enteric pathogens reported to NESP between 2017 and 2022 are shown in Table 2 and Appendix A. Isolates of O157 STEC, non-O157 STEC, Listeria monocytogenes, Salmonella spp., Shigella spp., and Vibrio cholerae are routinely forwarded to provincial public health laboratories, while isolates of Campylobacter spp., non-cholerae Vibrio, Yersinia spp., enteric parasites (Giardia spp., Cryptosporidium spp., Entamoeba histolytica/dispar and Cyclospora cayetanensis) and enteric viruses (Norovirus, Rotavirus, Adenovirus, Astrovirus, Sapovirus and Enterovirus) are not routinely forwarded to the provincial public health or central reference laboratories. As such, NESP incidence rates are considered to be reflective of the true incidence rate for those pathogens that are routinely reported, enabling the calculation of provincial and territorial incidence rates as shown in Table 3.
| Group | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | RateFootnote 1 | Total | RateFootnote 1 | Total | RateFootnote 1 | Total | RateFootnote 1 | Total | RateFootnote 1 | Total | RateFootnote 1 | |
| O157 STEC | 348 | 0.95 | 426 | 1.15 | 397 | 1.06 | 237 | 0.62 | 260 | 0.68 | 299 | 0.77 |
| Non-O157 STECFootnote 2 | 361 | 0.99 | 525 | 1.42 | 595 | 1.58 | 320 | 0.84 | 373 | 0.98 | 513 | 1.32 |
| Listeria | 109 | 0.30 | 150 | 0.40 | 174 | 0.46 | 158 | 0.42 | 154 | 0.40 | 183 | 0.47 |
| Salmonella | 7,313 | 20.01 | 7,300 | 19.70 | 6,350 | 16.89 | 4,919 | 12.94 | 3,360 | 8.79 | 4,826 | 12.40 |
| Shigella | 699 | 1.91 | 784 | 2.12 | 828 | 2.20 | 393 | 1.03 | 416 | 1.09 | 978 | 2.51 |
| Campylobacter | 1,287 | 3.52 | 1,333 | 3.60 | 1,664 | 4.43 | 1,289 | 3.39 | 1,255 | 3.28 | 1,088 | 2.79 |
| Vibrio | 54 | 0.15 | 67 | 0.18 | 52 | 0.14 | 44 | 0.12 | 51 | 0.13 | 68 | 0.17 |
| Yersinia | 387 | 1.06 | 404 | 1.09 | 318 | 0.85 | 283 | 0.74 | 298 | 0.78 | 233 | 0.60 |
| Parasites | 1,679 | 4.59 | 1,675 | 4.52 | 1,639 | 4.36 | 1,017 | 2.68 | 1,020 | 2.67 | 1,030 | 2.65 |
| Viruses | 2,593 | 7.10 | 2,273 | 6.13 | 2,564 | 6.82 | 1,035 | 2.72 | 938 | 2.45 | 2,789 | 7.16 |
|
||||||||||||
| GroupFootnote 2 | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O157 STEC | 0.24 | 2.22 | 0.75 | 0.28 | 0.78 | 0.48 | 0.74 | 0.10 | 2.93 | 0.00 | 0.00 | 0.00 | 0.00 |
| Non-O157 STEC | 1.75 | 4.89 | 4.10 | 2.55 | 0.43 | 0.47 | 0.74 | 0.00 | 0.00 | 0.19 | 0.00 | 0.00 | 0.00 |
| Listeria | 0.26 | 0.31 | 0.08 | 0.35 | 0.46 | 0.68 | 0.49 | 1.18 | 0.59 | 0.76 | 0.00 | 0.00 | 0.00 |
| Salmonella | 14.68 | 13.16 | 12.22 | 7.88 | 13.08 | 10.91 | 14.90 | 7.85 | 4.10 | 8.75 | 0.00 | 17.54 | 4.94 |
| Shigella | 5.72 | 6.05 | 0.25 | 0.35 | 1.53 | 1.68 | 0.49 | 0.10 | 0.00 | 1.33 | 2.28 | 2.19 | 0.00 |
|
|||||||||||||
Salmonella
A total of 4,826 Salmonella isolates representing 217 serotypes were reported to NESP in 2022. Salmonella Enteritidis accounted for 38% of all human salmonellosis, and together with the nine remaining most common serotypes (Figure 1), they made up 70% of all Salmonella infections reported. National, provincial and territorial case counts for Salmonella reported in 2022 are shown in Table 4 and Appendix B.
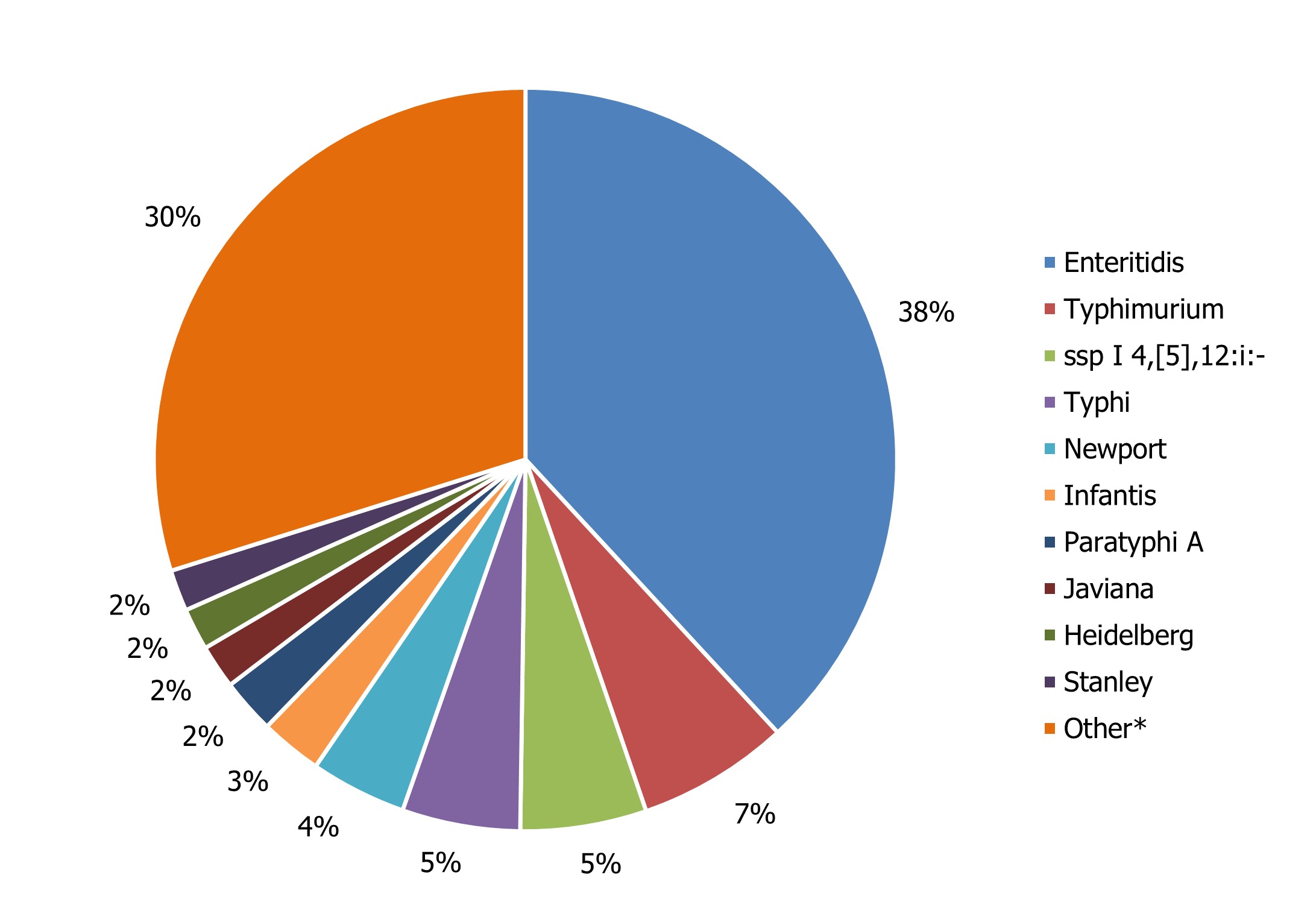
Figure 1 : Descriptive text
| Salmonella Species | Percentage of total Salmonella |
| Enteritidis | 38% |
| Typhimurium | 7% |
| ssp I 4,[5],12:i:- | 5% |
| Typhi | 5% |
| Newport | 4% |
| Infantis | 3% |
| Paratyphi A | 2% |
| Javiana | 2% |
| Heidelberg | 2% |
| Stanley | 2% |
| OtherFootnote * | 30% |
|
|
| Serotype | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total | % of total Salmonella (n=4,826) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enteritidis | 365 | 221 | 34 | 32 | 661 | 380 | 78 | 43 | 4 | 20 | 0 | 2 | 0 | 1,840 | 38.13% |
| Typhimurium | 29 | 66 | 7 | 10 | 113 | 81 | 3 | 7 | 0 | 2 | 0 | 1 | 0 | 319 | 6.61% |
| ssp I 4,[5],12:i:- | 19 | 20 | 3 | 6 | 109 | 96 | 9 | 1 | 0 | 0 | 0 | 1 | 1 | 265 | 5.49% |
| Typhi | 46 | 33 | 6 | 10 | 134 | 13 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 248 | 5.14% |
| Newport | 39 | 37 | 2 | 4 | 86 | 30 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 202 | 4.18% |
| Infantis | 11 | 16 | 6 | 4 | 56 | 26 | 5 | 4 | 1 | 1 | 0 | 0 | 0 | 130 | 2.69% |
| Paratyphi A | 26 | 15 | 2 | 5 | 60 | 5 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 115 | 2.38% |
| Javiana | 8 | 10 | 0 | 4 | 39 | 26 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 92 | 1.91% |
| Heidelberg | 5 | 5 | 4 | 0 | 34 | 32 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 88 | 1.82% |
| Stanley | 9 | 6 | 44 | 2 | 17 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 87 | 1.80% |
| Total | 557 | 429 | 108 | 77 | 1,309 | 697 | 105 | 61 | 6 | 31 | 0 | 4 | 2 | 3,386 | 70.16% |
Compared to the average number of Salmonella notifications received from 2017 through 2021 (5,848 cases), there was a 17% decrease observed in 2022 (4,826 cases). The national incidence rate of Salmonella (12.40 cases per 100,000 population) increased in comparison to 2021 (8.79 cases per 100,000 population), but remained lower than the previous five years, likely due to the impacts of the COVID-19 pandemic and the continued impact of the CFIA regulationFootnote 6 implemented in April 2019 to address Salmonella in raw frozen breaded chicken products (Figure 2). While S. Enteritidis remained the most common serotype over this time period, changes were observed among the other most commonly reported Salmonella serotypes (Table 5).
In 2022, five provinces/territories reported incidence rates of Salmonella higher than the national reported incidence rate: British Columbia (14.68 cases per 100,000 population), Alberta (13.16 cases per 100,000 population), Ontario (13.08 cases per 100,000 population), New Brunswick (14.90 cases per 100,000 population), and Northwest Territories (17.54 cases per 100,000 population) (Table 3).
In May 2017, PulseNet Canada began performing WGS on all Salmonella isolates submitted for routine laboratory-based surveillance, providing highly discriminatory genomic subtype data for outbreak detection and response.
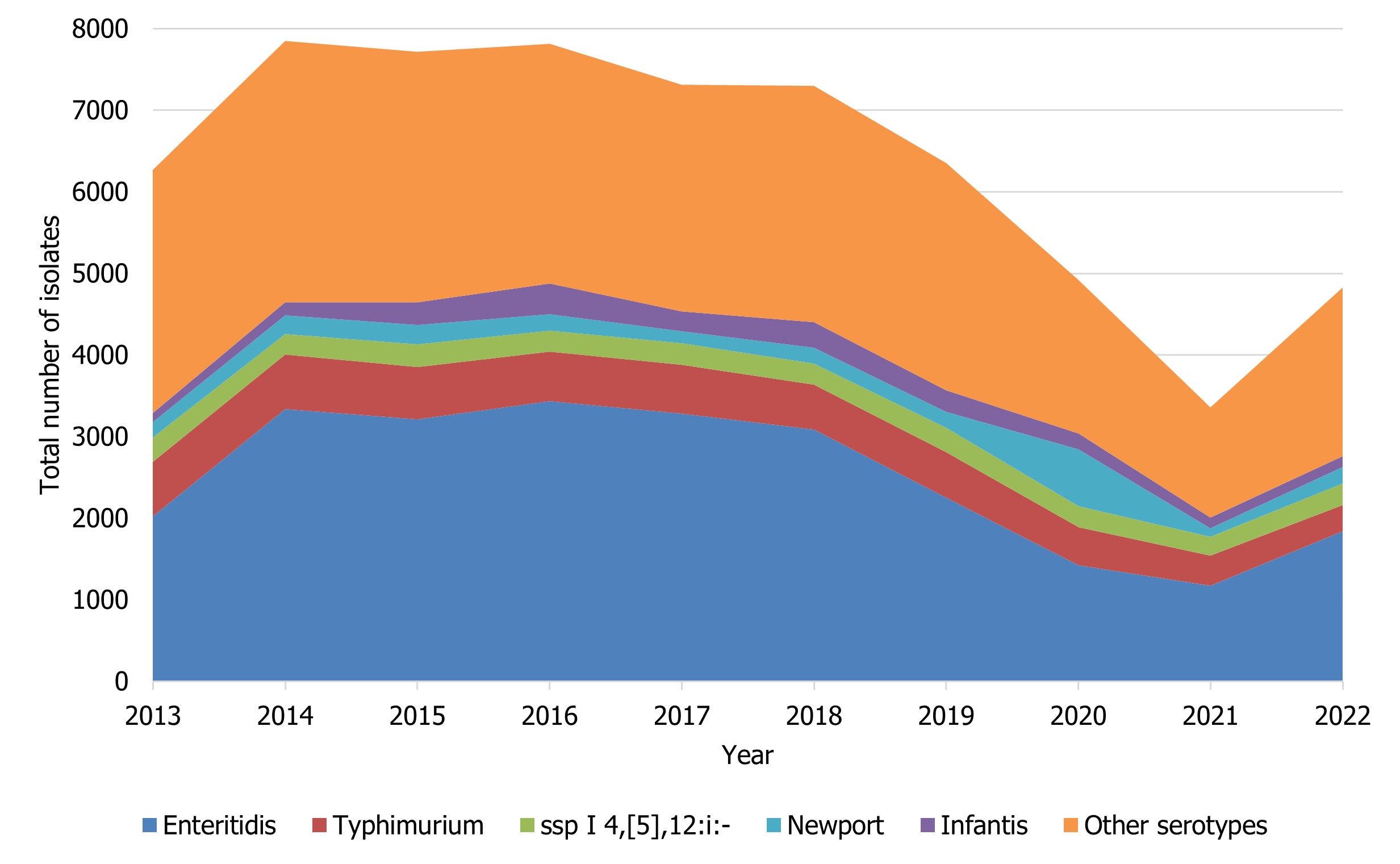
Figure 2 : Descriptive text
| Salmonella serovar | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| Enteritidis | 2019 | 3337 | 3209 | 3433 | 3278 | 3083 | 2254 | 1422 | 1172 | 1840 |
| Typhimurium | 668 | 671 | 642 | 607 | 602 | 551 | 557 | 468 | 370 | 319 |
| ssp I 4,[5],12:i:- | 299 | 251 | 280 | 259 | 265 | 263 | 294 | 256 | 231 | 265 |
| Newport | 189 | 224 | 235 | 198 | 143 | 192 | 200 | 693 | 99 | 202 |
| Infantis | 116 | 164 | 279 | 378 | 244 | 313 | 264 | 198 | 138 | 130 |
| Other serotypes | 2979 | 3203 | 3072 | 2941 | 2781 | 2898 | 2781 | 1882 | 1350 | 2072 |
| Serotypes | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | Average no. of isolates (2017-2021) |
|---|---|---|---|---|---|---|---|
| Enteritidis | 3,278 (1) | 3,083 (1) | 2,254 (1) | 1,422 (1) | 1,172 (1) | 1,840 (1) | 2,242 |
| Typhimurium | 602 (2) | 551 (2) | 557 (2) | 468 (3) | 370 (2) | 319 (2) | 510 |
| ssp I 4,[5],12:i:- | 265 (4) | 263 (5) | 294 (3) | 256 (4) | 231 (3) | 265 (3) | 262 |
| Typhi | 181 (6) | 198 (6) | 232 (6) | 113 (8) | 57 (8) | 248 (4) | 156 |
| Newport | 143 (8) | 192 (7) | 200 (7) | 693 (2) | 99 (5) | 202 (5) | 265 |
| Infantis | 244 (5) | 313 (4) | 264 (5) | 198 (6) | 138 (4) | 130 (6) | 231 |
| Paratyphi A | 62 | 56 | 116 (9) | 59 | 19 | 115 (7) | 62 |
| Javiana | 111 (10) | 118 | 143 (8) | 50 | 49 | 92 (8) | 94 |
| Heidelberg | 444 (3) | 390 (3) | 267 (4) | 207 (5) | 97 (6) | 88 (9) | 281 |
| Stanley | 65 | 101 | 87 | 29 | 31 | 87 (10) | 63 |
| Thompson | 135 (9) | 148 (8) | 98 | 126 (7) | 95 (7) | 85 | 120 |
| Muenchen | 89 | 53 | 80 | 55 | 56 (9) | 44 | 67 |
| Oranienburg | 53 | 113 | 104 (10) | 71 (10) | 55 (10) | 56 | 79 |
| Braenderup | 145 (7) | 127 (9) | 102 | 81 (9) | 42 | 73 | 99 |
| Agona | 103 | 125 (10) | 101 | 35 | 24 | 50 | 78 |
Salmonella Enteritidis
In 2022, 1,840 isolates of S. Enteritidis, 38% of all Salmonella submissions, were reported to NESP. The incidence rate observed in 2022 was 45% lower (4.73 cases per 100,000 population) relative to the 2013-2017 baseline period (8.53 cases per 100,000 population). A general decrease in incidence can be seen from 2018-2021, and the incidence rate in 2022 increased slightly in comparison to 2020 and 2021. However, the rate observed in 2022 remains lower than that seen in 2018 and 2019, suggesting that this is part of an ongoing trend unrelated to the impacts of COVID-19 (Figure 3) and was also likely a continuation of impacts of relatively recent CFIA poultry product regulations6.
Salmonella Typhimurium
Compared to the 2013-2017 baseline period (1.78 cases per 100,000 population), a 54% decrease in the incidence of S. Typhimurium cases was noted in 2022 (0.82 cases per 100,000 population). From 2018-2022, a slight decreasing trend can be seen in the incidence of S. Typhimurium (Figure 3). Although S. Typhimurium continues to rank among the top 3 most common serotypes causing human salmonellosis in Canada, it represents only 6.61% of all Salmonella isolates reported to NESP in 2022 (Figure 1 and Table 5).
Salmonella ssp I 4,[5],12:i:-
Salmonella ssp I 4,[5],12:i:-, for the third time since NESP was launched in 1997 (first time being 2019 and second time being 2021), was the third most common serotype in Canada, representing 5.49% of all human Salmonella isolates reported to NESP in 2022. The 2022 overall incidence (0.68 cases per 100,000 population) was 11% lower than the 2013-2017 baseline period (0.76 cases per 100,000 population).
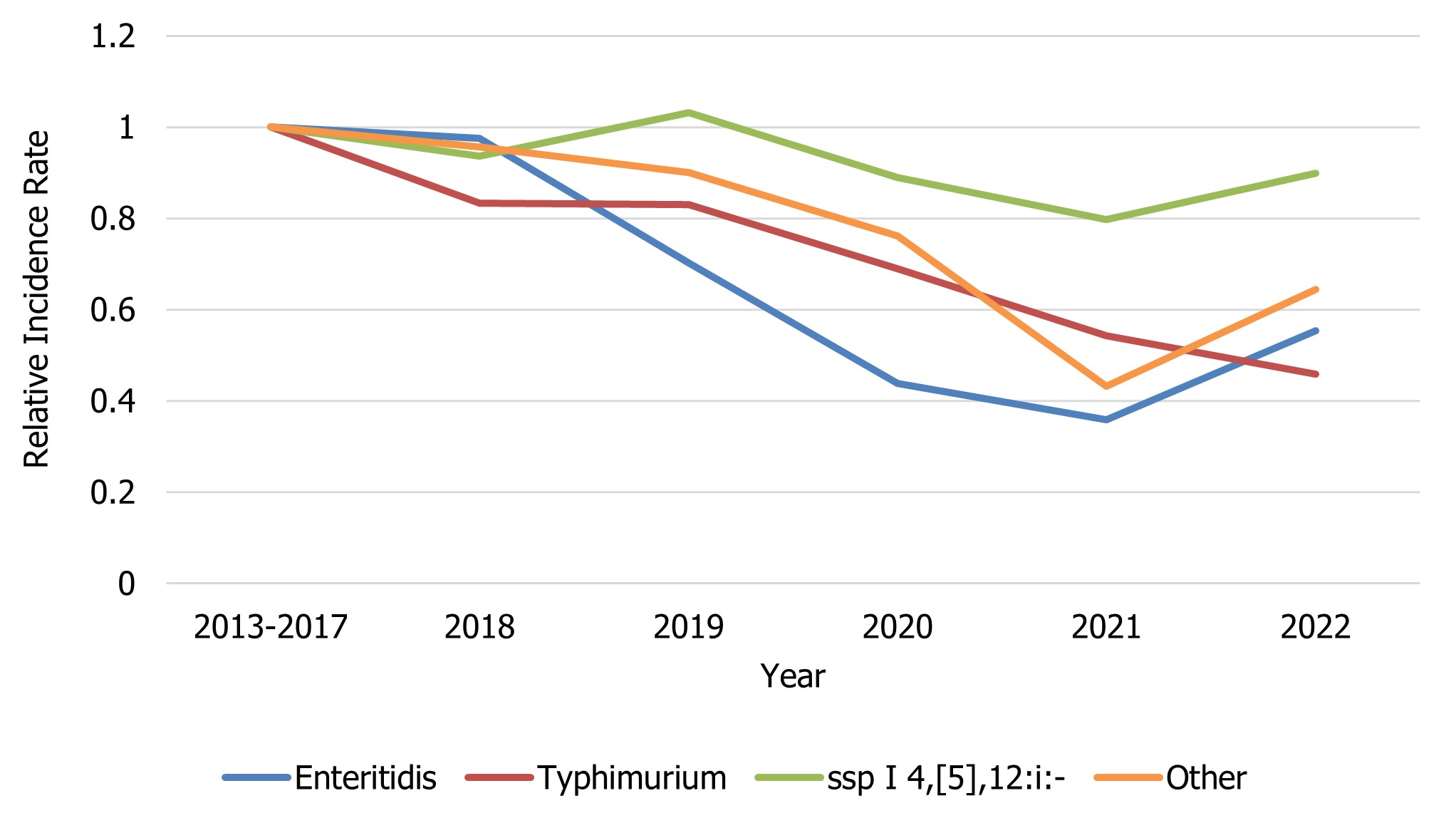
Figure 3 : Descriptive text
| Salmonella serovar | Year | |||||
|---|---|---|---|---|---|---|
| 2013-2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| Enteritidis | 1 | 0.98 | 0.70 | 0.44 | 0.36 | 0.55 |
| Typhimurium | 1 | 0.83 | 0.83 | 0.69 | 0.54 | 0.46 |
| ssp I 4,[5],12:i:- | 1 | 0.94 | 1.03 | 0.89 | 0.80 | 0.90 |
| Other | 1 | 0.96 | 0.90 | 0.76 | 0.43 | 0.64 |
- Footnote 1
-
Rates are compared to the 2013 to 2017 baseline period.
Escherichia coli
Unless otherwise indicated, it is assumed that all E. coli isolates reported to NESP from the provinces and territories are Shiga toxin-producing Escherichia coli (STEC). The 2022 rate of O157 STEC (0.77 cases per 100,000 population) is slightly higher than the 2021 rate of O157 STEC, but lower than the relatively stable rates seen between 2010 to 2019, which is likely due to the impacts of COVID-19 during 2020 to 2022 (Figure 4). In 2022, three provinces reported incidence rates of O157 STEC higher than the national reported incidence rate: Alberta (2.22 cases per 100,000 population), Ontario (0.78 cases per 100,000 population), and Prince Edward Island (2.93 cases per 100,000 population) (Table 3). The incidence rate of non-O157 STEC increased slightly in 2022 (1.32 cases per 100,000 population) from 2021 (0.98 cases per 100,000 population). However, this rate remains lower than in comparison to 2019 (1.58 cases per 100,000 population) likely due to the impacts of the pandemic (Figure 4). Data from 2022 represent the sixth consecutive year where the proportion of non-O157 STEC reported has exceeded the proportion of O157 STEC isolates. It should be noted that non-O157 STECare suspected to be reported less consistently than O157 STEC to NESP due to laboratory testing biases that select for E. coli O157. Therefore, any changes observed over time may also be a reflection in testing practices by some provincial public health laboratoriesFootnote 7.
Further, 178 E. coli cases reported to NESP by 4 provinces were identified using culture-independent diagnostic tests (CIDT), and were not later updated with reflex culture, as seen in Appendix B. CIDTs can detect a specific antigen or genetic sequence of the organism, without isolating or culturing the living organismFootnote 8. According to national guidanceFootnote 9, reflex cultures are to be obtained from CIDT-positive samples for public health and clinical management, especially when the Shiga toxin type is unknown (i.e., unable to differentiate between stx1 and stx2). However, sometimes organisms may not grow upon reflex culture. Reflex culture of a CIDT-positive sample can help obtain an isolate for further sub-typing, which would be updated in NESP.
Among non-O157 STEC isolates serotyped in 2022, 52% of these were represented by five O-antigen serogroups: O26, O103, O121, O111, and O5 (Figure 5). In 2022, 20% of non-O157 STEC did not have additional serotype information.
Of the top 5 serogroups from the broader list of the non-O157 STEC isolates where a serotype result was available, O26, O111 and O5 showed an increased rate per 100,000 population in 2022 compared to 2021. With the exception of O121 and O5, the top five serogroups among serotyped E. coli isolates showed a decreased rate in 2022 compared to 2019, likely due to the impacts of COVID-19 (Figure 6). All E. coli serotypes, including confirmed non-O157 STEC isolates, and any other reported pathotypes are summarized in Appendix B.
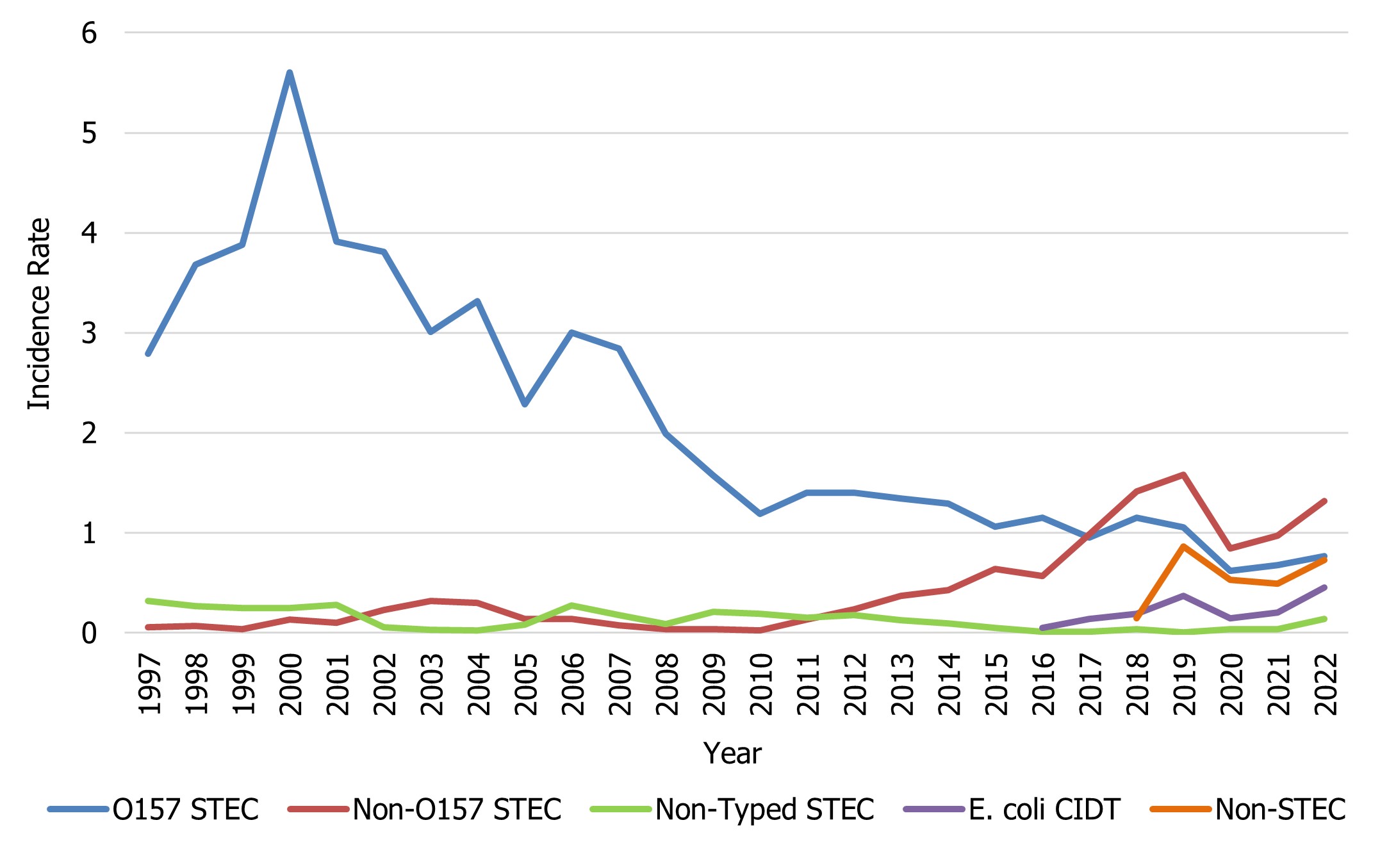
Figure 4 : Descriptive text
| E. coli group | Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
| O157 STEC | 2.79 | 3.68 | 3.88 | 5.60 | 3.91 | 3.81 | 3.01 | 3.32 | 2.28 | 3.00 | 2.84 | 1.99 | 1.57 |
| Non-O157 STEC | 0.06 | 0.07 | 0.04 | 0.13 | 0.10 | 0.23 | 0.32 | 0.30 | 0.14 | 0.14 | 0.08 | 0.04 | 0.04 |
| Non-Typed STEC | 0.32 | 0.27 | 0.25 | 0.25 | 0.28 | 0.05 | 0.03 | 0.03 | 0.08 | 0.28 | 0.18 | 0.09 | 0.21 |
| E. coli CIDT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Non-STEC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. coli group | Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| O157 STEC | 1.19 | 1.40 | 1.40 | 1.35 | 1.29 | 1.06 | 1.15 | 0.95 | 1.15 | 1.06 | 0.62 | 0.68 | 0.77 |
| Non-O157 STEC | 0.02 | 0.13 | 0.24 | 0.37 | 0.43 | 0.64 | 0.57 | 0.99 | 1.42 | 1.58 | 0.84 | 0.98 | 1.32 |
| Non-Typed STEC | 0.19 | 0.15 | 0.18 | 0.13 | 0.10 | 0.05 | 0.01 | 0.01 | 0.04 | 0.01 | 0.04 | 0.04 | 0.14 |
| E. coli CIDT | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0.14 | 0.19 | 0.37 | 0.14 | 0.20 | 0.46 |
| Non-STEC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.15 | 0.87 | 0.53 | 0.49 | 0.73 |
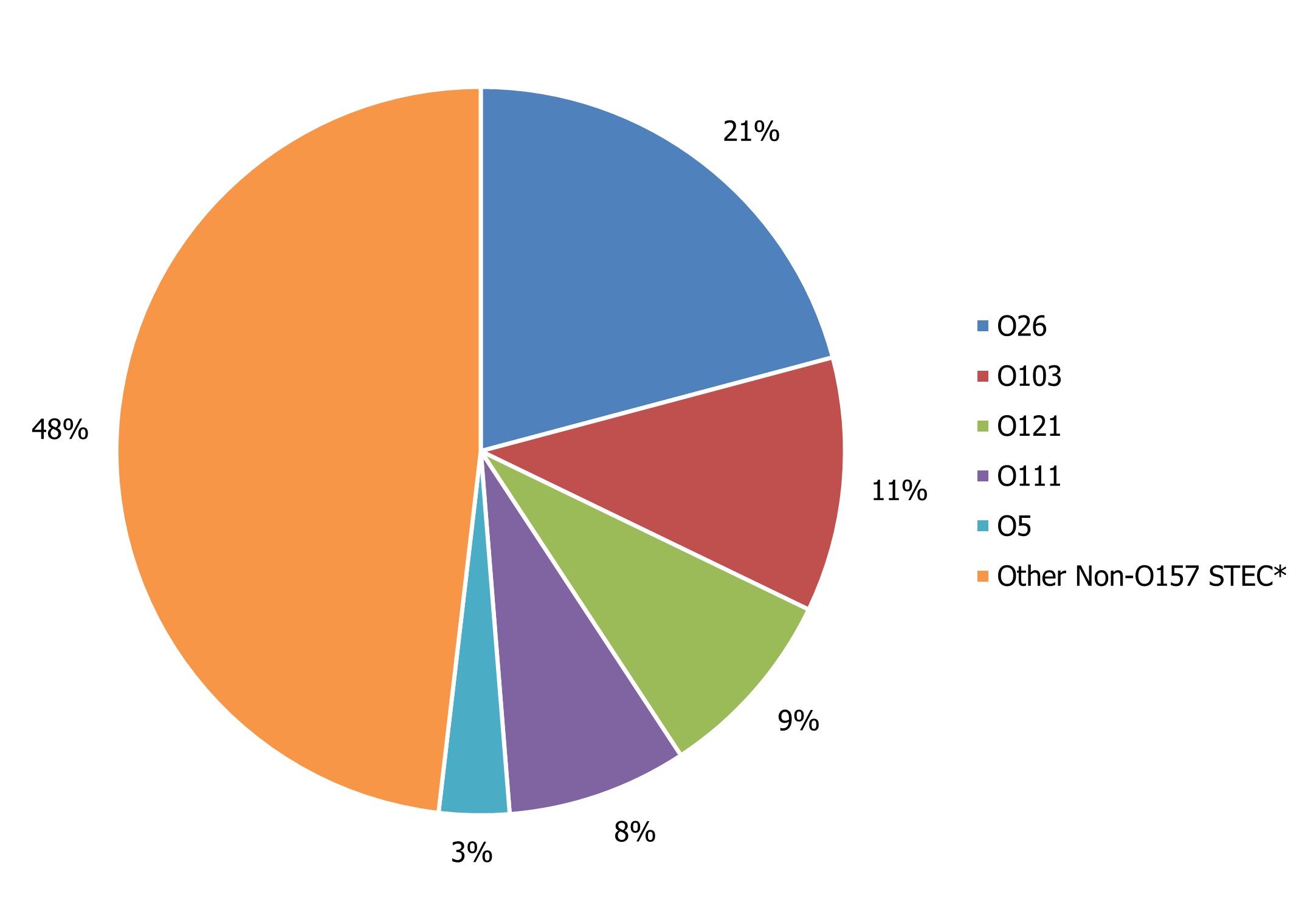
Figure 5 : Descriptive text
| Non-O157 serotype | Percentage of total non-O157 STEC |
|---|---|
| O26 | 21% |
| O103 | 11% |
| O121 | 9% |
| O111 | 8% |
| O5 | 3% |
| Other Non-O157 STECFootnote * | 48% |
|
|
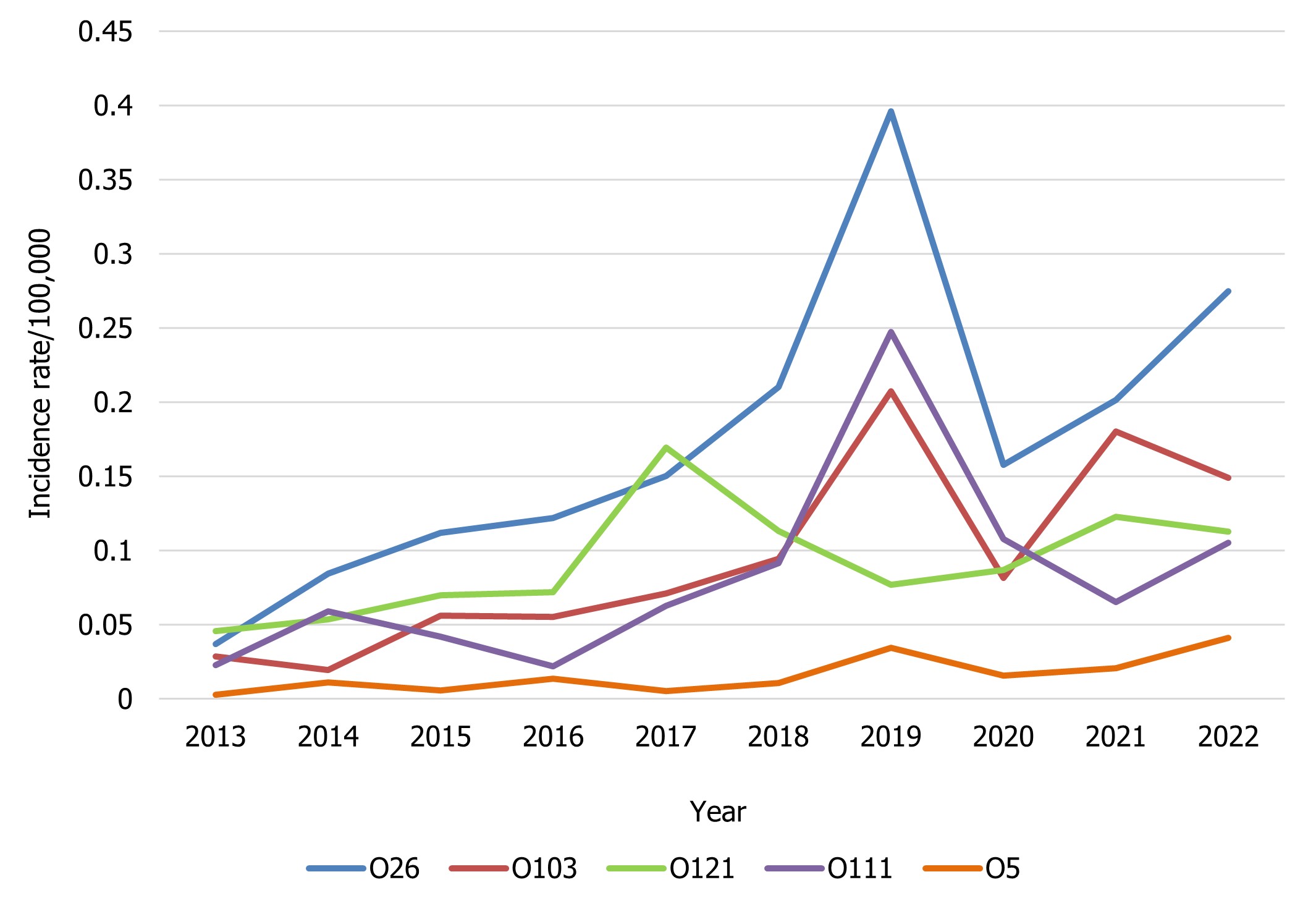
Figure 6 : Descriptive text
| Non-O157 STEC serotype | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| O26 | 0.04 | 0.08 | 0.11 | 0.12 | 0.15 | 0.21 | 0.40 | 0.16 | 0.20 | 0.27 |
| O103 | 0.03 | 0.02 | 0.06 | 0.06 | 0.07 | 0.09 | 0.21 | 0.08 | 0.18 | 0.15 |
| O121 | 0.05 | 0.05 | 0.07 | 0.07 | 0.17 | 0.11 | 0.08 | 0.09 | 0.12 | 0.11 |
| O111 | 0.02 | 0.06 | 0.04 | 0.02 | 0.06 | 0.09 | 0.25 | 0.11 | 0.07 | 0.11 |
| O5 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.02 | 0.04 |
Listeria monocytogenes
As per the case definition for invasive listeriosis, only isolates obtained from a normally sterile site or placental/fetal tissues should be reported. The national incidence rate of Listeria monocytogenes increased in 2022 (0.47 cases per 100,000 population) compared to 2021 (0.40 cases per 100,000 population). However, the 2022 rate is similar to the rate seen in 2019 pre-COVID-19 pandemic (0.46 cases per 100,000 population). As there are small numbers of cases of invasive listeriosis within most jurisdictions, the magnitude of the change is greatly affected with a difference of even one case (Figure 7). There remain wide differences in the incidence rate of invasive listeriosis across the country, with some provinces reporting an incidence rate more than ten times that of others. In 2022, five provinces reported incidence rates of Listeria monocytogenes higher than the national reported incidence rate: Québec (0.68 cases per 100,000 population), New Brunswick (0.49 cases per 100,000 population), Nova Scotia (1.18 cases per 100,000 population), Prince Edward Island (0.59 cases per 100,000 population), and Newfoundland and Labrador (0.76 cases per 100,000 population) (Table 3).
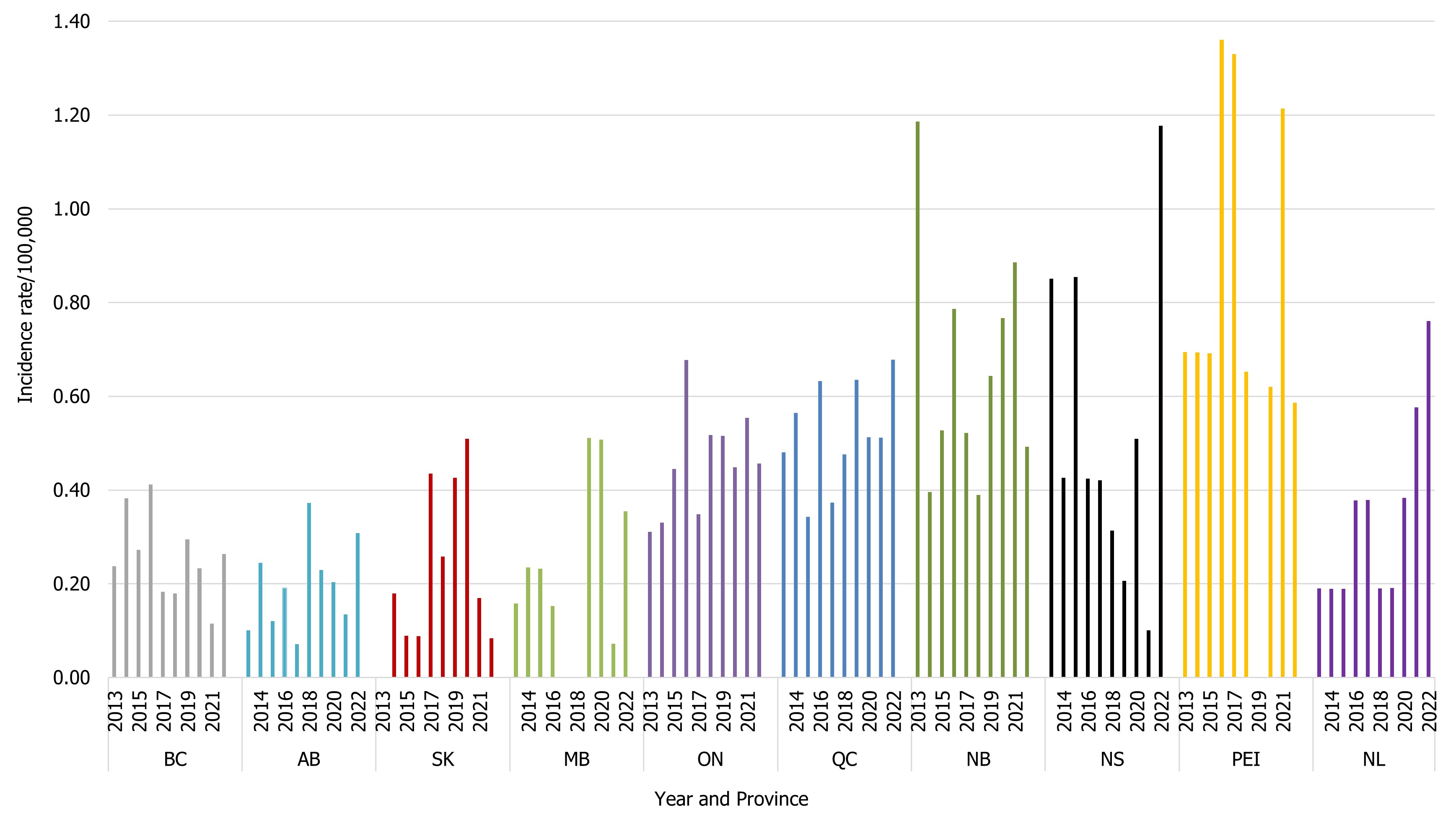
Figure 7 : Descriptive text
| ProvinceFootnote 1 | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| BC | 0.24 | 0.38 | 0.27 | 0.41 | 0.18 | 0.18 | 0.29 | 0.23 | 0.12 | 0.26 |
| AB | 0.10 | 0.24 | 0.12 | 0.19 | 0.07 | 0.37 | 0.23 | 0.20 | 0.14 | 0.31 |
| SK | 0 | 0.18 | 0.09 | 0.09 | 0.43 | 0.26 | 0.43 | 0.51 | 0.17 | 0.08 |
| MB | 0.16 | 0.23 | 0.23 | 0.15 | 0 | 0 | 0.51 | 0.51 | 0.07 | 0.35 |
| ON | 0.31 | 0.33 | 0.45 | 0.68 | 0.35 | 0.52 | 0.52 | 0.45 | 0.55 | 0.46 |
| QC | 0.48 | 0.56 | 0.34 | 0.63 | 0.37 | 0.48 | 0.64 | 0.51 | 0.51 | 0.68 |
| NB | 1.19 | 0.40 | 0.53 | 0.79 | 0.52 | 0.39 | 0.64 | 0.77 | 0.89 | 0.49 |
| NS | 0.85 | 0.43 | 0.85 | 0.42 | 0.42 | 0.31 | 0.21 | 0.51 | 0.10 | 1.18 |
| PEI | 0.69 | 0.69 | 0.69 | 1.36 | 1.33 | 0.65 | 0 | 0.62 | 1.21 | 0.59 |
| NL | 0.19 | 0.19 | 0.19 | 0.38 | 0.38 | 0.19 | 0.19 | 0.38 | 0.58 | 0.76 |
- Footnote 1
-
There were no cases of invasive listeriosis reported in 2022 by Yukon, Northwest Territories, and Nunavut.
Shigella
There were 978 isolates of Shigella reported in 2022, representing a rate of 2.51 cases per 100,000 population which is over double the 2021 rate of 1.09 cases per 100,000 population. This rate is higher than the average of 2.11 cases per 100,000 population reported between 2015 and 2019 (Figure 8). In 2022, two provinces reported an incidence rate of Shigella higher than the national reported incidence rate: British Columbia with 5.72 cases per 100,000 population and Alberta with 6.05 cases per 100,000 population.
Isolates of Shigella sonnei and Shigella flexneri comprised 38% and 58% of total notifications respectively. Overall trends for Shigella have historically been driven by the incidence of S. sonnei (0.96 cases per 100,000 population in 2022). However, the rate of S. flexneri (1.45 cases per 100,000 population in 2022) surpassed that of S. sonnei in 2020 and has remained higher since then (Figure 8). Among the other Shigella species, incidence trends over time have remained relatively unchanged with an incidence of 0.05 cases per 100,000 population for Shigella boydii and 0.02 cases per 100,000 population for Shigella dysenteriae observed in 2022 (Figure 8). Rates of all species of Shigella increased in 2022 compared to 2020 and 2021, likely due to the impacts of COVID-19 (Figure 8).
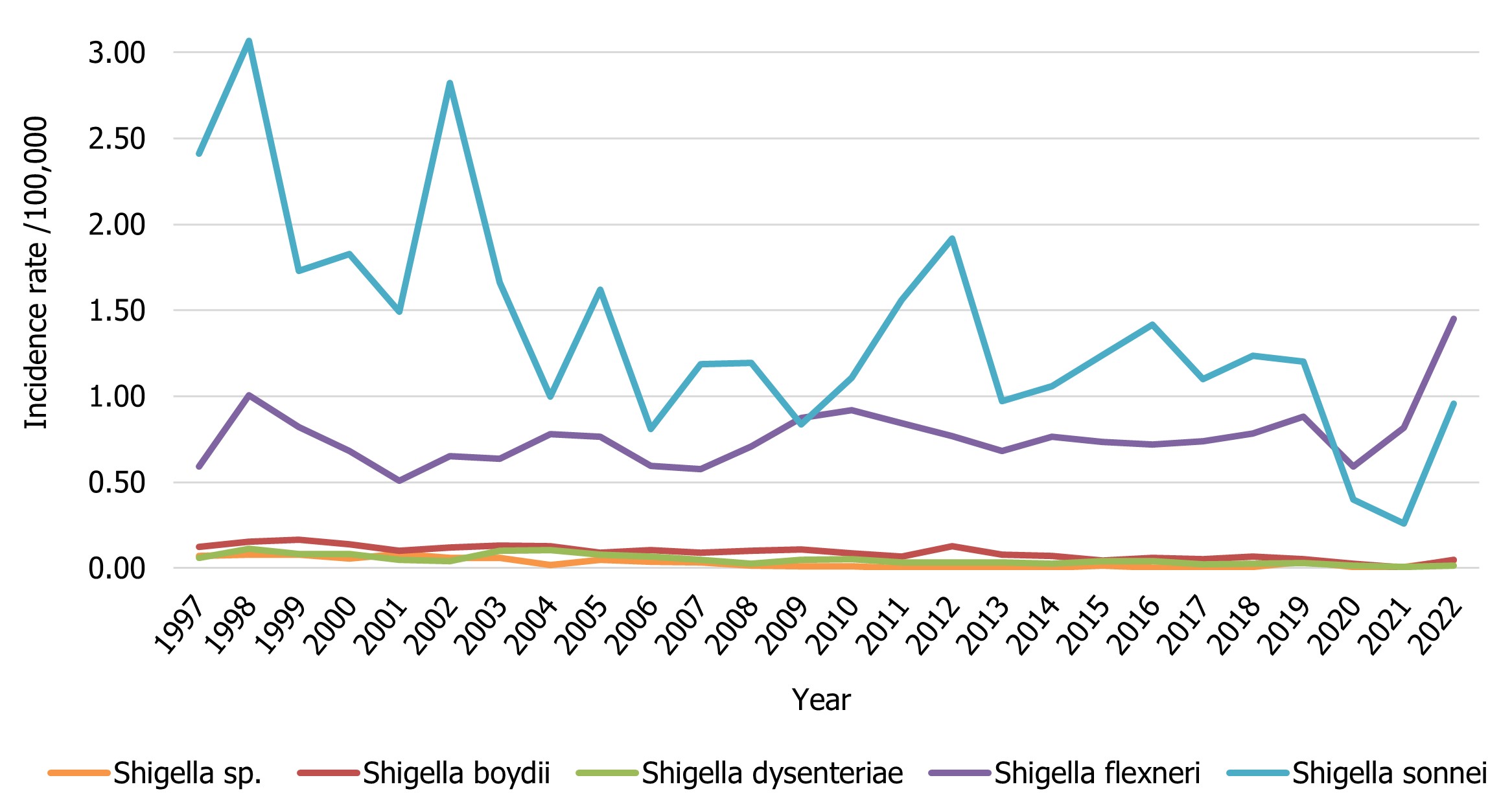
Figure 8 : Descriptive text
| Shigella group | Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
| Shigella sp. | 0.07 | 0.08 | 0.08 | 0.06 | 0.08 | 0.06 | 0.06 | 0.02 | 0.05 | 0.04 | 0.03 | 0.02 | 0.01 |
| Shigella boydii | 0.12 | 0.15 | 0.16 | 0.14 | 0.10 | 0.12 | 0.13 | 0.13 | 0.09 | 0.10 | 0.09 | 0.10 | 0.11 |
| Shigella dysenteriae | 0.06 | 0.11 | 0.08 | 0.08 | 0.05 | 0.04 | 0.10 | 0.11 | 0.08 | 0.07 | 0.05 | 0.03 | 0.05 |
| Shigella flexneri | 0.59 | 1.00 | 0.82 | 0.68 | 0.51 | 0.65 | 0.64 | 0.78 | 0.76 | 0.60 | 0.57 | 0.71 | 0.87 |
| Shigella sonnei | 2.41 | 3.07 | 1.73 | 1.83 | 1.49 | 2.82 | 1.66 | 1.00 | 1.62 | 0.81 | 1.19 | 1.19 | 0.84 |
| Shigella group | Year | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| Shigella sp. | 0.01 | 0 | 0 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.04 | 0.01 | 0.01 | 0.04 |
| Shigella boydii | 0.09 | 0.07 | 0.13 | 0.08 | 0.07 | 0.04 | 0.06 | 0.05 | 0.07 | 0.05 | 0.03 | 0.00 | 0.05 |
| Shigella dysenteriae | 0.05 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | 0.04 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 |
| Shigella flexneri | 0.92 | 0.84 | 0.77 | 0.68 | 0.76 | 0.73 | 0.72 | 0.74 | 0.78 | 0.88 | 0.59 | 0.82 | 1.45 |
| Shigella sonnei | 1.11 | 1.56 | 1.92 | 0.97 | 1.06 | 1.24 | 1.42 | 1.10 | 1.24 | 1.20 | 0.40 | 0.26 | 0.96 |
Hepatitis A
The national incidence rate for Hepatitis A in 2022 was higher than in 2021 (0.80 cases per 100,000 population in 2022 compared to 0.69 in 2021). However, this is still lower than the 2019 rate (1.30 cases per 100,000 population) (Figure 9). In 2022, three provinces reported incidence rates of Hepatitis A higher than the national reported incidence rate: Alberta (1.76 cases per 100,000 population), Manitoba (1.17 cases per 100,000 population), and Saskatchewan (2.13 cases per 100,000 population) (Figure 9).
Each provincial and territorial laboratory determines whether to report a case based solely on laboratory IgM testing, without public health follow-up. Considering that IgM testing can result in false positive results or indicate recent immunization, positive results are further investigated by local public health to determine if the case meets the definition of a "confirmed case". If the case does not meet this definition upon follow-up, the data is not always relayed back to the laboratory. Therefore, Hepatitis A data reported through NESP would not be corrected in this scenario and may result in over-reporting of confirmed cases for this virus. Hepatitis A rates appear to be increasing in some provinces since 2018, as seen in Figure 9. These increases observed in Figure 9 could be a result of change of laboratory detection methods, or over-reporting. Conversely, since not all specimens are referred from the regional and local laboratories to the provincial public health laboratories, viruses, including Hepatitis A,are under-represented in NESP and reported case counts are not representative of the true incidence of the disease in Canada.
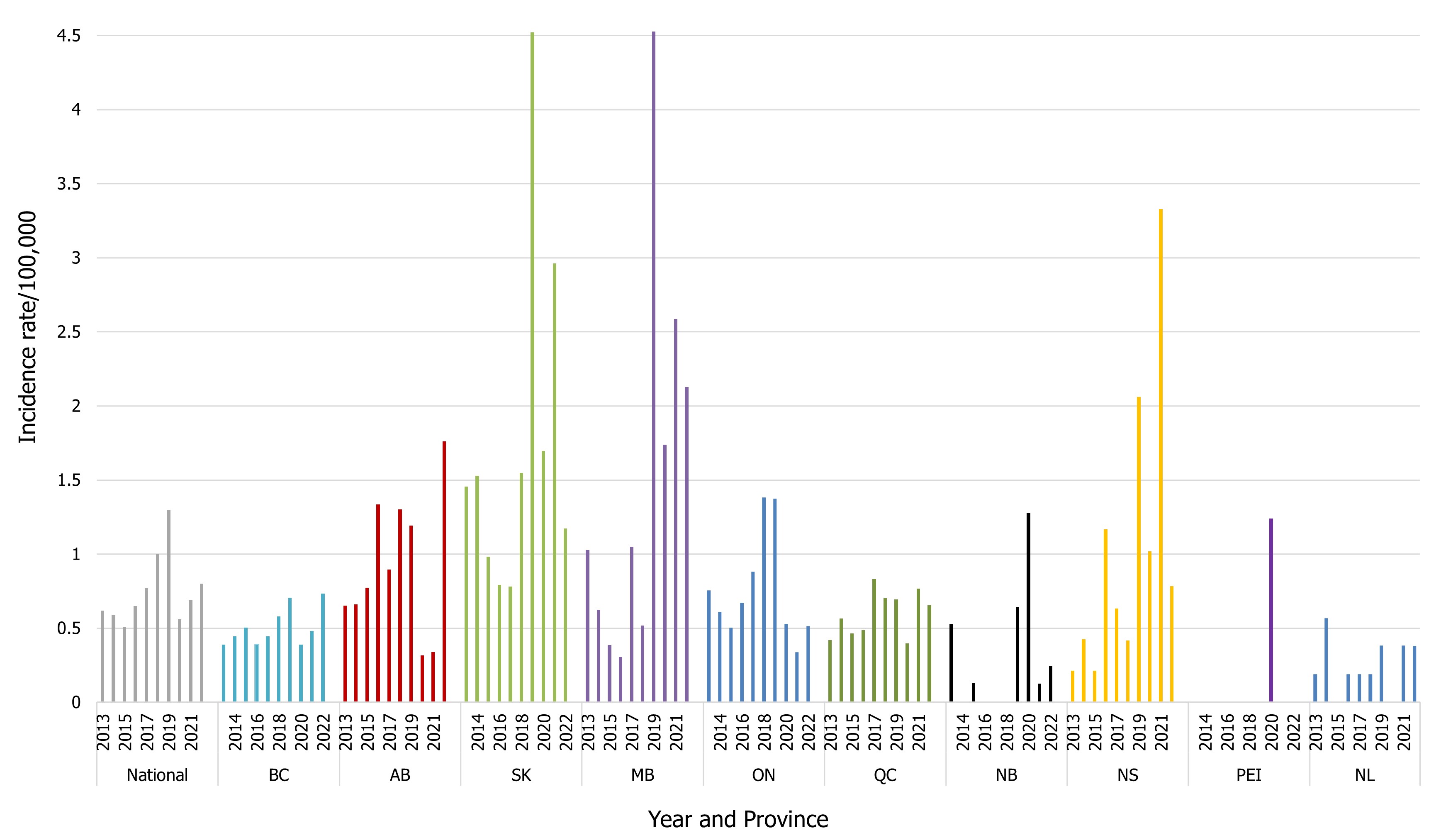
Figure 9 : Descriptive text
| Province | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
| CAD | 0.62 | 0.59 | 0.51 | 0.65 | 0.77 | 1.00 | 1.3 | 0.56 | 0.69 | 0.8 |
| BC | 0.39 | 0.45 | 0.50 | 0.39 | 0.45 | 0.58 | 0.71 | 0.39 | 0.48 | 0.73 |
| AB | 0.65 | 0.66 | 0.77 | 1.33 | 0.90 | 1.30 | 1.19 | 0.32 | 0.34 | 1.76 |
| SK | 1.45 | 1.53 | 0.98 | 0.79 | 0.78 | 1.55 | 4.52 | 1.70 | 2.96 | 1.17 |
| MB | 1.03 | 0.63 | 0.39 | 0.30 | 1.05 | 0.52 | 4.53 | 1.74 | 2.59 | 2.13 |
| ON | 0.75 | 0.61 | 0.50 | 0.67 | 0.88 | 1.38 | 1.38 | 0.53 | 0.34 | 0.52 |
| QC | 0.42 | 0.56 | 0.46 | 0.49 | 0.83 | 0.70 | 0.69 | 0.40 | 0.77 | 0.66 |
| NB | 0.53 | 0 | 0.13 | 0 | 0 | 0 | 0.64 | 1.28 | 0.13 | 0.25 |
| NS | 0.21 | 0.43 | 0.21 | 1.17 | 0.63 | 0.42 | 2.06 | 1.02 | 3.33 | 0.78 |
| PEI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.24 | 0 | 0 |
| NL | 0.19 | 0.57 | 0 | 0.19 | 0.19 | 0.19 | 0.38 | 0 | 0.38 | 0.38 |
Appendix A. Canadian Notifiable Disease Surveillance System (CNDSS) and the National Enteric Surveillance Program (NESP)
| Enteric, Food and Waterborne Diseases | Canadian Notifiable Disease Surveillance System (CNDSS) | National Enteric Surveillance Program (NESP) | % of CNDSS cases captured in NESP (NESP isolations / CNDSS casesFootnote 7) | ||
|---|---|---|---|---|---|
| 2021 | N | Rate per 100,000 population |
N | Rate per 100,000 population |
|
| Botulism | 6 | 0.02 | - | - | N/A |
| CampylobacteriosisFootnote 3 | 7,796 | 20.39 | 1,255 | - | 16.1 |
| CholeraFootnote 4 | 2 | 0.01 | 1 | 0.003 | 50.0 |
| CryptosporidiosisFootnote 3 | 963 | 2.52 | 307 | - | 31.9 |
| CyclosporiasisFootnote 3 | 121 | 0.32 | 32 | - | 26.4 |
| GiardiasisFootnote 3 | 2,320 | 6.07 | 515 | - | 22.2 |
| Hepatitis A | 177 | 0.46 | 263 | 0.69 | 148.67 |
| Invasive Listeriosis | 158 | 0.41 | 154 | 0.40 | 97.5 |
| NorovirusFootnote 3Footnote 5 | 199 | 4.4 | 443 | - | N/A |
| Paralytic Shellfish Poisoning | 0 | 0 | - | - | N/A |
| Salmonellosis | 3,318 | 8.68 | 3,303 | 8.64 | 99.5 |
| Shigellosis | 492 | 1.29 | 416 | 1.09 | 84.6 |
| TyphoidFootnote 6 | 57 | 0.15 | 57 | 0.15 | 100.0 |
| Shiga toxigenic Escherichia coli Infection | 772 | 2.02 | 633Footnote 8 | 1.66 | 82.0 |
|
|||||
Appendix B. Species and serotype data reported to NESP by province and territory, 2022
Cases visiting a different province or territory are captured in the total count for the province or territory where the case was detected.
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Campylobacter coli | 3 | 10 | 6 | 9 | 7 | 21 | 9 | 2 | 0 | 0 | 1 | 0 | 0 | 68 |
| Campylobacter concisus | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Campylobacter fetus | 1 | 0 | 1 | 0 | 8 | 5 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 17 |
| Campylobacter gracilis | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Campylobacter hyointestinalis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Campylobacter jejuni | 1 | 147 | 112 | 126 | 47 | 130 | 125 | 73 | 31 | 0 | 0 | 0 | 0 | 792 |
| Campylobacter lari | 0 | 0 | 0 | 0 | 2 | 7 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 13 |
| Campylobacter rectus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Campylobacter sp | 0 | 0 | 0 | 0 | 0 | 2 | 45 | 1 | 0 | 105 | 0 | 0 | 0 | 153 |
| Campylobacter sputorum | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Campylobacter upsaliensis | 0 | 5 | 0 | 0 | 5 | 6 | 6 | 5 | 0 | 0 | 0 | 0 | 0 | 27 |
| Campylobacter ureolyticus | 1 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Total Campylobacter | 6 | 164 | 119 | 135 | 69 | 186 | 187 | 84 | 32 | 105 | 1 | 0 | 0 | 1,088 |
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli CIDT Positive for STX/STEC | 27 | 0 | 0 | 78 | 0 | 71 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 178 |
| Non-O157 STEC | 2 | 0 | 49 | 36 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 93 |
| E. coli Non-Typed EAEC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 0 | 0 | 0 | 31 |
| E. coli Non-Typed EPEC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 222 | 0 | 0 | 0 | 222 |
| E. coli Non-Typed ETEC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 30 | 0 | 0 | 0 | 31 |
| Non-Typed STEC | 0 | 0 | 0 | 38 | 0 | 0 | 2 | 0 | 0 | 12 | 0 | 0 | 0 | 52 |
| E. coli O undetermined:H Rough | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O undetermined:H2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| E. coli O undetermined:H34 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O undetermined:H45 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O-Rough:HNM | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O-Rough:H2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O1:H20 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O5:H9 | 2 | 9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| E. coli O5:H19 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O5:HNM | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| E. coli O6:H1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O7:H18 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O8:H8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O8:H9 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O8:H19 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O17:H45 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| E. coli O24:H30 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O25:H12 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O26 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O26:H11 | 27 | 62 | 0 | 0 | 3 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| E. coli O26:HNM | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| E. coli O34:H40 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O38:H21 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O41:H19 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O44:H18 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O45:H2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O45:HNM | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O49:HNM | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O52:H45 | 0 | 1 | 0 | 0 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| E. coli O68:H18 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O69:H11 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O71:H2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O71:H8 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O71:H11 | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| E. coli O73:H18 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O75:H38 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O76:HNM | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O81:H21 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O84:H2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O84:HNM | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O85:H2 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| E. coli O85:H4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O87:H16 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O88:H25 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O91:H14 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| E. coli O91:H21 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O93:H46 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O98:HNM | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O100:H20 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O103:H2 | 3 | 21 | 0 | 0 | 10 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 39 |
| E. coli O103:H11 | 0 | 4 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| E. coli O103:H25 | 0 | 7 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| E. coli O103:HNM | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O111:H8 | 2 | 22 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 |
| E. coli O111:HNM | 3 | 6 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| E. coli O113:H4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O116:H21 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O116:H49 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O117:H7 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| E. coli O117:HNM | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O118:H2 | 0 | 4 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| E. coli O118:H16 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O119:H4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O121:H19 | 7 | 29 | 0 | 0 | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43 |
| E. coli O121:HNM | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O123:H11 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O128:H2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O128ab:H2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O130:H11 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O136:H12 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O140:H45 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O145:H7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O145:H12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O145:H undetermined | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O145:HNM | 1 | 5 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| E. coli O146:H21 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| E. coli O146:HNM | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O150:H8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O156:H25 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O156:HNM | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O157 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 10 |
| E. coli O157:H7 | 12 | 99 | 9 | 0 | 114 | 42 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 282 |
| E. coli O157:H16 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| E. coli O157:HNM | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| E. coli O166:H15 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O168:H8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O169:H8 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O169:H9 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O174:H21 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O177:H11 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O177:H25 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| E. coli O177:H45 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O177:HNM | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O178:H19 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O181:H49 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O183:H18 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O186:H2 | 2 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| E. coli O187:H52 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O2/O50:H4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O123/O186:H2 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| E. coli O123:H2/O186:H2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E. coli O151/O118:H2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O151/O118:H16 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Total E. coli | 133 | 325 | 58 | 156 | 183 | 154 | 17 | 1 | 5 | 296 | 0 | 0 | 0 | 1,328 |
|
||||||||||||||
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Listeria monocytogenes | 14 | 14 | 1 | 5 | 69 | 59 | 4 | 12 | 1 | 4 | 0 | 0 | 0 | 183 |
| Total Listeria | 14 | 14 | 1 | 5 | 69 | 59 | 4 | 12 | 1 | 4 | 0 | 0 | 0 | 183 |
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryptosporidium | 7 | 0 | 17 | 16 | 146 | 0 | 34 | 7 | 13 | 20 | 0 | 0 | 0 | 260 |
| Cyclospora | 4 | 3 | 0 | 0 | 57 | 0 | 0 | 1 | 0 | 7 | 0 | 0 | 0 | 72 |
| Entamoeba histolytica/dispar | 93 | 3 | 9 | 0 | 83 | 0 | 0 | 1 | 0 | 0 | 8 | 0 | 0 | 197 |
| Giardia | 43 | 1 | 62 | 51 | 146 | 0 | 68 | 59 | 6 | 52 | 13 | 0 | 0 | 501 |
| Total Parasites | 147 | 7 | 88 | 67 | 432 | 0 | 102 | 68 | 19 | 79 | 21 | 0 | 0 | 1,030 |
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella Aberdeen | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Salmonella Abony | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Adelaide | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Agbeni | 0 | 0 | 0 | 0 | 4 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 8 |
| Salmonella Agona | 11 | 7 | 0 | 0 | 24 | 5 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 50 |
| Salmonella Agoueve | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Alachua | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Albany | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Altona | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 |
| Salmonella Amager | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Amsterdam | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Anatum | 4 | 3 | 0 | 1 | 6 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 |
| Salmonella Arechavaleta | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Bareilly | 9 | 3 | 1 | 0 | 12 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 31 |
| Salmonella Benin | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Berta | 0 | 1 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Salmonella Bonariensis | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Bonn | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Bovismorbificans | 2 | 2 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Salmonella Braenderup | 11 | 11 | 3 | 4 | 32 | 11 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 73 |
| Salmonella Brancaster | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Brandenburg | 11 | 3 | 2 | 2 | 23 | 8 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 52 |
| Salmonella Bredeney | 1 | 1 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Salmonella Cerro | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Chester | 0 | 0 | 0 | 0 | 7 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Salmonella Choleraesuis | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella Choleraesuis var Kunzendorf | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Colindale | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Concord | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Corvallis | 2 | 2 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Salmonella Cotham | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Cubana | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Daytona | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Derby | 2 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella Dublin | 1 | 2 | 1 | 0 | 6 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 |
| Salmonella Durban | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella Durham | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Ealing | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Eastbourne | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Emek | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Enteritidis | 365 | 221 | 34 | 32 | 661 | 380 | 78 | 43 | 4 | 20 | 0 | 2 | 0 | 1,840 |
| Salmonella Florida | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Gaminara | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella Gateshead | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Gatuni | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Give | 2 | 1 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Salmonella Glostrup | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Goldcoast | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Hadar | 16 | 5 | 1 | 0 | 25 | 12 | 2 | 2 | 0 | 4 | 0 | 0 | 0 | 67 |
| Salmonella Haifa | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Salmonella Hartford | 0 | 1 | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Salmonella Hato | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Havana | 3 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Salmonella Heidelberg | 5 | 5 | 4 | 0 | 34 | 32 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 88 |
| Salmonella Hull | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Hvittingfoss | 3 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Salmonella Idikan | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Indiana | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Infantis | 11 | 16 | 6 | 4 | 56 | 26 | 5 | 4 | 1 | 1 | 0 | 0 | 0 | 130 |
| Salmonella Isangi | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Itami | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Ivrysurseine | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Javiana | 8 | 10 | 0 | 4 | 39 | 26 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 92 |
| Salmonella Kaevlinge | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Kedougou | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Kentucky | 4 | 1 | 1 | 1 | 6 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 |
| Salmonella Kiambu | 0 | 2 | 1 | 0 | 7 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 12 |
| Salmonella Kisii | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Kottbus | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella Linton | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Litchfield | 1 | 0 | 0 | 1 | 11 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 |
| Salmonella Liverpool | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Livingstone | 0 | 1 | 0 | 0 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Salmonella Llandoff | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Lomalinda | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Lome | 1 | 1 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella London | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Manhattan | 1 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Salmonella Mbandaka | 0 | 1 | 2 | 1 | 15 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 |
| Salmonella Meleagridis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Menston | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella Miami | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Michigan | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Mikawasima | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Minnesota | 2 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella Mishmarhaemek | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Mississippi | 0 | 1 | 0 | 0 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Salmonella Molade | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Montevideo | 5 | 2 | 0 | 3 | 16 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Salmonella Mountpleasant | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Muenchen | 2 | 6 | 2 | 0 | 22 | 5 | 1 | 4 | 1 | 1 | 0 | 0 | 0 | 44 |
| Salmonella Muenster | 2 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella Napoli | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Nessziona | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Newport | 39 | 37 | 2 | 4 | 86 | 30 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 202 |
| Salmonella Norwich | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella Ohio | 0 | 1 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Salmonella Oranienburg | 5 | 13 | 0 | 0 | 24 | 12 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 56 |
| Salmonella Orientalis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Orion | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Oslo | 2 | 0 | 0 | 0 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| Salmonella Panama | 8 | 3 | 0 | 0 | 15 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 |
| Salmonella Papuana | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Paratyphi A | 26 | 15 | 2 | 5 | 60 | 5 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 115 |
| Salmonella Paratyphi B | 2 | 1 | 1 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Salmonella Paratyphi B var. Java | 11 | 4 | 1 | 0 | 13 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 36 |
| Salmonella Pomona | 2 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella Poona | 1 | 2 | 0 | 3 | 7 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 |
| Salmonella Presov | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Putten | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella Reading | 11 | 16 | 10 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 43 |
| Salmonella Remiremont | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Rissen | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Rubislaw | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Saintpaul | 12 | 7 | 2 | 1 | 30 | 19 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 73 |
| Salmonella Sandiego | 2 | 3 | 1 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Salmonella Sandiego/Chester | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella Sangera | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Saphra | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Schwarzengrund | 3 | 2 | 1 | 1 | 5 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 14 |
| Salmonella Senftenberg | 5 | 8 | 1 | 0 | 6 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 21 |
| Salmonella Shubra | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Singapore | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Soerenga | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Stanley | 9 | 6 | 44 | 2 | 17 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 87 |
| Salmonella Stanleyville | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Salmonella Strathcona | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Takoradi | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Telelkebir | 0 | 0 | 0 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella Tennessee | 2 | 3 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 |
| Salmonella Thompson | 4 | 3 | 0 | 1 | 57 | 16 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 85 |
| Salmonella Tilene | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Tudu | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Typhi | 46 | 33 | 6 | 10 | 134 | 13 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 248 |
| Salmonella Typhimurium | 29 | 66 | 7 | 10 | 113 | 81 | 3 | 7 | 0 | 2 | 0 | 1 | 0 | 319 |
| Salmonella Uganda | 2 | 1 | 0 | 0 | 12 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 18 |
| Salmonella Urbana | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Salmonella Veneziana | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Virchow | 2 | 2 | 0 | 1 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Salmonella Vitkin | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella Wandsworth | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Waral | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Waycross | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Weltevreden | 4 | 1 | 0 | 0 | 15 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| Salmonella Widemarsh | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella Worthington | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella bongori 48:z81:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella sp | 10 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 7 | 0 | 0 | 0 | 22 |
| Salmonella ssp I 11:i:- | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 13,23:-:l,w | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 13,23:b:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 16:-:1,5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 18:z4,z23:- | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 3,10:-:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 4,[5],12,27:-:- | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 4,[5],12,[27]:HNM | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella ssp I 4,[5],12:-:- | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 4,[5],12:-:1,7 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 4,[5],12:b:- | 1 | 4 | 1 | 0 | 19 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Salmonella ssp I 4,[5],12:d:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 4,[5],12:i:- | 19 | 20 | 3 | 6 | 109 | 96 | 9 | 1 | 0 | 0 | 0 | 1 | 1 | 265 |
| Salmonella ssp I 4,[5],12:l,v:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 44:z10:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,7,14:-:- | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,7,[14]:r:- | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,7:-:1,5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,7:c:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,7:e,h:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,8,[20]:HNM | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,8:-:1,5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,8:d:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 6,8:e,h:- | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 9,12:-:- | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella ssp I 9,12:-:1,5 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella ssp I 9,12:HNM | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 9,12:a:- | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 9,12:l,v:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I 9,12:r:- | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I Rough-O:-:1,5 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella ssp I Rough-O:H undetermined | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I Rough-O:HNM | 1 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Salmonella ssp I Rough-O:b:- | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella ssp I Rough-O:g,m:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I Rough-O:m,t:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I Rough-O:r:1,5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp I Rough-O:z4,z23:- | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp II 17:b:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp II 42:b:e,n,x,z15 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp II 6,14:m,t:e | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp II 9,12:e,n,x:1,[5],7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIa 41:z4,z23:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIa 44:z4,z23:- | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 16:z10:e,n,x,z15 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 38:z53:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 48:i:z | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella ssp IIIb 48:i:z:[z72] | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 48:k:1,5,7 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 50:k:z | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella ssp IIIb 50:l,v:z35 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 53:z10:z35 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:l,v:- | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:l,v:1,5,7 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:l,v:1,5,7:[z57] | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:l,v:z | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:l,v:z35 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:r:z53 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:z52:1,5,7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IIIb 61:z52:z53 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella ssp IV 11:z4,z23:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IV 44:z4,z23:- | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Salmonella ssp IV 48:g,z51:- | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella ssp IV 48:z4,z32:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IV 50:g,z51:- | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salmonella ssp IV 50:z4,z23:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IV Rough-O:HNM | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IV Rough-O:g,z51:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Salmonella ssp IV Rough-O:g:- | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total Salmonella | 781 | 598 | 146 | 111 | 1,977 | 949 | 121 | 80 | 7 | 46 | 0 | 8 | 2 | 4,826 |
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shigella | 7 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 7 | 0 | 0 | 0 | 17 |
| Shigella boydii 10 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Shigella boydii 12 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella boydii 14 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Shigella boydii 18 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella boydii 20 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Shigella boydii 4 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Shigella boydii 5 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Shigella boydii 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella boydii 8 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Shigella boydii | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Shigella dysenteriae 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella dysenteriae 12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella dysenteriae 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella dysenteriae 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella dysenteriae 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella dysenteriae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella flexneri 1a | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Shigella flexneri 1b | 14 | 1 | 0 | 0 | 19 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 |
| Shigella flexneri 2a | 66 | 7 | 0 | 0 | 43 | 49 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 166 |
| Shigella flexneri 2b | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Shigella flexneri 3a | 3 | 236 | 0 | 0 | 7 | 15 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 262 |
| Shigella flexneri 3b | 2 | 4 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| Shigella flexneri 4a | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Shigella flexneri 4c | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Shigella flexneri 6 | 4 | 0 | 0 | 0 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 |
| Shigella flexneri | 0 | 0 | 2 | 3 | 0 | 8 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 15 |
| Shigella flexneri Prov. SH-104 | 7 | 1 | 0 | 0 | 14 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 |
| Shigella flexneri var. Y | 0 | 2 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| Shigella sonnei | 190 | 20 | 1 | 2 | 117 | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 372 |
| Total Shigella | 304 | 275 | 3 | 5 | 231 | 146 | 4 | 1 | 0 | 7 | 1 | 1 | 0 | 978 |
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vibrio alginolyticus | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Vibrio cholerae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Vibrio cholerae O1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Vibrio cholerae O1 Ogawa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Vibrio cholerae O1 bio El Tor sero | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Vibrio cholerae O139 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Vibrio cholerae non-O1/O139 | 2 | 0 | 2 | 0 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Vibrio fluvialis | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Vibrio parahaemolyticus | 17 | 0 | 0 | 0 | 9 | 0 | 8 | 0 | 3 | 0 | 0 | 0 | 0 | 37 |
| Vibrio sp | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Vibrio vulnificus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total Vibrio | 27 | 3 | 3 | 0 | 17 | 4 | 11 | 0 | 3 | 0 | 0 | 0 | 0 | 68 |
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenovirus | 33 | 198 | 0 | 51 | 123 | 0 | 0 | 4 | 0 | 111 | 0 | 0 | 1 | 521 |
| Astrovirus | 23 | 41 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 61 | 0 | 0 | 0 | 144 |
| Enterovirus | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Hepatitis A | 39 | 80 | 14 | 30 | 78 | 57 | 2 | 8 | 0 | 2 | 0 | 0 | 0 | 310 |
| Norovirus | 141 | 324 | 138 | 88 | 339 | 0 | 136 | 53 | 26 | 182 | 0 | 1 | 1 | 1,429 |
| Rotavirus | 6 | 67 | 71 | 23 | 29 | 0 | 12 | 2 | 4 | 7 | 0 | 0 | 0 | 221 |
| Sapovirus | 16 | 101 | 0 | 18 | 0 | 0 | 1 | 0 | 0 | 20 | 0 | 0 | 1 | 157 |
| Total Virus | 258 | 811 | 223 | 236 | 569 | 57 | 151 | 67 | 30 | 383 | 0 | 1 | 3 | 2,789 |
| Organism | BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | YT | NT | NU | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yersinia enterocolitica | 36 | 32 | 4 | 4 | 95 | 28 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 201 |
| Yersinia frederiksenii | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 15 |
| Yersinia intermedia | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| Yersinia kristensenii | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Yersinia massiliensis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Yersinia mollaretii | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Yersinia pseudotuberculosis | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Yersinia sp | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Total Yersinia | 65 | 33 | 4 | 4 | 95 | 30 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 233 |
Appendix C. NESP support for outbreak investigations
NESP data supported 5 out of 7 National Outbreak Investigation Coordinating Committees (OICCs) in 2022. More information about the OICCs that NESP supported can be seen below in Table 16.
| Multi-jurisdictional Outbreak Investigations | Outbreak Source | Number of cases-final (Canada only) | Date of first case onset | Date of last case onset | Provinces and Territories with Cases |
|---|---|---|---|---|---|
| [2022-091] [OICC: Norovirus and GI reports associated with Spot Prawns in AB, BC, MB and ON] [May, 2022 - June, 2022] |
Spot Prawns | 60 | 2022-05-12 | 2022-05-23 | BC 18 AB 12 MB 19 ON 11 |
| [2022-065] [OICC: Hepatitis A in AB and SK] [May, 2022 - July, 2022] |
Fresh Organic Strawberries | 10 | 2022-04-04 | 2022-04-17 | AB 4 SK 6 |
| [2022-029] [OICC: GI reports associated with raw oysters in BC] [March, 2022 - May, 2022] |
Oysters | 339 | 2022-01-21 | 2022-04-05 | BC 301 AB 3 SK 1 ON 19 MB 15 |
| [2022-017] [OICC: E. coli O157:H7 in AB, SK, and the US] [January, 2022 - March, 2022] |
Kimchi | 14 | 2021-12-11 | 2022-01-07 | AB 13 SK 1 |
| [2021-180] [OICC: WGS Cluster of S. Enteritidis in BC, AB, SK, MB & ON] [October, 2021 - March, 2022] |
Frozen corn | 118 | 2021-09-06 | 2022-01-27 (isolation date) | BC 44 AB 55 SK 4 MB 13 ON 2 |
Appendix D. Impacts of COVID-19: Comparison of NESP weekly isolate counts from 2022, 2021 and 2020 compared to the 2015 to 2019 historical average for select pathogens
In March of 2020, the COVID-19 pandemic was declaredFootnote 5 and global public health action was taken to address it. Across Canada and within specific provinces/territories and regions, various public health measures were put in place. These included internationalFootnote 10 and domestic travel restrictions, closing of non-essential businesses and activities (including restaurants, gyms, salons, places of worship, etc.), closing of in-person schools and initiating virtual learning, and mandating of face coverings in public and indoor spaces. Additionally, increased public health messaging related to hand washing and cough and sneeze etiquette, reminders about staying home if you were feeling unwell and to get tested for COVID-19 were implemented. These public health measures and the adaptations Canadians made to combat COVID-19 not only helped to reduce the transmission of COVID-19 but have also impacted other reported infectious diseases to varying degrees in various ways. These measures were first implemented in 2020, and many remained in place or were re-instated in 2021 and 2022. Similar to the 2020 and 2021 NESP Annual Summaries, interpretation of the data and findings in the 2022 NESP Annual Summary must be interpreted with caution, as the public health measures invoked to help limit the spread of COVID-19 likely impacted disease incidence as well as data collection and reporting to NESP. Figures 10-17 compare 2022, 2021 and 2020 NESP data with the pre-pandemic period five-year (2015-2019) average for select pathogens.
The impact that the public health measures for COVID-19 had on the pathogens reported through NESP in 2022 was variable. For all Salmonella serotypes (Figure 10), the 2022 NESP counts remain lower than the 2015-2019 historical average throughout the year. However, generally counts appear higher in 2022 than in 2020 and 2021, excluding the early months in 2020. This is likely due to the absence of COVID-19 public health measures prior to March 2020.
Focusing on certain Salmonella serotypes and groupings, the magnitude of the difference between the 2022 NESP data and the pre-pandemic historical average is greater for Salmonella Enteritidis (Figure 12) compared to all other Salmonella serotypes excluding S. Enteritidis (Figure 11). In 2022, Salmonella Enteritidis case counts increased in comparison to 2020 and 2021, but still remain lower than the 2015 to 2019 historical average. The decrease in S. Enteritidis data is likely related to both the impacts of COVID-19 public health measures as well as the continued positive impact related to the Canadian Food Inspection Agency's regulation implemented in April 2019 to address Salmonella in raw frozen breaded chicken productsFootnote 6.
Regarding O157 STEC (Figure 13) and non-O157 STEC (Figure 14), 2022 case counts appear generally comparable to the pre-pandemic historical average. Cases of non-O157 STEC in 2022 surpass the pre-pandemic historical average overall, whereas cases of O157 STEC remain similar to the historical average over the same time period. The divergence of trends in O157 STEC and non-O157 STEC over this time period may be related to the overall increased frequency of non-O157 STEC reported to NESP over the last several years (i.e. since 2018; data not shown).
Trends in data for Listeria monocytogenes reported to NESP in 2022 (Figure 15) are comparable to trends observed in 2020, 2021, and to the pre-pandemic historical average for 2015-2019. This suggests that the impact of the public health measures put in place to combat COVID-19 may have had less of an effect on L. monocytogenes. Possible explanations for this are that L. monocytogenes is generally less associated with travel-acquired infection (i.e. less travel in the pandemic period) and considering the severe illness this organism often causes – reporting of cases may have been less affected than other organisms that often cause less-severe illnessFootnote 11.
For Shigella, the number of cases reported in 2022 generally rebounded back to levels similar to and sometimes higher than the pre-pandemic historical average (Figure 16). These trends are higher than the reduced counts of Shigella reported in 2020 and 2021. Shigellosis is frequently travel-related and this microorganism is often transmitted through person-to-person contact, and thus the impact of COVID-19 public health measures in place likely played a role in the lower levels of Shigella observed in 2020 and 2021.
In general, compared to pre-pandemic counts, larger decreases were seen in 2020 and 2021 for pathogens that typically are more often associated with travel, are typically milder symptom-wise or less invasive, or are more frequently transmitted via person-to-person contact. The main exception to this finding relates to Listeria monocytogenes which is typically more severe and is much less likely to be associated with travel or acquired through person-to-person contact; thus, the frequency of cases of Listeria reported to NESP during 2020, 2021 and 2022 did not appear to differ compared to the pre-pandemic period.
The public health measures that were implemented in response to the COVID-19 pandemic were multifaceted; thus, it is challenging to attribute specific measures to specific enteric disease impacts (perhaps with the exception of the impacts of travel restrictions)Footnote 11. Considering that some public health measures also caused a major shift in food consumption patterns (i.e., decreasing food purchased and consumed outside the home) further complicates our ability to easily discern these individual impacts. Finally, possible deviations in medical care-seeking behaviour among Canadians as a result of stay-at-home orders and local healthcare system changes may have also influenced specimen submission and thus, reporting of isolates to NESP during the pandemic period.
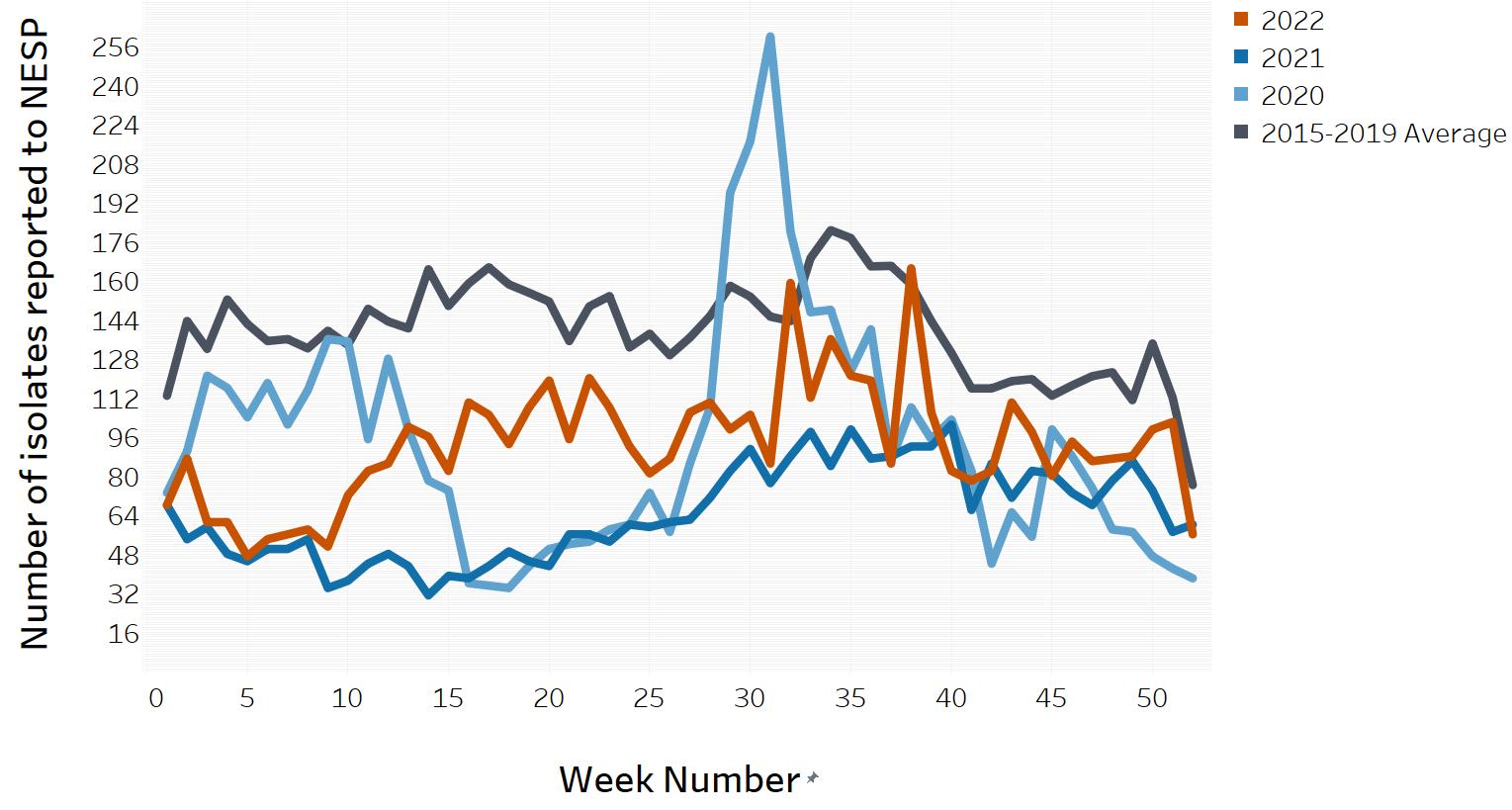
Figure 10 : Descriptive text
| Week Number | 2020 count | 2021 count | 2022 count | 2015-2019 Average |
|---|---|---|---|---|
| 1 | 73 | 68 | 68 | 112.8 |
| 2 | 90 | 54 | 87 | 143.4 |
| 3 | 121 | 59 | 61 | 132 |
| 4 | 116 | 48 | 61 | 152.2 |
| 5 | 104 | 45 | 47 | 142.2 |
| 6 | 118 | 50 | 54 | 135.2 |
| 7 | 101 | 50 | 56 | 136 |
| 8 | 115 | 54 | 58 | 132.2 |
| 9 | 136 | 34 | 51 | 139.4 |
| 10 | 135 | 37 | 72 | 133.6 |
| 11 | 95 | 44 | 82 | 148.4 |
| 12 | 128 | 48 | 85 | 143.2 |
| 13 | 99 | 43 | 100 | 140.4 |
| 14 | 78 | 31 | 96 | 164.6 |
| 15 | 74 | 39 | 82 | 149.6 |
| 16 | 36 | 38 | 110 | 158.8 |
| 17 | 35 | 43 | 105 | 165.4 |
| 18 | 34 | 49 | 93 | 158.4 |
| 19 | 43 | 45 | 108 | 155 |
| 20 | 50 | 43 | 119 | 151.4 |
| 21 | 52 | 56 | 95 | 135.2 |
| 22 | 53 | 56 | 120 | 149.4 |
| 23 | 58 | 53 | 108 | 153.6 |
| 24 | 60 | 60 | 92 | 132.6 |
| 25 | 73 | 59 | 81 | 138.2 |
| 26 | 57 | 61 | 87 | 129.4 |
| 27 | 85 | 62 | 106 | 136.6 |
| 28 | 108 | 71 | 110 | 145.4 |
| 29 | 196 | 82 | 99 | 157.8 |
| 30 | 217 | 91 | 105 | 153.4 |
| 31 | 260 | 77 | 85 | 145.2 |
| 32 | 180 | 88 | 159 | 143.4 |
| 33 | 147 | 98 | 112 | 169.2 |
| 34 | 148 | 84 | 136 | 180.6 |
| 35 | 123 | 99 | 121 | 177.4 |
| 36 | 140 | 87 | 119 | 165.8 |
| 37 | 86 | 88 | 85 | 166 |
| 38 | 108 | 92 | 165 | 158.4 |
| 39 | 95 | 92 | 106 | 143.4 |
| 40 | 103 | 101 | 82 | 130.4 |
| 41 | 82 | 66 | 78 | 115.8 |
| 42 | 44 | 85 | 82 | 115.8 |
| 43 | 65 | 71 | 110 | 118.8 |
| 44 | 55 | 82 | 98 | 119.6 |
| 45 | 99 | 81 | 80 | 112.8 |
| 46 | 88 | 73 | 94 | 117 |
| 47 | 75 | 68 | 86 | 120.8 |
| 48 | 58 | 78 | 87 | 122.4 |
| 49 | 57 | 86 | 88 | 111 |
| 50 | 47 | 74 | 99 | 134.2 |
| 51 | 42 | 57 | 102 | 112 |
| 52 | 38 | 60 | 56 | 76.2 |
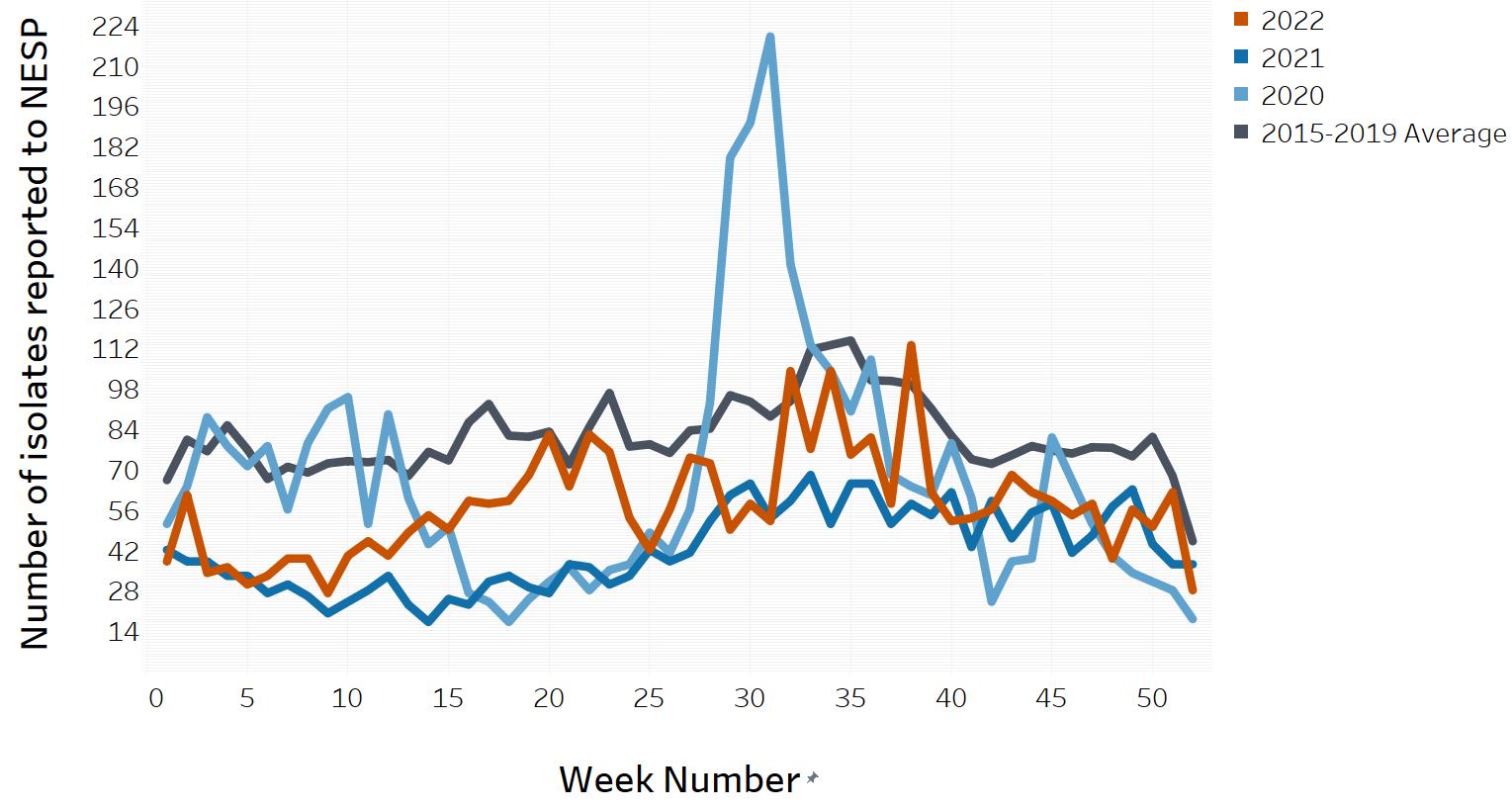
Figure 11 : Descriptive text
| Week Number | 2020 count | 2021 count | 2022 count | 2015-2019 Average |
|---|---|---|---|---|
| 1 | 51 | 42 | 38 | 66.2 |
| 2 | 64 | 38 | 61 | 80.2 |
| 3 | 88 | 38 | 34 | 76.2 |
| 4 | 78 | 33 | 36 | 85.2 |
| 5 | 71 | 33 | 30 | 76.8 |
| 6 | 78 | 27 | 33 | 66.6 |
| 7 | 56 | 30 | 39 | 70.8 |
| 8 | 79 | 26 | 39 | 68.8 |
| 9 | 91 | 20 | 27 | 72 |
| 10 | 95 | 24 | 40 | 72.8 |
| 11 | 51 | 28 | 45 | 72.4 |
| 12 | 89 | 33 | 40 | 73.2 |
| 13 | 60 | 23 | 48 | 67.6 |
| 14 | 44 | 17 | 54 | 76 |
| 15 | 50 | 25 | 49 | 73 |
| 16 | 27 | 23 | 59 | 86.2 |
| 17 | 24 | 31 | 58 | 92.6 |
| 18 | 17 | 33 | 59 | 81.6 |
| 19 | 25 | 29 | 68 | 81.2 |
| 20 | 31 | 27 | 82 | 83 |
| 21 | 36 | 37 | 64 | 71.6 |
| 22 | 28 | 36 | 82 | 84.6 |
| 23 | 35 | 30 | 76 | 96.4 |
| 24 | 37 | 33 | 53 | 77.8 |
| 25 | 48 | 42 | 42 | 78.6 |
| 26 | 41 | 38 | 56 | 75.6 |
| 27 | 56 | 41 | 74 | 83.4 |
| 28 | 93 | 52 | 72 | 84 |
| 29 | 178 | 61 | 49 | 95.6 |
| 30 | 190 | 65 | 58 | 93.4 |
| 31 | 220 | 53 | 52 | 88.2 |
| 32 | 141 | 59 | 104 | 93.6 |
| 33 | 113 | 68 | 77 | 111.4 |
| 34 | 104 | 51 | 104 | 113 |
| 35 | 90 | 65 | 75 | 114.6 |
| 36 | 108 | 65 | 81 | 100.8 |
| 37 | 68 | 51 | 58 | 100.6 |
| 38 | 64 | 58 | 113 | 99.4 |
| 39 | 61 | 54 | 62 | 91 |
| 40 | 79 | 62 | 52 | 81.6 |
| 41 | 60 | 43 | 53 | 73.4 |
| 42 | 24 | 59 | 56 | 71.8 |
| 43 | 38 | 46 | 68 | 74.8 |
| 44 | 39 | 55 | 62 | 78 |
| 45 | 81 | 58 | 59 | 76.4 |
| 46 | 66 | 41 | 54 | 75.4 |
| 47 | 51 | 47 | 58 | 77.6 |
| 48 | 40 | 57 | 39 | 77.4 |
| 49 | 34 | 63 | 56 | 74.4 |
| 50 | 31 | 44 | 50 | 81.2 |
| 51 | 28 | 37 | 62 | 67.6 |
| 52 | 18 | 37 | 28 | 45 |
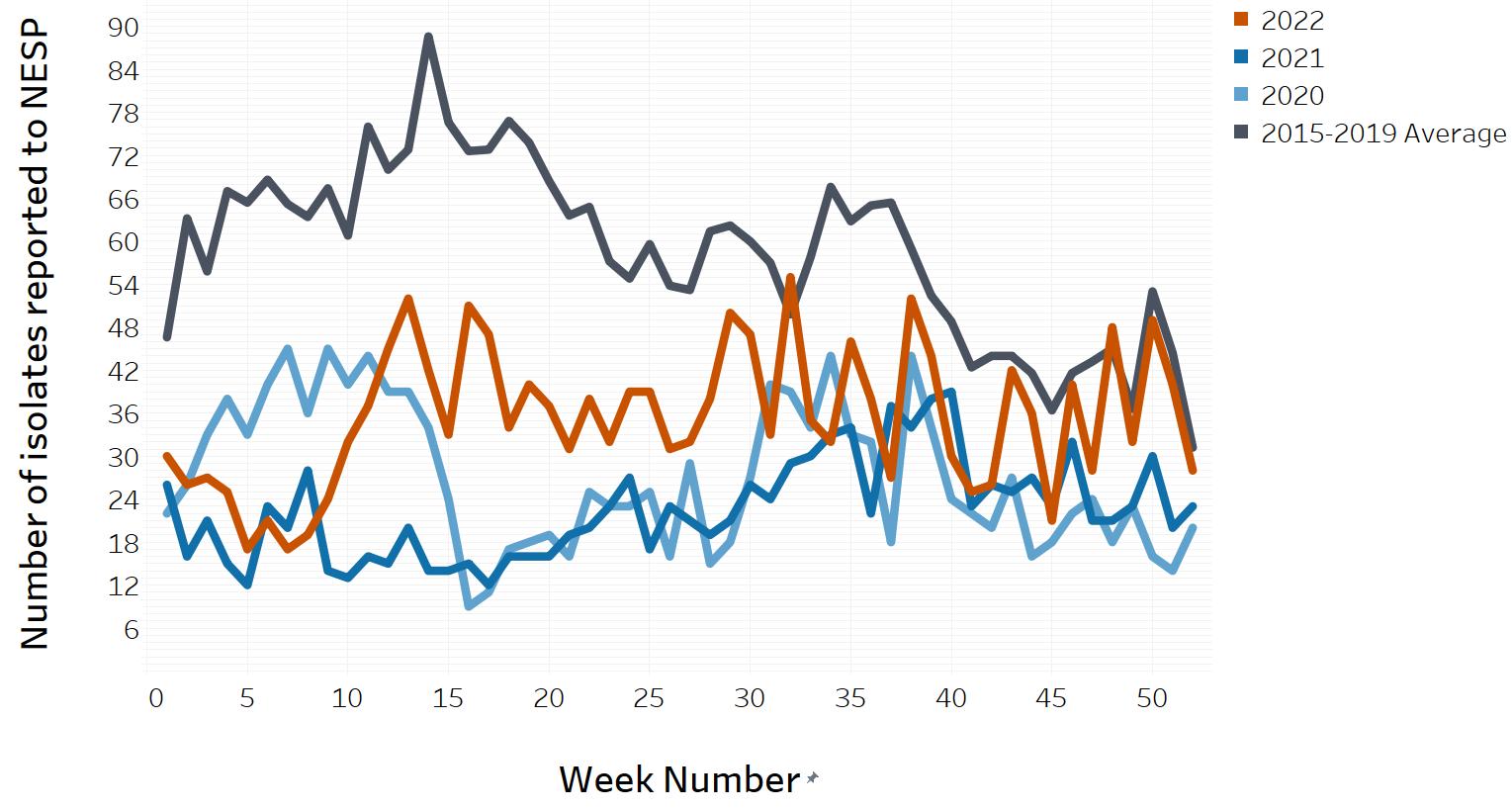
Figure 12 : Descriptive text
| Week Number | 2020 count | 2021 count | 2022 count | 2015-2019 Average |
|---|---|---|---|---|
| 1 | 22 | 26 | 30 | 46.6 |
| 2 | 26 | 16 | 26 | 63.2 |
| 3 | 33 | 21 | 27 | 55.8 |
| 4 | 38 | 15 | 25 | 67 |
| 5 | 33 | 12 | 17 | 65.4 |
| 6 | 40 | 23 | 21 | 68.6 |
| 7 | 45 | 20 | 17 | 65.2 |
| 8 | 36 | 28 | 19 | 63.4 |
| 9 | 45 | 14 | 24 | 67.4 |
| 10 | 40 | 13 | 32 | 60.8 |
| 11 | 44 | 16 | 37 | 76 |
| 12 | 39 | 15 | 45 | 70 |
| 13 | 39 | 20 | 52 | 72.8 |
| 14 | 34 | 14 | 42 | 88.6 |
| 15 | 24 | 14 | 33 | 76.6 |
| 16 | 9 | 15 | 51 | 72.6 |
| 17 | 11 | 12 | 47 | 72.8 |
| 18 | 17 | 16 | 34 | 76.8 |
| 19 | 18 | 16 | 40 | 73.8 |
| 20 | 19 | 16 | 37 | 68.4 |
| 21 | 16 | 19 | 31 | 63.6 |
| 22 | 25 | 20 | 38 | 64.8 |
| 23 | 23 | 23 | 32 | 57.2 |
| 24 | 23 | 27 | 39 | 54.8 |
| 25 | 25 | 17 | 39 | 59.6 |
| 26 | 16 | 23 | 31 | 53.8 |
| 27 | 29 | 21 | 32 | 53.2 |
| 28 | 15 | 19 | 38 | 61.4 |
| 29 | 18 | 21 | 50 | 62.2 |
| 30 | 27 | 26 | 47 | 60 |
| 31 | 40 | 24 | 33 | 57 |
| 32 | 39 | 29 | 55 | 49.8 |
| 33 | 34 | 30 | 35 | 57.8 |
| 34 | 44 | 33 | 32 | 67.6 |
| 35 | 33 | 34 | 46 | 62.8 |
| 36 | 32 | 22 | 38 | 65 |
| 37 | 18 | 37 | 27 | 65.4 |
| 38 | 44 | 34 | 52 | 59 |
| 39 | 34 | 38 | 44 | 52.4 |
| 40 | 24 | 39 | 30 | 48.8 |
| 41 | 22 | 23 | 25 | 42.4 |
| 42 | 20 | 26 | 26 | 44 |
| 43 | 27 | 25 | 42 | 44 |
| 44 | 16 | 27 | 36 | 41.6 |
| 45 | 18 | 23 | 21 | 36.4 |
| 46 | 22 | 32 | 40 | 41.6 |
| 47 | 24 | 21 | 28 | 43.2 |
| 48 | 18 | 21 | 48 | 45 |
| 49 | 23 | 23 | 32 | 36.6 |
| 50 | 16 | 30 | 49 | 53 |
| 51 | 14 | 20 | 40 | 44.4 |
| 52 | 20 | 23 | 28 | 31.2 |
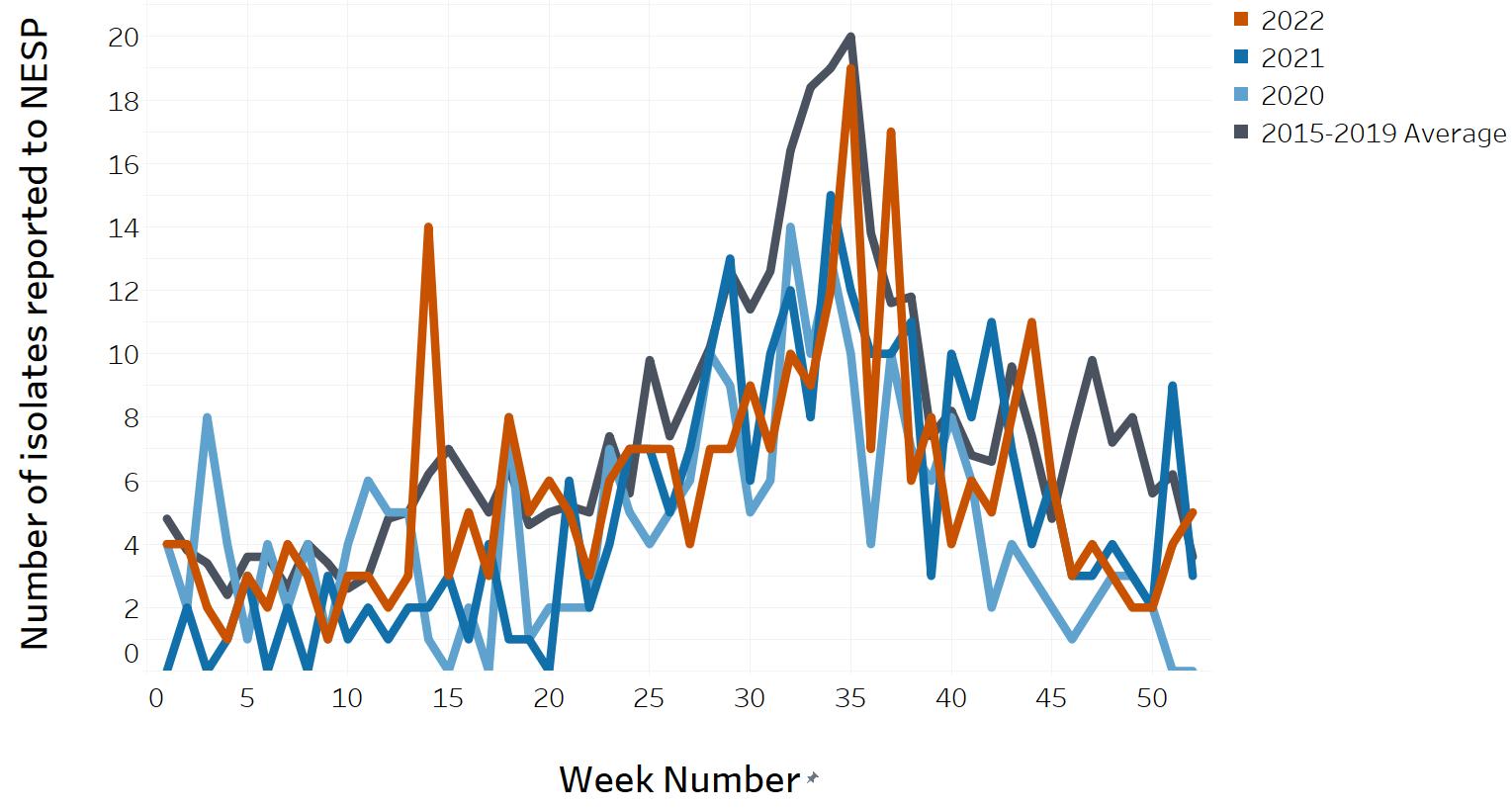
Figure 13 : Descriptive text
| Week Number | 2020 count | 2021 count | 2022 count | 2015-2019 Average |
|---|---|---|---|---|
| 1 | 4 | 0 | 4 | 4.8 |
| 2 | 2 | 2 | 4 | 3.8 |
| 3 | 8 | 0 | 2 | 3.4 |
| 4 | 4 | 1 | 1 | 2.4 |
| 5 | 1 | 3 | 3 | 3.6 |
| 6 | 4 | 0 | 2 | 3.6 |
| 7 | 2 | 2 | 4 | 2.6 |
| 8 | 4 | 0 | 3 | 4 |
| 9 | 1 | 3 | 1 | 3.4 |
| 10 | 4 | 1 | 3 | 2.6 |
| 11 | 6 | 2 | 3 | 3 |
| 12 | 5 | 1 | 2 | 4.8 |
| 13 | 5 | 2 | 3 | 5 |
| 14 | 1 | 2 | 14 | 6.2 |
| 15 | 0 | 3 | 3 | 7 |
| 16 | 2 | 1 | 5 | 6 |
| 17 | 0 | 4 | 3 | 5 |
| 18 | 8 | 1 | 8 | 6.4 |
| 19 | 1 | 1 | 5 | 4.6 |
| 20 | 2 | 0 | 6 | 5 |
| 21 | 2 | 6 | 5 | 5.2 |
| 22 | 2 | 2 | 3 | 5 |
| 23 | 7 | 4 | 6 | 7.4 |
| 24 | 5 | 7 | 7 | 5.6 |
| 25 | 4 | 7 | 7 | 9.8 |
| 26 | 5 | 5 | 7 | 7.4 |
| 27 | 6 | 7 | 4 | 8.8 |
| 28 | 10 | 10 | 7 | 10.2 |
| 29 | 9 | 13 | 7 | 12.6 |
| 30 | 5 | 6 | 9 | 11.4 |
| 31 | 6 | 10 | 7 | 12.6 |
| 32 | 14 | 12 | 10 | 16.4 |
| 33 | 10 | 8 | 9 | 18.4 |
| 34 | 13 | 15 | 12 | 19 |
| 35 | 10 | 12 | 19 | 20 |
| 36 | 4 | 10 | 7 | 13.8 |
| 37 | 10 | 10 | 17 | 11.6 |
| 38 | 7 | 11 | 6 | 11.8 |
| 39 | 6 | 3 | 8 | 7.4 |
| 40 | 8 | 10 | 4 | 8.2 |
| 41 | 6 | 8 | 6 | 6.8 |
| 42 | 2 | 11 | 5 | 6.6 |
| 43 | 4 | 7 | 8 | 9.6 |
| 44 | 3 | 4 | 11 | 7.4 |
| 45 | 2 | 6 | 6 | 4.8 |
| 46 | 1 | 3 | 3 | 7.4 |
| 47 | 2 | 3 | 4 | 9.8 |
| 48 | 3 | 4 | 3 | 7.2 |
| 49 | 3 | 3 | 2 | 8 |
| 50 | 2 | 2 | 2 | 5.6 |
| 51 | 0 | 9 | 4 | 6.2 |
| 52 | 0 | 3 | 5 | 3.6 |
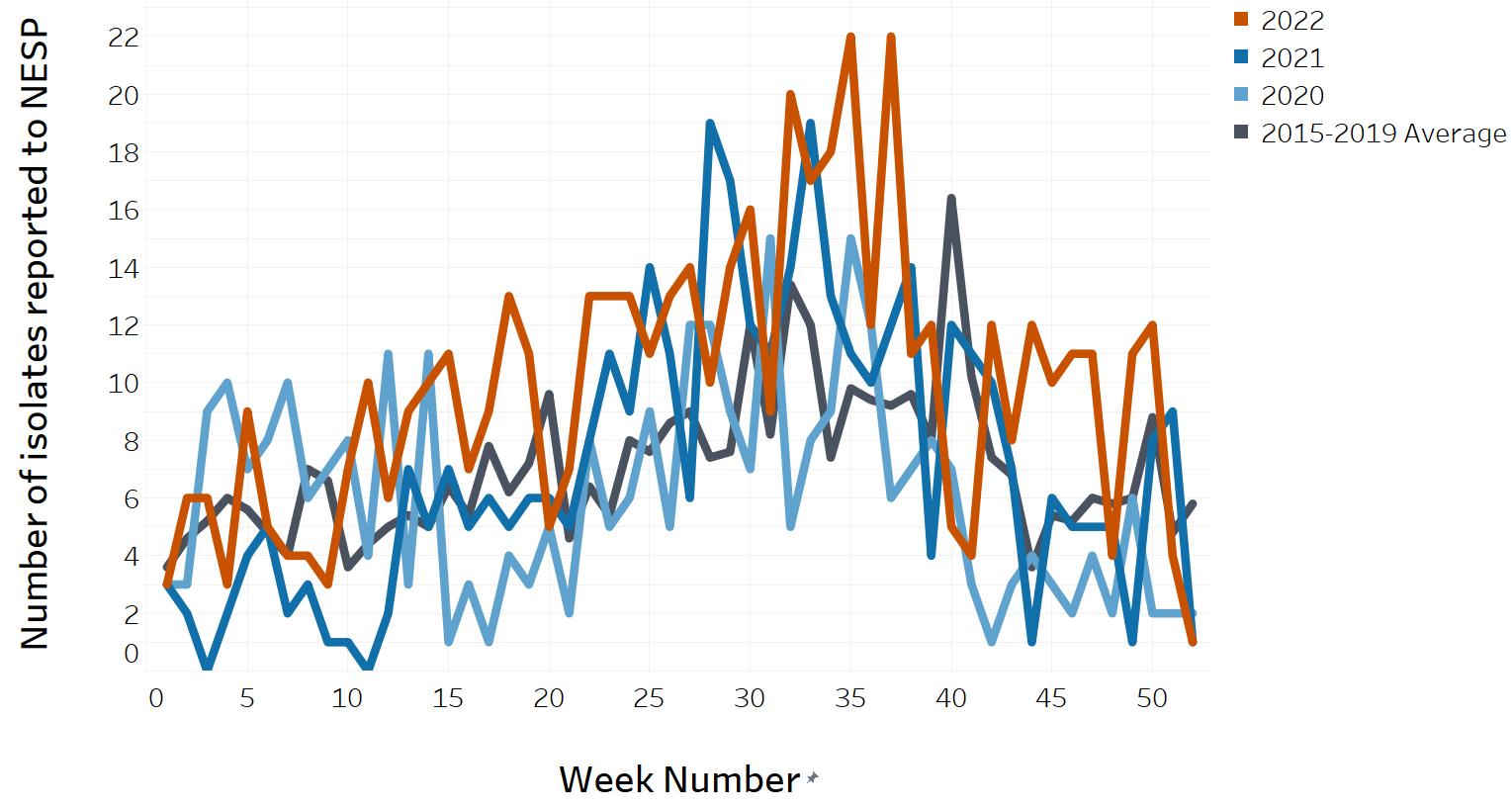
Figure 14 : Descriptive text
| Week Number | 2020 count | 2021 count | 2022 count | 2015-2019 Average |
|---|---|---|---|---|
| 1 | 3 | 3 | 3 | 3.6 |
| 2 | 3 | 2 | 6 | 4.6 |
| 3 | 9 | 0 | 6 | 5.2 |
| 4 | 10 | 2 | 3 | 6 |
| 5 | 7 | 4 | 9 | 5.6 |
| 6 | 8 | 5 | 5 | 4.8 |
| 7 | 10 | 2 | 4 | 4 |
| 8 | 6 | 3 | 4 | 7 |
| 9 | 7 | 1 | 3 | 6.6 |
| 10 | 8 | 1 | 7 | 3.6 |
| 11 | 4 | 0 | 10 | 4.4 |
| 12 | 11 | 2 | 6 | 5 |
| 13 | 3 | 7 | 9 | 5.4 |
| 14 | 11 | 5 | 10 | 5 |
| 15 | 1 | 7 | 11 | 6.4 |
| 16 | 3 | 5 | 7 | 5.4 |
| 17 | 1 | 6 | 9 | 7.8 |
| 18 | 4 | 5 | 13 | 6.2 |
| 19 | 3 | 6 | 11 | 7.2 |
| 20 | 5 | 6 | 5 | 9.6 |
| 21 | 2 | 5 | 7 | 4.6 |
| 22 | 8 | 8 | 13 | 6.4 |
| 23 | 5 | 11 | 13 | 5.4 |
| 24 | 6 | 9 | 13 | 8 |
| 25 | 9 | 14 | 11 | 7.6 |
| 26 | 5 | 11 | 13 | 8.6 |
| 27 | 12 | 6 | 14 | 9 |
| 28 | 12 | 19 | 10 | 7.4 |
| 29 | 9 | 17 | 14 | 7.6 |
| 30 | 7 | 12 | 16 | 12 |
| 31 | 15 | 11 | 9 | 8.2 |
| 32 | 5 | 14 | 20 | 13.4 |
| 33 | 8 | 19 | 17 | 12 |
| 34 | 9 | 13 | 18 | 7.4 |
| 35 | 15 | 11 | 22 | 9.8 |
| 36 | 12 | 10 | 12 | 9.4 |
| 37 | 6 | 12 | 22 | 9.2 |
| 38 | 7 | 14 | 11 | 9.6 |
| 39 | 8 | 4 | 12 | 8 |
| 40 | 7 | 12 | 5 | 16.4 |
| 41 | 3 | 11 | 4 | 10.2 |
| 42 | 1 | 10 | 12 | 7.4 |
| 43 | 3 | 7 | 8 | 6.8 |
| 44 | 4 | 1 | 12 | 3.6 |
| 45 | 3 | 6 | 10 | 5.4 |
| 46 | 2 | 5 | 11 | 5.2 |
| 47 | 4 | 5 | 11 | 6 |
| 48 | 2 | 5 | 4 | 5.8 |
| 49 | 6 | 1 | 11 | 6 |
| 50 | 2 | 8 | 12 | 8.8 |
| 51 | 2 | 9 | 4 | 4.8 |
| 52 | 2 | 1 | 1 | 5.8 |
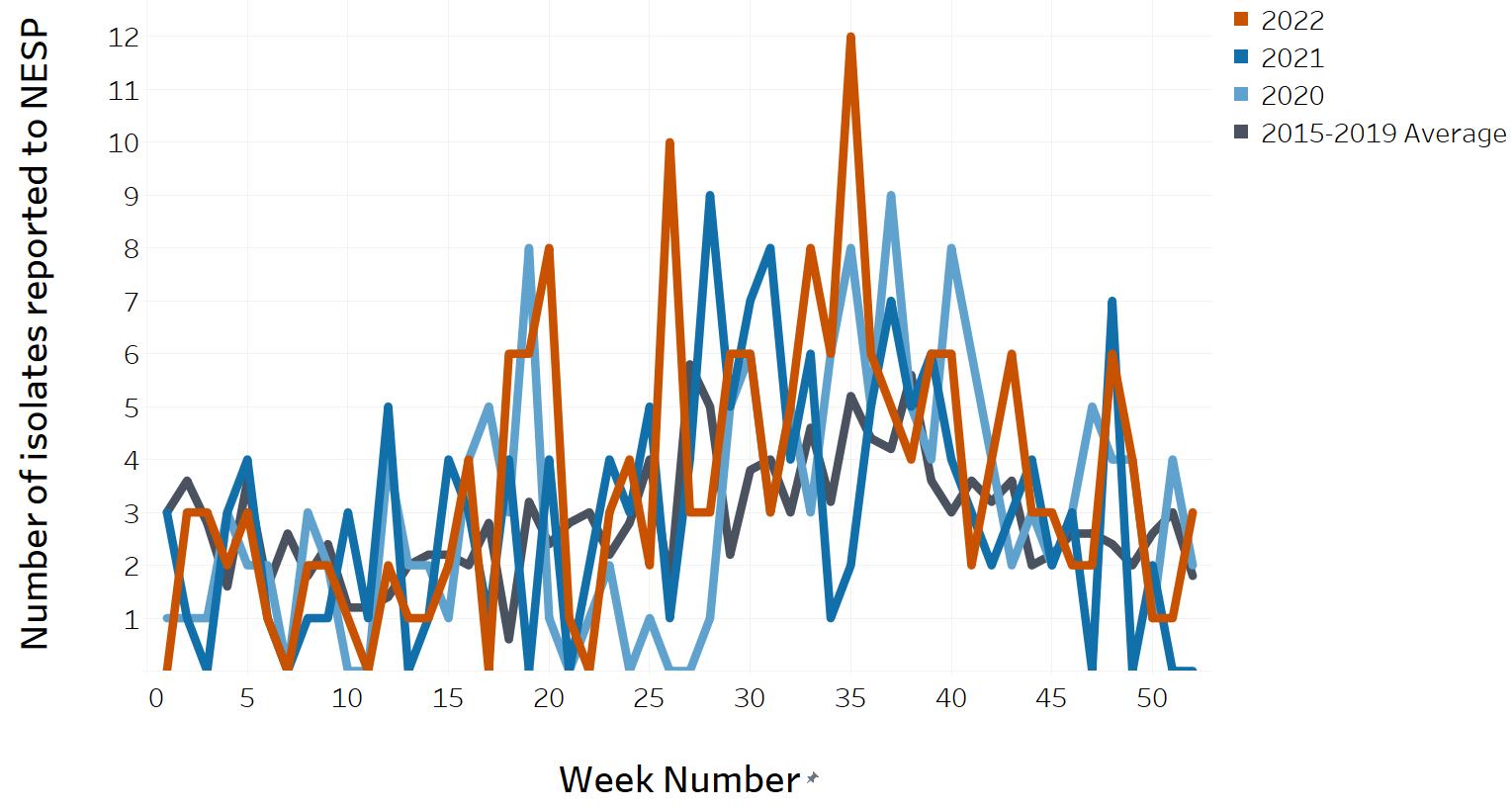
Figure 15 : Descriptive text
| Week Number | 2020 count | 2021 count | 2022 count | 2015-2019 Average |
|---|---|---|---|---|
| 1 | 1 | 3 | 0 | 3 |
| 2 | 1 | 1 | 3 | 3.6 |
| 3 | 1 | 0 | 3 | 2.8 |
| 4 | 3 | 3 | 2 | 1.6 |
| 5 | 2 | 4 | 3 | 3.6 |
| 6 | 2 | 1 | 1 | 1.6 |
| 7 | 0 | 0 | 0 | 2.6 |
| 8 | 3 | 1 | 2 | 1.8 |
| 9 | 2 | 1 | 2 | 2.4 |
| 10 | 0 | 3 | 1 | 1.2 |
| 11 | 0 | 1 | 0 | 1.2 |
| 12 | 4 | 5 | 2 | 1.4 |
| 13 | 2 | 0 | 1 | 2 |
| 14 | 2 | 1 | 1 | 2.2 |
| 15 | 1 | 4 | 2 | 2.2 |
| 16 | 4 | 3 | 4 | 2 |
| 17 | 5 | 1 | 0 | 2.8 |
| 18 | 3 | 4 | 6 | 0.6 |
| 19 | 8 | 0 | 6 | 3.2 |
| 20 | 1 | 4 | 8 | 2.4 |
| 21 | 0 | 0 | 1 | 2.8 |
| 22 | 1 | 2 | 0 | 3 |
| 23 | 2 | 4 | 3 | 2.2 |
| 24 | 0 | 3 | 4 | 2.8 |
| 25 | 1 | 5 | 2 | 4 |
| 26 | 0 | 1 | 10 | 1.6 |
| 27 | 0 | 4 | 3 | 5.8 |
| 28 | 1 | 9 | 3 | 5 |
| 29 | 5 | 5 | 6 | 2.2 |
| 30 | 6 | 7 | 6 | 3.8 |
| 31 | 3 | 8 | 3 | 4 |
| 32 | 5 | 4 | 5 | 3 |
| 33 | 3 | 6 | 8 | 4.6 |
| 34 | 6 | 1 | 6 | 3.2 |
| 35 | 8 | 2 | 12 | 5.2 |
| 36 | 5 | 5 | 6 | 4.4 |
| 37 | 9 | 7 | 5 | 4.2 |
| 38 | 5 | 5 | 4 | 5.6 |
| 39 | 4 | 6 | 6 | 3.6 |
| 40 | 8 | 4 | 6 | 3 |
| 41 | 6 | 3 | 2 | 3.6 |
| 42 | 4 | 2 | 4 | 3.2 |
| 43 | 2 | 3 | 6 | 3.6 |
| 44 | 3 | 4 | 3 | 2 |
| 45 | 2 | 2 | 3 | 2.2 |
| 46 | 3 | 3 | 2 | 2.6 |
| 47 | 5 | 0 | 2 | 2.6 |
| 48 | 4 | 7 | 6 | 2.4 |
| 49 | 4 | 0 | 4 | 2 |
| 50 | 1 | 2 | 1 | 2.6 |
| 51 | 4 | 0 | 1 | 3 |
| 52 | 2 | 0 | 3 | 1.8 |
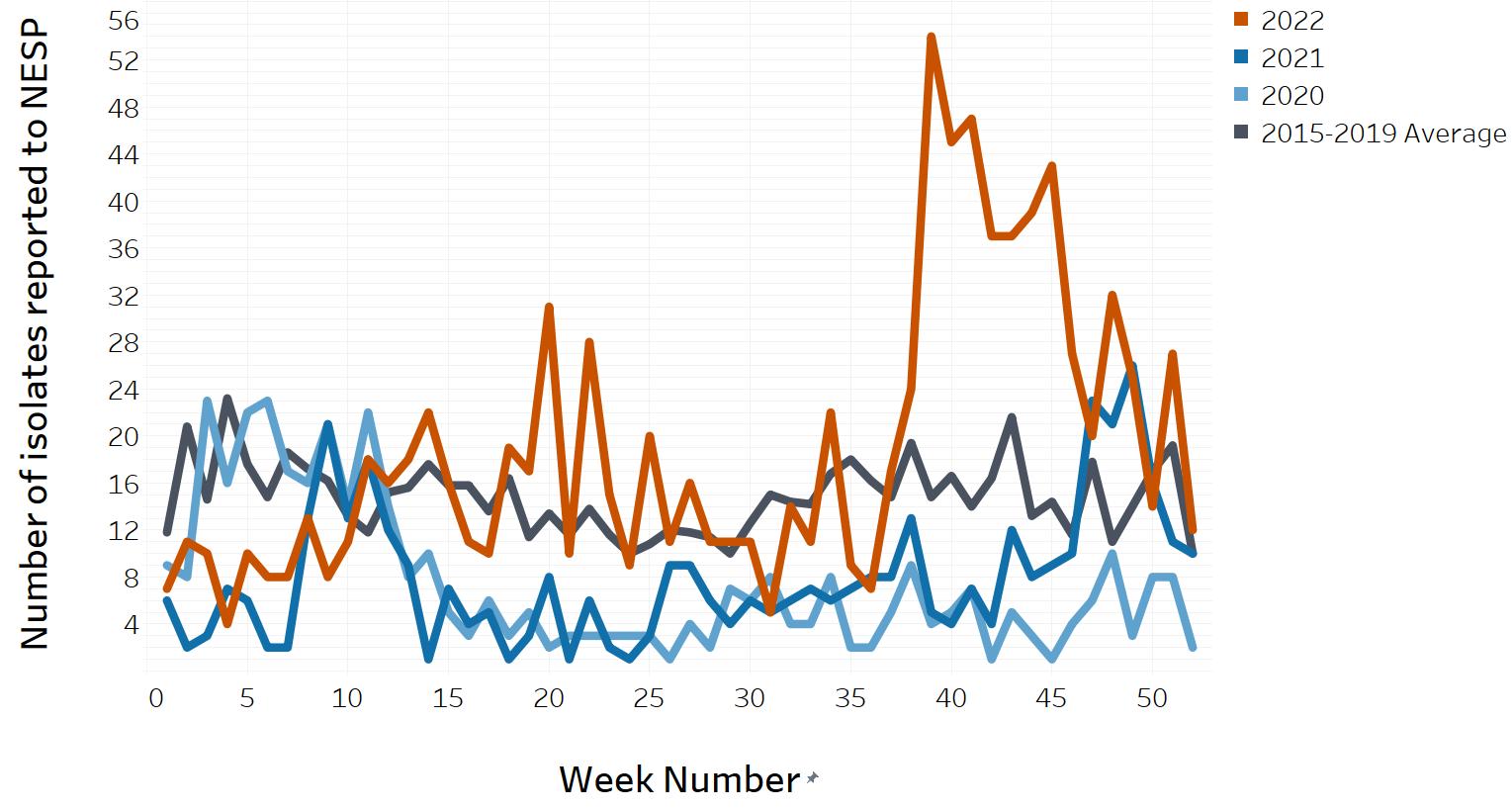
Figure 16 : Descriptive text
| Week Number | 2020 count | 2021 count | 2022 count | 2015-2019 Average |
|---|---|---|---|---|
| 1 | 9 | 6 | 7 | 11.8 |
| 2 | 8 | 2 | 11 | 20.8 |
| 3 | 23 | 3 | 10 | 14.6 |
| 4 | 16 | 7 | 4 | 23.2 |
| 5 | 22 | 6 | 10 | 17.6 |
| 6 | 23 | 2 | 8 | 14.8 |
| 7 | 17 | 2 | 8 | 18.6 |
| 8 | 16 | 13 | 13 | 17.2 |
| 9 | 21 | 21 | 8 | 16.2 |
| 10 | 14 | 13 | 11 | 13.2 |
| 11 | 22 | 18 | 18 | 11.8 |
| 12 | 14 | 12 | 16 | 15.2 |
| 13 | 8 | 9 | 18 | 15.6 |
| 14 | 10 | 1 | 22 | 17.6 |
| 15 | 5 | 7 | 16 | 15.8 |
| 16 | 3 | 4 | 11 | 15.8 |
| 17 | 6 | 5 | 10 | 13.6 |
| 18 | 3 | 1 | 19 | 16.4 |
| 19 | 5 | 3 | 17 | 11.4 |
| 20 | 2 | 8 | 31 | 13.4 |
| 21 | 3 | 1 | 10 | 11.6 |
| 22 | 3 | 6 | 28 | 13.8 |
| 23 | 3 | 2 | 15 | 11.6 |
| 24 | 3 | 1 | 9 | 10 |
| 25 | 3 | 3 | 20 | 10.8 |
| 26 | 1 | 9 | 11 | 12 |
| 27 | 4 | 9 | 16 | 11.8 |
| 28 | 2 | 6 | 11 | 11.4 |
| 29 | 7 | 4 | 11 | 10 |
| 30 | 6 | 6 | 11 | 12.6 |
| 31 | 8 | 5 | 5 | 15 |
| 32 | 4 | 6 | 14 | 14.4 |
| 33 | 4 | 7 | 11 | 14.2 |
| 34 | 8 | 6 | 22 | 16.8 |
| 35 | 2 | 7 | 9 | 18 |
| 36 | 2 | 8 | 7 | 16.2 |
| 37 | 5 | 8 | 17 | 14.8 |
| 38 | 9 | 13 | 24 | 19.4 |
| 39 | 4 | 5 | 54 | 14.8 |
| 40 | 5 | 4 | 45 | 16.6 |
| 41 | 7 | 7 | 47 | 14 |
| 42 | 1 | 4 | 37 | 16.4 |
| 43 | 5 | 12 | 37 | 21.6 |
| 44 | 3 | 8 | 39 | 13.2 |
| 45 | 1 | 9 | 43 | 14.4 |
| 46 | 4 | 10 | 27 | 11.6 |
| 47 | 6 | 23 | 20 | 17.8 |
| 48 | 10 | 21 | 32 | 11 |
| 49 | 3 | 26 | 25 | 14 |
| 50 | 8 | 16 | 14 | 17 |
| 51 | 8 | 11 | 27 | 19.2 |
| 52 | 2 | 10 | 12 | 10 |
Footnotes
- Footnote 1
-
Canadian Notifiable Diseases Surveillance System, Public Health Agency of Canada: https://diseases.canada.ca/notifiable/
- Footnote 2
-
PulseNet Canada, National Microbiology Laboratory, Public Health Agency of Canada: https://www.canada.ca/en/public-health/programs/pulsenet-canada.html
- Footnote 3
-
Food-borne Illness Outbreak Response Protocol (FIORP) 2017: To guide a multi-jurisdictional response. Public Health Agency of Canada: https://www.canada.ca/en/public-health/services/publications/health-risks-safety/canadas-foodborne-illness-outbreak-response-protocol-fiorp-guide-multi-jurisdictional-enteric-outbreak-response.html
- Footnote 4
-
SAS software, Version 9.4 of the SAS System for Windows. Copyright © 2016 SAS Institute Inc.
- Footnote 5
-
https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed September 13, 2021)
- Footnote 6
-
Salmonella control options in frozen raw breaded chicken products. Canadian Food Inspection Agency: https://inspection.canada.ca/preventive-controls/meat/salmonella-in-frozen-raw-breaded-chicken/eng/1531254524193/1531254524999
- Footnote 7
-
Public Health Agency of Canada. CPHLN recommendations for the laboratory detection of Shiga toxin-producing Escherichia coli (O157 and non-O157). Can Commun Dis Rep 2018;44(11):304-7. https://doi.org/10.14745/ccdr.v44i11a06
- Footnote 8
-
Centers for Disease Control and Prevention (CDC). Foodborne illness and culture-independent diagnostic tests (CIDTs). 2015 May 14. Available from: https://www.cdc.gov/foodnet/reports/cidt-questions-and-answers-2015.html
- Footnote 9
-
Public Health Agency of Canada. National case definition: Shiga toxin-producing Escherichia coil (STEC) infection. December 2023. Available from: https://www.canada.ca/en/public-health/services/diseases/e-coli/health-professionals-e-coli/national-case-definition.html
- Footnote 10
-
https://pm.gc.ca/en/news/news-releases/2020/03/16/prime-minister-announces-new-actions-under-canadas-covid-19-response (accessed October 20, 2021).
- Footnote 11
-
Dougherty et al., 2023. Impact of the COVID-19 Pandemic on the Reported Incidence of Select Bacterial Enteric Diseases in Canada, 2020. DOI:10.1089/fpd.2022.0064.
