Industry Guide to Vaping Products Subject to the Canada Consumer Product Safety Act
1. Introduction
This guide provides information to industry on requirements under the Canada Consumer Product Safety Act (CCPSA) for vaping products that are manufactured, imported, advertised or sold in Canada. These requirements apply to vaping productsFootnote 1 that are consumer productsFootnote 2, within the meaning of the CCPSA. In particular, this guide provides information on the requirements set out in Part 2 of the Vaping Products Labelling and Packaging Regulations (VPLPR). In addition, section 5 of this guide discusses authorities under the CCPSA that can be used to address a danger to human health or safety posed by a vaping product not marketed for a therapeutic use or by a cannabis accessoryFootnote 3 (such as a vaporizer represented to be used in the consumption of cannabis) not marketed for a therapeutic use.
The information in this guide is not intended to substitute for, supersede, or limit the requirements under the applicable legislation. In case of any discrepancy between this guide and the legislation, the legislation will prevail.
This guide may be updated from time to time without notice. For the most recent version of the guide, consult the Consumer product safety reports page.
2. Legislation
Vaping products supplied on the Canadian market are subject to, among others, the following Acts and their Regulations:
- Canada Consumer Product Safety Act (CCPSA)
- Tobacco and Vaping Products Act (TVPA)
- Food and Drugs Act (FDA)
- Non-smokers' Health Act
- Vaping Products Labelling and Packaging Regulations (VPLPR)
- Other vaping-product specific regulations
In addition to the CCPSA, TVPA and FDA, vaping products also have to meet requirements under the Non-smokers' Health Act. Additional legislation, such as the Consumer Packaging and Labelling Act, may apply to vaping products. Industry members are advised to consult with relevant federal, provincial, territorial, municipal and other legislation for more information on specific requirements.
3. Canada Consumer Product Safety Act
Vaping products and cannabis accessories that are manufactured, imported, advertised or sold in Canada are subject to provisions under the CCPSA, which include:
- a prohibition on the manufacture, import, advertisement or sale of any consumer product that is a "danger to human health or safety"Footnote 4 as defined in the CCPSA (see paragraphs 7(a) and 8(a)),
- requirements for preparing and maintaining documents relating to suppliers and the location and duration of retail sale of a product (see section 13), and
- requirements for mandatory incident reporting (see section 14).
Under the CCPSA, Health Canada has the authority to:
- order tests or studies on a product (see paragraph 12(a)), and
- order recalls and other measures (see sections 31 and 32).
Enforcement actions taken by Health Canada on a non-compliant consumer product, such as a vaping product that does not comply with a requirement under Part 2 of the VPLPR (see section 4 of this guide), are authorized by the CCPSA and may include:
- commitment to product correction by industry;
- negotiation with industry for the voluntary removal of the product from the market;
- orders by Health Canada to take specified actions such as a recall;
- product seizure by Health Canada; and
- prosecution.
A Quick Reference Guide for the CCPSA is available on the Government of Canada website. The reader is directed to the legislation for full information on the requirements and prohibitions. See Appendix A - Information Resources of this document for links to references.
4. Vaping Products Labelling and Packaging Regulations
The VPLPR were published in Canada Gazette, Part II, on December 25, 2019. The requirements of the VPLPR came into force on July 1, 2020, with one exception. The child-resistant container requirements for refillable vaping devices and refillable vaping parts come into force on January 1, 2021.
The VPLPR are jointly authorized by the TVPA and the CCPSA.
- The requirements under Part 1 of the VPLPR are authorized by the TVPA. Part 1 applies to vaping products, as defined in the TVPA, and vaping products as set out in sections 2 and 3 of the VPLPR.
- The requirements under Part 2 of the VPLPR are authorized by the CCPSA. Part 2 applies to vaping products, as defined in the TVPA, and vaping products that are consumer products as set out in section 44 of the VPLPR.
The CCPSA and its associated regulations, such as the VPLPR, do not apply to a vaping product that is subject to the FDA. Therefore, vaping products marketed for a therapeutic use are out of the scope of the CCPSA and this guide.
The VPLPR do not apply to a tobacco product or accessory, as defined in the TVPA.
The VPLPR also do not apply to cannabis or a cannabis accessory, as defined in subsection 2(1) of the Cannabis Act.
4.1 Structure and Objectives of the VPLPR
The VPLPR are divided into three Parts. Part 1 of the VPLPR sets out labelling requirements pursuant to the TVPA. Part 2 of the VPLPR sets out labelling and packaging requirements pursuant to the CCPSA. Part 3 of the VPLPR sets out the coming-into-force provisions, transitional provision and related amendment. The legislative authority used for taking enforcement action on non-compliance will depend on which Part the requirement is set out in the VPLPR.
4.1.1 Part 1
The objective of Part 1 of the VPLPR is to use the authorities set out in the TVPA to help protect young persons and non-users of tobacco from exposure to, and dependence on, nicotine and to help prevent vaping product use from leading to the use of tobacco products. More specifically, Part 1 of the VPLPR aims to enhance awareness of the health hazards of using vaping products and prevent the public from being deceived or misled with respect to the health hazards posed by their use. In addition to other requirements, Part 1 of the VPLPR:
- requires that vaping products containing nicotine display a health warning that nicotine is highly addictive;
- requires that vaping products containing nicotine display a nicotine concentration statement; and
- sets out three permitted expressions that may be displayed on the product or package to indicate when a vaping substance does not contain nicotine.
The reader is directed to the VPLPR for full information on the requirements in Part 1.
4.1.2 Part 2
The objective of Part 2 of the VPLPR is to use the authorities set out in the CCPSA to help protect the health and safety of young children by reducing the risk that they will ingest vaping substances containing toxic concentrations of nicotine. In addition to other requirements, Part 2 of the VPLPR:
- requires every vaping substance to display a list of ingredients;
- requires that a vaping product must not contain nicotine in a concentration of 66 mg/mL or more. Regulated parties are directed to the Nicotine Concentration in Vaping Products Regulations under the TVPA as these regulations prescribe a limit of 20 mg/mL;
- requires every immediate container of vaping substance (including stand-alone refill containers) containing nicotine in a concentration of 0.1 mg/mL or more to be child-resistant and to display toxicity information; and
- requires every refillable vaping device or refillable vaping part to be child-resistant.
The requirements in Part 2 of the VPLPR are discussed in subsection 4.2 of this guide. The reader is directed to the VPLPR for full information on the requirements in Part 2.
4.1.3 Part 3
Part 3 of the VLPLR sets out a transitional provision, a related amendment and coming-into-force dates. There are two coming-into-force dates that apply to specific sections of the VPLPR: July 1, 2020 and January 1, 2021. As of January 1, 2021, Part 3 of the VPLPR has served the purpose for which it was enacted.
The reader is directed to the VPLPR for full information on the requirements in Part 3.
4.1.4 Defined Terms and Undefined Terms
The definitions in Part 1 of the VPLPR apply only to the requirements set out in Part 1. Additionally, the VPLPR state that all words and expressions that are not defined in Part 1 have the same meaning as those in the TVPA. Therefore, the Part 1 requirements must be read in conjunction with the TVPA.
The definitions in Part 2 of the VPLPR apply only to the requirements set out in Part 2. Additionally, the VPLPR state that all words and expressions that are not defined in Part 2 have the same meaning as those in the CCPSA. Therefore, the Part 2 requirements must be read in conjunction with the CCPSA.
It is important to note that the same terms may be defined differently across the two Parts of the VPLPR. For example, "display surface" and "main display panel" are defined differently across the two Parts of the VPLPR.
In some cases, the same word may be defined differently across the two Acts. For example, the term "manufacture" in the TVPA (relevant for the Part 1 requirements) is defined differently than the term "manufacture" in the CCPSA (relevant for the Part 2 requirements). Part 1 further defines the term "manufacturer" to limit the scope of the definition from the TVPA for the purpose of the VPLPR. Part 2 describes the term "manufacturer" within the definition of a "responsible person". The term "sell" is another example where the same word is defined differently in the respective Acts. The reader is encouraged to consult the CCPSA and TVPA to familiarize themselves with these differences.
The definitions in the VPLPR for "vaping product", "vaping device", "vaping part" and "vaping substance" reference the definitions for the same terms in section 2 of the TVPA. Vaping products include a vaping device (commonly referred to as an e-cigarette, vape pen, vaporizer or vape mod), a vaping part (e.g., cartridge, pod, tank, mouth piece, etc.) as well as a vaping substance (commonly referred to as an e-liquid).
A definition for the term "immediate container" is set out in subsection 43(1) of Part 2 of the VPLPR. This definition includes the terms vaping device and vaping part. It is frequently referenced in Part 2 and should be carefully noted.
"immediate container means the container, including a vaping device or vaping part, that is in direct contact with a vaping substance or which it is reasonably foreseeable will be in direct contact with such a substance."
An immediate container can be a stand-alone container of vaping substance, for example, one that is used to refill a vaping device or part. In the case of a vaping device or a vaping part, an immediate container can be an empty container, for example, a refillable vaping device or vaping part.
4.2. Requirements for Vaping Products under Part 2 of the Vaping Products Labelling and Packaging Regulations
4.2.1 Responsibility for Complying with the VPLPR
The requirements apply to any person who manufactures, imports, advertises or sells a vaping product, as defined in Part 2 of the VPLPR, that is a consumer product (CCPSA, see section 6).
A responsible person, as defined in Part 2 of the VPLPR, must meet additional specific requirements related to the evaluation of a child-resistant container (see section 53) and to the preparation and retention of child-resistant container documents (see section 54). The definition for responsible person can be found in subsection 43(1).
4.2.2 Requirement for a List of Ingredients
Every vaping substance, even if it is without nicotine, must display a list of ingredients in English and French (see subsection 47(1)). This list must set out the common name, without abbreviation, of each ingredient that is present in the vaping substance. For example, propylene glycol or vegetable glycerin must be set out with their common names. The use of an abbreviation such as PG, for propylene glycol, or VG, for vegetable glycerin, is not permitted. The requirement does not prescribe the common name for vaping substance ingredients. The common name of an ingredient is the name that would be known to Canadians. It is generally not the chemical name, but in some cases it may be. For example, for a vaping substance that contains nicotine salts, the common name that must be set out in the ingredient list can include: nicotine salt, nicotine salts, or a similar common name.
4.2.2.1 Flavour Ingredients
If any ingredient or combination of ingredients is added to a vaping substance solely to produce a particular flavour or combination of flavours, those ingredients must be denoted only by the single term "flavour" in the English list of ingredients, and by the single term "arôme" in the French list of ingredients (see subsection 47(2)). The use of common names within the list of ingredients is not permitted for ingredients that are added solely to produce a particular flavour.
4.2.2.2 Order of Ingredients in the List of Ingredients
Any ingredients that are present in a vaping substance in a concentration of 1% or more must be set out in descending order of their proportion in the list of ingredients (see paragraph 61(1)(a)). Any ingredients that are present in a vaping substance in a concentration of less than 1% may be set out in any order immediately following the ingredients present at a concentration of 1% or more (see paragraph 61(1)(b)). The percentage is obtained from the ratio of the weight of an ingredient to the volume of the vaping substance (see subsection 61(2)). The concentration of an ingredient is not required to be displayed within the list of ingredients.
As discussed in the preceding section, "flavour" and "arôme" is to appear only once in the English list of ingredients and only once in the French list, respectively, even if multiple ingredients are added solely to produce a particular flavour or flavours. The total proportion of all flavour ingredients must be calculated in order to determine the placement of the term "flavour" and "arôme" in the list of ingredients (see subsection 61(3)).
4.2.2.3 Placement of the List of Ingredients
Every vaping substance is required to display a list of ingredients (see subsection 47(1)). The list of ingredients must be displayed on the display surface of the immediate container of the vaping substance and on the display surface of any exterior package (see subsection 48(1)). There are two exceptions:
- If the immediate container is a vaping device or vaping part and it is not packaged, then the list of ingredients must be displayed on a tag attached to the vaping device or vaping part (see subsection 48(2)).
- For small immediate containers:
- If the main display panel of the immediate container has an area of greater than 15 cm2 but less than 45 cm2, then the list of ingredients must be displayed either on the display surface of the exterior package, the display surface of the immediate container or on a tag attached to the immediate container (see paragraph 48(3)(a)); and
- If the main display panel of the immediate container has an area equal to or less than 15 cm2, then the list of ingredients must be displayed on the display surface of the exterior package or on a tag attached to the immediate container (see paragraph 48(3)(b)).
The main display panel is defined in subsection 43(1) of the VPLPR. The area of the main display panel is used for determining whether or not the immediate container or the exterior package is subject to certain labelling exceptions.
The main display panel is a portion of the overall display surface. The display surface refers to the portion of an immediate container or an exterior package where information can be displayed. These terms are defined in subsection 43(1) of the VPLPR.
4.2.3 Nicotine Toxicity
Nicotine is highly toxic when ingested. It is found in many vaping substances. Nicotine ingestion can adversely affect the cardiovascular and central nervous systems, even when it is ingested in very small quantities. Young children are particularly susceptible to the harmful effects of nicotine poisoning through ingestion due to their physiological status.
Flavours may be added to vaping substances to make them more appealing to users. These flavours may also make vaping substances more appealing to young children and increase the risk that children will gain access to a vaping substance, swallow it and be poisoned. A number of cases of acute nicotine poisoning have occurred when vaping substances containing nicotine were ingested. A child may experience serious and potentially life-threatening health effects following ingestion of even a small amount of vaping substance that contains nicotine.
Part 2 of the VPLPR sets out requirements that help to protect the health and safety of young children by reducing the risk that they will ingest vaping substances containing toxic concentrations of nicotine.
4.2.3.1 Maximum Allowable Nicotine Concentration
The VPLPR requires that a vaping product must not contain nicotine in a concentration of 66 mg/mL or more. The maximum allowable nicotine concentration requirement applies to all vaping products containing nicotine, even if exposure to the vaping substance that is in a form other than an aerosol is impossible during the reasonably foreseeable use of the container. An example of this would be an immediate container that is permanently sealed and not refillable.
It is important to note that the Nicotine Concentration in Vaping Products Regulations under the TVPA imposes a nicotine concentration limit of 20 mg/mL for vaping products marketed in Canada. For more information, see the Nicotine Concentration in Vaping Products Regulations under the TVPA.
For vaping products intended only for export, the VPLPR continues to prohibit products with a nicotine concentration of 66 mg/mL or more.
4.2.4 Child-Resistant Containers
To help protect young children from ingesting vaping substances containing toxic concentrations of nicotine and being poisoned, the VPLPR set out requirements for child-resistant containers.
The child-resistant container requirements apply to immediate containers that contain a vaping substance with a nicotine concentration of 0.1 mg/mL or more (see subsection 50(1)). For example, a stand-alone container of vaping substance containing nicotine in a concentration of 0.1 mg/mL or more must meet the requirements.
The child-resistant container requirements also apply to immediate containers that are vaping devices or parts whether or not they contain a vaping substance (see subsection 50(1)). The requirements apply to refillable vaping devices and refillable vaping parts, including components, tanks or reservoirs that may hold a vaping substance. That is, they apply even if the refillable vaping device or vaping part is not prefilled with a substance that has a nicotine concentration of 0.1 mg/mL or more.
The child-resistant container requirements do not apply if exposure to the vaping substance in a form other than its aerosol form is impossible during reasonably foreseeable use of the container (see subsection 50(2)). One example where this exception could be applied is to a permanently sealed pre-filled vaping part that is not refillable.
4.2.4.1 Explanation of Child-Resistant Container Requirements
In order for a container to meet the child-resistant container requirements set out in the VPLPR, at least one of two things must be true. The container must be either constructed so that it can only be opened by the use of a tool not supplied with the container (see paragraph 51(1)(a)), or it must meet the child test protocol requirements in one of the standards specified in paragraph 51(1)(b) of the VPLPR, or a standard that is at least equivalent. Note that the standards specified in paragraph 51(1)(b) are incorporated by reference on an ambulatory basis. If an amended version of the standard is published, the responsible person must apply the requirements of the amended version within 180 days of the publication date.
4.2.4.2 Child-Resistant Container Requirements
The VPLPR set out requirements for child-resistant containers in sections 51 through 56. These requirements include:
- Where an immediate container is prefilled with a vaping substance that has a nicotine concentration of 0.1 mg/mL or more, such as a stand-alone container, it must maintain the characteristics that make it child-resistant throughout the useful life of the vaping substance it contains (see section 52).
- For a vaping device or vaping part that is not prefilled, a child-resistant container must maintain the characteristics that make it child-resistant throughout its useful life (see section 52).
- The responsible person for the container must evaluate the compatibility of the vaping substance with its child-resistant container to ensure that the substance will not compromise or interfere with the proper functioning of the container (see paragraph 53(1)(a)).
- The responsible person for the container must determine that the container will maintain its proper functioning over the number of reasonably foreseeable openings and closings for the size and contents of the container (see paragraph 53(1)(b)).
- The responsible person for the container must be able to demonstrate compliance with the child-resistant container requirements by providing all required documentation to an inspector within 15 days of receipt of a request. This documentation includes, among other things, a test report indicating the container's results when tested according to the child test protocol of one of the three referenced standards, or that of an equivalent standard, if applicable, as well as documentation relating to the compatibility of the container and closure system with the vaping substance that is to be placed in it and the endurance of the child-resistant closure. Relevant documents must be kept for at least three years after the date of manufacture in Canada or import (see section 54).
- A child-resistant container must display directions for opening the container, and if applicable, closing it. Directions for closing are applicable when the container is not a single-use immediate container. The opening and closing directions must be displayed on the closure system of the immediate container or the display surface of the immediate container (see section 55). The directions can be displayed using either words, or a diagram or self-explanatory symbol, or both (see section 62).
- In the case of an immediate container that is a vaping device or vaping part, the opening and closing directions can be displayed on a package or on a leaflet inserted into the package. If the vaping device or vaping part is not packaged, the opening and closing directions must be displayed on a tag attached to the vaping device or vaping part (see subsection 55(2)).
- In the case of a single-use immediate container, a statement must be displayed in English and French that instructs the user to use the entire contents on opening because the container is not child-resistant after it has been opened. The mandatory statement is provided in subsection 56(1). In the case that the immediate container has an exterior package, the mandatory statement must also appear on the package (see subsection 56(2)). There are exceptions for small immediate containers (see subsection 56(3)) and small exterior packages (see subsection 56(4)). Small immediate containers, that are not packaged, with a main display panel greater than 15 cm2 but less than 45 cm2 must display the statement on the display surface of the immediate container or display the statement on a tag attached to the immediate container (see paragraph 56(5)(a)). If the main display panel area is equal to or less than 15 cm2, then the statement must be on a tag attached to the immediate container (see paragraph 56(5)(b)). Note that single-use immediate containers are required to be child-resistant on opening (see subsection 50(1)).
4.2.5 Requirements for Toxicity Information
The VPLPR set out a requirement to display toxicity information on an immediate container of a vaping substance containing nicotine in a concentration of 0.1 mg/mL or more, including those that are prefilled refillable vaping devices and their parts (see subsection 57(1)).
The toxicity information that must be displayed includes:
- the toxic hazard symbol (see paragraph 57(1)(a) and Schedule 1), and
- a toxicity warning and a first aid treatment statement (see paragraph 57(1)(b)).
If the immediate container is packaged, the above toxicity information must be displayed on the exterior package as well (see subsection 57(3)).
Please refer to section 4.2.6 of this guide for specific exceptions for this requirement.
4.2.5.1 Placement, Size and Format of the Toxicity Information
The toxic hazard symbol is the same as the one found in Schedule 2 of the Consumer Chemicals and Containers Regulations, 2001 under the CCPSA. The symbol must appear on the display surface as an exact reproduction, except with respect to size, as it appears in Schedule 1 of the VPLPR (see paragraph 57(1)(a)). See Figure 1 for a reproduction of the symbol from Schedule 1 of the VPLPR:
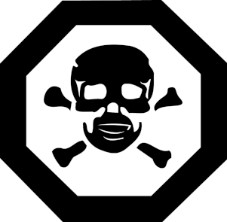
Figure 1. Hazard symbol in Schedule 1 of the VPLPR.
The size of the hazard symbol required on the display surface is based on the area of the main display panel of the immediate container and the area of the main display panel of the exterior package, where there is one. If the main display panel area is equal to or greater than 45 cm2, the diameter of the symbol must be at least as large as the diameter of an imaginary circle that has an area equal to 3% of the main display panel area (see paragraph 63(a)). If the main display panel area is less than 45 cm2, the diameter of the hazard symbol must be at least 6 mm (see paragraph 63(b)).
The toxicity warning and first aid treatment statement must be set out in English and French, exactly as worded in the VPLPR (see paragraph 57(1)(b)):
POISON: if swallowed, call a Poison Control Centre or doctor immediately.
POISON : en cas d'ingestion, appeler immédiatement un centre antipoison ou un médecin.
The above noted statement must appear either immediately beside or below the hazard symbol and the word poison must be in upper case bold-faced letters (see section 64).
Note that all of the information in English required in Part 2 (not only the toxicity information) can appear in one location on the display surface and all of the corresponding information in French can appear in another location on the display surface. With respect to the toxicity information, where the information is displayed in different locations on the display surface, two hazard symbols would be required, one for each official language.
4.2.6 Exceptions
The VPLPR establish various exceptions to the requirements laid out above. The child-resistant container requirements and toxicity information requirements are not applicable when exposure to the vaping substance in a form other than an aerosol is impossible during the reasonably foreseeable use of the container (see subsections 50(2) and 57(4)). One example where this exception could be applied is to a permanently sealed pre-filled vaping part that is not refillable.
In addition, refillable vaping devices and their parts are not subject to toxicity information labelling requirements, unless they are prefilled with a vaping substance containing nicotine in a concentration of 0.1 mg/mL or more (see subsection 57(1)). In such cases, the toxicity information is still required on the exterior package, and where there is no exterior package, the information is required to appear on a tag attached to the product (see subsection 57(2), 57(3) and section 58).
The Part 2 requirements of the VPLPR in general do not require labelling information to appear on the display surface of a vaping device or vaping part (see section 58). Information is required to be displayed instead on exterior packaging or attached as a tag.
4.2.7 Requirements for the Presentation of Information
4.2.7.1 Official Languages
All of the information required in Part 2 of the VPLPR, as outlined in the preceding sections, must be displayed in both official languages, English and French (see paragraph 59(a)).
The Part 2 labelling requirements of the VPLPR do not specify that English and French must appear side-by-side, or in the same location of the display surface. If industry members wish to present all required English information in one area and all required French information in another area of the display surface, they may choose to do so.
4.2.7.2 Legibility and Durability
The information required in Part 2 of the VPLPR must remain clear and legible throughout the useful life of the vaping substance, or in the case of a refillable immediate container, throughout its useful life, under normal conditions of transportation, storage, sale and use (see paragraph 59(b)).
4.2.7.3 Contrast
All information required in Part 2 of the VPLPR must be displayed in black type on a white background. This requirement also applies to the hazard symbol (see paragraph 59(c)).
4.2.7.4 Font Type, Format and Size
The VPLPR specify rules for the presentation of the information in words required by Part 2.
- These words must be displayed in sans-serif type (see subsection 60(1)), that is not compressed, expanded or decorative (see paragraph 60(1)(a)).
- The sans-serif type must have proportions as set out in Schedule 2 (see paragraph 60(1)(b)).
- The minimum font size allowable for type on the immediate container is based on the area of its main display panel. Similarly, the minimum font size allowable for the type on any exterior package is based on the area of its main display panel.
- In the case of an immediate container or an exterior package, if the area of the main display panel is equal to or greater than 45 cm2 the information must be displayed in a type with a minimum height of 3 mm and a minimum body size of 8 points (see paragraph 60(1)(c)). Industry members may elect to use a larger font size.
- In the case of an immediate container or an exterior package, if the area of the main display panel is less than 45 cm2, the information must be displayed in a type with a minimum height of 2 mm and a minimum body size of 6 points (see paragraph 60(1)(d)). Industry members may elect to use a larger font size.
4.2.7.5 Display of Additional Information
Additional information on an aspect of the vaping product may be displayed provided that the vaping product, package, tag and leaflet, as the case may be, comply with all applicable requirements of the VPLPR and applicable legislation.
5. Additional Health or Safety Considerations
The CCPSA applies to vaping products that are consumer products that are not marketed for a therapeutic use. The CCPSA also applies to cannabis accessories, as defined in subsection 2(1) of the Cannabis Act, when they are consumer products that are not marketed for a therapeutic use.
There are other regulations under the CCPSA that may be applicable to vaping products or cannabis accessories that are consumer products. For example, the Consumer Products Containing Lead Regulations set out a 90 mg/kg total lead limit for each accessible part of a consumer product that is brought in contact with the user's mouth during normal use. This includes accessible parts of a vaping device or cannabis accessory, such as a mouthpiece.
Furthermore, the CCPSA prohibits the manufacture, import, advertisement or sale of any consumer product that is a danger to human health or safety as defined in the CCPSA (see paragraphs 7(a) and 8(a)). The sections below outline considerations that industry members can apply to help mitigate the risk that their products pose a danger to human health or safety. The sections apply to consumer products that are vaping products and consumer products that are cannabis accessories (such as a vaporizer represented to be used in the consumption of cannabis). Other considerations regarding dangers to human health or safety that are not set out below may also apply. Further information on danger to human health or safety can be found in the Industry Guidance - "Danger to Human Health or Safety" Posed by Consumer Products. See Appendix A – Information Resources of this document for link to the guidance. The CCPSA authorizes Health Canada to order anyone who manufacturers or imports a consumer product into Canada for commercial purposes to carry out tests or studies, or to compile and provide information relating to the safety of the product (see paragraph 12(a)).
5.1 General Considerations
Vaping establishments are encouraged to have a quality management system in place to ensure regulatory compliance under the CCPSA. Manufacturers are recommended to consider any available and relevant vaping product safety standards and guidelines during the design and manufacture of their products. It is Health Canada's expectation that establishments implement necessary controls and set product specifications including acceptance limits to ensure that products are safe and compliant.
5.2 Electrical and Mechanical Considerations
Most vaping devices consist of a battery, a heating element, a reservoir and a mouthpiece. The following provides a summary of the health or safety considerations related to certain electrical and mechanical hazards of vaping devices. Many of the considerations outlined below are addressed in the standard ANSI/CAN/UL 8139 Electrical Systems of Electronic Cigarettes and Vaping Devices, and manufacturers are strongly encouraged to conform to this standard or an equivalent standard. Information about the burn, fire or explosion hazards associated with vaping devices may also be found in the Information for Regulated Parties on the Enforcement Approach for the General Prohibitions under the Canada Consumer Product Safety Act.
5.2.1 Devices
Consideration should be given to the electrical and mechanical integrity of vaping devices. Vaping devices should be manufactured to withstand the maximum anticipated current, voltage and temperature specifications of the product. The mechanical integrity of internal connections should be sufficient to withstand conditions of reasonably foreseeable misuse. Accessories for the vaping device and replacements should specify their operating characteristics and compatibility to avoid the risk of rupture, fire or explosion.
Vaping devices should not be susceptible to external short circuits, and should incorporate a pressure-relief mechanism so that they will discharge excessive internal pressure in a direction where the harm is minimized, and at a rate that will preclude rupture, explosion or self-ignition.
Other considerations for vaping device manufacturers are set out in Section 5.3.5 of this guide, entitled Thermal Degradation of Products.
5.2.2 Batteries
Manufacturers should consider battery quality and performance. While Lithium-ion (Li-ion) battery failures are uncommon, they do occur; battery failures are responsible for the majority of hardware incidents with vaping devices. In a typical vaping device, a Li-ion battery powers a heating element (e.g., a coil) that heats a vaping substance to produce emissions in the form of an aerosol for inhalation through the mouth. Li-ion batteries may possess an inherent burn, fire or explosion hazard due to their electrochemical design. This inherent hazard is not unique to vaping devices; however, the heating element in these products requires a larger current than most other portable electronic devices. This current may place an enormous strain on the battery that can cause overheating and catastrophic failure, posing a burn, fire or explosion hazard. The potential for injury is increased if the battery failure is confined within a metal enclosure that can allow pressure to build up. It can then rupture while in close contact with an individual's leg, hand, face or neck and cause severe injury or death.
Integrated Li-ion batteries should meet the requirements of the standard CAN/CSA C22.2 No. 62133, or the equivalent, and manufacturers should ensure these batteries are not overstressed during foreseeable use. A battery management system should be incorporated into a vaping device so that integrated cells cannot be overcharged or discharged. When a vaping device allows consumers to replace batteries, the battery compartment should only permit replacement batteries specified by the manufacturer and prohibit direct replacement of single cells. Approximately half of known injuries involve vaping device batteries or cells catching fire or exploding while in a pocket. Vaping device manufacturers should include the DC voltage and peak current rating on their device. This will help the consumer choose replacement batteries that are appropriate for the device.
5.2.3 Chargers
The manufacturer's charging specifications and instructions should clearly identify appropriate voltage, current and temperature limits. This information should be provided with each vaping device whether or not it includes a charger. All electrical products that plug into an electrical outlet, including AC chargers and USB chargers, are already required to be certified to the applicable Canadian national safety standard to comply with provincial and territorial legislation. That certification must be issued by an accredited certification body and the certified product must carry the recognized certification mark of that certification body.
5.3 Vaping Substance Considerations
The following subsections provide a summary of the health or safety considerations that industry members can apply to mitigate the risks that consumer products that are vaping substances may pose to users. The CCPSA does not apply to cannabis as defined in subsection 2(1) the Cannabis Act.
5.3.1 Nicotine Quality
Nicotine may contain various impurities. It is recommended that the nicotine used in vaping substances be within the specifications of an accepted pharmacopoeia, such as those outlined in Schedule B of the Food and Drugs Act.
5.3.2 Diluents
Vaping substances that are subject to the CCPSA, that is those that are not marketed for a therapeutic use and those that are not cannabis, typically utilize propylene glycol, glycerol, or a combination of both as a diluent, alternatively referred to as a 'base', 'carrier solvent' or 'vehicle'. The available data suggest that propylene glycol is likely to have low systemic toxicity, but may have irritant effects on the respiratory tract. Although widely used in food, cosmetics and pharmaceuticals, there are few publicly available inhalation studies on glycerol and the long-term health consequences associated with heating and inhaling this substance are unknown. Also, diluents may undergo thermal degradation under elevated operating temperatures and may produce other compounds that may be harmful if inhaled (see section 5.3.5 of this guide). It is recommended that the diluents used in vaping substances be within the specifications of an accepted pharmacopoeia, such as those outlined in Schedule B of the Food and Drugs Act.
When selecting a diluent, solvents known to be toxic to humans, such as ethylene glycol or diethylene glycolFootnote 5, should never be added to vaping substances.
5.3.3 Flavour Ingredients and Other Additives
Flavour ingredients are widely utilized in vaping substances. However, to date, limited information exists on the inhalation safety of the majority of flavour ingredients used in vaping substances, and certain flavour ingredients have been associated with pulmonary diseaseFootnote 6. Schedule 3 of the TVPA prohibits certain flavours. All permissible flavour ingredients added to vaping substances are recommended to be of food grade or higher purity.
Substances intentionally added to vaping substances, including flavourings, preservatives and fragrances, should be evaluated for their potential to cause both acute and chronic adverse human health effects via inhalation.
Substances with known inhalation risks (e.g., diacetyl and 2,3-pentanedione) should never be added to vaping substances.
5.3.4 Impurities
Published research studiesFootnote 7 Footnote 8 Footnote 9 have shown that commercially available vaping substances may contain a wide range of potentially harmful impurities, including certain chemical elements, benzene, toluene, naphthalene, 1,3-butadiene, propylene oxide and various tobacco-specific nitrosamines. It is recommended that manufacturers source ingredients of suitable quality, purity or both from reliable suppliers and take steps to minimize the presence of impurities in vaping substances through manufacturing specifications.
5.3.5 Thermal Degradation of Products
Under intended conditions of use, users of vaping products inhale the emissions (i.e., aerosol) generated by vaping products and not the vaping substance in liquid form. For a typical vaping device, the vaping substance is heated to form a vapour, which is drawn through the device and condenses into an aerosol that is then inhaled. The heat used to vapourize the vaping substance may alter the proportion of different ingredients in the aerosol compared to the liquid, trigger additional chemical reactions and may result in thermal decomposition of the diluent or other constituents. For example, it is known that the thermal degradation of propylene glycol and glycerol during vaping can lead to the emission of thermal degradants such as formaldehyde, acetaldehyde, and acrolein, among other potential toxicants.
Studies have demonstrated that these thermal degradants are produced as a result of the so-called 'dry hit' or 'dry puff' phenomenaFootnote 10 as well as under other conditions that are still pleasant to the user. It is recommended that vaping device manufacturers establish limits on operating parameters for devices such that the generation of harmful emissions due to thermal decomposition is as low as reasonably achievable.
Studies have suggested that factors such as coil type and composition, vaping device power setting, device type, and user behaviour could contribute to the release of certain elements, including toxic metals, from the device to the aerosol, which would subsequently be inhaled by the userFootnote 11. In general, the amounts of toxic metals such as cadmium and lead that are released into the aerosol generated by a vaping device are lower than those found in mainstream cigarette smokeFootnote 12. However, vaping devices and parts could be a potential source of exposure to a wide variety of elements that may be toxic when inhaled (including essential elements such as manganese and zinc)Footnote 13 Footnote 14. It is recommended that manufacturers control the presence of harmful elements in vaping devices and vaping parts so that their transmission to aerosol under normal use conditions is minimized.
5.3.6 Microbial Contamination
It is recommended that manufacturers have controls in place to minimize microbial contamination during the production of vaping substances.
Appendix A – Information Resources
NOTICE: For further information, refer to the links below or contact the Health Canada's Tobacco and Vaping Compliance and Enforcement Program via email (hc.tcp.questions-plt.sc@canada.ca) or telephone at 1-866-318-1116 (toll-free within Canada and the United States).
- Vaping Products Labelling and Packaging Regulations
- Nicotine Concentration in Vaping Products Regulations
- Canada Consumer Product Safety Act
- Health Canada - Vaping Product Page
- To subscribe for email updates about the Canada Consumer Product Safety Act
- Canada Consumer Product Safety Act Quick Reference Guide
- Canada Consumer Product Safety Act– Information for Retailers
- Canada Consumer Product Safety Act - Information for Direct Sellers (Suppliers and Independent Sales Contractors)
- Guidance on Mandatory Incident Reporting under the Canada Consumer Product Safety Act - Section 14 Duties in the Event of an Incident
- Guidance on Preparing and Maintaining Documents under the Canada Consumer Product Safety Act (CCPSA) - Section 13
- Industry Guidance - "Danger to Human Health or Safety" Posed by Consumer Products
- Information for Regulated Parties on the Enforcement Approach for the General Prohibitions under the Canada Consumer Product Safety Act
- Recalling Consumer Products - A Guide for Industry
- Guide to Notices of Violation and Administrative Monetary Penalties under the Canada Consumer Product Safety Act
- Tobacco and Vaping Products Act
- Non-smokers' Health Act
- Food and Drugs Act
- Cannabis Act
- Consumer Packaging and Labelling Act