How to Test for Chloride Ions in Iron Treatment Solutions Using Silver Nitrate – Canadian Conservation Institute (CCI) Notes 4/5
List of abbreviations and symbols
Abbreviations
- CCI
- Canadian Conservation Institute
- portable document format
- ppm
- part per million
Symbols
- μS/cm
- microsiemens per centimetre
- Ag
- silver
- AgCl
- silver chloride
- Cl-
- chloride ion
- g
- gram
- g/mL
- gram per milliliter
- HNO3
- nitric acid
- M
- molarity
- mg
- milligram
- mL
- millilitre
- mm
- millimetre
- pH
- potential of hydrogen
- v/v
- volume per volume
- w/v
- weight per volume
Introduction
Objects recovered from marine or burial sites may be contaminated with high concentrations of chloride ions, as described in Sources of salts in objects. This contamination is particularly dangerous for objects that contain iron (Selwyn et al. 1999). Chloride ions accelerate the corrosion of iron, as is all too familiar to anyone who drives a car on roads that are de-iced with road salt (mainly sodium chloride). Removing the chloride ions is an important step for conservators in treating chloride-contaminated objects. The treatment is straightforward, in principle: the objects are soaked in an alkaline bath until the chloride ions from the objects are dissolved into the bath (Selwyn 2004). In practice, the bath has to be replaced regularly, because it becomes less effective as chloride ions build up in it. Rimmer et al. (2013) recommend stopping iron treatments when the chloride ion concentration is less than 5 parts per million (ppm). A relatively simple test for chloride ions is needed to decide when the bath needs replacing or when the treatment can be stopped.
The test for chloride ions described here is based on precipitation of an insoluble chloride salt. When a few drops of a silver nitrate solution are added to a slightly acidic aqueous solution that contains chloride ions, a white precipitate of silver chloride will form. If standard solutions with known chloride ion concentration are available for comparison, the amount of precipitate can be used to estimate the concentration of chloride ions. With this test, users should be able to detect chloride ion concentrations as low as 1 ppm. For more information on the reactions involved, see The science behind the chloride ion test.
This CCI Note describes the procedure and the required materials to detect chloride ions in a solution. The first step in the procedure involves testing solutions of known chloride ion concentrations to get experience using silver nitrate and to confirm that the test is working properly. Then actual treatment solutions or other solutions of unknown chloride ion concentration can be tested. A laboratory and ventilation are not required for this procedure unless nitric acid is required to adjust the acidity of the solution. If nitric acid is to be used, then consult its safety data sheet (SDS) for health and safety information prior to use.
Procedure: how to detect chloride ions in a solution with silver nitrate
Equipment and materials required to test for chloride ions using silver nitrate
- Solutions with known and unknown chloride ion concentrations
- Solutions with known chloride ion concentrations should be purchased or prepared. For a procedure to prepare chloride ion solutions with 5 ppm, 30 ppm, 300 ppm and 3000 ppm, consult Preparation of chloride ion sample solutions.
- Solutions with unknown chloride ion concentrations can include a desalination treatment solution or any other solution requiring testing.
- Test tubes, glass (e.g. 16 mm outer diameter x 125 mm length; holds about 20 mL)
- Test tube rack
- Dark material to use as a background (e.g. black Coroplast or black Bristol board)
- Bright light (e.g. desk lamp)
- Pasteur pipettes or eyedroppers, one per solution
- Distilled or deionized water
- Dilute nitric acid solution (HNO3, 5% [v/v]); for preparation instructions, see Preparation of solutions
- Graduated cylinder (10 mL)
- Volumetric flasks (100 mL)
- Silver nitrate solution (2% [w/v]); for preparation instructions, see Preparation of solutions
- Small beaker for transferring the silver nitrate solution
- Brown bottle for storing the silver nitrate solution (e.g. 125 mL). Silver nitrate is light sensitive; if a brown bottle is not available, use a clear glass bottle and cover it with metal foil.
- Glass storage bottle (e.g. 125 mL) for the nitric acid solution
- Balance (to weigh to at least 0.01 g)
- pH indicator paper
- Sodium chloride (NaCl)
- Filter paper (optional)
- Funnel (optional)
Procedure for detecting chloride ions
Exercise caution while using chemicals such as nitric acid and silver nitrate, and wear personal protective equipment such as gloves, goggles and protective clothing while handling them. Silver nitrate can turn skin and clothing black. Never draw a sample directly out of the stock solutions. Transfer the desired amount to a small beaker then take the sample. Use proper ventilation, especially when working with concentrated nitric acid. For more information on making up chemical solutions, consult Odegaard et al. (2005) and Skoog et al. (2014).
Preparation of solutions
- Nitric acid solution (HNO3, 5% [v/v])
- Work in a fume hood
- Partially fill a 100 mL volumetric flask with distilled water
- Measure 5 mL of concentrated nitric acid (68–70% HNO3 by weight) into a 10 mL graduated cylinder
- Transfer the nitric acid to the volumetric flask (Important: always add concentrated acid to water)
- Swirl to mix
- Add more distilled water to the volumetric flask to fill it to the 100 mL mark
- Transfer to a glass bottle for storage and label the bottle
- This produces a solution that is approximately 0.8 M HNO3
- Silver nitrate solution (AgNO3, 2% [w/v])
- Partially fill a 100 mL volumetric flask with distilled water
- Weigh 2.0 grams of silver nitrate
- Transfer the silver nitrate to the flask and swirl to dissolve the solid
- Add more distilled water to fill the flask to the 100 mL mark
- Transfer the solution to a 125 mL brown bottle for storage and label the bottle
Preparation of chloride ion sample solutions
Two sample solutions, with 30 ppm and 5 ppm chloride ion, can be prepared as described below. These two concentrations are chosen to be near the low end and near the midpoint of the 1–100 ppm range, but other values could be used. Standard chloride ion solutions can also be purchased.
The required sample solutions have such a low concentration of chloride ions that they have to be prepared by dilution. Prepare a starting solution with an amount of sodium chloride that is easy to measure. The procedure assumes a balance that measures to 0.01 g. Therefore, about half a gram of sodium chloride is needed in the starting solution to achieve reasonable accuracy in the weighing. Prepare a second solution by diluting 10 mL of the first solution with water to make 100 mL. Similarly, prepare a third solution by diluting the second solution. Make sure the glassware is clean to prevent contamination by chloride ions from previous use.
- Solution A: 3000 ppm chloride ion solution
- Partially fill a 100 mL volumetric flask with distilled water
- Weigh out 0.49 g of sodium chloride
- Transfer the sodium chloride to the flask and swirl to dissolve the solid
- Add distilled water to fill the flask to the 100 mL mark
- Label the flask as A: 3000 ppm chloride ion
- Solution B: 300 ppm chloride ion solution
- Partially fill a 100 mL volumetric flask with distilled water
- Use a 10 mL graduated cylinder to measure 10 mL of solution A (3000 ppm)
- Transfer this solution A to the volumetric flask
- Swirl to mix
- Add more distilled water to fill the flask to the 100 mL mark
- Label the flask as B: 300 ppm chloride ion
- Solution C: 30 ppm chloride ion solution
- Partially fill a 100 mL volumetric flask with distilled water
- Use a 10 mL graduated cylinder to measure 10 mL of solution B (300 ppm)
- Transfer this solution B to the volumetric flask
- Swirl to mix
- Add more distilled water to fill the flask to the 100 mL mark
- Label the flask as C: 30 ppm chloride ion
- Solution D: 5 ppm chloride ion solution (Note: made from Solution B)
- Partially fill a 100 mL volumetric flask with distilled water
- Use a 10 mL graduated cylinder to measure 1.7 mL of solution B (300 ppm)
- Transfer this solution B to the volumetric flask
- Swirl to mix
- Add more distilled water to fill the flask to the 100 mL mark
- Label the flask as D: 5 ppm chloride ion
Chloride ion analysis
- If the chloride ion solution being tested contains suspended particulate, filter the solution using a funnel and filter paper.
- Add 10 mL of distilled water to a 10 mL graduated cylinder.
- Transfer the water from the graduated cylinder to a clean test tube. This is the "blank" solution and it should not contain any chloride ions.
- Similarly, add 10 mL of solution C (30 ppm), prepared above, to a second clean test tube. This is the "sample."
- Add one or two drops of 5% (v/v) nitric acid to both test tubes and swirl to mix. Test the pH with a pH indicator paper; the pH should be between 2 and 3. Add more nitric acid to the sample if the sample is alkaline (e.g. contains sodium hydroxide).
- Use a strong light to illuminate the test tubes from the side. Use a dark background behind and below the test tubes, if possible.
- Add two drops of the silver nitrate solution to each test tube and observe the solutions for white cloudiness.
- Repeat steps 1 to 7 with a 10 mL sample of solution D (5 ppm).
Results of this procedure
When chloride ions are present at a concentration of less than 1 ppm, such as in the blank, the test solution should not change from its appearance before the test, as shown in Figure 1a. When chloride ions are present at a concentration above about 1 ppm in the sample, a cloudy white precipitate (silver chloride) will become visible. The precipitate will be more intense in solution C (30 ppm, Figure 1b) than in solution D (5 ppm, Figure 1c). (In an alkaline test solution, brown silver oxide will precipitate, interfering with the test for chloride ions. Adjusting the pH to the 2–3 range prevents this.)

© Government of Canada, Canadian Conservation Institute. CCI 120260-0385
Figure 1a. Transparent test solution before adding a drop of silver nitrate.
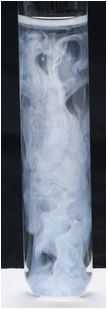
© Government of Canada, Canadian Conservation Institute. CCI 120260-0394
Figure 1b. Test solution showing precipitate of white silver chloride 40 seconds after adding silver nitrate to a solution with a chloride ion concentration of 30 ppm.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0394
Figure 1c. Test solution showing precipitate of white silver chloride 40 seconds after adding silver nitrate to a solution with a chloride ion concentration of 5 ppm.
The silver nitrate concentration used in this procedure is 2% (w/v) (0.1 M), as recommended by Plenderleith and Werner (1971) and Riss (1993). This concentration works well, so it is not necessary to use the higher concentration of 0.2 M (3.4% [w/v]) recommended by Lagowski and Sorum (2005) and Odegaard et al. (2005), pp. 108–109.
Additional information
Estimating chloride ion concentration
Between about 1 ppm and 100 ppm, a rough estimate of the chloride ion concentration of a sample can be made by visually comparing the density of the white precipitate in the sample with the densities of white precipitates in chloride ion standards (Semczak 1977). The degree of cloudiness after adding two drops of 2% (w/v) silver nitrate is roughly proportional to the chloride ion concentration. The cloudier the solution is, the higher the chloride ion content. Above 100 ppm, all the solutions look equally cloudy; below 1 ppm, the precipitate is too faint to see. The other solutions chosen for the illustration in Figure 2 can be made up by following the steps for solution D (5 ppm), but instead by using 5 mL of solution B to make 15 ppm, or 3.3 mL of solution A to make 100 ppm.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0394
Figure 2. Test tubes containing chloride ion concentrations (from left to right) of 0, 5, 15, 30 and 100 ppm about 40 seconds after adding two drops of 2% (w/v) silver nitrate.
The appearance of the test tubes during the forty seconds after adding the silver nitrate is shown in a video.
Other ways to test for chloride ions
There are other, more accurate, ways to measure the chloride ion concentration in aqueous solutions. One way to measure chloride ion concentrations quantitatively is with commercial test strips, as outlined in CCI Note 4/4 How to Test for Chloride Ions in Iron Treatment Solutions using Quantab Test Strips. Semi-quantitative analysis can be done using EM Quant Chloride Test Strips (Odegaard et al. 2011). Another more accurate way is to carry out a titration with silver nitrate (Selwyn 1999). Other methods are mentioned and studied by Wang et al. (2008) and Rimmer et al. (2012).
Monitoring desalination using conductivity
Conductivity is the electrical property of a material that determines its ability to conduct electricity. The higher the conductivity value, the more easily an ionic current flows in the liquid. The SI unit for conductivity is siemens per metre (S/m). Measurements of conductivity are usually reported in millisiemens per centimetre (mS/cm) or microsiemens per centimetre (μS/cm).
The conductivity of an aqueous solution depends on the movement of ions from dissolved salts and other charged particles. Relatively pure water has a lower conductivity than solutions containing high levels of dissolved salts. Ultra-pure water has a conductivity of 0.055 μS/cm, whereas the value for water purified by reverse osmosis is 3.8 μS/cm, and for drinking water, 5 to 500 μS/cm. A solution of sodium chloride with 100 ppm chloride ions has a conductivity of 340 μS/cm, increasing to 3200 μS/cm for 1000 ppm. The conductivity of seawater is 50,000 to 53,000 μS/cm, which is similar to a 1% solution of sodium hydroxide (NaOH), at 48,600 μS/cm.
A solution's conductivity can be measured quickly and easily with a conductivity meter. In some cases, taking a conductivity measurement is a simpler way to monitor the extraction of salts from an object than using a test for chloride ions. But it can only be used in this way if the object can be soaked in reasonably pure water. Ceramics can be soaked in pure water. Iron, on the other hand, corrodes in pure water, so an alkaline solution of sodium hydroxide must be used. The high conductivity of this solution masks any change in conductivity caused by the extracted salts. As a result, a conductivity measurement cannot be used to monitor the progress of the treatment of iron.
The science behind the chloride ion test
The reaction between silver nitrate and chloride ions
When a salt, such as sodium chloride (NaCl), dissolves in water, the ions that make up the salt disperse in the solution, but only up to a certain limit (called the solubility). If we try to produce a solution where that limit is exceeded, the ions will combine to form the solid salt. The solid formed in this way is called a precipitate. The key to the test in this procedure is the low solubility of silver chloride in water.
Most chloride salts are highly soluble in water (Weast 1974). Sodium chloride, for example, has a solubility of 357 g/L at 0°C, which corresponds to a chloride ion concentration of 220,000 ppm. Silver chloride, on the other hand, is one of the few chloride salts that have low solubility: 0.89 mg/L at 10°C, or 0.22 ppm chloride ion concentration.
When a solution containing silver ions (Ag+) is added to a solution containing chloride ions (Cl-), a white precipitate of silver chloride (AgCl) will form unless the concentration of chloride ions is very low. The reaction is written as:
Cl- (aqueous) + Ag+ (aqueous) → AgCl (white solid)
The higher the starting concentrations of silver and chloride ions are, the more precipitate will form.
Definition of parts per million (ppm)
Chloride ion concentrations are often given in parts per million (ppm). Parts per million is a unit of concentration defined as the weight of the solute (the ion or compound being added) divided by the weight of the solution (after the ion or compound is added) and then multiplied by one million (106). A common assumption for dilute aqueous solutions is that the added ions do not change the density of the water, so that the solution has the same density as pure water at room temperature (approximately 1 g/mL) (Skoog et al. 2014, p. 72). With this assumption, the definition of ppm simplifies to:
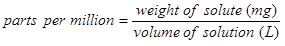
Note that the solute is the chloride ion. This means that a 1 ppm chloride ion solution contains 1 milligram (mg) chloride ion per litre (L) of solution.
In making up 100 mL (0.1 L) of solution A (3000 ppm) in this procedure, 0.49 g of sodium chloride is measured out. Sodium chloride contains 39.3% sodium and 60.7% chloride by weight, as determined from the atomic weight of sodium (22.99 g/mol) and chlorine (35.45 g/mol). This means that when sodium chloride is dissolved in solution, there are 0.30 g (300 mg) of chloride ions and 0.19 g of sodium ions. The concentration of chloride ions in ppm is therefore 300 mg divided by 0.1 L, which equals 3000 ppm (and not 4900 ppm).
To get a feeling for the scale of parts per million, consider how much sodium chloride there is in 10 mL of a solution with 10 ppm chloride ions. This volume contains 0.1 mg of chloride ions, and 0.065 mg of sodium ions, or 0.165 mg of sodium chloride, too small to weigh out with a laboratory balance. That mass corresponds to a cube of sodium chloride 0.42 mm on a side, which is roughly the size of a grain of salt.
Why sample solutions are acidified
The solutions are acidified with dilute nitric acid to prevent precipitation of some silver salts other than silver chloride when silver nitrate is added. In neutral solutions, silver phosphate or silver carbonate could form if enough phosphate or carbonate ions are present (Bassett et al. 1978). If a test solution has a pH greater than about 8, the silver ions from the silver nitrate react with the hydroxyl (OH-) ions to form silver hydroxide (AgOH) or silver oxide (Ag2O). The reaction with hydroxyl ions is:
AgNO3 + OH- → AgOH (light brown)
followed by
2AgOH → H2O + Ag2O (dark brown or black)
Figure 3 shows what happens when silver nitrate is added to an alkaline solution that contains about 200 ppm chloride ions. This sample was taken from a 1% (w/v) sodium hydroxide treatment bath for archeological iron.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0413
Figure 3. Silver oxide precipitates in an alkaline solution about 20 seconds after two drops of 2% (w/v) silver nitrate were added to an alkaline solution containing 1% (w/v) sodium hydroxide and a chloride ion concentration of about 200 ppm.
The appearance of the silver hydroxide, silver oxide, and silver chloride precipitates in the test tube during the 20 seconds after adding silver nitrate is shown in a video.
Sources of salts in objects
Objects recovered from a burial environment are contaminated with material from that environment. This often includes a variety of salts, especially if objects are recovered from the sea. The dominant ions in seawater (Weast 1974) are chloride ions (Cl-, 19,000 ppm) and sodium ions (Na+, 10,600 ppm), but also present are magnesium (Mg2+, 1270 ppm), sulfur (mainly in the form of sulfate ions SO42-, 880 ppm), calcium (Ca2+, 400 ppm), potassium (K+, 380 ppm) and various other ions with concentrations below 100 ppm.
These ions, or the salts from these ions, can damage objects if they are not removed. Chloride ions are particularly damaging to metal objects, especially iron. When iron corrodes, it becomes covered with a layer of iron oxides and hydroxides. This layer provides some protection against further corrosion. Chloride ions, however, interfere with the layer, greatly increasing the corrosion rate (Selwyn et al. 1999).
Other objects that are damaged by salts are porous materials, such as ceramics (Odegaard et al. 2011). The damage results as the salts change from ions dissolved in solution into solid crystals as the water evaporates. Some salts (sodium sulfate in particular) undergo such high-volume expansion on crystallization that they cause significant damage to the surrounding material if the crystallization occurs within a porous structure (Waller 1992).
Acknowledgements
Special thanks to Ute Werner, Lucy 't Hart, Catherine Machado and Meaghan Whalley, former CCI interns, for their help in developing this Note.
Suppliers
Note: The following information is provided only to assist the reader. Inclusion of a company in this list does not in any way imply endorsement by the Canadian Conservation Institute.
Chemicals
Silver nitrate, concentrated nitric acid and pH paper are available from chemical supply companies such as Fisher Scientific.
Chloride standards
Three Orion brand chloride standards (0.1 M, 1000 ppm, 100 ppm) are made by Thermo Scientific. Fourteen different chloride standards ranging from 1 ppm to 100,000 ppm are made by Ricca Chemical. These are distributed by chemical supply companies such as Fisher Scientific.
References
Bassett, J., R.C. Denney, G.H. Jeffery and J. Mendham. Vogel's Textbook of Quantitative Inorganic Analysis, 4th ed. Harlow, UK: Longman Group, 1978.
Lagowski, J.J., and C.H. Sorum. Introduction to Semimicro Qualitative Analysis, 8th ed. Upper Saddle River, NJ: Pearson Prentice Hall, 2005.
Odegaard, N., S. Carroll and W.S. Zimmt. Material Characterization Tests for Objects of Art and Archaeology, 2nd ed. London, UK: Archetype Publications, 2005.
Odegaard, N., P. Hill, B. Santarelli and W. Zimmt. "Detecting and Identifying Salts During the Desalination Process with Spot Test Papers." WAAC (Western Association for Art Conservation) Newsletter 33 (2011), pp. 14–17.
Plenderleith, H.J., and A.E.A. Werner. The Conservation of Antiquities and Works of Art, 2nd ed. London, UK: Oxford University Press, 1971, p. 201.
Rimmer, M., D. Watkinson and Q. Wang. "The Efficiency of Chloride Extraction from Archaeological Iron Objects Using Deoxygenated Alkaline Solutions." Studies in Conservation 57 (2012), pp. 29–41.
Rimmer, M., D. Watkinson and Q. Wang. "The Impact of Chloride Desalination on the Corrosion Rate of Archaeological Iron." Studies in Conservation 58 (2013), pp. 326–337.
Riss, D. Testing for Chlorides with Silver Nitrate (PDF format). Conserve O Gram 6/3. Washington, D.C.: National Park Service, 1993.
Semczak, C.M. "A Comparison of Chloride Tests." Studies in Conservation 22 (1977), pp. 40–41.
Selwyn, L. Analysis of the Chloride Ion Concentration in Aqueous Solutions by Potentiometric Titration. Canadian Conservation Institute Research Report No. 2. Ottawa, ON: Canadian Conservation Institute, 2001.
Selwyn, L. "Overview of Archaeological Iron: The Corrosion Problem, Key Factors Affecting Treatment, and Gaps in Current Knowledge." (PDF format) In J. Ashton and D. Hallam, eds., Metal 2004: Proceedings of the International Conference on Metals Conservation. Canberra, Australia: National Museum of Australia, 2004, pp. 294–306.
Selwyn, L.S., P.J. Sirois and V. Argyropoulos. "The Corrosion of Excavated Archaeological Iron with Details on Weeping and Akaganéite." Studies in Conservation 44 (1999), pp. 217–232.
Skoog, D.A., D.M. West, F.J. Holler and S.R. Crouch. Fundamentals of Analytical Chemistry, 9th ed. Belmont, CA: Brooks/Cole, 2014.
Waller, R. "Temperature- and Humidity-sensitive Mineralogical and Petrological Specimens." In F.M. Howie, ed., The Care and Conservation of Geological Material: Minerals, Rocks, Meteorites and Lunar Finds. Oxford, UK: Butterworth-Heinemann, 1992, pp. 25–50.
Wang, Q., S. Dove, F. Shearman and M. Smirniou. "Evaluation of Methods of Chloride Ion Concentration Determination and Effectiveness of Desalination Treatments Using Sodium Hydroxide and Alkaline Sulphite Solutions." The Conservator 31 (2008), pp. 67–74.
Weast, R.C., ed. Handbook of Chemistry and Physics, 54th ed. Cleveland, OH: CRC Press, 1974.
Written by Lyndsie Selwyn
Également publié en version française.
© Government of Canada, Canadian Conservation Institute, 2016
ISSN 1928-1455