Vaccine uptake in Canadian adults 2019
About
This report summarizes the results from the 2018-2019 Seasonal Influenza Vaccination Coverage Survey. Respondents aged 18 years and older were asked if they had been vaccinated against influenza for the 2018-2019 season; reasons for vaccination or non-vaccination; knowledge, attitudes, and beliefs (KAB) regarding vaccination; and select demographic information. This year, the participants were also questioned regarding four vaccines routinely offered to adults: pertussis, tetanus, pneumococcal (participants 65 years of age and older) and shingles (participants 50 years of age and older).
Key findings
Influenza
- Overall, influenza vaccination coverage in the 2018-2019 season (42%) was significantly higher than the 2017-2018 season (38%) and the 2016-2017 season (36%).
- Coverage was higher in females (47%) than in males (37%).
- Among high-risk groups, coverage for seniors 65 years of age and older (70%) and adults aged 18-64 years with a chronic medical condition (CMC) (43%) remained below the national coverage goals of 80%.
- The majority of vaccinated respondents had received their vaccine in October or November (79 %).
- The main vaccination places were pharmacies (35%) or doctor's offices (33%).
- The most commonly reported reason for receiving the vaccine was to prevent infection or to avoid getting sick (45%), whereas the most common reason for non-vaccination was the perception that the vaccine was not necessary (20%).
- Most of respondents agreed that the flu vaccine is safe and that getting sick with the flu can be serious.
- Four in ten respondents believed that the flu vaccine does not protect them against the flu (41%) and about the same proportion believed they might get the flu from the vaccine (43%).
- 69% agreed that the opinion of their family doctor, general practitioner or nurse practitioner is an important part of their decision for getting the flu vaccine.
Other vaccines: pertussis, tetanus, pneumococcal and shingles
- One in three respondents (33%) reported having received a pertussis-containing vaccine in adulthood.
- Two thirds of respondents (69%) reported having received a vaccine against tetanus in the previous 10 years.
- A greater proportion of older adults (58%) reported having received a pneumococcal vaccine in adulthood as compared to younger adults (18-64 years of age) with CMC (25%).
- Among participants 50 years of age and older, 28% reported having received the shingles vaccine.
- Across all groups, the most frequent reasons for non-vaccination was the perception that these vaccines are not necessary and the lack of awareness of the availability of vaccines.
- More effort is required to increase vaccination coverage in Canadian adults, particularly among high-risk groups.
Introduction
Vaccination is one of public health's greatest achievements. Vaccines are effective in preventing morbidity and mortality associated with certain infectious diseases. While the majority of routine vaccinations occur during childhood and adolescence, some vaccines are also recommended in adulthood. Adult vaccines are important for the following reasons:
- Immunity against certain vaccine-preventable diseases wanes over time and requires boosting;
- Some vaccine-preventable infections are more virulent in adults and can cause serious health complications and even death (e.g. varicella);
- Adult vaccination can help boost immunity against certain diseases that are more common in adulthood (e.g. herpes zoster);
- Adult vaccination helps prevent individual infection, decreasing the risk of transmission to those who are unable to be vaccinated for medical reasons, not yet fully vaccinated, or unable to build a strong immunity following vaccination.
Measuring vaccination coverage is necessary to track Canada's progress towards achieving its vaccination coverage goals by 2025, as well as to identify under-vaccinated populations. The national vaccination coverage goals for adults include:
- Achieving increased vaccination coverage (one dose per season) for the seasonal influenza vaccine as follows:
- 80% vaccination coverage among adults 65 years of age and older
- 80% vaccination coverage among adults 18-64 years of age with chronic medical conditions (CMC)
- Achieving 80% vaccination coverage (one dose) of a pneumococcal vaccine among adults 65 years of age and older Footnote 1.
Influenza, also known as the flu, is a significant cause of morbidity and mortality in Canada, averaging 12,200 hospitalizations and 3,500 deaths in Canada each yearFootnote 2. It is important to get the influenza vaccine every year because the virus is constantly changing, and a new vaccine is developed for each influenza season based on the circulating virus strain expected to be dominant during the seasonFootnote 3. The best time to get the influenza vaccine is between October and December, before the virus begins spreading in the community. The National Advisory Committee on Immunization (NACI) recommends that all individuals aged six months and older get the annual seasonal influenza vaccine, especially for populations at increased risk for influenza-related complications or hospitalization including:
- Children 6-59 months of age;
- Persons with certain CMC, such as heart conditions, cancer, diabetes, lung/liver diseases and immune disorder;
- Seniors 65 years of age and older; and
- Pregnant womenFootnote 4.
Pertussis (also known as "whooping cough") is a highly communicable bacterial infection caused by the bacterium Bordetella pertussis. The infection can cause a debilitating cough that may persist for over two weeks in adults. For infants, the infection can be life threatening. It is often unrecognized and undiagnosed in adults. It is important to know that infected adults can act as a source of infection for infants and other children. In Canada, pertussis vaccine is available only in combination with tetanus and diphtheria vaccines. To reduce the risk of transmission to children, NACI recommends that all adults should receive one dose of the tetanus-diphtheria-acellular pertussis (Tdap) vaccine if they have not previously received a dose of pertussis-containing vaccines in adulthood. Additionally, one dose of Tdap vaccine should be administered in every pregnancy to allow for the production and transfer of protective antibodies to the baby before birthFootnote 5.
Tetanus (also known as "lockjaw") is caused by a neurotoxin produced by the bacterium Clostridium tetani. The spores of the bacteria are found in soil worldwide. If tetanus spores enter the body, they can cause serious muscle spasms and complications which can lead to death. Tetanus vaccine is only available in combination vaccines along with diphtheria. Tetanus vaccination takes place in infancy and childhood. In adulthood, a booster dose of a tetanus toxoid-containing vaccine is recommended every 10 yearsFootnote 6.
The bacterium Streptococcus pneumoniae is a common cause of pneumonia, and can also cause blood infections referred to as invasive pneumococcal disease (IPD). Individuals with some underlying medical conditions and seniors aged 65 and older are at higher risk of IPD. NACI recommends these persons receive the pneumococcal vaccine, if not previously vaccinatedFootnote 7.
Primary varicella-zoster virus infection causes varicella (also known as "chickenpox") and reactivated infection results in herpes zoster (also known as "shingles"). Herpes zoster causes a rash and neuropathic pain. This illness occurs most frequently among older adults and immunocompromised persons. Immunization with a shingles vaccine is recommended by NACI for individuals 50 years of age and older without contraindicationsFootnote 8.
Besides vaccination coverage, this report also describes knowledge, attitudes and beliefs (KAB) regarding vaccines in general and the influenza vaccine in particular. Positive or negative perceptions regarding vaccination could serve as a barrier or facilitator to vaccinationFootnote 9. Understanding these elements can help inform vaccination promotion efforts in order to increase uptake within the Canadian population.
Methodology
Survey sampling
The survey was conducted by Léger Marketing. A comprehensive description of the quantitative methodology is described elsewhereFootnote 10. Briefly, a stratified regional sampling approach was used, with survey respondents from each province and territory selected using random digit dialling of landlines and known cellphone-only household numbers.
Sample weights were calculated by Léger based on region, sex, age, language (mother tongue), education, presence of minor children in the household, and whether the respondent lives in a cellphone-only household.
Data collection
Interviews were conducted between January 21 and February 24, 2019, using a computer-assisted telephone interviewing (CATI) system. A total of 3,737 adults were interviewed regarding their vaccination status, reasons for vaccination or non-vaccination, KAB regarding vaccination, and select demographic information. Respondents who were unsure of their vaccination status for a specific vaccine were excluded from any subsequent analyses for the vaccine(s) they were unsure of.
Statistical analysis
Vaccination coverage was estimated as the number of respondents who reported they received a given vaccine, expressed as a weighted proportion of the respondents who provided a definitive response (i.e. reported did or did not receive the vaccine). Coverage for each antigen was calculated for either all adults or for specific sub-populations defined by age or by health condition, depending on NACI recommendations for specific vaccines. Simple weighted proportions and 95% confidence intervals were calculated for categorical variables. Chi-squared tests with a p-value <0.05 were used to determine significant differences in vaccination coverage between genders within each age or risk group.
Results
A total of 3,737 eligible adults completed the telephone interview. The overall response rate calculated using the Marketing Research Intelligence Association's standard calculation method for the response rate of a telephone survey was 20%Footnote 10.
All the rates reported hereafter are weighted, whereas the sample sizes are unweighted.
Seasonal influenza vaccination
1. Vaccination coverage
After exclusion of 11 individuals who did not recall whether they had been vaccinated or not, a total of 3,726 adult respondents were included in the vaccination coverage calculations.
Approximately four in ten (42%) adults aged 18 years and older reported receiving the 2018-2019 influenza vaccine. In general, influenza vaccine uptake was significantly higher in females (47%) than in males (37%, p<0.001). (Table 1.1).
The vaccination rate was lowest among adults 18-64 years of age without any CMC (31%). For those 18-64 years of age, with or without CMC, a significant difference in influenza vaccine uptake between females and males was also observed. However, this difference was not significant among seniors aged 65 years and older, which is consistent with other studiesFootnote 11,Footnote 12. (Table 1.1).
Although the national influenza vaccination coverage goal for those at high risk of influenza-related complications or hospitalization (80%) has not been achieved, vaccine uptake among seniors 65 years of age and older is approaching this goal (70%). (Table 1.1).
| N/A | All | Male | Female | N/A | |||
|---|---|---|---|---|---|---|---|
| Age group (years) | n | Vaccination coverage, % (95% CI) |
n | Vaccination coverage, % (95% CI) |
n | Vaccination coverage, % (95% CI) |
p |
| All adults ≥18 | 3726 | 41.8 (39.8-43.8) | 1568 | 36.6 (33.6-39.5) | 2150 | 46.8 (44.2-49.4) | <0.001* |
| 18-64 | 2898 | 34.3 (32.0-36.5) | 1252 | 28.6 (25.4-31.8) | 1640 | 39.9 (36.9-43.0) | <0.001* |
| 18-64 with CMC | 770 | 42.8 (38.5-47.2) | 304 | 36.3 (29.8-42.8) | 465 | 48.5 (42.9-54.1) | 0.006* |
| 18-64 without CMC | 2124 | 30.8 (28.2-33.4) | 948 | 25.8 (22.1-29.5) | 1171 | 36.1 (32.5-39.7) | <0.001* |
| ≥65 | 828 | 69.9 (66.5-73.4) | 316 | 69.0 (63.4-74.6) | 510 | 70.9 (66.6-75.2) | 0.598 |
Totals do not add up to 3726 because of missing gender or age information. *Significant difference between males and females (p<0.05). n = number of respondents (unweighted). |
|||||||
Overall, the influenza vaccination coverage rates for the 2018-2019 flu season are greater than the vaccination coverage estimates for previous seasons in CanadaFootnote 13,Footnote 14 and the estimates for the 2018-2019 season in the USA (37%)Footnote 15,Footnote 16,Footnote 17. (Figure 1.1).
Figure 1.1. Seasonal influenza vaccination coverage, by risk group and influenza season
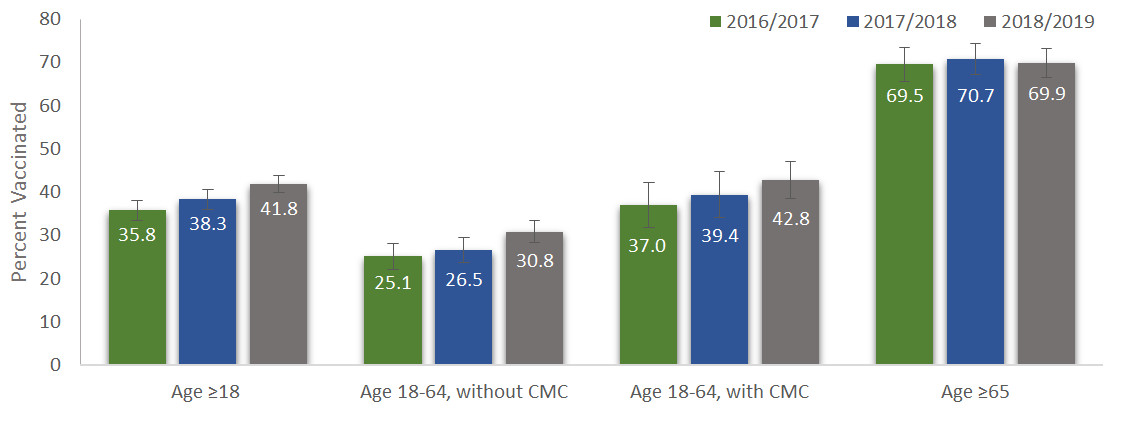
Figure 1: Text description
| Flu season | Percent vaccinated (%) |
|---|---|
| 2016-2017 | 35.8 |
| 2017-2018 | 38.3 |
| 2018-2019 | 41.8 |
| Flu season | Percent vaccinated (%) |
|---|---|
| 2016-2017 | 25.1 |
| 2017-2018 | 26.5 |
| 2018-2019 | 30.8 |
| Flu season | Percent vaccinated (%) |
|---|---|
| 2016-2017 | 37.0 |
| 2017-2018 | 39.4 |
| 2018-2019 | 42.8 |
| Flu season | Percent vaccinated (%) |
|---|---|
| 2016-2017 | 69.5 |
| 2017-2018 | 70.7 |
| 2018-2019 | 69.9 |
Influenza vaccination coverage increased mostly among adults 18-64 years of age without any CMC. Among high-risk groups, vaccination coverage rates for adults 18-64 years of age with CMC and seniors 65 years of age and older remained steady over the past three seasons. Consistent with the previous cycles of the survey, the proportion of vaccinated respondents was highest among seniors aged 65 years and older (70%), lower among those 18-64 years of age with CMC (43%), and lowest in those 18-64 years of age without CMC (31%). (Figure 1.1).
2. Month and place of vaccination
Among respondents who recalled the month they received their influenza vaccination (n=1570), the majority (79%) received the vaccine in October or November 2018. (Table 2.1). Vaccination early in the influenza season allows time for the development of antibodies against the influenza virus. Optimal antibody levels, which correlate with vaccine protection, are generally achieved by two weeks following vaccination.Footnote 1
| Month | Proportion vaccinated in this month, % (95% CI) |
|---|---|
| September 2018 | 5.5 (4.1-6.9) |
| October 2018 | 37.9 (35.0-40.9) |
| November 2018 | 41.6 (38.5-44.6) |
| December 2018 | 9.9 (8.2-11.7) |
| January 2019 | 5.1 (3.4-6.7)** |
| February 2019 | 0.0 (0.0-0.1)** |
A total of 1570 respondents recalled the month of influenza vaccination. **Coefficient of variation >16%; therefore, estimates should be interpreted with caution due to a higher level of error. CI - Confidence interval. |
|
Consistent with previous years, the most commonly reported places of vaccination among adults were pharmacies (35%) and doctor's offices (33%). (Table 2.2). The proportion of respondents vaccinated in pharmacies in 2018-2019 was significantly higher than the 2016-2017 season (28%). This may be due in part to the increasing number of jurisdictions allowing pharmacists to administer the influenza vaccine. It should be noted, however, that a proportion of the vaccines administered in pharmacies are given by nurses, not pharmacists.
| Place of vaccination | Proportion vaccinated by place, % (95% CI) |
|---|---|
| Pharmacy | 35.4 (32.5-38.3) |
| Doctor's office | 32.7 (29.9-35.4) |
| Work | 7.5 (5.7-9.3) |
| CLSC/Community Health Centre | 7.5 (5.7-9.2) |
| Hospital | 5.6 (4.2-6.9) |
| Temporary vaccine clinic | 4.9 (3.8-6.0) |
| Retirement residence | 1.4 (0.8-2.1)** |
| Other | 5.1 (3.8-6.4) |
A total of 1641 respondents recalled their place of influenza vaccination. **Coefficient of variation >16%; therefore, estimates should be interpreted with caution due to a higher level of error. CI - Confidence interval. |
|
3. Reasons for vaccination
Among adults who provided a reason for receiving the vaccine, 45% were vaccinated because they wanted to prevent infection or to avoid getting sick (Table 3.1). It was the most common reason for having received the influenza vaccine among high-risk groups, including seniors (44%) and those aged 18-64 years with CMC (46%).
| Age group (years) | Reason | % (95% CI) |
|---|---|---|
All adults ≥18 (n=1640) |
1. To prevent infection/don't want to get sick | 45.3 (42.4-48.3) |
| 2. Receive it yearly (no specific reason) | 20.4 (18.1-22.8) | |
| 3. If I don't, I can transmit to others (family, at-risk people, friends) | 12.1 (10.1-14.2) | |
18-64 without CMC (n=709) |
1. To prevent infection/don't want to get sick | 45.9 (40.9-50.9) |
| 2. If I don't, I can transmit to others (family, at-risk people, friends) | 16.9 (13.2-20.7) | |
| 3. Receive it yearly (no specific reason) | 14.5 (11.2-17.7) | |
18-64 with CMC (n=355) |
1. To prevent infection/don't want to get sick | 45.8 (39.5-52.1) |
| 2. Receive it yearly (no specific reason) | 16.8 (12.1-21.5) | |
| 3. At risk because of health condition | 15.6 (11.1-20.2) | |
≥65 (n=575) |
1. To prevent infection/don't want to get sick | 44.4 (40.0-48.9) |
| 2. Receive it yearly (no specific reason) | 29.8 (25.7-34.0) | |
| 3. At risk because of age | 14.8 (11.6-17.9) | |
Note: Respondents could provide more than one reason. n = number of respondents (unweighted). |
||
4. Reasons for non-vaccination
Among all survey respondents who provided a reason for choosing not to be vaccinated, the most common answer was the perception that the vaccine was not necessary (20%). However, among those aged 18-64 years with CMC, non-specific reasons for not receiving it was the most commonly provided response (20%). Concerns about vaccine safety was the most common reason for not receiving the 2018-2019 influenza vaccine among seniors (20%). (Table 4.1).
| Age group (years) | Reason | % (95% CI) |
|---|---|---|
All adults ≥18 (n=2074) |
1. I don't need the flu shot/it isn't necessary | 19.8 (17.6-22.0) |
| 2. No specific reason, just didn't get it | 18.4 (16.2-20.5) | |
| 3. I did not get around to it | 15.6 (13.5-17.7) | |
18-64 without CMC (n=1407) |
1. I don't need the flu shot/it isn't necessary | 20.7 (18.0-23.4) |
| 2. No specific reason, just didn't get it | 18.4 (15.8-21.1) | |
| 3. I did not get around to it | 16.4 (13.8-19.0) | |
18-64 with CMC (n=412) |
1. No specific reason, just didn't get it | 20.4 (15.4-25.5) |
| 2. I don't need the flu shot/it isn't necessary | 17.3 (12.4-22.2) | |
| 3. I did not get around to it | 16.9 (12.0-21.7) | |
≥65 (n=252) |
1. I do have concerns about the influenza vaccine, and/or its side effects | 20.1 (14.5-25.8) |
| 2. I don't need the flu shot/it isn't necessary | 19.2 (13.6-24.9) | |
| 3. No specific reason, just didn't get it | 13.9 (9.2-18.6)** | |
Note: Respondents could provide more than one reason. **Coefficient of variation >16%; therefore, estimates should be interpreted with caution due to a higher level of error. n = number of respondents (unweighted). |
||
For those at increased risk for influenza-related complications (i.e. seniors and those aged 18-64 years with CMC), one of the most commonly cited reasons for non-vaccination was the perception that the vaccine is not necessary (19% among seniors and 17% among adults with CMC). Low perceived risk of influenza among unvaccinated respondentsFootnote 18,Footnote 19 and recent media reports regarding low effectiveness of the influenza vaccineFootnote 20,Footnote 21 may have contributed towards this belief.
Among respondents who were prompted to clarify their response as to why they did not need the vaccine (n=428), the most common clarification provided was that they never get the flu or are healthy (55%). (Table 4.2).
| Clarification responses | % (95% CI) |
|---|---|
| I never get the flu/I'm healthy | 54.7 (48.6-60.9) |
| I am not a person at high risk/It's not recommended for me | 21.9 (16.8-27.0) |
| I am not likely to get very sick from the flu | 13.1 (8.6-17.5)** |
| The flu vaccine doesn't work | 3.4 (1.2-5.6)** |
| Other | 6.9 (4.0-9.8)** |
A total of 428 respondents clarified "I don't need the flu shot/it isn't necessary" as a reason for influenza non-vaccination. **Coefficient of variation >16%; therefore, estimates should be interpreted with caution due to a higher level of error. CI - Confidence interval. |
|
5. Knowledge, attitudes and beliefs regarding vaccination
The large majority of respondents (91%) agreed that vaccines are important for their health and the same proportion thought that they know enough about vaccines to make a decision about getting vaccinated. Regarding influenza vaccination, most of the respondents believed that getting sick with the flu can be serious and the flu can affect many people. One in three adults (30%) assumed that they would probably get sick with the flu during the influenza season. (Table 5.1).
| Statements | n | Strongly or somewhat agree % (95% CI) |
|---|---|---|
| All vaccines in general | ||
| In general, I consider vaccines to be important for my health. | 3712 | 91.4 (90.3-92.5) |
| I know enough about vaccines to make an informed decision about getting vaccinated. | 3704 | 90.6 (89.3-91.9) |
| Influenza vaccine | ||
| Getting sick with the flu can be serious. | 3716 | 94.0 (93.1-95.0) |
| I will probably get sick with the flu this season. | 3588 | 29.6 (27.7-31.5) |
| The flu does not really affect that many people. | 3597 | 20.1 (18.5-21.8) |
| The flu vaccine does not protect you against the flu. | 3551 | 40.7 (38.6-42.7) |
| Sometimes you can get the flu from the flu vaccine. | 3424 | 43.1 (40.9-45.2) |
| The flu vaccine is safe. | 3601 | 86.7 (85.3-88.2) |
| My family doctor, general practitioner, or nurse practitioner is an important part of my decision when it comes to getting the flu vaccine. | 3616 | 68.7 (66.8-70.7) |
n = number of respondents (unweighted). |
||
A majority (87%) of respondents believed that the flu vaccine is safe. However, approximately four in ten respondents (41%) strongly or somewhat agreed that the flu vaccine does not protect them against the flu; about the same proportion of respondents (43%) believed that they might get the flu from the flu vaccine - which is not true for any influenza vaccine. (Table 5.1).
Among those respondents who strongly agreed that the flu vaccine is safe (53%), 4% strongly agreed and 9% somewhat agreed with the statement that the flu shot could give them the flu, which seems to contradict the agreement of flu vaccine's safety. These results suggest the need to dispel the myth that the influenza vaccine can transmit the disease and to educate the Canadian population about the importance of getting the influenza vaccine and the safety of influenza vaccines.
About seven in ten respondents (69%) strongly or somewhat agreed that the opinion of their family doctor, general practitioner or nurse practitioner is an important part of their decision for getting the flu vaccine. This outcome shows that there is public trust in health care professionals and suggests that the advice by a health care provider and the frequency of interaction with the health system may play a large role in influencing influenza vaccine uptake.
Pertussis and tetanus vaccinations
For adults, the pertussis booster is given in combination with tetanus and diphtheria (Tdap) in CanadaFootnote 5. Approximately, one in three (33%) respondents reported having received a pertussis-containing vaccine in adulthood. The uptake is higher among adults aged 18-44 years (41%), and it declines as the age increases. (Table 6.1). The questions on pertussis vaccination were very different from what was previously asked in the earlier Adult National Immunization Coverage Survey (aNICS). For the first time, it was mentioned that pertussis vaccination was administered in a combination vaccine with tetanus. This change aimed to address the suspected under-reporting in the previous aNICS, and therefore results cannot be compared. Overall, pertussis vaccine uptake was significantly higher in females (37%) than in males (28%, p<0.001). The gender difference in pertussis vaccine uptake decreases as the age increases. (Table 6.1).
| N/A | All | Male | Female | N/A | |||
|---|---|---|---|---|---|---|---|
| Age group (years) | n | Vaccination coverage, % (95% CI) | n | Vaccination coverage, % (95% CI) | n | Vaccination coverage, % (95% CI | p |
| All adults ≥18 | 3184 | 33.1 (31.0-35.1) | 1306 | 28.2 (25.2-31.3) | 1878 | 37.4 (34.6-40.1) | <0.001* |
| 18-44 | 1446 | 40.8 (37.3-44.4) | 601 | 34.6 (29.3-39.8) | 845 | 46.4 (41.7-51.1) | 0.001* |
| 45-64 | 1001 | 29.1 (25.8-32.4) | 423 | 24.0 (19.2-28.8) | 578 | 33.9 (29.5-38.3) | 0.004* |
| ≥65 | 739 | 24.7 (21.4-28.1) | 282 | 23.0 (17.8-28.2) | 455 | 26.2 (21.9-30.4) | 0.361 |
Totals do not add up to 3726 because of missing gender or age information; and participants who did not recall whether they had received the pertussis vaccine were excluded from the pertussis coverage estimates. *Significant difference between males and females (p<0.05). n = number of respondents (unweighted). |
|||||||
For tetanus, NACI recommends that all adults receive a booster dose of tetanus toxoid-containing vaccine every 10 yearsFootnote 22. 69% of Canadian adults reported receiving a vaccine against tetanus in the previous 10 years. (Table 6.2). No difference in gender specific coverage was observed. Lower pertussis and tetanus vaccination coverage were observed among senior groups. (Table 6.1 and Table 6.2).
| N/A | All | Male | Female | N/A | |||
|---|---|---|---|---|---|---|---|
| Age group (years) | n | Vaccination coverage, % (95% CI) | n | Vaccination coverage, % (95% CI) | n | Vaccination coverage, % (95% CI) | p |
| All adults ≥18 | 3488 | 68.9 (66.9-70.8) | 1461 | 70.2 (67.2-73.2) | 2021 | 67.8 (65.3-70.3) | 0.227 |
| 18-64 | 2721 | 71.6 (69.4-73.8) | 1173 | 72.5 (69.2-75.9) | 1544 | 70.9 (68.0-73.7) | 0.461 |
| ≥65 | 767 | 58.2 (54.4-62.1) | 288 | 60.2 (54.0-66.5) | 477 | 56.8 (51.9-61.6) | 0.391 |
Totals do not add up to 3726 because of missing gender or age information; and participants who did not recall whether they had received the tetanus vaccine were excluded from the tetanus coverage estimates. n = number of respondents (unweighted). |
|||||||
Among adults who provided a reason for not receiving pertussis or tetanus vaccine, the most commonly cited reason was the perception that the vaccine was not necessary (30% and 38%, respectively). (Table 6.3).
| Reason | Pertussis (n=2136) % (95% CI) | Tetanus (n=1101) % (95% CI) |
|---|---|---|
| It isn't necessary | 30.1 (27.5-32.6) | 38.3 (34.6-42.1) |
| I never heard of this vaccine | 26.9 (24.4-29.5) | 14.7 (11.7-17.7) |
| Doctor did not mention it | 21.4 (19.1-23.7) | 11.6 (9.1-14.0) |
Note: Respondents could provide more than one reason for non-vaccination. n = number of respondents (unweighted). |
||
Pneumococcal vaccination
NACI recommends one dose of pneumococcal polysaccharide (Pneu-P-23) vaccine for all older adults (65 years of age and older) and adults with certain chronic medical conditions including heart conditions, asthma, other lung condition, diabetes, immune disorder and liver disease that are at increased risk for invasive pneumococcal disease (IPD).Footnote 7 The Pneu-P-23 vaccine is publicly-funded for both of these target groupsFootnote 23. A greater proportion of older adults (58%) reported having received a pneumococcal vaccine in adulthood as compared to younger adults (18-64 years of age) with CMC (25%). (Table 7.1). Pneumococcal vaccination coverage was significantly higher for females compared to males in the 65 years and older age group (47% for males vs. 66% for females). The difference was not significant among those aged 18-64 years with CMC.
| N/A | All | Male | Female | N/A | |||
|---|---|---|---|---|---|---|---|
| Risk group (years) | n | Vaccination coverage, % (95% CI) | n | Vaccination coverage, % (95% CI) | n | Vaccination coverage, % (95% CI) | p |
| 18-64 with CMC | 633 | 25.4 (21.3-29.6) | 249 | 22.6 (16.2-28.9) | 383 | 27.5 (22.1-32.8) | 0.256 |
| ≥65 | 798 | 58.1 (54.3-61.9) | 297 | 47.0 (40.7-53.3) | 499 | 66.5 (62.0-70.9) | <0.001* |
*Significant difference between males and females (p<0.05). n = number of respondents (unweighted). |
|||||||
Among respondents who provided a reason for not receiving a pneumococcal vaccine, the most commonly cited reason among adults 18-64 years of age with CMC was that they never heard of this vaccine (23%). In contrast, the most common reason for seniors was the perception that the pneumococcal vaccine is not necessary (10%). (Table 7.2).
| Risk group (years) | Reason | % (95% CI) |
|---|---|---|
18-64 with CMC (n=715) |
1. I never heard of this vaccine | 22.7 (18.8-26.6) |
| 2. I didn't think it was necessary | 10.8 (8.1-13.4) | |
| 3. Doctor did not mention it | 10.4 (7.7-13.1) | |
≥65 (n=833) |
1. I didn't think it was necessary | 10.5 (8.2-12.8) |
| 2. I never heard of this vaccine | 7.7 (5.7-9.7) | |
| 3. Doctor did not mention it | 6.7 (4.8-8.5) | |
Note: Respondents could provide more than one reason. n = number of respondents (unweighted). |
||
Shingles vaccination
NACI recommends that adults 50 years of age and older receive one dose of shingles vaccineFootnote 24. However, shingles vaccine is not publicly funded in all provincesFootnote 23. Among participants 50 years of age and older, 28% reported receiving their shingles vaccine. No difference in gender specific coverage was observed (p=0.086). (Table 8.1).
| Vaccination coverage | n | % (95% CI) |
|---|---|---|
| All | 1721 | 27.6 (25.3-30.0) |
| Male | 685 | 25.3 (21.6-29.0) |
| Female | 1034 | 29.5 (26.5-32.6) |
n = number of respondents (unweighted). CI - Confidence interval. |
||
Among adults 50 years of age and older who provided a reason for not receiving a shingles vaccine (n=1753), the most commonly cited reason was the perception that the vaccine was not necessary (17%). Approximately, one in ten respondents (9%) did not get the shingles vaccine because of the cost of the vaccine. (Table 8.2).
| Reason | % (95% CI) |
|---|---|
| I didn't think it was necessary | 16.9 (14.8-18.9) |
| I have not gotten around to it | 11.3 (9.6-13.1) |
| Cost of the vaccine | 9.4 (7.9-11.0) |
A total of 1753 respondents provided a reason for not receiving the shingles vaccine. Note: Respondents could provide more than one reason. CI - Confidence interval. |
|
Discussion
In the 2018-2019 Seasonal Influenza Vaccination Coverage Survey, reported vaccination coverage among Canadian adults remained below the national vaccination coverage goals for influenza and pneumococcal vaccinesFootnote 1.
In the general adult population, vaccination coverage against pertussis was the lowest among all vaccines covered by publicly-funded programs. As identified by another study, there is low awareness among Canadian adults regarding the need for pertussis vaccination and its existence in the Tdap vaccineFootnote 25.
Very low shingles vaccination coverage might partly be due to the fact that the vaccine is not publicly funded in all jurisdictions. Almost one in ten respondents aged 50 years and older identified the cost of the vaccine as their reason for not getting vaccinated. Moreover, the fact this vaccine is not funded by governments whereas others are, may contribute to the perception that this vaccine is not necessary.
Vaccination coverage for target groups at high risk of severe complications was also suboptimal. Adults aged 65 years and older reported higher coverage for the seasonal influenza and pneumococcal vaccines as compared to individuals aged 18-64 years with CMC, but coverage remains below the national goals. Individuals unaware that they are considered at high risk of influenza-related complications may contribute to low coverageFootnote 26.
This survey also provides clues for actions to consider in vaccination promotion campaigns, including: dispelling the myth that influenza vaccine can cause influenza and increasing the Canadian population's awareness of the importance and usefulness of influenza vaccination-despite its relatively low efficacy compared to other recommended vaccines.
Strengths and limitations
The major strength of this survey was the timely reporting of seasonal influenza vaccination coverage across Canada. The timeliness of this survey allows Canada to meet its international reporting obligations and help identify priorities for future vaccination program planning. Additionally, the Seasonal Influenza Vaccination Coverage Survey is flexible in allowing question modules to be added or removed on an annual basis in light of changing priorities.
Though consistent with previous cyclesFootnote 13,Footnote 14, limitations of this survey included the relatively low response rate of 20%, below the 45% achieved by a similar survey in the USAFootnote 27. This response rate can increase the potential for non-response bias, as survey respondents may differ from those who chose not to complete the survey.
Additionally, survey respondents were interviewed within six months of the beginning of the seasonal influenza vaccination campaign to further mitigate recall bias. Other vaccinations, such as pertussis, tetanus and pneumococcal vaccines, may have been administered more than 10 years before the survey was administered, thereby increasing the likelihood of inaccurate self-reporting. Recent studies have found that self-reported pneumococcal vaccination may underestimate the true rate due to unawareness of pneumococcal vaccinationFootnote 28. However, it appears in some studies that self-reported influenza vaccination status is a valid measure of vaccine exposure when medical records or registry data are not availableFootnote 28,Footnote 29.
This year, the respondents were told that pertussis vaccine is given in combination with tetanus. The substantial increase in reported coverage compared to previous years most likely reflects the change in questions on pertussis vaccination rather than a real change in vaccination coverage.
Conclusion
Adult influenza vaccination coverage estimated from the 2018-2019 Seasonal Influenza Vaccination Coverage Survey was considerably higher compared to previous influenza seasons. Nonetheless, the national vaccination coverage goal of 80% for those who are at increased risk of influenza-related complications, namely seniors and adults 18-64 years of age with CMC, remain unmet. A greater proportion of seniors reported having received a pneumococcal vaccine in their adulthood compared to younger adults, but again, the national vaccination coverage goal of pneumococcal vaccine among adults 65 years of age and older is still unmet.
The most common reported reasons for influenza vaccination was to prevent infection or to avoid getting sick, whereas the most common reason for non-vaccination was the perception that the vaccine was not necessary.
Ongoing efforts to promote and educate the adult population on the benefits of recommended vaccines is required in order to increase uptake, particularly among those who are considered at high risk of severe complications. We must continue to improve our understanding of the factors influencing vaccination coverage rates, and to identify and evaluate effective strategies and interventions to increase uptake.
References
- Footnote 1
-
Public Health Agency of Canada. Vaccination Coverage Goals and Vaccine Preventable Disease Reduction Targets by 2025. 2019; Available at: https://www.canada.ca/en/public-health/services/immunization-vaccine-priorities/national-immunization-strategy/vaccination-coverage-goals-vaccine-preventable-diseases-reduction-targets-2025.html
- Footnote 2
-
Schanzer DL, Sevenhuysen C, Winchester B, Mersereau T. Estimating influenza deaths in Canada, 1992-2009. PLoS One 2013;8(11);e80481.
- Footnote 3
-
Petrova VN, Russell CA. The evolution of seasonal influenza viruses. Nature Reviews Microbiology 2017;16:47.
- Footnote 4
-
An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2018-2019. 2018.
- Footnote 5
-
Public Health Agency of Canada. Canadian Vaccination Guide - PART 4: Active Vaccines - Pertussis Vaccine. 2016; Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines.html
- Footnote 6
-
Public Health Agency of Canada. Canadian Vaccination Guide - PART 4: Active Vaccines - Tetanus Toxoid. 2014; Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-22-tetanus-toxoid.html
- Footnote 7
-
Public Health Agency of Canada. Canadian Vaccination Guide - PART 4: Active Vaccines - Pneumococcal Vaccine. 2016; Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-16-pneumococcal-vaccine.html
- Footnote 8
-
Public Health Agency of Canada. Canadian Vaccination Guide - PART 4: Active Vaccines - Herpes Zoster (Shingles). 2018; Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-8-herpes-zoster-(shingles)-vaccine.html
- Footnote 9
-
Nowak GJ, Sheedy K, Bursey K, Smith TM, Basket M. Promoting influenza vaccination: Insights from a qualitative meta-analysis of 14 years of influenza-related communications research by U.S. Centers for Disease Control and Prevention (CDC). Vaccine 2015;33:2741-2756.
- Footnote 10
-
Léger Marketing. Seasonal Influenza Vaccination Coverage Survey, 2018-2019. POR 065-18. 2019; Available at: http://epe.lac-bac.gc.ca/100/200/301/pwgsc-tpsgc/por-ef/public_health_agency_canada/2019/065-18-e/report.pdf
- Footnote 11
-
Roy M, Sherrard L, Dubé È, Gilbert NL. Determinants of non-vaccination against seasonal influenza. Health Reports 2018; 29:13-23.
- Footnote 12
-
Farmanara N, Sherrard L, Dubé È, Gilbert NL. Determinants of non-vaccination against seasonal influenza in Canadian adults: findings from the 2015-2016 Influenza Immunization Coverage Survey. Canadian Journal of Public Health. 2018;109(3):369-78.
- Footnote 13
-
Public Health Agency of Canada. 2016/17 Seasonal Influenza Vaccine Coverage in Canada. 2018; Available at: http://publications.gc.ca/collections/collection_2018/aspc-phac/HP40-198-2017-eng.pdf
- Footnote 14
-
Public Health Agency of Canada. 2017/18 Seasonal Influenza Vaccine Coverage in Canada. 2019; Available at: http://publications.gc.ca/collections/collection_2019/aspc-phac/HP40-198-2018-eng.pdf
- Footnote 15
-
Santibanez TA, Zhai Y, O'Halloran A, Kahn KE, Srivastav A, Liu L, et al. Flu Vaccination Coverage, United States, 2016-17 Influenza Season. 2017.
- Footnote 16
-
Srivastav A, Williams WW, Santibanez TA, Kahn KE, Zhai Y, Lu P, et al. National Early-Season Flu Vaccination Coverage, United States, November 2017. 2017.
- Footnote 17
-
Centers for Disease Control and Prevention. Estimates of Influenza Vaccination Coverage among Adults-United States, 2017-18 Flu Season. 2018; Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1718estimates.htm
- Footnote 18
-
Kan T, Zhang J. Factors influencing seasonal influenza vaccination behaviour among elderly people: a systematic review. Public Health 2018;156:67-78.
- Footnote 19
-
Schmid P, Rauber D, Betsch C, Lidolt G, Denker M. Barriers of influenza vaccination intention and behavior-A systematic review of influenza vaccine hesitancy, 2005-2016. PLoS One 2017; 12(1);e0170550.
- Footnote 20
-
Ireland N. Flu vaccine may have low effectiveness against dominant strain, Canada's top public health doctor says. 2018; Available at: https://www.cbc.ca/news/health/flu-vaccine-potential-low-effectiveness-h3n2-1.4476100
- Footnote 21
-
Belluz J. The flu shot offers lousy protection against this year's worst strain. Blame eggs 2018; Available at: https://www.vox.com/science-and-health/2018/2/1/16960758/flu-vaccine-effectiveness
- Footnote 22
-
Public Health Agency of Canada. Canadian Immunization Guide - PART 3: Vaccination of Specific Population - Immunization of Adults. 2016; Available at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-2-immunization-of-adults.html
- Footnote 23
-
Public Health Agency of Canada. Canada's Provincial and Territorial Routine Vaccination Programs for Healthy, Previously Immunized Adults. 2019; Available at: https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/routine-vaccination-healthy-previously-immunized-adult.html
- Footnote 24
-
An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Updated Recommendations on the Use of Herpes Zoster Vaccines. 2018; Available at: https://www.canada.ca/en/services/health/publications/healthy-living/updated-recommendations-use-herpes-zoster-vaccines.html
- Footnote 25
-
Halperin BA, MacDougall D, MacKinnon-Cameron D, Li L, McNeil SA, Langley JM, et al. Universal Tetanus, diphtheria, acellular pertussis (Tdap) vaccination of adults: What the Canadian public knows and wants to know. Vaccine. 2015;33(48):6840-48.
- Footnote 26
-
Schoefer Y, Schaberg T, Raspe H, Schaefer T. Determinants of influenza and pneumococcal vaccination in patients with chronic lung diseases. J Infect 2007;55(4):347-52.
- Footnote 27
-
Centers for Disease Control and Prevention. The Behavioral Risk Factor Surveillance System 2017: Summary Data Quality Report. 2018; Available at: https://www.cdc.gov/brfss/annual_data/2017/pdf/2017-sdqr-508.pdf
- Footnote 28
-
Laurence A, Lewis P, Gately C, Dixon A. Influenza and pneumococcal vaccination: do older people know if they have been vaccinated?. Aust N Z J Public Health 2016;40(3):279-280.
- Footnote 29
-
King JP, McLean HQ, Belongia EA. Validation of self-reported influenza vaccination in the current and prior season. Influenza Other Respi Viruses 2018 07/20; 2018/08;0(0).