How to Test For Sulfur in Materials Using Lead Acetate Test Paper – Canadian Conservation Institute (CCI) Notes 17/5
- Introduction
- Procedure: how to use lead acetate paper to test for the presence of sulfur
- The science behind the lead acetate test
- Acknowledgements
- Suppliers
- References
Introduction
Materials that contain sulfur can give off gases such as hydrogen sulfide or carbonyl sulfide, which cause silver and copper to tarnish. If possible, such materials should not be placed near objects that contain silver and copper, especially in a closed space like a display case. But what materials should be avoided? Some materials made from animal proteins contain sulfur—wool, hair and feathers, for example—but others, such as silk, contain almost none (Mills and White 1994). Rubber contains sulfur, but many plastics do not, although plastics with sulfur are being developed and used more extensively, especially since the 1990s (Kultys 2007). Furthermore, elemental sulfur (which can also cause silver to tarnish) can turn up in unexpected places (Benson 2012): as an adhesive, in inlays in furniture, as a strengthening material in hollow jewellery or in cement.
This Note describes a test for the presence of sulfur in materials. The test involves heating a small sample of the material in a glass pipette with a piece of moistened lead-acetate test paper. It is a destructive test, but it requires only a small sample, about 10 mg. The test is particularly useful when choosing materials for exhibits and display cases. When possible, the sulfur-containing material should be avoided in display cases that contain silver or copper, but if the exhibit requires that the material and the metals be placed together, protective measures can be taken (Tétreault 2003).
In the test, a flame is used to heat the pipette until the material under test gives off fumes. Any hydrogen sulfide in the fumes will produce lead sulfide in the test paper and turn the paper black. This is a sensitive test for hydrogen sulfide, so only a small sample is needed to produce detectable levels of hydrogen sulfide, and there is only a small amount of lead in the test paper. (Nevertheless, it is recommended that the test be carried out in a well-ventilated area.) The procedure should be used first to test a sample known to contain sulfur and a sample known not to contain sulfur, to gain experience doing the test. Then actual samples can be tested.
Procedure: how to use lead acetate paper to test for the presence of sulfur
Equipment and materials required to test for sulfur
- Pasteur pipettes, disposable glass (one pipette per sample to be tested)
- Parafilm M (plastic laboratory film) or another kind of plastic wrap
- Wood applicator sticks
- Source of heat (e.g. alcohol lamp, Bunsen burner or butane torch)
- Matches
- Lead acetate test paper
- Tweezers (stainless steel or plastic)
- Scalpel (for sampling hard materials)
- Scissors (for sampling soft materials)
- Distilled water (if available, otherwise tap water)
- Eyedropper or extra Pasteur pipette
- Hydrogen peroxide (3% v/v solution, preferably in a spray bottle)
- Disposable gloves (e.g. nitrile)
- Heat resistant gloves (e.g. leather)
- Eye protection
- Samples (about 10 mg)
- Known to contain sulfur (e.g. wool, hair)
- Known not to contain sulfur (e.g. plain white cotton, filter paper)
- Unknown samples
Procedure for testing for sulfur
Wear disposable gloves when handling lead acetate paper or when using hydrogen peroxide; wear heat-resistant gloves when heating the pipette or the samples; and wear eye protection. Use caution when using any type of open flame, and follow the instructions for any flame source. Perform this test in a well-ventilated area or under a fume hood if possible. Treat used lead acetate papers as hazardous waste and dispose of them accordingly.
- Seal the tapered tip of a Pasteur pipette by melting it in a flame. (A butane torch works well for this, but an alcohol lamp will also work.)
- Cut off a sample of about 10 mg (i.e. 0.01 g) from the material to be tested using a scalpel or scissors.
- With a wooden stick, push the sample to the tapered part of the pipette. Do not pack it tightly.
- If using lead acetate test paper from a roll, cut a piece about 5 cm long. If the test paper is wider than the pipette, fold the piece of test paper in half lengthwise so it can be inserted into the pipette.
- Add a drop or two of water to the folded test paper with another pipette or an eyedropper. The water should wick along the entire length of the paper. Do not add too much water.
- Use tweezers to place the test paper inside the pipette, near the open end. The test paper should not touch the sample.
- Cover the open end of the pipette with a piece of Parafilm M.
- Gently heat the sample above a flame (not directly in it) until it fumes and the fumes reach the test paper. Since the fumes are denser than air, hold the tube horizontally, or tilt it slightly so the paper is lower than the sample (but do not tilt so much that the sample moves).
- Leave the tube horizontal for several minutes to give the reaction time to proceed.
- Remove the test paper from the pipette with tweezers.
- Record whether or not there has been any colour change on the test paper.
- Lightly spray or place a drop of hydrogen peroxide (3% v/v) on the test paper.
- Record any colour change.
- The test is positive for sulfur if the paper turns dark brown or black after exposure to the fumes from the sample and then turns white after exposure to hydrogen peroxide.
- The test should be done first on a sample known to contain sulfur and a sample known not to contain sulfur and then repeated with unknown samples.
Results of this procedure
A butane torch is a convenient source of heat to seal the tapered tip of a glass pipette (Figure 1).

© Government of Canada, Canadian Conservation Institute. CCI 120260-0206
Figure 1. The tapered tip of a glass pipette is being sealed with a butane torch.
A sample pushed into the tapered part of a pipette is shown in Figure 2.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0209
Figure 2. A sample from a purple disposable nitrile glove has been pushed to the tapered part of a glass pipette with a wood applicator stick.
A wool sample is shown in Figure 3 at the narrow end of a pipette, with lead acetate test paper at the other end. The pipette has been sealed with Parafilm M. Figure 4 shows a more detailed view of the Parafilm M covering the end of the glass pipette.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0210
Figure 3. An example of a prepared pipette containing a blue wool sample and a piece of damp lead acetate test paper; the open end is covered with Parafilm M.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0211
Figure 4. A more detailed image of Parafilm M sealing the open end of a glass pipette with a piece of lead acetate test paper inside.
Figure 5 shows a pipette just after being inserted above the flame from an alcohol lamp. During heating, the sample may shrink, gradually change colour and sometimes appear to be boiling. At the stage of the test shown in Figure 6, the blue wool sample has changed colour and produced fumes that have turned the lead acetate test paper black. After the test, the inside of the pipette nearest the sample is coated with a dark brown residue from the degraded sample.
The appearance of a blue wool sample during heating for 5 minutes is shown in a video.

© Government of Canada, Canadian Conservation Institute. CCI 127994-0001
Figure 5. A pipette containing a blue wool sample just after being inserted above the flame from an alcohol lamp.

© Government of Canada, Canadian Conservation Institute. CCI 127994-0002
Figure 6. A pipette containing a blue wool sample after being heated enough with an alcohol lamp for a reaction to happen. The heat has caused the blue wool to darken and give off fumes, and the lead acetate test paper has turned black.
The dark brown and black discolouration on the lead acetate test paper in Figure 7 illustrates the strong reaction of the fumes from ebonite with the lead acetate test paper.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0216
Figure 7. The dark brown and black discolouration formed on this piece of lead acetate test paper after it was exposed to the fumes from about 10 mg of ebonite.
The last step in the procedure is to remove the test paper from the pipette and add hydrogen peroxide (3% v/v). The hydrogen peroxide is used to distinguish lead sulfide from carbon soot. Hydrogen peroxide will oxidize the dark lead sulfide to white lead sulfate, turning the darkened paper white (as shown in Figure 8), but will not change the colour of soot.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0215
Figure 8. Three pieces of lead acetate test paper: left, new (dry, untested); middle, after reacting with reduced sulfur compounds and turning brown; and right, the middle test paper after applying 3% v/v hydrogen peroxide and converting the brown to white.
Additional information
Sample size
Tétreault (2003) suggests a sample weight of 10 mg. This is a small amount of material: a chunk of rubber a few millimetres across, a piece of nitrile or latex glove about a centimetre across or a piece of wool yarn about 2 cm long. Tétreault (2003) also recommends drying liquid samples (white glue, for example) on a piece of aluminum foil for one day before testing.
Alcohol burner
The heat source shown in Figure 5 is an alcohol burner. The recommended fuel for this burner is some form of ethanol (ethyl alcohol), such as 95% ethanol or denatured ethanol. Denatured ethanol is ethanol that has been made poisonous or undrinkable through additives (such as methanol or a bittering agent). The alcohol burner is a convenient gentle heat source for heating the sample. It can also be used to seal the pipette, although a butane torch (Figure 1) is faster.
Parafilm M
Parafilm M (as shown in Figure 9) is a stretchy laboratory film that is used for sealing containers such as the glass pipette.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0212
Figure 9. A box of Parafilm M plastic laboratory film.
Storage
These test papers need to be stored below 30°C in a dry place away from sunlight. If stored properly, they last several years. Nevertheless, it is important to check that the test paper still works by testing a known sample containing sulfur.
Materials containing sulfur
Sulfur-containing gases may originate from a wide range of materials. Some natural materials contain sulfur, such as:
- Wool, wool felt
- Hair
- Feathers
Elemental sulfur has been used, especially in the past, for various purposes, such as (Benson 2012):
- Adhesives
- Cement
- Grout
- Inlays in furniture
- Munitions
Some rubber-like objects are made by adding sulfur to polymers to modify their properties by forming bridges between individual chains. Some examples are:
- Ebonite (formerly called vulcanite)
- Elastic bands
- Gaskets
- Gloves (e.g. latex)
- Molding material (polysulfide rubber)
- O-rings
- Pencil erasers
- Stoppers
Sulfur can also be found in a surprising number of other materials (Benson 2012), such as:
- Clays (e.g. certain modelling clays)
- Drywall (poor quality)
- Glues (protein-based)
- Paints (certain ones)
- Plaster casts (made with gypsum)
- Wood (recovered from anaerobic environments)
If a commercial product is being considered for use, first check its safety data sheet (SDS). Look for the section on hazardous combustion products and check if any contain sulfur (such as hydrogen sulfide, sulfur dioxide or sulfuric acid). If present, then it is not necessary to carry out the lead acetate test because these combustion products indicate the commercial product must contain sulfur (Tétreault 2003).
Other tests for sulfur
Lead is used to make the test papers because of two important properties: it has a soluble salt (lead acetate), and lead sulfide is insoluble and dark. Other metals can be used instead of lead. One example is the Hydrogen Sulfide Test Paper made by Hach that contains copper sulfate. Another sulfide test paper, made by Macherey-Nagel, is said to be lead-free and not containing any hazardous materials. (The composition of the Macherey-Nagel sulfide test paper is unknown, but it worked as well as the lead acetate test paper in preliminary tests using the glass pipette.)
A different kind of test, based on a sodium azide solution, can also be used to test materials for sulfur (Daniels and Ward 1982). In this test, a drop of a solution containing sodium azide and iodine is placed on the material to be tested. Rapid evolution of nitrogen gas from the decomposition of the azide indicates the presence of sulfur.
The science behind the lead acetate test
The purpose of this test is to identify materials that contain sulfur, because these materials might emit gases that will tarnish silver, copper and alloys that contain these metals (such as sterling silver, brass and bronze). Tarnishing occurs when corrosion products form a thin film of discolouration on a metal's surface. One of the main causes of tarnishing is the reaction between the metal and sulfur-containing compounds such as hydrogen sulfide (H2S) and carbonyl sulfide (COS) (Selwyn 2004). Tarnish on silver is mainly composed of silver sulfide (Ag2S). Tarnish on copper is made up of copper sulfides (e.g. CuS, Cu2S) as well as copper oxide (e.g. cuprite, Cu2O).
More information on the lead acetate test can be found in Odegaard et al. (2005), Rémillard (2007) and Tétreault (2003).
Lead acetate test paper
These papers are coated with lead(II) acetate [Pb(CH3COO)2], a white crystalline lead compound that is soluble in water. Lead test papers can be prepared by soaking paper in a solution of lead acetate, but it is more convenient to buy commercial test papers (as shown in Figure 10). During the test, a drop or two of water is added to the test paper to dissolve the lead acetate and produce lead ions (Pb2+) in solution.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0205
Figure 10. Lead acetate test papers in their container.
Lead acetate test paper is generally regarded as selective for detection of hydrogen sulfide (ASTM 2006). Hydrogen sulfide reacts with lead ions from the lead acetate to form solid lead sulfide PbS, a black solid.
H2S + Pb2+ → PbS + 2H+
Hydrogen sulfide is released in the first few seconds after wool begins to burn (Spurgeon et al. 1977).
The selectivity for hydrogen sulfide is not perfect. Methyl mercaptan can turn the test paper a yellow colour, which fades after a few minutes (ASTM 2006). Carbonyl sulfide (COS) can react with the water in the test paper to form hydrogen sulfide, which in turn reacts with the test paper (Feigl et al. 1972).
Like other lead compounds, lead acetate and lead sulfide are toxic (Selwyn 2005), although there is so little lead acetate in these papers that some safety data sheets do not label the papers as hazardous. Lead acetate is also known as sugar of lead and was used historically to sweeten (and inadvertently poison) wine (Nriagu 1992). Lead sulfide is the mineral galena.
Reduced sulfur
Materials with sulfur that cause silver and copper to tarnish contain reduced sulfur (Selwyn 2004). Reduced sulfur is a term used to describe sulfur compounds in which the sulfur is in a reduced oxidation state, such as an oxidation state of zero [written as S(0) or S0], minus one [written as S(-I) or S-] or minus two [written as S(-II) or S2-]. An example with an oxidation state of zero is elemental sulfur (S). Examples with oxidation state minus one are keratin-containing materials (e.g. hair, wool). These contain disulfide (S-S) bonds in compounds of the form R-S-S-R, where R is a carbon-containing chain. Examples with oxidation state minus two are hydrogen sulfide (H2S), carbonyl sulfide (COS), organic thiols (RSH) and organic sulfides (R-S-R).
Rubber
Natural rubber is obtained from the latex (sap) of the rubber tree. The latex can be hardened by vulcanization, a process where the latex is mixed with sulfur and heated (Nebergall et al. 1972), causing the sulfur to form bridges between latex molecules. Latex gloves, hockey pucks, Minibrix and ebonite are some examples of objects made from rubber. Neoprene is an example of a synthetic rubber that is sometimes vulcanized with sulfur.
Minibrix (as shown in Figure 11) are children's building blocks made from rubber between 1935 and 1976 (Hanson 2012). Ebonite is a type of hard rubber. It is a good electrical insulator and is often found in association with copper alloys in historic scientific instruments, telegraphy and radio collections. An example of ebonite in a telephone receiver is shown in Figure 12.
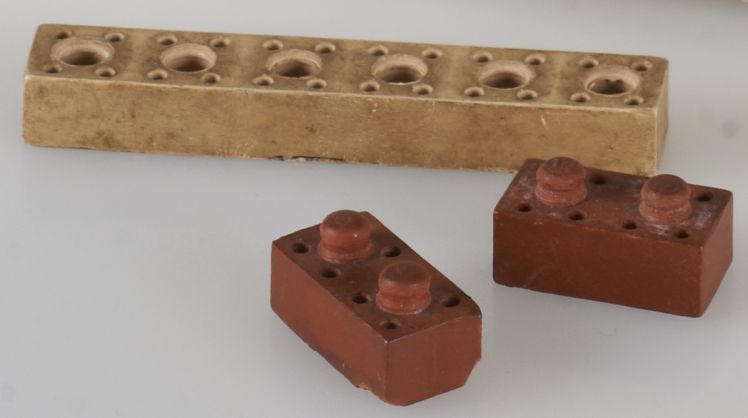
© Government of Canada, Canadian Conservation Institute. CCI 120260-0218
Figure 11. Three pieces from a set of Minibrix. The upper piece is 75 mm long.

© Government of Canada, Canadian Conservation Institute. CCI 120260-0217
Figure 12. The large earpiece at the left end of this telephone receiver is made of ebonite. Beside the receiver is a clear container holding small samples of the ebonite suitable for testing with the lead acetate test paper.
Ebonite deteriorates in the presence of air, moisture and light. The sulfur in the ebonite oxidizes to sulfuric acid, which can corrode metals or damage other adjacent material (Selwyn 2004).
Materials based on proteins
Wool, feathers, hair, skin and silk are all composed primarily of proteins, but whereas wool, feathers and hair contain a significant amount of sulfur in the protein keratin, skin contains less sulfur and silk contains almost none.
At room temperature, keratin does not release much sulfur (Tétreault 2003), but at 50°C or under illumination it can. In an open room, sulfur from wool is probably insignificant compared to sulfur coming from other sources, so wool rugs are generally not a problem there. In a sealed display case, however, it is better to avoid wool where possible (Brimblecombe et al.1992).
Proteins are made from amino acids. Of the 20 amino acids commonly found in protein, two contain sulfur—cysteine and methionine (Mills and White 1994). In cysteine, the sulfur is present as a sulfhydryl group (SH) bonded to a carbon atom (sometimes written as -C-SH). Two cysteine molecules can join together through a sulfur-sulfur bond (a disulfide bond) to form cystine. It is these disulfide bonds that hold the molecular coils together in keratin, the main protein in wool, feathers and hair. (The sulfur-sulfur bonds are broken and then reformed when a perm is done on someone's hair.) There is much less sulfur in fibroin, the main protein in silk, or in collagen, the main protein in skin or muscle tissue; these proteins are held together through hydrogen bonds. (Collagen does contain some sulfur in methionine.)
In contrast to these protein-based materials, the plant-based materials cotton and linen are made of cellulose, which does not contain sulfur.
Hydrogen peroxide
It is possible that the dark brown discolouration on the test paper is carbon (i.e. soot) from the heated material. In addition, if the pipette is heated too much, the paper itself may char. Hydrogen peroxide is used to distinguish between black from lead sulfide and black from carbon soot or charred paper.
Hydrogen peroxide (H2O2) is an oxidizing agent. If the dark brown is lead sulfide, then the hydrogen peroxide (3% v/v) will oxidize the lead sulfide to white lead sulfate (PbSO4, mineral name anglesite) (Vogel 1945).
PbS + 4H2O2 → PbSO4 + 4H2O
In this case, the brown stain will disappear and the paper will turn white, because the white lead sulfate is indistinguishable from the white of the paper. If the dark brown is soot, on the other hand, the colour will not change after the hydrogen peroxide is applied.
Hydrogen sulfide
Hydrogen sulfide, a colourless, heavier-than-air gas, has a foul smell and is extremely toxic. Fortunately the smell of hydrogen sulfide is detectable well below toxic concentrations; many people can recognize the smell at concentrations as low as 0.0047 parts per million (ppm) (Powers 2004). Levels of about 1 ppm can cause eye irritation; above 100 ppm, the gas deadens the sense of smell; and above about 200 ppm, hydrogen sulfide becomes deadly (Weil et al. 2007).
Acknowledgements
Special thanks to Avital Lang, CCI intern, for her help with developing this Note.
Suppliers
Note: The following information is provided only to assist the reader. Inclusion of a company in this list does not in any way imply endorsement by the Canadian Conservation Institute.
Lead acetate paper
Lead acetate paper is available from chemical supply companies such as Fisher Scientific, Macherey-Nagel distributed in Canada by Aldert Chemicals, Sigma-Aldrich and VWR International. Fisher test papers are supplied in a box of 24 vials; each vial contains 100 strips 4.8 cm long and 0.65 cm wide. Other brands usually come in a roll.
Chemicals and laboratory equipment
Alcohol burners, disposable nitrile gloves, ethanol, wood applicator sticks, Parafilm M film and Pasteur pipettes are available from chemical supply companies such as Fisher Scientific and Canadawide Scientific. An alcohol burner is also available from Lee Valley. Ethanol is also available from hardware stores such as Home Hardware.
Butane torch
Butane torches are available at hardware stores. The handheld micro torch used in this procedure is available from Lee Valley.
References
ASTM D2420-91. "Standard Test Method for Hydrogen Sulfide in Liquefied Petroleum (LP) Gases (Lead Acetate Method)." In Annual Book of ASTM Standards, vol. 05.01. West Conshohocken, PA: American Society for Testing and Materials, 2006, pp. 881–882.
Benson, P.L. "Some Unusual, Hidden, Surprising, or Forgotten Sources of (Possible) Sulfur Contamination in Museums and Historic Structures." AIC Objects Specialty Group Postprints 19 (2012), pp. 85–107.
Brimblecombe, P., D. Shooter and A. Kaur. "Wool and Reduced Sulphur Gases in Museum Air." Studies in Conservation 37,1 (1992), pp. 53–60.
Daniels, V., and S. Ward. "A Rapid Test for the Detection of Substances Which Will Tarnish Silver." Studies in Conservation 27,2 (1982), pp. 58–60.
Feigl, F., and V. Anger. Spot Tests in Inorganic Analysis, 6th ed. Amsterdam, Netherlands: Elsevier, 1972, p. 465.
Hanson, M. The History of Minibrix.
Kultys, A. "Sulfur-containing Polymers." In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed., vol. 23. Hoboken, NJ: John Wiley & Sons, 2007, pp. 702–753.
Mills, J.S., and R. White. The Organic Chemistry of Museum Objects, 2nd ed. Oxford, UK: Butterworth-Heinemann, 1994.
Nebergall, W.H., F.C. Schmidt and H.F. Holtzclaw. General Chemistry, 4th ed. Lexington, MA: D.C. Heath, 1974, pp. 581–582.
Nriagu, J.O. "Saturnine Drugs and Medicinal Exposure to Lead: An Historical Outline." In H.L. Needleman, ed., Human Lead Exposure. Boca Raton, FL: CRC Press, 1992, pp. 3–21.
Odegaard, N., S. Carroll and W.S. Zimmt. "Test for Sulfur Using Lead Acetate Paper and Pyrolysis." In Material Characterization Tests for Objects of Art and Archaeology, 2nd ed. London, UK: Archetype Publications, 2005, pp. 146–147.
Powers, W. The Science of Smell Part 1: Odor Perception and Physiological Response. Ames, USA: Iowa State University, 2004.
Rémillard, F. "Lead Acetate Test." In Identification of Plastics and Elastomers: Miniaturized Tests (PDF version). Québec, QC: Centre de conservation du Québec, 2007, pp. 12–13.
Selwyn, L. Metals and Corrosion: A Handbook for the Conservation Professional. Ottawa, ON: Canadian Conservation Institute, 2004.
Selwyn, L. "Health and Safety Concerns Relating to Lead and Lead Compounds in Conservation." Journal of the Canadian Association for Conservation 30 (2005), pp. 18–37.
Spurgeon, J.C., L.C. Speitel and R.E. Feher. "Thermal Decomposition Products of Aircraft Interior Materials." Report No. FAA-RD-77-20. Washington, D.C.: U.S. Department of Transportation, Federal Aviation Administration, 1977.
Tétreault, J. Airborne Pollutants in Museums, Galleries, and Archives: Risk Assessment, Control Strategies, and Preservation Management. Ottawa, ON: Canadian Conservation Institute, 2003.
Vogel, A.I. A Text-book of Qualitative Chemical Analysis Including Semimicro Qualitative Analysis, 3rd ed. London, UK: Longmans, Green and Co. Ltd., 1945, p. 323.
Weil, E.D., S.R. Sandler and M. Gernon. "Sulfur Compounds." In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed., vol. 23. Hoboken, NJ: John Wiley & Sons, 2007, pp. 621–701.
Written by Lyndsie Selwyn
Également publié en version française.
© Government of Canada, Canadian Conservation Institute, 2017
ISSN 1928-1455