Human health risk characterization document for the assessment of triphenyl phosphate (TPHP) and tris(2-butoxyethyl) phosphate (TBOEP)
Official title: Human Health Risk Characterization Document for the Assessment of Triphenyl phosphate (TPHP) and Tris(2-butoxyethyl) phosphate (TBOEP)
Chemical Abstracts Service Registry Numbers:
- 78-51-3
- 115-86-6
Health Canada
November 2025
Synopsis
A Draft Screening Assessment for the Flame Retardants Group was published on November 6, 2021. This current document contains additional information to support the assessment of phosphoric acid, triphenyl ester (CAS RNFootnote 1 115-86-6) and ethanol, 2-butoxy-, phosphate (3:1) (CAS RN 78-51-3), hereinafter referred to as TPHP and TBOEP, respectively, which are 2 of the 10 substances in the Flame Retardants Group. Data identified or generated since the publication of the draft assessment are included herein.
The scope of this risk characterization document is limited to assessing potential human health concerns with respect to TPHP and TBOEP. For TPHP, significant new critical health effects were identified and exposures were re-examined. For TBOEP, updated exposure parameters used to estimate dermal intakes to substances found in foam-containing mattresses or upholstered furniture and infant or child restraint systems were incorporated. This document contains an updated characterization and an updated draft conclusion of the human health risks associated with exposure to TPHP and TBOEP. The public has the opportunity to comment on data and analysis included herein prior to it being considered in the finalization of the assessment of TPHP and TBOEP, and, if appropriate, the corresponding risk management approach document.
In Canada, TPHP and TBOEP are primarily used as either additive flame retardants or plasticizers in various applications including paints and coatings (TPHP and TBOEP), foam (TPHP and TBOEP), plastics and rubber products (TPHP), lubricants and greases (TPHP), adhesives and sealants (TPHP), and floor coverings (TBOEP). These substances are also used in food packaging applications. TPHP is also used as a formulant in pest control products and in nail care products in Canada.
Significant new information on the critical effects for TPHP were identified through the scientific literature since the publication of the draft assessment resulting in a change in the critical endpoint used to characterize risk. The critical effects associated with exposure to TPHP are developmental effects. People living in Canada may be exposed to TPHP from dust, soil, indoor air, drinking water, food, human milk, and the use of products available to consumers, including nail care products, lubricants and greases, foam-containing mattresses or furniture, and infant and child restraint systems (including booster seats). Children may also be exposed from mouthing foam toys or other products available to consumers containing TPHP. For TPHP, the margins of exposure associated with environmental media, food, and mouthing of foam objects are considered adequate to account for uncertainties in the exposure and health effects data used to characterize risk. However, the margins for dermal exposure to nail care products, lubricants and greases, as well as for prolonged skin contact from lying on foam-containing mattresses or upholstered furniture or from sitting in infant or child restraint systems (0 to 13 years), are considered potentially inadequate to account for uncertainties in the exposure and health effects data used to characterize risk.
For TBOEP, no significant changes were identified for the health effects (liver effects in males), the exposures to environmental media and food, or the exposures to products available to consumers (for example, rust paint). Updates to exposure parameters used to estimate dermal exposures while lying on foam-containing mattresses or upholstered furniture and sitting in an infant or child restraint system (including boosters) were incorporated. As a result, the calculated margins associated with prolonged skin contact to TBOEP from lying on foam-containing mattresses or upholstered furniture (all age groups) and sitting in infant or child restraint systems (0 to 13 years) are considered potentially inadequate to account for uncertainties in the exposure and health effects data used to characterize risk.
The human health assessment took into consideration those groups of individuals living in Canada who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. The potential for increased susceptibility during reproduction and development were assessed and age-specific exposure estimates were derived. Generally, infants and children were found to have higher exposure than adults. All of these populations were taken into consideration while assessing the potential harm to human health.
Considering all the information presented in this document, it is proposed to conclude that TPHP and TBOEP meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health conduct assessments of substances to determine whether they present or may present a risk to the environment or to human health.
The Draft Screening Assessment of the Flame Retardants Group was published in November 2021, hereinafter referred to as the draft assessment (ECCC, HC 2021). It proposed that TPHP was harmful to the environment but not for human health. It proposed that TBOEP was not harmful to human health and not harmful for the environment. Since the publication of that assessment, significant new critical human health effects were identified for TPHP resulting in the re-examination of exposures. For TBOEP, updated exposure parameters used to estimate dermal intakes to substances found in the foam-containing mattresses or upholstered furniture, and infant or child restraint systems (including booster seats) were incorporated. This current document contains an updated characterization and an updated draft conclusion of the human health risk associated with exposure to TPHP and TBOEP to inform the Flame Retardants Group assessment.
The scope of this document is limited to assessing potential human health concerns for TPHP and TBOEP. The data and analysis herein provide the opportunity for public comment on the updated risk characterization prior to it being considered in the finalization of the assessment of TPHP and TBOEP, and if appropriate, the corresponding risk management approach document. No significant changes to the ecological assessment of TPHP and TBOEP were identified from the publication of the updated draft assessment (ECCC, HC 2021) that warrant further public consultation.
This document includes data identified or generated since November 2021, when the draft assessment was published. Targeted literature searches were conducted up to September 2023 for the human health component of the assessment. More recent studies or information provided via internal and external peer consultation for human health components may also be cited. Empirical data from key studies, as well as some results from models were used to reach the proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
TPHP and TBOEP have been identified in vaping products, also known as electronic cigarettes (Wei et al. 2020). The assessment of risk to the general population from this use, including risk relative to that associated with conventional cigarettes, and possible options to mitigate risk associated with these products are being addressed through a separate legislative framework (HC [modified 2024a,b]).
This additional document was prepared by staff in the Existing Substances Program at Health Canada and incorporates input from other programs within this department. This document has undergone external written peer review or consultation. Comments on the technical portions relevant to human health were received from Tetra Tech Inc. Additionally, the draft assessment (published November 6, 2021) was subject to a 60-day public comment period. On the basis of these comments as well as new information identified through the scientific literature and updated exposure parameters, an amendment of the draft human health risk characterization is presented here. While external comments were taken into consideration, the final content of the document remain the responsibility of Health Canada.
Assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by considering scientific information, including information, if available, on subpopulations who may have greater susceptibility or greater exposure, vulnerable environments and cumulative effectsFootnote 2, and by incorporating a weight of evidence approach and precautionFootnote 3. This assessment presents the critical information and considerations on which the conclusions are based.
2. Assessment of TPHP
2.1 Identity of substance
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) name, common name and abbreviations for TPHP are presented in Table 2‑1. Information on the identity of the substances and their components is presented below.
| CAS RN (abbreviation) | DSL name (common name) | Representative chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 115-86-6 (TPHP) | phosphoric acid, triphenyl ester (triphenyl phosphate) | 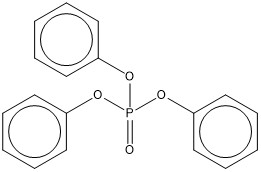 C18H15O4P |
326.29 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
2.2 Physical and chemical properties
summary of experimental and modelled physical and chemical properties of TPHP is provided in the draft assessment (ECCC, HC 2021).
2.3 Sources and uses
Details on the many sources and uses of TPHP are provided in the draft assessment of the Flame Retardants Group (ECCC, HC 2021). A summary of key data is provided below.
TPHP does not occur naturally in the environment.
TPHP, as an organophosphate flame retardant, is most commonly used as a flame retardant in electronics, lubricants, plastics, rubbers, resins, textiles, elastomers, adhesives and sealants. However, it is also commonly used as a plasticizer in many of the same applications (US EPA 2020a, 2020b). As a plasticizer, TPHP is applied to improve the flexibility and durability of certain materials, such as polyvinylchloride (PVC), flexible and rigid polyurethane foams (PUF) and thermoplastic materials (Marklund 2005). TPHP has also been identified in infant clothing and textiles/fabrics in the U.S. (Zhu et al. 2020).
TPHP was included in surveys issued pursuant to section 71 of CEPA (Canada 2012, 2016). According to information submitted to these surveys, TPHP was not manufactured in Canada in 2011 and 2015; however, a total of 100,000 kg to 10,000,000 kg of TPHP was imported into Canada in each of 2011 and 2015 (Environment Canada 2013; ECCC 2016)Footnote 4. The extent to which the reported values represent quantities present in manufactured goods entering Canada from other parts of the world is unknown, as these uses would be unlikely to meet the reporting criteria for these surveys. Data collected from 2021 indicated that import quantities for TPHP may be slightly lower than the reported quantities from section 71 and voluntary follow-up surveys (ECCC 2022).
In Canada, according to information submitted in response to CEPA section 71 surveys (ECCC 2016; Environment Canada 2013), TPHP is primarily used as either an additive flame retardant and/or as a plasticizer in products available to consumers and commercial products, such as adhesives and sealants, paints and coatings, lubricants and greases, and in plastic and rubber formulation. TPHP has been detected in foam-containing products available to consumers in Canada such as mattresses, upholstered furniture and children’s products including infant and child restraint seats (CEC 2015; Health Canada 2019).
In Canada, TPHP may be used in food packaging as a component in the manufacture of certain printing inks that may be applied on the outside layer of laminated plastic structures for food packaging applications (personal communication, e-mail from the Food and Nutrition Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 28, 2018; unreferenced). TPHP may also be used in the formulations of lubricants; these will not have contact with food and thus there are no potential exposures to these substances from this use (personal communication, e-mail from the Food and Nutrition Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 20, 2022; unreferenced).
On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, TPHP is used in cosmetics in Canada, primarily nail care products (personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 16, 2022; unreferenced).
In Canada, TPHP can also be used as a formulant in pest control products and is currently present in 2 registered domestic class pest control products, both of which are anti-fouling paints with marine applications (personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 18, 2022; unreferenced).
2.4 Potential to cause harm to human health
2.4.1 Exposure assessment of TPHP
2.4.1.1 Environmental media and food
There were no significant changes from exposure to TPHP in ambient and indoor air, drinking water, soil and dust as described in section 2.7.1.1 of the draft assessment (ECCC, HC 2021). Appendix A contains the data used from the various media to estimate daily intake for various age groups. Additional data on concentrations of TPHP measured in food and in human milk were identified and summarized below.
In Canada, TPHP has been identified as an additive in a small number of printing inks that may be applied on the outside layer of laminated plastic structures for food packaging applications. Potential exposure to TPHP from food packaging uses, for which there is no direct contact with food, is expected to be negligible. Any contribution to total dietary exposure from these uses would be accounted for in the occurrence data for processed foods that were employed in the assessment (personal communication, e-mail from the Food and Nutrition Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated Feb. 2, 2023; unreferenced).
As a result of various anthropogenic uses, TPHP can enter into the environment and has been found at generally low levels in foods. Overall, very little Canadian occurrence data for TPHP in foods are available. There are some data for infant foods from the Canadian Food Inspection Agency’s Children’s Food Project Survey (CFIA 2019-2021), however, these are quite limited (n=13). Therefore, data employed in the dietary exposure assessment were predominantly from a U.S. study (Wang and Kannan 2018), and, to a lesser extent, from studies conducted in Australia (He et al. 2018), Belgium (Poma et al. 2018), China (Zhao et al. 2019a), and Sweden (Poma et al. 2017) (Appendix B, Table B-1). The maximum TPHP concentrations in foods and beverages were conservatively used to estimate exposures in the assessment and are presented in Appendix B (Appendix B, Table B-1). Mean “all person” exposures to TPHP across all age groups ranged from 0.021 to 0.64 µg/kg bw/day (Appendix B, Table B-2).
There are no Canadian data for TPHP in human milk, but TPHP has been detected in human milk in the U.S., Sweden, Spain, Japan, Vietnam, China and the Philippines (Beser et al. 2020; Chen et al. 2021; Kim et al. 2014; Ma et al. 2019; Sundkvist et al. 2010; Zheng et al. 2021) (Appendix C, Table C‑1). The U.S. studies are considered most relevant to the general population of Canada, so the maximum value of 0.760 ng/ml from the Ma et al. (2019) study was conservatively used to estimate exposure to human milk-fed infants aged 0 to 5 months (0.094 µg/kg bw/day) and 6 to 11 months (0.053 µg/kg bw/day; Appendix A, Table A-2).
There are no Canadian data for TPHP in infant formula, but TPHP has been detected in formula samples purchased and analyzed in China in 2 studies (Chen et al. 2022; Zhou et al. 2022). The Chen et al. (2022) study analyzed 75 powdered infant formula samples that were purchased in March 2021 from supermarkets in Beijing. Several of the formulas purchased were imported from Europe and New Zealand (53 of 75). Of the 75 formulas tested, 25 were considered “stage 1 formulas” that were meant for 0 to 6 month olds while 25 each were also tested for stage 2 (7 to 12 month olds) and stage 3 formulas (13 to 36 month olds). TPHP was detected in 100% of the samples for all stages (n=75). Means of 19.53 ng/g, 24.07 ng/g, and 23.71 ng/g TPHP in infant stage 1, stage 2, and stage 3 formulas, respectively, were reported (Chen et al. 2022).TPHP levels in the Zhou et al. (2022) study (reported under the acronym TPPA) for the 54 infant formula samples tested fell within the range of TPHP concentrations reported in the Chen et al. (2022) study, with lower means and a detection frequency of 85.2%. The means for infant stage 1 and stage 2 formulas reported in Chen et al. (2022) were therefore used to estimate daily intakes of TPHP by formula-fed 0 to 5 and 6 to 11 month olds.
Estimates of total exposure for TPHP from environmental media and food for the general population of Canada ranged from 0.079 µg/kg bw/day for those aged 14 to 18 years to 1.2 µg/kg bw/day for formula-fed infants aged 6 to 11 months (Appendix A).
2.4.1.2 Products available to consumers
Cosmetics
On the basis of notifications submitted under the Cosmetic Regulations to Health Canada, TPHP is used in various nail care products in Canada such as base coats, top coats, and nail polish, and nail kits with concentrations ranging from <0.1% to 30% (personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 16, 2022; unreferenced). The function of TPHP in these products is as a plasticizer (Mendelsohn et al. 2016). Given that TPHP has a low vapour pressure (1.68 x 10-4 Pa), the primary route of exposure is considered to be through the skin (Estill et al. 2021; Mendelsohn et al. 2016). This is supported by studies that measured air concentrations of TPHP in nail salons (Estill et al. 2021; Nguyen et al. 2022) that resulted in estimates of inhalation exposure (data not shown) that are orders of magnitude lower than those presented below (Table 2‑2) for dermal exposure. Therefore, only dermal estimates are presented.
Limited dermal absorption data were identified for TPHP in 2 infinite dose studies that included several organophosphate ester flame retardants (Frederiksen et al. 2018; Zhang et al. 2022). Exposures to TPHP from products and manufactured items discussed herein are all finite dose scenarios to which infinite dose studies have limited applicability. Furthermore, these studies do not incorporate all pertinent skin bound residues into reported permeability coefficients and may underestimate exposure by the dermal route. The Scientific Committee on Consumer Safety (SCCS) also acknowledged the lack of relevant dermal absorption data for TPHP in their draft “Opinion on Triphenyl phosphate” and stated that a default dermal absorption of 50% would therefore be used in their exposure estimates (SCCS 2024). The same approach has been taken herein.
Table 2‑2 summarizes the dermal doses for nail care products containing TPHP that are available to consumers. Given that the base coat, top coat, and nail polish products may be used together, the estimated dermal exposures for the 3 products were aggregated for individuals 14 years and older. Dermal exposure estimates are presented for the nail kit alone and an aggregate exposure of the kit with 2 coats of nail polish. Details on the method and parameters used to estimate external dermal exposures to TPHP from nail care products are available in Appendix D, Table D-1.
| Age group | Base coatb | Nail polish | Top coatb | Aggregated (base, top, nail polish) total | Nail kitc | Nail kit with polishc,d |
|---|---|---|---|---|---|---|
| 19+ years | 0.027 | 0.16 | 0.047 | 0.24 | 0.16 | 0.24 |
| 14 to 18 years | 0.032 | 0.19 | 0.056 | 0.28 | 0.19 | 0.29 |
| 9 to 13 years | N/A | 0.28 | N/A | N/A | 0.28 | 0.43 |
| 4 to 8 years | N/A | 0.20 | N/A | N/A | N/A | N/A |
Abbreviations: N/A, not applicable
a Dermal absorption of 50%.
b Product not anticipated to be used by children younger than 14 years old.
c Product not anticipated to be used by children younger than 9 years old.
d Aggregated estimate of TPHP exposure from nail kit, and 2 coats of nail polish on fingernails (for example for adults: 0.16 [nail kit exposure] + (0.16/2) [2 coats of polish on fingernails only] = 0.24 mg/kg bw per event).
Other products
In Canada, TPHP was reported as an ingredient in lubricants and greases available to consumers (for example, power steering fluid, engine oils, synthetic greases) with concentrations ranging from 0.1% to 1% (Environment Canada 2013; SDS 2017; SDS 2021; US EPA 2020b). Dermal exposures were estimated for adult users and ranged from 4.2×10-4 to 4.2×10-3 mg/kg-bw/event (see Appendix D for more details).
Manufactured items
TPHP is one of several flame retardants that is incorporated into flexible PUF during foam production (Marklund 2005). TPHP is commonly found in commercial flame retardant mixtures, one of which is Firemaster 550 (or FM550) (McGee et al. 2013; Phillips et al. 2017). Some studies report the level of FM550 as a sum of TPHP and the 2 brominated substances (benzoic acid, 2,3,4,5-tetrabromo-, 2-ethylhexyl ester or TBB and 1,2-benzenedicarboxylic acid, 3,4,5,6-tetrabromo-, bis(2-ethylhexyl) ester or TBPH) that make up most of this blend (Stapleton et al. 2012), while others used an authentic standard of FM550 for its quantification (Cooper et al. 2016). TPHP has been detected in foam-containing products available to consumers in Canada, including furniture such as couches, and children’s products such as nap mats, baby slings, baby mattresses, infant and child restraint systems, changing table pads, portable mattresses, and rocking chairs (CEH 2013a,b; CEC 2015; Cooper et al. 2016; Danish EPA 2015; Health Canada 2019; Stapleton et al. 2011; Rodgers et al. 2021). Table 2‑3 summarizes available information on the concentration of TPHP measured in various products.
| Manufactured item(s) | Concentration of TPHP | Reference |
|---|---|---|
| Foam from child restraint systems (n=10), other children’s products (n=10), upholstered furniture (n=10), foam mattresses (n=10), mattress toppers (n=9), and pillows (n=1) purchased in Canada | Not detected (LOQ = 0.0012%) to 1.8% w/w (that is, detected above LOQ in PUF foam from 4 child restraint systems, 4 other children’s products, 1 article of upholstered furniture, 3 foam mattresses) | Health Canada 2019 |
| Children’s products including foam and fabric samples (for example, foam chairs, pajamas, nap mat, mattress topper, blanket, crib sheets, toys, foam tiles, crib mattresses), (n=111 samples from 41 products) purchased in Canada | Not detected above the LOD (0.0004% w/w) | Health Canada 2023a |
| Building material and foam pillows (for example, flooring, wallpaper, carpet, ceiling tiles, blinds, nursing pillow, toddler pillow) (n=38 samples from 23 products) purchased in Canada | 0.007% (blind) 0.017% (wallpaper) Detected in 1 blind and 1 wallpaper sample; LOD = 0.0004% w/w |
Health Canada 2023b |
| Foam from children’s products (n=27 samples from 19 products) purchased in Canada | 0.0056% w/w (bath book) Detected in 1 sample; LOD = 0.0004% w/w |
Health Canada 2023c |
| Foam, fabric, upholstery, padding, and stuffing of various furniture products (n=132; for example, chairs, sofas, ottoman) purchased in 2014 to 2015 in Canada, the U.S. and Mexico | 0.02% to 1.15% w/w (reported as 200 to 11,500 ppm) TPHP detected in 82% of foam samples, 9% of fabric samples, and 18% of “other” samples (from 11 products) TPHP was detected in 5%, 0.1% and 1% of products purchased in Canada, Mexico and the U.S., respectively |
CEC 2015 |
| Child restraint systems (n=387 material samples from 15 systems/seats) purchased in U.S. | (reported as 11,000 ppm) Detected in the foam of 1 child restraint system |
Miller and Gearhart 2016 |
| Child restraint systems (n = 36 samples including foam, fabric and composites of foam and fabric, 18 systems/seats) purchased in the U.S. in 2018 | Not detected (LOD = 0.0002%) to 0.041% TPHP was detected in 72% samples |
Wu et al. 2019 |
Abbreviations: LOD, limit of detection; LOQ, limit of quantification
Prolonged dermal exposure to TPHP from lying on foam-containing mattresses or upholstered furniture and sitting in an infant or child restraint system (including boosters seats) containing TPHP are described in Table 2‑4. Details for the scenario are described in Appendix D.
TPHP has also been identified in toys made of plastic, rubber, wood, foam and textiles in Antwerp, Belgium (Ionas et al. 2014) and in various children’s products in the U.S. (Stapleton et al. 2011). It is expected that some of the same types of children’s products as those found to contain TPHP in Europe and the U.S. would be present in Canada. Therefore, estimates of exposure to TPHP via mouthing were also derived (Table 2‑4) as described in Appendix D.
A study by Davis et al. 2021, evaluated chairs with different fire-resistant technologies for flame retardant exposures via inhalation, ingestion, and dermal contact exposure routes; for the scenario of one chair in a living room with an average ventilation, inhalation was predicted to be the route resulting in the least flame retardant exposure, accounting for less than 1% of the total average daily dose for TPHP. As such, exposures via inhalation were not derived.
| Exposure route | Source | Age group | Exposure estimate (mg/kg bw/day) |
|---|---|---|---|
| Dermal | Lying on foam-containing mattress or upholstered furniture | 0 to 5 months | 1.3×10-2 to 8.0×10-2 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 6 to 11 months | 1.1×10-2 to 7.1×10-2 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 1 year old | 1.1×10-2 to 7.1×10-2 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 2 to 3 years old | 6.8×10-3 to 5.8×10-2 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 4 to 8 years old | 5.5×10-3 to 4.9×10-2 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 9 to 13 years old | 4.2×10-3 to 3.9×10-2 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 14 to 18 years old | 3.0×10-3 to 2.9×10-2 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 19+ years old | 2.7×10-3 to 2.3×10-2 |
| Dermal | Foam/fabric in infant and child restraint systems | 0 to 5 months | 3.0×10-3 to 5.6×10-3 |
| Dermal | Foam/fabric in infant and child restraint systems | 6 to 11 months | 2.7×10-3 to 5.0×10-3 |
| Dermal | Foam/fabric in infant and child restraint systems | 1 year old | 2.5×10-3 to 4.7×10-3 |
| Dermal | Foam/fabric in infant and child restraint systems | 2 to 3 years old | 1.7×10-3 to 3.2×10-3 |
| Dermal | Foam/fabric in infant and child restraint systems | 4 to 8 years old | 1.5×10-3 to 2.7×10-3 |
| Dermal | Foam/fabric in infant and child restraint systems | 9 to 13 years oldb | 1.2×10-3 to 2.2×10-3 |
| Oral | Foam in children’s products | 0 to 5 months | 1.6×10-4 to 1.5×10-3 |
| Oral | Foam in children’s products | 6 to 11 months | 1.1×10-4 to 1.0×10-3 |
| Oral | Foam in children’s products | 1 year old | 9.1×10-5 to 8.4×10-4 |
| Oral | Foam in children’s products | 2 to 3 years old | 6.7×10-5 to 6.2×10-4 |
a Dermal absorption was assumed to be 50%.
b Infant and child restraint system regulations in Canada vary by province and territory (CPSAC 2019). On the basis of relevant age and weight considerations, dermal exposure estimates were derived for various age groups up to and including 9 to 13 year olds.
TPHP was not detected above the limit of detection (LOD = 0.0004% w/w or 4000 ng/g) in fabrics tested from children’s products including pajamas purchased in Canada (Health Canada 2023a; see Table 2‑3 ). TPHP was measured in various textiles purchased in the US, including infant clothing (for example, socks, bodysuits, pants, trousers, skirts, shirts) and raw fabrics (that is, fabrics available prior to sewing marketed for various applications including curtains, cushions, toss pillows and patio furniture) (Zhu et al. 2020). The method detection limit (MDL) in the Zhu et al. (2020) study was lower than that of the Health Canada (2023a) study, at 0.24 ng/g. Concentrations of TPHP in conventional textiles tested by Zhu et al. (2020), which included infant clothing (n=52) and raw fabrics (n=29) made of cotton, polyester, or nylon, ranged from <0.8 ng/g (method quantification limit or MQL) to 3350 ng/g. Estimated dermal exposures to TPHP derived in Zhu et al. (2020) for infants from clothing based on the 95th percentile concentration ranged from 2860 to 3560 pg/kg bw/day.
2.4.1.3 Biomonitoring
Diphenyl phosphate (DPHP), a metabolite of TPHP as well as other organophosphate flame retardants (Kosarac et al. 2016; He et al. 2018; Bjornsdotter et al. 2018), has been measured in urine in numerous human biomonitoring studies in Canada, the U.S. and elsewhere, including the Canadian Health Measures Survey (CHMS), a study from Maternal-Infant Research on Environmental Chemicals (MIREC), and the US National Health and Nutrition Examination Survey (NHANES) (NHANES 2011-2018; Butt et al. 2014, 2016; Fromme et al. 2014; Cequier et al. 2015; Kosarac et al. 2016; Thomas et al. 2016; Hoffman et al. 2017; Yang et al. 2017; He et al. 2018; Ospina et al. 2018; Phillips et al. 2018; Wang et al. 2019; Hammel et al. 2020; Siddique et al. 2020; Schoeters et al. 2022; Siddique et al. 2022; Health Canada 2023d; Ashley-Martin et al. 2023). DPHP has also been measured in serum (Li et al. 2017; Ma et al. 2017), hair and nails (Alves et al. 2017; Liu et al. 2016).
Given DPHP is a metabolite for several OPFRs and may also be used itself in various industrial applications, the origin of the compound in urine is unknown (Kosarac et al. 2016; Bjornsdotter et al. 2018). In addition to DPHP not being a unique biomarker for TPHP, there is insufficient information on the kinetics and metabolism of TPHP to derive exposure estimates (see section 2.4.2.1). A more specific biomarker for TPHP has been identified by Su et al. (2016) and Zhao (2019b); however, more information and measured levels of this metabolite in humans are required before it can be used to estimate human exposures.
2.4.1.4 Consideration of subpopulations who may have greater exposure
There are groups of individuals living in Canada who, due to greater exposure, may be more vulnerable to experiencing adverse health effects from exposure to substances. The potential for elevated exposure within the Canadian population was examined. Exposure estimates are routinely assessed by age to take into consideration physical and behavioural differences during different stages of life. In the assessment of background exposure from environmental media, indoor air, food, drinking water, dust and soil, young children had higher exposures than adults. Human-milk fed infants had higher exposure than formula-fed infants and adults. In addition, infants and children also had higher exposure to TPHP from manufactured items (for example, foam-containing furniture or mattresses), as compared to adults. In the assessment of exposure to TPHP in products available to consumers, products used by children that were assessed included foam in toys and children’s products and nail polish. The potential for elevated exposure to TPHP by people who frequent nail salons was also considered, including evaluation of studies performed in nail salons in Canada (Nguyen et al. 2022) and the USA (Estill et al. 2021; Mendelsohn et al. 2016).
2.4.2 Health effects assessment of TPHP
TPHP has been assessed by ATSDR (2012) and OECD (2002). Targeted literature searches were conducted from a year prior to the publication of the draft assessment to February 2023. Health effects studies that could impact the risk characterization (that is, result in different critical endpoints or lower points of departure than those stated in ECCC, HC [2021]) were identified.
A safety assessment of TPHP as used in cosmetics was also available (CIR 2018).
2.4.2.1 Toxicokinetics
Triphenyl phosphate (TPHP) is degraded by hydrolysis in rat liver homogenate to DPHP as the major metabolite (OECD 2002). Additional metabolites have been identified through in vitro exposure of human liver microsomes, which converted TPHP to the diester (DPHP) and mono- and dehydroxylated metabolites through cytochrome P450 action (Zhang et al. 2018). A study by Selmi-Ruby et al. (2020) suggested that DPHP may not be the primary metabolite of TPHP after administration by gavage or drinking water. The dermal uptake and percutaneous penetration of TPHP was studied using human skin in Franz diffusion cells. TPHP tended to build up in the skin tissues, primarily in the upper layers. Only “smaller amounts” of TPHP permeated the skin and reached the receptor fluid within 72 hours (Frederiksen et al. 2018). TPHP has the potential to be absorbed dermally following cosmetic application in humans (Mendelsohn et al. 2016, as cited in CIR 2018). A mean of 41% TPHP is retained in the lungs when inhaled at a flow rate of 18 L/minute. This retention rate is increased with increasing particle size and flow rate (Landhal et al. 1951, 1952, as cited in ATSDR 2012).
Multiple possible routes of elimination in humans have been indicated for TPHP. The renal clearance rate of TPHP was estimated to be 68.9 mL/kg/day through comparison of the paired human plasma and urine samples of TPHP and DPHP (diester metabolite of TPHP), respectively, from 30 people in China. The hepatic clearance of TPHP in humans was extrapolated from in vitro human liver microsome clearance, resulting in an estimated value of 166 ml/kg/day. As the clearance from the liver was higher than the kidney for TPHP, the authors suggest other methods of elimination are possible. Clearance of TPHP could also be affected by binding with plasma proteins, as the binding affinity for TPHP was 97.4% (Wang et al. 2020).
From paired maternal and cord whole blood samples from pregnant women in China, TPHP was found to have a transplacental transfer efficiency value of 1.06 (cord blood to maternal blood concentration ratio). From this ratio and the log Kow (4.70), the authors consider that passive diffusion is the main mode of transfer across the placenta, but that active transport may also play a role (Wang et al. 2021).
2.4.2.2 Carcinogenicity and genetic toxicity
No carcinogenicity studies were identified for TPHP. TPHP is not considered to be genotoxic on the basis of negative evidence of mutagenicity in in vitro tests with Salmonella typhimurium, with and without metabolic activation (ATSDR 2012).
2.4.2.3 Repeated dose toxicity
Two 4-month oral studies were available. Rats were exposed to TPHP at doses of 0, 161, 345, 517 or 711 mg/kg bw/day in diet. A statistically significant reduction in growth weight was detected at levels of 345 mg/kg bw/day and higher in male rats (only sex tested in this study) in Sobotka et al. (1986, as cited in OECD 2002). This change was only observed at 711 mg/kg bw/day in Hinton et al. (1987, as cited in OECD 2002). However, only limited data are reported and a number of standard parameters of repeated dose toxicity are missing, such as organ weight measurement and histopathology of organs other than lymphoid organs (spleen, thymus, lymph nodes) as well as hematology and clinical chemistry other than serum proteins.
In a 90-day oral study, according to OECD guideline 408, Wistar rats were fed TPHP in diet at doses of 0, 20, 105 and 583 mg/kg bw/day for males and 0, 22, 117 and 632 mg/kg bw/day for females (ECHA c2007-2018). Treatment-related increases in liver weight in the high dose groups (approximately 30% and 21% for males and females, respectively) were considered adverse in nature, although no supportive adverse histopathological changes were noted in the liver. The authors noted a lowest observed effect level (LOEL) for this study was 105 mg/kg bw/day based on liver weight increase at the next dose level of 583 mg/kg-bw/day.
In a short-term oral study, according to OECD guideline 407, Wistar rats were exposed in the diet to TPHP at doses of 0, 23.5, 161.4 or 701 mg/kg bw/day for 28 days (ECHA c2007-2018). In males, decreased body weight gain was observed at 161.4 mg/kg bw/day. At 701 mg/kg bw/day, there was an increase in food consumption in both males and females. A statistically significant increase in liver weights was also observed at 701 mg/kg bw/day in both sexes. Males displayed a higher frequency of enlarged livers. This trend correlates with the occurrence of slight hypertrophy/cytoplasmic change of periportal hepatocytes observed in males at 161.4 mg/kg bw/day, compared to females at 701 mg/kg bw/day. Changes in the liver marked by minimal to slight hypertrophy/cytoplasmic change of periportal hepatocytes and hepatocytes in the periportal and partly midzonal areas showed a swollen eosinophillic appearance with a homogenous dust like granulated cytoplasm were also observed at 701 mg/kg bw/day in females. The no observed adverse effect level (NOAEL) was established to be 161.4 mg/kg bw/day, on the basis of decrease in body weight gain and liver effects at the next tested dose. In another study, only a slight depression of body weight gain and an increase of liver weights at a level of 350 mg/kg bw/day were observed after 35 days of treatment (Sutton et al. 1960, as cited in OECD 2002).
In a non-guideline short-term toxicity study conducted by the National Toxicology Program (NTP), groups of male rats (Sprague-Dawley; 5 per group) were administered TPHP via gavage at doses of 0, 55, 110, 220, 441 and 881 mg/kg bw/day once a day for 4 consecutive days (NTP 2018). Animals were sacrificed one day after their final exposure. Clinical observations, body weight, organ weight and clinical chemistry were evaluated. Transcriptomic changes in the liver were also evaluated in the study and it was noted that further testing at lower doses would be needed to refine estimates of the transcriptional point of departure. All animals survived through the study. No differences in incidence of clinical observations between treated and control animals were noted. The most sensitive apical endpoints for which Benchmark dose (BMD) values could be obtained were serum HDL (high-density lipoprotein) cholesterol levels, absolute liver weights, relative liver weights, and serum cholesterol levels. The benchmark dose lower confidence limit BMDL1SD (that is, one standard deviation increase over the background) (and BMD) were 39 (79), 48 (136), 71 (103), and 90 (142) mg/kg, respectively. Although serum cholinesterase appeared to be a sensitive endpoint (35 to 70% decrease) at all doses, beginning with 55 mg/kg (the lowest-observed-effect level), its BMD could not be determined due to poor model fit.
In a non-guideline study, kidney and gut microflora were evaluated with and without a high fructose and fat diet and TPHP. The results of TPHP exposure alone are presented in this assessment. Groups of 8 male mice (C57BL/6J) were fed a control diet, 0.01 mg/kg bw/day TPHP in diet and 1 mg/kg bw/day TPHP in diet for 12 weeks. At both doses of TPHP, mice were observed to have gut microbiota disorders. Significant inflammation based on increased cytokine levels was reported starting at 0.01 mg/kg bw/day. Structural damage in the kidney was noted in the 1 mg/kg bw/day group (vacuolation of the kidney tubular epithelial cells, glomerular atrophy, cytosolic vacuole formation in the cortex), in addition to increased urine total protein and urine protein/creatinine levels, which indicate kidney damage. In addition, kidney tissue fibrosis significantly increased at this dose level (Cui et al. 2020). Given that these kidney effects were not seen in the 2 available guideline studies conducted in rats (ECHA c2007-2018), the results of this study (Cui et al. 2020) were not further considered for risk characterization.
In a non-guideline study, intestinal health was investigated in a 28-day study in BALB/c mice. Groups of 10 mice/sex/group were orally gavaged with corn oil (control) or TPHP (dissolved in corn oil) at 2, 10, or 50 mg/kg bw/day (Peng et al. 2023). At 2 mg/kg bw/day, mice of both sexes were observed to have shortened colon length, infiltration of inflammatory cells in the colon and decreased villus length in the ileum, crypt deformation in the ileum, appearance of vacuoles in the ileum, and infiltration of immune cells in the ileum.
In a non-guideline study, effects on mouse liver were evaluated with and without a high fructose and fat diet and TPHP (Cui et al, 2022). The results of TPHP exposure alone are presented in this assessment. Groups of 15 male mice (C57BL/6J) were fed a control diet, 0.01 mg/kg bw/day and 1 mg/kg bw/day TPHP in diet for 12 weeks. At both doses of TPHP, mice were observed to have liver histopathological damage including squeezing and narrowing of liver sinusoids, edema and degeneration of liver cells, cytoplasmic looseness, vacuole formation, and inflammatory infiltration, compared with the control group. In addition, at both doses of TPHP, there were significant levels of chromatin condensation of the nuclei, cytoplasm sparsity, mitochondrial expansion, endoplasmic reticulum dispersion, and a reduction in cytoplasmic organelles in the liver as well as increased levels of alanine aminotransferase (ALT/GPT) and aspartate aminotransferase (AST/GOT) in serum as compared to the control group. At 1 mg/kg bw/day TPHP, there was a significant increase in liver lipid accumulation compared with the control group. An oral glucose tolerance test and an insulin tolerance test were conducted in the last week of exposure and mice treated with TPHP were shown to experience an aberrant glucose recovery mechanism as compared with control (Cui et al, 2022).
In a short-term dermal study, rabbits were exposed to TPHP on clipped and intact or abraded skin at doses of 0, 100 or 1000 mg/kg bw/day for 3 weeks. No effect on body weight, hematology and clinical chemistry, organ weights or histopathology was observed. The only treatment-related effect was a dose-related depression (only statistically significant at 1000 mg/kg bw/day) of acetyl cholinesterase in plasma, erythrocytes and the brain of the TPHP treated rabbits, with no clinical signs of increased cholinergic activity. No effects were observed in the reproductive organs of rabbits administered TPHP up to the highest dose of 1000 mg/kg bw/day. The NOAEL was considered to be 1000 mg/kg bw/day (Monsanto 1979, as cited in OECD 2002).
2.4.2.4 Reproductive/developmental toxicity
No developmental or reproductive effects were observed in a non-guideline 4-month oral fertility and developmental study in rats up to doses of 690 mg/kg bw/day (Welsh et al. 1987, as cited in OECD 2002).
In a uterotrophic assay, immature female mice and rats (17 days old) were found to have no increased uterine weight when TPHP was administered by subcutaneous injection at doses of 0, 200 or 600 mg/kg TPHP once daily for 3 consecutive days (Wang et al. 2018).
In a non-guideline study, pregnant C57BL/6J mice (n =11-14) were orally gavaged with corn oil (control) or TPHP (dissolved in corn oil) at 1 or 5 mg/kg bw/day from embryonic day 0 (E0) that is gestational day 0 to delivery (Hong et al 2022a). At E18, 5 dams from each group were randomly selected to evaluate the accumulation of TPHP/DPHP and effects of TPHP on placenta. The authors only reported results for the mice exposed to 1 mg/kg bw/day and noted that TPHP and DPHP accumulated in the placenta and this accumulation was not seen in control mice. The remaining dams continued to be dosed until natural delivery (n = 6–9). There were no significant differences observed in maternal food consumption or body weight between control and treatment groups. There were no significant differences in the number of offspring, offspring weight or body length between control and treatment groups. However, the number of live offspring per litter decreased in both TPHP exposed groups as compared to the control group. The gestation period for mice exposed to 5 mg/kg bw/day was significantly shorter as compared with both the control and the 1 mg/kg bw/day exposed mice. The still birth rate in 1 mg/kg bw/day exposed mice was significantly higher as compared to the control group. However, there was no significant difference in still birth rate for mice exposed to 5 mg/kg bw/day TPHP as compared to the control group (Hong et al 2022a).
In a non-guideline study, pregnant C57BL/6J mice were orally gavaged with corn oil (control) or TPHP (dissolved in corn oil) at 1 mg/kg bw/day from gestational day 0 to postnatal day 21 (PND21) (Liu et al. 2023). At PND 56, serum, liver, ileum and colon were collected from 6 pups from each group for investigation. Compared to the control group, the birth weight of offspring in the TPHP group was not significantly different, but in lactation, the weight of offspring in the TPHP group was significantly lower than that in the control group. There was no significant difference in body weight between the 2 groups after weaning when offspring began to consume standard feed. In male offspring, tissue necrosis in colon and ileum, as well as congestion of capillaries in the ileum were observed. In female offspring, tissue necrosis in ileum, and lymph node hyperplasia and increased alkaline mucus secretion in colon were observed. In male offspring, mass lipid droplets and inflammation were observed in the liver, and liver weight was significantly increased and lipid accumulation was observed in male offspring of TPHP exposed mice. In addition, there was a significant increase in total cholesterol, total triglycerides and total bile acid in the serum of male offspring only. The study also noted that TPHP exposed mice had disturbances in gut microbiota homeostasis, with this being more pronounced in male offspring (Liu et al. 2023).
In a non-guideline 30-day study, groups of 18 C57BL/6J male mice were orally gavaged with corn oil (control) or TPHP (dissolved in corn oil) at 5, 50, or 200 mg/kg bw/day (Wang et al. 2023). In all TPHP treated groups, observations included loosened germ cells in the seminiferous tubules, a decrease in sperm in the lumen of seminiferous tubules, a decrease in Leydig cells in the interstitial connective tissue, significantly decreased sperm count in cauda epididymides, abnormal sperm morphology. A significant decrease in serum testosterone levels was also noted in the mice administered 50, or 200 mg/kg bw/day TPHP as compared to the control group. The study further examines a potential mechanism by which these effects might occur and the authors note that this is likely via Leydig cell apoptosis (Wang et al. 2023).
A study by Ma et al. (2021) was conducted to investigate the role of TPHP on ovarian function, through evaluation of pubertal timing and follicular development. In this study, groups of immature female mice (postnatal day (PND) 21; n=6-15) were administered 0, 2, 10 or 50 mg/kg bw TPHP by gavage daily for 40 days. To evaluate serum hormone levels, groups of mice (n=12) were administered 0, 2, 20 or 50 mg/kg bw/day TPHP from PND 21-PND28. No significant changes in body weight or ovary weight were reported over the study period (PND 21-61). A significant increase in the average days to vaginal opening was reported in the 50 mg/kg bw/day group – a delay of 2 days from control animals. At all doses of TPHP, the total number of follicles in the ovary at all stages (primordial, pre-antral, small antral, large antral) were decreased from 16% to 47% with increasing doses compared to the control group. These decreases were statistically significant for all follicle types at 10 mg/kg bw/day and 50 mg/kg bw/day, but only for pre-antral follicles at 2 mg/kg bw/day. After 40-days of TPHP administration, the level of 17β-estradiol in serum was significantly decreased in 10 and 50 mg/kg bw/day mice, without significant change after 7 days of exposure. A significant increase in follicle-stimulating hormone (FSH) level (2.5-fold) was reported in 50 mg/kg bw/day mice after 40-days of administration of TPHP. After 7 days of TPHP administration, the serum level of FSH in mice was significantly increased by 2.8-fold and 5.4-fold in 10 mg/kg bw/day and 50 mg/kg bw/day groups, respectively. The serum level of luteinizing hormone was increased by 2.5-fold in the 50 mg/kg bw/day group after 7-days of TPHP administration (Ma et al. 2021). A lowest observed adverse effect level (LOAEL) of 2 mg/kg bw/day was considered for this study, based on the significantly decreased number of pre-antral follicles in the ovary of mice starting at 2 mg/kg bw/day.
In a non-guideline combined repeated dose toxicity study groups of time-mated Sprague-Dawley rats (n=15-22) were fed 0, 1000, 3000, 10,000, 15,000, 30,000 ppm (equivalent to approximately 50, 150, 500, 750, 1500 mg/kg bw/dayFootnote 5, respectively) TPHP in their feed from gestational day (GD) 6 to PND 28; pups were weaned at PND 28 and fed the corresponding diet to their dam from PND 28 to 56 (Witchey et al. 2023). All dams in the 1500 mg/kg bw/day group were removed from the study on GD12 due to clinical signs and lack of body weight gain, while 3 were removed from the 750 mg/kg bw/day group during the lactation phase. The remaining dams were reported to have clinical observations in the 500 mg/kg bw/day group (hunched posture, thin appearance and/or ruffled coats) as well as decreased body weight (6% to 25%) and increased food consumption (17% to 50%) starting at 500 mg/kg bw/day during gestation and/or lactation. TPHP exposure to dams was found to increase relative liver weight (starting at 150 mg/kg bw/day) and relative brain size (750 mg/kg bw/day), while significantly decreasing relative thymus weight (500, 750 mg/kg bw/day) without gross pathology changes. In terms of developmental effects, decreases in the number of live pups and offspring survival were reported for the 750 mg/kg bw/day group. Pup weights at PND1 were significantly decreased at 500 mg/kg bw/day and 750 mg/kg bw/day TPHP exposure. Onset of puberty was delayed in male and female offspring starting at 50 mg/kg bw/day and 150 mg/kg bw/day, respectively (increased days of balanopreputial separation and vaginal opening). Adjustment for body weight at weaning was reported to reduce the impact of the observed delay. Offspring organ weights were also affected by TPHP exposure – significant decrease in relative thymus in males and increased relative brain weight starting at 500 mg/kg bw/day. Additional evaluation noted offspring experienced maternal transfer through gestation and lactation, and exposure to TPHP had reduced cholinesterase activity in a dose-dependant manner in offspring starting in the low-dose group. The authors did not determine a NOAEL based on the effects observed in all exposure levels (Witchey et al. 2023). For this assessment, a LOAEL of 50 mg/kg bw/day is considered for this study, based on the significant delay in onset of puberty (significant increase in days to balanopreputial separation) in male offspring exposed to TPHP.
2.4.2.5 Neurological
In a non-guideline study to investigate the permeability of the blood-brain barrier and brain histopathology, groups of male mice were orally administered 0, 50 or 150 mg/kg bw/day TPHP by gavage for 30 days. After exposure for 30 days, TPHP and its metabolite DPHP were detected in the brain tissue analyzed using ultra-performance liquid chromatography-mass spectrometry (UPLC/MS). Histopathology of the brain showed microglial invasion of the hippocampus and cortex at both doses, along with neural cell loss at 150 mg/kg bw/day in the dentate gyrus of the hippocampus. The authors reported edema in the thalamus tissue, along with dilation and congestion of blood vessels at 150 mg/kg bw/day (Liu et al. 2020).
Groups of mouse pups were orally administered 0, 0.5, 5 or 50 mg/kg bw/day of TPHP from postnatal day (PND) 10-70 in a non-guideline study to investigate neurotoxicity and potential modes of action. Behavioural tests were conducted to test spatial memory (Y-maze test) and recognition memory (Novel object recognition test). In the Y-maze test, the number of entries into each arm and the number of correct spontaneous alternations (entering the 3 arms of the maze consecutively) were recorded to determine a percent alternation behaviour. In the novel object recognition test, the time exploring a new object and a familiar object are recorded and used to derive a discrimination index. At 5 mg/kg and 50 mg/kg significant decreases were reported for spontaneous alternation in the Y-maze test, and significant decreases in exploration time and discrimination index in the novel object recognition test. Staining of mouse hippocampus axons with the neuronal axon marker TUBB3 show reduced axon thickness and weaker fluorescence with TPHP exposure. Exposure to TPHP was also reported to have changes in expression of proteins and genes in the hippocampus relating to axon guidance, synapse function, neurotransmitter transport, and exocytosis compared to controls (Zhong et al. 2021).
In a non-guideline study, pregnant C57BL/6J mice (n =11-14) were orally gavaged with corn oil (control) or TPHP (dissolved in corn oil) at 1 or 5 mg/kg bw/day from gestational day 0 to delivery (Hong et al 2022b). At E18, 5 dams from each group were randomly selected and sacrificed to collect placenta, fetal brain, and maternal serum. The remaining dams were dosed until delivery. Then, male offspring were normally fed to post-natal day 56 (PND 56) for neurobehavioral testing, including open field test, novel object recognition and contextual fear conditioning (n = 6). There was a significant increase noted in IL-6, TNFα and NFκB levels in the placenta of TPHP treated mice as compared to control mice. There were no significant differences noted in these levels in the fetal brain. In TPHP treated mice, the recognition index was lower in the novel object recognition, the freezing level in recent and remote fear memory retrieval was lower in the contextual fear conditioning test and treated mice had decreased time in the center in the open field test which the authors attribute to anxiety like behaviour. There were no significant histopathological changes in the hippocampus in TPHP treated mice but the number of Nissl bodies in the hippocampus of treatment group was significantly reduced. Transcriptome analysis of fetal hippocampus showed that several neuronal cell signaling pathways (that is, axon guidance signaling pathway, neurotrophin signaling pathway, dopaminergic synapse signaling pathway and the cholinergic synapse signaling pathway) were disturbed by TPHP exposure (Hong et al 2022b).
2.4.2.6 Consideration of subpopulations who may have greater susceptibility
There are groups of individuals living in Canada who, due to greater susceptibility, may be more vulnerable to experiencing adverse health effects from exposure to substances. The potential for susceptibility during different life stages or by sex are considered from available studies. Available data for TPHP consists of kinetic, short-term, reproductive and developmental, genotoxicity data in experimental animals. In this health effects assessment, several studies were conducted in both male and female experimental animals for TPHP. Consideration was also given to developmental and neurological effects in the young, reproductive effects in males and females, and pregnant female animals through developmental and reproductive toxicity studies in animals. These considerations were taken into account in the selection of the critical health effects for risk characterization.
2.4.3 Characterization of risk to human health TPHP
No carcinogenicity studies were identified for TPHP. TPHP is not expected to be genotoxic. Health effects studies that could impact the risk characterization (that is, result in different critical endpoints or lower points of departure than those stated in [ECCC, HC 2021]) were identified. On the basis of the available studies, the LOAEL for developmental effects of 2 mg/kg bw/day based on the significant decrease in the number of pre-antral follicles in the ovary of mice (Ma et al. 2021) was selected as the critical effect level to characterize the risk to human health from exposures to TPHP. At all doses of TPHP tested, the total number of follicles in the ovary at all stages were decreased with increasing doses compared to the control group and there was a significant delay in vaginal opening in mice in the 50 mg/kg bw/day group (Ma et al. 2021). In rats, onset of puberty was delayed in male and female offspring starting at 50 mg/kg bw/day and 150 mg/kg bw/day, respectively (increased days of balanopreputial separation and vaginal opening) (Witchey et al. 2023). Studies have reported altered behaviour with respect to memory and learning in mice following early life exposure to TPHP (Zhong et al. 2021; Hong et al 2022b). The selected endpoint (Ma et al. 2021) is considered to be protective of the effects seen in the available studies as described in the health effects assessment.
Table 2‑5 provides all relevant exposure estimates and hazard points of departure as well as the resultant margins of exposure (MOEs) for the characterization of risk for exposures to TPHP.
| Exposure scenario | Systemic exposure (mg/kg bw/day) | Margins of exposure based on the LOAELa of 2 mg/kg bw/day |
|---|---|---|
| Environmental media and food (all age groups) | 7.9×10-5 to 1.2×10-3 | 1,670 to 25,300 |
| Nail polish (4 to 19+ years) | 0.16 to 0.28 | 7 to 13 |
| Nail polish, base coat, top coat (including when all 3 products used together) (14 to 19+ years; dermal)b | 0.24 to 0.28 | 7 to 8 |
| Nail kit, nail kit with polish (9 to 19+ years; dermal)b | 0.16 to 0.43 | 5 to 13 |
| Lubricants and greases (for example, power steering fluid) (19+ years; dermal) | 4.2×10-4 to 4.2×10-3 | 480 to 4,760 |
| Mouthing a foam object (for example, toy) (0 to 3 years; daily, intermittent; oral) | 6.7×10-5 to 1.5×10-3 | 1,330 to 29,900 |
| Dermal contact from lying on foam-containing mattresses or upholstered furniture (all age groups; daily)b | 2.7×10-3 to 8.0×10-2 | 25 to 7,403 |
| Dermal contact from sitting in an infant or child restraint system (0 to 13 years; daily)b,c | 1.2×10-3 to 5.6×10-3 | 357 to 1,670 |
| Dermal exposures from infant clothing (0 to 11 months) | 2.86×10-6 to 3.56×10-6 | 562,000 to 699,000 |
Abbreviations: LOAEL, lowest observed adverse effect level
a Based on a significant decrease in the number of pre-antral follicles in the ovary of mice observed in a 40-day mouse study (Ma et al, 2021). Target MOE = 1000 (x10 for interspecies extrapolation; x10 for intraspecies variation; x10 for use of a LOAEL, considering severity of effect [developmental toxicity]).
b Dermal absorption was assumed to be 50%.
c Infant and child restraint system regulations in Canada vary by province and territory (CPSAC 2019). On the basis of relevant age and weight considerations, dermal exposure estimates were derived for various age groups up to and including 9 to 13 year olds.
Comparisons between levels associated with critical effects in animal studies and estimates of exposure from environmental media, food, mouthing of foam objects, and dermal exposures from infant clothing are considered adequate to account for uncertainties in the exposure and health effects data used to characterize risk. However, the margins of exposure associated with dermal contact from lying on foam-containing mattresses or upholstered furniture and sitting in foam-containing infant and child restraints systems (including booster seats), as well as dermal contact to nail care products and lubricants and greases are considered potentially inadequate to account for uncertainties in the health effects and exposure data used to characterize risk.
The human health assessment took into consideration those groups of individuals living in Canada who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. The potential for increased susceptibility during reproduction and development were assessed and age-specific exposure estimates were derived. Generally, infants and children were found to have higher exposure than adults. All of these populations were taken into consideration while assessing the potential harm to human health.
2.4.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Limited occurrence data for TPHP in foods sold in Canada were available. | +/- |
| Maximum TPHP concentrations were applied to all food categories (except for infant formula). | + |
| Certain food categories only reported ND concentrations of TPHP and, in these cases, a concentration equivalent to the LOD was assumed. | + |
| The maximum concentrations reported in a given food item were assumed to be representative of a broader category as a whole. | +/- |
| Some of the source studies utilized for estimating exposure reported very low positive detection rates for TPHP. | +/- |
| Limited data measuring TPHP in foam-containing mattresses and/or furniture sold in Canada. Data from other countries were used to support derivation of exposure estimates. | +/- |
| Absence of data on migration of TPHP out of foam materials. | +/- |
| Dermal absorption data for TPHP were limited and therefore a default value was used in agreement with another jurisdiction. | +/- |
| There are no chronic toxicity studies for TPHP. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; +/- = unknown potential to cause over or under estimation of risk.
3. Assessment of TBOEP
3.1 Identity of substance
The CAS RN, DSL name and common names and/or acronyms for TBOEP are presented in Table 3‑1.
| CAS RN (abbreviation) | DSL name (common name) | Representative chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 78-51-3 (TBOEP) | ethanol, 2-butoxy-, phosphate (3:1) (tris(2-butoxyethyl) phosphate) | 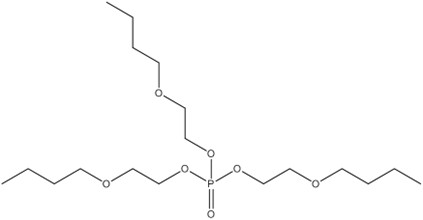 C18H39O7P |
398.47 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
3.2 Physical and chemical properties
A summary of experimental and modelled physical and chemical properties of TBOEP is provided in the draft assessment (ECCC, HC 2021).
3.3 Sources and uses
Details on the sources and uses of TBOEP are provided in the draft assessment of the Flame Retardants Group (ECCC, HC 2021). A summary of key data is provided below.
TBOEP does not occur naturally in the environment.
TBOEP was included in a survey issued pursuant to section 71 of CEPA (Canada 2012). In 2011, a total of 1000 kg to 10,000 kg of TBOEP were manufactured and between 10,000 kg and 100,000 kg were imported into Canada (Environment Canada 2013).Footnote 6
In Canada, according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013), TBOEP is used in paints and coatings, and in floor coverings.
TBOEP may also be used as a component in the manufacture of a limited number of adhesives used in the middle layers of food packaging materials in Canada (personal communication, e-mails from the Food and Nutrition Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated March 28, 2018 and May 20, 2022; unreferenced).
In Canada, TBOEP can be used as a formulant in pest control products but is currently not registered in any pest control products (Personal communication, e-mail from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated May 18, 2022; unreferenced).
TBOEP can also be found in rust paint (SDS 2014), leather repair solution (SDS 2011), shoe waterproofing spray (SDS 2018), marine mildew block spray (SDS 2019), and glass marker (SDS 2015) products in Canada.
TBOEP has been measured in foam sampled from products including pillows, upholstered furniture, child restraint systems, and building materials in Canada, the US, and elsewhere (CEC 2015; Health Canada 2023b; Miller and Gearhart 2016; Wu et al. 2019). TBOEP has also been identified in infant clothing and textiles/fabrics in the U.S. (Zhu et al. 2020).
3.4 Potential to cause harm to human health
3.4.1 Exposure assessment TBOEP
There were no significant changes from exposure to TBOEP in ambient and indoor air, drinking water, soil, dust, food, human milk and from the use of products available to consumers that contain TBOEP (for example, rust paint) as described in section 3.7.1.2 of the draft assessment (ECCC, HC 2021). However, updated exposure parameters used to estimate dermal intakes to TBOEP in the foam or fabric of infant and child restraint systems or upholstered furniture such as sofas were incorporated as described below.
3.4.1.1 Products available to consumers
Manufactured items
TBOEP was found in various foam products in Canada, the U.S. and elsewhere. Table 3‑2 summarizes available information on the concentration of TBOEP measured in various products.
| Manufactured item(s) | Concentration of TBOEP | Reference |
|---|---|---|
| Building material and foam pillows (for example, flooring, wallpaper, carpet, ceiling tiles, blinds, nursing pillow, toddler pillow) (n=38 samples from 23 products) purchased in Canada | 0.04% Detected in 1 wallpaper sample; LOD = 8 mg/kg |
Health Canada 2023b |
| Foam, fabric, upholstery, padding, and stuffing of various furniture products (n=132; for example, chairs, sofas, ottoman) purchased in 2014 to 2015 in Canada, the U.S. and Mexico | 0.08 to 0.17% w/w (reported as 750 to 1700 ppm) Detected in foam of 2 sofas purchased in Canada |
CEC 2015 |
| Child restraint systems (n=387 material samples from 15 systems/seats) purchased in U.S. | 0.009 to 2.5% w/w (reported as 98 ppm to 25,000 ppm) Detected in the foam and/or fabric of 9 child restraint systems |
Miller and Gearhart 2016 |
| Child restraint systems (n=36 samples including foam, fabric and composites of foam and fabric, 18 systems/seats) purchased in the U.S. in 2018 | Not detected (LOD =0.0003%) to 0.35% w/w TBOEP was detected in 58% of samples |
Wu et al. 2019 |
Abbreviation: LOD, limit of detection
Prolonged dermal exposure to TBOEP from lying on foam-containing mattresses or upholstered furniture and sitting in an infant or child restraint system (including booster seats) were estimated and are summarized in Table 3‑3 (see Appendix D, Table D-5, for details on parameters).
Limited dermal absorption data were identified for TBOEP in 2 infinite dose studies that included several organophosphate ester flame retardants (Frederiksen et al. 2018; Zhang et al. 2022). Exposures to TBOEP from the manufactured items discussed herein are all finite dose scenarios to which infinite dose studies have limited applicability. Frederiksen et al. (2018) also reported difficulty quantifying TBOEP. Furthermore, these studies do not incorporate all pertinent skin bound residues into reported permeability coefficients and may underestimate exposure by the dermal route. Given the lack of relevant dermal absorption data for TBOEP, including a dearth of information from other jurisdictions, 100% dermal absorption was assumed when calculating estimates of dermal exposure to TBOEP from foam-containing mattresses or upholstered furniture and infant and child restraint systems (including booster seats).
In Europe, TBOEP has also been measured in toys made of plastic, rubber, wood, and foam and textiles (Ionas et al. 2014). No studies of children’s products in Canada that included TBOEP were identified, but it could be expected that some of the same types of toys as those found to contain TBOEP in Europe may be present in Canada. There were no significant changes to the estimates of oral exposure via mouthing as described in section 3.7.1.2 of the draft assessment (ECCC, HC 2021).
| Exposure route | Source | Age group | Exposure estimate (mg/kg bw/day) |
|---|---|---|---|
| Dermal | Lying on foam-containing mattress or upholstered furniture | 0 to 5 months | 6.5×10-1 to 5.0 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 6 to 11 months | 5.8×10-1 to 4.5 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 1 year old | 5.6×10-1 to 4.5 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 2 to 3 years old | 3.5×10-1 to 3.6 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 4 to 8 years old | 2.8×10-1 to 3.1 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 9 to 13 years old | 2.1×10-1 to 2.5 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 14 to 18 years old | 1.5×10-1 to 1.8 |
| Dermal | Lying on foam-containing mattress or upholstered furniture | 19+ years old | 1.4×10-1 to 1.4 |
| Dermal | Foam/fabric in infant and child restraint systems | 0 to 5 months | 1.5×10-1 to 3.5×10-1 |
| Dermal | Foam/fabric in infant and child restraint systems | 6 to 11 months | 1.4×10-1 to 3.2×10-1 |
| Dermal | Foam/fabric in infant and child restraint systems | 1 year old | 1.3×10-1 to 2.9×10-1 |
| Dermal | Foam/fabric in infant and child restraint systems | 2 to 3 years old | 8.8×10-2 to 2.0×10-1 |
| Dermal | Foam/fabric in infant and child restraint systems | 4 to 8 years old | 7.5×10-2 to 1.7×10-1 |
| Dermal | Foam/fabric in infant and child restraint systemsb | 9 to 13 years old | 5.9×10-2 to 1.4×10-1 |
| Oral | Foam in children’s products | 0 to 5 months | 4.0×10-3 to 4.6×10-2 |
| Oral | Foam in children’s products | 6 to 11 months | 2.8×10-3 to 3.2×10-2 |
| Oral | Foam in children’s products | 1 year old | 2.3×10-3 to 2.7×10-2 |
| Oral | Foam in children’s products | 2 to 3 years old | 1.7×10-3 to 1.9×10-2 |
a Dermal absorption was assumed to be 100% when estimating dermal exposures to TBOEP.
b Infant and child restraint system regulations in Canada vary by province and territory (CPSAC 2019). On the basis of relevant age and weight considerations, dermal exposure estimates were derived for various age groups up to and including 9 to 13 year olds.
TBOEP was also identified in various textiles purchased in the US including infant clothing (for example, socks, bodysuits, pants, trousers, skirts, shirts) and raw fabrics (that is, fabrics available prior to sewing marketed for various applications including curtains, cushions, toss pillows and patio furniture) (Zhu et al. 2020). It is expected that some of the same types of textiles containing TBOEP could be present in Canada. Concentrations of TBOEP in conventional textiles, which included infant clothing (n=52) and raw fabrics (n=29) made of cotton, polyester, or nylon, ranged from 0.108 ng/g to 1 083 ng/g (method quantification limit=0.1 ng/g). Estimated dermal exposures to TBOEP derived in Zhu et al. (2020) for infants from clothing based on the 95th percentile concentration ranged from 795 to 988 pg/kg bw/day. These exposure estimates are considered negligible (≤2.5 ng/kg bw/day).
3.4.1.2 Biomonitoring
There were no significant changes to the estimated daily intakes derived from the available human biomonitoring data for TBOEP as described in section 3.7.1.5 of the draft assessment (ECCC, HC 2021).
3.4.1.3 Consideration of subpopulations who may have greater exposure
There are groups of individuals living in Canada who, due to greater exposure, may be more vulnerable to experiencing adverse health effects from exposure to substances. The potential for elevated exposure within the Canadian population was examined. Exposure estimates are routinely assessed by age to take into consideration physical and behavioural differences during different stages of life. In the assessment of background exposure from environmental media, indoor air, food, drinking water and dust, young children had higher exposures than adults. Human-milk fed infants had higher exposure than formula-fed infants. Exposure to TBOEP from manufactured products is similar across all age groups.
3.4.2 Health effects assessment TBOEP
Hazard characterization for TBOEP is described in the draft assessment (ECCC, HC 2021). There were no significant changes in the effects of concern identified. No health effect studies that would impact the risk characterization (that is, result in different critical endpoints or lower points of departure than those stated in ECCC, HC (2021)) were identified.
3.4.2.1 Consideration of subpopulations who may have greater susceptibility
There are groups of individuals living in Canada who, due to greater susceptibility, may be more vulnerable to experiencing adverse health effects from exposure to substances. The potential for susceptibility during different life stages or by sex are considered from available studies. Available data for TBOEP consists of kinetic, short-term, repeated-dose, reproductive and developmental and genotoxicity data in experimental animals. In this health effects assessment, several studies were conducted in both male and female experimental animals for TBOEP. Consideration was also given to developmental effects in the young through developmental toxicity studies in animals. These considerations were taken into account in the selection of the critical health effects for risk characterization.
3.4.3 Characterization of risk to human health TBOEP
As indicated in the draft assessment (ECCC, HC 2021), additional sources of exposure to TBOEP include through indoor air, drinking water, indoor dust, soil, food, human milk and use of products available to consumers containing TBOEP (for example, rust paint). Section 3.7.3.2 of the draft assessment addressed the risk characterization of TBOEP from these sources and uses (ECCC, HC 2021).
No carcinogenicity studies were identified for TBOEP. TBOEP is not expected to be genotoxic (ATSDR 2012). An 18-week oral study was selected as the most relevant study to characterize the risk to human health from exposures to TBOEP (Reyna and Thake 1987, as cited in ATSDR 2002). A BMDL10 of 8.88 mg/kg bw/day was derived by the ATSDR (2012) on the basis of periportal hepatocellular vacuolization in males at 173 to 209 mg/kg bw/day (NOAEL = 17 to 21 mg/kg bw/day). Since no long-term dermal study was available, this BMDL10 was used as the point of departure for characterization of the risk from daily exposures via the dermal route.
Table 3‑4 provides the updated exposure estimates from dermal contact with mattresses or upholstered furniture and infant or child restraint systems (including booster seats) as well as relevant hazard points of departure and resulting MOEs for TBOEP.
| Exposure scenarioa | Systemic exposure (mg/kg bw/day) | Margins of exposures based on BMDL10 of 8.88 mg/kg-bw/dayb |
|---|---|---|
| Dermal contact from lying on foam-containing mattresses or upholstered furniture (all age groups; daily) | 1.3×10-1 to 5.0 | 2 to 68 |
| Dermal contact from sitting in an infant or child restraint system (0 to 13 years; daily)c | 5.9×10-2 to 3.5×10-1 | 25 to 150 |
| Mouthing a foam object (for example, toy) (0 to 3 years; daily, intermittent; oral) | 1.7x10-3 to 4.6x10-2 | 190 to 5220 |
Abbreviations: BMDL, benchmark dose level
a Dermal absorption was assumed to be 100% when estimating dermal exposures to TBOEP.
b Based on periportal hepatocellular vacuolization in males in a 18-week rat study (Reyna and Thake 1987, as cited in ATSDR 2012). Target MOE = 100 (x10 for interspecies extrapolation; x10 for intraspecies variation).
c Infant and child restraint system regulations in Canada vary by province and territory (CPSAC 2019). On the basis of relevant age and weight considerations, dermal exposure estimates were derived for various age groups up to and including 9 to 13 year olds.
The calculated MOEs from mouthing foam objects containing TBOEP are considered adequate to account for uncertainties in the exposure and health effect data used to characterize risk. However, the MOEs derived for prolonged dermal exposure to TBOEP from lying on foam-containing mattresses or upholstered furniture (all age groups) and from sitting in infant or child restraint systems (0 to 13 years) are considered potentially inadequate to account for uncertainties in the data used to characterize risk.
The human health assessment took into consideration those groups of individuals living in Canada who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects. The potential for increased susceptibility during reproduction and development were assessed and age-specific exposure estimates were derived. Generally, infants and children were found to have higher exposure than adults. All of these populations were taken into consideration while assessing the potential harm to human health.
3.4.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Limited available data on the presence of TBOEP in foam in infant and child restraint systems and in foam-containing mattresses and upholstered furniture in Canada. | +/- |
| Absence of data on migration of TBOEP out of foam materials. | +/- |
| Dermal absorption data for TBOEP are unavailable, so 100% absorption is assumed. | + |
+ = uncertainty with potential to cause over-estimation of exposure/risk; +/- = unknown potential to cause over or under estimation of risk.
4. Conclusion
On the basis of the inadequacy of the margins between estimates of exposure and critical effect levels in experimental animals presented in this document, it is proposed to conclude that TPHP and TBOEP meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that TPHP and TBOEP meets one or more of the criteria set out in section 64 of CEPA.
References
Abbott Nutrition. 2024. Infant formula preparation guidelines and dilution charts [PDF]. Saint-Laurent (QC): Abbott. [accessed 2024 Apr 23].
Arcus-Art A, Krowech G, Zeise L. 2005. Breast milk and lipid intake distributions for assessing cumulative exposure and risk. J Expo Anal Environ Epidemiol. 15:357-365.
Alves A, Covaci A, Voorspoels S. 2017. Method development for assessing the human exposure to organophosphate flame retardants in hair and nails. Chemosphere 168:692-698.
Ashley-Martin J, MacPherson S, Zhao Z, Gaudreau É, Provencher G, Fisher M, Borghese M.M, Bouchard M.F, Booij L, Arbuckle T.E. 2023. Descriptive analysis of organophosphate ester metabolites in a pan-Canadian pregnancy cohort. Sci Total Environ. 163327.
[ATSDR] Agency for Toxic Substances and Disease Registry. 2012. Toxicological profile for Phosphate Ester Flame Retardants [PDF]. Atlanta (GA): US Department of Health and Human Services, Public Health Service [accessed 2017 Oct 26].
Beser MI, Pardo O, Beltrán J, Yusà V. 2020. Determination of 21 perfluoroalkyl substances and organophosphorus compounds in breast milk by liquid chromatography coupled to orbitrap high-resolution mass spectrometry. Anal Chim Acta. 1049:123-132.
Bjornsdotter MK, Romera-Garcia E, Borrull J, de Boer J, Rubio S, Ballesteros-Gomez A. 2018. Presence of diphenyl phosphate and aryl-phosphate flame retardants in indoor dust from different microenvironments in Spain and the Netherlands and estimation of human exposure. Environ Int. 112:59-67.
Bremmer HJ, Prud’homme de Lodder LCH, and van Engelen JGM. 2006. Cosmetics Fact Sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.:320104001/2006.
Butt C, Congleton J, Hoffman K, Fang M, Stapleton H. 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate (EH-TBB) in urine from paired mothers and toddlers. Environ Sci Technol. 48:10432-10438.
Butt C, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton H. 2016. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int. 94:627-634.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canada, Dept. of the Environment, Dept. of Health. 2016. Canadian Environmental Protection Act, 1999: Early stakeholder engagement to help inform the plan to address the remaining 1 550 substances under the Chemicals Management Plan [PDF]. Canada Gazette, Part I, vol. 150, no. 6, p. 152-154.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
[CEC] Commission for Environmental Cooperation. 2015. Enhancing Trilateral Understanding of Flame Retardants and Their Use in Manufactured Items: Analysis of Select Flame Retardants Contained in Office and Household Furniture [PDF]. Montreal (QC): CEC. 16 p. [accessed 2018 Mar 23].
[CEH] Center for Environmental Health. 2013a. Playing on poisons: Harmful flame retardants in children’s furniture [PDF]. Oakland (CA): Center for Environmental Health. 19 p. [accessed 2016 Mar 17].
[CEH] Center for Environmental Health. 2013b. Naptime nightmares: toxic flame retardants in child care nap mats [PDF]. Oakland (CA): CEH. 18 p. [accessed 2016 Mar 17].
Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C. 2015. Human exposure pathways to organophosphate triesters — A biomonitoring study of mother–child pairs. Environ Int. 75:159-165.
[CFIA] Canadian Food Inspection Agency. 2019-2021. Children’s Food Project Survey. Survey Results from 2019 – 2021.
Chen N, Fan S, Zhang N, Zhao Y, Yao S, Chen X, Liu X, Shi Z. 2022. Organophosphate esters and their diester metabolites in infant formulas and baby supplementary foods collected in Beijing, China: Occurrence and the implications for infant exposure. Sci Total Environ. 827:154272
Chen X, Zhao X, Shi Z. 2021. Organophosphorus flame retardants in breast milk from Beijing. Occurrence, nursing infant's exposure and risk assessment. Sci Total Environ. 771:145404.
[CIR] Cosmetic Ingredient Review. 2018. Safety Assessment of Triphenyl Phosphate as Used in Cosmetics. Final Report. September 18, 2018. Washington (DC): CIR. [accessed 2018 Oct 23].
[ConsExpo Web] Consumer Exposure Web Model. 2021. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Cooper EM, Kroeger G, Davis K, Clark C, Ferguson PL, Stapleton H. 2016. Results from screening polyurethane foam based consumer products for flame retardant chemicals: Assessing impacts on the change in the furniture flammability standards. Environ Sci Technol. 50:10653-10660.
[CPSAC] Child Passenger Association of Canada. 2019. Provincial & Territorial Legislation Summary [PDF]. Ottawa (ON): Child Passenger Association of Canada. [accessed 2024 Apr 24].
Cui K, Wen J, Zeng F, Li S, Zhou X, Zeng Z. 2017. Occurrence and distribution of organophosphate esters in urban soils of the subtropical city, Guangzhou, China. Chemosphere. 175:514-520.
Cui H, Chang Y, Jiang X, Li M. 2020. Triphenyl phosphate exposure induces kidney structural damage and gut microbiota disorders in mice under different diets. Environ Int. 144: 106054.
Cui H, Chang Y, Cao J, Jiang X, Li M. 2022. Liver immune and lipid metabolism disorders in mice induced by triphenyl phosphate with or without high fructose and high fat diet. Chemosphere.308(Pt 3):136543
[Danish EPA] Danish Environmental Protection Agency. 2015. Chemical substances in car safety seats and other textile products for children. Survey of chemical substances in consumer products No. 135. Copenhagen (DK): Danish Ministry of the Environment. 159 p. [accessed 2018 Feb 13].
Davis A., Ryan P.B., Cohn J.A., Harris D., and Black M. 2021. Chemical exposures from upholstered furniture with various flame retardant technologies. Indoor Air. 31(5):1473-1483.
[ECCC] Environment and Climate Change Canada. 2016. Data collected under the Canadian Environmental Protection Act, 1999: Early stakeholder engagement to help inform the plan to address the remaining 1 550 substances under the Chemicals Management Plan. Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2022. Unpublished confidential technical background study on products containing aryl organophosphate substances in Canada. Gatineau (QC): ECCC, Industrial Sectors and Chemicals Directorate.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2021. Draft screening assessment flame retardants group. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2018. Registered substances database; search results for CAS RN 115-86-6. Helsinki (FI): ECHA. [accessed 2018 Apr 4].
[ECHA] European Chemicals Agency. 2018. Candidate List of Substances of Very High Concern for Authorisation [Internet]. Helsinki (FI): European Chemicals Agency.
Environment Canada. 2013. DSL Inventory Update data collected under theCanadian Environmental Protection Act, 1999, section71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Estill CF, Mayer A, Slone J, Chen I-C, Zhou M, La Guardia MJ, Jayatilaka N, Ospina M, Calafat A. 2021. Assessment of triphenyl phosphate (TPHP) exposure to nail salon workers by air, hand wipe, and urine analysis. Int J Hyg Environ Health. 231:113630.
Ficheux AS, Morriset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food Chem Toxicol. 66:36-43.
Frederiksen M, Stapleton HM, Vorkamp K, Webster TF, Jensen NM, Sørensen JA, Nielsen F, Knudsen LE, Sørensen LS, Clausen PA, Nielsen JB. 2018. Dermal uptake and percutaneous penetration of organophosphate esters in a human skin ex vivo model. Chemosphere. 197:185-192.
Fromme H, Lahrz T, Kraft M, Fembacher L, Mach C, Dietrich S, Burkardt R, Völkel W, Göen T. 2014. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ Int. 71:158-163.
Hammel SC, Zhang S, Lorenzo AM, Eichner B, Stapleton HM, Hoffman K. 2020. Young infants' exposure to organophosphate esters: Breast milk as a potential source of exposure. Environ Int. 143:106009.
He C, Wang X, Tang S, Thai P, Li Z, Baduel C, Mueller JF. 2018. Concentrations of organophosphate esters and their specific metabolites in food in Southeast Queensland, Australia: is dietary exposure an important pathway of organophosphate esters and their metabolites? Environ Sci Technol. 52(21):12765-12773.
Health Canada. 1994. Human health risk assessment for priority substances [PDF, 213.5 KB]. Ottawa (ON): Minister of Supply and Services Canada. Cat. No.: En40-215/41E
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2018a. Draft backgrounder document on default values for breast milk and formula intakes. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada 2018b. Food Consumption Table derived from Statistics Canada's 2015 Canadian Community Health Survey, Nutrition, Share file. Ottawa. Available from: Food Consumption Table (2015 CCHS Nutrition) - Open Government Portal (canada.ca).
Health Canada. 2019. Determination of Flame Retardants in a Survey of Consumer Products: Isopropylphenyl phosphate (IPPP). Project Report number P2019-00015A5; 27 June, 2019. Ottawa (ON): Product Safety Laboratory. 35 pp.
Health Canada. [modified 2022 Feb 11]. Canadian exposure factors used in human health risk assessments. Ottawa (ON): Government of Canada. [accessed 2024 January 4].
Health Canada. 2023a. 2022-2023 Chemicals Management Plan Flame Retardants in Children’s Products Survey. Project Report number P2022-00074; 27 June 2023. Ottawa (ON): Product Safety Laboratory. 55 pp.
Health Canada. 2023b. 2022-2023 Chemicals Management Plan Flame Retardants in Building Material and Foam Pillows Survey. Project Report number P2022-00050; 18 May 2023. Ottawa (ON): Product Safety Laboratory. 48 pp.
Health Canada. 2023c. Screening for Organic Flame Retardants in Foam Children’s Products. Project Report number P2023-00051; 27 October 2023. Ottawa (ON): Product Safety Laboratory. 23 pp.
Health Canada. 2023d. Canadian Biomonitoring Dashboard. Ottawa (ON): Health Canada.
[HC] Health Canada.[modified 2024a Jan 22]. Industry Guide to Vaping Products Subject to the Canada Consumer Product Safety Act. Ottawa (ON): Government of Canada.
[HC] Health Canada.[modified 2024b Jul 22]. Regulating tobacco and vaping products. Ottawa (ON): Government of Canada.
Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, Daniels JL. 2017. Predictors of urinary flame retardant concentration among pregnant women. Environ Int. 98:96-101.
Hong J, Jiang M, Guo L, Lin J, Wang Y, Tang H, Liu X. 2022a. Prenatal exposure to triphenyl phosphate activated PPARγ in placental trophoblasts and impaired pregnancy outcomes. Environ Pollut. 301:119039.
Hong J, Lu X, Wang J, Jiang M, Liu Q, Lin J, Sun W, Zhang J, Shi Y, Liu X. 2022b. Triphenyl phosphate disturbs placental tryptophan metabolism and induces neurobehavior abnormal in male offspring. Ecotoxicol Environ Saf. 243:113978.
Ionas AC, Dirtu AC, Anthonissen T, Neels H, Covaci A. 2014. Downsides of the recycling process: Harmful organic chemicals in children’s toys. Environ Int. 65:54-62.
Kim JW, Isobe T, Muto M, Tue NM, Katsura K, Marlarvannan G, Sudaryanto A, Chang KH, Prudente M, Viet PH, et al. 2014. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere. 116:91-97.
Kosarac I, Kubwabo C, Foster WG. 2016. Quantitative determination of 9 urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J Chromatogr B. 1014:24-30.
Li P, Jin Y, Hu J, Xu J, Sun Y, Ma Y. 2017. Concentrations of organophosphorus, polybromobenzene, and polybrominated diphenyl ether flame retardants in human serum, and relationships between concentrations and donor ages. Chemosphere. 171:654-660.
Liu LY, He K, Hites R, Salamova A. 2016. Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ Sci Technol. 50:3065−3073.
Liu X, Zhao X, Wang Y, Hong J, Shi M, Pfaff D, Guo L, Tang H. 2020. Triphenyl phosphate permeates the blood brain barrier and induces neurotoxicity in mouse brain. Chemosphere. 252:126470.
Liu X, Lin J, Chen Y, Jiang M, Liu Q, Zhang J, Lu X, Hong J, Sun W, Sun Y, Guo L. 2023. Gestation and lactation triphenyl phosphate exposure disturbs offspring gut microbiota in a sex-dependent pathway. Food Chem Toxicol. 172:113579
Ma H, Ishida K, Xu C, Takahashi K, Li Y, Zhang C, Kang Q, Jia Y, Hu W, Matsumaru D, Nakanishi T, Hu J. 2021. Triphenyl phosphate delayed pubertal timing and induced decline of ovarian reserve in mice as an estrogen receptor antagonist. Environ Pollut. 290:118096.
Ma J, Zhu H, Kannan K. 2019. Organophosphorus Flame Retardants and Plasticizers in Breast Milk from the United States. Environ Sci Technol Lett. 6(9):525-531
Ma Y, Jun J, Li P, Xu M, Sun Y, Wang Y, Yuan H. 2017. Organophosphate ester flame retardant concentrations and distributions in serum from inhabitants of Shandong, China, and changes between 2011 and 2015. Environ Toxicol Chem. 36:414-421.
Marklund A. 2005. Levels and sources of organophosphorus flame retardants and plasticizers in indoor and outdoor environments [PDF]. Umeå (SE): Umeå University. [accessed 2016 Mar 23].
Matz CJ, Stieb DM, Davis K, Egyed M, Rose A, Chou B, Brion O. 2014. Effects of age, season, gender and urban-rural status on time-activity: Canadian Human Activity Pattern Survey 2 (CHAPS 2). Int J Environ Res Public Health. 11:2108-2124.
McGee SP, Konstantinov A, Stapleton HM, Volz DC. 2013. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: Potential role of the aryl hydrocarbon receptor. Toxicol Sci. 133(1):144-156.
Mead Johnson Nutrition. 2020. Product information & resources: Scoop Information for Powder Products. Kanata (ON): Mead Johnson & Company, LLC. [accessed 2024 Apr 23].
Mead Johnson Nutrition. 2023. Pediatric Products Handbook [PDF]. Kanata (ON): Mead Johnson & Company, LLC. [accessed 2024 Apr 23].
Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, Webster TF, Stapleton HM. 2016. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. 86:45-51.
Miller GZ, Gearhart J. 2016. Traveling with toxics: Flame retardants & other chemicals in children’s car seats. Michigan (US): HealthyStuff, Ecology Center. [accessed 2018 Mar 29].
Navaranjan G, Jantunene LM, Diamond ML, Harris SA, Bernstein S, Scott JA, Takaro TK, Dai R, Lefebvre DL, Mandhane PJ, et al. 2021. Early life exposure to tris(2-butoxyethyl) phosphate (TBOEP) is related to the development of childhood asthma. Environ Sci Technol Lett. 8:531-537.
Nguyen LV, Diamond ML, Kalenge S, Kirkham TL, Holness DL, Arrandale VH. 2022. Occupational Exposure of Canadian Nail Salon Workers to Plasticizers Including Phthalates and Organophosphate Esters. Environ Sci Technol. 56: 3193-3203.
[NHANES 2011-2018] Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, [2011-2018].
Norris B, Smith S. 2002. Research into the mouthing behaviour of children up to 5 years old. London (GB): Consumer and Competition Policy Directorate, Department of Trade and Industry.
[NTP] National Toxicology Program (US). 2018. NTP Research Report on In Vivo Repeat Dose Biological Potency Study of Triphenyl Phosphate (CAS No. 115-86-6) in Male Sprague Dawley Rats (Hsd: Sprague Dawley SD) (Gavage Studies). Research Triangle Park (NC): National Toxicology Program. NTP RR 8.
[OECD] Organisation for Economic Co-operation and Development. 2002. SIDS initial assessment profile Triphenyl phosphate: CAS No. 115-86-6. SIAM [SIDS Initial Assessment Meeting] 15, 2002; Germany. [accessed 2018 Apr 5].
Ospina M, Jayatilaka N, Wong LY, Restrepo P, Calafat A. 2018. Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int. 110:32-41.
Peng C, Zhang X, Chen Y, Wang L. 2023. Toxicity assessment of organophosphate flame retardant triphenyl phosphate (TPHP) on intestines in mice. Ecotoxicol Environ Saf. 268, 115685.
Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM. 2018. Children's residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ Int. 116:176-185.
Phillips AL, Hammel SC, Konstantinov A, Stapleton HM. 2017.Characterization of individual isopropylated and tert-butylated triarylphosphate (ITP and TBPP) isomers in several commercial flame retardant mixtures and house dust standard reference material SRM 2585. Environ Sci Technol. 51:13443-13449.
Poma G, Glynn A, Malarvannan G, Covaci A, Darnerud PO. 2017. Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem Toxicol. 100:1-7.
Poma G, Malysheva SV, Goscinny S, Malarvannan G, Voorspoels S, Covaci A, Van Loco J. 2018. Occurrence of selected halogenated flame retardants in Belgian foodstuff. Chemosphere. 194:256-265.
Rodgers KM, Bennett D, Moran R, Knox K, Stoiber T, Gill R, Young TM, Blum A, Dodson RE. 2021. Do flame retardant concentrations change in dust after older upholstered furniture is replaced? Environ Int.153:106513.
[SCCS] Scientific Committee on Consumer Safety. 2024. Opinion on Triphenyl phosphate. Luxembourg: European Commission. [accessed 2024 April 4].
Schoeters G, Verheyen V. J, Colles A, Remy S, Rodriguez M. L, Govarts E, Nelen V, Den Hond E, De Decker A, Franken C, et al. Internal exposure of Flemish teenagers to environmental pollutants: Results of the Flemish Environment and Health Study 2016–2020 (FLEHS IV). Int J Hyg Environ Health. 242: 2022: 113972.
[SDS] Material Safety Data Sheet. 2011. Trade Secret Pro Leather Touch Up. Lachine (QC): Dover Finishing Products Inc. [accessed 2022 September 20].
[SDS] Material Safety Data Sheet. 2014. Trmcld 2X3.78L water base gloss black. Concord (ON): Rust-Oleum Consumer Brands Canada. [accessed 2016 Jun 20].
[SDS] Safety Data Sheet. 2015. R-O 4X20ml glass marker black [PDF]. Concord (ON): Rust-Oleum Consumer Brands Canada. [accessed 2022 September 20].
[SDS] Safety Data Sheet. 2017. Wynn's Engine Tune-Up. Ontario (CA). ITW Professional Automotive Products. [accessed 2023 Nov 05].
[SDS] Safety Data Sheet. 2018. Scotchgard shoe guard (Cat. No 1007). St. Paul (MN): 3M. [accessed 2022 September 20].
[SDS] Safety Data Sheet. 2019. 3M Marine mildew block, PN 09065. St. Paul (MN): 3M. [accessed 2022 September 20].
[SDS] Safety Data Sheet. 2021. Mobil Steering Fluid. Alberta (CA) . Imperial Oil Downstream. [accessed 2023 Nov 05].
Selmi-Ruby S, Marín-Sáez J, Fildier A, Buleté A, Abdallah M, Garcia J, Deverchère J, Spinner L, Giroud B, Ibanez S, Granjon T. 2020. In vivo characterization of the toxicological properties of DPhP, one of the main degradation products of aryl phosphate esters. Environ Health Persp. 128(12): 127006.
Shoeib M, Ahrens L, Jantunen L, Harner T. 2014. Concentrations in air of organobromine, organochlorine and organophosphate flame retardants in Toronto Canada. Atmos Environ. 99:140-147.
Siddique S, Harris SA, Kosarac I, Latifovic L, Kubwabo C. 2020. Urinary metabolites of organophosphate esters in women and their relationship with serum lipids: An exploratory analysis. Environ Pollut. 263: 114110.
Siddique S, Farhat I, Kubwabo C, Chan P, Goodyer CG, Robaire B, Chevrier J, Hales BF. 2022. Exposure of men living in the greater Montreal area to organophosphate esters: association with hormonal balance and semen quality. Environ Int. 166:107402.
Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 45:5323-5331.
Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. 2012. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 46:13432-13439.
Statistics Canada. 2015. Canadian Community Health Survey (CCHS). Ottawa (ON): Statistics Canada.
Su G, Letcher RJ, Yu H. 2016. Organophosphate flame retardants and plasticizers in aqueous solution: pH-dependent hydrolysis, kinetics, and pathways. Environ Sci Technol. 50:8103-8111.
Sundkvist A, Olofsson U, Haglund P. 2010. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit. 12:943-951.
Thomas MB, Stapleton H, Dills RL, Violtte H, Christakis D, Sathyanarayama S. 2016. Demographic and dietary risk factors in relation to urinary metabolites of organophosphate flame retardants in toddlers. Chemosphere. 185:918-925.
[US CPSC] US Consumer Product Safety Commission, 2005. Migration of Flame Retardant Chemicals in Upholstered Furniture Foam [PDF]. Washington (DC): Division of Chemistry, US CPSC. [accessed 2018 Mar 29].
[US CPSC] US Consumer Product Safety Commission. 2006. CPSC Staff Preliminary risk assessment of flame retardant chemicals in upholstered furniture foam [PDF]. Bethesda (MD): US CPCS. [accessed 2018 Mar 29].
[US EPA] United States Environmental Protection Agency. 2012. Chemical Data Reporting data collected under the Environmental Protection Agency’s Toxic Substances Control Act. Washington (DC): US EPA. [accessed 2020 Jun 4].
[US EPA] US Environmental Protection Agency. 2011. Exposure Factors Handbook 2011 Edition (Final Report). Washington, DC: Office of Research and Development, National Center for Environmental Assessment. [accessed 2022 April 29].
[US EPA] US Environmental Protection Agency. 2020a. Final Scope of the Risk Evaluation for Triphenyl Phosphate (TPP) CASRN 115-86-6 [PDF]. Washington (DC); Office of Chemical Safety and Pollution Prevention. EPA-740-R-20-010. [accessed 2024 January 4].
[US EPA] US Environmental Protection Agency. 2020b. Use Report for TPP (CASRN 115-86-6) [PDF]. Washington (DC); Office of Pollution, Prevention and Toxics. [accessed 2024 January 4].
Wang D, Zhu W, Chen L, Yan J, Teng M, Zhou Z. 2018. Neonatal triphenyl phosphate and its metabolite diphenyl phosphate exposure induce sex-and dose-dependent metabolic disruptions in adult mice. Environ pollut. 237:10-7.
Wang Y, Kannan K. 2018. Concentrations and Dietary Exposure to Organophosphate Esters in Foodstuffs from Albany, New York, United States. J Agric Food Chem. 66(51):13525-13532.
Wang Y, Li W, Martínez-Moral MP, Sun H, Kannan K. 2019. Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. Environ Int. 122:213-221.
Wang X, Liu Q, Zhong W, Yang L, Yang J, Covaci A, Zhu L. 2020. Estimating renal and hepatic clearance rates of organophosphate esters in humans: Impacts of intrinsic metabolism and binding affinity with plasma proteins. Environ Int. 134: 105321.
Wang X, Chen P, Zhao L, Zhu L, Wu F. 2021. Transplacental behaviors of organophosphate tri-and diesters based on paired human maternal and cord whole blood: efficiencies and impact factors. Environ Sci Technol. 55(5): 3091-100.
Wang M, Xu J, Zhao Z, Gong L, Su Y, Fang Z, Chen P, Liu Y, Zhang L, Xu F. 2023. Triphenyl phosphate induced apoptosis of mice testicular Leydig cells and TM3 cells through ROS-mediated mitochondrial fusion inhibition. Ecotoxicol Environ Saf. 256:114876
Wei B, O’Connor RJ, Goniewicz ML, Hyland A. 2020. Emerging chemicals of health concern in electronic nicotine delivery systems. Chem Res Toxicol. 33(10):2637-2646.
Witchey SK, Sutherland V, Collins B, Roberts G, Shockley KR, Vallant M, Krause J, Cunny H, Waidyanatha S, Mylchreest E, Sparrow B, Moyer R, Behl M. 2023. Reproductive and developmental toxicity following exposure to organophosphate ester flame retardants and plasticizers, triphenyl phosphate and isopropylated phenyl phosphate, in Sprague Dawley rats. Toxicol Sci. 191(2):374-386.
Wu Y, Miller G. Z, Gearhart J, Romanak K, Lopez-Avila V, Venir M. 2019. Children’s car seats contain legacy and novel flame retardants [PDF]. Environ Sci Technol. 6(1) :14-20.
Yang C, Diamond ML, Latifovic L, Tsirlin D, Harris SA, Jantunen L. 2017. Semivolatile organic compounds in Canadian homes; A comprehensive household exposure study. Unpublished report. Toronto (ON): University of Toronto, Cancer Care Ontario, and Environment and Climate Chage Canada.
Yang C, Harris SA, Jantunen LM, Sidiqque S, Kubwabo C, Tsirlin D, Latifovic L, Fraser B, St-Jean M, De La Campa R, You H, Kulka R, Diamond ML. 2019. Are cell phones an indicator of personal exposure to organophosphate flame retardants and plasticizers? Environ Int. 122:104-116.
Zhang Q, Ji S, Chai L, Yang F, Zhao M, Liu W, Schüürmann G, Ji L. 2018. Metabolic mechanism of aryl phosphorus flame retardants by cytochromes P450: a combined experimental and computational study on triphenyl phosphate. Environ Sci Technol. 52(24):14411-21.
Zhang R, Li N, Li J, Zhao C, Luo Y, Wang Y, Jiang G. 2022. Percutaneous absorption and exposure risk assessment of organophosphate esters in children’s toys. J Hazard Mater. 440:129728.
Zhao L, Jian K, Su H, Zhang Y, Li J, Letcher RJ, Su G. 2019a. Organophosphate esters (OPEs) in Chinese foodstuffs: Dietary intake estimation via a market basket method, and suspect screening using high-resolution mass spectrometry. Environ Int. 128:343-352.
Zhao F, Kang Q, Zhang X, Liu J, Hu J. 2019b. Urinary biomarkers for assessment of human exposure to monomeric aryl phosphate flame retardants. Environ Int. 124:259-264.
Zheng G, Schreder E, Dempsey JC, Uding N, Chu V, Andres G, Sathyanarayana S, Salamova A. 2021. Organophosphate Esters and Their Metabolites in Breast Milk from the United States: Breastfeeding Is an Important Exposure Pathway for Infants. Environ Sci Technol Lett. 8:224-230
Zhong X, Yu Y, Wang C, Zhu Q, Wu J, Ke W, Ji D, Niu C, Yang X, Wei Y. 2021. Hippocampal proteomic analysis reveals the disturbance of synaptogenesis and neurotransmission induced by developmental exposure to organophosphate flame retardant triphenyl phosphate. J Hazard Mater. 404: 124111.
Zhou M, Wang J, Yang H, Ji X, Qian M, Li Z. 2022. Organophosphate ester concentrations in infant food and dietary risk assessment for the infant population in China. Food Control. 139:109107.
Zhu H, Al-Bazi MM, Kumosani TA, Kannan K. 2020. Occurrence and Profiles of Organophosphate Esters in Infant Clothing and Raw Textiles Collected from the United States. Environ Sci Technol Lett. 7:415-420.
Appendix A. Estimates of daily intake by various age groups within the general population of Canada
| Age groups | Body weight (kg) | Inhalation rate (m3/day) | Drinking water (L/day) | Soil ingestion rate (µg/day) | Dust ingestion rate (µg/day) |
|---|---|---|---|---|---|
| 0 to 5 months | 6.3 | 3.7 | 0.83 | N/A | 21.6 |
| 6 to 11 months | 9.1 | 5.4 | 0.76 | 7.3 | 27.0 |
| 1 year | 11 | 8.0 | 0.36 | 8.8 | 35.0 |
| 2 to 3 years | 15 | 9.2 | 0.43 | 6.2 | 21.4 |
| 4 to 8 years | 23 | 11.1 | 0.53 | 8.7 | 24.4 |
| 9 to 13 years | 42 | 13.9 | 0.74 | 6.9 | 23.8 |
| 14 to 18 years | 62 | 15.9 | 1.09 | 1.4 | 2.1 |
| Adults (19+) | 74 | 15.1 | 1.53 | 1.6 | 2.6 |
a Health Canada [modified 2022].
| Route of exposure | 0 to 5 months(human milk-fedb) | 0 to 5 months (formula fedc) | 6 to 11 months (human milk-fedb) | 6 to 11 months (formula fedc) | 1 year | 2 to 3 years | 4 to 8 years | 9 to 13 years | 14 to 18 years | Greater than or equal to 19 years |
|---|---|---|---|---|---|---|---|---|---|---|
| Indoor aird | 7.8x10-3 | 7.8x10-3 | 7.9x10-3 | 7.9x10-3 | 9.7x10-3 | 8.2x10-3 | 6.4x10-3 | 4.4x10-3 | 3.4x10-3 | 2.7x10-3 |
| Drinking watere | N/A | 0.35 | 0.23 | 0.23 | 8.8x10-2 | 7.7x10-2 | 6.2x10-2 | 4.8x10-2 | 4.8x10-2 | 5.6x10-2 |
| Food and beveragesf | 9.4x10-2 | 0.38 | 0.69 | 0.94 | 0.64 | 0.64 | 6.4x10-2 | 4.1x10-2 | 2.7x10-2 | 2.4x10-2 |
| Dustg | 8.2x10-2 | 8.2x10-2 | 7.1x10-2 | 7.1x10-2 | 7.6x10-2 | 3.4x10-2 | 2.5x10-2 | 1.4x10-2 | 8.1x10-4 | 8.4x10-4 |
| Total intake | 0.18 | 0.83 | 1.0 | 1.2 | 0.81 | 0.76 | 0.16 | 0.11 | 0.079 | 0.083 |
Abbreviations: N/A, not applicable
a Estimated intakes of TPHP from ambient air were negligible when using the maximum concentration of TPHP in outdoor air (0.0022 µg/m3; Shoeib et al. 2014). No monitoring data of soil in North America were identified. An estimated predicted environmental concentration (PEC) for soil representing the worst case scenario (that is, the maximum PEC for all the aryl OPs of 2400 ng/g) and a maximum concentration of 46 µg/kg from a soil study for commercial areas in China (Cui et al. 2017) were available, but resulted in negligible exposures.
b Human milk-fed infants are assumed to consume solely human milk for 6 months. No Canadian data on the concentration of TPHP in human milk were identified. The maximum concentration of TPHP in human milk of 0.76 ng/mL (7.6x10-4 µg/g) measured from 100 women in the U.S. (Ma et al. 2019) was used to derive estimates of daily intake of TPHP for 0 to 5 month olds and 6 to 11 month olds. The median human milk consumption of 127.95 g/kg bw/day for 0 to 6 month olds reported by Arcus-Arth et al. (2005) was used to derive estimates of daily intake for exclusively human milk-fed 0 to 5 month olds. Infants 6 to 11 months old were assumed to consume 632 mL of human milk per day, in addition to some solid foods (Health Canada 2018a). See the footnote on daily intake from food for details on estimates of dietary exposure to TPHP from solid foods for 6 to 11 month olds.
c Exclusively formula-fed infants 0 to 5 months old are assumed to consume 826 mL of infant formula per day and formula is assumed to be the only dietary source for infants under 6 months (Health Canada 2018a). Formula-fed infants 6 to 11 months old are assumed to consume 764 mL of formula per day, in addition to some solid foods (Health Canada 2018a). Drinking water is used to reconstitute formula, so, therefore, infants 0 to 5 months and 6 to 11 months are assumed to consume 826 mL and 764 mL of drinking water per day, respectively. See footnotes on daily intake from food for details on estimates of dietary exposure to TPHP from solid foods and on drinking water for details on levels of TPHP in water used for estimates for 6 to 11 month olds. Daily intake of powdered formula was estimated assuming 9 g of dry formula is reconstituted with 60 mL of water (Abbott Nutrition 2024; Mead Johnson Nutrition 2020, 2023). The means of 19.53 and 24.07 ng/g TPHP in powdered stage 1 (for 0 to 6 month olds) and stage 2 (for 7 to 12 month olds) formulas, respectively, reported by Chen et al. (2022) were used to derive estimates of daily intake for each age group.
d The 95th percentile indoor air concentration of TPHP (0.0152 µg/m3, from Toronto and Ottawa, ON) (Yang et al. 2019) was used for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
e The maximum estimated aquatic PEC for all the aryl OPs of 2.7 µg/L was chosen as a conservative approach for deriving general population intakes of TPHP from drinking water (ECCC, HC 2021).
f Unless specified otherwise (for example, human milk, powdered formula), refer to Appendix B for details of the assessment of dietary exposure to TPHP. For 6 to 11 month olds, dietary exposure from solid foods was assumed to be the same as for 1 to 3 year olds; however consumption information for this exposure scenario was based on the broader food category “baby food products” which may include infant formula in addition to various infant foods and, therefore, may overestimate exposure from solid foods for 6 to 11 month olds. Dietary exposure for adults 19 years of age and older was calculated using the dietary exposure to TPHP estimated for 19 to 30 year olds, which may overestimate exposure from food for those 31 years of age and older.
g The highest 95th percentile concentration of TPHP (23,972 ng/g) in the CHILD Cohort Study (Navaranjan et al. 2021) measured in various Canadian cities was selected for deriving estimates of daily intake for dust exposure.
Appendix B. Data used in dietary exposure estimates for TPHP
The food consumption data used in the subject assessment were based on the Food Consumption Table (Health Canada 2018b), derived from 24-hour dietary recall survey results from the 2015 Canadian Community Health Survey (Statistics Canada 2015). Health Canada’s Food and Nutrition Directorate conservatively and deterministically estimated single-day mean ‘all persons (AP)’ total dietary exposures by multiplying the assumed maximum concentration of a given food category by the mean quantity of that food reportedly consumed by each age group. Exposures across food categories for each age group were then summed to obtain a mean ‘total’ exposure estimate for TPHP at each age group (Table B-2).
| Food Category Used in Dietary Exposure Assessment | Maximum TPHP Concentration (ng/g) | Reference |
|---|---|---|
| Pasta, rice, cereal grains & flours; White breads; Whole meal breads; Other breads; Whole grain, oats and high fibre breakfast cereals; Breakfast cereals | 4.55 | Wang and Kannan 2018 |
| Cookies, Biscuits & Granola bars; and Cakes, pies, danishes and other pastries, commercial | 1.24 | Poma et al. 2017 |
| Milk; and Creams; Frozen Dairy products | 0.15 | Wang and Kannan 2018 |
| Cheeses | 0.15 | Wang and Kannan 2018 |
| Yogurts | 0.15 | Wang and Kannan 2018 |
| Fats (butter, margarine, vegetable oil, shortening, and animal fats) | 0.15 | Wang and Kannan 2018 |
| Beef; Veal; Lamb; Pork, fresh and ham; Chicken, Turkey & Other birds; Livers & Liver pates; Sausages (fresh & cured); Game meats; and Luncheon meats (canned & cold cuts) | 0.18 | Wang and Kannan 2018 |
| Fish | 0.28 | Wang and Kannan 2018 |
| Shellfish | 0.15 | Wang and Kannan 2018 |
| Egg | 0.17 | Poma et al. 2018 |
| Vegetables (excluding potatoes) | 1.00 | He et al. 2018 |
| Potatoes (cooked and fried, incl. potato chips) | 1.29 | Poma et al. 2018 |
| Fruits | 1.4 | Zhao et al. 2019a |
| Fruit juices; and Liqueurs | 0.0013 | He et al. 2018 |
| Non-alcoholic beverages (soft & fruit drinks, etc.) | 0.50 | Poma et al. 2017 |
| Beer | 0.0013 | He et al. 2018 |
| Tea; Coffee | 0.0013 | He et al. 2018 |
| Baby food (including infant formula) | 423 | CFIA 2019 - 2021 |
| Sugars (white and brown); and Candies, gums, etc.; Jams, jellies and marmalade; Other sugars (syrups, molasses, honey, etc.); Popsicle, sherbet; Jello, dessert toppings and pudding mixes, commercial; and chocolate bars | 0.50 | Poma et al. 2017 |
| Soups with vegetables; and soups without vegetables | 2.41 | Poma et al. 2018 |
| Gravies; Sauces (white, béarnaise, soya, tartar, ketchup, etc.); and salad dressings (with or without oil) | 0.50 | Poma et al. 2017 |
| Age Group, Females & Males (years) | Mean ‘All Persons’ Dietary Exposure to TPHPa (µg/kg bw per day) |
|---|---|
| 1 to 3 | 0.64 |
| 4 to 8 | 0.064 |
| 9 to 13 | 0.041 |
| 14 to 18 | 0.027 |
| 19 to 30 | 0.024 |
| 31 to 50 | 0.023 |
| 51 to 70 | 0.021 |
| 71+ | 0.021 |
a Since consumption figures in the 2015 CCHS Food Consumption Table are on an age-group or age-sex group basis, and exposures were summed across age-groups rather than on an individual basis, it is not appropriate to sum high percentile consumption figures or high percentile exposures estimates across food categories since they likely represent different micro populations. In this regard, upper percentile exposure estimates using upper percentile consumption figures were not calculated.
Appendix C. Summary of data for TPHP measured in human milk
| Location | n | Detection frequency | Mean | Concentration range | Study |
|---|---|---|---|---|---|
| USA | 100 | 55% | 0.149 ng/mL | ND (LOQ=0.109 ng/mL) to 0.760 ng/mL | Ma et al. 2019 |
| USA (Seattle area) | 50 | 61% | 0.0820 ng/mL | <0.062 (MDL) to 0.241 ng/mL | Zheng et al. 2021 |
| Valencia, Spain | 20a | NR | 12.3 ng/g lw (median=9.9 ng/g lw) | 17.3 ng/g lw (max) | Beser et al. 2020 |
| Swedenb | 5 pooled samples and 1 single person sample | N/A | N/A (median 8.5 ng/g lipids) | 5.0 to 11 ng/g lipids | Sundkvist et al. 2010 |
| Beijing, China | 105c | 99% | 13.1 ng/mL (median 1.07 ng/mL) | 87.1 ng/mL (max) | Chen et al. 2021 |
| Japan, Philippines, and Vietnamd | 87 | 86%e | NR (medians 1.4 to 19 ng/g lw) | NDf to 140 ng/g lw | Kim et al. 2014 |
Abbreviations: LOQ, limit of quantification; lw, lipid weight; MDL, method detection limit; N/A, not applicable; ND, not detected; NR, not reported
a Samples were obtained from 14 women aged 30-39.
b Samples were obtained from 286 women in 4 Swedish towns. Pooled composites of samples collected from 1997 to 2003 were tested. The highest concentration for a pooled sample was 10 ng/g lipids for 90 women in Uppsala in 1998. Only one sample collected from one woman in Umeå in 2007 gave a higher level of TPHP in this study, at 11 ng/g lipids.
c Samples collected from women aged 25 to 40.
d Samples from Kanagawa, Japan were mainly collected in urban areas. In the Philippines, samples were collected in Malate (an urban area) and Payatas (located near a municipal waste dumping site). In Vietnam, samples were collected in Hanoi (a densely populated city) and in Bui Dau and Trang Minh (e-waste recycling sites).
e The Kim et al. (2014) study reported an overall detection frequency of TPHP in samples of 86% for all locations.
f The Kim et al. (2014) study reported the MDLs for all substances included in the study as in the range of 0.01 to 0.08 ng/g lw.
Appendix D. Parameters used to estimate human exposure from use of products and manufactured items available to consumers
Products
Sentinel exposure scenarios were used to estimate the potential exposure to TPHP and TBOEP from products available to consumers. Dermal exposure to nail polish, lubricants and greases, and paints were estimated using ConsExpo Web (2021) or algorithms (see below for more details). Body weights used in the exposure estimates are presented in Table A-1. Scenario-specific assumptions are provided in Table D-1.
| Product (substance) | Assumptions |
|---|---|
| Top coat (assume 1 coat applied to finger- and toenails)b (TPHP) | Maximum Concentration of TPHP: 10% ConsExpo Web: Dermal – instant application Frequency (use/day): per event exposures derived |
| Nail polish (assume 2 coats applied to finger- and toenails)b (TPHP) | Maximum Concentration of TPHP: 15% ConsExpo Web: Dermal – instant application Frequency (use/day): per event exposures derived |
| Base coat (assume 1 coat applied to finger- and toenails)b (TPHP) | Maximum Concentration of TPHP: 10% ConsExpo Web: Dermal – instant application Frequency (use/day): per event exposures derived |
| Nail kit (assume 2 coats of activator applied to fingernails)b (TPHP) | Maximum Concentration of TPHP: 30% in Activator ConsExpo Web: Dermal – instant application Frequency (use/day): per event exposures derived |
| Lubricants and greases (for example, power steering fluid) (TPHP) | Concentration of TPHP: 0.1% to 1% (SDS 2017, 2021; US EPA 2020b) Dermal: Intake = (concentration × surface area × film thickness × density × dermal absorption)/body weight Exposed surface area: 6 cm2 (surface area of fingertips of fingers and thumb, 19+ years) |
a Dermal absorption of 50% used in all dermal estimates (refer to section 2.4.1.2).
b Assume exposure does not occur through the nail but only the skin surrounding the nail (Bremmer et al. 2006).
Manufactured Items
On the basis of the available information, dermal exposure intakes were estimated for direct contact with foam-containing mattresses and related manufactured items for all age groups and with foam-containing infant or child restraint systems for 0 to 13 year olds (includes booster seats). Oral exposure estimates were also derived for 0 to 3 year olds from mouthing (sucking) on foam-containing manufactured items intended for children, as well as textiles. The exposure parameters and values used to estimate exposures are presented below and are on the basis of conservative assumptions. Body weights used in the exposure estimates are presented in Table A-1. Limited data were identified on the relationship between migration rates from foam and the concentrations in foam tested; this aspect could not be quantified for any of the flame retardants included herein.
Dermal exposure intake estimates:
Intake (mg/kg-bw/day) = [surface area, SA (cm2) × skin contact factor, SCF × textile penetration factor, TPF × migration rate, M (mg/cm2/hr) × exposure duration, ED (hr/day) × dermal absorption, DA] / body weight, BW (kg)
| Age group | Surface area of skin contacta (SA) (cm2) | Exposure duration for lying on foam-containing mattresses or upholstered furnitureb (ED) (hr/d) | Exposure duration for sitting in an infant or child restraint system (including booster seats) (ED)c,d (hr/d) |
|---|---|---|---|
| 0 to 5 months | 520 to 1750 | 12.7 | 3 |
| 6 to 11 months | 668 to 2250 | 12.7 | 3 |
| 1 year | 753 to 2650 | 13 | 3 |
| 2 to 3 years | 705 to 3250 | 11.8 | 3 |
| 4 to 8 years | 922 to 4450 | 11.25 | 3 |
| 9 to 13 years | 1332 to 6700 | 10.83 | 3 |
| 14 to 18 years | 1642 to 8600 | 9.17 | n/a |
| 19+ years | 2005 to 9350 | 8 | n/a |
a A range in surface areas (SA) was used to represent dermal contact with mattresses and upholstered furniture while only the lower SAs were used to represent dermal contact with the foam/fabric of infant or child restraint systems (including booster seats). For the lower SAs used, it was assumed that an individual is wearing shorts and a t-shirt that cover half of the limbs. The surface area of exposure is a fraction of the lower half of the limbs (arms and legs) and the back of the head. The surface areas of the limbs (Health Canada [modified 2022]) were multiplied by one half to account for clothing coverage and then were multiplied by one third to account for the triangular shape of limbs, where only one side is directly in contact with the foam-containing manufactured item (US CPSC 2006). The surface area of the head (Health Canada [modified 2022]) was multiplied by a factor of 0.5 to represent exposure to the back of the head only. For the higher SA used, it was assumed that half of the body was in dermal contact with the mattress or upholstered furniture (US EPA 2012).
b Median sleep times reported in US EPA (2011), Tables 16-25 (0 to 6 months) and 16-26 (all other age groups), were used as the exposure durations for lying on foam-containing mattresses or upholstered furniture.
c An exposure duration of 3 hours per day was selected for sitting in an infant or child restraint system (including booster seats) based on the leisure sitting duration reported by the US CPSC (2006) and the highest 95th percentile daily time spent in vehicles for children aged <1 year to 11 years in the Canadian Human Activity Patter Survey 2 (CHAPS 2) (2 hr 50 min for 5 to 11 year olds) (Matz et al. 2014).
d Infant and child restraint system regulations in Canada vary by province and territory (CPSAC 2019). On the basis of relevant age and weight considerations, dermal exposure estimates were derived for various age groups up to and including 9 to 13 year olds.
| Parameter | TDCPP | TBB | TPHP |
|---|---|---|---|
| Water solubility (mg/L) | 18.1 | 0.00282 | 2.25 |
| Log Kow (dimensionless) | 3.69 | 7.71 | 4.42 |
| Rate of migration from covered foam (mg/cm2/hr)a | 5.62x10-5 | 1.97x10-5 | 2.45x10-5 b 4.54x10-5 c |
a The migration rates for TDCPP and TBB were determined in migration studies performed on treated furniture foam by the US CPSC (US CPSC 2005). In the study, a furniture mini-seat mock-up consisting of a block of foam covered with cotton fabric and attached to plywood was prepared. The mini-seat was wetted with a saline solution, to mimic sweat, and pressure was applied to imitate the action of sitting. The migration rates of TDCPP and TBB were determined on the basis of the reported maximum daily amount extracted for each substance (8 and 2.8 µg, respectively) onto a filter paper (5-cm diameter) over the course of the migration testing period (6 hours) (US CPSC 2005).
b Calculated on the basis of plotting a straight line between water solubilities and migration rates for TDCPP and TBB with equation y = (2×10-6) × water solubility + 2×10-5
c Calculated using differential equations on the basis of TDCPP and TBB migration rates, water solubilities, and log Kow values using the equation migration rate = x (log of water solubility) + y (log Kow). For TDCPP this is 0.0000562 mg/cm2/hr = x (1.26) + y (3.69) and for TBB this is 0.0000197 = x (-2.55) + y (7.71). Solving for x and y results in: x = 0.0000188 and y = 0.0000088. For TPHP, migration rate = 0.0000188 (log of water solubility) + 0.0000088 (log Kow) = 0.0000188 (0.35) + 0.0000088 (4.42) = 4.54 × 10-5 mg/cm2/hr.
| Parameter | TDCPP | TCEP | TBOEP |
|---|---|---|---|
| Water solubility (mg/L) | 18.1 | 7820 | 1670 |
| log Kow (dimensionless) | 3.69 | 1.78 | 3.81 |
| Rate of migration from covered foam (mg/cm2/hr)a | 0.00297 | 0.0207 | 0.00624b 0.0143c |
a The migration rates for TDCPP and TCEP were determined in migration studies performed on treated furniture foam by the Danish EPA (2015) as reported by ECHA (2018). The migration rates of TCEP and TDCPP were determined using children’s products (that is, infant or child restraint systems, baby slings, baby mattresses) by submerging pieces of foam from these products (usually with some of the fabric covering included in the samples) in sweat simulant and incubating them at 37°C for 3 hours (Danish EPA 2015). The migration rate for TDCPP used here is the average of the rates found across all samples for this flame retardant while the migration rate for TCEP was from a single item (ECHA 2018).
b Calculated on the basis of plotting a straight line between water solubilities and migration rates for TDCPP and TCEP with equation y = (2×10-6) × water solubility + 0.0029
c Calculated using differential equations on the basis of TDCPP and TCEP migration rates, water solubilities, and log Kow values using the equation migration rate = x (log of water solubility) + y (log Kow). For TDCPP this is 0.00297 mg/cm2/hr = x (1.26) + y (3.69) and for TCEP this is 0.0207 = x (3.89) + y (1.78). Solving for x and y results in: x = 0.0059 and y = -0.0012. For TBOEP, migration rate = 0.0059 (log of water solubility) – 0.0012 (log Kow) = 0.0059 (3.22) – 0.0012 (3.81) = 1.43 × 10-2 mg/cm2/hr.
| Parameter | TPHP | TBOEP |
|---|---|---|
| Skin contact factora (SCF) | 1 | 1 |
| Textile penetration factorb (TPF) | N/A | 0.1 |
| Migration ratec (M) (mg/cm2/hr) | 2.45×10-5 to 4.54×10-5 | 6.24×10-3 to 1.43×10-2 |
| Dermal absorption (DA) | 50%d | 100%e |
Abbreviations: N/A, not applicable
a No substance-specific skin contact factors, that is, the fraction of substance on a surface adhering to skin, were identified in the literature for TPHP or TBOEP. A value of 1 was therefore selected for both substances, with the implicit assumption that all of the chemical in contact with the skin is available for absorption.
b A textile penetration factor (TPF) was not applied for TPHP since the migration rates used for extrapolation were found using covered foam samples (see Table D-3). A TPF was applied for TBOEP to account for the migration rates used for extrapolation (that is, TDCPP and TCEP) being determined using uncovered foam (see Table D-4). No substance-specific textile penetration data were identified in the literature. A value of 0.1 (Driver et al. 2007 as cited in ECHA 2018) was therefore used for the TPF for TBOEP.
c Refer to Tables D-3 and D-4 for derivation of migration rates.
d Dermal absorption of TPHP was adjusted to 50% (refer to section 2.4.1.2).
e Dermal absorption of TBOEP was assumed to be 100% (refer to section 3.4.1.1).
Oral exposure intake estimates:
Intake (mg/kg bw/day) = (SA [cm2] × M [mg/cm2/hr] × ED [hr/day]) / BW (kg)
It is assumed that TPHP and TBOEP are completely absorbed through the oral route and that a textile covering on a foam object would not affect migration. The estimated migration rates for TPHP were extrapolated from experimental data from covered foam (see Table D-3), therefore the migration rates were divided by a textile penetration factor (TPF) of 0.1 (see Table D-4) when estimating oral exposure. For TBOEP, the estimated migration rates were extrapolated from experimental data from uncovered foam (see Table D-4), therefore no adjustment was necessary to account for a textile cover.
| Age Group | Surface area moutheda (SA) (cm2) | Exposure durationb (ED) (min/d) |
|---|---|---|
| 0 to 5 months | 10 | 24.5 |
| 6 months to 3 years | 10 to 50 | 24.5 |
a Surface area of object that is mouthed estimated to range from 10 to 50 cm2 based on information from U.S. CPSC 2006 and US EPA 2012. For 0 to 5 month olds, a single value of 10 cm2 was assumed given their smaller size and limited ability to move around.
b The mouthing duration of 24.5 min/d for children’s foam products such as nap mats, infant and child restraint systems, and small furniture was based on the highest mean duration for “other objects” (for 6–9 month olds) in Norris and Smith (2002) [cited in U.S. EPA (2011)].
