How to Determine Metal Density – Canadian Conservation Institute (CCI) Notes 9/10
- Introduction
- Procedure: determining metal density
- The science behind density measurements
- Acknowledgements
- References
Introduction
The density of an object is the object’s mass divided by its volume. The density is characteristic of the material that the object is made of, and its value can help to identify the material.
Except for objects with simple shapes, it is difficult to determine the volume directly. A simple way to determine the density of a metal object is to weigh it in air and then weigh it again when it is immersed in a liquid, as explained in the section The science behind density measurements. Water is the most convenient liquid to use, but if an object cannot be immersed in water, then organic solvents such as ethanol or acetone can be used. The density of the object can be calculated from the two weight measurements and the density of the liquid.
With the right balance and the right size container, this method can be used on a variety of objects: large or small, metal or non-metal. The method works for complicated shapes, even objects with holes through them, as long as the liquid can penetrate and fill the holes. Once the density is determined, it can be compared to densities of known materials to help narrow down what the object might be made of.
This Note describes the procedure and the required materials for determining the density of a metal object. The first step is to carry out the procedure on one or more metal objects of known composition, either a pure metal or an alloy, to gain experience using the method and to confirm that it is being used correctly. Then the density of unknown metals can be determined.
Procedure: determining metal density
Equipment and materials required to determine density
- Small metal objects that can be immersed in water
- Balance with below balance weighing capability (that is, can weigh objects suspended underneath it) and that can measure to a resolution of at least 0.01 grams (see the section Balance without below balance weighing capability for how to adapt the procedure for weighing below the balance)
- Metal wire to attach to hook inside balance (a bent paperclip works well)
- Support stand or platform to hold the balance so objects can be hung underneath it from the hook
- Beakers large enough so that the objects can be totally immersed without the liquid overflowing
- Supports to hold the beakers at the correct height underneath the balance
- Tap water
- Calculator
- Nylon thread (e.g. fishing line or an equivalent lightweight material) for suspending the objects under the balance
- Disposable nitrile gloves
- Optional: Clamps to attach the balance support to the edge of a counter
Procedure to determine density with below balance weighing capability
- Remove the cover from the underside of the balance to expose the hook inside.
- Place the balance on a support with a hole that allows access to the internal hook.
- Attach a wire hook to the internal hook and then tare the balance (set it to zero).
- Hang an object on the hook beneath the balance using nylon thread or equivalent and weigh it in air. Wear gloves when handling metal objects, especially those suspected of containing lead.
- Fill the beaker with water and place it under the balance.
- Lift the beaker until the object is completely immersed. Place a support under the beaker to hold it at the correct height. Make sure there are no bubbles trapped under the object or in voids within the object.
- Weigh the immersed object.
- Calculate the density using the equation below.
- Compare the calculated density with the known densities of metals and alloys, using the table given below or the more comprehensive lists available in the references.
- Repeat steps 4–9 with the remaining objects.
Calculating the density
The density ρ of an object or a material is defined as the mass m divided by the volume V; in symbols, ρ = m/V. If the object is weighed in air to determine its actual mass and weighed in a liquid to determine its (apparent) mass in the liquid, then the density of the object is given by:

The density of water is 0.998 g/cm3 at 20°C and 0.997 g/cm3 at 25°C.
Results of this procedure
Example objects
Figure 1 shows examples of eight different metal samples used to demonstrate this procedure.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0358
Figure 1. Metal objects used to demonstrate the procedure.
The measured densities of the metal samples in Figure 1 are provided below.
In the top row, from left to right:
- Probably cast iron (7.13 g/cm3)
- High purity aluminum (2.70 g/cm3)
- Reddish copper alloy (possibly 85% copper and 15% zinc, 8.23 g/cm3)
- High purity copper (8.88 g/cm3)
In the bottom row, from left to right:
- Cast zinc (alloy unknown, 7.09 g/cm3)
- High purity lead (11.20 g/cm3)
- High purity tin (7.27 g/cm3)
- Yellow cartridge brass (70% copper and 30% zinc, 8.45 g/cm3)
In each sample, the density was determined from the above formula. For example, for the aluminum object (b) the mass was found to be 110.18 g in air and 69.45 g in water, giving a density of 2.70 g/cm3. For the cast iron object (a), the mass was 209.47 g in air and 180.13 g in water, giving 7.13 g/cm3. For the lead object (f), the mass was 102.44 g in air and 93.31 g in water, giving 11.20 g/cm3.
The measured densities of aluminum, cast iron and lead (2.70, 7.13 and 11.20 g/cm3) are close to the known densities (2.71, 7.20 and 11.33 g/cm3 from Table 1). The aluminum and lead objects are, thus, easily identified by the density.
For the cast iron object, the density alone is not enough to rule out other metals, such as zinc (known density 7.13 g/cm3). When the density of an unknown metal falls close to several metals and alloys (e.g. zinc, iron and tin), then other properties, such as magnetism and colour, will need to be determined to help identify it.
Known density of selected metals and alloys
The known density of selected metals and alloys is given in Table 1, listed in order of increasing density (ASTM 2006, Lide 1998).
| Metal or alloy | Density (g/cm3) |
|---|---|
| Aluminum | 2.71 |
| Aluminum alloys | 2.66–2.84 |
| Zinc | 7.13 |
| Iron (grey cast) | 7.20 |
| Tin | 7.30 |
| Steel (carbon) | 7.86 |
| Stainless steels | 7.65–8.03 |
| Brass (cartridge: 70% copper, 30% zinc) | 8.52 |
| Brass (red: 85% copper, 15% zinc) | 8.75 |
| Nickel silver (65% copper, 18% nickel, 17% zinc) | 8.75 |
| Bronze (85% copper, 5% tin, 5% zinc, 5% lead) | 8.80 |
| Nickel | 8.89 |
| Copper | 8.94 |
| Silver | 10.49 |
| Lead | 11.33 |
| Gold | 19.30 |
Details of the balance
A balance with below balance weighing capability usually comes with a cover under the internal hook. Figure 2 shows an example of the location of the cover on the bottom of a balance.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0359
Figure 2. Balance with below balance weighing capability.
Figure 3 shows an expanded view with the cover closed; in Figure 4, the cover is rotated open to reveal the internal hook.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0360
Figure 3. Detail of underside of balance, showing the movable metal lid covering the internal hook.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0361
Figure 4. Detail of underside of balance, showing the internal hook after the metal lid has been rotated.
Figure 5 shows a metal wire bent to form hooks on both ends. Figure 6 shows the hook on one end of the wire attached to the internal hook inside the balance.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0363
Figure 5. Wire with ends bent into the shape of a hook.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0362
Figure 6. Detail of wire bent into hooks at either end. The top end of the hook is attached to another hook inside the balance.
Figure 7 shows the balance being placed over a Plexiglas stand with a hole cut in the top. The hole allows access to the hook on the underside of the balance.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0365
Figure 7. Balance being placed on a Plexiglas stand with the hook about to pass through the hole in the stand.
Figure 8 shows the balance on a Plexiglas stand with a rectangular coupon of pure copper being weighed in air. Figure 9 shows the balance on a Plexiglas stand with a rectangular coupon of pure copper being weighed in water. A smaller Plexiglas stand is used to support the beaker at the correct height.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0366
Figure 8. Rectangular coupon of pure copper being weighed in air.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0367
Figure 9. Rectangular coupon of pure copper immersed in water.
Figure 10 shows an example of an object with an opening that has trapped air bubbles. Be careful not to trap air bubbles in the object as this will result in an inaccurate reading.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0375
Figure 10. Three air bubbles trapped in an opening.
Additional information
Using solvents other than water
If it is not appropriate to immerse an object in water, such as iron because it is so susceptible to rusting, then an organic solvent such as acetone or anhydrous ethanol can be used. Proper ventilation and appropriate personal protective equipment needs to be used. Refer to the safety data sheet (SDS) of the specific solvent for the recommended equipment. The density of acetone is 0.790 g/cm3 and the density of anhydrous ethanol is 0.789 g/cm3, both at 20°C. For someone who might need to use one of these other liquids, try measuring the density of an object using both water and one of these liquids, and compare the results.
Tips on adapting balances
Alternate stand for the balance
A plywood sheet with a hole in it can be clamped to the edge of a counter if a stand is not available to hold the balance (Figure 11).
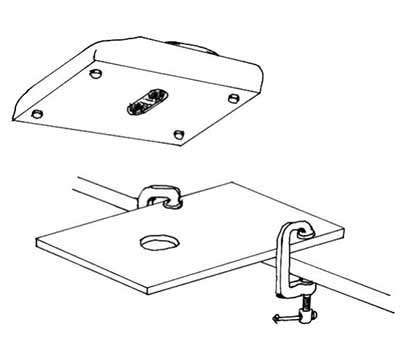
© Government of Canada, Canadian Conservation Institute. CCI 120260-0296
Figure 11: A platform for a balance made with plywood and clamps.
Balance without below balance weighing capability
A balance without a weighing hook can be used to determine density, but it requires a frame to suspend the object under the balance and transfer the weight of the object to the balance. The balance must be set on a platform; a setup similar to the one in Figure 11 could be used. (In this case, the hole in the wood in Figure 11 is not needed.) A four-sided frame (shaped like a picture frame) is then fitted around the balance and the platform, resting only on the weighing pan and not touching any other part of the balance (Figure 12). The balance is tared with the frame and hook in place, and then the object is attached to the hook on the frame and weighed in air and in a liquid, just as in steps 4–9 of Procedure: determining metal density.
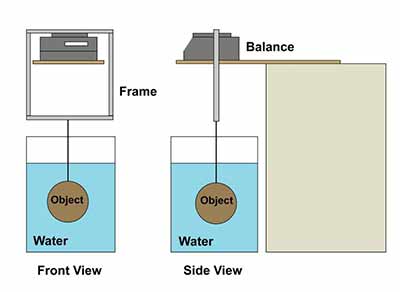
© Government of Canada, Canadian Conservation Institute. CCI 120260-0298
Figure 12. Front view (left side of figure) and side view (right side) showing a balance without below balance weighing capability. The top segment of the rectangular frame rests on the pan of the balance, and the object is attached to the bottom segment.
The science behind density measurements
Buoyancy and Archimedes’ principle
The techniques in this procedure date back to the third century BC. In his book On Floating Bodies, Archimedes of Syracuse proposed that if an object is submerged in a liquid and weighed, it will be measured to be lighter than its true weight by the weight of the liquid that it displaces. The story goes that Archimedes used this idea to show that a crown was not pure gold, but rather a mixture of gold and silver (Heath 1920).
The object appears lighter in the liquid because there is a force pushing up on the object, called the buoyant force. The force comes about because the pressure in a liquid increases with depth, so the pressure on the bottom of the object (pushing the object up) is higher than the pressure on the top (pushing it down). The difference between the upward and downward pressures produces the buoyant force. The buoyant force, pushing the object upwards, acts against gravity, which is pulling the object down. If the buoyant force is less than the force of gravity, the object will sink, but it will appear to weigh less in the liquid than in air. If the buoyant force is larger than the force of gravity, the object will float up to the surface of the liquid.
The density of the object is calculated according to the formula given earlier

Once the density is known, it can be used to calculate the volume of the object through the following formula:
Volume of object = (mass in air) / (density of object)
Like water, air also produces a buoyant force. (That’s why helium balloons float upwards.) The buoyant force of air is too small to matter in this procedure but must be taken into account when high accuracy is needed in weighing (Skoog et al. 2014).Density determined from displaced volume
A simpler but less accurate way to measure the density is to place the object in a liquid and measure the volume of liquid displaced. This can be used on small objects that fit into a graduated cylinder, for example, to decide if the object is made of lead or a less dense metal.
The procedure is as follows. Find a graduated cylinder with a diameter not much larger than the object. Determine the mass of the object with a suitable balance. Add water to the graduated cylinder, and record the initial volume. Submerge the object fully in the water, being careful to avoid bubbles, and then record the volume a second time. The volume of the object is equal to the difference in the final and initial volumes read from the graduated cylinder, and the density is the mass divided by the volume of the object.
As an example, a figurine of a moose was measured. The mass was 4.088 g. Figure 13 shows the figurine outside the graduated cylinder, and Figure 14 shows it immersed. The water in the graduated cylinder rose from 5.0 mL to 5.6 mL when the figurine was immersed, giving a change in volume of 0.6 mL. Ignoring any errors in measuring the volume, the density is calculated to be 4.088 g / 0.6 mL = 6.8 g/cm3. (Note: 1 mL = 1 cm3.) That is less than the density of zinc and could suggest an alloy of zinc and a lighter metal, perhaps magnesium or aluminum. But given the small volume, there are uncertainties in the measurement. The volume can be measured only to the nearest 0.1 mL with the graduated cylinder used, so the volume could be between about 0.5 mL and 0.7 mL. Thus the density could be anywhere from 4.088 g / 0.7 mL = 5.8 g/cm3 to 4.088 g / 0.5 mL = 8.2 g/cm3. From this range of measurements, the figurine could be zinc, iron, tin, steel or other alloys, but it is not pure aluminum or pure lead. In fact, analysis showed it to be tin, which has a density of 7.30 g/cm3.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0373
Figure 13. Small metallic object before immersing in water in a 25 mL graduated cylinder. Note the level of the water.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0374
Figure 14. Small metallic object after immersing in water in a 25 mL graduated cylinder. The level of the water is about 0.6 mL more than before the object was immersed.
Other uses
The above procedures can be used for more than just identifying metals through their density.
Weight for casting metals
In casting a sculpture, one needs to estimate the amount of metal needed to fill a mold of a model of the sculpture. If the model being cast can be immersed, the volume of the model can be determined by the techniques above. Then the mass m of metal needed can be calculated from the volume V of the model and the density ρ of the metal by m = ρV. (Keep in mind that extra metal is usually needed to fill the channels that guide the molten metal into the mold.)
Acknowledgements
Special thanks to Meaghan Whalley, Lucy ‘t Hart and Catherine Machado, former CCI interns, for their help with developing this Note.
References
ASTM G1-03. “Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens.” In Annual Book of ASTM Standards, vol. 03.02. West Conshohocken, PA: American Society for Testing and Materials, 2006, pp. 17–25.
Heath, T.L. Archimedes. New York, NY: MacMillan, 1920.
Lide, D.R., ed. CRC Handbook of Chemistry and Physics, 79th ed. Boca Raton, FL: CRC Press, 1998, pp. 12-191–12-192.
Skoog, D.A., D.M. West, F.J. Holler and S.R. Crouch. Fundamentals of Analytical Chemistry, 9th ed. Belmont, CA: Brooks/Cole, 2014, pp. 22–23.
Written by Lyndsie Selwyn
Également publié en version française.
© Government of Canada, Canadian Conservation Institute, 2016
ISSN 1928-1455