Preparing Silica Gel for Contained Storage of Metal Objects – Canadian Conservation Institute (CCI) Notes 9/14
Introduction
Many metal objects in museum collections will have already corroded due to exposure to moisture before becoming a part of the collection. The extent to which this happens will vary depending on several factors that include the type of metal, the amount and type of previous corrosion, the presence of surface contamination like dirt or salts and the relative humidity (RH) of the surrounding air. Not all of these factors can be controlled, such as the type of metal in the object or the amount or type of corrosion that has occurred in the past. However, RH, the key factor, can be reduced for museum objects made only of metal (for more information, consult CCI Note 9/2 Storage of Metals).
Generally, only a part of a collection is made up of metal objects; of those, only a few will be at risk of ongoing corrosion. As a result, few institutions can provide a collection space specifically tailored for the dry storage of metal objects. Nevertheless, there is a solution for smaller metal objects that consists of a plastic box and a dry moisture sorbent. The idea is to establish dry conditions as a microenvironment in a container that will slow down or halt active corrosion initiated or accelerated by humidity in the air. Whatever system is chosen, it should be designed for the storage of metals over many years with no need for frequent maintenance. This type of storage is often not suitable for non-metallic materials.
Moisture sorbents for museum use
Traditionally, the most common moisture sorbent used in the museum community has been silica gel (amorphous silicon dioxide). When sealed in packets of polyethylene non-woven sheet (such as Tyvek) or sewn into unbleached cotton bags, silica gel still provides a cost-effective moisture sorbent alternative that is readily available from museum, office and scientific suppliers. More recently, there have been other products, such as aluminum silicate clay, molecular sieves as well as various formulations of silica gel (Art-Sorb, PROsorb and RHapid Gel), that have provided varying degrees of performance. The gel Artsorb should not be used, however, since it can corrode metals (Robinet 2007).
To keep things simple, this Note will use regular density silica gel with a mesh size of 6 to 12 as an example of a moisture sorbent that has been used in museums for many decades and that performs well at an RH below 45%. Further information on the use of silica gel to control humidity can be found in Tétreault and Bégin (2018).
Calculating silica gel quantity
Determining how much of a moisture sorbent to use is not a difficult calculation. If the moisture sorbent comes pre-packaged, then a certain number of units will be needed.
Calculating the interior volume of a square or rectangular box is done by multiplying the length by the width by the height (L × W × H). For cylindrical containers, the formula is the height multiplied by the radius (half of the diameter) squared, multiplied by π (pi), which is (H × r2 × 3.14). Using metric values and measuring your lengths in centimetres will make the calculations easier because you can divide the calculated volume by 1000 to get the number of litres.
To keep the calculation simple, the volume of the objects and the volume of the silica gel can be ignored. The volume of moisture sorbent is not critical for storing metals because a humidity-indicating card (available from museum and conservation suppliers) placed in the container will gradually show increasing humidity and will signal when the silica gel needs to be regenerated or replaced. Many cards change from blue to pink as the humidity starts to climb. Too little gel will require more frequent replacement, and an excess of gel will extend the time before replacement.
An example calculation for a storage box could be:
A container that is L = 30 cm, W = 20 cm and H = 10 cm would have a volume of 6000 cm3.
To convert to litres, 6000 cm3 × 1 litre/1000 cm3 = 6 litres.
If we use 50 g/litre of silica gel, then 6 litres × 50 g/litre = 300 g of regular density silica gel.
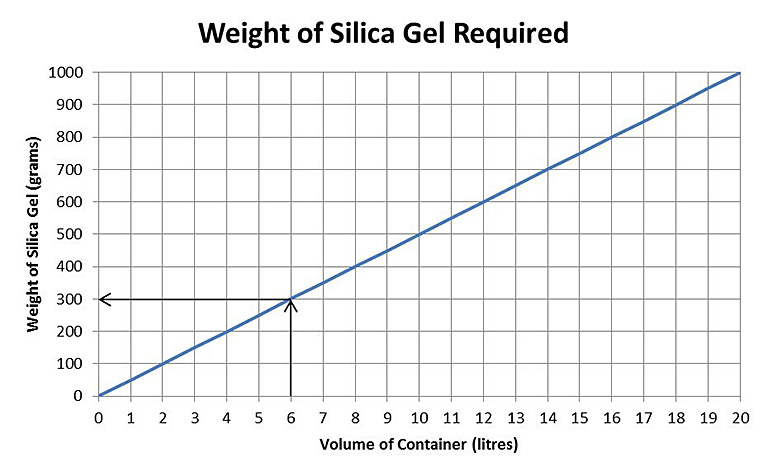
© Government of Canada, Canadian Conservation Institute. CCI 126258-0009
Figure 1. Calculating the weight of silica gel required at the rate of 50 g/litre.
Figure 1 can be used to determine how much silica gel to use. Find the volume of the container on the x axis and follow up to the slanted line. Move left from this point to find the volume of silica gel required on the y axis. For the above example, a six-litre container will need 300 g of gel. If the container chosen is a well-sealed polyethylene or polypropylene food storage box, then this amount of dry silica gel should keep the RH in the unopened box below 5% for several months. Additional silica gel in the container (such as 600 g, 10% of the box volume) can extend the length of time to several years, depending on the size and type of box used. With continued use, the above graph can be modified to meet the explicit iron storage needs for any museum, based on the containers used and the RH in the collection storage space.
Tests conducted with Lock & Lock food storage boxes (Figure 2) showed that larger boxes maintained dry conditions longer then smaller boxes when the same ratio of silica gel to box volume was used (for example, 10% of the internal volume or 100 g/litre). The increase in length of time was proportional to the increase in box size. As well, there was a direct relationship between the amount of silica gel and the length of time the boxes stayed completely dry.
Indicating humidity in the container
There are examples of pre-packaged moisture sorbents that have a built-in indicator to show when they can no longer keep the interior of the container dry. These packages are very convenient to use because any change in colour shows that the container is no longer dry. The simplest method to indicate dry conditions for the storage of corroded metals is to use a humidity-indicating card that can be seen inside a clear food storage container. Many versions of these cards will indicate an RH as low as 10%, which is suitable for the storage of most metal objects. These cards have a limited lifetime and should be replaced according to the manufacturer’s instructions.
With time, water vapour will slowly penetrate the container and the card will indicate the gradual increase in RH in the box and the eventual need to regenerate or replace the silica gel. If the storage container is opaque like a can or barrier film, then it will have to be opened every few months to check on the indicated humidity level, then resealed. This action will introduce small amounts of humid air and shorten the time until the silica gel needs to be replaced or regenerated.
Drying silica gel
Before the silica gel can be used to protect metal objects, it must be conditioned to be dry. This is usually done by heating in an oven at a temperature of 60°C (140°F) for six to seven hours or 120°C (250°F) for one to two hours. Silica gel in polyethylene non-woven packets should be conditioned at a temperature below 90°C (194°F) to prevent shrinking or melting of the packet material. Laying the gel on a cookie sheet is the best method for drying. There should be no need to open the cloth pouches to dry the silica gel inside. After several hours of drying, monitoring the drying progress can be done by weighing the pouches once an hour until they do not lose any more water and the weight does not change.
After drying, the pouches should be stored in an airtight container to prevent absorption of moisture from the air. Metal cookie tins with tight fitting lids or unused paint cans are ideal choices to contain the pouches until they are needed for metal storage. Care should be taken when handling the pouches of hot silica gel when they come out of the oven. Silica gel can be regenerated many times before it becomes less effective, although heating silica gel at temperatures over 100°C (212°F) may cause it to decay. With long use, the cloth pouches may wear out, but they can be replaced as needed.
Health and safety
Silica gel is a chemical product; thus, its safety data sheet should be read before use. It is considered to be slightly hazardous, and it is recommended that skin and eye contact as well as ingestion and inhalation be avoided.
Indicating gel that includes cobalt chloride should be avoided. These indicators are now considered to be a health hazard in some jurisdictions (Goldberg et al. 2005). Since 2005, it has been recommended that silica gel that does not contain a cobalt chloride indicator be used in museum situations.
Silica gel can be placed in a sealed plastic bag for disposal as regular waste.
How to assemble the container
One of the simplest containers in which to house smaller objects is a polyethylene or polypropylene food storage box with a tight-fitting lid (Figure 1). These types of boxes are usually square or rectangular, which makes it easier to calculate the interior volume. Alternatives to a food storage box could be bags made from a heat-sealable barrier film with a foil layer or metal cans with tight-fitting lids. For larger objects, plastic storage bins may be suitable if the lids can be well sealed by taping them closed, for example.
In some cases, the size and shape of the objects will determine the correct container to use. Choosing polyethylene or polypropylene containers is made easier because the manufacturers mark the type of polymer used on the container, below the recycle symbol.
The storage container should be lined with a layer of polyethylene foam to provide a cushion before adding small metal objects packaged in individual polyethylene bags. Larger objects can be supported on pieces of fluted plastic sheet and separated from each other with pieces of polyethylene foam. Fragile objects can be protected by placing them in cut-outs in polyethylene foam. Suggestions for how to work with plastic sheet and foam have been described in Schlichting (1994).
Numerous labelled objects in small, labelled polyethylene bags with zipper closures can be placed in one box, leaving room for the silica gel that can be sewn into cloth bags.

© Government of Canada, Canadian Conservation Institute. CCI 126258-0001
Figure 2. Lock & Lock box with iron objects, silica gel and humidity indicator card.
Depending on the sizes of containers used, it can be helpful to create pouches that contain a standard weight of silica gel, like 100 or 1000 g. If storage boxes of a standard size are used, they can be stacked on shelves to save space in collection storage areas.
Conclusion
Silica gel and easily purchased food storage containers provide a simple, cost-effective storage method for metals that are actively corroding. Removing the moisture by creating a dry storage environment will reduce or even halt further deterioration of the objects, and once packed in the boxes, the metal objects are immune to the detrimental effects of even the poorest museum environment.
Bibliography
- Goldberg, L., S. Weintraub and F. Sterniolo. Cobalt Indicating Silica Gel Health and Safety Update (PDF format). Conserve O Gram 2/15. Washington, D.C.: National Park Service, September 2005.
- Logan, J., and L. Selwyn. Recognizing Active Corrosion, revised. CCI Notes 9/1. Ottawa, ON: Canadian Conservation Institute, 2007.
- Robinet, L. “ArtSorb.” Conservation DistList. Instance 21:7, May 2007.
- Schlichting, C. Working with Polyethylene Foam and Fluted Plastic Sheet . CCI Technical Bulletin 14. Ottawa, ON: Canadian Conservation Institute, 1994.
- Tétreault, J., and P. Bégin. Silica Gel: Passive Control of Relative Humidity. CCI Technical Bulletin 33. Ottawa, ON: Canadian Conservation Institute, 2018.
- Thomson, G. The Museum Environment. 2nd ed. London, UK: Butterworths, 1986.
- Waller, C. “ArtSorb.” Conservation DistList. Instance 19:39, February 2006.
- Watkinson, D., and V. Neal. First Aid for Finds. 3rd ed. Hertford, UK: RESCUE – The British Archaeological Trust/UKIC Archaeology Section, 1998.
By Cliff Cook
© Government of Canada, Canadian Conservation Institute, 2019
Cat. No.: NM95-57/9-14-2019E-PDF
ISSN 1928-1455
ISBN 978-0-660-30578-3