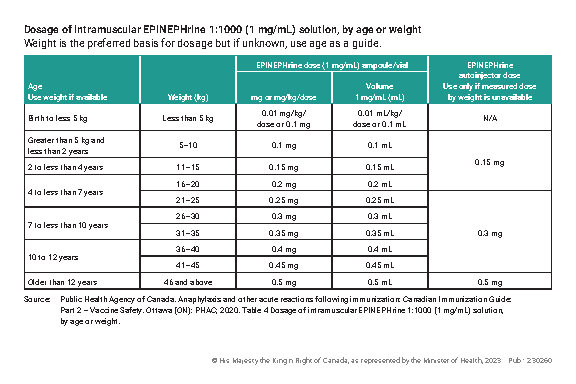
Dosage of intramuscular EPINEPHrine 1:1000 (1 mg/mL) solution, by age or weight
Download in PDF format
(65 KB, 1 page)
Organization: Public Health Agency of Canada
Date published: 2023-09-19
Weight is the preferred basis for dosage but if unknown, use age as a guide.
| Age Use weight if available |
Weight (kg) | EPINEPHrine dose (1 mg/mL) ampoule/vial |
EPINEPHrine autoinjector dose Use only if measured dose by weight is unavailable |
|
|---|---|---|---|---|
| mg or mg/kg/dose |
Volume 1 mg/mL (mL) |
|||
| Birth to less 5 kg |
Less than 5 kg | 0.01 mg/kg/dose or 0.1 mg |
0.01 mL/kg/dose or 0.1 mL |
N/A |
| Greater than 5 kg and less than 2 years | 5 - 10 | 0.1 mg | 0.1 mL | 0.15 mg |
| 2 to less than 4 years | 11 - 15 | 0.15 mg | 0.15 mL | |
| 4 to less than 7 years | 16 - 20 | 0.2 mg | 0.2 mL | |
| 21 - 25 | 0.25 mg | 0.25 mL | 0.3 mg | |
| 7 to less than 10 years | 26 - 30 | 0.3 mg | 0.3 mL | |
| 31 - 35 | 0.35 mg | 0.35 mL | ||
| 10 to 12 years | 36 - 40 | 0.4 mg | 0.4 mL | |
| 41 - 45 | 0.45 mg | 0.45 mL | ||
| Older than 12 years | 46 and above | 0.5 mg | 0.5 mL | 0.5 mg |
Reference
Public Health Agency of Canada. Anaphylaxis and other acute reactions following immunization: Canadian Immunization Guide: Part 2 – Vaccine Safety. Ottawa (ON): PHAC; 2020. Table 4 Dosage of intramuscular EPINEPHrine 1:1000 (1 mg/mL) solution, by age or weight.