COVID-19 Call for Proposals

Long description of The Four COVID-19 Challenges graphic
IDEaS received 469 proposals to strengthen the country’s response to COVID-19, as well as future pandemics. 48 projects were selected and funded up to $200,000, which will help in the development of strategies to reuse protective gear, clean equipment and workspaces, gain real-time insight for decision-making, and care for frontline workers.
In addition to the four challenges issued, the program worked with other government departments including the National Research Council (NRC) to address other pandemic issues of importance to Canadians. Three contribution agreements were signed for solicited projects related to pandemic response. These projects were managed and primarily funded by IDEaS.
Funding valued at just over $1 million was awarded to innovators to develop COVID-19 fast testing kits. A third contribution agreement, valued at $2.1 million, was awarded to advance the clinical development of a broad-spectrum antiviral drug.
The benefits of these projects will aid in decision-making and future approaches to similar threats. But more broadly, they will have long-term benefits for defence capability and civilian response to future pandemics.
Additional Projects:
Custom Biologics
Custom Biologics has developed diagnostic point-of-care kits that are more stable for shipping and storage and will not require trained personnel for administering. Saliva-based tests are used to detect SARS-CoV-2 instead of a nose swab, a much simpler and more agreeable procedure. And finally, point-of-care readers that can be deployed in diverse community-based settings such as long-term care facilities, mobile clinics, airports, and seagoing vessels.
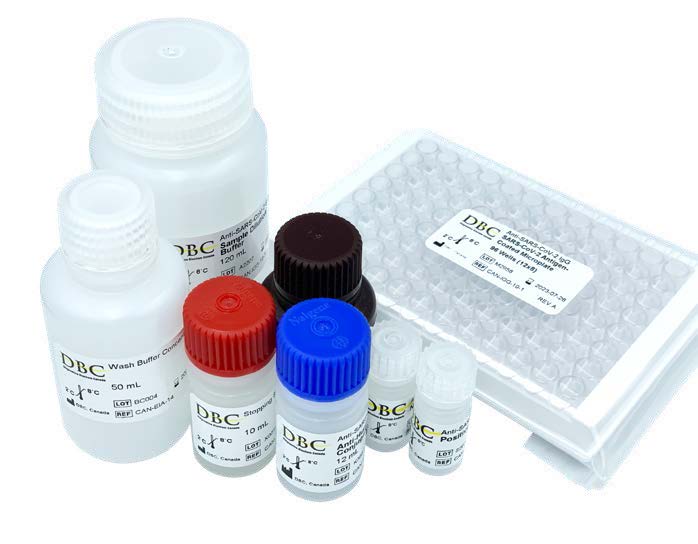
Diagnostic Biochem Canada Inc.
Diagnostic Biochem Canada Inc. successfully developed four Anti-SARS-Cov-2 serological tests. Two of them, the total antibody and IgG tests, were the first Canadian COVID-19 serological tests to be authorized for in vitro diagnostics in Canada. The tests aid in the detection of an adaptive immune response to the virus, and verify vaccination response.
What has been done to date
- IDEaS invested $1million in Diagnostic Biochem Canada’s project which developed a dry blood spot sampling method and testing kit to measure COVID-19 antibodies.
- Diagnostic Biochem Canada went on to receive Health Canada COVID-19 Medical Device Authorization for its ELISA Kit in Canada.
Pandemic Challenges by the Numbers:

Long description of Pandemic Challenges by the Numbers infographic
- Four Challenges issued
- 469 proposals received
- 48 projects funded @ $200,000
- Two $1 M direct contributions awarded to advance rapid testing
- One $2.1M contribution agreement awarded to advance the research and development of a broad-spectrum anti-viral drug candidate through clinical trial testing
- Total Funding: Over $11 million