Cost-Effectiveness of Palivizumab Prophylaxis for Respiratory Syncytial Virus (RSV): A Systematic Review
Published: February 1, 2023
On this page
- Conflict of interest
- Abbreviations
- Table of contents summary
- Executive summary
- Introduction
- Methods
- Results
- Study characteristics
- Quality appraisal
- Study population
- Study outcomes
- Economic evaluations with outcomes expressed in cost per QALY
- Economic evaluations with outcomes expressed in cost per hospitalization avoided
- Economic evaluations with outcomes expressed in other ratios
- Economic evaluations from a Canadian setting
- Cost-effectiveness in preterm infants
- Key model parameters
- Influential parameters
- Discussion
- Conclusion
- Figures and Tables
- Figure 1. Literature search and study selection
- Figure 2. Quality appraisal results
- Figure 3. Cost-effectiveness of PVZ in preterm infants where the ICER was less than $200,000 per QALY
- Figure 4. Most influential parameters reported
- Table 1. Summary of study and model characteristics
- Table 2. Summary of study cost-effectiveness (unadjusted and adjusted to 2017 Canadian dollars) outcomes
- Table 3. Summary of cost-effectiveness estimates by health condition and perspective
- Table 4. Characteristics and conclusions from Canadian-context studies
- Supplementary materials
- References
Conflict of interest
The authors have no conflicts of interest relevant to this report to disclose.
Abbreviations
BPD, bronchopulmonary dysplasia; CAD, Canadian dollar; CE, cost-effective; CF, cystic fibrosis; CHD, congenital heart disease; CLD, chronic lung disease; GA, gestational age; HA, hospitalizations avoided; ICER, Incremental cost-effectiveness ratio; LYG, life years gained; OECD, Organisation for Economic Co-operation and Development; PPP, purchasing power parity; QALY, quality-adjusted life year; RSV, respiratory syncytial virus; RSVH, respiratory syncytial virus hospitalization; PVZ, palivizumab; UK, United Kingdom; US, United States; USD, United States dollar; wGA, weeks gestational age (GA)
Table of contents summary
This systematic review investigates the cost-effectiveness of palivizumab prophylaxis for respiratory syncytial virus, stratified by setting and infant population subgroups relevant to health policy decision-making.
Executive summary
Background: Palivizumab (PVZ) prophylaxis is used as passive immunization for respiratory syncytial virus (RSV). However, due to its high acquisition costs, the value of this intervention is unclear. The objective of this study was to systematically review the cost-effectiveness of PVZ prophylaxis compared to no prophylaxis in infants under 24 months of age.
Methods: The systematic review followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Three databases were searched: Medline, Embase, and the Cochrane Library for terms related to RSV, PVZ, cost-effectiveness, health economics, and economic evaluations. The following were included: economic evaluations (e.g., cost-benefit, cost-effectiveness, and cost-utility analyses) conducted from Organization for Economic Co-operation and Development (OECD) countries, published between 2000 and 2018. Quality appraisal was completed using the Joanna Briggs Institute checklist for economic evaluations. Costs were adjusted to 2017 Canadian dollars (CAD), using purchasing power parity and inflation rates. Results were stratified based on outcomes used, study perspective and other risk factors (e.g., prematurity, chronic lung disease, and congenital heart disease) for RSV. Parameters affecting cost-effectiveness were summarized.
Results: A total of 28 economic evaluations met the inclusion criteria, of which 20 were cost-utility analyses, and 8 studies were cost-effectiveness analyses. Most studies were conducted in the United States (n=6), Canada (n=5), Netherlands (n=3), the United Kingdom (n=3) and Spain (n=3). Overall, included studies were considered good to high quality; 23 studies met over 80% of the checklist criteria. PVZ prophylaxis ranged from being a dominant strategy (i.e., less costly and more effective) to having an incremental cost-effectiveness ratio (ICER) of $2,975,489/quality-adjusted life years (QALY) depending on the study setting, perspective, population (risk factors, weeks GA at birth), and key model input parameters such as reduction in RSV hospitalizations (39%-96%), RSV-related mortality (1%-8.1%), and PVZ costs ($1,099-$2,198 per 100-mg vial). From the payer perspective, the cost-effectiveness of PVZ prophylaxis was estimated for infants born prematurely at 29 to 35 weeks GA (ICER: $6,216/QALY to $938,623/QALY, n=21), with 82% of estimates below $50,000/QALY. The top 3 influential parameters reported were: reduction in RSV hospitalization (RSVH) rates, PVZ cost, and the discounting rate.
Conclusions: Cost-effectiveness results of PVZ as a RSV prophylaxis were heterogeneous across studies, ranging from being dominant (i.e., less costly and more effective) to highly not cost-effective. Results varied due to study setting, population of interest, local RSV epidemiology, and healthcare setup, as well as key model parameters such as reduction in hospitalization rates, RSV acquisition costs, dosage schemes and vial usage. Palivizumab may be considered cost-effective in specific subgroups: infants with bronchopulmonary dysplasia / chronic lung disease, infants with congenital heart disease, term infants from specific remote communities with high baseline RSVH rates, and preterm infants with and without lung complications. No overall trends were detected between GA thresholds and cost-effectiveness results. No trends were seen either when stratified by perspective.
Introduction
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections in infants and young children worldwide Footnote 1. It is a ubiquitous virus that nearly 100% of infants will contract within 2 years after birth Footnote 2Footnote 3Footnote 4. RSV is seasonal respiratory infection that is a significant cause of morbidity and mortality, with the virus estimated to cause up to 90% of pediatric bronchiolitis hospitalizations and up to 50% of pediatric hospitalizations for pneumonia Footnote 1Footnote 5. Risk factors for severe RSV in infants include: preterm birth, congenital heart disease (CHD), bronchopulmonary dysplasia (BPD)/chronic lung disease (CLD), cystic fibrosis (CF), Down syndrome, and a weakened immune systemFootnote 6Footnote 7Footnote 8.
Although there is currently no vaccine available to prevent RSV infection, since 1998, passive prophylaxis has been available with palivizumab (PVZ)Footnote 9. PVZ is a humanized murine monoclonal antibody administered monthly as an intramuscular injection, and has shown a significant reduction in overall rate of RSVH infection Footnote 10. However, due to its high acquisition costs, there has been considerable debate surrounding the cost-effectiveness of this intervention. Since 2000, 8 reviews have summarized the cost-effectiveness of PVZ, of which half were completed over 10 years ago Footnote 11Footnote 12Footnote 13Footnote 14. A recent study by Andabaka et al. in 2013 reported that the economic evaluation results are inconsistent across studies, ranging from highly cost-effective to not cost-effective depending on the scenario Footnote 15.
The objective of this study was to provide an update on the cost-effectiveness of PVZ passive immunization for prevention of RSV in infants and children up to 24 months of age, and where possible, to stratify results by risk populations to inform policy decisions for these groups. Economic evaluations conducted in high income countries from the Organization for Economic Co-operation and Development (OECD) after the year 2000 were included in order to limit heterogeneity in population baseline health, healthcare systems and quality of care. This review provides a much needed update to support health policy decision-making for PVZ prophylaxis with particular emphasis on cost-effectiveness results according to GA at birth for preterm infants, which has historically been an area of clinical and policy uncertainty Footnote 16Footnote 17Footnote 18.
Methods
This systematic review was completed to inform the National Advisory Committee on Immunization's evidence-informed recommendations on PVZ prophylaxis for RSV, which are presented in the NACI Statement entitled "Recommended Use of Palivizumab to Reduce Complications of Respiratory Syncytial Virus Infection in Infants" published on June 1, 2022. The original systematic review was published in the journal of Pediatrics in 2019 Footnote 19. For the purposes of this NACI supplement, changes were made to the reporting and discussion of the original review to meet the needs of NACI's decision-making, including having currency reported in CAD, a section on Canadian studies, alternate subgroups reported, and additional commentary.
Search strategy
The systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Appendix 1) Footnote 20. The search strategy was developed with a Public Health Agency of Canada librarian (LG). The scientific literature search included English and French language studies published in 3 electronic databases: Medline and E-pub Ahead of Print, In-Process & Other Non-Indexed Citations (Ovid interface), Embase (Ovid interface), and the Cochrane Library, which included the Health Technology Assessment Database (HTA), National Health Service Economic Evaluation Database Economic Evaluation Database (NHS EED) and Database of Abstracts of Reviews of Effects (DARE). The search used medical subject headings and text words related to the following concepts: respiratory syncytial virus, palivizumab, economic evaluations, and cost-effectiveness. The primary search strategy was developed in Medline and adapted to other databases for account for database-specific vocabulary and functionality. A complete list of search terms and the full search strategy for Medline are summarized in Appendix 2. Reference lists were manually searched from relevant articles and systematic reviews.
Eligibility criteria
The protocol and eligibility criteria for studies are published on PROSPERO (CRD42018104977). The following were included: full economic evaluations (e.g., cost-benefit analysis, cost-effectiveness analysis and cost-utility analysis) comparing palivizumab prophylaxis for RSV to any comparator (e.g., no prophylaxis) for infants up to 24 months of age, based on current NACI guidelines Footnote 21. Economic evaluations were included if they were conducted in OECD countries between 2000 and 2018 (the time the review was conducted), and reported outcomes related to an incremental ratio of cost per unit of effect (e.g., cost per quality-adjusted life year (QALY), cost per cases avoided, cost per life year gained (LYG), and cost-benefit ratio). Cost-minimization studies, cost-of-illness studies, and budget impact analyses were excluded. Studies conducted outside of the OECD countries, studies published in a language other than English or French, and studies published prior to 2000 were also excluded.
Data extraction and analysis
All levels of screening, data extraction and quality appraisal were completed in duplicate. Conflicts were discussed and resolved through consensus. Data extraction was guided by CHEERS (Consolidated Health Economics Evaluation and Reporting Statement) Footnote 22. The following were collected: study characteristics (publication year, country, study design, study perspective, time horizon, discounting, primary and secondary outcomes, use of cost-effectiveness thresholds, funding sources), study population characteristics (age range, GA, health conditions, setting), key parameters (RSV incidence/hospitalization rates, mortality rates, sequelae, cost of PVZ, number of doses), and results (base-case incremental cost-effectiveness ratios (ICER), scenario analyses, type of sensitivity analysis, and influential parameters). The quality of included studies were assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Economic Evaluations Footnote 23. A study was "high-quality" if it met over 80% of the JBI checklist criteria Footnote 24. The World Health Organization checklist for immunization programmes to assess economic evaluations was not used since PVZ is not considered a vaccine.
Study and population characteristics were summarized descriptively. Cost-effectiveness outcomes were adjusted to 2017 CAD using purchasing power parity (PPP) rates from the OECD Footnote 25 and inflation rates from the Bank of Canada. Unadjusted and adjusted ICERs were summarized. In this supplement, currency was not updated to the year 2022 to retain the prices upon which NACI had deliberated upon. Subgroup analyses were conducted to summarize the cost-effectiveness for studies conducted from a Canadian perspective, and studies reporting cost-effectiveness in costs/QALY for preterm infants. For studies that included preterm infants, ICERs were stratified based on GA at birth (weeks) and plotted to visually identify the spread of ICER estimates and possible trends related to GA. The number of estimates and the proportion of them being cost-effective at various thresholds were summarized. Meta-analysis of cost-effectiveness was not appropriate due to the heterogeneity of the study setting, model designs, parameters used, population, and perspective taken in the studies.
Results
Study characteristics
The systematic literature search identified 237 unique records, of which 30 met the eligibility criteria and were included in this review (Figure 1) Footnote 14Footnote 26Footnote 27Footnote 28Footnote 29Footnote 30Footnote 31Footnote 32Footnote 33Footnote 34Footnote 35Footnote 36Footnote 37Footnote 38Footnote 39Footnote 40Footnote 41Footnote 42Footnote 43Footnote 44Footnote 45Footnote 46Footnote 47Footnote 48Footnote 49Footnote 50Footnote 51Footnote 52Footnote 53Footnote 54 . Conclusions from 2 studies Footnote 33Footnote 53 were updated using more recent data Footnote 34Footnote 41; hence, only the more recent studies were included in this review. The 28 studies included were published between 2000 and 2018, with most conducted from the United States (US) (n=6), Canada (n=5), Netherlands (n=3), United Kingdom (UK) (n=3), and Spain (n=3). The rest of the studies were conducted from: Austria (n=2), Germany (n=2), Italy (n=1), Mexico (n=1), New Zealand (n=1), and Sweden (n=1) Study characteristics are summarized in Table 1.
Of the 28 economic evaluations (20 cost-utility analyses, 8 cost-effectiveness analyses), 23 studies used decision-tree models, 4 used Markov cohort models, and 1 conducted a microsimulation. Two economic evaluations were piggy-backed with a clinical trial, and the rest were considered model-based. All studies conducted deterministic sensitivity analysis; 15 studies conducted probabilistic sensitivity analyses.
Quality appraisal
Most studies (83%) met over 80% of the JBI quality appraisal checklist criteria (Appendix 3). The 2 checklist items that were least met were: (1) whether the study results included all issues of concerns to users (39%); and (2) whether all relevant costs and outcomes were identified (75%). Overall, studies included in this review were considered relatively high-quality (Figure 2).
Study population
In 14 studies, the chronological patient age was explicitly reported to be <24 months, while the other 14 studies were assumed to assess the cost-effectiveness of PVZ in infants <24 months of age based on their respective country guidelines on PVZ use. High-risk infant populations were often studied, and in some cases overlapped: preterm infants (< 35 weeks gestational age, wGA) (n=19), BPD or CLD (n=13), CHD (n=11), and other risk factors (n=6).
Study outcomes
Base-case analyses were almost equally conducted from a societal perspective (n=13) or health system payer perspective (n=15). Eight of the 15 payer perspective studies performed additional analyses from a societal perspective. Time horizon ranged from 6 months to lifetime, and 1 studyFootnote 50 did not report a time horizon (Table 1). Discount rates ranged between 3% and 5%; 5 studies did not discount due to time horizon of 1 year or less Footnote 26Footnote 35Footnote 36Footnote 47Footnote 48 and 3 studies did not report a discount rateFootnote 43Footnote 50Footnote 15. The majority of studies were industry sponsored (n=17, 61%). Cost-effectiveness was reported as ICERs, mostly represented as the incremental cost per additional QALY (n=20) and cost per hospitalization avoirded (HA) (n=6). For the remainder of this review, results are reported as adjusted ICERs (2017 CAD); original unadjusted ICERs are summarized in Table 2.
Economic evaluations with outcomes expressed in cost per QALY
For studies reporting cost-effectiveness in incremental cost per QALY units, Table 3 summarizes the number of estimates, the ICER ranges, and proportion of estimates under selected thresholds of $50,000/QALY to $200,000/QALY, stratified by population subgroups and study perspective. From a health system payer perspective, there were 22 variable cost-effectiveness estimates for preterm infants, ranging between $6,216/QALY and $938,623 per QALYFootnote 14Footnote 29Footnote 30Footnote 31Footnote 34Footnote 38Footnote 39Footnote 41Footnote 49. The subgroups with the next highest number of estimates were preterm infants with risk factors (n=14)Footnote 27Footnote 39Footnote 41Footnote 42 where the ICER was between $215/QALY and $205,563/QALY, infants with CHD (n=10) Footnote 14Footnote 29Footnote 31Footnote 32Footnote 34Footnote 37 where the ICER was between $11,668/QALY and $164,946/QALY, and infants with BPD/CLD (n=6)Footnote 29Footnote 31Footnote 34Footnote 37Footnote 49 where the ICER was between $4,786/QALY and $46,821/QALY. At a $100,000/QALY threshold, 86% of estimates for preterm infants, 86% of estimates for preterm infants with risk factors, 90% of estimates for infants with CHD, and 100% of estimates for infants with BPD/CLD were considered cost-effective. Other risk factors considered in preterm infants included chronological age at the beginning of the RSV season, school age siblings, day-care attendance, smoking during pregnancy, male sex, and CF (in term infants only)Footnote 27Footnote 39Footnote 41Footnote 44Footnote 54. From a societal perspective, PVZ prophylaxis was considered a dominant strategy (i.e., less costly and more effective) in some instances for preterm infantsFootnote 30Footnote 44Footnote 54, term infants (with and without other risk factors)Footnote 42 and infants with CHDFootnote 31.
Economic evaluations with outcomes expressed in cost per hospitalization avoided
There were 6 studies that reported cost-effectiveness as cost per hospitalization avoided (HA)Footnote 14Footnote 26Footnote 35Footnote 36Footnote 43Footnote 50 of which 3 were industry fundedFootnote 26Footnote 36Footnote 43. The study by Banerji et al. studied healthy term infants from a payer perspective in different regions of the Canadian Arctic, and compared 2 scenarios of PVZ prophylaxis for infants who were <6 months of age. The ICER for PVZ prophylaxis ranged from being dominant (in specific Arctic regions) up to $593,250/HA in the Northwest TerritoriesFootnote 26. Also from the payer perspective, Hampp et al. was conducted in a Florida (US) setting, and studied cost-effectiveness in preterm infants (<32 wGA) and term infants with CHD, CLD and combinations of all 3 risk factors. The ICERs were between $413,127/HA (preterm infants) and $2,924,911/HA (healthy term infants without CLD or CHD)Footnote 50.
From a societal perspective, the study by Rietveld et al. in 2010 from southwest Netherlands studied preterm infants (< 28 wGA) with additional risk factors (male sex, birth weight < 2,500 grams, and BPD). The ICER ranged between $24,875/HA and $1,572,268/HA depending on the month of the prophylaxis. PVZ prophylaxis had the lowest ICER) during the month of December while October had the highest ICER (indicating poor value for money). This study recommended a restricted immunization policy based on their resultsFootnote 35. Roeckl-Wiedmann et al. conducted a study in 2003 from southern Germany on preterm infants (<35 wGA) with additional risk factors. ICERs ranged between $11,821/HA and $364,462/HA for preterm infants with CLD and preterm with risk factors (male, no CLD, no siblings in school), respectively. This study also recommended a restricted use of PVZ in preterm infants with CLD 36 . In New Zealand, Vogel et al. studied preterm infants (<28, 29-31 wGA) and infants with CLD. The ICER ranged between $33,376/HA for preterm infants discharged home on oxygen and $193,859/HA for preterm (29-31 wGA) infants with CLD. The authors concluded that the intervention was more cost-effective for preterm infants discharged home on oxygen, followed by preterm infants of 28 weeks’ gestation or lessFootnote 43.
Economic evaluations with outcomes expressed in other ratios
Three studies reported cost-effectiveness of PVZ prophylaxis in other units: cost to prevent 1 day of hospitalizationFootnote 51. cost per life-year gained (LYG)Footnote 52, and cost per RSV infection episode avoidedFootnote 47. Two of 3 studies conducted analyses from a societal perspectiveFootnote 51Footnote 52. Harris et al. conducted an economic evaluation on term infants with CHD in western Canada. The base-case ICER was $18,155 per 1 day of hospitalization preventedFootnote 51. Hascoet et al. studied preterm infants (< 32 wGA) with BPD and infants with significant CHD in France. The base-case ICER was $43,856 per LYG and $33,450/LYG for preterm infants with BPD, and preterm infants with cardiopathy (CHD), respectively. Originally, this study used a cost-effectiveness threshold (unadjusted) of 45,000 Euros/LYG, and considered prophylaxis cost-effective for both subgroups in FranceFootnote 52. Lastly, a study by Lofland et al. studied preterm infants with CLD in the US. Their model used a reduction in incidence of RSV infection instead of a hospitalization reduction approach, ranging from 50% ($66,494 per RSV infection episode avoided) to 83% reduction, where PVZ prophylaxis was considered a dominant strategy (i.e. less costly and more effective)Footnote 47.
Economic evaluations from a Canadian setting
From a Canadian setting, there were 5 studies included in this reviewFootnote 26Footnote 27Footnote 41Footnote 42Footnote 51. Three studies investigated cost-effectiveness of PVZ in term infants (1 with CFFootnote 27, 2 in Canadian Arctic settings)Footnote 26Footnote 42 1 in preterm infantsFootnote 41, and 1 in infants with CHDFootnote 51. Most studies used a lifetime horizon (60%), reported outcomes from a healthcare payer perspective (80%), in units of cost/QALY (60%), conducted scenario analysis (100%), and deterministic sensitivity analysis (100%). The study on term infants with CF by McGirr et al., was the only study not funded by industryFootnote 27.
These studies assumed 4.5 to 6 doses of PVZ per RSV season at a cost of $1,599 - $1,718 (2017 CAD) per 100mg of PVZ. The effectiveness of PVZ was measured in reduction in RSVH, which ranged between 42% and 96%. Mortality rates were incorporated into only 2 models, at 1% and 8.1%Footnote 41Footnote 42. Sequelae was incorporated into 2 models (1 related to RSV, one associated with CF) 27 41. Study characteristics and conclusions are summarized in Table 4.
Overall, 4 of the 5 studies concluded that the use of PVZ passive immunization was cost-effective based on their models and target populationFootnote 26Footnote 41Footnote 42Footnote 51. Tam et al. considered PVZ as cost-effective for all Baffin Island infants < 1 year chronological age ($46,151/QALY), infants at high-risk for RSV ($391/QALY), infants from remote areas ($28,965/QALY), infants < 6 months of age from remote areas, or remote areas with high rates of RSV (dominant—that is, less costly and more effective). However, when compared to a $100,000/QALY threshold, it was not cost-effective for infants < 6 months, or infants < 1 year of age residing in IqaluitFootnote 42. Similarly, Banerji et al. concluded that their proposed PVZ programs would be cost-effective in some but not all Arctic regions. Both studies attribute the likelihood of these results to the high costs of hospitalizations in these regionsFootnote 26Footnote 42. From the payer perspective, PVZ was considered cost-effective for preterm infants ($35,119/QALYFootnote 41, while from a societal perspective, PVZ yielded an ICER of $18,155/day of HA in the study by Harris et al. Although no cost-effectiveness threshold was used, Harris et al. concluded that PVZ prophylaxis was likely cost-effectiveFootnote 51. PVZ for high-risk infants with CF was determined to unlikely be cost-effective ($167,107/QALY)Footnote 27.
The most influential parameters in the 5 Canadian studies were: RSVH ratesFootnote 26Footnote 42, cost of palivizumabFootnote 27Footnote 51, and cost for hospitalizationFootnote 26Footnote 42, which includes inpatient medical costs and transportation costs to the medical centre.
Cost-effectiveness in preterm infants
The cost-effectiveness of PVZ prophylaxis compared to no PVZ prophylaxis ranged widely. Some studies found PVZ to be a dominant strategy (i.e., less costly and more effective than no PVZ), whereas one study found an ICER of $2,975,489/QALY in preterm infants. Since studies estimated cost-effectiveness for varying ranges of wGA, not all estimates could be grouped into pre-defined intervals. For example, <29 wGA estimates were not grouped under the <32 wGA estimates as the breakdown of the wGA in each preterm group could not be inferred, or reasonably assumed. From the payer perspective, the ICER for PVZ prophylaxis for infants born < 29 wGA (n=3) ranged between $6,216/QALY and $24,009/QALYFootnote 37Footnote 38. For infants born 29-32 wGA, the ICER (n=3) ranged between $9,989/QALY and $58,872/QALYFootnote 37Footnote 38. At < 32 wGA and < 33 wGA, 2 estimates ($12,710/QALY to $25,065/QALY)Footnote 30, and 3 estimates ($16,434/QALY to $42,730/QALY) were identified, respectivelyFootnote 34Footnote 49. In the 32-35 wGA range (includes 2 estimates at 32-35 wGA and 4 estimates at 33-35 wGA), there were 6 ICER estimates for preterm infants ($26,170/QALY to $919,073/QALY)Footnote 34Footnote 37Footnote 39Footnote 41Footnote 49, and 14 ICER estimates for preterm infants with additional risk factors ($215/QALY to $205,563/QALY) 39 41. For preterm infants born < 35 wGA, there were 5 estimates between $30,650/QALY and $938,623/QALYFootnote 14Footnote 29Footnote 31Footnote 34. In subgroup analyses of preterm infants born <35 wGA with BPD/ CLD, 4 estimates ranged between $15,202/QALY and $131,874/QALYFootnote 14Footnote 29Footnote 31Footnote 49.
From a societal perspective, estimates of preterm infants born at 26-28 wGA were entirely extracted from El-Hassan et al. with ICERs between $165,301/QALY and $2,406,619/QALYFootnote 46. For preterm infants born < 29 wGA, there were 5 ICER estimates between $22,765/QALY and $1,359,641/QALYFootnote 28. PVZ cost-effectiveness from a societal perspective varied across studies for preterm infants born between 29 and 35 wGA, with ICER estimates between $449,264/QALY and $1,083,976/QALY (29-30 wGA)Footnote 46, being a dominant strategy (i.e., less costly and more effective for < 32 wGA)Footnote 30Footnote 44Footnote 54, $32,390/QALY and $338,823/QALY (32-35 wGA)Footnote 39Footnote 48, and $25,678/QALY and $983,064/QALY (<35 wGA)Footnote 14Footnote 34. In preterm infants (< 35 wGA) with lung complications, 3 studies reported separate ICER estimates between $18,717/QALY and $138,282/QALY Footnote 14Footnote 29Footnote 31. Six estimates were reported for preterm infants with risk factors between $21,931/QALY and $635,172/QALY ($21,931/QALY to $61,229/QALY for 2 32-34 wGA estimates, $52,299/QALY to $635,172/QALY for 4 32-35 wGA estimates)Footnote 44Footnote 54.
ICERs were stratified and plotted for PVZ prophylaxis expressed in cost/QALY in preterm infants by wGA and Figure 3 presents 57 of 72 ICER estimates, stratified by study perspective, that were estimated under $200,000/QALYFootnote 14Footnote 28Footnote 29Footnote 30Footnote 31Footnote 33Footnote 34Footnote 37Footnote 38Footnote 39Footnote 41Footnote 44Footnote 46Footnote 49Footnote 53Footnote 54. In Figure 3, 50 of the 56 (89%) ICER estimates for preterm infants (with or without other health conditions) were below the $100,000 per QALY threshold. Of the 16 ICER estimates excluded from Figure 3, 8 (i.e., half of all estimates) were from a single study by El-Hassan et alFootnote 46. while the rest were single estimates from other studiesFootnote 28Footnote 37Footnote 41Footnote 44Footnote 48Footnote 54.
Key model parameters
Reduction in RSVH used in models ranged between 39% for infants with CLD in the UKFootnote 37, and 96% in healthy infants in a Canadian Arctic settingFootnote 26. Mortality was reported in 19 studies, ranging between 1% Footnote 42Footnote 52 and 8.11% Footnote 34 for various infant populations. Number of PVZ doses per season were between an average of 3.88 doses in a 5-month season in SpainFootnote 39, and 6 doses in a 6-month RSV season in the Canadian ArcticFootnote 26. Most studies evaluated cost-effectiveness assuming 5 PVZ doses per RSV season (n=17), while 3 studies did not report the dose scheduleFootnote 37Footnote 48Footnote 50. The cost of a 100-mg vial of PVZ in 2017 CAD ranged between $1,099 (from UK study) Footnote 37 and $2,198 (from US study)Footnote 46.
Influential parameters
The most influential parameters reported across the 28 studies (Figure 4) were: RSVH rates (43%)Footnote 26Footnote 29Footnote 31Footnote 32Footnote 35Footnote 36Footnote 37Footnote 41Footnote 42Footnote 44Footnote 50Footnote 54, cost of PVZ (36%)Footnote 27Footnote 32Footnote 35Footnote 36Footnote 44Footnote 46Footnote 47Footnote 50Footnote 51Footnote 54, discount rate (32%)Footnote 28Footnote 29Footnote 30Footnote 31Footnote 32Footnote 34Footnote 37Footnote 38Footnote 40, and efficacy of PVZ (29%)Footnote 35Footnote 36Footnote 37Footnote 43Footnote 44Footnote 48Footnote 50Footnote 54. Other parameters that were influential in multiple studies included: mortality rate reduction, incidence of RSV (and/or sequelae), drug wastage resulting from vial usage, utility values (health-related quality of life) and dosage scheme.
Discussion
Summary of results
The systematic review identified 28 relevant economic evaluations from OECD countries assessing the cost-effectiveness of PVZ prophylaxis compared to no prophylaxis. The most cost-effectiveness estimates were for preterm infants, consistent with their higher risk for RSVFootnote 6Footnote 8. Almost all categories of infants (term, preterm, with and without additional risk factors) had a majority (>50%) of their cost-effectiveness estimates below the $100,000/QALY threshold. The only exception to this was the estimates for preterm infants from the societal perspective, where only 48% of the estimates were below $100,000/QALY. This exception was likely a result of a large group of estimates (n=8, 35% of all for this subgroup) extracted from one study that were between $347,803/QALY and $2,975,489/QALYFootnote 46. Possible reasons for the higher ICERs in this study may be having used the highest adjusted cost for a 100-mg vial of PVZ at $2,198, following infants only up to 8 years of age, and as suggested by the authors, overestimating the impact of subsequent asthma onset on health-related quality of life (utility). Sensitivity analysis reducing the PVZ cost by 25%, or reducing the impact of asthma on utility afforded ICERs under $200,000/QALY (USD), and under $100,000/QALY (USD), respectivelyFootnote 46.
Based on this review, PVZ prophylaxis cost-effectiveness varies depending on the population and setting. In order to facilitate comparisons and summarize the findings, all ICERs were adjusted to 2017 CAD per QALY, and stratified based on GA at birth and risk-factors for RSV in Figure 3. For term and preterm infants with BPD/CLD, the ICER was under $50,000/QALY in 9 of the 10 estimates from a payer perspective. All other subgroups of infants (term, preterm, CHD, other risk factors) resulted in inconsistent results for PVZ prophylaxis with the intervention being dominant at times (i.e., less costly and more effective), and having an ICER up to $938,623/QALY in other scenarios. When stratifying for preterm births by wGA, evidence was lacking for infants born < 28 wGA, especially from the payer perspective. No specific trend was depicted between the wGA and the ICER, overall or stratified by perspective. However, while preterm estimates were available across 26-35 wGA, preterm infants with additional risk factors or BPD/CLD were limited to 33-35 wGA. Generally, one would expect ICERs from a societal perspective to be lower than those from a payer perspective, but based on this review, this trend does not exist for 2 reasons: 1) payer and societal perspective estimates were coming from different studies and; 2) due to the heterogeneity in model designs and differences between setting-specific costs, and RSV epidemiology.
Economic evaluations from a Canadian setting
There were 5 economic evaluations conducted from a Canadian setting. While 4 of 5 studies used a healthcare payer perspective, the time horizon, discount rate and cost-effectiveness outcome report varied. Populations were different between all studies: term infants with CF, term infants from the Canadian Arctic, preterm infants, and infants with CHD. The studies by Tam et al. and Banerji et al. similarly concluded that PVZ prophylaxis was cost-effective for most subgroups of infants in the Canadian Arctic due to high costs of hospitalizations (e.g., transportation). Study conclusions from Tam et al. were for Baffin region infants, while Banerji et al. included populations from 8 Arctic regions: the Northwest Territories, Nunavut, Nunavut without Iqaluit, the 3 sub-regions of Nunavut (Kitikmeot, Kivalliq and Qikiqtaaluk), the Qikiqtaaluk Region without Iqaluit, and Nunavik (northern Quebec). Both studies were conducted from a healthcare payer perspective, used similar PVZ costs ($220 - $226 per kilogram weight of the infant, original cost). Given the study populations, clinical settings, and costing of drugs and resources, the studies by Tam et al. and Banerji et al. can be considered generalizable to other territories or remote areas in Canada for infants at high-risk for RSV.
The remaining 3 studies are also considered generalizable to most Canadian provinces given that they used similar PVZ costs ($1,468 - $1,505 per 100 mg vial, original costs) available to Canadian provinces, used dosing schedules close to 5 injections per season (4.5 to 5.39 vials per season), used healthcare costs from British Columbia and Ontario, and included model parameters of relevance to the Canadian healthcare system. While there was evidence for varying subpopulations from large provinces and territories, cost-effectiveness of PVZ prophylaxis for infants from smaller Canadian provinces (e.g., Maritimes provinces) were lacking.
Heterogeneity in results: Key parameters
Since PVZ prophylaxis was determined to be cost-effective in some settings but not cost-effective in others, this review summarized the most frequently reported influential parameters affecting the ICER. They included RSVH rates and cost of PVZ used. Reduction in RSVH varied drastically between 39% and 96% depending on the population of interest and the data source. The cost of a 100mg vial of PVZ also ranged between $1,099 and $2,198 (2017 CAD). Both parameters' influential nature was expected given the reduction in RSV and RSVH is essential to reduction in costs, and future sequelae, while the costs of PVZ is directly related to the ICER. However, it was interesting to note that vial usage and dosage scheme only affected the ICER in 4Footnote 29Footnote 30Footnote 40Footnote 43, and 3 studiesFootnote 39Footnote 43Footnote 49, respectively.
In studies addressing drug wastage through vial usage, ICERs fluctuated up to 50% depending on the assumed vial usage. In a New Zealand study, assuming no vial sharing (entire 100mg vial is used per injection) increased costs of up to 50%Footnote 43, while another study in Spain concluded a lower ICER when 50-mg vials were used instead of 100mgFootnote 30. It has been suggested in the literature and by physicians that vial usage efficiency can be achieved for PVZFootnote 56. Many studies did not assess scenarios in which the vial usage becomes more efficient or the number of assumed doses is reduced, which remains a question that should be addressed in future studies.
Comparison to the literature
The cost-effectiveness of PVZ prophylaxis has been explored in multiple reviews in the past 2 decadesFootnote 11Footnote 12Footnote 14, but only 4 have been published between 2010 and 2013 Footnote 15Footnote 57Footnote 58Footnote 59. These results and conclusions are consistent with other reviews, and are most comparable to a systematic review by Smart et al. published in 2010, where the authors reported a range of ICERs (in 2009 CAD) for PVZ prophylaxis: from being dominant (i.e., less costly and more effective), up to being $3,365,768/QALY depending on the study population, outcomes, and model parameters Footnote 59. This present work added onto the Smart review by capturing studies from 2010 to mid-2018, but limited the scope to OECD countries, and adjusted for inflation differences by using the PPP rates from the OECD. Reviews by Andabaka et al. and Prescott et al. similarly concluded that cost-effectiveness of PVZ was inconsistentFootnote 15Footnote 58. Hussman et al. conducted a review on RSV prophylaxis overall and included studies comparing PVZ and other interventions (e.g., Respiratory syncytial virus immune globulin (RSV-IGIV)Footnote 57. This present review is the first to update PVZ prophylaxis cost-effectiveness compared to no prophylaxis since the 2014 American Academy of Pediatrics (AAP) guideline updateFootnote 60.
Generalizability of included studies to Canadian setting
Most study results may be broadly generalizable to the Canadian healthcare system since the eligibility criteria screened for economic evaluations conducted from OECD countries, of which all members except for the US and Switzerland have healthcare components similar to Canada Footnote 61. The only exception were the 6 studies from the US. The remaining studies from the Netherlands, UK, Spain, Austria, Germany, Italy, New Zealand and Sweden have public system financing in parts by general tax revenueFootnote 61. However, choice of payer or societal perspective may influence the included costs in the analysis. For example, cost-effectiveness from a societal perspective includes possible indirect, out-of-pocket, or productivity loss costs, which can vary across different countries regardless of healthcare system financing.
From 3 Canadian studies, the cost per 100-mg vial of PVZ used in models were between $1,599 and $1,718 (2017 CAD). Models from the UK used a lower PVZ cost of $1,099 to $1,240 per 100-mg vial, and the remaining studies (with the exception of US studies) scattered between $1,386 and $2,035 (2017 CAD). The number of doses per season assumed or calculated in these economic evaluations ranged between 3.88 and 6 doses. The Canadian Pediatric Society recommends up to 5 doses of PVZ per season for infants up to 24 months of ageFootnote 62. In the subset of countries with similar healthcare structure to Canada, almost all models assumed 5 doses of PVZ per season, except for Resch et al (Austria)Footnote 34, Banerji et al. (Canada)Footnote 26, Nuijten et al, Sanchez-Luna et al, and Schmidt et al (all from Spain), where the average number of doses was 4 per seasonFootnote 30Footnote 39Footnote 40. Additionally, the RSV risk factors modeled are consistent with those published in the Canadian literature: preterm birth, CHD, BPD/CLD, male sexFootnote 8.
Despite the similarities in PVZ prophylaxis cost and dosage schedule, reduction rates of RSVH varied from 39% to 96% depending on the infant population, and literature referenced. Many studies cited the IMpact-RSV trial for their model parameters, a trial that included Canada and concluded that reduction in RSVH was 78% for preterm infants, 39% for children with BPD/CLD and 55% overall. The subgroup of Canadian subjects in the IMpact-RSV trial reportedly showed a 40% overall reduction in RSVH. The trend was similar to that seen in US (56%), and UK subjects (64%)Footnote 63. Question 11 of the JBI quality appraisal checklist gauges for transferability in which 2 independent reviewers concluded that 24 of 28 studies were generalizable based on their model attributes (structure, parameters) and reported outcomes. Results from the US may have limited generalizability when evaluating this part of the checklist.
Study limitations
This review has several limitations. Differences in model designs, RSVH rates used, disease progress, perspectives and settings prevented us from providing definitive conclusions on the value of this intervention. The review attempted to summarize cost-effectiveness of this intervention from 2000 to 2018, but changes in American Academy Pediatrics recommendations in the US (and decision-makers in other respective countries) over time can affect model design and input data. Lastly, the review may be subject to publication and language bias since it did not search the grey literature or include articles not in English or French.
Study strengths
Despite these limitations, this review provides a comprehensive summary of the cost-effectiveness of PVZ prophylaxis from OECD countries to inform decision-makers of the estimated value for this intervention in term infants, preterm infants, and infants at high-risk for RSV (e.g., CHD, BPD/CLD). Figure 3 shows all base-case results and scenario analyses, which gives a sense of the number of studies (and estimates) that fall under specific cost-effectiveness thresholds from both payer and societal perspectives. All estimates were standardized to 2017 CAD, which allowed us to group, stratify and compare the cost-effectiveness estimates. These adjusted ICERs should be useful for program decision-makers where costs can be significantly underestimated if not appropriately adjusted.
Conclusion
Cost-effectiveness results of PVZ as a RSV prophylaxis were heterogeneous across studies, ranging from being dominant (i.e., less costly and more effective) to highly not cost-effective. Results varied due to study setting, population of interest, local RSV epidemiology, and healthcare setup, as well as key model parameters such as reduction in hospitalization rates, RSV acquisition costs, dosage schemes and vial usage. Based on several authors' conclusions, PVZ prophylaxis for RSV may be considered cost-effective in certain subgroups of infants. From a payer perspective, authors concluded PVZ was considered cost-effective in infants with BPD/CLD, infants with CHD, term infants from specific remote communities, and preterm infants with and without lung complications. No overall trends were detected between specific GA thresholds and cost-effectiveness results, overall or stratified by perspective. Among the 2 studies in the Canadian North, authors concluded PVZ was considered cost-effective for some settings where baseline RSVH rates were very high, thus preventing high hospitalization and medical evacuation costs.
Figures and tables
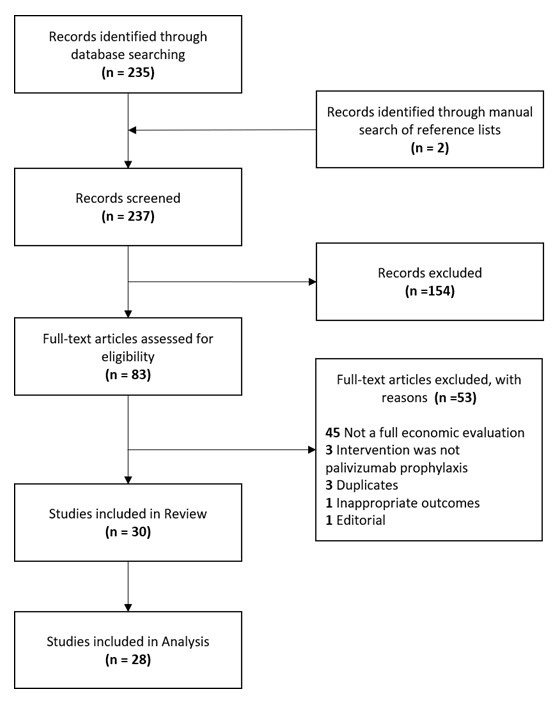
Figure 1 - Text description
The flow diagram shows the number of records searched and selected. There were 235 records identified through database searching and two records identified through a manual search of reference lists for a total of 237 records. The 237 records were screened, and 154 records were excluded, with a remainder of 83 full-texts. After assessing the full-texts for eligibility, 53 articles were excluded for the following reasons: not a full economic evaluation (n = 45), intervention was not palivizumab prophylaxis (n = 3), duplicates (n = 3), inappropriate outcomes (n = 1), editorial (n = 1). Hence, 30 full-texts were included in the review, of which, only 28 were included in analysis.
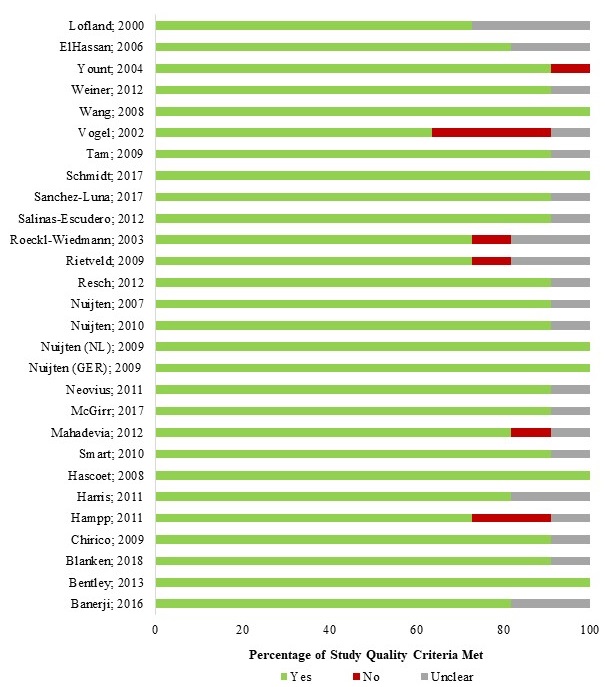
Figure 2 - Text description
| Author; Year | Yes (N) | No (N) | Unclear (N) | Yes (%) | No (%) | Unclear (%) |
|---|---|---|---|---|---|---|
| Banerji; 2016 | 9.00 | 0.00 | 2.00 | 81.82 | 0.00 | 18.18 |
| Bentley; 2013 | 11.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| Blanken; 2018 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Chirico; 2009 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Hampp; 2011 | 8.00 | 2.00 | 1.00 | 72.73 | 18.18 | 9.09 |
| Harris; 2011 | 9.00 | 0.00 | 2.00 | 81.82 | 0.00 | 18.18 |
| Hascoet; 2008 | 11.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| Smart; 2010 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Mahadevia; 2012 | 9.00 | 1.00 | 1.00 | 81.82 | 9.09 | 9.09 |
| McGirr; 2017 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Neovius; 2011 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Nuijten (GER); 2009 | 11.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| Nuijten (NL); 2009 | 11.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| Nuijten; 2010 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Nuijten; 2007 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Resch; 2012 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Rietveld; 2009 | 8.00 | 1.00 | 2.00 | 72.73 | 9.09 | 18.18 |
| Roeckl-Wiedmann; 2003 | 8.00 | 1.00 | 2.00 | 72.73 | 9.09 | 18.18 |
| Salinas-Escudero; 2012 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Sanchez-Luna; 2017 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Schmidt; 2017 | 11.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| Tam; 2009 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Vogel; 2002 | 7.00 | 3.00 | 1.00 | 63.64 | 27.27 | 9.09 |
| Wang; 2008 | 11.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| Weiner; 2012 | 10.00 | 0.00 | 1.00 | 90.91 | 0.00 | 9.09 |
| Yount; 2004 | 10.00 | 1.00 | 0.00 | 90.91 | 9.09 | 0.00 |
| ElHassan; 2006 | 9.00 | 0.00 | 2.00 | 81.82 | 0.00 | 18.18 |
| Lofland; 2000 | 8.00 | 0.00 | 3.00 | 72.73 | 0.00 | 27.27 |

Figure 3 - Text description
| wGA | Perspective | ICER (2017 CAD per QALY) |
|---|---|---|
Pre-term |
||
| 29 | Payer | 6,216 |
| 29 | Payer | 7,494 |
| 29 | Payer | 24,009 |
| 31.5 | Payer | 9,989 |
| 31.5 | Payer | 28,429 |
| 31.5 | Payer | 58,872 |
| 32 | Payer | 12,710 |
| 32 | Payer | 25,065 |
| 33 | Payer | 16,434 |
| 33 | Payer | 38,733 |
| 33 | Payer | 42,730 |
| 33.5 | Payer | 37,815 |
| 34 | Payer | 26,170 |
| 34 | Payer | 35,530 |
| 34 | Payer | 39,642 |
| 34 | Payer | 193,068 |
| 35 | Payer | 30,650 |
| 35 | Payer | 33,257 |
| 35 | Payer | 35,216 |
| 35 | Payer | 42,600 |
| 35 | Payer | 938,623 |
| 26 | Societal | 165,301 |
| 26 | Societal | 1,331,595 |
| 27 | Societal | 2,078,481 |
| 28 | Societal | 347,803 |
| 28 | Societal | 2,406,619 |
| 29 | Societal | 22,765 |
| 29 | Societal | 30,000 |
| 29 | Societal | 58,922 |
| 29 | Societal | 75,595 |
| 29 | Societal | 1,359,641 |
| 29.5 | Societal | 449,264 |
| 29.5 | Societal | 1,083,976 |
| 31 | Societal | 1,944,890 |
| 32 | Societal | - |
| 32 | Societal | - |
| 32 | Societal | - |
| 32 | Societal | 2,975,489 |
| 33 | Societal | 29,470 |
| 33.5 | Societal | 32,930 |
| 33.5 | Societal | 338,823 |
| 34 | Societal | 25,990 |
| 35 | Societal | 25,678 |
| 35 | Societal | 983,064 |
Pre-term and BPD |
||
| 35 | Payer | 15,202 |
| 35 | Payer | 33,412 |
| 35 | Payer | 37,362 |
| 35 | Societal | 18,717 |
| 35 | Societal | 25,684 |
Pre-term and CLD |
||
| 35 | Payer | 131,874 |
| 35 | Societal | 138,282 |
Pre-term and risk factors |
||
| 33.5 | Payer | 215 |
| 33.5 | Payer | 5,906 |
| 33.5 | Payer | 22,173 |
| 33.5 | Payer | 23,309 |
| 33.5 | Payer | 27,216 |
| 33.5 | Payer | 29,901 |
| 33.5 | Payer | 35,119 |
| 33.5 | Payer | 36,356 |
| 33.5 | Payer | 38,566 |
| 33.5 | Payer | 54,308 |
| 33.5 | Payer | 56,479 |
| 33.5 | Payer | 92,649 |
| 33.5 | Payer | 163,744 |
| 33.5 | Payer | 205,563 |
| 33.5 | Payer | 919,073 |
| 33 | Societal | 21,931 |
| 33 | Societal | 61,229 |
| 33.5 | Societal | 52,299 |
| 33.5 | Societal | 108,685 |
| 33.5 | Societal | 385,488 |
| 33.5 | Societal | 635,172 |
Note: Estimates included (n=56), ICERs > $200,000/QALY were not captured (n=16). Payer perspective: preterm (32-35 wGA) w/RF: $205,563/QALY, 41 preterm (<35 wGA): $938,623/QALY, 14 preterm (32-35 wGA): $919,073/QALY 41. Societal: preterm (26 wGA): $1,331,595/QALY, 46 preterm (27 wGA): $2,078,481/QALY, 46 preterm (28 wGA): $2,406,619/QALY, 46 preterm (28 wGA): $347,803/QALY, 46 preterm (<29 wGA): $1,359,641/QALY, 28 preterm (29-30w GA): $1,083,976/QALY, 46 preterm (29-30 wGA): $449,264/QALY, 46 preterm (31w GA): $1,944,890/QALY, 46 preterm (32 wGA): $2,975,489/QALY, 46 preterm (32-35 wGA): $338,823/QALY, 48 preterm (<35 wGA): $983,064/QALY, 14 preterm (32-35 wGA) w/RF: $635,172/QALY 54 and $385,488/QALY. 44 |
||

Figure 4 - Text description
| Influential parameters | Frequency of studies reporting this parameter |
| RSV hospitalization rates | 12 |
| Cost of palivizumab | 10 |
| Discount rate | 9 |
| Efficacy of palivizumab | 8 |
| Mortality rate reduction | 5 |
| Incidence of RSV (or sequelae) | 5 |
| Cost for hospitalization (inpatient or transportation) | 5 |
| Drug wastage from vial usage | 4 |
| Utility of sequelae | 4 |
| Dosage scheme | 3 |
| Authors, year | Country | Perspective | Type of analysis | Piggy-back or model-based?; Type of model | Outcome measure | Population | Time horizon | Discount rate | Industry funding |
| Banerji 2016Footnote 26 | Canada | Payer | CEA | Model-based; Decision-analysis | Cost per HA | Term infants | 6-month | N/Ap | Abbott/MedImmune (Grants) |
| Bentley 2011Footnote 37 | UK | Payer | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm, CHD, CLD infants | Lifetime | 3.5% | AbbVie |
| Blanken 2018Footnote 48 | Netherlands | Societal | CUA | Piggy-back; Decision-analysis | Cost per QALY | Preterm infants | 1-year | N/Ap | Unknown: Grants for investigator-initiated studies from MedImmune and AbbVie |
| Chirico 2009Footnote 49 | Italy | Payer | CUA | Model-based; Simulation | Cost per QALY | Preterm, BPD infants | Lifetime | 3% | Abbott |
| ElHassan 2006Footnote 46 | US | Societal | CUA; CBA | Model-based; Markov cohort | Cost per QALY | Preterm infants without CLD | 8 years | 3% | None |
| Hampp 2011Footnote 50 | US | Payer | CEA | Model-based; Decision-analysis | Cost per HA | Preterm and term infants (both with and without CHD, CLD) | NR | NR | None |
| Harris 2011Footnote 51 | Canada | Societal | CEA; CBA | Model-based; Decision-analysis | Cost per day of HA | CHD infants | 5-yearFootnote a | NR | Unknown: Honorarium (< $1,000) from Abbott |
| Hascoet 2008Footnote 52 | France | Societal (BC) and payer | CEA; CBA | Model-based; Decision-analysis | Cost per LYG | Preterm infants with CHD or BPD | Lifetime | 3% | Abbott France |
| Lofland 2000Footnote 57 | US | Payer | CEA | Piggy-back; Decision-analysis | Cost per RSV infection avoided | Preterm infants with CLD | 6 months | N/Ap | MedImmune, Inc. |
| Mahadevia 2012Footnote 54 | US | Societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm infants | Lifetime | 3% | MedImmune LLC |
| McGirr 2017Footnote 27 | Canada | Payer | CUA | Model-based; Markov cohort | Cost per QALY | Term infants with CF | Lifetime | 5% | None |
| Neovius 2011Footnote 28 | Sweden | Societal | CUA | Model-based; Markov cohort | Cost per QALY | Preterm infants | Lifetime | 3% | Abbott Scandinavia |
| Nuijten 2009Footnote 32 | Germany | Societal (BC), and payer | CUA | Model-based; Decision-analysis | Cost per QALY | CHD infants | Lifetime | 5% | Abbott |
| Nuijten 2009Footnote 31 | Netherlands | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm, BPD, CHD infants | Lifetime | 4%, 1.5%Footnote a | Abbott GmbH & Co. Germany |
| Nuijten 2010Footnote 30 | Spain | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm infants | Lifetime | 3% | Abbott GmbH & Co. Germany |
| Nuijten 2007Footnote 29 | UK | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY; Cost per HA | Preterm, BPD, CHD infants | Lifetime | 3.5% | Abbott GmbH & Co. Germany |
| Resch 2012Footnote 33Footnote 34 | Austria | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm, BPD, CHD infants | Lifetime | 5% | None |
| Rietveld 2010Footnote 35 | Netherlands | Societal | CEA | Model-based; Decision-analysis | Cost per HA | Preterm, BPD infants | 1-year | N/Ap | None |
| Roeckl-Wiedmann 2003Footnote 36 | Germany | Societal | CEA | Model-based; Decision-analysis | Cost per HA | Preterm infants with RF | 1-year | N/Ap | Abbott Laboratories, Germany. |
| Salinas-Escudero 2012Footnote 38 | Mexico | Payer | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm infants | Lifetime | 3% | Abbott Laboratories of Mexico |
| Sanchez-Luna 2017Footnote 39 | Spain | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm infants with RF | 6-year | 3% | None |
| Schmidt 2017Footnote 40 | Spain | Societal | CUA | Model-based; Markov state (Decision-tree structure in first year and Markov structure in later years) | Cost per QALY | CHD infants | Lifetime | 3% | AbbVie (Grant) |
| Smart 2010Footnote 41Footnote 53 | Canada | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm infants with RF | Lifetime | 5% | Unknown: Other relationships with Abbott |
| Tam 2009Footnote 42 | Canada | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY | NR | Lifetime | 5% | Abbott Laboratories/ Abbott International (Grant) |
| Vogel 2002Footnote 43 | New Zealand | Societal | CEA; CBA | Model-based; Decision-analysis | Cost per case averted | Preterm, CLD infants | 3-yearFootnote b | NR | Abbott (Grant) |
| Wang 2008Footnote 14 | UK | Payer (BC) and societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm, BPD, CHD, CLD, term infants with RF | Lifetime | 3.5% | None |
| Weiner 2012Footnote 44 | US | Societal | CUA | Model-based; Decision-analysis | Cost per QALY | Preterm infants with RF | Lifetime | 3% | MedImmune LLC |
| Yount 2004Footnote 45 | US | Societal | CUA; CBA | Model-based; Decision-analysis | Cost per QALY | CHD infants | Lifetime | 3% | None |
Definitions: BC, base-case; BPD, bronchopulmonary dysplasia; CBA, Cost-benefit analysis; CEA, Cost-effectiveness analysis; CF, Cystic fibrosis; CHD, congenital heart disease; CLD, chronic lung disease; CUA, Cost-utility analysis; LYG, life years gained; N/Ap, not applicable; NR, not reported; QALY, quality-adjusted life years; UK, United Kingdom; US, United States. Footnotes
|
|||||||||
| Author, year | Original currency, WTP used | ICER (original) | ICER (adjusted, 2017 CAD) |
Results (context) | Sensitivity analysis | Study conclusions | |
|---|---|---|---|---|---|---|---|
Banerji 2016Footnote 26 |
CAN 2011; $50,000 per hospital admission |
4,633 | 5,042 | Cost per hospitalization avoided |
Scenario BFootnote a Nunavut without Iqaluit | Deterministic |
PVZ was CE in the Kitikmeot and Kivalliq regions and Nunavik. Scenario B (compared to Scenario A) was more CE in all regions except the Kitikmeot region. |
| 14,545 | 15,829 | Scenario BFootnote a, Nunavut | |||||
| 15,601 | 16,979 | Scenario BFootnote a, Nunavik | |||||
| 22,954 | 24,981 | Scenario AFootnote b, Kivalliq Region | |||||
| 28,580 | 31,104 | Scenario AFootnote b, Nunavut without Iqaluit | |||||
| 30,230 | 32,899 | Scenario AFootnote b, Nunavik | |||||
| 41,404 | 45,060 | Scenario AFootnote b, Nunavut | |||||
| 105,259 | 114,554 | Scenario BFootnote a, Qikiqtaaluk Region w/o Iqaluit | |||||
| 133,407 | 145,187 | Scenario BFootnote a, Qikiqtaaluk Region | |||||
| 166,600 | 181,311 | Scenario AFootnote b, Qikiqtaaluk Region w/o Iqaluit | |||||
| 211,444 | 230,115 | Scenario AFootnote b, Qikiqtaaluk Region | |||||
| 326,441 | 355,267 | Scenario BFootnote a, NWT | |||||
| 545,115 | 593,250 | Scenario AFootnote b, NWT | |||||
| Dominant | Dominant | Scenario AFootnote b, Kitikmeot Region | |||||
| Dominant | Dominant | Scenario BFootnote a, Kitikmeot Region | |||||
| Dominant | Dominant | Scenario BFootnote a, Kivalliq Region | |||||
Bentley 2011Footnote 37 |
GBR 2010; £20,000 per QALY and |
3,845 | 7,494 | Cost per QALY gained |
Preterm infants (<29 wGA) | Deterministic; probabilistic |
Prophylactic PVZ represents an economically viable use of NHS resources for infants (aged under 24 months) with CHD, infants (aged under 24 months) with CLD and preterm infants born at 32 wGA or below and preterm infants born 33–35 wGA when additional RF are considered. |
| 19,168 | 37,360 | CLD infants | |||||
| 30,205 | 58,872 | Preterm infants (29-32 wGA) | |||||
| 33,216 | 64,741 | CHD Infants | |||||
| 99,056 | 193,068 | Preterm infants (33-35 wGA) | |||||
| Blanken 2018Footnote 48 | NLD 2015; €80,000 per QALY | 214,748 | 338,823 | Cost per QALY gained | Preterm (32-35 wGA) | Deterministic; probabilistic | |
Chirico 2009Footnote 49 |
ITA 2007; €50,000 per QALY |
2,732 | 4,786 | Cost per QALY gained |
BPD | Deterministic |
Compared with no prophylaxis, PVZ is CE in the prevention of RSV infection among high risk preterm infants. |
| 8,677 | 15,202 | Preterm (<35 wGA, mix) with BPD | |||||
| 9,380 | 16,434 | Preterm (<33 wGA) | |||||
| 14,937 | 26,170 | Preterm (33-35 wGA) | |||||
ElHassan 2006Footnote 46 |
USA 2002; $200,000 per QALY |
103,053 | 165,301 | Cost per QALY gained |
BC, preterm (26 wGA), targeted use policy | Deterministic |
Our model supports implementing more restrictive guidelines for PVZ prophylaxis. PVZ was CE for some infants in an analysis that accounted for increased risk of severe asthma following RSV infection. We found evidence that long-term health consequences of RSV are central to the determination of the CEness of the intervention. |
| 216,830 | 347,803 | BC, preterm (28 wGA), targeted use policy | |||||
| 280,083 | 449,264 | BC, preterm (29-30 wGA), targeted use policy | |||||
| 675,780 | 1,083,976 | BC, preterm (29-30 wGA) | |||||
| 830,152 | 1,331,595 | BC, preterm (26 wGA) | |||||
| 1,212,497 | 1,944,890 | BC, preterm (31 wGA) | |||||
| 1,295,781 | 2,078,481 | BC, preterm (27 wGA) | |||||
| 1,500,351 | 2,406,619 | BC, preterm (28 wGA) | |||||
| 1,855,000 | 2,975,489 | BC, preterm (32 wGA) | |||||
Hampp 2011Footnote 50 |
USA 2010; NR (compared to $8,910 per hospitalization) |
302,103 | 413,127 | Cost per hospitalization avoided |
Preterm (<32 wGA) | Deterministic; probabilistic |
The cost of immunoprophylaxis with PVZ far exceeded the economic benefit of preventing hospitalizations, even in infants at highest risk for RSV infection. |
| 361,727 | 494,663 | Preterm (<32 wGA) and CHD | |||||
| 368,048 | 503,307 | Preterm (<32 wGA) and CLD | |||||
| 522,490 | 714,507 | Term, CLD and CHD | |||||
| 823,868 | 1,126,642 | Term, CHD only | |||||
| 920,033 | 1,258,148 | Any risk factor (indication) | |||||
| 1,322,422 | 1,808,416 | Term, CLD only | |||||
| 2,138,870 | 2,924,911 | No risk factor (indication) | |||||
Harris 2011Footnote 51 |
CAN 2007; NR |
8,292 | 9,704 | Cost to treat 1 child per RSV season | BC | Deterministic |
Our study contributes to the growing body of literature that suggests PVZ is not CE in children < 2 years old with hsCHD. |
| 15,513 | 18,155 | Cost to prevent 1 day of hospitalization | BC | ||||
Hascoet 2008Footnote 52 |
FRA 2006; €45,000 per QALY |
10,172 | 16,368 | Cost per LYG |
Preterm (<32 wGA) with BPD (healthcare) | Deterministic; probabilistic |
RSV prophylaxis using PVZ in premature children with BPD or hsCHD can be considered CE in France. |
| 20,788 | 33,450 | Preterm (<32 wGA) with cardiopathy (societal) | |||||
| 27,255 | 43,856 | Preterm (<32 wGA) with BPD (Societal) | |||||
Lofland 2000Footnote 47 |
USA 2000; NR |
1,008 | 1,693 | Cost per RSV infection episode avoided |
BC, preterm (NR wGA), 81% reduction incidence of RSV infection (5% vs. 26%) | Deterministic |
The incremental Cost per RSV infection episode avoided ranged from $0 (cost savings) to $39,591 for PVZ prophylaxis costs of $2,500 and from $2,702 to $79,706 for PVZ prophylaxis costs of $4,500. Clinicians may use this information to help determine whether prophylactic PVZ therapy is CE in their clinical practice setting. |
| 39,591 | 66,494 | Preterm (NR wGA), 50% reduction incidence of RSV infection (5% vs. 10%) | |||||
| Dominant | Dominant | Preterm (NR wGA), 83% reduction incidence of RSV infection (5% vs. 28%) | |||||
Mahadevia 2012Footnote 54 |
USA 2010; NR (Compared to $157,000 per QALY, from meningococcal vaccine) |
44,774 | 61,229 | Cost per QALY gained |
Group 2, preterm (32-35 wGA) with RFFootnote c | Deterministic |
PVZ remained CE for guideline-eligible high-risk infants across both public and private sectors. Guideline-eligible infants included infants of <32 wGA, 32–34 wGA with 2009 AAP RF, and 32–35 wGA with 2006 AAP RF. PVZ was not CE in infants of 32– 35 wGA with 1 RF. |
| 79,477 | 108,685 | Group 3, preterm (32-35 wGA) with RFFootnote d | |||||
| 464,476 | 635,172 | Group 4, preterm (32-35 wGA) with RFFootnote e | |||||
| Dominant | Dominant | Group 1, preterm (<32 wGA)Footnote f | |||||
McGirr 2017Footnote 27 |
CAN 2013; $50,000 per QALY |
157,332 | 167,107 | Cost per QALY gained |
High risk CF < 2 yrs. (high risk for severe RSV disease) | Deterministic |
PVZ is not CE in CAD by commonly used thresholds. However, given the rarity of CF and relatively small budget impact, consideration may be given. |
| 652,560 | 693,105 | All CF < 2 yrs. | |||||
Neovius 2011Footnote 28 |
SWE 2009; 500,000 SEK per QALY |
148,293 | 22,765 | Cost per QALY gained |
Preterm (<29 wGA) adding wheezing to asthma | Deterministic; probabilistic |
Based on a WTP of 500 000 SEK ⁄ QALY, PVZ was found to be cost–effective compared with no prophylaxis for infants born at <29 weeks if severe RSV infection was assumed to increase subsequent asthma or mortality risk. |
| 195,420 | 30,000 | BC, Preterm (<29 wGA) | |||||
| 383,825 | 58,922 | Preterm (<29 wGA) excluding indirect effect on asthma | |||||
| 492,430 | 75,595 | Preterm (<29 wGA) excluding indirect effect on mortality | |||||
| 8,856,829 | 1,359,641 | Preterm (<29 wGA) excluding the indirect effect of mortality and asthma | |||||
Nuijten 2009_DEUFootnote 32 |
DEU 2006; €20,000 per QALY |
2,221 | 3,772 | Cost per QALY gained |
BC, CARDIAC Study parameters, societal | Deterministic; probabilistic |
This analysis showed that PVZ represents a CE means of prophylaxis against severe RSV infection requiring hospitalisation in infants with hsCHD. |
| 9,528 | 16,184 | BC, CARDIAC Study parameters, including asthma, payer | |||||
| 9,529 | 16,185 | BC, societal | |||||
| 11,126 | 18,898 | BC, CARDIAC Study parameters, excluding asthma, payer | |||||
| 16,673 | 28,320 | BC, direct medical costs (including asthma), payer | |||||
| 18,266 | 31,025 | BC, direct medical costs (excluding asthma), payer | |||||
| 123,439 | 209,666 | BC, excluding mortality, societal | |||||
Nuijten 2009_NLDFootnote 31 |
NLD 2006; €30,000 per QALY |
7,067 | 11,668 | Cost per QALY gained |
BC - CHD | Deterministic; probabilistic |
PVZ provides CE prophylaxis against RSV in high-risk infants. The use of PVZ in these children results in short- and long-term health-economic benefits. |
| 11,336 | 18,717 | BC – preterm (<35 wGA, mix) with BPD (total costs, societal) | |||||
| 18,563 | 30,650 | Preterm (<35 wGA, mix) | |||||
| 20,236 | 33,412 | BC, preterm (<35 wGA, mix) with BPD | |||||
| 23,461 | 38,737 | BPD sub-populations | |||||
| Dominant | Dominant | BC - CHD (total costs, societal) | |||||
Nuijten 2010Footnote 30 |
ESP 2006; €30,000 per QALY |
6,498 | 12,710 | Cost per QALY gained |
BC, preterm (<32 wGA) inclusion of costs of sequelae treatment | Deterministic; probabilistic |
PVZ provides a CE method of prophylaxis against severe RSV disease among preterm infants in Spain. |
| 12,814 | 25,065 | BC, preterm (<32 wGA) | |||||
| Dominant | Dominant | BC, preterm (<32 wGA), societal perspective | |||||
Nuijten 2007Footnote 29 |
GBR 2003; £25,000 per QALY |
6,664 | 14,891 | Cost per QALY gained |
CHD | Deterministic; probabilistic |
This study suggests that PVZ prophylaxis against severe RSV infection in children at high risk may be CE from the NHS perspective. |
| 11,494 | 25,684 | BC, preterm (<35 wGA) with indirect costs (societal) | |||||
| 14,883 | 33,257 | Preterm (<35 wGA) | |||||
| 16,720 | 37,362 | BC, preterm (<35 wGA) with BPD | |||||
| 20,953 | 46,821 | BPD only | |||||
Resch 2012Footnote 33Footnote 34 |
AUT 2010; €40,693 per QALY (based on the £30,000 per QALY from NICE in 2010) |
3,045 | 4,949 | Cost per QALY gained |
BC, CHD, including recurrent wheezing treatment, societal | Deterministic |
Our results based on nationwide long-term epidemiologic data suggest that PVZ is CE in prevention of RSV disease in high-risk infants. |
| 7,818 | 12,706 | BC, CHD, including recurrent wheezing treatment | |||||
| 8,484 | 13,788 | BC, CHD | |||||
| 15,800 | 25,678 | BC, for all preterm (<35 wGA, mix), including recurrent wheezing treatment, societal | |||||
| 15,992 | 25,990 | BC, preterm (33-35 wGA), including recurrent wheezing treatment, societal | |||||
| 17,554 | 28,529 | BC, BPD, including recurrent wheezing treatment, societal | |||||
| 18,133 | 29,470 | BC, preterm (<33 wGA), including recurrent wheezing treatment, societal | |||||
| 21,669 | 35,216 | BC, for all preterm (<35 wGA, mix), including recurrent wheezing treatment | |||||
| 21,862 | 35,530 | BC, preterm (33-35 wGA), including recurrent wheezing treatment | |||||
| 22,515 | 36,591 | BC, BPD, including recurrent wheezing treatment | |||||
| 23,833 | 38,733 | BC, preterm (<33 wGA), including recurrent wheezing treatment | |||||
| 24,392 | 39,642 | BC, preterm (33-35 wGA) | |||||
| 24,654 | 40,068 | BC, BPD | |||||
| 26,212 | 42,600 | BC, for all preterm (<35 wGA, mix) | |||||
| 26,292 | 42,730 | BC, preterm (<33 wGA) | |||||
Rietveld 2010Footnote 35 |
NLD 2000; NR ($1325 to $8700, mean of $5,787 per hospitalization) |
13,190 | 24,875 | Cost per hospitalization avoided |
Male infant, preterm (< 28 wGA), birth weight < 2,500g, with BPD (December) | Deterministic |
Every month costs per hospitalisation avoided were higher for children without BPD and children with higher GAs. Incremental costs per hospitalisation avoided were always high. Passive immunisation was always most cost effective in December. A restrictive immunisation policy only immunising children with BPD in high-risk months is therefore recommended. The costs of passive immunisation would have to be considerably reduced to achieve cost-effectiveness. |
| 30,795 | 58,076 | Male infant, preterm (< 28 wGA), birth weight < 2,500g, with BPD (January) | |||||
| 31,055 | 58,567 | Male infant, preterm (< 28 wGA), birth weight < 2,500g, with BPD (November) | |||||
| 47,145 | 88,911 | Male infant, preterm (< 28 wGA), birth weight < 2,500g, with BPD (February) | |||||
| 105,120 | 198,246 | Male infant, preterm (< 28 wGA), birth weight < 2,500g, with BPD (March) | |||||
| 395,860 | 746,554 | Male infant, preterm (< 28 wGA), birth weight < 2,500g, with BPD (April) | |||||
| 833,695 | 1,572,268 | Male infant, preterm (< 28 wGA), birth weight < 2,500g, with BPD (October) | |||||
Roeckl-Wiedmann 2003Footnote 36 |
DEU 2000; NR |
6,639 | 11,821 | Cost per hospitalization avoided |
Group A, preterm (<35 wGA)Footnote g | Deterministic |
Because of the findings of our cost-effectiveness analysis, we would recommend a restricted use of PVZ prophylaxis in premature infants with CLD in their risk combination. The results of this cost-effectiveness analysis do not justify the widespread use of PVZ among preterm infants. PVZ was most cost-effective among male infants with CLD who had siblings visiting day-care groups, and who were discharged between October and December. |
| 25,288 | 45,028 | Group B, preterm (<35 wGA) with RFFootnote h | |||||
| 52,838 | 94,084 | Group C, preterm (<35 wGA) with RFFootnote i | |||||
| 204,684 | 364,462 | Group D, preterm (<35 wGA) with RFFootnote j | |||||
Salinas-Escudero 2012Footnote 38 |
USA 2009; $50,000 per QALY, and 3x GDP per capita |
4,539 | 6,216 | Cost per QALY gained |
Partial coverage, preterm (< 29 wGA) | Deterministic; probabilistic |
PVZ prophylaxis for preterm newborn patients born ≤ 32 weeks of age resulted in a CE alternative. When evaluating the ICER per QALY and LYG against the USD $50,000 threshold, all age groups within the prophylaxis group are CE. |
| 7,294 | 9,989 | Partial coverage, preterm (29-32 wGA) | |||||
| 17,532 | 24,009 | Full coverage, preterm (<29 wGA) | |||||
| 20,760 | 28,429 | Full coverage, preterm (29-32 wGA) | |||||
Sanchez-Luna 2017Footnote 39 |
ESP 2016; €30,000 per QALY |
11,550 | 22,173 | Cost per QALY gained |
Subgroup A (payer), preterm (32-35 wGA) with RF: 2 major 2 minorFootnote k | Deterministic; probabilistic |
Out of 1,000 Monte Carlo simulations, 85.70% of the cases presented an ICUR under a €30,000/QALY. PVZ is efficient for preventing from RSV infections in preterm infants 32-35 wGA in Spain, including specific high risk subgroups. |
| 14,177 | 27,216 | Subgroup B (payer), preterm (32-35 wGA) with RF: 2 major 1 minorFootnote l | |||||
| 17,153 | 32,930 | BC (societal), preterm (32-35 wGA) | |||||
| 18,938 | 36,356 | Subgroup C (payer), preterm (32-35 wGA) with RF: 2 major RFFootnote m | |||||
| 19,698 | 37,815 | BC (payer), preterm (32-35 wGA) | |||||
| Schmidt 2017Footnote 40 | ESP 2016; €30,000 per QALY | 15,748 | 30,232 | Cost per QALY gained | BC | Deterministic; probabilistic | PSA demonstrated that the probability of PVZ prophylaxis being CE at a € 30,000 per QALY threshold was 92.7%. The ICER remained below this threshold for most extreme scenario analyses. PVZ prophylaxis was shown to be a CE health care intervention according to the commonly accepted standards of CEness in Spain (ICER below the threshold of € 30,000 per QALY). |
Smart 2010Footnote 41Footnote 53 |
CAN 2010; $50,000 per QALY |
192 | 215 | Cost per QALY gained |
Preterm (32-35 wGA), 4 or more RF | Deterministic; probabilistic |
PVZ ICERs remained fairly stable from 2007 to 2010. The original recommendation stating that PVZ is cost effective in infants born between 32 and 35 wGA with 2 or more RF, or who are at moderate-to-high risk based on a risk assessment model, does not change. |
| 5,274 | 5,906 | Preterm (32-35 wGA), Risk scoring tool, high risk (65-100) | |||||
| 20,814 | 23,309 | BC Preterm (32-35 wGA), (including asthma) | |||||
| 26,701 | 29,901 | Preterm (32-35 wGA), 3 RF | |||||
| 31,360 | 35,119 | BC Preterm (32-35 wGA), (excluding asthma) | |||||
| 34,438 | 38,566 | Preterm (32-35 wGA), Risk scoring tool, medium risk (49-64 score) | |||||
| 48,495 | 54,308 | Base case, Preterm (32-35 wGA), mortality rate (1.2%) | |||||
| 50,434 | 56,479 | Base case, Preterm (32-35 wGA), mortality rate (1.0%) | |||||
| 82,732 | 92,649 | Preterm (32-35 wGA), 2 RF | |||||
| 146,218 | 163,744 | Preterm (32-35 wGA), 1 RF | |||||
| 183,561 | 205,563 | Preterm (32-35 wGA), Risk scoring tool, low risk (0-48 score) | |||||
| 820,701 | 919,073 | Preterm (32-35 wGA), Zero RF (Preterm only) | |||||
Tam 2009Footnote 42 |
CAN 2007; $50,000 to $75,000 per QALY |
334 | 391 | Cost per QALY gained |
High risk <1 year | Deterministic; probabilistic |
PVZ is a CE option for the prevention of RSV for Inuit infants on Baffin Island, is highly cost effective in Arctic infants <1 year of age specifically residing outside of Iqaluit and is a dominant strategy for those under 6 months of age in remote areas. However, PVZ is not cost effective compared to no treatment for infants of all ages residing in Iqaluit. |
| 7,822 | 9,154 | Baffin < 6 mo., societal | |||||
| 10,190 | 11,925 | Baffin < 6 mo. | |||||
| 22,383 | 26,195 | Outside of Iqaluit (remote areas) <1 year, societal | |||||
| 24,750 | 28,965 | Outside of Iqaluit (remote areas) <1 year | |||||
| 37,070 | 43,383 | All Baffin Island infants <1 year, societal | |||||
| 39,435 | 46,151 | All Baffin Island infants < 1 year | |||||
| 100,872 | 118,052 | Residing in Iqaluit <6 mo., societal | |||||
| 103,235 | 120,817 | Residing in Iqaluit <6 mo. | |||||
| 149,782 | 175,291 | Residing in Iqaluit <1 year, societal | |||||
| 152,145 | 178,057 | Residing in Iqaluit <1 year | |||||
| Dominant | Dominant | remote infants < 6 mo. | |||||
| Dominant | Dominant | high risk < 6 mo. | |||||
| Dominant | Dominant | High risk < 1 year, societal | |||||
| Dominant | Dominant | remote infants < 6 mo., societal | |||||
| Dominant | Dominant | high risk < 6 mo., societal | |||||
Vogel 2002Footnote 43 |
NZL 2000; NR |
28,700 | 33,376 | Cost per case avoided |
Preterm (32-35 wGA) with CLD, discharged home on oxygen | Deterministic |
If value is placed on preventing morbidity, the priority groups for PVZ prophylaxis are preterm infants discharged home on oxygen, followed by preterm infants of 28 wGA or less. |
| 32,000 | 37,213 | Preterm (<=28 wGA), no CLD | |||||
| 60,000 | 69,775 | Total cohort, Preterm (32-35 wGA) with CLD, societal | |||||
| 65,000 | 75,590 | Preterm (<=28 wGA) with CLD | |||||
| 98,000 | 113,966 | Preterm (29-31 wGA), no CLD | |||||
| 166,700 | 193,859 | Preterm (29-31 wGA) with CLD | |||||
Wang 2008Footnote 14 |
GBR 2006; £30,000 per QALY |
51,800 | 107,070 | Cost per hospitalization avoided | Preterm infants (<35 wGA) with children without CLD | Deterministic |
According to this model, prophylaxis with PVZ is not a CE strategy for preterm infants and children with CHD compared with no prophylaxis from both an NHS perspective and societal perspective. These findings are robust to probabilistic and other sensitivity analyses. Prophylaxis with PVZ is also not a CE strategy for preterm infants or infants with CLD who have no other RF. Subgroup analyses showed that prophylaxis with PVZ for children with CLD may be CE, at a WTP threshold of £30,000/QALY. |
| 63,800 | 131,874 | Cost per QALY gained |
Preterm infants (<35 wGA) and children with CLD | ||||
| 66,900 | 138,282 | Preterm infants (<35 wGA) and children with CLD (societal) | |||||
| 67,600 | 139,729 | Cost per hospitalization avoided |
Preterm infants (<35 wGA) and children with CLD | ||||
| 78,600 | 162,466 | CHD | |||||
| 79,800 | 164,946 | Cost per QALY gained |
CHD | ||||
| 83,200 | 171,974 | CHD (societal) | |||||
| 454,100 | 938,623 | Preterm infants (<35 wGA) and children without CLD | |||||
| 475,600 | 983,064 | Preterm infants (<35 wGA) and children without CLD (societal) | |||||
Weiner 2012Footnote 44 |
USA 2010; $157,000 per QALY (highest ICER for vaccine - meningo-coccal vaccine) |
16,037 | 21,931 | Cost per QALY gained |
BC Group 2, preterm (32-34 wGA) with RFFootnote n | Deterministic; probabilistic |
PVZ, when dosed consistent with the FDA-approved labeling, was either cost-saving or CE among current guideline-eligible infants in the Medicaid population. PVZ did not demonstrate CEness in 32–35 wGA infants with <=1 RF. |
| 38,244 | 52,299 | BC Group 3, preterm (32-35 wGA) with RFFootnote o | |||||
| 281,892 | 385,488 | BC Group 4, preterm (32-35 wGA) with RFFootnote p | |||||
| Dominant | Dominant | BC Group 1, preterm (<32 wGA)Footnote q | |||||
| Yount 2004Footnote 45 | USA 2002; NR | 114,337 | 183,401 | Cost per QALY gained | BC, term, CHD | Deterministic | The cost of PVZ prophylaxis was high relative to benefits realized. Given the large number of CHD patients who might be considered candidates for RSV prophylaxis (>6000 patients per year in the US) routine use of PVZ in young children with CHD needs to be evaluated further. |
Definitions: AUT, Austria; BC, base-case; BPD, bronchopulmonary dysplasia; CA, chronological age; CAD, Canadian dollar; CAN, Canada; CE, cost-effective; CHD, congenital heart disease; CLD, chronic lung disease; DEU, Germany; ESP, Spain; EUR, Euros; FRA, France; GA, gestational age; GBP, Great Britain Pounds; GBR: Great Britain; HA, hospitalization avoided; hsCHD, hemodynamically significant heart disease; ICER, incremental cost-effectiveness ratio; ITA, Italy; LYG, life years gained; NLD, Netherlands; NR, not reported; NWT, Northwest Territories; NZD, New Zealand dollars; NZL, New Zealand; PVZ, palivizumab;QALY, quality-adjusted life years; RF, risk factors; RSV, respiratory syncytial virus; SEK, Swedish Krona; SWE, Sweden; wGA, weeks' gestation age; USA, United States of America; USD, US dollars; WTP, Willingness-to-pay. Footnotes
|
|||||||
Perspective |
BPD/CLD |
CHD |
Preterm |
Preterm with BPD/CLD |
Preterm with risk factorsFootnote a |
CF |
Canadian ArcticFootnote b |
|
|---|---|---|---|---|---|---|---|---|
| All infants | High-risk areas | |||||||
Payer perspective |
||||||||
| Number of estimates | 6 | 10 | 22 | 4 | 14 | 2 | 6 | 2 |
| ICER (Minimum) | 4,786 | 11,668 | 6,216 | 15,202 | 215 | 167,107 | Dominant | Dominant |
| ICER (Maximum) | 46,821 | 164,946 | 938,623 | 131,874 | 205,563 | 693,105 | 178,057 | 391 |
| Proportion of estimates that are cost saving | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.17 | 0.50 |
| Proportion of estimates CE below $50,000/QALY | 1.00 | 0.80 | 0.82 | 0.75 | 0.64 | 0.0 | 0.67 | 1.00 |
| Proportion of estimates CE below $100,000/QALY | 1.00 | 0.90 | 0.86 | 0.75 | 0.86 | 0.0 | 0.67 | 1.00 |
| Proportion of estimates CE below $200,000/QALY | 1.00 | 1.00 | 0.91 | 1.00 | 0.92 | 0.50 | 1.00 | 1.00 |
Societal perspective |
||||||||
| Number of estimates | 1 | 8 | 23 | 3 | 6 | 0 | 6 | 2 |
| ICER (Minimum) | 28,529 | Dominant | Dominant | 18,717 | 21,931 | N/A | Dominant | Dominant |
| ICER (Maximum) | 28,529 | 209,666 | 2,975,489 | 138,282 | 635,172 | N/A | 175,291 | Dominant |
| Proportion of estimates that are cost saving | 0.0 | 0.13 | 0.13 | 0.0 | 0.0 | N/A | 0.17 | 1.00 |
| Proportion of estimates CE below $50,000/QALY | 1.00 | 0.63 | 0.39 | 0.67 | 0.17 | N/A | 0.67 | 1.00 |
| Proportion of estimates CE below $100,000/QALY | 1.00 | 0.63 | 0.48 | 0.67 | 0.50 | N/A | 0.67 | 1.00 |
| Proportion of estimates CE below $200,000/QALY | 1.00 | 0.88 | 0.52 | 1.00 | 0.67 | N/A | 1.00 | 1.00 |
Notes: All ICERs are reported in 2017 Canadian dollars per QALY. Definitions: BPD, Bronchopulmonary dysplasia; CE, Cost-effective; CHD, Congenital heart disease; CLD, Chronic lung disease; CF: cystic fibrosis; ICER, Incremental cost-effectiveness ratio; N/A, not applicable; QALY, Quality-adjusted life years. Footnotes
|
||||||||
Authors, year |
Dosage |
Cost per unit (unadjusted) |
Hospitalization |
Mortality |
RSV-sequelae |
Conclusions |
||
|---|---|---|---|---|---|---|---|---|
| PVZ | No PVZ | Reduction (%) | ||||||
| Tam et al., 2009 | 5 (per season) | Vial unit cost: NR; $220/kg infant |
1.4 - 11.4% | 6.3 - 51.2% | 78% | 1% (both groups) | N | From the payer perspective, PVZ is CE for all Baffin Island infants <1 year CA (ICER: $46,151/QALY), infants at high-risk for RSV ($391/QALY), infants from remote areas ($28,965/QALY), infants < 6 months of age from remote areas, or at high-risk for RSV (dominant). It was not CE for infants < 12 mos residing in Iqaluit. |
| Smart et al., 2010 | 5.39 vials (per season) | 50mg: $752 100mg: $1,505 |
1.8% | 10.0% | NR (82% back calculation) | 3.9% (both groups)Footnote a | Y | From the payer perspective, PVZ is CE for preterm infants when excluding asthma ($35,119/QALY), and when including asthma ($23,309/QALY). |
| Harris et al., 2011 | 4.5 (per season) | 100mg: $1,468 | 1.7% | 2.9% | NR (42% back calculation) | NR; 0.2% (1/41, no PVZ); 0% (0/292, PVZ) | N | From a societal perspective, PVZ in CHD infants: ICER of $18,155/day of hospitalization avoided. No threshold was used but the study concluded this strategy was CE for the CHD infant population. |
| Banerji et al., 2016 | 6; (per mo, max assump.) | Vial unit cost: NR; $226/kg infant | NR | NR | 96% | NR | N | From a payer perspective, 2 PVZ program scenarios proposed were both CE in some, but not all, Canadian Arctic regions (ICER range, dominant to $45,060/QALY). PVZ is CE in these regions for term infants due to high costs of hospitalizations. |
| McGirr et al., 2017 | 5 (monthly over 5-mo season) | 100mg: $1,505 | 1.7% (assuming 55% reduction)Footnote b | 3.8% | 55% | NR (Used CF-related death) | Y (CF) | PVZ is unlikely to be CE for all infants with CF (ICER: $693,105/QALY) and for high-risk infants with CF (ICER: $167,107/QALY). |
Definitions: CE, cost-effective; CF, cystic fibrosis; CHD, congenital heart disease; ICER, incremental cost-effectiveness ratio; mo, months; N, noNR, Not reported; PVZ, Palivizumab; QALY, quality-adjusted life years; RSV, respiratory syncytial virus; Y, yes. Footnotes
|
||||||||
Supplementary materials
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 4-5 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 6 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 6 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 7 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 7 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 6-7 |
| Search | 8 | Present full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | Supplementary 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 7-8 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 7-8 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 7-8 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 7-8 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 7-8 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 7-8 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | N/Ap |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 7-8 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 8 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 8 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 8 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group; (b) effect estimates and confidence intervals, ideally with a forest plot. | 8-13 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | N/Ap |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | N/Ap |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 9-13 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 13-15 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 15 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 15-16 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 3 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097 |
|||
Appendix 2. Search strategy
| Number | Searches | Results |
|---|---|---|
| 1 | exp Antibodies, Monoclonal/ or exp antivaral agents/ or exp immunoglobulins/ or (antibody protein or anti viral agent* or anti viral drug* or antiviral agent* or antiviral drug* or antiviral substance or antivirals or antivirus agent* or antivirus drug* or anti-RSV or clonal antibody or endobulin or flebogamma or flebogammadif or gamastan or gamimmune n or gamimune or gamma globulin* or gamma-globulin* or gammaglobulin* or gammar or gamulin or globuman or humanized antibody or humanized monoclonal antibody or hybridoma antibody or Ig or igam or igc or immune gamma globulin or immune globin or immune globulin* or immune serum globulin* or immuno gamma globulin* or immuno globulin* or immunogammaglobulin* or immunoglobin* or immunoglobulin* or immunoprotein* or intragam or intraglobin f or isiven or iveegam or ivega or mAbs or MEDI 493 or monoclonal antibodies or monoclonal antibody or palivizumab or panglobulin* or passive immunization or sandoglobin* or sandoglobulin* or synagis or tegelin* or veinoglobulin* or venoglobulin* or viral inhibitor or virostatic agent* or virucidal agent* or virucide agent* or virustatic agent* or vivaglobin).tw,kf,nm. | 1043332 |
| 2 | respiratory syncytial virus infections/pc | 1417 |
| 3 | respiratory syncytial virus infections/ or Respiratory Syncytial Virus, Human/ or Respiratory Syncytial Viruses/ or (respiratory syncytial vir* or RSV*).tw. | 18025 |
| 4 | prophylaxis/ or (control or health protection or immunoprophylaxis or prevention or preventive measures or preventive medication or preventive therapy or preventive treatment or prophylactic institution or prophylactic management or prophylactic medication or prophylactic therapy or prophylactic treatment or prophylaxis).tw. | 2762004 |
| 5 | 2 or (3 and 4) | 4221 |
| 6 | and/1,5 | 1760 |
| 7 | exp Infant/ or exp Child/ or GA/ or exp Pediatrics/ or Schools, Nursery/ or (infant* or infancy or newborn* or baby* or babies or neonat* or preterm* or prematur* or preemie* or gestation* or GA or child or children or preschool* or kid or kids or toddler* or pediatric* or paediatric* or peadiatric* or nursery school*).tw. | 3053948 |
| 8 | Economics/ | 26937 |
| 9 | exp "Costs and Cost Analysis"/ | 217482 |
| 10 | Economics, Nursing/ | 3981 |
| 11 | Economics, Medical/ | 8964 |
| 12 | Economics, Pharmaceutical/ | 2794 |
| 13 | exp Economics, Hospital/ | 23019 |
| 14 | Economics, Dental/ | 1897 |
| 15 | exp "Fees and Charges"/ | 29368 |
| 16 | exp Budgets/ | 13335 |
| 17 | budget*.ti,ab,kf. | 26174 |
| 18 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf. | 202619 |
| 19 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab. /freq=2 | 246788 |
| 20 | (cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf. | 138081 |
| 21 | (value adj2 (money or monetary)).ti,ab,kf. | 2025 |
| 22 | exp models, economic/ | 13464 |
| 23 | economic model*.ab,kf. | 2837 |
| 24 | markov chains/ | 12909 |
| 25 | markov.ti,ab,kf. | 19178 |
| 26 | monte carlo method/ | 25573 |
| 27 | monte carlo.ti,ab,kf. | 43481 |
| 28 | exp Decision Theory/ | 11145 |
| 29 | (decision* adj2 (tree* or analy* or model*)).ti,ab,kf. | 19525 |
| 30 | or/8-29 | 647482 |
| 31 | and/6-7,30 | 230 |
| 32 | exp australia/ or austria/ or exp baltic states/ or exp belgium/ or exp canada/ or chile/ or czech republic/ or exp "scandinavian and nordic countries"/ or exp france/ or exp germany/ or greece/ or hungary/ or exp ireland/ or israel/ or exp italy/ or exp japan/ or exp republic of korea/ or luxembourg/ or mexico/ or exp netherlands/ or exp new zealand/ or poland/ or exp portugal/ or slovakia/ or slovenia/ or exp spain/ or exp switzerland/ or turkey/ or exp united kingdom/ or exp united states/ or (australia* or new south wales or queensland or tasmania or victoria or sydney or melbourne or brisbane or austria* or vienna or viennese* or belgium* or belgian* or brussels or flemish* or canad* or ottawa* or british columbia* or colombie britannique* or vancouver* or alberta* or edmonton* or calgar* or saskatchewan* or regina* or saskatoon* or manitoba* or winnipeg* or ontari* or toronto* or quebec* or montreal* or new brunswick* or nouveau brunswick* or fredericton* or nova scotia* or nouvelle ecosse* or halifax* or haligonian* or prince edward island* or ile du prince edouard* or pei or charlottetown* or newfoundland* or terre neuve* or labrador* or nfld or yukon* or whitehorse* or northwest territor* or territoires du nord ouest* or nwt or yellowknife* or nunavut* or iqaluit* or chile* or santiago or czech* or prague or denmark* or danish or dane* or faroe* or copenhagen or estonia* or tallinn or finland* or finnish* or helsinki* or france* or french* or paris* or marseille or lyon or lille or nice or toulouse or bordeaux or german* or deutschland* or berlin* or hamburg or munich or cologne or frankfurt or stuttgart or dusseldorf or greece* or hellenic* or greek* or athens or macedonia* or hungary* or hungarian* or budapest or iceland* or reykjavik or ireland* or irish* or dublin* or israel* or jerusalem or tel aviv or italy or italian* or rome or milan or naples or turin or sicily or japan* or tokyo or yokohama or osaka or nagoya or sapporo or kobe or kyoto or korea* or seoul or busan or daegu or daejeon or gwangju or incheon or ulsan or latvia* or riga or lithuania* or vilnius or luxembourg* or netherland* or holland* or dutch* or amsterdam or rotterdam or hague or new zealand* or aotearoa or wellington or auckland or maori or mexic* or norway* or norwegian* or oslo or poland* or polish or warsaw or krakow or wroclaw or lodz or portug* or lisbon or slovak* or bratislava or slovenia* or slovene* or ljubljana or spain* or spanish* or spaniard* or madrid or barcelona or catalonia* or valencia* or seville or zaragoza or malaga or basque or scandinavia* or sweden or swedish or swede* or stockholm or switzerland* or swiss* or zurich or geneva or bern or turkey or turkish or istanbul or constantinople or britain* or british* or united kingdom* or scotland* or scottish or wales* or welsh or england* or belfast or london or manchester or glasgow or birmingham or leeds or bradford or liverpool or alabama* or alaska* or arizona* or arkansas* or california* or colorado* or connecticut* or delaware* or florida* or georgia* or hawaii* or idaho* or illinois* or indiana* or iowa* or kansas* or kentucky* or louisiana* or maine* or maryland* or massachusetts* or michigan* or minnesota* or mississippi* or missouri* or montana* or nebraska* or nevada* or new hampshire* or new jersey* or new mexico* or new york* or north carolina* or north dakota* or ohio* or oklahoma* or oregon* or pennsylvania* or rhode island* or south carolina* or south dakota* or tennessee* or texas* or utah* or vermont* or virginia* or washington* or west virginia* or wisconsin* or wyoming* or montgomery* or juneau* or anchorage* or phoenix* or little rock* or sacramento* or los angeles* or san diego* or san francisco* or denver* or hartford* or dover* or tallahassee* or miami* or orlando* or atlanta* or honolulu* or boise* or springfield* or chicago* or des moines* or topeka* or frankfort* or baton rouge* or new orleans* or augusta* or annapolis* or boston* or lansing* or detroit* or st?paul* or jackson* or jefferson city* or helena* or lincoln* or carson city* or reno* or las vegas* or concord* or trenton* or santa fe* or albany* or raleigh* or bismarck* or columbus* or oklahoma city* or salem* or harrisburg* or providence* or columbia* or peirre* or nashville* or austin* or dallas* or salt lake city* or montpelier* or richmond* or olympia* or seattle* or charleston* or madison* or cheyenne* or district of columbia* or usa or united states).tw,kf. | 4128242 |
| 33 | 31 and 32 | 117 |
| 34 | limit 33 to (yr="2000 -Current" and (english or french)) | 104 |
| 35 | (comment or editorial or letter or news).pt. | 1825216 |
| 36 | 34 not 35 | 91 |
Critical appraisal: Joanna Briggs Institute checklist |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author; year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Banerji; 2016 | Y | U | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Bentley; 2013 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Blanken; 2018 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Chirico; 2009 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Hampp; 2011 | Y | Y | N | Y | U | Y | N | Y | Y | Y | Y |
| Harris; 2011 | Y | Y | U | Y | Y | Y | U | Y | Y | Y | Y |
| Hascoet; 2008 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Smart; 2010 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Mahadevia; 2012 | Y | Y | N | Y | Y | Y | Y | Y | Y | U | Y |
| McGirr; 2017 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Neovius; 2011 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Nuijten; 2009_DEU | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Nuijten; 2009_NLD | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Nuijten; 2010 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Nuijten; 2007 | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y |
| Resch; 2012 | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y |
| Rietveld; 2009 | Y | Y | N | Y | Y | Y | Y | Y | Y | U | U |
| Roeckl-Wiedmann; 2003 | Y | Y | N | Y | Y | Y | Y | Y | Y | U | U |
| Salinas-Escudero; 2012 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Sanchez-Luna; 2017 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Schmidt; 2017 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Tam; 2009 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Vogel; 2002 | Y | Y | N | Y | Y | Y | N | Y | Y | U | N |
| Wang; 2008 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Weiner; 2012 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Yount; 2004 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N |
| ElHassan; 2006 | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y |
| Lofland; 2000 | Y | Y | U | Y | U | U | Y | Y | Y | U | Y |
Definitions: |
|||||||||||
Questions 23: 1. Is there a well-defined question? 2. Is there comprehensive description of alternatives? 3. Are all important and relevant costs and outcomes for each alternative identified? 4. Has clinical effectiveness been established? 5. Are costs and outcomes measured accurately? 6. Are costs and outcomes valued credibly? 7. Are costs and outcomes adjusted for differential timing? 8. Is there an incremental analysis of costs and consequences? 9. Were sensitivity analyses conducted to investigate uncertainty in estimates of cost or consequences? 10. Do study results include all issues of concern to users? 11. Are the results generalizable to the setting of interest in the review?
References
- Footnote 1
-
Meates-Dennis M. Bronchiolitis. Arch Dis Child Educ Pr Ed. 2005 Nov 21;90:81-86. http://dx.doi.org/10.1136/adc.2004.067660.
- Footnote 2
-
Collins CL, Pollard AJ. Respiratory syncytial virus infections in children and adults. J Infect. 2002 Jul;45(1):10-17. doi: 10.1053/jinf.2001.1016.
- Footnote 3
-
Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981 May;98(5):708-715. doi: 10.1016/s0022-3476(81)80829-3.
- Footnote 4
-
Hall CB. Respiratory Syncytial Virus and Parainfluenza Virus. N Engl J Med. 2001 Jun 21;344(25):1917-1928. doi: 10.1056/NEJM200106213442507.
- Footnote 5
-
Paes BA, Frcpc M, Mitchell I, et al. A decade of respiratory syncytial virus epidemiology and prophylaxis: Translating evidence into everyday clinical practice Case presentation. Can Respir J. 2011 Mar-Apr;18(2):e10-e19. doi: 10.1155/2011/493056.
- Footnote 6
-
Simoes EA. Respiratory syncytial virus infection. Lancet. 1999 Sep 4;354(9181):847-852. doi:10.1016/S0140-6736(99)80040-3.
- Footnote 7
-
Sánchez-Solis M, Gartner S, Bosch-Gimenez V, Garcia-Marcos L. Is palivizumab effective as a prophylaxis of respiratory syncytial virus infections in cystic fibrosis patients? A meta-analysis. Allergol Immunopathol (Madr). 2015 May-Jun;43(3):298-303. doi:10.1016/J.ALLER.2013.09.003.
- Footnote 8
-
Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1995 Feb;126(2):212-219. doi: 10.1016/s0022-3476(95)70547-3.
- Footnote 9
-
American Academy of Pediatrics. Palivizumab granted licensure for prophylaxis of RSV disease. AAP News; 1998 Oct 1 [cited 2018 Dec 19]. Available from: http://www.aappublications.org/content/14/10/10.3.
- Footnote 10
-
The IMpact-RSV Study Group. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics. 1998;102(3):531-537. https://doi.org/10.1542/peds.102.3.531.
- Footnote 11
-
Embleton ND, Harkensee C, Mckean MC. Palivizumab for preterm infants. Is it worth it? Arch Dis Child Fetal Neonatal Ed. 2005 Jul;90(4):286-289. doi: 10.1136/adc.2004.058081.
- Footnote 12
-
Fenton C, Scott LJ, Plosker GL. Palivizumab: a review of its use as prophylaxis for serious respiratory syncytial virus infection. Paediatr Drugs. 2004;6(3):177-197. doi: 10.2165/00148581-200406030-00004.
- Footnote 13
-
Harkensee C, Brodlie M, Embleton ND, Mckean M. Passive immunisation of preterm infants with palivizumab against RSV infection. J Infect. 2006 Jan;52(1):2-8. doi:10.1016/j.jinf.2005.08.003.
- Footnote 14
-
Wang D, Cummins C, Bayliss S, Sandercock J, Burls A. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess. 2008 Dec;12(36):1-86. doi: 10.3310/hta12360.
- Footnote 15
-
Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Vrca VB, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013 Apr 30;(4):1-106. doi: 10.1002/14651858.CD006602.pub4.
- Footnote 16
-
Byington CL, Munoz FM. Palivizumab Prophylaxis for Healthy Preterm Infants: More Data Supporting American Academy of Pediatrics Guidelines. 2016 Aug. doi: 10.1542/peds.2016-1494
- Footnote 17
-
Chabra S. Precise Gestational Age Definitions Needed for Palivizumab Prophylaxis in Preterm Infants. Am J Respir Crit Care Med. 2018 Mar 1;197(5):680-680. doi: 10.1164/rccm.201708-1605LE
- Footnote 18
-
Embleton N, Harkensee C, Mckean MC. Palivizumab for preterm infants. Is it worth it? Arch Dis Child Fetal Neonatal Ed. 2005 Jul;90:F286-F289. doi: 10.1136/adc.2004.05808
- Footnote 19
-
Mac S, Sumner A, Duchesne-Belanger S, Stirling R, Tunis M, Sander B. Cost-Effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics. 2019 May;143:e20184064 doi: 10.1542/peds.2018-4064.
- Footnote 20
-
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009 Jul 21;6(7):1-6. doi: 10.1371/journal.pmed.1000097.
- Footnote 21
-
National Advisory Committee on Immunization (NACI). Statement on the recommended use of monoclonal anti-RSV antibody (palivizumab). Can Commun Dis Rep. 2003 Sep 15;29(ACS-7):1-13.
- Footnote 22
-
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--Explanation and elaboration: A report of the ISPOR Health Economic Evaluations Publication Guidelines Task Force. Value Heal. 2013 Mar-Apr;16:231-250. doi: 10.1016/j.jval.2013.02.002.
- Footnote 23
-
Gomersall J, Jadote Y, Xue Y, Lockwood S, Riddle D, Preda A. Conducting systematic reviews of economic evaluations. Int J Evid Based Heal. 2015 Sep;13(3):170-178. doi: 10.1097/XEB.0000000000000063.
- Footnote 24
-
Patel H, Sander B, Nelder MP. Long-term sequelae of West Nile virus-related illness: A systematic review. Lancet Infect Dis. 2015 Aug;15(8):951-959. doi: 10.1016/S1473-3099(15)00134-6.
- Footnote 25
-
OECD. Purchasing power parities (PPP) (indicator). 2022. doi:10.1787/1290ee5a-en.
- Footnote 26
-
Banerji A, Panzov V, Young M, Robinson J, Lee B, Moraes T, et al. Hospital admissions for lower respiratory tract infections among infants in the Canadian Arctic: a cohort study. C Open. 2016 Oct 17;4(4):E615-E622. doi: 10.9778/cmajo.20150051.
- Footnote 27
-
McGirr AA, Schwartz KL, Allen U, Solomon M, Sander B. The cost-effectiveness of palivizumab in infants with cystic fibrosis in the Canadian setting: A decision analysis model. Hum Vaccines Immunother. 2017 Mar 4;13(3):599-606. doi: 10.1080/21645515.2016.1235670.
- Footnote 28
-
Neovius K, Buesch K, Sandström K, Neovius M. Cost-effectiveness analysis of palivizumab as respiratory syncytial virus prophylaxis in preterm infants in Sweden. Acta Paediatr Int J Paediatr. 2011 Oct;100(10):1306-1314. doi: 10.1111/j.1651-2227.2011.02309.x.
- Footnote 29
-
Nuijten MJC, Wittenberg W, Lebmeier M. Cost Effectiveness of Palivizumab for Respiratory Syncytial Virus Prophylaxis in High-Risk Children. Pharmacoeconomics. 2007;25(1):55-71. doi: 10.2165/00019053-200725010-00006.
- Footnote 30
-
Nuijten MJ, Wittenberg W. Cost effectiveness of palivizumab in Spain: An analysis using observational data. Eur J Heal Econ. 2010 Feb;11(1):105-115. doi: 10.1007/s10198-009-0206-x.
- Footnote 31
-
Nuijten M, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab for RSV prevention in high-risk children in the Netherlands. J Med Econ. 2009;12(4):291-300. doi: 10.3111/13696990903316961.
- Footnote 32
-
Nuijten M, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab in children with congenital heart disease in Germany. J Med Econ. 2009;12(4):301-308. doi: 10.3111/13696990903347172.
- Footnote 33
-
Resch B, Gusenleitner W, Nuijten MJC, Lebmeier M, Wittenberg W. Cost-effectiveness of palivizumab against respiratory syncytial viral infection in high-risk children in Austria. Clin Ther. 2008 Apr;30(4):749-760. doi: 10.1016/j.clinthera.2008.03.014.
- Footnote 34
-
Resch B, Sommer C, Nuijten MJC, et al. Cost-effectiveness of Palivizumab for Respiratory Syncytial Virus Infection in High-risk Children, Based on Long-term Epidemiologic Data From Austria. Pediatr Infect Dis J. 2012 Jan;31(1):e1-e8. doi: 10.1097/INF.0b013e318235455b.
- Footnote 35
-
Rietveld E, Steyerberg EW, Polder JJ, Veeze HJ, Vergouwe Y, Huysman MW, et al. Passive immunisation against respiratory syncytial virus: A cost-effectiveness analysis. Arch Dis Child. 2010 Jul;95(7):493-498. doi: 10.1136/adc.2008.155556.
- Footnote 36
-
Roeckl-Wiedmann I, Liese JG, Grill E, Fischer B, Carr D, Belohradsky BH. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr. 2003 Apr;162(4):237-244. doi: 10.1007/s00431-002-1106-6.
- Footnote 37
-
Bentley A, Filipovic I, Gooch K, Büsch K. A cost-effectiveness analysis of respiratory syncytial virus (RSV) prophylaxis in infants in the United Kingdom. Health Econ Rev. 2013 Aug 6;3(1):1-12. doi: 10.1186/2191-1991-3-18.
- Footnote 38
-
Salinas-Escudero G, Martínez-Valverde S, Reyes-López A, Garduño-Espinosa J, Muñoz-Hernández O, Granados-Garciá, et al. Cost-effectiveness analysis of the use of palivizumab in the prophylaxis of preterm patients in Mexico. Salud Publica Mex. 2012 Jan-Feb;54(1):47-59. [cited 2022 Aug 10]. Available from: https://www.scielosp.org/article/spm/2012.v54n1/47-59/en/.
- Footnote 39
-
Sanchez-Luna M, Burgos-Pol R, Oyagüez I, Figueras-Aloy J, Sánchez-Solís M, Martinón-Torres F, et al. Cost-utility analysis of Palivizumab for Respiratory Syncytial Virus infection prophylaxis in preterm infants: Update based on the clinical evidence in Spain. BMC Infect Dis. 2017 Oct 17;17(1):1-11. doi: 10.1186/s12879-017-2803-0.
- Footnote 40
-
Schmidt R, Majer I, García Román N, Rivas Basterra A, Grubb EB, Medrano López C. Palivizumab in the prevention of severe respiratory syncytial virus infection in children with congenital heart disease; a novel cost-utility modeling study reflecting evidence-based clinical pathways in Spain. Health Econ Rev. 2017 Dec 19;7(1):47. doi: 10.1186/s13561-017-0181-3.
- Footnote 41
-
Smart KA, Paes BA, Lanctôt KL. Changing costs and the impact on RSV prophylaxis. J Med Econ. 2010;13(4):705-708. doi: 10.3111/13696998.2010.535577.
- Footnote 42
-
Tam DY, Banerji A, Paes BA, Hui C, Tarride J-E, Lanctôt KL. The cost effectiveness of palivizumab in term Inuit infants in the Eastern Canadian Arctic. J Med Econ. 2009;12(4):361-370. doi: 10.3111/13696990903442155.
- Footnote 43
-
Vogel AM, McKinlay MJ, Ashton T, Lennon DR, Harding JE, Pinnock R, et al. Cost-effectiveness of palivizumab in New Zealand. J Paediatr Child Health. 2002 Aug;38(4):352-357. doi: 10.1046/j.1440-1754.2002.00790.x.
- Footnote 44
-
Weiner LB, Masaquel AS, Polak MJ, Mahadevia PJ. Cost-effectiveness analysis of palivizumab among pre-term infant populations covered by Medicaid in the United States. J Med Econ. 2012;15(5):997-1018. doi: 10.3111/13696998.2012.672942.
- Footnote 45
-
Yount LE, Mahle WT. Economic Analysis of Palivizumab in Infants With Congenital Heart Disease. Pediatrics. 2004 Dec;114(6):1606-1611. doi: 10.1542/peds.2004-0224.
- Footnote 46
-
Elhassan NO, Sorbero MES, Hall CB, Stevens TP, Dick AW. Cost-effectiveness analysis of palivizumab in premature infants without chronic lung disease. Arch Pediatr Adolesc Med. 2006 Oct;160(10):1070-1076. doi: 10.1001/archpedi.160.10.1070.
- Footnote 47
-
Lofland JH, Touch SM, O'Connor JP, Chatterton ML, Moxey ED, Paddock LE, et al. Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: A cost-effectiveness analysis. Clin Ther. 2000 Nov;22(11):1357-1369. doi: 10.1016/S0149-2918(00)83032-5.
- Footnote 48
-
Blanken MO, Frederix GW, Nibbelke EE, Koffijberg H, Sanders EAM, Rovers MM, et al. Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants. Eur J Pediatr. 2018 Jan;177:133-144. doi: 10.1007/s00431-017-3046-1.
- Footnote 49
-
Chirico G, Ravasio R, Sbarigia U. Cost-utility analysis of palivizumab in Italy: Results from a simulation model in the prophylaxis of respiratory syncytial virus infection (RSV) among high-risk preterm infants. Ital J Pediatr. 2009 Feb 25;35(4):1-12 doi: 10.1186/1824-7288-35-4.
- Footnote 50
-
Hampp C, Kauf TL, Saidi AS, Winterstein AG. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med. 2011 Jun;165(6):498-505. doi: 10.1001/archpediatrics.2010.298.
- Footnote 51
-
Harris KC, Anis AH, Crosby MC, Cender LM, Potts JE, Human DG. Economic Evaluation of Palivizumab in Children With Congenital Heart Disease: A Canadian Perspective. Can J Cardiol. 2011 Jul-Aug;27(4):523.e11-523.e15. doi: 10.1016/j.cjca.2010.12.064.
- Footnote 52
-
Hascoet JM, Fagnani F, Charlemagne A, Vieux R, Roze JC, Bendjenana H. [Methodological aspects of economic evaluation in pediatrics: illustration by RSV infection prophylaxis in the French setting]. Arch Pediatr. 2008 Dec;15(12):1739-1748. doi: 10.1016/j.arcped.2008.09.024.
- Footnote 53
-
Lanctôt K, Masoud S, Paes B, Tarride JE, Chiu A, Hui C, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32–35 weeks: a Canadian-based analysis. Curr Med Res Opin. 2008 Nov;24(11):3223-3237. doi: 10.1185/03007990802484234.
- Footnote 54
-
Mahadevia PJ, Masaquel AS, Polak MJ, Weiner LB. Cost utility of palivizumab prophylaxis among pre-term infants in the United States: a national policy perspective. J Med Econ. 2012;15(5):987-996. doi: 10.3111/13696998.2012.690013.
- Footnote 55
-
Wang T, Sangha O, Phillips C, Wright EA, Lew RA, Fossel AH, et al. Outcomes of Children Treated for Lyme Disease. J Rheumatogy. 1998 Nov 1;25(11):2249-2253. [cited 2022 Aug 10]. Available from: https://europepmc.org/article/med/9818672.
- Footnote 56
-
Seeler RA, Schatz B. Palivizumab (Synagis)--cohorting babies to reduce waste. Pediatrics. 1999 Nov;104(5 Pt 1):1170-1171. doi: 10.1542/PEDS.104.5.1170.
- Footnote 57
-
Hussman JM, Li A, Paes B, Lanctôt KL. A review of cost-effectiveness of palivizumab for respiratory syncytial virus. Expert Rev Pharmacoeconomics Outcomes Res. 2012 Oct;12(5):553-568. doi: 10.1586/erp.12.45.
- Footnote 58
-
Prescott WA, Doloresco F, Brown J, Paladino JA. Cost Effectiveness of Respiratory Syncytial Virus Prophylaxis. Pharmacoeconomics. 2010;28(4):279-293. doi: 10.2165/11531860-000000000-00000.
- Footnote 59
-
Smart KA, Lanctôt KL, Paes BA. The cost effectiveness of palivizumab: a systematic review of the evidence. J Med Econ. 2010;13(3):453-463. doi: 10.3111/13696998.2010.499749.
- Footnote 60
-
American Academy of Pediatrics Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics. 2014 Aug;134(2):415-420. doi: 10.1542/peds.2014-1665.
- Footnote 61
-
The Commonwealth Fund. 2015 International Profiles of Health Care Systems.; 2015. [cited 2022 Aug 10]. Available from: http://www.commonwealthfund.org/~/media/files/publications/fund-report/2016/jan/1857_mossialos_intl_profiles_2015_v7.pdf.
- Footnote 62
-
Robinson J, Le Saux N, Canadian Pediatric Society, Infectious Diseases and Immunization Committee. Preventing hospitalizations for respiratory syncytial virus infection. Pediatr Child Heal. 2015 Sep 8;20(6):321-326. [cited 2022 Aug 10]. Available from: https://www.cps.ca/en/documents/position/preventing-hospitalizations-for-rsv-infections
The IMpa. - Footnote 63
-
The IMpact-RSV Study Group. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics. 1998;102(3):531-537. https://doi.org/10.1542/peds.102.3.531.
