Basic care – Recognizing metals and their corrosion products
by Bart Ankersmit, Martina Griesser-Stermscheg, Lyndsie Selwyn and Susanne Sutherland
Introduction and authors
Introduction
The PDF format of this text is also available for purchase as a spiral-bound colour-print booklet from Lana Chan, Parks Canada.
Objectives
- Help staff responsible for historic houses, museums, churches
- Act as a visual guide to the problems and damage occasionally observed in metal collections
- Offer tips for the proper care of metal objects
- Provide reference material for further reading
Authors

Cultural Heritage Agency of the Netherlands
PO Box 1600
3800 BP Amersfoort
Telephone: +31 033 – 421 71 89
E-mail: b.ankersmit@cultureelerfgoed.nl

University of Applied Arts Vienna, Conservation Department (in German only)
Salzgries 14/ 3-5
A-1010 Vienna
Austria
Telephone: 0043-1-71133-4815
E-mail: martina.griesser@uni-ak.ac.at
Lyndsie Selwyn , Senior Conservation Scientist
Canadian Conservation Institute
1030 Innes Road
Ottawa ON K1B 4S7
Canada
Telephone: 613-998-3721 or 1-866-998-3721
Fax: 613-998-4721
E-mail: lyndsie.selwyn@canada.ca

Retired from Parks Canada
Recognizing metals and their corrosion products
Recognizing copper
Copper and Copper Alloys (Brass and Bronze)
- colour of bare metal: various shades of yellow (pure copper is reddish)
- not magnetic (but brass-plated iron will be magnetic)
- brass is an alloy of copper plus zinc
- bronze is an alloy of copper plus tin
Copper objects

Brass objects

Bronze objects

Copper corrosion
Copper and Copper Alloys (Brass and Bronze)
- tarnish (or patina) is black
- more serious corrosion is green or blue-green (may be red underneath)
- fingerprints easily stain polished metal
- organic acids react with copper to form green corrosion products
Tarnished copper

Tarnished brass

Fingerprints on copper

Polish residue on copper alloys

Residue in crevices of copper alloys
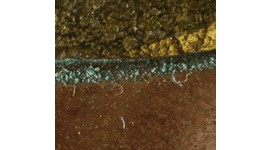
Local spots of green corrosion on copper alloys

Green corrosion on outdoor copper alloys

Bronze disease on archaeological copper alloys

Recognizing iron
Iron and Iron Alloys (Wrought and Cast)
- colour of bare metal: silvery-gray
- almost always magnetic (certain stainless steels are not magnetic)
- historic wrought iron is almost pure iron (<0.1% carbon) with glass inclusions
- steel is iron plus 0.2 to 2% carbon
- cast iron is iron plus 2 to 4% carbon
- stainless steel is iron plus chromium and nickel
Wrought iron objects

Steel objects

Cast iron objects


Stainless steel objects

Iron corrosion
Iron and Iron Alloys (Wrought and Cast)
- corrosion is rust-coloured (red, yellow, red-brown)
- rapid rusting (flash rusting) caused by sudden increase in relative humidity
- drops of liquid on iron or dry, hollow shells are evidence of contamination by salt (chlorides)
Stable corrosion (stable rust)

Damage by ongoing rusting

Local rusting
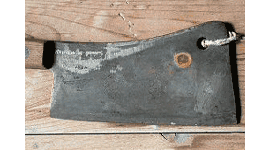
Fingerprints on bare iron
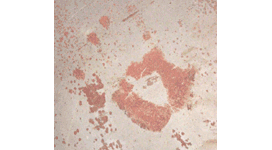
Flash rusting on iron

Delamination of iron

Weeping iron (droplets of water)

Weeping iron (dry, hollow shells)
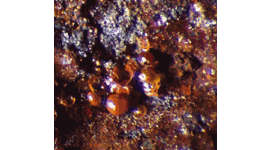
Recognizing silver
Silver Plate and Silver Alloys (Sterling Silver)
- colour of bare metal: silvery-gray (highly reflective when polished)
- not magnetic
- possibly stamped to indicate sterling silver or silver plate
- sterling silver is an alloy of 92.5% silver and 7.5% copper
- silver plate stamped E.P.B.M. is silver plated onto Britannia metal (an alloy of mainly tin with some antimony and copper)
- silver plate stamped E.P.N.S. is silver plated onto nickel silver (an alloy of mainly copper with some nickel and zinc)
Sterling silver objects

Silver-plated objects

Silver corrosion
Silver Plate and Silver Alloys (Sterling Silver)
- tarnish is black if thick
- tarnish can be the colours of the rainbow if thin
- fingerprints easily stain polished silver
Tarnish on silver alloys

Interference colours on silver

Fingerprints

Loss of plating
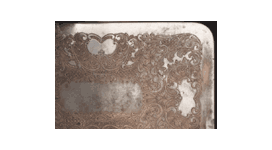
Residue in crevices after polishing silver
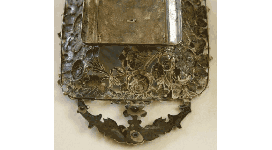
Recognizing tin
Tin Plate and Tin Alloys (Pewter)
- colour of bare metal: silvery-gray
- not magnetic (but tin-plated iron will be magnetic)
- modern pewter is mainly tin plus some antimony and copper
- old pewter may also contain lead
Tin-plated objects

Modern pewter

Tin corrosion
Tin Plate and Tin Alloys (Pewter)
- tarnish (patina) is a darkening of bare metal
- tarnish on leaded pewter is dark gray
- tin corrosion products are either white or black
- rust on tin plate is corrosion of the underlying iron
Rusting of tin plate

White corrosion on tin

Pewter objects

Recognizing lead
Lead and Lead Alloys (Solder)
- colour of bare metal: dull silvery-gray (cannot be polished)
- not magnetic
- objects are relatively heavy
- soft solder (mixtures of lead and tin)

Lead corrosion
Lead and Lead Alloys (Solder)
- tarnish (or patina) is dark gray
- corrosion products are usually white
- lead is susceptible to corrosion by acetic acid (often from wood products)
White corrosion versus stable patina on lead
White corrosion on lead



Gray crystals on lead alloys

Problems and solutions
When in doubt, consult a conservator.
| Problems | Solutions |
|---|---|
| Damage caused by handling | Minimize handling, wear cotton gloves |
| Damage caused by abrasive cleaning | Clean only when necessary; use softest abrasive for the job (e.g. paste of precipitated calcium carbonate and water) |
| New corrosion of metal under plating | Isolate object in a dry environment |
| Loss of plating layer | Leave alone to save information about history of use |
| Condensation inside sealed plastic bag |
|
| Blue-green corrosion on copper alloys inside wood case | Add ventilation to case; consider painting case |
| Tarnish on silver | Store inside a sealed plastic bag; better yet seal inside a plastic bag containing dry silica gel and activated charcoal |
| White or gray corrosion on lead | Provide better ventilation to dilute source of problem (volatile organic acids) and consult a conservator about safe corrosion removal and disposal |
| Difficulty finding conservation resources | See reference section below. See Canadian Conservation Institute (CCI) Notes (9 series) available on the CCI website. |
| Difficulty finding conservation advice | When in doubt, consult a conservator. For more information, see the Canadian Association of Professional Conservators (CAPC) website. |
Appendix: Factors of deterioration
The following factors can affect the deterioration of objects in collections. They are often used as a basis for assessing the risks to a collection:
Contaminants and pollutants
- accelerate metal corrosion
- contaminants may be from:
- sulfur-containing gases (e.g. food, polluted air)
- cleaning chemicals (especially aerosols)
- soot, dust and dirt
- degrading plastics
Fire
- loss of collection
Handling (and other physical forces)
- corrodes metals (from salts and acids on bare hands)
- damages objects (e.g. scratching, dents, breakage)
Incorrect relative humidity
- corrodes metals above about 65% relative humidity
Incorrect temperature
- damages sensitive material (e.g. wood) associated with metals
Light
- fades light-sensitive material (e.g. ribbons) associated with metals
Pests
- attack organic material associated with metals
Security
- loss of portable, valuable or rare objects
Water
- corrodes metals
- water may come from:
- burst pipes
- melting ice
- leaks because of heavy rain and wind
- floods
- condensation
References and acknowledgements
References
- Canadian Conservation Institute. Recognizing Active Corrosion . CCI Notes 9/1. Ottawa: Canadian Conservation Institute, .
- Canadian Conservation Institute. Storage of Metals . CCI Notes 9/2. Ottawa: Canadian Conservation Institute, .
- Canadian Conservation Institute. The Cleaning, Polishing and Protective Waxing of Brass and Copper . CCI Notes 9/3. Ottawa: Canadian Conservation Institute, .
- Canadian Conservation Institute. Basic Care of Coins, Medals and Medallic Art . CCI Notes 9/4. Ottawa: Canadian Conservation Institute, .
- Canadian Conservation Institute. Care and Cleaning of Iron . CCI Notes 9/6. Ottawa: Canadian Conservation Institute, .
- Canadian Conservation Institute. Silver – Care and Tarnish Removal . CCI Notes 9/7. Ottawa: Canadian Conservation Institute, .
- Canadian Conservation Institute. Mechanical Removal of Rust from Machined Ferrous Surfaces . CCI Notes 9/8. Ottawa: Canadian Conservation Institute, .
- Drayman-Weisser, T. "Metal Objects." pp. 108-121 in Caring for Your Collections (editor H. Whelchel). New York: Harry N. Abrams, .
- Selwyn, L. Metals and Corrosion: A Handbook for the Conservation Professional . Ottawa: Canadian Conservation Institute, .
- Tétreault, J. "Display Materials: The Good, The Bad and The Ugly." pp. 79-87 in Exhibitions and Conservation . Edinburgh: Scottish Society for Conservation and Restoration, .
Acknowledgements
The authors thank Lana Chan and Liz Croome from the Western and Northern Service Centre of Parks Canada in Winnipeg for their ideas, comments and help.
Contact information for this web page
These resources were published by the Canadian Conservation Institute (CCI). For comments or questions, including reproduction requests, contact the CCI.