Screening assessment - Pigments and Dyes Group
Official title: Screening assessment - Pigments and Dyes Group
Chemical Abstracts Service Registry Numbers
596-03-2, 1326-03-0, 8005-03-6, 12224-98-5, 26694-69-9, 42373-04-6
Environment and Climate Change Canada
Health Canada
August 2020
Cat. No.: En14-414/2020E-PDF
ISBN: 978-0-660-35221-3
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 6 of 25 substances referred to collectively under the Chemicals Management Plan as the Pigments and Dyes Group. These 6 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. Nineteen of the 25 substances were determined to be of low concern through other approaches, and decisions for these substances are provided in separate reports.Footnote 1Footnote 2 Accordingly, this screening assessment addresses the 6 substances listed in the table below, hereinafter referred to as the Pigments and Dyes Group.
CAS RNa |
Domestic Substances List (DSL) name |
Common name |
|---|---|---|
596-03-2 |
Spiro[isobenzofuran-1(3H),9’-[9H]xanthen]-3-one, 4’,5’-dibromo-3’,6’-dihydroxy- |
D&C Orange 5 |
1326-03-0 |
Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, molybdatetungstatephosphate |
Pigment Violet 1 |
8005-03-6b,c |
C.I. Acid Black 2 |
Acid Black 2 |
12224-98-5 |
Xanthylium, 9-[2-(ethoxycarbonyl)phenyl] -3,6-bis(ethylamino)-2,7-dimethyl-, molybdatetungstatephosphate |
Pigment Red 81 |
26694-69-9 |
Xanthylium, 9-[2-(ethoxycarbonyl)phenyl] -3,6-bis(ethylamino)-2,7-dimethyl-, ethyl sulfate |
NA |
42373-04-6 |
Thiazolium, 3-methyl-2-[(1-methyl-2-phenyl- 1H-indol-3-yl)azo]-, chloride |
Basic Red 29 |
Abbreviations: NA, not available.
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior written permission of the American Chemical Society.
b This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
c This substance was not identified under subsection 73(1) of CEPA but was included in this screening assessment as it was considered a priority based on other human health concerns.
The substances in the Pigments and Dyes Group are used as colouring agents, spanning a variety of potential applications. Substances in this group are used in products available to consumers, including cosmetics (e.g., hair products, lipstick/lip balm, make-up, face paint, nail polish), food packaging materials, inks (e.g., printing inks, ink pads), textiles, and children’s arts and crafts materials (e.g., crayons, chalk). According to information submitted under section 71 of CEPA in the 2011 calendar year, no manufacturing was reported above the 100 kg threshold for the substances in the Pigments and Dyes Group. Reported import quantities were not above the 100 kg threshold for D&C Orange 5, were in the range of 1 000 to 10 000 kg for each of Pigment Violet 1 and Acid Black 2, were not above 100 kg for each of Pigment Red 81 and CAS RN 26694-69-9, and were in the range of 100 to 1 000 kg for Basic Red 29.
The ecological risks of the substances in the Pigments and Dyes Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the substances in the Pigments and Dyes Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29. It is concluded that D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the general population of Canada, the predominant source of exposure to substances in the Pigments and Dyes Group is from use of products available to consumers that contain these substances. The predominant routes of exposure are oral and dermal. Potential dermal and oral exposures to D&C Orange 5 and Acid Black 2 were based on use of cosmetics. Potential exposures to Pigment Violet 1, Pigment Red 81, and CAS RN 26694-69-9 were derived for toddlers from the use of children’s arts and crafts materials. Potential dermal and oral exposures to Basic Red 29 were derived from contact with textiles. Potential inhalation exposure to Pigment Violet 1 from use of chalk was low relative to oral exposures. Inhalation exposure to the remaining substances in the Pigments and Dyes Group was not considered to be of concern given their very low volatility and their uses.
In laboratory studies, no treatment-related or consistent dose-dependent health effects were observed for D&C Orange 5. The critical health effects for Pigment Violet 1, based on its dye component Basic Violet 10, were an increase in the incidence of astrocytomas of the brain and/or spinal cord and increased mortality, organ weights and food consumption. No treatment-related or consistent dose-dependent health effects were observed in the key studies used for risk characterization for Acid Black 2 based on the structural analogue Solvent Black 5. Decreased body weight was the critical health effect for the substance bearing CAS RN 26694-69-9 as well as for Pigment Red 81, based on its dye component, Basic Red 1. A threshold of toxicological concern (TTC)-based approach was taken for Basic Red 29.
Margins of exposure comparing levels at which critical health effects occur (or in their absence, the highest tested dose in key studies) and the estimates of exposure from the use of products available to consumers were considered adequate to address uncertainties in the health effects and exposure databases for D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81 and CAS RN 26694-69-9. For Basic Red 29, the estimate for exposure from products available to consumers was lower than the TTC value based on its Cramer Class and overall negative genotoxicity, indicating a low probability of risk to human health. Basic Red 29 is considered to be a low concern for human health at current levels of exposure.
On the basis of the information presented in this screening assessment, it is concluded that D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29 do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 6 of 25 substances referred to collectively under the Chemicals Management Plan as the Pigments and Dyes Group, to determine whether they present or may present a risk to the environment or to human health. These 6 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The other 19 substances (listed in Table 1-1, below) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and in either the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016) or via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018a) and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these 19 substances are provided in Substances Identified as Being of Low Concern using the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018b) and the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018a).
CAS RN |
Domestic Substances List name |
Approach under which the substance was addressed |
References |
|---|---|---|---|
2387-03-3 |
1-Naphthalenecarboxaldehyde, 2-hydroxy-, [(2-hydroxy-1-naphthalenyl)methylene]hydrazone |
ERC/Rapid Screening |
ECCC, HC 2018a |
2478-20-8 |
1H-Benz[de]isoquinoline-1,3(2H)-dione, 6-amino-2-(2,4-dimethylphenyl)- |
ERC/Rapid Screening |
ECCC, HC 2018a |
4378-61-4 |
Dibenzo[def,mno]chrysene-6,12-dione, 4,10-dibromo- |
ERC/Rapid Screening |
ECCC, HC 2018a |
5521-31-3 |
Anthra[2,1,9-def:6,5,10-d’e’f’]diisoquinoline-1,3,8,10(2H,9H)-tetrone, 2,9-dimethyl- |
ERC/Rapid Screening |
ECCC, HC 2018a |
5718-26-3 |
1H-Indole-5-carboxylic acid, 2-[(1,5-dihydro-3-methyl-5-oxo-1-phenyl-4H-pyrazol-4-ylidene)ethylidene]-2,3-dihydro-1,3,3-trimethyl-, methyl ester |
ERC/Rapid Screening |
ECCC, HC 2018a |
6858-49-7 |
Propanedinitrile, [[4-[ethyl[2-[[(phenylamino)carbonyl]oxy]ethyl]amino]-2-methylphenyl]methylene]- |
ERC/TTC |
ECCC, HC 2018b |
7576-65-0 |
1H-Indene-1,3(2H)-dione, 2-(3-hydroxy-2-quinolinyl)- |
ERC/Rapid Screening |
ECCC, HC 2018a |
13082-47-8 |
Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, hydroxide |
ERC/TTC |
ECCC, HC 2018b |
16294-75-0 |
14H-Anthra[2,1,9-mna]thioxanthen-14-one |
ERC/Rapid Screening |
ECCC, HC 2018a |
62973-79-9 |
Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, molybdatesilicate |
ERC/Rapid Screening |
ECCC, HC 2018a |
63022-09-3
|
Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, molybdatephosphate |
ERC/Rapid Screening |
ECCC, HC 2018a |
66241-11-0 |
C.I. Leuco Sulphur Black 1 |
ERC/Rapid Screening |
ECCC, HC 2018a |
68310-07-6 |
Xanthylium, 3,6-bis(ethylamino)-9-[2-(methoxycarbonyl)phenyl]-2,7-dimethyl-, molybdatephosphate |
ERC/Rapid Screening |
ECCC, HC 2018a |
68409-66-5 |
Ethanaminium, N-[4-[[4-(diethylamino)phenyl][4-(ethylamino)-1-naphthalenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, molybdatephosphate |
ERC/Rapid Screening |
ECCC, HC 2018a |
68814-02-8
|
Ethanaminium, N-[4-[bis[4-(diethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, molybdatephosphate |
ERC/Rapid Screening |
ECCC, HC 2018a |
75627-12-2 |
Xanthylium, 3,6-bis(ethylamino)-9-[2-(methoxycarbonyl)phenyl]-2,7-dimethyl-, molybdatesilicate |
ERC/Rapid Screening |
ECCC, HC 2018a |
80083-40-5
|
Xanthylium, 9-[2-(ethoxycarbonyl)phenyl]-3,6-bis(ethylamino)-2,7-dimethyl-, molybdatetungstatesilicate |
ERC/Rapid Screening |
ECCC, HC 2018a |
102082-92-8 |
Xanthylium, 3,6-bis(diethylamino)-9-[2-(methoxycarbonyl)phenyl]-, molybdatesilicate |
ERC/Rapid Screening |
ECCC, HC 2018a |
106276-80-6 |
Benzoic acid, 2,3,4,5-tetrachloro-6-cyano-, methyl ester, reaction products with p-phenylenediamine and sodium methoxide |
ERC/Rapid Screening |
ECCC, HC 2018a |
The six substances addressed in this screening assessment report will hereinafter be referred to as the Pigments and Dyes Group.
The ecological risks of the substances in the Pigments and Dyes Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
While each of the substances in the Pigments and Dyes Group was assessed individually in this screening assessment, the same substance was used to inform the health effects of Pigment Red 81 and CAS RN 26694-69-9 as a component and as a structural analogue, respectively. The substances in the Pigments and Dyes Group were generally not grouped together on the basis of chemical structure because, although it contains some structurally-related xanthene pigments, not all members are structurally related.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to October 2016. Targeted literature searches were conducted up to April 2017, and additional data were submitted by stakeholders up to May 2017. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Theresa Lopez, Jennifer Flippin, and Joan Garey of Tetra Tech. Additionally, the draft of this screening assessment (published on January 5, 2019) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 3 This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Identity of substances
The CAS RN, DSL names and common names for the individual substances and representative structures in the Pigments and Dyes Group are presented in Table 2‑1. Pigment Violet 1 and Pigment Red 81 were categorized as discrete substances under CEPA (ECCC, HC [modified 2017]) and the primary concerns relating these substances are with their organic moiety; however, it is recognized that these substances possess UVCB-type characteristics due to the variation in their inorganic moiety (Table 2-1). Although some of the substances in the Pigments and Dyes Group are structurally related xanthenes (i.e., Pigment Violet 1, Pigment Red 81, and CAS RN 26694-69-9), there is notable diversity among the substances with respect to chemical structure and application class (e.g., pigments, basic dye, and acid dye).
| CAS RN | DSL name(common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 596-03-2 | Spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one, 4',5'-dibromo-3',6'-dihydroxy-(D&C Orange 5) |
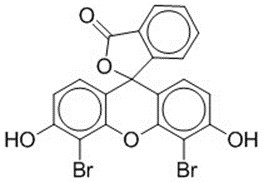 C20H10Br2O5 C20H10Br2O5 | 490.10 |
| 1326-03-0a,b | Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, molybdatetungstatephosphate(Pigment Violet 1) |
![Representative chemical structure of Pigment Violet 1, with SMILES notation: CCN(CC)C1=CC2=[O+]C3=CC(N(CC)CC)=CC=C3C(C4=CC=CC=C4C(O)=O)=C2C=C1](/content/dam/eccc/images/pded/pigments-and-dyes/t2-1b.jpg) [C28H31N2O3]n . PTMA [C28H31N2O3]n . PTMA
n = 6 PTMA = O3.P2O5.xWO3.yMoO3 x = 12 – 32 y = 1 – 12 | 7361 – 8328 |
| 8005-03-6a | C.I. Acid Black 2(Acid Black 2; also called Nigrosine, water-soluble) |
![Representative chemical structure of Acid Black 2, with SMILES notation: [O-]S(C(C=C1)=CC=C1NC(C(NC2=CC=C(S([O-])(=O)=O)C=C2)=CC3=N4)=CC3=[N+](C5=C4C(NC6=CC=CC=C6)=CC=C5)C7=CC=CC=C7)(=O)=O.[Na+]](/content/dam/eccc/images/pded/pigments-and-dyes/t2-1c.jpg) C36H26N5NaO6S2 C36H26N5NaO6S2 | 711.74 |
| 12224-98-5a,b | Xanthylium, 9-[2-(ethoxycarbonyl)phenyl] -3,6-bis(ethylamino)-2,7-dimethyl-, molybdatetungstatephosphate(Pigment Red 81) |
![Representative chemical structure of Pigment Red 81, with SMILES notation: CCNC1=CC2=[O+]C3=CC(NCC)=C(C)C=C3C(C4=CC=CC=C4C(OCC)=O)=C2C=C1C](/content/dam/eccc/images/pded/pigments-and-dyes/t2-1d.jpg) [C28H31N2O3]n. PTMA
[C28H31N2O3]n. PTMA
n = 6 PTMA = O3.P2O5.xWO3.yMoO3 x = 12 – 32 y = 1 – 12 |
7361 – 8328 |
| 26694-69-9 | Xanthylium, 9-[2-(ethoxycarbonyl)phenyl] -3,6-bis(ethylamino)-2,7-dimethyl-, ethyl sulfate |
![Representative chemical structure of CAS RN 26694-69-9, with SMILES notation: CCOC(c1ccccc1c2c(cc(C)c(NCC)c3)c3[o+]c4c2cc(C)c(NCC)c4)=O.O=S([O-])(OCC)=O](/content/dam/eccc/images/pded/pigments-and-dyes/t2-1e.jpg) C30H36N2O7S C30H36N2O7S | 568.68 |
| 42373-04-6 | Thiazolium, 3-methyl-2-[(1-methyl-2-phenyl- 1H-indol-3-yl)azo]-, chloride(Basic Red 29) |
C=CS3)C4=C1C=CC=C4.[Cl-]](/content/dam/eccc/images/pded/pigments-and-dyes/t2-1f.jpg) C19H17N4SCl C19H17N4SCl | 368.89 |
Abbreviations: PTMA, phosphosphatetungstatemolybdate.
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials), and the chemical structure shown is a representative structure. The molecular formula and molecular weight correspond to the structure shown.
b Exact structure (e.g., stoichiometric ratio between the xanthene moiety and molybdatetungstatephosphate counterion) is dependent on the pH and precipitation temperature during manufacturing (Herbst and Hunger 2004). The representative structure corresponding to n = 6 for the cation dye moiety and the anionic salt moiety PTMA = O3.P2O5.xWO3.yMoO3 is consistent with Laden (1997).
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected that were structurally and/or functionally similar to substances within this group (e.g., in terms of physical-chemical properties, toxicokinetics) and that had relevant empirical data that could be used to read-across to substances with limited empirical data. Details of the read-across data and (Q)SAR models chosen to inform the ecological and human health assessments of the Pigments and Dyes Group are further discussed in the relevant sections of this report. A list of the analogues as well as the dye components of certain pigments used to inform this assessment is presented in Table 2-2.
| CAS RN for analogue and/or component | DSL or other name(common name) | Chemical structure and molecular formula | Molecular weight (g/mol) | Target substance(s) |
|---|---|---|---|---|
| 81-88-9a,b | Xanthylium, 9-(2-carboxyphenyl)-3,6-bis(diethylamino)-, chloride (Basic Violet 10; also called Rhodamine B, D&C Red 19) |
\CC)C=C1)CC.[Cl-]](/content/dam/eccc/images/pded/pigments-and-dyes/t2-2a.jpg) C28H31ClN2O3 C28H31ClN2O3 | 479.017 | Pigment Violet 1a, CAS RN 26694-69-9b |
| 989-38-8a | Xanthylium, 9-[2-(ethoxycarbonyl)phenyl]-3,6-bis(ethylamino)-2,7-dimethyl-, chloride (Basic Red 1; also called Rhodamine 6G) |
![Representative chemical structure of Basic Red 1, with SMILES notation: CCNc1c(C)cc2c(c3c(C(OCC)=O)cccc3)c(cc(C)c(NCC)c4)c4[o+]c2c1.[Cl-]](/content/dam/eccc/images/pded/pigments-and-dyes/t2-2b.jpg) C28H31ClN2O3 C28H31ClN2O3 | 479.0169 | Pigment Red 81a, CAS RN 26694-69-9 |
| 11099-03-9c | C.I. Solvent Black 5 (Solvent Black 5; also called Nigrosine, spirit soluble) |
![Representative chemical structure of Solvent Black 5, with SMILES notation: [H]N1C2=C(C=CC=C2)N(C3=CC=CC=C3)C(C1=C4)=CC5=C4N=C6C(N5C7=CC=CC=C7)=C/C(C(N(C8=CC=CC=C8)[H])=C6)=N/C9=CC=CC=C9](/content/dam/eccc/images/pded/pigments-and-dyes/t2-2c.jpg) (where integer = 1)C42H30N6 (where integer = 1)C42H30N6 | 618.73 | Acid Black 2 |
| 36877-69-7b | Xanthylium, 9-[2-carboxy-5(or 6)-isothiocyanatophenyl]-3,6-bis(diethylamino)-, chloride(Rhodamine B isothiocyanate) |
\CC)C=C1)CC.[Cl-]](/content/dam/eccc/images/pded/pigments-and-dyes/t2-2d.jpg) C29H30ClN3O3S C29H30ClN3O3S | 536.087 | CAS RN 26694-69-9b |
Abbreviations: NA, not available.
a Basic Violet 10 and Basic Red 1 are not considered to be analogues of Pigment Violet 1 and Pigment Red 81, respectively, but they are the dye components that were used to inform the potential health effects of the respective related pigments (see section 6.2 for additional information).
b Basic Violet 10 and Rhodamine B isothiocyanate were used for read-across to CAS RN 26694-69-9 for the dermal absorption endpoint only.
c This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials). Read-across to Acid Black 2 on the basis that both substances are nigrosine; Solvent Black 5 is its alcohol-soluble form while Acid Black 2 is its water-soluble form.
3. Physical and chemical properties
A summary of available physical and chemical property data of the substances in the Pigments and Dyes Group is presented in Table 3‑1. Experimental information regarding the physical and chemical properties of these substances is limited. Additional physical and chemical properties are reported in ECCC 2016b. The reported vapour pressure of D&C Orange 5 was very low (1.18 × 10-16 mmHg at 25ºC; LookChem 2008). Vapour pressure data for other substances were not found in the literature. The vapour pressure of Basic Red 29 was estimated by the MPBPWIN module in EPI Suite (EPI Suite c2000-2012) and was also found to be very low (1.23 × 10-10 mmHg at 25ºC). Other substances in this group are all expected to have very low vapour pressures on the basis of their relatively high molecular weights and their molecular structures (e.g., ionization).
Substance |
Water solubility |
log Kow |
Key references |
|---|---|---|---|
D&C Orange 5 |
“Slightly soluble”; 0.5 mg/L (estimated) |
5.29 (estimated) |
LookChem 2008; ECCC 2016b |
Pigment Violet 1 |
“Insoluble”; <0.1 mg/mL |
NA |
Chemicalland21 2016; Keith and Walters as cited in Cameo Chemicals 2017a |
Acid Black 2 |
10 – 50 mg/mL at 18ºC |
NA |
Keith and Walters 1992 as cited in NCBI 2005 |
Pigment Red 81 |
“Slightly soluble”; 1-5 mg/mL |
NA |
Keith and Walters 1992 as cited in Cameo Chemicals 2017b; Ash and Ash 2013b |
CAS RN 26694-69-9 |
<0.01 mg/L (estimated) |
4.15 (estimated) |
ECCC 2016b |
Basic Red 29 |
1.88 mg/L (estimated) |
4.76 (estimated) |
ECCC 2016b |
Abbreviations: NA, not available.
4. Sources and uses
None of the substances in the Pigments and Dyes Group occur naturally. These substances have been included in a survey issued pursuant to a CEPA section 71 notice (Canada 2012). No imports above the reporting threshold of 100 kg were reported for D&C Orange 5, Pigment Red 81, or CAS RN 26694-69-9 in the 2011 calendar year. Table 4‑1 presents a summary of the total import quantities for the remaining substances in the Pigments and Dyes Group. No manufacturing activities were reported above the reporting threshold.
Common name |
Total imports (kg) |
Reporting year |
|---|---|---|
Pigment Violet 1 |
3 005 |
2011 |
Acid Black 2 |
1 000 – 10 000 |
2011 |
Basic Red 29 |
100 – 1 000 |
2011 |
a Values reflect quantities reported in response to a survey conducted under CEPA section 71 (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
In Canada, as well as globally, substances in the Pigments and Dyes Group are used as colourants, spanning a variety of potential applications as outlined in Table 4‑2. Confirmed uses in Canada, as based on use codes reported by submitters through a survey issued pursuant to a CEPA section 71 notice, information provided to Health Canada, or other publicly available information, are indicated by a footnote.
Substance |
Uses |
Reference |
|---|---|---|
D&C Orange 5 |
Cosmeticsa,b (e.g., bath products, cleansers, face paint, lipstick, make-up, nail polish and tanning products), drugs, dye for paper, permanent make-up/tattoo ink,b textiles |
Ash and Ash c2013a, c2013b; CPSD 2016;c EWG c2007-2016; MSDS 2009a; US FDA 2015 |
Pigment Violet 1 |
Children’s arts and crafts,d food packaging materials,e inks |
CPMA 2016;f ECCC 2016c; Environment Canada 2013; FD 2016e |
Acid Black 2 |
Cosmeticsa (i.e., hair gel and eye make-up), food packaging materials,e inks, leather |
CPSD 2016;c ETAD 2016;h FD 2016;e Hunger 2003 |
Pigment Red 81 |
Children’s arts and crafts,a food packaging materials,e inks, paints and/or coatings, plastics, textiles |
Ash and Ash c2013a; CPMA 2016;f Dionisio et al. 2015; FD 2016;e MSDS 2007 |
CAS RN 26694-69-9 |
Children’s arts and crafts,g inks |
CPMA 2016;f MSDS 2009b |
Basic Red 29 |
Textiles |
ETAD 2016;h Hunger 2003 |
a Confirmed use in Canada based on publicly available information.
b According to notifications submitted under the Cosmetic Regulations to Health Canada, these substances are used in certain cosmetic products in Canada.
c Personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced.
d Non-confidential uses reported in response to the survey issued pursuant to a CEPA section 71notice (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
e Identified to be used in the manufacture of some food packaging materials in Canada (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced).
f Personal communication, email from Color Pigments Manufacturers Association, Inc., to Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced.
g Although direct evidence is limited, use in Canada is assumed based on publicly available information.
h Personal communication, email from Ecological and Toxicological Association of Dyers to Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced.
No uses were identified for substances in the Pigments and Dyes Group in pesticides, drugs, or natural health products, and none of the substances are permitted food additives in Canada, although D&C Orange 5 is a colouring agent permitted in drugs for external use (Canada [1978]; Health Canada [modified 2015]; LNHPD [modified 2016]; NHPID [modified 2017]; personal communication, emails from the Risk Management Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced; Pesticide Label Search [modified 2016]).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Pigments and Dyes Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, on the basis of weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (e.g., OECD QSAR Toolbox 2014), and responses to surveys issued pursuant to CEPA section 71 notices, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate, or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Pigments and Dyes Group, as well as the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for the substances in the Pigments and Dyes Group are summarized in Table 5-1.
Substance |
ERC hazard classification |
ERC exposure classification |
ERC risk classification |
|---|---|---|---|
D&C Orange 5 |
high |
low |
moderate |
Pigment Violet 1 |
high |
low |
moderate |
Acid Black 2 |
high |
low |
moderate |
Pigment Red 81 |
high |
low |
moderate |
CAS RN 26694-69-9 |
high |
low |
low |
Basic Red 29 |
high |
low |
low |
According to information considered under ERC, D&C Orange 5 was classified as having a low exposure potential. While D&C Orange 5 was profiled to have a moderate potential to cause adverse effects in aquatic food webs given its bioaccumulation potential, it was classified as having a high hazard potential due to structural alerts from the OECD (Q)SAR toolbox (OECD 2014), which identified this substance as being a potential endocrine receptor binder. D&C Orange 5 was classified as having a moderate potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, Pigment Violet 1 and Acid Black 2 were classified as having a low exposure potential, but also as having a high hazard potential based on the agreement between reactive mode of action and elevated toxicity ratio, both of which suggest that these chemicals are likely of high potency. Pigment Violet 1 and Acid Black 2 were profiled to have a high potential to cause adverse effects in aquatic and terrestrial food webs given their bioaccumulation potential and were classified as having a moderate potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of these substances. On the basis of current use patterns, these substances are unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, Pigment Red 81 was classified as having a low exposure potential, but also as having a high hazard potential on the basis of an elevated toxicity ratio and a high potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. Pigment Red 81 was classified as having a moderate potential for ecological risk. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, CAS RN 26694-69-9 and Basic Red 29 were classified as having low exposure potential. While CAS RN 26694-69-9 and Basic Red 29 were profiled to have moderate and high potentials, respectively, to cause adverse effects in aquatic food webs given their bioaccumulation potential, they were both classified as having high hazard potential on the basis of the agreement between reactive mode of action and high toxic ratio, both of which suggest that these chemicals are likely of high potency. CAS RN 26694-69-9 and Basic Red 29 were classified as having a moderate potential for ecological risk; however, the ecological risk classification was decreased to low potential following the adjustment of risk classification based on current use quantities (see section 7.1.1. of the ERC science approach document [ECCC 2016a]). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of these substances. On the basis of current use patterns, these substances are unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Substances in the Pigments and Dyes Group were not identified or measured in any environmental media in Canada or elsewhere. Overall, given the limited commercial quantities of these substances in Canada, their very low volatility, the very low to low water solubility of the pigments and some dyes, the dilution of the dye substances in aqueous media, and the expected removal by water treatment systems (mainly of pigments), exposure from environmental media is either not expected or is considered to be minimal for substances in the Pigments and Dyes Group.
In Canada, while there are no approved food additive uses of any of the substances in the Pigments and Dyes Group, some of them can be used in the manufacture of food packaging materials. However, there is no direct contact with the packaged foods. Therefore, exposure to substances in this group from food is not expected (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016–2017; unreferenced).
Exposures from use of products available to consumers were evaluated. Exposure estimates for uses that result in the highest levels of potential exposure for each substance by the oral and dermal routes are presented in Table 6-1 and 6-2, respectively. Potential exposures were estimated using conservative assumptions and default values, as presented in Appendix A.
Substance |
Product scenario |
Age group |
Per-event systemic exposure (mg/kg bw) |
Daily systemic exposure (mg/kg bw per day) |
|---|---|---|---|---|
D&C Orange 5 |
Lip gloss |
Child |
NA |
0.029 |
D&C Orange 5 |
Lipstick |
Adult |
NA |
0.034 |
D&C Orange 5 |
Face paint |
Toddler |
0.95 |
NA |
Pigment Violet 1 |
Crayonsa |
Toddler |
NA |
0.00076 |
Pigment Violet 1 |
Chalka |
Toddler |
NA |
0.000239 |
Pigment Red 81 |
Body art ink stamp |
Toddler |
0.048 |
NA |
CAS RN 26694-69-9 |
Paint pens |
Toddler |
0.419 |
0.021 |
Basic Red 29 |
Textiles, mouthing |
Infant |
NA |
0.0000013 |
Abbreviations: NA, not applicable
a The measured bioaccessible fraction of Basic Violet 10 from Pigment Violet 1 was used to characterize the potential risk to human health from exposure to Pigment Violet 1 in crayons (see Appendix A) as a refined approach, whereas it was conservatively assumed (i.e., as a first tier estimation) that the entire amount of Pigment Violet 1 present in chalk was bioaccessible as the component Basic Violet 10 (although the bioaccessibility of Basic Violet 10 is expected to be lower than 100% in chalk).
For estimated potential exposures via the dermal route, dermal absorption was conservatively assumed to be equivalent to absorption by the gastrointestinal tract for Acid Black 2 and Basic Red 29. On the basis of results in a skin absorption study conducted by the United States Food and Drug Administration (US FDA 1984), the dermal absorption of D&C Orange 5 ranged from 0.06% to 0.5% (depending on the vehicle). In this screening assessment, 0.5% was used in the estimation of potential dermal exposures to D&C Orange 5. No dermal absorption data were identified for CAS RN 26694-69-9. However, a dermal flux study by Gomaa et al. (2012) showed the skin absorption of structurally similar xanthene dyes (that were subsaturated in a pH 7.4 phosphate-buffered saline solution) to be low. In this study, the cumulative amount of Basic Violet 10 that permeated through a sample of full thickness porcine ear skin (1164 µm) over 48 hours was 0.54 (± 0.17) µg/cm2. Of the dyes studied by Gomaa et al., Basic Violet 10 is the most structurally similar to CAS RN 26694-69-9. However, the use of full thickness porcine skin could reduce dermal absorption because of the skin path length. When dermatomed human skin (330 µm) was used, which is considered to be a more conservative approach than using full thickness porcine skin, the cumulative amount of Rhodamine B isothiocyanate (CAS RN 36877-69-7) that permeated over 48 hours was 4.99 µg/cm2. It is assumed that the skin absorption of CAS RN 26694-69-9 is similar to that of Basic Violet 10 and Rhodamine B isothiocyanate, and dermal exposure was therefore estimated to be 0.54 to 4.99 µg/cm2.
Although dermal contact with Pigment Violet 1 and Pigment Red 81 is possible via use of certain children’s arts and craft products, absorption through the skin is not expected because these pigments are large complexes with molecular weights greater than 1000 g/mol. Furthermore, since these pigments are not readily soluble in water and since pigments in general are formulated to be insoluble in their intended matrix (Herbst and Hunger 2004), they cannot readily solubilize in perspiration and hence cannot readily penetrate intact skin (BfR 2007). Therefore, systemic exposure via the dermal route to Pigment Violet 1 and Pigment Red 81 is not expected.
Substance |
Product scenario |
Age group |
Per-event systemic exposure (mg/kg bw)a |
Daily systemic exposure (mg/kg bw per day)a |
|---|---|---|---|---|
D&C Orange 5 |
Face make-up |
Adult |
NA |
0.0048 |
D&C Orange 5 |
Face paint |
Toddler |
0.032 |
NA |
Acid Black 2 |
Hair gel |
Adult |
NA |
0.0016 |
CAS RN 26694-69-9 |
Paint pens |
Toddler |
0.0017 – 0.016 |
0.0017 – 0.016 |
Basic Red 29 |
Textiles, wearing clothing |
Infant |
NA |
0.0002 |
Basic Red 29 |
Textiles, wearing clothing |
Adult |
NA |
0.00013 |
Abbreviations: NA, not applicable
a Dermal absorption of Acid Black 2 and Basic Red 29 was conservatively assumed to be equivalent to absorption by the gastrointestinal tract. Dermal absorption of D&C Orange 5 was assumed to be 0.5%. Dermal exposure of CAS RN 26694-69-9 was estimated to be 0.54 to 4.99 µg/cm2.
The use of children’s chalk containing Pigment Violet 1 may also lead to inhalation exposure, but potential exposures via this route are considered to be low relative to oral exposures.
One permanent make-up/tattoo ink with 1% to 3% by weight D&C Orange 5 was reported in a notification submitted under the Cosmetic Regulations to Health Canada (email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced). Potential systemic exposure to D&C Orange 5 in tattoo inks is acknowledged, but not quantified since exposure to the general population to D&C Orange 5 from other uses is expected to be more prevalent.
6.2 Health effects assessment
Limited information is available on the toxicity of the substances in the Pigments and Dyes Group. Structural analogues were selected for hazard read-across for CAS RN 26694-69-9 and Acid Black 2, and the respective dye component of Pigment Violet 1 and Pigment Red 81 was used to inform the characterization of potential health effects.
6.2.1 Pigment Violet 1
Pigment Violet 1 was found to be not mutagenic with or without metabolic activation in an in vitro bacterial mutagenicity assay (Zeiger et al. 1987). A search for structural analogues with data on repeated-dose toxicity, including carcinogenicity, was conducted, and there were no suitable analogues from both a structural and physical chemical properties perspective. Available information indicates that Pigment Violet 1 can dissociate, releasing the component Basic Violet 10. This is consistent with the manner in which complex pigments like Pigment Violet 1 are manufactured (Herbst and Hunger 2004). Also, data from the Duke University Toxicology Program confirmed that a small amount of Basic Violet 10 would be bioaccessible in the gastrointestinal tract upon ingestion of a product containing Pigment Violet 1 (see Appendix A, footnote c for additional information; personal communication from the Duke University Toxicology Program to the Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced). Therefore, in the absence of substance-specific health effects data for Pigment Violet 1, the toxicity of Basic Violet 10 was considered relevant and has been used to inform the health effects characterization of Pigment Violet 1.
The available health effects information for Basic Violet 10 is summarized in assessments conducted by the European Food Safety Authority’s Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (EFSA 2005), the US FDA (Lipman 1995), the International Agency for Research on Cancer (IARC 1978), the American Federal Color Additive Scientific Review Panel (Hart et al. 1986), and the European Commission Scientific Committee on Cosmetology (SCC 1993).
Basic Violet 10 is extensively absorbed from the gastrointestinal tract and is extensively metabolized, with only 3% to 5% of the administered dose being recovered unchanged in urine and feces in dogs, cats and rabbits (IARC 1978).
No cancer effects were noted in chronic dietary studies in rats and dogs conducted in the 1950s and 1960s and summarized in Hart et al. (1986), but the results of three more recent rodent oral carcinogenicity studies submitted to the US EPA indicate a carcinogenic potential for Basic Violet 10 (purity 90–92%).
In the first study, CD rats (70/sex/group) were administered 0% or 0.075% of Basic Violet 10 in their diet for 29 months (corresponding to 43 and 56 mg/kg bw per day in males and females, respectively, based on the average compound consumption values provided in the study report). Basic Violet 10 was administered for 2 months prior to mating, and rats were thus exposed in utero (Bio/dynamics Inc. 1981a, 1981b; Hart et al. 1986; EFSA 2005). A significant decrease in body weight between weeks 6 to 38, an increase in food consumption, and an increase in absolute and relative organ weights (i.e., adrenals, thyroid, kidney, and liver) were observed in both sexes (Bio/dynamics Inc. 1981a, 1981b). A significant increase in follicular cell tumours of the thyroid in treated males (adenomas: 12/56 compared to 2/57 in controls; carcinoma: 5/56 compared to 1/57 in controls) was also observed (Bio/dynamics Inc. 1981b; Hart et al. 1986; EFSA 2005). In the second study, CD rats (70/sex/treatment group, 140/sex for controls) were administered lower doses of Basic Violet 10, i.e. 0%, 0.002%, 0.005% or 0.02% (0, 1, 3 or 11 mg/kg bw per day in males and 0, 1, 3 or 16 mg/kg bw per day in femalesFootnote 4), in their diet for 27 or 29 months (males and females, respectively) (Bio/dynamics Inc. 1980a, 1981c; Hart et al. 1996; EFSA 2005). No histopathological changes were observed in the thyroid. Decreased body weight in low- and high-dose females, increased organ weights (i.e., thyroid, liver, kidney, spleen, and adrenals) and food consumption in high-dose females, and increased mortality in high-dose males were observed. A no observed adverse effect level (NOAEL) for non-cancer effects of 3 mg/kg bw per day was selected for Pigment Violet 1 for risk characterization on the basis of increased mortality in males and increased organ weights and food consumption in females at the highest tested dose. A slight but significant increase in the incidence of astrocytomas of the brain and/or spinal cord in high-dose males (incidences were 1, 1, 0 and 5 for control, low-, mid- and high- dose, respectively) and of granular cell tumours of the brain in mid- and high-dose males (incidences were 0, 0, 2 and 2 for control, low-, mid- and high- dose, respectively) was noted (Bio/dynamics Inc. 1980a, 1981c).
In the third study, CD-1 mice (60/sex/treatment group, 120/sex for controls) were administered 0%, 0.005%, 0.02% or 0.10% (0, 9, 33 or 166 mg/kg bw per day in males and 0, 10, 40, 196 mg/kg bw per day in femalesFootnote 5) of Basic Violet 10 in their diet for 22 and 25 months for males and females, respectively (Bio/dynamics Inc. 1980b, 1981d; Hart et al. 1986; EFSA 2005). An increase in the total number of hepatocellular tumours (adenoma and carcinoma) was observed at all doses in both sexes, but only female mice showed a dose-dependent increase in hepatocellular carcinomas (0/115, 2/60, 5/60, 14/60). The increase in hepatocellular tumours in male mice is not expected to be significant given the lack of a dose-response, the absence of any treatment-related histopathology in liver (or any other tissue) except for an increase in the incidence of hepatocytomegaly in high-dose males (Bio/dynamics Inc. 1980b, 1981d), and the similar incidences of hepatocellular tumours observed in historical controls from other laboratories during a similar time period (Lang 1995).
Additionally, no increase in the incidence of neoplasia was observed in a limited skin painting study, where ICR mice were exposed to 9.3 mg/kg bw per dayFootnote 6 of Basic Violet 10 twice a week for 18 months (Carson 1984; Hart et al. 1986).
On the basis of the above-noted dietary studies showing an increased incidence of liver tumours in mice and thyroid tumours in rats, the US FDA considered Basic Violet 10 to be an animal carcinogen by the oral route (Lipman 1995). The European Commission Scientific Committee on Cosmetology (SCC) also considered there to be a potential health concern for this substance given the evidence of carcinogenicity in mice (SCC 1993). IARC has classified Basic Violet 10 as a Group 3 (not classifiable as to its carcinogenicity to humans) (IARC 1987). More recent reviews have challenged the human relevance of mouse hepatocellular carcinomas, in part due to the fact that hepatocellular carcinoma in humans, particularly chemically induced, is rare (Velazquez et al. 1996; Carmichael et al. 1997; EFSA 2011). While the relevance of the dose-dependent increase in hepatocellular carcinoma in female mice is uncertain, particularly in the absence of any associated treatment-related liver histopathology (Bio/dynamics Inc. 1980b, 1981d; Hart et al. 1986; EFSA 2005), astrocytomas of the brain and spinal cord were observed in male rats at lower doses (Bio/dynamics Inc. 1980a, 1981c), and a benchmark dose lower bound (BMDL10) of 10.2 mg/kg bw per day was derived for this tumour type (email from the Environmental Health Science and Research Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced).
Results of genotoxicity studies on Basic Violet 10 are mixed. The mutagenicity observed in some Ames assays was attributed to the presence of impurities in the test samples (Hart et al. 1986; EFSA 2005). Similarly, the positive genotoxicity results observed in some chromosomal aberration and DNA damage assays were attributed to impurities and colour phototoxicity (Hart et al. 1986). In an in vivo assay, an increase in wing spot frequency and induction of sex-linked recessive lethal mutations were observed in Drosophila and were interpreted as an indication of genotoxicity (Tripathy et al. 1995 as cited in EFSA 2005).
No adverse effects on reproductive performance were reported in rats administered Basic Violet 10 in the diet, and no skeletal or soft tissue abnormalities were observed in rats and rabbits administered the test substance (no doses reported) by gavage (Burnett et al. 1974; Pierce et al. 1974). Similarly, in the two rat studies described above, no developmental toxicity was reported when parental rats were treated with up to 0.075% (corresponding to 43 to 56 mg/kg bw per day) of Basic Violet 10 from 2 months prior to mating until the end of lactation (Bio/dynamics Inc. 1981b, 1981c). At a dose of 43 to 56 mg/kg bw per day, a decrease in litter size was observed, but only in the presence of decreased body weight in parental rats (Bio/dynamics Inc. 1981b).
6.2.2 Pigment Red 81 and CAS RN 26694-69-9
As with Pigment Violet 1, the health effects characterization for Pigment Red 81 is based on a component, whereas that for CAS RN 26694-69-9 is based on a structural analogue, which in both cases is Basic Red 1. In an in vitro bacterial mutagenicity assay, Pigment Red 81 was not mutagenic with or without metabolic activation (Zeiger et al. 1992). However, if Pigment Red 81 were to dissociate, it would result in the same organic moiety as Basic Red 1, and data from the Duke University Toxicology Program confirmed that a small amount of Basic Red 1 would be bioaccessible in the gastrointestinal tract upon ingestion of a product containing Pigment Red 81 (personal communication from Duke University Toxicology Program to Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced). The cationic organic dye moiety of Basic Red 1 is considered to be a component of Pigment Red 81, and in the absence of substance-specific hazard data for the pigment, the toxicity of Basic Red 1 has been used to inform the potential health effects of Pigment Red 81.
No health effects studies were found for CAS RN 26694-69-9. Basic Red 1 was selected as the most suitable analogue with hazard data for read-across to CAS RN 26694-69-9 on the basis of chemical structure (OECD QSAR Toolbox 2016) and was considered appropriate from a physical-chemical properties perspective as well. Basic Red 1 is also structurally more closely related to CAS RN 26694-69-9 than Basic Violet 10, one of the analogues used in the Exposure Assessment section of this report for dermal absorption given the lack of empirical dermal absorption data from which to conduct read-across based on Basic Red 1.
Overall, Basic Red 1 is considered genotoxic in in vitro genotoxicity assays. While, as in the case of Pigment Red 81 (Zeiger et al. 1992), the available information indicates that Basic Red 1 is not mutagenic in bacterial reverse mutation assays (Nestmann et al. 1979; Matula et al. 1982; Wuebbles and Felton 1985; Zeiger et al. 1987; NTP 1989), it is mutagenic in the mouse lymphoma assay without metabolic activation (NTP 1989). It is also positive for sister chromatid exchanges in cultured Chinese hamster ovary cells with metabolic activation, and mixed results were obtained for chromosomal aberrations in the same cells (Au and Hsu 1979; NTP 1989; Loveday et al. 1990).
The potential toxicity and carcinogenicity of Basic Red 1 (purity 97.4%) was investigated in F344/N rats and B6C3F1 mice (NTP1989). The animals were administered the test substance in their diet for 14 days, 13 weeks and 2 years.Footnote 7
Rats (5/sex/group) were administered 0, 310, 620, 1250, 2500 or 5000 ppm (corresponding to 0, 37, 76, 150, 400 or 750 mg/kg bw per day in males and 0, 47, 100, 209, 486 or 909 mg/kg bw per day in females) of Basic Red 1 in their diet for 14 days. A NOAEL of 76 to 100 mg/kg bw per day and a lowest observed adverse effect level (LOAEL) of 150 to 209 mg/kg bw per day were identified based on a decrease in body weight in both sexes. At higher doses, mortality, diarrhea, ruffled fur, decreased activity and uncoordinated gait were also observed. Decreased body weight was also observed in mice of both sexes at the highest dose tested when mice (5/sex/group) were administered 0, 310, 620, 1250, 2500 or 5000 ppm (corresponding to 0, 48, 100, 199, 418 or 870 mg/kg bw per day in males and 0, 66, 133, 263, 532 or 1081 mg/kg bw per day in females) of the test substance in their diet for 14 days. No substance-specific clinical signs were observed.
In the 13-week study, rats (10/sex/group) were administered 0, 120, 250, 500, 1000 or 2000 ppm (corresponding to 0, 9, 18, 40, 81 or 190 mg/kg bw per day in males and 0, 14, 28, 60, 123 or 268 mg/kg bw per day in females) of Basic Red 1 in their diet. A NOAEL of 18 to 28 mg/kg bw per day and a LOAEL of 40 to 60 mg/kg bw per day were identified on the basis of decreased body weight in males and a dose-dependent increase in the incidence and severity of bone marrow atrophy in both sexes. Similarly, mice (10/sex/group) were administered 0, 500, 1000, 2000, 4000 or 8000 ppm (corresponding to 0, 68, 135, 270, 600 or 1460 mg/kg bw per day in males and 0, 88, 182, 375, 775 or 1758 mg/kg bw per day in females) of Basic Red 1 in their diet for 13 weeks. Decreased body weight was observed in females at the LOAEL of 375 mg/kg bw per day. At higher doses, decreased body weight, minimal to moderate cytoplasmic vacuolization of hepatocytes, and mortality (1/10) were observed in male mice.
When rats (50/sex/group) were administered 0, 120 or 250 ppm (corresponding to 0, 5 or 10 mg/kg bw per day in males and 0, 5 or 12 mg/kg bw per day in females) of Basic Red 1 in their diet for 2 years, a marginal but significant increase in focal hyperplasia of the adrenal medulla was observed in females at the high dose (8%, 12%, 16%). At the high dose, there was also an increased incidence of neoplastic lesions in adrenal glands in females (pheochromocytoma [6%, 6%, 16%; historical control values of the laboratory 6% +/- 5%] and malignant pheochromocytomas [combined; 6%, 6%, 20%]) and in keratoacanthomas in males (2%, 4%, 16%; historical control values of the laboratory 3% +/- 5%). The relevance of these tumours is unclear; the epithelium and adrenal glands were not identified as target tissues in the 13-week study described above, and the tumours were observed in one sex only. On the basis of these effects, a NOAEL of 5 mg/kg bw per day and a LOAEL of 10 to 12 mg/kg bw per day were established.
Male and female mice (50/sex/group) were administered 0, 1000 or 2000 ppm (corresponding to 0, 210 or 440 mg/kg bw per day) and 0, 500 or 1000 ppm (corresponding to 0, 125 or 250 mg/kg bw per day), respectively, of Basic Red 1 in their diet for 103 weeks. A LOAEL of 125 mg/kg bw per day was established on the basis of decreased body weight gain in females after 35 weeks. No neoplastic effects were observed in male or female mice.
There was no evidence of carcinogenicity of Basic Red 1 in mice and only equivocal evidence of carcinogenicity in male and female rats (i.e., increases in the incidence of neoplastic lesions in the adrenal glands in females and of keratoacanthomas in males) (NTP 1989). Similarly, on the basis of an earlier evaluation, IARC has classified Basic Red 1 as Group 3—not classifiable as to its carcinogenicity to humans (IARC 1987).
6.2.3 Acid Black 2
On the basis of limited data, there is no evidence that Acid Black 2 is genotoxic or carcinogenic (Allmark et al. 1957; Au and Hsu 1979; Kubinski et al. 1981; CEC 1988; Yamaguchi 1988; BIBRA 1993). The lack of observed genotoxicity is further supported by the absence of genotoxicity in studies conducted on the structural analogue Solvent Black 5 (CAS RN 11099-03-9) (ETAD 1988; SNBL 2011a, 2011b, 2011c, 2011d; Zeiger et al. 1988). Although they are of different dye application classes, both are nigrosine dyes; Acid Black 2 is the water soluble form, while Solvent Black is the alcohol soluble form.
Although a repeated-dose study was identified for Acid Black 2, it was not considered in this assessment because of the lack of information on purity, the low number of animals, and the high mortality in all groups (Allmark et al. 1957). A combined repeated-dose and reproductive/developmental toxicity study on the structural analogue, Solvent Black 5, was therefore considered and is described below. No other repeated-dose study was identified.
In a combined repeated-dose toxicity study with a reproduction/developmental toxicity screening test, CD rats (12/sex/group) were administered 0, 40, 200 or 1000 mg/kg bw per day of Solvent Black 5 (purity unknown) by gavage for 42 days. Males were treated 14 days prior to mating, throughout mating until 1 day before necropsy, while females were treated 14 days prior to mating, throughout mating until day 4 of lactation. Satellite groups consisting of rats (5/sex) from the vehicle control and high-dose groups were subjected to a 14-day recovery period. Offspring were sacrificed on post-natal day (PND) 4. No treatment-related changes were observed in parents or in their offspring, and the highest dose tested (1000 mg/kg bw per day) was therefore selected for risk characterization (SNBL 2011e, 2011f).
6.2.4 D&C Orange 5
In a three-generation study, rats (10 males, 20 females/group) were exposed to four parallel control groups (one for each dose) or to 5, 50, 150 or 300 mg/kg bw/day of D&C Orange 5 (purity 93%) in their diet. The parental rats started treatment 2 weeks prior to mating and were mated twice to produce the F1a and F1b generations; they were sacrificed after the F1b rats were weaned. The F1a rats were sacrificed on PND 21, at the end of the lactation period, and the F1b rats were mated to produce the F2a, F2b and F2c generations. The F2a rats were sacrificed after weaning and the F2b rats were mated to produce the F3a generation. For the third mating, half of the F1b dams were sacrificed on gestation day 19 and the other half delivered their offspring and were sacrificed along with their offspring after weaning. F3a rats were also sacrificed after weaning. Given the absence of consistent, dose-dependent effects, the highest dose tested of 300 mg/kg bw per day was selected for risk characterization for systemic, reproductive and developmental toxicity (CTFA 1972a; Study Submission 1972).
Two other studies of shorter duration were identified (CTFA 1972b, c), but were found to be of poor quality (e.g., low number of animals, limited details, multiple parallel control groups, and lack of statistics) and were therefore not considered further.
Overall, D&C Orange 5 is not mutagenic in in vitro bacterial assays (Brown et al. 1979; Muzall and Cook 1979; Green and Pastewka 1979, 1980). Similarly, no increase in the incidence of neoplasia was observed in a limited skin painting study, where ICR mice were exposed to 9.2 mg/kg bw per dayFootnote 8 D&C Orange 5 twice a week for 18 months (Carson 1984). The US FDA also does not consider D&C Orange 5 to be carcinogenic on the basis of unpublished chronic studies in rats and mice (Lipman 1995).
6.2.5 Basic Red 29
Carcinogenicity and genotoxicity are generally considered to be the critical health effects of potential concern for aromatic azo substances (Environment Canada, Health Canada 2013), such as Basic Red 29. One of the primary mechanisms by which aromatic azo substances exert their toxicity involves the reductive cleavage of the azo bonds and the subsequent release of the free aromatic amines. These aromatic amines can be converted to reactive electrophilic intermediates through metabolic oxidation (Environment Canada, Health Canada 2013).
Although there are no in vivo or in vitro data to suggest that Basic Red 29 undergoes azo bond cleavage, it is considered that such a potential exists and is consistent with the approach taken by the Certain Azo Basic Dyes screening assessment (ECCC, HC 2016) and the body of knowledge available for most aromatic azo substances in general (Environment Canada, Health Canada 2013). No hazard data were identified for the expected azo metabolites of Basic Red 29, i.e., 1-methyl-2-phenyl-1H-indol-3-amine (CAS RN not found) and 2-amino-3-methyl-thiazol-3-ium chloride (CAS RN not found), for any of the analogues of the thiazole metabolite (i.e., CAS RNs 56010-23-2, 6149-13-9, or 652152-19-7) or for CAS RN 23747-09-3, an analogue of the indole metabolite. However, there are no indications of effects of concern, such as classifications of carcinogenicity or genotoxicity, by national or international agencies for the azo reductive cleavage products. In addition, Basic Red 29 is not mutagenic in Salmonella typhimurium strains TA 98 and TA 100 under reductive conditions (Cameron et al. 1987).
The health effects database for Basic Red 29 is limited to several in vitro mutagenicity studies (Cameron et al. 1985, 1987). In the Ames assay, Basic Red 29 was mutagenic in Salmonella typhimurium strain TA 1537, with or without metabolic activation (hamster or rat S9), but was not mutagenic in Salmonella typhimurium strains TA1535, TA 1538, TA98 or TA100, with or without metabolic activation (hamster or rat S9). Equivocal results were obtained in the mouse lymphoma assay, with or without metabolic activation (Cameron et al. 1985). While there is some indication that Basic Red 29 may be mutagenic in these assays, the purity of the substance was only 21% and the positive response could be the result of one or more contaminants (although the contaminants were not specifically identified). (Q)SAR models were used to supplement the empirical in vitro genotoxicity results, and their predictions were interpreted to be negative to equivocal (Derek Nexus 2016; Leadscope Model Applier 2016; TIMES 2016). Overall, on the basis of available information, Basic Red 29 is not considered to be genotoxic.
Since no other toxicity study was identified for Basic Red 29 and since some azo basic dyes have been reported to be absorbed systemically as the parent compound prior to azo bond cleavage (ECCC, HC 2016), structural analogues of Basic Red 29 were also considered, but no suitable analogue with empirical hazard data was identified.
6.3 Characterization of risk to human health
Pigment Violet 1 is found in a limited number of products available to consumers. Basic Violet 10, the component of Pigment Violet 1, was used to inform the characterization of health effects for Pigment Violet 1. The critical health effect for risk characterization is a small but significant increase in the incidence of astrocytomas of the brain and/or spinal cord in male rats (Bio/dynamics Inc. 1980a, 1981c). A BMDL10 for astrocytomas of the brain and spinal cord in male rats was therefore derived. This point of departure is protective of the increased incidence of liver tumours in mice and thyroid tumours in rats. The relevant estimates of exposure of Pigment Violet 1, the critical effect levels for Basic Violet 10, and the resulting margins of exposure (MOEs) are provided in Table 6-3.
Exposure scenario |
Systemic exposure |
Critical effect level (mg/kg bw per day)a |
Critical health effect endpointa |
MOE |
|---|---|---|---|---|
Crayons (daily, oral, toddler) |
0.00076 mg/kg bw per day |
BMDL10 = 10.2 |
Astrocytomas of the brain and spinal cord in male rats. |
13 400 |
Chalk (daily, oral, toddler) |
0.000239 mg/kg bw per day |
BMDL10 = 10.2 |
Astrocytomas of the brain and spinal cord in male rats. |
42 700 |
Crayons (daily, oral, toddler) |
0.00076 mg/kg bw per day |
NOAEL = 3 |
Increased mortality in male rats and increased organ weights and food consumption in female rats at 11 and 16 mg/kg bw per day, respectively. |
3 950 |
Chalk (daily, oral, toddler) |
0.000239 mg/kg bw per day |
NOAEL = 3 |
Increased mortality in male rats and increased organ weights and food consumption in female rats at 11 and 16 mg/kg bw per day, respectively. |
12 500 |
a Based on the health effects of the dye component Basic Violet 10.
As shown in Table 6-3, comparison of estimated exposures to Pigment Violet 1 with the range of critical effect levels results in MOEs ranging from 3 950 to 42 700. These MOEs are considered adequate to address uncertainties in the exposure and health effects databases.
For the other substances in the Pigments and Dyes Group, characterization of risk is based on non-cancer effects only. Basic Red 1 was selected as the most suitable analogue with hazard data for read-across to CAS RN 26694-69-9 and its toxicity was used to inform the potential health effects of Pigment Red 81 given that the cationic, organic dye moiety of Basic Red 1 is considered to be a component of Pigment Red 81. Similarly, based on the available limited empirical data, there is no evidence to indicate that Acid Black 2, its structural analogue Solvent Black 5, or D&C Orange 5 is genotoxic or carcinogenic.
Table 6-4 provides the relevant estimates of exposure and critical effect levels for Pigment Red 81, CAS RN 26694-69-9, Acid Black 2, and D&C Orange 5, as well as the resulting MOEs.
Substance |
Exposure scenario |
Systemic exposure |
Critical effect level |
MOE |
|---|---|---|---|---|
Pigment Red 81 |
Body art ink stamp (per event, oral, toddler) |
0.048 mg/kg bw |
NOAEL = 76 mg/kg bw per day based on decreased body weight in rats at 150-209 mg/kg bw per day in both sexes.a |
1 583 |
CAS RN 26694-69-9 |
Paint pens (per event, oral, toddler) |
0.419 mg/kg bw |
NOAEL = 76 mg/kg bw per day based on decreased body weight in rats at 150-209 mg/kg bw per day in both sexes.a |
181 |
CAS RN 26694-69-9 |
Paint pens (daily, oral, toddler) |
0.021 mg/kg bw per day |
NOAEL = 5 mg/kg bw per day based on an increase in focal hyperplasia of the adrenal medulla in female rats and neoplastic lesions in the adrenal glands of female rats and keratoacanthomas in male rats at 10-12 mg/kg bw per day.a |
238 |
CAS RN 26694-69-9 |
Paint pens (per event, dermal, toddler) |
0.0017 – 0.016 mg/kg bw |
NOAEL = 76 mg/kg bw per day based on decreased body weight in rats at 150-209 mg/kg bw per day in both sexes.a |
4 750 – 45 000 |
CAS RN 26694-69-9 |
Paint pens (daily, dermal, toddler) |
0.0017 – 0.016 mg/kg bw per day |
NOAEL = 5 mg/kg bw per day based on an increase in focal hyperplasia of the adrenal medulla in female rats and neoplastic lesions in the adrenal glands of female rats and keratoacanthomas in male rats at 10–12 mg/kg bw per day.a |
313 – 2 941 |
Acid Black 2 |
Hair gel (daily, dermal, adult) |
0.0016 mg/kg bw per day |
Highest dose tested (1 000 mg/kg bw per day) in a 42-day rat combined repeated-dose and reproductive/developmental screen study.b |
625 000 |
D&C Orange 5 |
Lip gloss (daily, oral, child) |
0.029 mg/kg bw per day |
Highest dose tested (300 mg/kg bw per day) in a rat three-generation study. |
10 000 |
D&C Orange 5 |
Lipstick (daily, oral, adult) |
0.034 mg/kg bw per day |
Highest dose tested (300 mg/kg bw per day) in a rat three-generation study. |
8 824 |
D&C Orange 5 |
Face make-up (daily, dermal, adult) |
0.0048 mg/kg bw per day |
Highest dose tested (300 mg/kg bw per day) in a rat three-generation study. |
63 000 |
D&C Orange 5 |
Face paint (per event, oral, toddler) |
0.95 mg/kg bw |
Highest dose tested (300 mg/kg bw per day) in a rat three-generation study. |
316 |
D&C Orange 5 |
Face paint (per event, dermal, toddler) |
0.032 mg/kg bw |
Highest dose tested (300 mg/kg bw per day) in a rat three-generation study. |
9 375 |
a Based on the health effects of the dye component or analogue Basic Red 1.
b Based on the health effects of the analogue Solvent Black 5.
As shown in Table 6-4, a comparison of estimated exposures to Pigment Red 81 and Acid Black 2 with the range of critical effect levels results in MOEs of 1 583 and 625 000, respectively, and a comparison of estimated exposures to CAS RN 26694-69-9 and D&C Orange 5 with the range of critical effect levels results in MOEs ranging from 181 to 45 000 and from 316 to 63 000, respectively. These MOEs are considered adequate to address uncertainties in the exposure and health effects databases.
For Basic Red 29, chemical-specific empirical hazard data were lacking, and no suitable structural analogue with hazard data was identified. The highest exposure to Basic Red 29 of 0.0002 mg/kg bw per day (0.2 µg/kg bw per day) was for infants from daily dermal exposure to textiles. Given the lack of hazard information and the low exposure estimates, the threshold of toxicological concern (TTC)-based approach was deemed to be relevant and was adopted accordingly (Health Canada 2016). The TTC value of 0.0015 mg/kg bw per day was assigned on the basis that the chemical structure of Basic Red 29 is a Cramer Class III substance (OECD QSAR Toolbox 2016) and given the overall negative genotoxicity. Therefore, as exposure was less than the TTC value, Basic Red 29 was not considered to be a concern for human health at current levels of exposure.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
Key sources of uncertainty |
Impact |
|---|---|
Oral exposures to Pigment Violet 1, which were estimated from incidental ingestion of crayons by toddlers, were based on a measured average concentration of extracted Basic Violet 10 dye from various art products. Oral exposures to Pigment Violet 1 and Pigment Red 81 from incidental ingestion of other craft products were conservatively based on assumed 100% bioaccessibility of the dye component, although the bioaccessibility of the dye components from these pigments is expected to be much lower. |
+ |
There is uncertainty in the estimated dermal exposure to CAS RN 26694-69-9 based on the skin absorption data from analogues, Basic Violet 10 and Rhodamine B isothiocyanate. However, the estimated exposure may be considered to be conservative since the cumulative amount of Basic Violet 10 and Rhodamine B isothiocyanate that permeated through skin over 48 hours was used to estimate systemic exposure from paint, which would typically remain on the skin for less time. |
+/- |
There are no or limited empirical hazard data for Pigment Violet 1, Pigment Red 81, CAS RN 26694-69-9, Acid Black 2 and Basic Red 29. |
+/- |
The structural analogues identified did not have a complete hazard dataset to cover off all relevant exposure durations and routes of exposure (e.g., no short-term dermal study for Basic Red 1 and no chronic dermal study for Solvent Black 5). |
+/- |
No suitable analogue with empirical hazard data was identified for Basic Red 29. |
- |
There is uncertainty associated with the use of available toxicological information on the analogues to characterize risk of CAS RN 26694-69-9 and Acid Black 2 to human health. |
+/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29. It is concluded that D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that D&C Orange 5, Pigment Violet 1, Acid Black 2, Pigment Red 81, CAS RN 26694-69-9, and Basic Red 29 do not meet any of the criteria set out in section 64 of CEPA.
References
Allmark MG, Mannell WA, Grice HC. 1957. Chronic toxicity studies on food colours, part III: observations on the toxicity of malachite green, new coccine and nigrosine in rats. J Pharm Pharmacol. 9(9):622-628.
Ash M, Ash I. c2013a. Handbook of paper and pulp chemicals. Synapse Information Resources, Inc.
Ash M, Ash I. c2013b. Handbook of textile processing chemicals. Synapse Information Resources, Inc.
Au W, Hsu TC. 1979. Studies on the clastogenic effects of biologic stains and dyes. Environ Mutagen. 1(1):27-35.
[BfR] Federal Institute for Risk Assessment (DE). 2007. Introduction to the problems surrounding garment textiles. BfR Information No. 018/2007, 1 June 2007. Available upon request.
Bibra [database]. 1993. Toxicity Profile: Nigrosine. First edition. TNO BIBRA International Ltd. [accessed 2011 Dec 21].
Bio/dynamics Inc. 1980a. A long-term toxicity/carcinogenicity study of D&C Red #19 in rats. Study No. 1100-26834. East Millstone (NJ): Bio/dynamics.
Bio/dynamics Inc. 1980b. A long-term feeding study of D&C Red #19 in mice. Study No. 1100-26836. East Millstone (NJ): Bio/dynamics.
Bio/dynamics Inc. 1981a. Long-term oral toxicity/carcinogenicity study of 0.075% D&C Red #19 in rats. Study No. 1100-26835. East Millstone (NJ): Bio/dynamics.
Bio/dynamics Inc. 1981b. Long-term oral toxicity/carcinogenicity study of 0.075% D&C Red #19 in rats. Volume 1. Project No. 78-2243. East Millstone (NJ): Bio/dynamics.
Bio/dynamics Inc. 1981c. Long-term oral toxicity/carcinogenicity study of D&C Red #19 in rats. Volume 1. Project No. 77-1880. East Millstone (NJ): Bio/dynamics.
Bio/dynamics Inc. 1981d. A long-term feeding study of D&C Red #19 in mice. Volume 1. Project No. 77-1881. East Millstone (NJ): Bio/dynamics.
Bremmer HJ, van Veen MP. 2002. Children’s toys fact sheet to assess the risk for the consumer. RIVM report 612810012/2002 [PDF] . RIVM report 612810012/2002.
Bremmer HJ, Prudhomme de Lodder LCH, van Engelen JGM. 2006. Cosmetics fact sheet. RIVM report 320104001/2006 [PDF].
Brown JP, Dietrich PS, Bakner CM. 1979. Mutagenicity testing of some drug and cosmetic dye lakes with the Salmonella/mammalian microsome assay. Mutat Res. 66(2):181-185.
Burnett CM, Agerborg HPK Jr, Birzelleca JF, Eagle E, Abert AG, Pierce EC, Kirschman JC, Scala RA. 1974. Teratogenic studies with certified colors in rat and rabbits. Toxicol Appl Pharmacol. 29(1):121.
Cameo Chemicals [database on the Internet] . 2017a. Version 2.7. Washington (DC): National Oceanic and Atmospheric Administration. [modified 2016 Sep; accessed 2017 Mar 23].
Cameo Chemicals [database on the Internet]. 2017b. Version 2.7. Washington (DC): National Oceanic and Atmospheric Administration. [modified 2016 Sep; accessed 2017 Mar 23].
Cameron TP, Hughes TJ, Kirby PE, Palmer KA, Fung VA, Dunkel VC. 1985. Mutagenic activity of 5 thiazole compounds in the Salmonella/microsome and mouse lymphoma TK+/- assays. Mutat Res. 155(1-2):17-25.
Cameron TP, Hughes TJ, Kirby PE, Fung VA, Dunkel VC. 1987. Mutagenic activity of 27 dyes and related chemicals in the Salmonella/microsome and mouse lymphoma TK +/- assays. Mutat Res. 189(3):223-261.
Canada. [1978]. Food and Drug Regulations. C.R.C., c. 870, s. C.01.040.2.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette, Part III, vol. 22, no. 3
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Carmichael NG, Enzmann H, Pate I, Waechter F. 1997. The significance of mouse liver tumor formation for carcinogenic risk assessment: results and conclusions from a survey of ten years of testing by the agrochemical industry. Environ Health Perspect. 105(11):1196-1203.
Carson S. 1984. Skin painting studies in mice with 14 FD&C and D&C colors: FD&C Blue No. 1, Red No. 3, and Yellow No. 5, D&C Red No. 7, Red No. 9. Red No. 10, Red No. 19, Red No. 21, Red No. 27, Red No. 31, Red No. 36, Orange No. 5, Orange No. 10, and Orange No. 17. J Toxicol Cutaneous Ocul Toxicol. 3(4):357-370.
Chemicalland21. 2016. Rhodamine blue. [accessed 2016 Mar 23].
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[CPSC] Consumer Product Safety Commission (US). 1994. Brief description of the CPSC staff hazard assessment procedure for lead in crayons. US Consumer Product Safety Commission.
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1972a. D&C Orange No. 5: Results of inclusion in the diet of rats for three generations. Unpublished report submitted to the US FDA (Washington, DC). Washington (DC): Cosmetic, Toiletry and Fragrance Association, Inter-Industry Color Committee. pp. 17325-17455. Report No.: CMF000009. [restricted access].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1972b. Safety evaluation of D&C Orange No. 5 in a teratology study in the rat. Unpublished reported submitted to the US FDA (Washington, DC). Washington (DC): Cosmetic, Toiletry and Fragrance Association, Inter-Industry Color Committee. pp. 13525-13555. Report No.: CMF000009. [restricted access].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1972c. Safety evaluation of D&C Orange No. 5 in a teratology study in the rabbit. Unpublished reported submitted to the US FDA (Washington, DC). Washington (DC): Cosmetic, Toiletry and Fragrance Association, Inter-Industry Color Committee. pp. 13556-13577. Report No.: CMF000009. [restricted access].
Derek Nexus [toxicity prediction module] 2016. Ver. 5.0.2. Leeds (UK): Lhasa Limited.. [restricted access].
Dionisio KL, Frame AM, Goldsmith MR, Wambaugh JF, Liddell A, Cathey T, Smith D, Vail J, Ernstoff AS, Fantke P, et al. 2015. Exploring consumer exposure pathways and patterns of use for chemicals in the environment. Toxicol Rep. 2:228-237.
[ECCC] Environment and Climate Change Canada. 2016a. Science Approach Document: Ecological Risk Classification of Organic Substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016c. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (June 2016). Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada [accessed 2017 Apr 26].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2016. Screening assessment. Aromatic azo and benzidine-based substance grouping. Certain azo basic dyes [PDF].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid Screening of Substances with Limited General Population Exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening Assessment: Substances Identified as Being of Low Concern using the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. Ottawa (ON): Government of Canada.
[EFSA] European Food Safety Authority. 2005. Review of the toxicology of a number of dyes illegally present in food in the EU [PDF]. Question number EFSA-2005-082. EFSA J. 263. 71 p.
[EFSA] European Food Safety Authority. 2011. Statement of EFSA on the scientific evaluation of two studies related to the safety of artificial sweeteners [PDF]. EFSA J. 9(2):2089. 16 p.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2013. The Chemicals Management Plan Substance Grouping Initiative. Subgrouping Approach and Background Information for the Screening Assessment of Aromatic Azo and Benzidine-Based Substances. May 2013. Environment Canada, Health Canada.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1983. Final report on extractability of dyestuffs from textiles. Basel (CH): ETAD. Project No. A 4007.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1988. Executive summary on the ETAD Project T 2015. Toxicological testing of major colorants, with attachments and cover letter dated 06/03/88. Basel (CH): ETAD. TSCA OTS0000621, Doc #FYI-AX-0688-0621.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model] c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[EWG] SkinDeep Cosmetics Database [database]. c2007-2016. Washington (DC): Environmental Working Group. [accessed 2016 Oct 12].
Gomaa YA, Garland MJ, McInnes FJ, Donnelly RF, El-Khordagui LK, Wilson CG. 2012. Flux of ionic dyes across microneedle-treated skin: effect of molecular characteristics. Int J Pharm. 438(1-2):140-149.
Green MR, Pastewka JV. 1979. Mutagenicity of some lipsticks and their dyes for Salmonella typhimurium TA98. Environ Mutagen. 1(2):116.
Green MR, Pastewka JV. 1980. Mutagenicity of some lipsticks and their dyes. J Natl Cancer Inst. 64(3):665-669.
Hansen P, Tønning K, Malmgren-Hansen B. 2008. Survey and health assessment of chemical substances in hobby products for children [PDF]. Danish Ministry of the Environment, Environmental Protection Agency (Danish EPA). Survey of Chemical Substances in Consumer Products, No. 93.
Hart RW, Freni SC, Gaylor DW, Gillette JR, Lowry LK, Ward JM, Weisburger EK, Lepore P, Turturro A. 1986. Final report of the Color Additive Scientific Review Panel. Risk Anal. 6(2):117-154.
Health Canada. 1994. Human Health Risk Assessment for Priority Substances [PDF]. Minister of Supply and Services Canada. Cat. No. En40-215/41E. [accessed 2016 Nov 25].
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Government of Canada.
Health Canada 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. [modified 2015 Oct 7]. List of colouring agents. Ottawa (ON): Government of Canada. [accessed 2016 Sep 27].
Health Canada. 2016. Science Approach Document: Threshold of Toxicological Concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada. [accessed 2017 May].
Herbst W, Hunger K. 2004. Industrial organic pigments: production, properties, applications. 3rd ed. Weinheim (DE): Wiley-VCH.
Hunger K. 2003. Industrial dyes: chemistry, properties, applications. Weinheim (DE): Wiley-VCH.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1987. Agents classified by the IARC monographs, volumes 1-116. IARC Monogr Eval Carcinog Risks Hum.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Man. 1978. Some aromatic amines and related nitro compounds: hair dyes, colouring agents and miscellaneous industrial chemicals [PDF]. IARC Monogr Eval Carcinog Risks Hum. Volume 16. ISBN 92-832-1216-9.
Kubinski H, Gutzke GE, Kubinski ZO. 1981. DNA-cell-binding (DCB) assay for suspected carcinogens and mutagens. Mutat Res. 89(2):95-136.
Laden P, editor. 1997. Chemistry and Technology of Water Based Inks. London (UK): Blackie Academic and Professional, an imprint of Chapman & Hall. p. 150.
Lang PL. 1995. Spontaneous neoplastic lesions in the Crl:CD-1® BR Mouse. Wilmington (MA): Charles River Laboratories.
Leadscope Model Applier [prediction module]. 2016. Ver. 2.1. Columbus (OH): Leadscope, Inc. [restricted access].
Lipman AL. 1995. Safety of xanthene dyes according to the U.S. Food and Drug Administration. In: Heitz JR, Downum KR, editors. Light-activated pest control. Vol. 616. Washington (DC): American Chemistry Society. p. 34.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2017 Apr 21].
LookChem. 2008. 4’,5’-Dibromofluorescein: CasNO. 596-03-2. Hangzhou (CN): LookChem.com. [accessed 2016 Mar 23].
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44(12):2008-2018.
Loveday KS, Anderson BE, Resnick MA, Zeiger E. 1990. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. V: results with 46 chemicals. Environ Mol Mutagen. 16(4):272-303.
Matula TI, Downie R, Butterfield AG, Nestmann ER. 1982. Mutagenicity of Rhodamine dyes B and 6G and their impurities in Salmonella and Saccharomyces cerevisiae. Environ Mutagen. 4(3):378-379.
[MSDS] Material Safety Data Sheet. 2007. Body art ink pad. Warren (NJ): Horizon Group. [accessed 2016 Oct 12]. Available upon request.
[MSDS] Material Safety Data Sheet. 2009a. Wax Based Make Up/Face Paint Crayon Products. Orlando (FL): Wolfe Brothers Face Art & FX. [accessed 2016 Oct 4]. Available upon request.
[MSDS] Material Safety Data Sheet. 2009b. Paint pens. Warren (NJ): Horizon Group [accessed 2016 Oct 12]. Available upon request.
[NCBI] National Center for Biotechnology Information. 2005. [modified 2016]. PubChem compound database [database on the Internet]. CID 5359556. [accessed 2016 Mar 29].
Nestmann ER, Douglas GR, Matula TI, Grant CE, Kowbel DJ. 1979. Mutagenic activity of rhodamine dyes and their impurities as detected by mutation induction in Salmonella and DNA damage in Chinese hamster ovary cells. Cancer Res. 39(11):4412-4417.
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. [modified 2017 Jan 10]. Ottawa (ON): Government of Canada. [accessed 2017 Apr 21].
[NTP] National Toxicology Program (US). 1989. Toxicology and carcinogenesis studies of Rhodamine 6G (C.I. Basic Red 1) (CAS No. 989-38-8) in F344/N rats and B6C3F1 mice (feed studies) [PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Report No.: TR-364. NIH Publication No.: 89-2819.
OECD QSAR Toolbox. 2014 Ver. 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox. 2016. Ver. 3.4. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Pesticide Label Search [database]. [modified 2016 Jan 25]. Ottawa (ON): Health Canada. [accessed 2015 Sep 30].
Pierce EC, Agersborg HPK Jr, Borzelleca JF, Burnett CM, Eagle E, Ebert AG, Kirschman JC, Scala RA. 1974. Multi-generation reproduction studies with certified colors in rats. Toxicol Appl Pharmacol. 29(1):121-122.
[SCC] Scientific Committee on Cosmetology. 1993. CI 45170. Reports of the Scientific Committee on Cosmetology concerning the use of certain colourants in cosmetic products. Prepared by the 45th reunion of the SCC; Oct 2, 1990. Luxembourg (LU): Commission of the European Communities. p. 498-503.
[SCCS] Scientific Committee on Consumer Safety. 2012. The SCCS’s notes of guidance for the testing of cosmetic substances and their safety evaluation, 8th revision [PDF].
[SNBL] Shin Nippon Biomedical Laboratories Ltd. 2011a. Reverse mutation test of C.I. Solvent Black 5 in bacteria. Tokyo (JP): Shin Nippon Biomedical Laboratories. Study No.: SBL807-015. 35 p.
[SNBL] Shin Nippon Biomedical Laboratories Ltd. 2011b. C.I. sorubento burakku 5 no saikin o mochiiru kaifuku hen'i shiken = Reverse mutation test of C.I. Solvent Black 5 in bacteria. Tokyo (JP): Shin Nippon Biomedical Laboratories. 9 p.
[SNBL] Shin Nippon Biomedical Laboratories Ltd. 2011c. In vitro chromosomal aberration test of C.I. Solvent Black 5 in cultured mammalian cells. Tokyo (JP): Shin Nippon Biomedical Laboratories. Study No.: SBL807-010. 40 p.
[SNBL] Shin Nippon Biomedical Laboratories Ltd. 2011d. C.I. sorubento burakku 5 no chainīzu hamusutā baiyō saibō o mochiiru senshokutai hen'i shiken = In vitro chromosomal aberration test of C.I. Solvent Black 5 in cultured Chinese hamster cells. Tokyo (JP): Shin Nippon Biomedical Laboratories. 10 p.
[SNBL] Shin Nippon Biomedical Laboratories Ltd. 2011e. Combined oral repeated dose and reproductive/developmental toxicity test of C.I. Solvent Black 5. Tokyo (JP): Shin Nippon Biomedical Laboratories. Study No.: SBL807-006. 360 p.
[SNBL] Shin Nippon Biomedical Laboratories Ltd. 2011f. C.I. sorubento burakku 5 no ratto o mochiiru hanpuku keikō tōyo dokusei seishoku hassei dokusei heigō shiken = Combined repeated dose and reproductive/developmental toxicity screening test of C.I. Solvent Black 5 by oral administration in rats. Tokyo (JP): Shin Nippon Biomedical Laboratories. 18 p.
Study Submission. 1972. A multi-generation study in rats. Unpublished report submitted to the US FDA (Washington, DC). pp. 6312-6319. Report No.: CAP8C0061. [restricted access].
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2016. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry. [restricted access].
[US EPA] US Environmental Protection Agency. 2008. Child-Specific Exposure Factors Handbook. EPA/600/R-06/096F.
[US EPA] US Environmental Protection Agency. 2012. Standard operating procedures for residential exposure assessments [PDF]. Washington (DC): US EPA, Office of Chemical Safety and Pollution Prevention, Office of Pesticide Programs, Health Effects Division.
[US FDA] US Food and Drug Agency. 1984. Rules and Regulations: D & C Orange No. 5. Federal Register Vol. 49, No. 66, pp. 13339-13343.
[US FDA] US Food and Drug Administration. 2015. Color Additive Status List. Silver Springs (MD): U.S. Food and Drug Administration.
Velazquez SF, Schoeny R, Rice GE, Cogliano VJ. 1996. Cancer risk assessment: historical perspectives, current issues, and future directions. Drug Chem Toxicol. 19(3):161-185.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48(11):3109-3119.
Wuebbles BJY, Felton JS. 1985. Evaluation of laser dye mutagenicity using the Ames/Salmonella microsome test. Environ Mutagen. 7(4):511-522.
Yamaguchi T. 1988. Adsorption of carcinogenic and/or mutagenic pigments on DNA-binding Sepharose. Agric Biol Chem. 52(3):845-847.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. 1987. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 9(Suppl 9):1-110.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1988. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen. 11(Suppl 12):1-158.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen. 19(Suppl 21):2-141.
Zeilmaker MJ, Kroese ED, van Haperen P, van Veen MP, Bremmer HJ, van Kranen HJ, Wouters MFA, Janus JA. 1999. Cancer risk assessment of azo dyes and aromatic amines from garment and footwear. Bilthoven (NL): Rijkinstituut voor Volkgezondheid en Milieu [National Institute of Public Health and the Environment]. RIVM Report No.: 601503014.
Zeilmaker MJ, van Kranen HJ, van Veen MP, Janus JA. 2000. Cancer risk assessment of azo dyes and aromatic amines from tattoo bands, folders of paper, toys, bed clothes, watch straps and ink. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute of Public Health and the Environment]. RIVM Report No.: 601503019. 45 p.
Appendix A. Estimated potential human exposures to substances in the Pigments and Dyes Group
Sentinel exposure scenarios were used to estimate the potential human exposure to substances in the Pigments and Dyes Group; scenario assumptions are summarized in Table A-1. Exposures were estimated using ConsExpo version 4.1 or algorithms from the model (ConsExpo 2006), unless noted otherwise. Dermal absorption was assumed to be 100% unless noted otherwise.
Substance |
Sentinel exposure scenario |
Assumptionsa |
|---|---|---|
D&C Orange 5 |
Lip gloss |
Concentration: £10% (email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced) Age group: Child Body weight: 31.0 kg Frequency: 0.89/day (Wu et al. 2010; conservatively assuming same frequency of use as lip balm)Product amount: 0.01 g/application (Loretz et al. 2005) |
D&C Orange 5 |
Lipstick |
Concentration: £10% (email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced)Age group: Adult Body weight: 70.9 kg Frequency: 2.4/day (Loretz et al. 2005) Product amount: 0.01 g/application (Loretz et al. 2005) |
D&C Orange 5 |
Face make-up |
Concentration: £10% (email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced) Age group: Adult Body weight: 70.9 kg Frequency: 1.24/day (Loretz et al. 2006) Product amount: 0.54 g/application (Loretz et al. 2006)Dermal absorption: 0.5% (US FDA 1984) |
D&C Orange 5
|
Face paint |
Concentration: £7% (MSDS 2009a) For Estimated Per-Event Dermal Exposure: For Estimated Per-Event Oral Exposure: |
Pigment Violet 1 |
Crayonsb |
Concentration (Conc): 0.0205% for Pigment Violet 1c Estimated Daily Oral Exposure |
Pigment Violet 1 |
Chalk |
Concentration (Conc): 0.04% (ECCC 2016c) Estimated Daily Oral Exposure |
Acid Black 2 |
Hair gel |
Concentration: £0.1% (email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced) Age groups: Adult Body weight: 70.9 kg Frequency: 0.59/day (Bremmer et al. 2006)Product amount: 1.9 g/application (Bremmer et al. 2006) Retention factor (transfer factor from hair to scalp): 0.1 (SCCS 2012) |
Pigment Red 81 |
Body art ink stamp |
Concentration (Conc): 30% (MSDS 2007) Estimated Per-Event Oral Exposuree |
CAS RN 26694-69-9 |
Paint pens |
Age group: Toddlerd For Estimated Per-Event Oral Exposure For Estimated Daily Oral Exposure: |
Basic Red 29 |
Textiles, mouthing |
Concentration (Conc): 1% (BfR 2007) Estimated Daily Oral Exposure |
Basic Red 29 |
Textiles, wearing clothes |
Concentration (Conc): 1% (BfR 2007) Estimated Daily Dermal Exposure |
a The age ranges for an infant, a toddler, a child, and an adult were assumed to be newborn to 6 months, 0.5 to 4 years, 5 to 11 years, and 20 to 59 years, respectively. Default body weights were obtained from Health Canada 1998.
b Scenario was adapted from the US Consumer Product Safety Commission assessment on lead (CPSC 1994).
c The concentration used to estimate exposure to Pigment Violet 1 from the ingestion of crayons was derived from the measured bioaccessibility of the dye component, Basic Violet 10. The reported average content of Basic Violet 10 extracted from various art material products containing Pigment Violet 1 was 205 ppm, which was provided by the Duke University Toxicology Program (personal communication from Duke University Toxicology Program to Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced). The use of this concentration in this exposure estimate is considered to be conservative, as the reported concentration of Pigment Violet 1 in a crayon of 1.4% (ECCC 2016c) is at the lower end of the range of use-levels reported by ACMI (1% to 16%; same reference as above) and as the bioaccessibility of Pigment Violet 1 embedded within a crayon’s wax matrix is generally expected to be lower compared to other art and crafts materials.
d This age group is considered to be the most highly exposed through the use of markers or pens, particularly in terms of mouthing (US EPA 2008).
e Oral exposure resulting from hand-to-mouth and object-to-mouth ingestion of ink from a body art ink pad is conservatively assumed to be the same as that from a felt marker. The ingestion of ink from a body art ink stamp is limited to exposure on a per-event basis, as chronic/daily use is not expected. However, exposure is estimated using cumulative exposure to ink over the course of 8 hours that is representative of chronic/daily oral (or dermal) exposures to marker ink, during which it is presumed that an individual will absorb 25 cm of ink line/day. This estimate is considered to be sufficiently high to cover off potential per-event oral exposures from a body art ink pad. The 90th percentile level for ink laydown of writing instruments of 100 µg/cm was used (personal communication from the Art and Creative Materials Institute (ACMI), Duke University, to Existing Substances Risk Assessment Bureau, Health Canada, 2009; unreferenced).
f The consistency (i.e., density) of paint in paint pens (may also be referred to as paint markers) is estimated to be similar to that of felt marker ink, as opposed to acrylic paint that is applied with a brush.
g Oral exposure resulting from hand-to-mouth and object-to-mouth ingestion of ink from a paint marker is conservatively assumed to be the same as that from a felt marker. Exposure is estimated using cumulative exposure to ink over the course of 8 hours that is representative of chronic/daily oral (or dermal) exposures to marker ink, during which it is presumed that an individual will absorb 25 cm of line/day. The 90th percentile level for ink laydown of writing instruments of 100 µg/cm was used (personal communication from the Art and Creative Materials Institute (ACMI), Duke University, to Existing Substances Risk Assessment Bureau, Health Canada, 2009; unreferenced). Absorption of 100% is assumed.
h The area weight of textile corresponds to “all synthetics”, which is representative of acrylic fibres. Basic Dyes (such as Basic Red 29) are most commonly used for dyeing polyacrylonitrile (i.e., acrylic fibres), modified nylons, and modified polyesters (Hunger 2003), although historically they were used to dye many different fibres
i The migration of dyes from textiles varies considerably depending on the type of fibre, type of dye used, dye load, dyeing technology, colour intensity and after treatment process. The exposure from textiles is partly dictated by the amount of dye that migrates from textile material onto human skin (ETAD 1983) or via mouthing. The “Textiles” Working Group (BfR 2007) uses a peak initial migration of 0.5% to estimate exposure to dyes from newly bought unwashed garments, and the chronic migration rate is assumed to be one tenth of the value measured for the first migration to reflect exposure after initial washes. It is assumed that the sweat migration rate is similar to the salivary migration rate. This is consistent with observations of leaching behaviours of dyes from textiles reported by Zeilmaker et al. (1999). Accordingly, the fraction of dye that migrates from a textile material per day was assumed to be 0.0005 for estimating both dermal and oral exposures on a chronic basis.
j The assumed combined adjustment factor to account for market penetration rate and the likelihood of wearing fabric coloured with a red dye on a given day of 0.1 (10%) is considered to be conservative. The market penetration rate of any particular textile dye in the Canadian marketplace is relatively low (e.g., given the number of potential dyes available), and in the case of Basic Red 29, the total reported DSL IU import volume of 100 to 1000 kg for the 2011 reporting year was considered to be low (Environment Canada 2013). The portion of this 10% fraction attributed to the assumed likelihood of wearing fabric coloured with a red dye on a given day is based on professional judgement.
