Summary of Reports submitted to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) for Vaccines Administered in 2013-2016
What is an Adverse Event Following Immunization (AEFI)?
- An AEFI is defined as any medical event like a sign, symptom or a defined illness that follows immunization. These symptoms are not necessarily caused by or related to the vaccine.
What is the Canadian Adverse Events Surveillance system?
- The Public Health Agency of Canada monitors the safety of vaccines through the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS), the federal/provincial/territorial public health post-market vaccine adverse event monitoring system.
How are adverse events following immunization monitored?
- In Canada, vaccine safety surveillance takes place at local, provincial/territorial and national levels to monitor the safety profile of marketed vaccines to ensure that their benefits continue to outweigh the risks of serious events.
Key Results:
What did the results of reports submitted to CAEFISS between 2013 and 2016 tell us about vaccines administered in Canada?
- Over 80 million vaccine doses were distributed in Canada between 2013 and 2016.
- 11,080 AEFI reports from 12 provinces/territories were received by CAEFISS during this period.
- A very large majority (92%, n=10,188) of reports were non-serious, common and expected adverse events such as vaccination site reactions (e.g. pain and redness) as well as rash and allergic events such as hypersensitivity.
- Only 8% (n=892) of all AEFI reports were determined to be serious adverse events. This means that on average there were 1.1 serious adverse event reports submitted per 100,000 doses of vaccines distributed.
- The majority (73%, n=651) of serious adverse events had fully recovered at the time of reporting.
How are adverse events following immunization processed in CAEFISS?
When reports come in to CAEFISS, each is reviewed and assigned a primary reason for reporting such as:
- Vaccine site reactions (Local reactions)
- Rash
- Neurologic events
- Immunization anxiety
- Allergic events
- Infection/Syndrome/Symptom (ISS) – includes events involving many body systems often accompanied by fever and are classified under the following ISS subcategories: syndromes (e.g. Kawasaki syndrome, fibromyalgia, etc); fever alone; influenza-like illness, and systemic events (such as fatigue, malaise, and lethargy) and may include evidence of infection of one or more body parts
- Other events which includes arthralgia, arthritis, hypotonic-hyporesponsive episode (HHE), intussusception, gastrointestinal diseases, anaesthesia/paraesthesia, parotitis, persistent crying, thrombocytopenia, sudden infant death syndrome (SIDS) and sudden unexpected/unexplained death syndrome (SUDS).
Are adverse events the same in infants, children and adults?
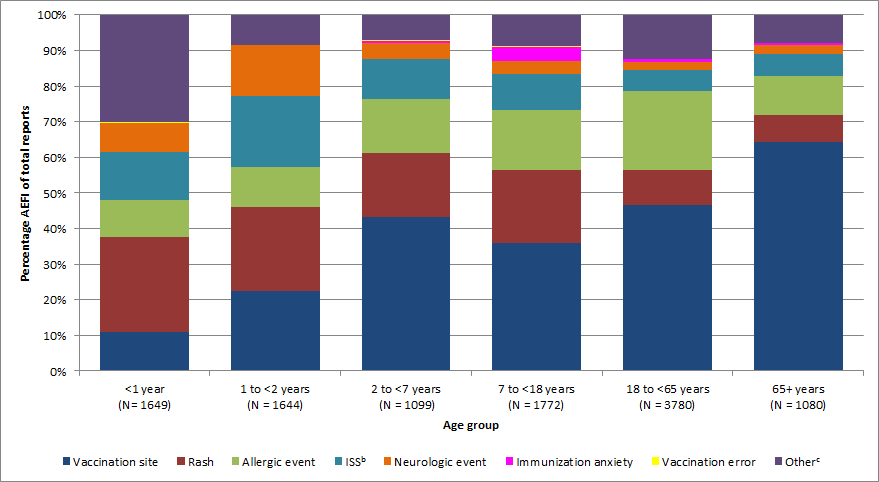
- Vaccination site reaction was the most common AEFI reported overall (37%, n=4,122), followed by rash (17%, n=1,834) and allergic event (16%, n=1,783).
- Vaccination site reaction represented the majority for all the age groups except for those under two years of age.
- For those less than one year of age, the most commonly reported AEFIs were of the 'Other category' (sub-categories such as gastrointestinal symptoms, hypotonic-hyporesponsive episode (HHE) and persistent crying) (30%, n=498), followed by rash (27%, n=440).
- For those between the ages of one to less than two years, the most commonly reported AEFI was rash (24%, n=388), followed by vaccination site reactions (23%, n=371)
- For those aged two years and older, vaccination site reactions represented the majority of AEFIs followed by rash and allergic events.
- For information on the Canadian vaccination schedule and which vaccines are administered at specific ages, please visit the Vaccines for Children and Vaccines for Adults websites.
Figure 1. Proportion of primary adverse events following immunization reported by age group, 2013-2016Figure 1 footnote a
- Figure 1 footnote a
-
56 reports with missing age are excluded from this figure.
- Figure 1 footnote b
-
ISS are primarily events involving many body systems often accompanied by fever. It includes subcategories such as recognized syndromes (e.g. Kawasaki syndrome, fibromyalgia, etc), fever alone, influenza-like illness, and systemic events (such as fatigue, malaise, and lethargy). It also includes evidence for infection of one or more body parts.
- Figure 1 footnote c
-
Other includes arthralgia, arthritis, hypotonic-hyporesponsive episode, intussusception, gastrointestinal diseases, anaesthesia/paraesthesia, parotitis, persistent crying, thrombocytopenia, sudden infant death syndrome (SIDS) and sudden unexpected/unexplained death syndrome (SUDS).
Figure 1: Proportion of primary adverse events following immunization reported by age group, 2013-2016 - Text Description
| no data | Primary Adverse Event Following Immunization | |||||||
|---|---|---|---|---|---|---|---|---|
| Age Group | Allergic event | ISSTable 1 footnote b | Immunization anxiety | Neurologic event | OtherTable 1 footnote c | Rash | Vaccination error | Vaccination site |
| Less than 1 year | 10% | 14% | 0.1% | 8% | 30% | 27% | 0.20% | 11% |
| 1 to less than 2 years | 11% | 20% | 0.1% | 14% | 9% | 24% | 0.10% | 23% |
| 2 to less than 7 years | 15% | 11% | 0.5% | 5% | 7% | 18% | 0.20% | 43% |
| 7 to less than 18 years | 17% | 10% | 4% | 4% | 9% | 21% | 0.10% | 36% |
| 18 to less than 65 years | 22% | 6% | 1% | 2% | 12% | 10% | 0.03% | 47% |
| 65 years and older | 11% | 6% | 0.7% | 2% | 8% | 7% | 0.10% | 64% |
Serious Adverse Events (SAE) reported during 2013-2016.
- A serious adverse event is defined as an event that results in death, is life-threatening, requires in-patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or a congenital anomaly or birth defect.
- Of the 8% (n=892) of AEFI reports that were found to be serious adverse events, the majority resulted from hospitalization (83.5%, n=745) followed by life threatening events (11.5%, n=103), fatal outcome (3.6%, n=32), residual disability (1.2%, n=11) and other reasons (0.1%, n=1).
- The greatest proportion of serious events was seen in the neurological event category (46%, n=275).
The graph below illustrates the percentage of serious and non-serious adverse events per primary reasons for reporting.
Figure 2. Primary adverse event following immunization category by seriousness, 2013-2016

- Figure 2 footnote a
-
ISS: infection/syndrome/systemic symptoms.
- Figure 2 footnote b
-
Vaccination errors included only a small number of reports (9 AEFI Reports) and no serious reports.
- Figure 2 footnote c
-
Other includes arthralgia, arthritis, hypotonic-hyporesponsive episode (HHE), intussusception, gastrointestinal diseases, anaesthesia/paraesthesia, parotitis, persistent crying, thrombocytopenia, sudden infant death syndrome (SIDS) and sudden unexpected/unexplained death syndrome (SUDS).
Figure 2: Primary adverse event following immunization category by seriousness, 2013-2016 - Text Description
| no data | Number of Adverse Event Reports | ||
|---|---|---|---|
| Primary Adverse Event Following Immunization Category | Number of Non-serious Reports | Number of Serious Reports | Percent Serious Reports |
| Vaccination site | 4075 | 74 | 2% |
| Rash alone | 1833 | 7 | 0% |
| Allergic event | 1664 | 131 | 7% |
| ISSTable 2 footnote a | 920 | 220 | 19% |
| Neurologic event | 324 | 275 | 46% |
| Immunization anxiety | 117 | 4 | 3% |
| Vaccination errorTable 2 footnote b | 9 | 0 | 0% |
| OtherTable 2 footnote c | 1244 | 183 | 13% |
Did you know?
- Vaccines are safe and protect you and those around you from vaccine-preventable diseases. They go through many tests before they can be used in Canada.
- Vaccines are not just for babies and children. Getting vaccinated gives adults the protection they need to stay healthy.
- Learn more about vaccine preventable diseases and vaccination for adults.
- Find out more about how vaccine works in Not just for Kids: An adult guide to vaccination
For additional information: