Risk management approach for the Decenes Group
Official title: Risk Management Approach for 1-Decene, dimer, hydrogenated (CAS RN 68649-11-6) and 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (CAS RN 68649-12-7) (The Decenes Group)
Environment and Climate Change Canada
Health Canada
November 2025
Summary of proposed risk management
This document outlines the proposed risk management actions for 1-Decene, dimer, hydrogenated (“hydrogenated didecene”), and 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (“HTTD”), known under the Chemicals Management Plan (CMP) as the Decenes Group, which has been found to be harmful to human health but not to the environment in Canada. For the purposes of subparagraph 77(6)(c)(i) of the Canadian Environmental Protection Act, 1999 (CEPA), the Government of Canada is recommending the following new risk management actions:
- Regulations under CEPA that would establish concentration limits for hydrogenated didecene and HTTD in cleaner/lubricant/preservative (CLP) spray/aerosol products available to consumers
Moreover, because certain data gaps remain, the following information should be provided (ideally on or before January 21, 2026, to the contact details identified in section 8 of this document) to inform risk management decision-making:
- Alternative substances that may be used in firearm-cleaning products as replacements for hydrogenated didecene and HTTD
- Product cost comparison if alternatives are used instead of hydrogenated didecene and/or HTTD
- Functionality of alternatives in products compared to hydrogenated didecene and HTTD
- Availability of CLP products that contain hydrogenated didecene and HTTD in non-spray/aerosol formats; and
- The quantities of firearm-cleaning products sold in spray/aerosol format containing hydrogenated didecene and/or HTTD and their concentrations
The risk management actions outlined in this Risk Management Approach document may evolve through consideration of assessments and risk management options published for other CMP substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of actions proposed to manage these substances and to seek information on identified information gaps and uncertainties. Refer to section 3 of this document for more complete details in this regard. It should be noted that the proposed risk management actions may evolve through consideration of additional information obtained from the public comment period, literature and other sources.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) provides the authority for the Minister of the Environment and the Minister of Health (the Ministers) to conduct assessments to determine if substances are toxic to the environment and/or to human health as set out in section 64 of CEPA,Footnote 1, Footnote 2 and, if so, to manage the associated risks.
The substances 1-Decene, dimer, hydrogenated (“hydrogenated didecene”, Chemical Abstracts Service Registry Number (CAS RNFootnote 3) 68649-11-6) and 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (“HTTD”, CAS RN 68649-12-7) are included in the final assessment of the Decenes Group as part of this third phase of the Chemicals Management Plan (CMP) (Canada 2025).
2. Issue
Health Canada and Environment and Climate Change Canada conducted a joint scientific assessment of hydrogenated didecene and HTTD in Canada. A notice summarizing the scientific considerations of the final assessment report for these substances was published in the Canada Gazette,Part I, on November 22, 2025 (Canada 2025). For further information, refer to the final assessment for the Decenes Group.
2.1 Final assessment report conclusion
On the basis of the information available, the final assessment concludes that hydrogenated didecene and HTTD meet the criteria set out in paragraph 64(c) of CEPA as they are entering or may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health (Canada 2025). However, it is concluded that hydrogenated didecene and HTTD do not meet the criteria set out in paragraphs 64(a) or 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The exposure source of concern to human health, as identified in the final assessment, is potential inhalation exposure to hydrogenated didecene and HTTD from the use of cleaner/lubricant/preservative (CLP) spray/aerosol products for firearm maintenance available to consumers.
2.2 Recommendation under CEPA
On the basis of the findings of the final assessment conducted pursuant to CEPA, the Ministers recommended that hydrogenated didecene and HTTD be added to Part 2 of Schedule 1 of the ActFootnote 4. Addition of a substance to Schedule 1 to CEPA enables the Government to propose certain risk management measures under CEPA to manage potential ecological and human health risks associated with the substance.
CEPA sets out a 2-track approach for managing risks.
Under sub-section 77(3), the Ministers are required to propose recommending the addition of a substance that poses the highest risk, as defined in paragraph (a), (b) or (c), to Part 1Footnote 5 of Schedule 1 of the Act and, in developing a proposed regulation or instrument respecting preventive or control actions, to give priority to the total, partial or conditional prohibition of activities in relation to the substance or to the release of the substance into the environment.
For other substances recommended for addition to Part 2 of Schedule 1 of the Act, the Ministers shall give priority to pollution prevention, and this could include regulatory or non-regulatory measures such as prohibition if warranted.
Substances in the Decenes group are proposed not to meet the criteria per sub-section 77(3) for addition to Part 1 of Schedule 1 of the Act.
The Ministers have taken into consideration comments made by stakeholders during the 60-day public comment period on the draft screening assessment for the hydrogenated didecene and HTTD (Canada 2021a) and the associated Risk Management Scope document (Canada 2021b).
As the Ministers finalize the recommendation to add hydrogenated didecene and HTTD to Part 2 of Schedule 1, risk management instruments must, unless an exception in section 91 of CEPA applies, be proposed within 24 months from the date on which the Ministers recommend that hydrogenated didecene and HTTD be added to Schedule 1 of CEPA, and finalized within 18 months from the date on which risk management instruments are proposed, as outlined in sections 91 and 92 of CEPA (refer to section 8 for publication timelines applicable to this group of substances).
2.3 Public comment period on the draft screening assessment and the risk management scope
The draft screening assessment report for hydrogenated didecene and HTTD and the associated Risk Management Scope document summarizing the proposed risk management options under consideration at that time were published on January 9, 2021. Industry and other interested stakeholders were invited to submit comments on both documents during a 60-day comment period.
Comments received on the draft screening assessment and the Risk Management Scope were taken into consideration in the development of this document. A summary of responses to public comments received is available.
3. Proposed risk management
3.1 Proposed human health objectives
Proposed human health objectives are quantitative or qualitative statements of what should be achieved in order to address human health concerns. As such, the proposed human health objective for these substances is:
- To reduce exposure of the general population to hydrogenated didecene and HTTD to levels that are protective of human health.
3.2 Proposed risk management objectives and options under consideration
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulation(s), instrument(s) and/or tool(s) for a given substance or substances. In this case, the proposed risk management objective for hydrogenated didecene and HTTD is to reduce inhalation exposure of the general population to hydrogenated didecene and HTTD in CLP spray/aerosol products available to consumers such as those used for firearm maintenance to levels that are protective of human health.
For the purposes of subparagraph 77(6)(c)(i) of CEPA, the Government of Canada is recommending the following new risk management actions:
- The development of regulations under CEPA that would establish concentration limits for hydrogenated didecene and HTTD in CLP spray/aerosol products available to consumers
Note that the proposed risk management action is preliminary and subject to change. Following the publication of this document, additional information obtained from the public comment period and from other sources will also be considered in the instrument selection and development processFootnote 6. The risk management action may also evolve through consideration of assessments and risk management options or actions published for other CMP substances to ensure effective, coordinated, and consistent risk management decision-making.
3.3 Performance measurement evaluation
Performance measurement evaluates the ongoing effectiveness and relevance of the actions taken to manage risks from toxic substancesFootnote 7. ECCC and HC have developed a Performance Measurement Evaluation Strategy that sets out the approach to evaluate the effectiveness of actions taken on substances found toxic under CEPA. The aim is to determine whether human health and/or environmental objectives have been met, and whether there is a need to revisit the risk management approach for those substances. Selection of a substance for performance measurement evaluation is conducted through readiness, prioritization and work planning as outlined in the Performance Measurement Evaluation Strategy. In evaluating progress and revisiting risk management, as warranted, these activities together will aim to manage risks effectively over time.
To do so, the Government of Canada may collect and analyze data, such asmonitoring data obtained from section 71 survey on the presence of hydrogenated didecene and HTTD in certain CLP spray/aerosol products available to consumers.
When undertaken, the results of performance measurement and evaluation are used to inform whether further risk management action is warranted and are made available to people in Canada along with recommendations for further action, if applicable.
3.4 Risk management information gaps
Interested stakeholders are invited to provide further information regarding the following, to inform risk assessment and management decision-making for hydrogenated didecene and HTTD:
- Alternative substances that may be used in firearm-maintenance products as replacements for hydrogenated didecene and HTTD
- Product cost comparison if alternatives are used instead of hydrogenated didecene and/or HTTD
- Functionality of alternatives in products compared to hydrogenated didecene and HTTD
- Availability of CLP products that contain hydrogenated didecene and HTTD in non-spray/aerosol formats
- Quantities of firearm-cleaning products sold in spray/aerosol format containing hydrogenated didecene and/or HTTD and their concentrations
Stakeholders that have information to help address these gaps should provide it on or before January 21, 2026 to the address identified in section 8.
4. Background
4.1 General information on hydrogenated didecene and HTTD
The Decenes Group substances are organic and they do not occur naturally. They are identified as 1-Decene, dimer, hydrogenated (“hydrogenated didecene”) and 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (“HTTD”).
4.2 Current uses and identified sectors
Hydrogenated didecene and HTTD were included in a survey issued pursuant to section 71 of CEPA (Environment Canada 2013). According to the 2013 data received for hydrogenated didecene, there was no reported manufacturing in Canada, and reported imports were in the range of 10 000 kg to 100 000 kg. For HTTD, 1 000 kg to 10 000 kg were reported to be manufactured in Canada, and 203 742 kg were reported to be imported into Canada.
Hydrogenated didecene is used in cosmetics (including lipsticks, moisturizers, cleansers and skin creams) and in mining applications.
HTTD is used in automotive care, aircraft care (hydraulic fluid, heat sink coolant fluid) and transportation sectors.
Both substances are used in greases and lubricants (gear oil, transmission oil and firearm maintenance oil sprays). They may also be used as components in incidental additives, specifically in lubricants used in food processing establishments with no potential for food contact; therefore, exposure to these substances via food is not expected (Canada 2021).
5. Exposure sources and identified health risk
In Canada, individuals may be exposed to hydrogenated didecene and HTTD via dermal exposure to cosmetics, and via oral exposure to lipstick. Individuals may be exposed to both substances via dermal exposure to greases and lubricants (gear oil, transmission oil, engine oil), and via dermal and inhalation exposure to CLP aerosol and trigger spray products used for firearm maintenance available to consumers.
With respect to the human health assessment, no critical health effects were identified for hydrogenated didecene or HTTD via the oral or dermal routes of exposure. This was based on the available health effects data on these substances as well as on analogues. Therefore, oral exposure to substances in the Decenes Group resulting from potential releases to surface water and exposure to lipsticks is not a concern. In addition, dermal exposure from use of automotive and cosmetics containing these substances is not of concern. However, critical human health effects were identified as a result of inhaling hydrogenated didecene and HTTD in CLP aerosol and trigger spray products used for firearm cleaning and maintenance.
The critical human health effects for hydrogenated didecene and HTTD were based on identified histopathological effects in the nasal cavity and lungs. No critical health effects were identified for hydrogenated didecene or HTTD via the oral and dermal routes of exposure.
A comparison of estimated inhalation exposure to hydrogenated didecene and HTTD from their use in CLP aerosol and trigger spray products used by consumers for firearm maintenance to the critical health effect level resulted in margins of exposure (MOEs) which were considered inadequate to address uncertainties in the health effects and exposure databases (Canada 2023) used to characterize risk.
No other sources of exposure of concern were identified.
6. Risk management considerations
6.1 Alternatives and alternate technologies
There are currently other application/delivery formats of CLP liquid products used for firearm maintenance available on the market (for example, squeeze bottles, liquid, pinpoint applicator, etc.), which do not expose users to droplets or aerosolized mixtures containing hydrogenated didecene and HTTD. These other formats would not produce the potentially respirable particles produced in spray/aerosol formats. It is not known whether there are alternative substances with the same functionality as hydrogenated didecene and HTTD in CLP products.
6.2 Socio-economic and technical considerations
Socio-economic factors have been considered in the selection process for a regulation respecting preventive or control actions, and in the development of the risk management objective as per the guidance provided in the Treasury Board document Policy on Regulatory Development (TBS 2018a).
In addition, socio-economic factors will be considered in the development of the regulations, instrument(s) or tool(s) to address risk management objective(s), as identified in the Cabinet Directive on Regulation (TBS 2018b), Red Tape Reduction Action Plan (TBS 2012), the Red Tape Reduction Act (Canada 2015), as well as in the objectives of the most recent federal Red Tape Review (TBS 2025).
7. Overview of existing risk management
7.1 Related Canadian risk management context
Domestically, relevant risk management actions for hydrogenated didecene include the following:
- Volatile Organic Compound Concentration Limits for Certain Products Regulations [PDF] – Hydrogenated didecene in CLP products available to consumers may be captured under these regulations (Canada 2022).
- Hydrogenated didecene is notified to be present in cosmetics in Canada under the Cosmetic RegulationsFootnote 8. It is not listed on the Cosmetic Ingredient Hotlist.
No relevant risk management actions specifically for HTTD have been identified in Canada.
7.2 Pertinent international risk management context
Internationally, no relevant risk management measures specific to hydrogenated didecene and HTTD were identified.
7.2.1 Risk management alignment
No relevant international risk management measures specifically for hydrogenated didecene or HTTD were identified. The measures proposed by the Government of Canada would make Canada the first jurisdiction, globally, to mitigate inhalation exposure of the general public to hydrogenated didecene and HTTD.
8. Next Steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Approach or other information that would help to inform decision-making (such as outlined in sections 3.2). Please submit additional information and comments prior to January 21, 2026.
Comments and information submissions on the Risk Management Approach should be submitted to the address provided below:
Environment and Climate Change Canada
Chemicals Management Division
Gatineau, Quebec K1A 0H3
Tel: 1-800-567-1999 | 819-938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies that have a business interest in hydrogenated didecene and HTTD are encouraged to identify themselves as stakeholders. Stakeholders will be informed of future decisions regarding hydrogenated didecene and HTTD and may be contacted for further information.
Stakeholders and members of the public who are interested in being notified of CMP publications are invited to subscribe for the latest news on the CMP. Stakeholders and members of the public who would like to receive CMP Publication Plans on a quarterly basis by email can contact: substances@ec.gc.ca.
Following the public comment period on the Risk Management Approach document, the Government of Canada will initiate the development of the specific risk management instrument(s), where necessary. Comments received on the Risk Management Approach document will be taken into consideration in the selection or development of these instrument(s). Consultation will also take place as instrument(s) are developed.
8.2 Timing of actions
Electronic consultation on the Risk Management Approach: November 22, 2025 to January 21, 2026.
Publication of responses to public comments on the Risk Management Approach document: Concurrent to the publication of the proposed instrument.
Publication of the proposed instrument(s): At the latest, 24 months from the date on which the Ministers recommended that hydrogenated didecene and HTTD be added to Schedule 1 of CEPA.
Consultation on the proposed instrument(s): 60-day public comment period starting upon publication of the proposed instrument(s).
Publication of the final instrument(s): At the latest, 18 months from the publication of the proposed instrument(s).
These are planned timelines and are subject to change.
9. References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2015. Red Tape Reduction Act. S.C. 2015, c.12.
Canada. 2016. Canada Gazette, Part I: Vol. 150, No. 25 – June 18, 2016.
Canada. 2022. Canada Gazette, Part II: Vol. 156, No. 1 – January 5, 2022
Department of the Environment, Department of Health. [2025]. Canadian Environmental Protection Act, 1999: Notice with respect to hydrogenated didecene and hydrogenated trimer and tetramer of decene. Canada Gazette, Part I, vol.159 #47.
Environment and Climate Change Canada, Health Canada. 2021a. Draft Screening Assessment for the Decenes Group. Ottawa (ON): Government of Canada. [accessed 2023 Aug 29].
Environment and Climate Change Canada, Health Canada. 2021b. Risk Management Scope for the Decenes Group. Ottawa (ON): Government of Canada. [accessed 2023 Aug 29].
Environment and Climate Change Canada, Health Canada. 2025 Final Assessment for Decenes Group. Available from: Final Assessment Report.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program. [accessed 2022 Jun 10].
[TBS] Treasury Board of Canada Secretariat. 2012. Red Tape Reduction Action Plan. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
[TBS] Treasury Board of Canada Secretariat. 2018a. Policy on Regulatory Development. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
[TBS] Treasury Board of Canada Secretariat. 2018b. Cabinet Directive on Regulation. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
[TBS] Treasury Board of Canada Secretariat. 2025. Red Tape Review. Ottawa (ON): Government of Canada. [accessed 2025 Sept 17].
Appendix A. Substances targeted for risk management
| CAS RN | DSL name (common name) | Common Name (and/or abbreviation) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|
| 68649-11-6a | 1-Decene, dimer, hydrogenated | Hydrogenated didecene | 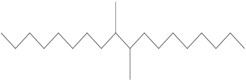 (Representative dimer structure) C20H42 |
282.556 |
| 68649-12-7a | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated | Hydrogenated trimer and tetramer of decene, or HTTD | 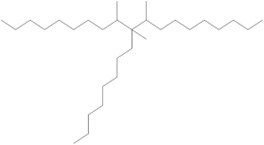 (Representative trimer structure) C30H62 |
422.81 |
a UVCB, substance of unknown or variable composition, complex reaction products or biological materials. These materials are derived from natural sources or complex reactions and cannot be characterized in terms of constituent chemical compounds because their composition is too complex or variable. A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
