Risk management scope for 1-decene, dimer, hydrogenated and 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (The Decenes Group)
Official title: Risk management scope for 1-decene, dimer, hydrogenated (CAS RN 68649-11-6) and 1-decene, tetramer, mixed with 1-decene trimer, hydrogenated (CAS RN 68649-12-7) (The Decenes Group)
Environment and Climate Change Canada
Health Canada
January 2021
Summary of proposed risk management
This document outlines the risk management options under consideration for 1-decene, dimer, hydrogenated (“hydrogenated didecene”) [CAS RN 68649-11-6], and 1-decene, tetramer, mixed with 1-decene trimer, hydrogenated (“HTTD”) [CAS RN 68649-12-7], known under the Chemicals Management Plan (CMP)as the Decenes Group, and which have been proposed to be harmful to human health but not to the environment.
In particular, the Government of Canada is considering:
- Regulatory and/or non-regulatory actions to help reduce inhalation exposure of the general population to hydrogenated didecene and HTTD in cleaner/lubricant/preservation spray products available to consumers and which are used for firearm maintenance, and to prevent exposure from other types of spray products available to consumers.
The risk management options outlined in this Risk Management Scope document may evolve through consideration of assessments and risk management options published for other CMP substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of options under consideration to manage these substances and to seek information on identified information gaps and uncertainties. Refer to section 3 of this document for more complete details in this regard.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) provides the authority for the Minister of the Environment and the Minister of Health (the ministers) to conduct assessments to determine if substances are harmfulto the environment and/or to human health as set out in section 64 of CEPA,Footnote 1,Footnote 2 and, if so, to manage the associated risks.
The substances 1-decene, dimer, hydrogenated (“hydrogenated didecene”) [Chemical Abstracts Service Registry Number (CAS RN) 68649-11-6] and 1-decene, tetramer, mixed with 1-decene trimer, hydrogenated (“HTTD”) [CAS RN 68649-12-7] are included in the screening assessment of the Decenes Group as part of the third phase of the Chemicals Management Plan (CMP) (Canada 2016).
2. Issue
Health Canada and Environment and Climate Change Canada conducted a joint scientific assessment relevant to the evaluation of hydrogenated didecene and HTTD in Canada. A notice summarizing the scientific considerations of the draft screening assessment for these substances was published in the Canada Gazette, Part I, on January 9, 2021 (Canada 2021). For further information, refer to the draft screening assessment for hydrogenated didecene and HTTD.
2.1 Draft screening assessment conclusion
On the basis of the information available, the draft screening assessment proposes hydrogenated didecene and HTTD are harmful to human health under section 64(c) of CEPA because they may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health (Canada 2021). However, it is proposed to conclude hydrogenated didecene and HTTD are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends under paragraphs 64(a) or 64(b) of CEPA.
The draft screening assessment also proposes that HTTD meets the persistence criteria, but not the bioaccumulation criteria, and that hydrogenated didecene does not meet the criteria for persistence or bioaccumulation, as defined in the Persistence and Bioaccumulation Regulations of CEPA (Canada 2000).
The exposure source of concern, as identified in the draft screening assessment, is potential inhalation exposure to hydrogenated didecene and HTTD from the use of cleaner/lubricant/preservation spray products available to consumers and which are used for firearm maintenance.
2.2 Proposed recommendation under CEPA
On the basis of the findings of the draft screening assessment, the ministers propose to recommend that hydrogenated didecene and HTTD be added to the List of Toxic Substances in Schedule 1 of the ActFootnote 3.
The ministers will take into consideration comments made by stakeholders during the 60-day public comment period on the draft screening assessment for hydrogenated didecene and HTTD and its associated Risk Management Scope document.
If the ministers finalize the recommendation to add hydrogenated didecene and HTTD to Schedule 1, risk management instruments must be proposed and finalized within a set period of time, as outlined in sections 91 and 92 of CEPA (refer to section 8 for publication timelines applicable to this group of substances).
3. Proposed risk management
3.1 Proposed human health objective
Proposed human health objectives are quantitative or qualitative statements of what should be achieved in order to address human health concerns.
For hydrogenated didecene and HTTD, the proposed human health objective is focused on addressing the risks and exposure sources of concern outlined in section 5 of this document. As such, the proposed human health objective for these substances is to reduce exposure of the general population to levels that are protective of human health.
3.2 Proposed risk management objective
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulations, instrument(s) and/or tool(s) for a given substance or substances.
The proposed risk management objectives for hydrogenated didecene and HTTD are:
- to reduce inhalation exposure of the general population to hydrogenated didecene and HTTD in cleaner/lubricant/preservation spray products available to consumers and which are used for firearm maintenance, to levels that are protective of human health; and
- to prevent exposure from other types of spray products available to consumers.
Should the final screening assessment confirm that hydrogenated didecene and HTTD are harmful to human health, the proposed risk management objectives may be revised in the Risk Management Approach document. This document would be published concurrently with the final screening assessment for these substances, or in subsequent risk management documents (e.g., consultation document on proposed instrument), as the case may be.
3.3 Proposed risk management options under consideration
To achieve the proposed risk management objective and to work towards achieving the proposed human health objective, the risk management options under consideration for hydrogenated didecene and HTTD are as follows:
- Regulatory and/or non-regulatory measures to reduce inhalation exposure of the general population to hydrogenated didecene and HTTD in cleaner/lubricant/preservation spray products available to consumers and which are used for firearm maintenance, and to prevent exposure from other types of spray products available to consumers.
Following the publication of this Risk Management Scope document, additional information obtained during the public comment period and from other sources will be considered, along with the information presented in this document, in the instrument selection and development process.Footnote 4 The risk management options outlined in this document may evolve through consideration of assessments and risk management options published for other CMP substances to ensure effective, coordinated, and consistent risk management decision-making.
3.4 Performance Measurement and Evaluation
Performance measurement evaluates the ongoing effectiveness and relevance of the actions taken to manage risks from toxic substances.Footnote 5 The aim is to determine whether human health and/or environmental objectives have been met, and whether there is a need to revisit the risk management approach for that substance, to ensure that risks are managed effectively over time. To achieve this, the Government of Canada will review the effectiveness of the risk management action(s) for hydrogenated didecene and HTTD.
The Government of Canada is considering the collection and analysis of data, such as data obtained from the mandatory or voluntary surveys on the presence of hydrogenated didecene and HTTD in products available to Canadian consumers.
The results of performance measurement and evaluation will be used to inform whether further risk management action is warranted. These results will be made available to Canadians along with recommendations for further action, if applicable.
3.5 Risk management information gaps
Interested stakeholders are invited to provide further information regarding the following, to inform risk assessment and management decision-making for hydrogenated didecene and HTTD:
- Amount and size of respirable particles released during use of cleaner/lubricant/preservation spray products available to consumers and which are used for firearm maintenance containing hydrogenated didecene and/or HTTD; and
- Other spray products containing hydrogenated didecene and/or HTTD, including intended use and substance concentrations.
Should stakeholders have further information to help address these gaps, they should provide it to the contact identified in section 8 of this document on or before March 9, 2021 to inform the risk management decision-making process.
4. Background
4.1 General information on hydrogenated didecene and HTTD
Hydrogenated didecene and HTTD were included in a survey issued pursuant to a CEPA section 71 notice (Environment Canada 2013). According to information submitted for hydrogenated didecene, there was no manufacturing in Canada above the reporting threshold of 100 kg, and import volumes ranged from 10 000 – 100 000 kg in the year 2011. For HTTD, manufacturing volumes were reported in the range of 1000 – 10 000 kg, and an import volume of 203 742 kg was reported in the year 2011.
4.2 Current uses and identified sectors
Hydrogenated didecene is used in self-care products, including cosmetics, and as a non-medicinal ingredient in natural health products as a skin conditioning agent. It is listed in the Natural Health Products Ingredients Database (NHPID) as a non-medicinal ingredient for use as a skin-conditioning agent for topical use only, with no restrictions. It is also listed in the Licensed Natural Health Products Database (LNHPD) as a non-medicinal ingredient in two currently licensed products. It is also used in mining applications.
HTTD is not listed in the NHPID or in the LNHPD. It is used in automotive care, aircraft (hydraulic fluid, heat sink coolant fluid) and transportation.
Both substances are used in greases and lubricants (gear oil, transmission oil and firearm maintenance oil sprays). They may also be used as components in incidental additives, specifically in lubricants used in food processing establishments with no potential for food contact; therefore, exposure to these substances via food is not expected (personal communication, email from the Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated August, 2018; unreferenced).
5. Exposure sources and identified risk
According to the draft screening assessment, hydrogenated didecene and HTTD do not occur naturally. In Canada, individuals may be exposed to hydrogenated didecene via dermal exposure to cosmetics and from use as non-medicinal ingredients in natural health products. Oral exposure may occur from the use of lipsticks. Individuals may be exposed to both substances via dermal exposure to greases and lubricants (gear oil, transmission oil, engine oil). Dermal and inhalation exposure may also occur from cleaner/lubricant/preservative spray products available to consumers and which are used for firearm maintenance.
The critical human health effects for hydrogenated didecene and HTTD were from an acute rat study on hydrogenated didecene which identified histopathological effects in the nasal cavity and lungs. A comparison of estimated inhalation exposure to hydrogenated didecene and HTTD, from their use in spray products used for firearm maintenance to the critical health effect level, resulted in margins of exposure (MOEs) which were considered inadequate to address uncertainties in the health effects and exposure databases (Canada 2021) for the general population.
No sources of exposure of concern, other than inhalation exposure to cleaner/lubricant/preservative spray products used for firearm maintenance, were identified.
6. Risk management considerations
6.1 Alternatives and alternate technologies
There are currently other application/delivery formats of cleaner/lubricant/ preservative products used for firearm maintenance available on the market which do not expose users to spray formats of mixtures containing hydrogenated didecene and/or HTTD (e.g., squeeze bottles, liquid, pinpoint applicator). These other formats would not produce the potentially respirable particles produced in spray formats.
6.2 Socio-economic and technical considerations
Socio-economic factors will be considered in the selection process for a regulation and/or instrument respecting preventive or control actions, and in the development of the risk management objectives. Socio-economic factors will also be considered in the development of regulations, instrument(s) and/or tool(s) as identified in the Cabinet Directive on Regulation (TBS 2018) and the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action (TBS 2007).
7. Overview of existing risk management
7.1 Related Canadian risk management context
Domestically, the following relevant risk management actions for hydrogenated didecene include:
- Natural Health Products Ingredients Database – Hydrogenated didecene is listed with a non-medicinal role as a skin conditioning agent for topical use only (NHPID, [modified 2016]), with no restrictions noted;
- Licensed Natural Health Products Database – Hydrogenated didecene is listed as a non-medicinal ingredient in a number of currently licensed topical natural health products (LNHPD [modified 2016]).
- Hydrogenated didecene is notified to be present in cosmetics in Canada under the Cosmetic RegulationsFootnote 6 . It is not listed on the Cosmetic Ingredient Hotlist.
No relevant risk management actions specific to HTTD have been identified in Canada.
7.2 Pertinent international risk management context
Internationally, no relevant risk management measures specific to hydrogenated didecene and HTTD were identified.
8. Next steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Scope or other information that would help to inform decision-making (such as outlined in sections 3.2).
Should the final screening assessment confirm that hydrogenated didecene and HTTD are harmful to human health, the Risk Management Approach document, which would outline and seek input on the proposed risk management instrument(s), would be published at the same time as the final screening assessment. At that time, there would be further opportunity for consultation.
Comments and information submissions on the Risk Management Scope should be submitted to the address provided below:
Environment and Climate Change Canada
Chemicals Management Division
Gatineau Quebec K1A 0H3
Tel: 1-800-567-1999 | 819- 938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies with a business interest in hydrogenated didecene and/or HTTD are encouraged to identify themselves as stakeholders. Stakeholders will be informed of future decisions regarding hydrogenated didecene and HTTD and may be contacted for further information.
8.2 Timing of actions
Electronic consultation on the draft screening assessment report and Risk Management Scope: January 9, 2021 to March 9, 2021.This should include the submission of public comments, additional studies and/or information on Decenes Group.
Publication of responses to public comments on the draft screening assessment report and Risk Management Scope: concurrent to the publication of the screening assessment and, if required, the Risk Management Approach document.
These are planned timelines, and are subject to change. Please consult the schedule of risk management activities and consultations for updated information on timelines.
References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107.
Canada, 2015. Red Tape Reduction Act.
Canada, 2016. Canada Gazette, Part I: Vol. 150, No. 25 – June 18, 2016 .
Canada. 2021. Dept. of the Environment, Dept. of Health. Draft Screening Assessment for Decenes Group.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[TBS] Treasury Board of Canada Secretariat. 2007. Assessing, Selecting, and Implementing Instruments for Government Action.
[TBS] Treasury Board of Canada Secretariat. 2012. Red Tape Reduction Action Plan.
[TBS] Treasury Board of Canada Secretariat. 2018. Cabinet Directive on Regulation.
Appendix A. Substances targeted for risk management
| CAS RN | DSL name (common name) | Common Name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|
| 68649-11-6a | 1-Decene, dimer, hydrogenated | Hydrogenated didecene | 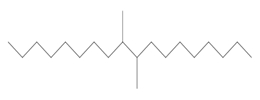 (Representative dimer structure) (Representative dimer structure)C20H42 | 282.556 |
| 68649-12-7a | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated | Hydrogenated trimer and tetramer of decene, or HTTD | 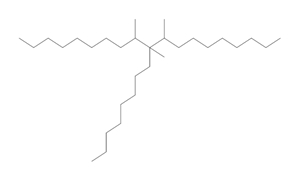 (Representative trimer structure) (Representative trimer structure)C30H62 | 422.81 |
a UVCB, Unknown or Variable composition, Complex reaction products and Biological material. These materials are derived from natural sources or complex reactions and cannot be characterized in terms of constituent chemical compounds because their composition is too complex or variable. A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
