Screening assessment - Fatty Acids and Derivatives Group
Official title: Screening Assessment - Fatty Acids and Derivatives Group
Environment and Climate Change Canada
Health Canada
August 2020
Cat No.: En14-418/2020E-PDF
ISBN 978-0-660-35504-7
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 9 of 16 substances referred to collectively under the Chemicals Management Plan as the Fatty Acids and Derivatives Group. The 9 substances in this assessment were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health or ecological considerations. Fats and glyceridic oils, margosa (Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1) 8002-65-1) was included in the draft screening assessment for the Fatty Acids and Derivatives Group published on August 18, 2018; however, based on additional information received, this UVCB (Unknown or Variable Composition, Complex Reaction Products or Biological Materials) substance requires further assessment. Further evaluation of this substance will be provided in a separate screening assessment. Four of the 16 substances were subsequently determined to be of low concern through other approaches, and decisions for these substances are provided in separate reports.Footnote 2Footnote 3 Additionally, two substances were placed into another substance group to which they are more appropriately suited on the basis of chemical structure and uses.Footnote 4 Accordingly, this screening assessment addresses the nine substances listed in the table below. The nine substances addressed in this screening assessment report will hereinafter be referred to as the Fatty Acids and Derivatives Group. The CAS RN, their Domestic Substances List (DSL) names and their common names and acronyms are listed in the table below.
CAS RN |
DSL name |
Common name and abbreviations |
|---|---|---|
112-38-9 |
10-Undecenoic acid |
Undecylenic acid |
463-40-1 |
9,12,15-Octadecatrienoic acid, (Z,Z,Z) |
α-Linolenic acid (ALA) |
8001-20-5 a,b |
Tung Oil |
Tung Oil |
61790-12-3 a |
Fatty acids, tall-oil |
Tall oil fatty acid |
61790-44-1a |
Fatty acids, tall-oil, potassium salts |
Tall oil fatty acids, potassium salts |
90028-66-3a,c |
Evening primrose, Oenothera biennis, ext. |
Evening primrose oil |
61788-89-4a |
Fatty acids, C18-unsaturated, dimers |
Dimer acid |
68937-90-6a,b |
Fatty acids, C18-unsaturated, trimers |
Trimer acid |
92044-87-6a,c |
Fatty acids, coco, 2-ethylhexyl esters |
Ethylhexyl cocoate |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
c This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other ecological considerations.
Four of the nine fatty acids and derivatives were reported, pursuant to a survey under section 71 of CEPA, to have been manufactured in Canada in 2011 at quantities of 1 430 kg for tall oil fatty acid, 10 000 to 100 000 kg for tall oil fatty acids, potassium salts, and 100 to 1 000 kg each for dimer and trimer acids. Seven of the nine substances were reported to have been imported into Canada the same year at quantities of 1 000 to 10 000 kg for ALA, 120 412 kg for tung oil, 6 317 473 kg for tall oil fatty acid, 47 992 kg for tall oil fatty acids, potassium salts, 293 472 kg for dimer acid, 1 088 638 kg for trimer acid and 6 470 kg for ethylhexyl cocoate. The remaining two substances, undecylenic acid, and evening primrose oil were not reported to be manufactured or imported into Canada in 2011 above the reporting threshold of 100 kg.
These substances occur naturally in the environment or are derived from natural sources, such as plants and animal fats and oils. The substances in the Fatty Acids and Derivatives Group have a number of reported uses, including lubricants and greases, adhesives and sealants, paints and coatings, fuels and related products, and food packaging. Some of these products are available to consumers. Several of the substances included in the Fatty Acids and Derivatives Group are used in cosmetics, as well as in natural and non-prescription health products.
The ecological risks of the substances in the Fatty Acids and Derivatives Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established principally on the basis of mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the substances in the Fatty Acids and Derivatives Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from the nine substances in the Fatty Acids and Derivatives Group. It is concluded that undecylenic acid; ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid; and ethylhexyl cocoate do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
ALA was assessed together with a group of aliphatic acids by the Organisation for Economic Co-operation and Development (OECD) in 2014. ALA and the major components of tall oil fatty acid, evening primrose oil, dimer/trimer acid and the free fatty acids of ethylhexyl cocoate were not identified by OECD as possessing properties indicating a hazard for human health for systemic health effects, as supported by the toxicity information of tung oil.
The European Food Safety Authority concluded in 2010 that laboratory studies on the conjugated form of a major component of tung oil did not indicate a risk for genotoxicity, reproductive toxicity or carcinogenicity.
In a Multi-Chemical Tiered I Human Health Risk Assessments carried out by the Australian Government Department of Health in 2017, dimer acid was considered as not to pose unreasonable risk to human health.
On the basis of information from the above-noted international assessments, ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; and trimer acid were not identified as having systemic health effects of concern and risk to human health is considered to be low.
General population exposure to undecylenic acid can occur from its use as a flavouring agent in certain foods, from cosmetics, as well as from natural health products. Exposure to ethylhexyl cocoate can occur from its use in cosmetics. The available health effects information on undecylenic acid and its sodium salt, as well as ethylhexyl cocoate and its hydrolyzed products, indicates effects on the body/organs weights and effects on the clinical chemistry parameters. The margins of exposure between estimated levels of exposure for both these substances and the critical effect levels in laboratory studies are considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this screening assessment, it is concluded that undecylenic acid; ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid and ethylhexyl cocoate do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that undecylenic acid; ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid; and ethylhexyl cocoate do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 9 of 16 substances, referred to collectively under the Chemicals Management Plan as the Fatty Acids and Derivatives Group, to determine whether they present or may present a risk to the environment or to human health. The nine substances in this assessment were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other health or ecological considerations (ECCC, HC [modified 2017]). Fats and glyceridic oils, margosa (Chemical Abstracts Service Registry Numbers (CAS RNFootnote 5) 8002-65-1) was included in the draft screening assessment for the Fatty Acids and Derivatives Group published on August 18, 2018; however, based on additional information received, this UVCB (Unknown or Variable composition, Complex Reaction Products or Biological Materials) substance requires further assessment. Further evaluation of this substance will be provided in a separate screening assessment.
Four other substances (listed in Table 1‑1) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and either in the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Science Approach Document (Health Canada 2016) or the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018a). The four substances were identified as being of low concern to both human health and the environment and are therefore not further addressed in this report. The conclusion for one substance is provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC, HC 2018b), while conclusions for the other three substances are provided in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018a).
CAS RN |
Domestic Substances List (DSL) name |
Approach under which the substance was addressed |
References |
|---|---|---|---|
68139-89-9 |
Fatty acids, tall-oil, maleated |
ERC/TTC |
ECCC, HC 2018b |
53980-88-4 |
2-Cyclohexene-1-octanoic acid, 5(or 6)-carboxy-4-hexyl- |
ERC/Rapid Screening |
ECCC, HC 2018a |
68647-55-2 |
Fatty acids, tall-oil, esters with triethanolamine |
ERC/Rapid Screening |
ECCC, HC 2018a |
CDSL #11556-0 |
Fatty acids, reaction products with maleic anhydride |
ERC/Rapid Screening |
ECCC, HC 2018a |
Additionally, two substances were placed into another substance group to which they are more appropriately suited on the basis of similar structural features and/or functionalities of toxicological significanceFootnote 6.
The nine substances addressed in this draft screening assessment report will hereinafter be referred to as the Fatty Acids and Derivatives Group.
The ecological risk of the substances in the Fatty Acids and Derivatives Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food-web derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and it considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
ALA and the major components of tall oil fatty acid, evening primrose oil, dimer/trimer acid and the free fatty acid of ethylhexyl cocoate were reviewed by the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme, and an OECD Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) is available. The OECD assessment undergoes rigorous review (including peer-review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process, and consider these assessments to be reliable. In addition, the conjugated form of a major component of tung oil was reviewed by the European Food Safety Authority (EFSA), and dimer acid was reviewed in a Multi-Chemical Tiered I Human Health Risk Assessment by the Australian Government Department of Health (AGDH). The OECD SIAR, US EPA, EFSA and AGDH reviews will be used to inform the health effects characterization in this screening assessment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to June 2017. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external peer-review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published on August 18, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 7. This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names and/or acronyms for the individual substances in the Fatty Acids and Derivatives Group are presented in Table 2‑1. Information on the identity of the components in the UVCB (Unknown or Variable Composition, Complex Reaction Products or Biological Materials) substances is presented in Appendix A. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2015).
Fatty acids are organic compounds that contain at least one terminal carboxylic group and whose derivatives contain at least one ester linkage. The substances in this screening assessment consist of discrete or single component fatty acids (undecylenic acid, α-Linolenic acid), complex substances that include a mixture of multi-component substances of saturated, unsaturated and undefined fatty acids (tung oil; tall oil fatty acid; evening primrose oil; trimer acid and dimer acid), and direct reaction products of fatty acids including a potassium salt (tall oil fatty acids, potassium salts) and a fatty acid ester (ethylhexyl cocoate). The degree of saturation and the carbon chain length distribution of the major components of this group of substances are presented in Appendix A.
| CAS RN(abbreviation) | DSL name(common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 112-38-9 | 10-Undecenoic acid(Undecylenic acid) |
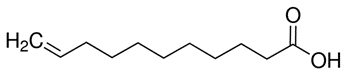 C11H20O2
C11H20O2 | 184.28 |
| 463-40-1(ALA) | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)(α-Linolenic acid) |
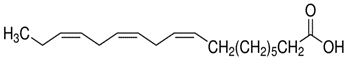 C18H30O2
C18H30O2 | 278.43 |
| 8001-20-5 | Tung oil | UVCB | Unspecified |
| 61790-12-3 | Fatty acids, tall-oil (tall-oil fatty acid) | UVCB | Unspecified |
| 61790-44-1 | Fatty acids, tall-oil, potassium salts ( Tall oil fatty acids, potassium salts) | UVCB | Unspecified |
| 90028-66-3 | Evening primrose, Oenothera biennis, ext.(Evening primrose oil) | UVCB | Unspecified |
| 68937-90-6 | Fatty acids, C18-unsaturated, trimers(Trimer acid) | UVCB | 801.03 |
| 61788-89-4 | Fatty acids, C18-unsaturated, dimers(Dimer acid) | UVCB | 564.92 |
| 92044-87-6 | Fatty acids, coco, 2-ethylhexyl esters(Ethylhexyl cocoate) | UVCB | Unspecified |
Abbreviations: UVCB, unknown or variable composition, complex reaction products, or biological materials.
2.1 Selection of analogues
A read-across approach using data from analogues or components of the target substances, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected that were structurally similar and/or functionally similar to substances within this group (similar physical-chemical properties, toxicokinetics) and that had relevant empirical data that could be used to read-across to substances with limited empirical data. Details of the read-across data chosen to inform the ecological and human health assessments of the Fatty Acids and Derivatives Group are further discussed in the relevant sections of this report.
3. Physical and chemical properties
A summary of physical and chemical properties of the substances in the Fatty Acids and Derivatives Group are presented in Table 3‑1. When experimental information was limited or not available for a property, (Q)SAR models were used to generate predicted values for the substance (OECD QSAR Toolbox 2016). Additional physical and chemical properties are reported in ECCC 2016b.
Physical and chemical property data were not available for all of the substances because many are UVCBs. Generally, the fatty acids have low water solubility and vapour pressures and moderate to high octanol-water partition coefficients (log Kow) and organic carbon-water partition coefficients (log Koc), suggesting that they are more likely to be found in soil and sediments. Given that tall oil fatty acids, potassium salts (CAS RN 61790-44-1) is a fatty acid salt, its water solubility is expected to be greater (HERA 2002). According to OECD (2014), two clear trends are evident with increasing alkyl chain length: (1) increasing melting point, boiling point, and partition coefficient, and (2) decreasing water solubility and vapour pressure. Furthermore, within a given carbon chain length, melting point increases with increasing saturation and decreases with increasing unsaturation (OECD 2014).
Fatty acid components of this group of substances are mainly linear fatty acids with a carbon chain length of C16 or C18, with some exceptions, such as undecylenic acid (CAS RN 112-38-9), a single component C11 fatty acid, and ethylhexyl cocoate (CAS RN 92044-87-61), a C12 and C14 predominant fatty acid and the dimerized or trimerized C18 of fatty acids (CAS RN 61788-89-45 and 68937-90-6 C18-unsaturated, dimers/trimers). While the chain length and number, location and isomer form of double bond(s) in carbon chains may alter their physical/chemical properties, the overall physical and chemical properties are expected to be similar among the complex substances, as they form a mixture of aforementioned single carbon chain fatty acids.
Property |
Undecylenic acida |
ALAb |
Dimer acidc |
Trimer acidd |
|---|---|---|---|---|
Physical state |
solid |
Liquid |
liquid |
liquid |
Vapour pressure (Pa) |
0.0192 |
7.2E-5 |
negligible (estimated) |
negligible (estimated) |
Henry’s law constant (Pa·m3/mol) |
0.53 (estimated) |
3.52 (estimated) |
NA |
NA |
Water solubility (mg/L) |
38.46 at pH 4.27 |
0.124 (estimated) |
> 0 - < 0.12 |
> 0 - < 0.37 |
log Kow (dimensionless) |
4.0 |
6.46 |
1 – 2.5 (pH 2) |
2.2 – 8.9 (pH 2) |
log Koc (dimensionless) |
2.84 |
4.068 (estimated) |
6.34 (estimated) |
6.73 (estimated) |
Abbreviations: NA, not available; Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient.
a ECHA (c2007-2017a), ChemIDPlus (1993-)
b ChemIDPlus (1993-), EPISuite c2000-2012
c ECHA (c2007-2017b)
d ECHA (c2007-2017c)
4. Sources and uses
Two of the fatty acids in this group occur naturally in the environment. Undecylenic acid is a natural component of human sweat (Alternative Medicine Review 2002) and occurs naturally in Rohdororula glutinis var. lusitanica, in the essential oils of Juniperus chinensis, and Thujopsis dolabrata and in skim milk powder (Burdock 2010). ALA is considered a dietary essential fatty acid and is found in certain vegetable oils (e.g. canola, soy), nuts (e.g., walnuts) and seeds (e.g., flaxseeds, chia) (IOM 2005; Dietitians of Canada 2017). The remaining 8 substances are derived from natural sources (from plant and animal fats and oils) with the source often clearly indicated by their name. Tung oil is derived from the seeds of the tung tree or China wood oil tree (Vernicia fordii and Vernicia montana) (Shockey et al. 2016) and evening primrose oil is derived from the evening primrose plant (Oenothera biennis) (NTP 2009). Ethylhexyl cocoate is derived from coconut oil. Tall oil fatty acid and tall oil fatty acids, potassium salts are derived from tall oil, a by-product of the pulp from resinous woods (Robinson et al. 2009). As part of the public comment period, industry clarified that dimer and trimer acids are produced mainly from tall oil fatty acid; a smaller volume is produced from vegetable fatty acids such as oleic acid.
All of the substances in the Fatty Acids and Derivatives Group have been included in surveys issued pursuant to a CEPA section 71 notice (Canada 2012). Table 4‑1 presents a summary of information reported on the total manufacture and total import quantities for the Fatty Acids and Derivatives Group.
Common name |
Total manufacture (kg) |
Total imports (kg) |
Reporting year |
Survey reference |
|---|---|---|---|---|
Undecylenic acid |
NR |
NR |
2011 |
Environment Canada 2013 |
ALA |
NR |
1 000 – 10 000 |
2011 |
Environment Canada 2013 |
Tung oil |
NR |
120 412 |
2011 |
Environment Canada 2013 |
Tall oil fatty acid |
1430 |
6 317 473 |
2011 |
Environment Canada 2013 |
Tall oil fatty acids, potassium salts |
10 000 – 100 000 |
47 992 |
2011 |
Environment Canada 2013 |
evening primrose oil |
NR |
NR |
2011 |
Environment Canada 2013 |
Trimer acid |
100 – 1 000 |
293 472 |
2011 |
Environment Canada 2013 |
Dimer acid |
100 – 1 000 |
1 088 638 |
2011 |
Environment Canada 2013 |
Ethylhexyl cocoate |
NR |
6 470 |
2011 |
Environment Canada 2013 |
Abbreviations: NR, not reported.
a Values reflect quantities reported in response to the survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
Table 4‑2 presents a summary of the major uses of the Fatty Acids and Derivatives Group according to information reported pursuant to a CEPA section 71 survey (Environment Canada 2013). Undecylenic acid and evening primrose oil did not have any reported uses above the reporting threshold of 100 kg. Table 4‑4 summarizes additional Canadian uses except for trimer acid which was not identified in products used in any of these applications.
Major usesa |
ALA |
Tung oil |
Tall oil fatty acid |
Tall oil fatty acids, potassium salts |
|---|---|---|---|---|
Lubricants and greases |
Y |
Y |
Y |
Y |
Adhesives and sealants |
Y |
N |
Y |
N |
Paper products |
N |
N |
Y |
Y |
Food packaging |
N |
N |
Y |
Y |
Fuels and related products |
Y |
N |
N |
N |
Paints and coatings |
Y |
Y |
Y |
N |
Personal care |
N |
N |
Y |
N |
Building and construction materials |
N |
N |
Y |
N |
Oil and natural gas extraction |
N |
N |
Y |
N |
Cleaning and furnishing care |
N |
N |
Y |
N |
Automotive care |
N |
N |
Y |
N |
Water treatment |
N |
N |
Y |
N |
Metal materials |
N |
N |
Y |
N |
Other |
Y |
Y |
Y |
Y |
Abbreviations: Y = use was reported for this substance; N= use was not reported for this substance.
a Non-confidential uses reported in response to the survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
Major usesa |
Trimer acid |
Dimer acid |
Ethylhexyl cocoate |
|---|---|---|---|
Lubricants and greases |
Y |
Y |
N |
Adhesives and sealants |
Y |
Y |
N |
Paper products |
N |
Y |
N |
Food packaging |
N |
Y |
N |
Fuels and related products |
Y |
Y |
N |
Paints and coatings |
Y |
Y |
N |
Personal care |
Y |
Y |
Y |
Building and construction materials |
N |
Y |
N |
Oil and natural gas extraction |
Y |
Y |
N |
Water treatment |
N |
Y |
N |
Metal materials |
Y |
Y |
N |
Floor coverings |
Y |
N |
N |
Other |
Y |
Y |
Y |
Abbreviations: Y = use was reported for this substance; N= use was not reported for this substance.
a Non-confidential uses reported in response to the survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
Use |
UA |
ALA |
Tung oil |
Tall oil fatty acid |
|---|---|---|---|---|
Food packaging materialsa |
N |
Y |
Y |
Y |
Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsb |
N |
Y |
N |
Y |
Natural Health Products Ingredients Databasec |
Y |
Y |
N |
Y |
Medicinal or non-medicinal ingredients in licensed natural health productsd |
Y |
Y |
N |
N |
Present in cosmetics, based on notifications submitted under the Cosmetic Regulationse |
Y |
Y |
N |
Y |
Formulant in registered pest control productsf |
Y |
Y |
Ng |
Y |
Abbreviations: UA, undecylenic acid; PT, potassium tallate; EhC, ethylhexyl cocoate; Y= use was reported for this substance; N= use was not reported for this substance.
a Personal communication, e-mail from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 25, 2016; unreferenced).
b DPD [modified 2016]; Personal communication, e-mail from Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated April 28, 2016; unreferenced).
c NHPID [modified 2019].
d LNHPD [modified 2018].
e Personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated April 22, 2016; unreferenced.
f Additionally, none of these substances are registered as active ingredients in pest control products in Canada (personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 9, 2016; unreferenced).
g Can be used as a formulant; however, it is currently not registered in any products (personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 9, 2016; unreferenced).
Use |
Tall oil fatty acids, potassium salts |
Evening primrose oil |
Dimer acid |
EhC |
|---|---|---|---|---|
Food packaging materialsa |
Y |
N |
Y |
N |
Incidental additivea |
Y |
N |
Y |
N |
Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsb |
N |
Y |
N |
N |
Natural Health Products Ingredients Databasec |
Y |
Y |
N |
Y |
Medicinal or non-medicinal ingredients in licensed natural health productsd |
N |
Y |
N |
N |
Present in cosmetics, based on notifications submitted under the Cosmetic Regulationse |
N |
Y |
N |
Y |
Formulant in registered pest control productsf |
Ng |
N |
Y |
N |
Abbreviations: UA, undecylenic acid; PT, potassium tallate; EhC, ethylhexyl cocoate; Y= use was reported for this substance; N= use was not reported for this substance.
a Personal communication, e-mail from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 25, 2016; unreferenced).
b DPD [modified 2016]; Personal communication, e-mail from Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated April 28, 2016; unreferenced).
c NHPID [modified 2019].
d LNHPD [modified 2018].
e Personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated April 22, 2016; unreferenced.
f Additionally, none of these substances are registered as active ingredients in pest control products in Canada (personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 9, 2016; unreferenced).
g Can be used as a formulant; however, it is currently not registered in any products (personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 9, 2016; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Fatty Acids and Derivatives Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. Since tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid and ethylhexyl cocoate are UVCB substances and could not be suitably represented by single chemical structures, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD (Q)SAR Toolbox 2016), and from responses to surveys under section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used either as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence and long-range transport potential, were also established. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of the UVCBs, hazard and exposure could not be fully profiled because of the lack of a representative structure to estimate needed properties and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining UVCB constituents and information submitted under section 71 surveys under CEPA was performed and decisions on the basis of consideration of similar substances and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in the use of a conservative (protective) tissue residue value for critical body residue (CBR) analysis. Error of underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Fatty Acids and Derivatives Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the substances in the Fatty Acids and Derivatives Group are summarized in Table 5-1.
Common name |
ERC hazard classification |
ERC exposure classification |
ERC risk classification |
|---|---|---|---|
Undecylenic acid |
low |
low |
low |
ALA |
high |
low |
low |
Tung Oil |
low |
high |
low |
Tall oil fatty acid |
low |
low |
low |
Tall oil fatty acids, potassium salts |
low |
low |
low |
Evening primrose oil |
high |
low |
low |
Trimer acid |
low |
low |
low |
Dimer acid |
low |
low |
low |
Ethylhexyl cocoate |
low |
low |
low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, undecylenic acid; tall oil fatty acid; tall oil fatty acids, potassium salts; trimer acid; dimer acid; and ethylhexyl cocoate were classified as having a low potential for ecological risk. Although the reported import/manufacture quantities of tall oil fatty acid and tall oil fatty acids, potassium salts were high and moderate, respectively, the ERC classified the exposure potential of these substances as low on the basis of low overall persistence. Additionally, new information was considered following the public comment period of the draft screening assessment for tall oil fatty acid and tall oil fatty acids, potassium salts. Consequently, the ERC hazard classifications of tall oil fatty acid and tall oil fatty acids, potassium salts were lowered from high to low on the basis of a narcotic mode of action and a low potential to cause adverse effects in aquatic food webs based on bioaccumulation potential. It is therefore unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, ALA and evening primrose oil were classified as having low ecological exposure potential. ALA and evening primrose oil were classified as having a high hazard potential on the basis of a reactive mode of actionFootnote 8 and increased potential to cause adverse effects in aquatic food webs given their bioaccumulation potential. Structural alerts from OECD toolbox identified ALA and evening primrose oil also identified these substances as being potential DNA and/or protein binders. ALA and evening primrose oil were initially classified as having a moderate potential for ecological risk; however, the risk classifications were decreased to low potential for ecological risk following the adjustment of risk classification on the basis of current use quantities (see section 7.1.1. of ECCC 2016a). The potential effects and how they may manifest in the environment were not further investigated given the low exposure of these substances. On the basis of current use patterns, these substances are unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, tung oil was classified as having a high exposure potential on the basis of moderate use quantities and a high margin of exposure. The ERC classified tung oil as having a low hazard potential and subsequently a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Overview and approach
The available toxicity studies demonstrate the low acute toxicity of several of the fatty acids and their salts. The estimated LD50s for seven of the substances in this group are greater than 2000 mg/kg bw via oral route and greater than 3000 mg/kg bw via dermal route of exposure in laboratory animals (HERA 2002). Although the OECD toolbox prediction profiles identified ALA and evening primrose oil as being potential DNA binders based on structure, the (Q)SAR genotoxicity predictions from the Danish (Q)SAR database (2015) for ALA and for the major component of evening primrose oil were negative. In addition, ALA, an essential fatty acid for humans and the major component of evening primrose oil, along with 78 other fatty acids, was not identified by OECD (2014) as possessing properties indicating a hazard for human health for systemic health effects or for mutagenic or clastogenic activities. Given the apparently low toxicity of some of the fatty acids and derivatives in this assessment as identified in acute and repeated dose toxicity studies, ALA; tung oil; evening primrose oil; tall oil fatty acid; tall oil fatty acids, potassium salts; dimer acid and trimer acid are addressed qualitatively in section 6.3. The remaining two substances, undecylenic acid and ethylhexyl cocoate, are more complex and are described in more detail in section 6.4.
In the absence of data for UVCB substances, the information for the major constituents (as indicated in Table A-1 in Appendix A) and potential hydrolysis products was used to inform characterization of the potential health effects of the fatty acids and their derivatives.
6.2 General information on Fatty Acids and Derivatives Group
Environmental media
No empirical data on the presence of these substances in environmental media in Canada or elsewhere were identified. In general, considering their physical and chemical properties and current use patterns, the fatty acids in this group are not likely to be found in air, but may be found in water as a result of industrial releases or from the use of products available to consumers (down-the-drain releases). However, exposure of the general population to substances in the Fatty Acids and Derivatives Group via environmental media is not expected to be of concern.
General toxicokinetic and metabolism information for fatty acids
There is no substance-specific toxicokinetic information identified for the fatty acids and their derivatives covered by this group, except for limited information on ALA. Fatty acid chain length and unsaturation number influence fat absorption. Shorter chain length fatty acids are more extensively absorbed than longer chain fatty acids because they can be solubilized in the aqueous phase of intestinal contents (Ramírez et al. 2001).
The dermal absorption of fatty acids demonstrated a decreased trend with increasing chain length (Howes 1975). An in vitro study in human skin confirmed the penetration of several fatty acid components (e.g., oleic, linoleic, lauric and capric acid) (Kezutyte et al. 2013). A 100% dermal absorption of alpha-linolenic acid (CAS 463-40-1) was predicted by an in vitro model (Buist et al. 2010; Kim et al. 2014). However, the predicted dermal absorption of ethylhexyl cocoate was very low, i.e., in the range of 1.26E-05 to 4.46E-05 mg/cm²/event (ECHA dossier c2007-2017d). Differences in physicochemical properties of fatty acids might determine their different affinity to skin lipids and mechanisms of action (Kezutyte et al. 2013).
6.3 ALA; tung oil; evening primrose oil; tall oil fatty acid; tall oil fatty acids, potassium salts; dimer and trimer acids
There are no international classifications for carcinogenicity, genotoxicity, and developmental or reproductive toxicity for ALA; tung oil; evening primrose oil; tall oil fatty acid; tall oil fatty acids, potassium salts; dimer acid or trimer acid. The available oral and dermal repeated dose toxicity studies demonstrated the low toxicity of fatty acids and their salts (HERA 2002). OECD conducted a human health assessment on a group of 78 naturally derived homologous straight-chain fatty acids together with a few fatty acid salts and esters, including ALA and 8 major components of the fatty acid UVCBs assessed here (as indicated in Appendix A). In addition, tall oil fatty acid (CAS 61790-12-3) was included by the OECD (2014) as a supporting substance to inform the straight-chain fatty acids category assessment. Adverse effects from fatty acids and derivatives were only seen at high exposures (e.g., greater than 3000mg/kg bw/day). ALA; tung oil; evening primrose oil; tall oil fatty acid; tall oil fatty acids, potassium salts; dimer acid and trimer acid are therefore considered to have low hazard potential.
6.3.1 Alpha-linolenic acid (ALA)
The general population of Canada may be exposed to ALA from its natural presence in foods and from food packaging, cosmetics, natural and non-prescription health products, certain fungicides, fuels, lubricants and greases, and/or paints and coatings.
ALA is an essential n-3 or omega-3 polyunsaturated fatty acid (PUFA). It cannot be synthesized in humans (IOM 2005; FAO 2010).
A dietary reference intake (DRI) level, specifically, an adequate intake (AI), has been set for ALA by the Institute of Medicine (IOM) and has been adopted by Health Canada (Health Canada 2010). The AI for the various age groups ranges from 0.5 g/day for infants (0 to 12 months old) (based on intakes from human milk and complementary foods) to 1.1 and 1.6 g/day for females and males aged 14 years and older, respectively. These AIs were derived on the basis of an intake that supports normal growth and neural development and results in no nutrient deficiency. Currently, there is no tolerable upper intake level for ALA (IOM 2005).
There were no genotoxicity or repeated dose toxicity studies identified for ALA. However, there were numerous studies available investigating the beneficial effects of ALA in humans for the purpose of nutraceutical or pharmaceutical uses. The potential adverse human health effects of ALA, together with a group of 78 naturally derived homologous straight-chain fatty acids, were assessed by OECD (2014). ALA, a non-branched fatty acid with a carbon chain length of 18, was not identified by OECD as possessing properties indicating a hazard for human health for systemic health effects or for mutagenic or clastogenic activities based on the toxicity information of another C18 fatty acid, tung oil. Although structural alerts from the OECD QSAR Toolbox identified ALA and evening primrose oil as being potential DNA binders, the negative predictions from four main models in the Ames Salmonella typhimurium test for ALA obtained from the Danish (Q)SAR database (2015) indicate that its potential for mutagenicity is low.
Although the hazard database is limited, the available information indicates that ALA is considered to be of low hazard potential, and risk to human health is considered to be low.
6.3.2 Tung oil
The general population of Canada may be exposed to tung oil from its presence in food packaging, paints and coatings, and lubricants and greases.
The major constituent of tung oil, α-eleostearic acid (CAS RN 506-23-0), which makes up nearly 80% of the fatty acids in tung oil, was found to be converted to conjugated linoleic acid (CLA; 9Z, 11E-18:2) in the liver and the plasma in rats (Tsuzuki et al. 2004a). The conjugated linoleic acid (CLA)-rich oil was determined by EFSA (2010) as not indicating a risk for genotoxicity, reproductive toxicity or carcinogenicity based on animal studies. Moreover, α-eleostearic acid was shown to have antitumorigenic activity in cancer cells and in animal models. A recent in vitro study demonstrated that α-eleostearic acid inhibited growth and induced apoptosis in human breast cancer cells (Zhuo et al. 2014). In an in vivo study, a strong antitumorigenic effect of α-eleostearic acid was also reported by Tsuzuki et al. (2004b) in nude mice into which human colon cancer cells were transplanted.
Both sexes of weanling and adult rats were administered tung oil via gavage at 74 960 or 10 708 mg/kg bw/day, respectively, for 14 days. All weanling rats died by day 5 and 50% of the adult rats died at the end of the study. Immediate and significant suppression of diet intake occurred in weanling rats at the beginning of treatment. The same effect was reported in adult rats starting on day 3 of dosing. However, there were no gross lesions or pathological changes observed on autopsy of the dead rats. The lowest observed adverse effect level (LOAEL) for mortality in adult rats was 10 708 mg/kg bw/day (McPherson 1973). When rats were administered tung oil at 6 000 to 14 400 mg/kg bw/day in the diet for 28 days, an increase in serum cholesterol was reported. No other health effects were reported by the author (Hegsted et al. 1957).
Although the hazard database is limited, the available information indicates that tung oil is considered to be of low hazard potential, and risk to human health is considered to be low.
6.3.3 Evening primrose oil
The general population of Canada is exposed to evening primrose oil from its use in cosmetics and natural health products.
There were no genotoxicity studies identified for evening primrose oil. However, the major component of evening primrose oil, linoleic acid (CAS RN 60-33-3, 70-77% in evening primrose oil) was determined by OECD (2014) as not mutagenic based on an in vitro genotoxicity study.
In chronic studies in which both sexes of SD rats were administered Efamol, an evening primrose oil product containing 70 to 73% linoleic acid, via gavage at 0, 0.3, 1.0 or 2.5 mL/kg bw/day (equivalent to 0, 279, 928 or 2321 mg/kg bw/day of evening primrose oil) for 53 weeks, no significant adverse effects were found compared to the controls (Everett et al. 1988a, cited in NTP 2009; EMA 2011). In addition, Efamol did not induce significant differences in the nature or frequency of tumours between the treated and control animals when the same dosage regimen was used in SD rats for 104 weeks or in CD-1 mice for 78 weeks (NOAEL = 2321 mg/kg bw/day, Everett et al. 1988b, cited in NTP 2009; EMA 2011). When male F344/DuCrj rats were administered evening primrose oil at 5233 mg/kg bw/day in diet (only dose tested) for 15 weeks, the only effect observed was an increase in the cholesterol level (NOAEL = 5233 mg/kg bw/day, Fukushima et al. 2001).
Several animal studies showed that dietary supplementation of evening primrose oil had no effect on parturition, maternal or birth weight, postnatal growth rate, or fetal or placenta prostaglandin E2 levels (NTP 2009). When Wister rats were exposed to evening primrose oil in diet at 0 or 1543 mg/kg bw/day for 5 weeks until mating, there was no effect on parturition, birth weight, postnatal growth rate or maternal weight (NOAEL = 1543 mg/g bw/day, Leaver et al. 1986, cited in NTP 2009). Some studies showed that evening primrose oil exposure could benefit reproductive and developmental performance by enhancing male reproductive function and increasing neonate survival in animals (NTP 2009).
The major components of evening primrose oil—oleic acid (CAS RN112-80-1), palmitic acid (CAS RN 57-10-3) and linoleic acid (CAS RN 60-33-3)—were not identified by OECD (2014) as possessing properties indicating a hazard for human health for systemic health effects. Although the hazard database is limited, the available information indicates that evening primrose oil is considered to be of low hazard potential, and risk to human health is considered to be low.
6.3.4 Tall oil fatty acid and tall oil fatty acids, potassium salts
The general population of Canada may be exposed to tall oil fatty acid from its use in food packaging, a few drug products, and cosmetics. It is also used by Canadian consumers in adhesives and sealants, building or construction materials, cleaning and furniture care products, automotive care products, lubricants and greases, and agricultural products.
Tall oil fatty acids, potassium salts can be used in food packaging and in various commercial and industrial uses, and may be present as a component in an incidental additive (food contact surface cleaner with a potable water rinse).
No toxicity studies were identified for tall oil fatty acids, potassium salts, specifically. However, for fatty acids in general, their acid and alkali salt forms of the same fatty acid are expected to have many similar physicochemical and toxicological properties when they become bioavailable (HERA 2002). Thus, the potential health effect induced by tall oil fatty acids, potassium salts is expected to be similar to that of tall oil fatty acid. In addition, the contribution of the cation of fatty acid salt, in this case the potassium ion, is not expected to add excessively to the normal body load to induce health effects at current exposure levels. Therefore, the potential health effect induced by potassium ion released from tall oil fatty acids, potassium salts will not be considered in this assessment.
Tall oil fatty acid was not mutagenic in S. typhimurium strains (OECD 2014), and it was not clastogenic in either human lymphocytes or Chinese hamster ovary cells in the presence or absence of metabolic activation (ECHA c2007-2017e).
In a two-generation study, both sexes of rats (strain not specified) were administered 0, 5 or 10% of tall oil fatty acid in the diet (equivalent to 0, 2500 or 5000 mg/kg bw/day). The F0 generation was exposed from age of 80 days to 100 days and through the weaning period of the first generation (F1). After weaning, 20 F1 males and 20 F1 females per group were maintained on the parental diet. At 100 days of age, these rats were mated and allowed to deliver pups (F2). Treatment did not affect the number of live born or stillborn F1 litters and pups, or F1 weaning weight. No treatment-related changes in fertility, viability, lactation, or gestation indices were reported. Hematology, clinical chemistry and urinalysis parameters were unchanged, and gross and microscopic pathology revealed no treatment-related effects (NOAEL reproductive toxicity ≥ 5000 mg/kg bw/day, OECD 2014).
In a 90-day study, both sexes of rats (strain not specified) were administered tall oil acid in the diet at 0, 5, 10 or 25% (equivalent to 0, 2 500, 5 000, or 12 500 mg/kg bw/day). No treatment-related effects were noted in any treated groups. A NOAEL of 2 500 mg/kg bw/day was determined by OECD (2014) based on slightly decreased food consumption in the mid- and high- dose groups. Male SD rats were administered tall oil fatty acid distillate in the diet for 28 days at 0, 15, 30, or 60% (equivalent to 0, 7 500, 15 000 or 30 000 mg/kg bw/day). Significantly decreased growth rate accompanied by slightly decreased food consumption was reported in rats treated with 15 000 mg/kg bw/day of tall oil acid. All 10 animals in the high-dose group died in the first 4 days (Seppanen 1969). The major components of tall oil acid, oleic acid and linoleic acid were not identified by OECD (2014) as possessing properties indicating a hazard for human health for systemic health effects. Although the hazard database is limited, the available information indicates that tall oil fatty acid and tall oil fatty acids, potassium salts are considered to be of low hazard potential, and risk to human health is considered to be low.
6.3.5 Dimer and Trimer Acids
The general population of Canada may be exposed to dimer acid from its use in food packaging, in various industrial and commercial uses, and in certain personal care products, and in a specialized air filter oil that is available to consumers (SDS 2016). Dimer acid may also be used as a component in an incidental additive (lubricant for non-food contact surface) however exposure is not expected.
No consumer uses were identified for trimer acid in Canada. However, it is used in various industrial and commercial applications.
Dimer acid contains two fatty acid molecules, dicarboxylic acids, and is produced by dimerizing unsaturated fatty acids. The chemical reaction can be taken further to form a trimer acid, where the product consists of three fatty acid molecules. Although the commercial products contain predominantly a dimer (C16-18), dimer acids also comprise various ratios of fatty acids trimer (ECHA dossier c2007-2017c) and vice versa. As there were no toxicity studies identified for trimer acid, the toxicity information for dimer acid is used to inform the hazard of trimer acid given the similarity of their physical-chemical properties.
In in vitro assays, in the presence or absence of metabolic activation, dimer acid tested negative in gene mutation assays with S. typhimurium strains or with mouse lymphoma cell lines. It also tested negative in chromosome aberration with human lymphocytes (US EPA 2005).
In a reproductive toxicity study, both sexes of SD rats were administered dimer acid in the diet at concentrations of 0, 200, 2 000, or 20 000 ppm (equivalent to 0, 15/17, 145/169, or 1 450/1 692 mg/kg bw/day, male/female). Males were dosed for at least 4 weeks, starting from 2 weeks prior to mating, while females were dosed from 2 weeks prior to mating until at least day 6 of lactation. A slight decrease in weight gain (statistically non-significant) and an increase in piloerection (lack of dose relationship) in parent rats were observed in high-dose groups. There were no effects on birth and live birth index, litter size, litter weight, pup weight, viability index or externally visible anomalies in any of the pups. There were no obvious maternal effects reported in this study (NOAEL maternal /developmental = 1 450/1 692 mg/kg bw/day, US EPA 2005).
In a 90-day study, SD rats were administered dimer acid in the diet at concentrations of 0, 0.1, 1 or 5% (equivalent to 0, 74/90, 740/854, or 3591/4085 mg/kg bw/day, male/ female). At 3 591/4 085 mg/kg bw/day (male/female), histopathological changes, such as aggregations of macrophages in the mesenteric lymph nodes, statistically significant changes in multiple clinical chemistry parameters, and significant decreases in absolute and relative spleen and liver weights, were observed in both sexes of rats (NOAEL = 740/854 mg/kg bw/day, US EPA 2005).
In a multi-chemical Tier I human health risk assessment carried out by the Australian Government Department of Health (AGDH 2017), dimer acid was listed as one of the chemicals that were not considered to pose an unreasonable risk to the health of workers or the general public. Although the hazard database is limited, the available information indicates that dimer and trimer acids are considered to be of low hazard potential, and risk to human health is considered to be low.
6.4 Undecylenic acid and ethylhexyl cocoate
6.4.1 Undecylenic acid
6.4.1.1 Exposure assessment
Environmental media and food
No empirical data on the presence of undecylenic acid in air, water, soil, sediment or dust were identified in Canada or elsewhere, and undecylenic acid did not have any reported uses above the CEPA section 71 notice reporting threshold of 100 kg (Environment Canada 2013). Therefore, exposures to undecylenic acid from environmental media are not expected (see section 6.2).
Undecylenic acid can be used as a flavouring agent in alcoholic beverages, baked goods, frozen dairy products, gelatins and puddings, gravies, meat products, non-alcoholic beverages, and soft candy (Burdock 2010). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated 42 flavouring substances including linear and branched-chain aliphatic unsaturated and unconjugated alcohols, aldehydes, acids, and related esters (WHO 1999). As part of that evaluation, the Committee estimated the per capita intake of undecylenic acid from its use as a food flavouring agent to be 0.01 µg/kg bw/day for the US population and 0.5 µg/kg bw/day for the European population. In deriving these intakes which were estimated using a maximized survey-derived daily intake (MSDI) approach, it was assumed that the reported annual production amount of undecylenic acid in the United States and Europe was consumed by just 10% of the population (“eaters only”), and that only 60% of the annual production amount was reported in the poundage surveys (International Organization of the Flavor Industry 1995; US National Academy of Sciences 1989, both cited in WHO 1999).
Products available to consumers
Undecylenic acid is currently present as medicinal or non-medicinal ingredient in natural health products (LNHPD [modified 2018]). An oral product has been identified as containing undecylenic acid as non-medicinal ingredient, with an estimated daily exposure ranging from 0.42 to 0.63 mg/kg bw/day (see Appendix B). The topical products identified as containing undecylenic acid as a non-medicinal ingredient are considered to be covered by the cosmetic exposure estimates described below.
Undecylenic acid was identified in several cosmetics in Canada, including face and body moisturizers, shampoo, make-up, face and body cleansers, and nail conditioners (personal communication, e-mail from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 22, 2016; unreferenced). Table 6‑1 summarizes the sentinel exposure scenarios for cosmetics containing undecylenic acid. ConsExpo Web was used to estimate cosmetic exposures (ConsExpo Web 2016) and details on the parameters used in the model are found in Appendix B. The dermal exposure estimates presented in Table 6‑1 represent external exposure doses. Given that no information on the dermal absorption of undecylenic acid was identified and based on its physical and chemical properties (low molecular weight, moderate log Kow), a dermal absorption of 100% is assumed.
Product scenario |
Concentration range |
Dermal exposure estimate for adults (mg/kg bw/day) |
Dermal exposure estimate for infants (mg/kg bw/day) |
|---|---|---|---|
Body lotion |
0.0025 |
0.0017 |
0.0079 |
Specialized body lotiona |
0.3 – 1% |
0.093 – 0.31 |
N/A |
Face moisturizer |
0 – 0.1% |
0 – 0.03 |
N/A |
Facial make-up |
0.0011 – 0.3% |
0.0001 – 0.028 |
N/A |
Nail conditioner |
3 – 10% |
0.04 – 0.14 mg/kg bw per event |
N/A |
Abbreviations: N/A, not applicable
a Specialized body lotions for soothing and warming, for adult use only (for feet and legs)
6.4.1.2 Health effects assessment
Undecylenic acid did not induce gene mutations in in vitro studies in the presence or absence of metabolic activation with S. typhimurium strains or mouse lymphoma cell lines, nor did it induce chromosome aberrations in human lymphocytes or cause DNA damage in rat hepatocytes (ECHA c2007-dossier 2017a). In an in vivo study, undecylenic acid did not induce micronucleus formation in bone marrow of mice administered doses of up to 4000 mg/kg via gavage (ECHA dossier c2007-2017a). There were no carcinogenicity studies identified for undecylenic acid.
In a prenatal developmental toxicity study, pregnant SD rats were administered undecylenic acid at 0, 150, 450 or 750 mg/kg bw/day via gavage from gestation day 6 to 20 (15 days). All rats in the 450 mg/kg bw/day group exhibited hypersalivation and significantly reduced body weight gain compared to controls. There was no embryonic toxicity or teratogenicity observed. The highest dose of 750 mg/kg bw/day was removed from the study due to the high maternal mortality (NOAEL teratogenicity = 450 mg/kg bw/day, NOAEL maternal toxicity = 150 mg/kg bw/day, LOAEL maternal toxicity = 450 mg/kg bw/day for hypersalivaiton and reduced body weight gain, ECHA dossier c2007-2017a). In a reproductive and developmental toxicity study, undecylenic acid was administered to both sexes of SD rats at 0, 50, 150 or 450 mg/kg bw/day via gavage. Males were dosed 2 weeks before mating, during the mating period (2 weeks), until sacrifice (at least 4 weeks in total); females were dosed 2 weeks before mating, during the mating period (2 weeks), during pregnancy and lactation period until day 4 of post-partum. No reproductive or developmental parameters investigated were affected. Two males in the high-dose group died on day 3 and two died on day 35 without ante-mortem clinical signs of toxicity. A LOAEL of 450 mg/kg bw/day was determined based on the mortality in high-dose male group (NOAEL parental effect = 150 mg/kg bw/day, NOAELF1 = 450 mg/kg bw/day, NOAEL reproductive performance = 450 mg/kg bw/day; ECHA dossier c2007-2017a). When rats were administered orally with undecylenic acid at 0, 100, 200 or 400 mg/kg bw/day for 9 months via gavage, then mated, there were no abnormalities found in the litters and there was no sign of toxicity reported in parent rats (ECHA dossier c2007-2017a).
In a 90-day study, males and female SD rats were administered sodium salt of undecylenic acid (no CAS RN provided, presumably CAS RN 1002-62-6, ECHA dossier c2007-2017a) via gavage at 0, 20, 60 or 180 mg/kg bw/day for 50 days. The 180 mg/kg bw/day dose group was examined at 50 days then further administered a dose of 360 mg/kg bw/day for 40 days (as 180/360 mg/kg bw/day groups), with an additional 4 weeks of recovery. Dose-dependent clinical signs including ptyalism, loud breathing, respiratory difficulties and poor clinical condition were reported. Reduced body weight gain in males accompanied by reduced food consumption in the 180 mg/kg bw/day dose groups and in the 180/360 mg/kg bw/day dose groups was reported. Reduced glucose plasma levels (reversible) and reduced triglyceride-levels (not reversible) in females were observed in the 180 mg/kg bw/day dose groups as well as in the 180/360 mg/kg bw/day dose groups. Forestomach oedema and inflammatory cell infiltration were observed in the same dose groups. Cardiomyopathy, as reversible myocardial degeneration and monocellular aggregation, was reported in both sexes in the 180/360 mg/kg bw/day dose groups exclusively. A LOAEL of 180 mg/kg bw/day (NOAEL = 60 mg/kg bw/day) was derived for undecylenic acid sodium salt by ECHA dossier (c2007-2017a). The converted equivalent doses for undecylenic acid are 160 mg/kg bw/day (LOAEL) and 53 mg/kg bw/day (NOAEL), respectively. Given the paucity of relevant health effects studies for undecylenic acid, hazard data obtained from the ECHA dossier was utilized to inform critical health effects and subsequent risk characterization.
6.4.1.3 Characterization of risk to human health
The estimated per capita intake from the use of undecylenic acid as a possible flavouring agent in foods was derived by the JECFA to range from 0.01 µg/kg bw/day for the US population to 0.5 µg/kg bw/day for the European population. JECFA concluded that there is no safety concern for undecylenic acid used as a flavouring agent primarily on the basis of it being “expected to be oxidized to the corresponding aldehyde and carboxylic acid which is completely metabolized in the fatty acid and tricarboxylic acid pathways” (WHO 1999).
Undecylenic acid was not mutagenic in vitro or in vivo. It did not induce reproductive or developmental health effects at oral dose levels up to 450 mg/kg bw/day in rats. However, maternal health effects, such as hypersalivation, reduced body weight gain and death, occurred at this dose level. In addition, a 90-day oral study conducted with undecylenic acid sodium salt showed that the treatment related changes, such as altered multiple clinical chemistry parameters and reduced body weight gain, occurred at undecylenic acid equivalent dose level of 160 mg/kg bw/day (considered to be the LOAEL; NOAEL = 53 mg/kg bw/day). The use of critical effects levels derived from the 90-day oral toxicity study with undecylenic acid sodium salt is considered appropriate for characterization of the human health risk from exposure to undecylenic acid. This approach is considered to be conservative as the salts of the fatty acids tend to be more bioavailable given that they have greater water solubility than the free acids.
With respect to dermal toxicity, no studies were identified. The oral critical effect levels were therefore applied to the dermal external exposure scenarios presented in Table 6‑1, assuming 100% dermal absorption.
Table 6‑2 provides all relevant exposure and hazard values for undecylenic acid, as well as resultant margins of exposure, for determination of risk.
Exposure scenario |
Systemic exposure |
Critical effect level oral |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Non-medicinal ingredient in natural health product - oral |
0.42 – 0.63 mg/kg bw/day |
53 mg/kg bw/day (NOAEL) |
Changes in clinical parameter, body weight and clinical signs |
84 – 126 |
Body moisturizer (adult) |
0.0017 mg/kg bw/day |
53 mg/kg bw/day (NOAEL) |
Changes in clinical parameter, body weight and clinical signs |
31 176 |
Body moisturizer (infant) |
0.0079 mg/kg bw/day |
53 mg/kg bw/day (NOAEL) |
Changes in clinical parameter, body weight and clinical signs |
6 709 |
Specialized body moisturizer (adult) |
0.093 – 0.31 mg/kg bw/day |
53 mg/kg bw/day (NOAEL) |
Changes in clinical parameter, body weight and clinical signs |
172 – 570 |
Face moisturizer |
0 – 0.03 mg/kg bw/day |
53 mg/kg bw/day (NOAEL) |
Changes in clinical parameter, body weight and clinical signs |
1 767 |
Facial make-up |
0.0001 – 0.028 mg/kg bw/day |
53 mg/kg bw/day (NOAEL) |
Changes in clinical parameter, body weight and clinical signs |
1 893 – 530 000 |
Nail conditioner (per event) |
0.04 – 0.14 mg/kg bw/day |
150 mg/kg bw/day (NOAEL) |
Parental systemic effects |
1 071 – 3 750 |
Abbreviations: NOAEL, no-observed adverse effect level.
On the basis of the conservative parameters used in modelling exposure to products and their recommended conditions of use, as well as the use of conservative critical effect levels derived from undecylenic acid salt, which is considered to be absorbed to a much greater extent than undecylenic acid due to higher solubility, the calculated margins are considered adequate to address uncertainties in the health effects and exposure databases.
6.4.1.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
Key source of uncertainty |
Impact |
|---|---|
There is some uncertainty on the dermal absorption of undecylenic acid; however, it is assumed that 100% is considered reasonable given the molecular size and type of substance. |
+ |
No chronic oral studies, inhalation studies or dermal studies were identified. No dermal absorption data were identified. |
+/- |
There is some uncertainty regarding the use of critical effective level of undecylenic acid salt to characterize the risk of undecylenic acid. |
+/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
6.4.2 Ethylhexyl Cocoate
6.4.2.1 Exposure Assessment
Environmental media and food
No empirical data on the presence of ethylhexyl cocoate in air, water, soil, sediment, dust, or food were identified in Canada or elsewhere. Given the import quantities reported in Canada in 2011 (see Table 4-1), drinking water intakes were estimated using the Environmental Assessment Unit’s Drinking Water Workbook (Health Canada 2015), predicted wastewater treatment system removal rates (SimpleTreat 1997), and information on the quantities of the substance in Canada (Environment Canada 2013). The predicted drinking water intakes for ethylhexyl cocoate resulting from potential industrial and down-the-drain releases were less than 2.5 ng/kg bw/day and are therefore considered negligible.
Cosmetics
In Canada, ethylhexyl cocoate is used primarily as an emollient in cosmetics, including moisturizers, cleansers, conditioners, make-up, styling products, shaving products, bath products, and massage products (personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 22, 2016; unreferenced). Table 6‑4 summarizes the sentinel exposure scenarios for cosmetics containing ethylhexyl cocoate. ConsExpo Web was used to estimate cosmetic exposures (ConsExpo Web 2016), and details on the parameters used in the model are found in Appendix B.
Product scenario |
Concentration range |
Dermal exposure estimate for adults (mg/kg bw/day)a |
Systemic dermal exposure estimate for adults (mg/kg bw/day)b |
Oral exposure estimate for toddlers (mg/kg bw per event)c |
|---|---|---|---|---|
Body moisturizer |
1 – 10% |
0.68 – 6.8 |
0.041 – 0.41 |
N/A |
Face moisturizer |
0 – 30% |
9.1 |
0.55 |
N/A |
Facial make-up |
0.025 – 100% |
0.00024 – 9.4 |
1.4E-05 – 0.56 |
N/A |
Hair oil |
10 – 30% |
2 – 6.1 |
0.12 – 0.37 |
N/A |
Lipstick/lip gloss |
0 – 0.1% |
0.00034 |
N/A |
0.00065 |
Massage oil (body)d |
0.1 – 0.3% |
0.045 – 0.14 mg/kg bw per event |
0.0027 – 0.0084 mg/kg bw per event |
N/A |
Abbreviations: N/A, not applicable.
a Dermal exposure scenarios except for lipstick, which is an oral exposure scenario.
b Systemic exposures from the dermal route were derived using a dermal absorption value of 6%.
c Lip gloss assumed to be used occasionally by toddlers (not every day).
d Assume that use of massage oil does not occur daily, therefore exposure estimate is per event (not per day).
The dermal absorption rate of ethylhexyl cocoate was predicted to range between 1.26E-05 and 4.46E-05 mg/cm²/event (ECHA c2007-2017d). However, details on the method used to derive this prediction were not available. In an in vivo study in rats, the dermal absorption of 2-ethylhexanol, one of the hydrolysis products of ethylhexyl cocoate, was between 5% and 6% after 96 hours of dermal exposure (Deisinger et al. 1994, cited in EC, HC 2011). Given their physical and chemical properties, it is expected that the dermal absorption of ethylhexyl cocoate would be less than that of 2-ethylhexanol, which is a smaller molecule and more hydrophilic than ethylhexyl cocoate. In addition, ethylhexyl cocoate may be hydrolyzed in the stratum corneum (see section 6.4.2.2). As such, a dermal absorption of 6% from the in vivo rat study of 2-ethylhexanol was used to estimate systemic exposure of ethylhexyl cocoate from the dermal route (see Table 6‑4).
6.4.2.2 Health effects assessment
No studies were identified for ethylhexyl cocoate. However, as a fatty acid ester, ethylhexyl cocoate can be rapidly hydrolyzed in digestive fluids and yield the corresponding alcohol and free fatty acid within the gastrointestinal tract after oral exposure (Bookstaff et al. 2003, cited in ECHA c2007-2017f). Thus, the available health effects information of the hydrolyzed products of ethylhexyl cocoate and the information of the major components of ethylhexyl cocoate were used in this assessment to inform the potential health effects of ethylhexyl cocoate.
Toxicity data of hydrolyzed products of ethylhexyl cocoate
Ethylhexyl cocoate could be hydrolyzed into fatty acids and 2-ethylhexanol (2-EH, CAS RN 104-76-7) post absorption in the gastrointestinal tract. Following dermal exposure, the absorption rate of 2-EH was only 5% to 6 % in rats within 96 hours (Deisinger et al. 1994, cited in EC, HC 2011). Given that the current use patterns indicate dermal exposure, it is important to consider how ethylhexyl cocoate may be metabolized on the skin by esterases. Although the majority of the esterase activity in human skin is located in the epidermis and hair follicles (Tokudome et al. 2015), such activity has also been found in the stratum corneum (Beisson et al. 2001). This suggests that ethylhexyl cocoate may be hydrolyzed by esterases in the stratum corneum to form 2-EH.
For 2-EH, a LOAEL of 150 mg/kg bw/day (NOAEL = 50 mg/kg bw/day) was derived from a chronic study based on reduced body weight gain and increased relative weights of brain, stomach, kidneys and liver in both sexes of rats after 2-EH was administered via gavage at 0, 50, 150 or 500 mg/kg bw/day for 24 months (EC, HC 2011).
For dermal route of exposure, there was no evidence of toxicity to the developing young at up to a maximum tested dose of 2520 mg/kg bw/day in a developmental toxicity study, in which rats were dermally exposed to 2-EH at doses of 0, 252, 420, 840, 1680, or 2520 mg/kg bw/day during gestation day 6 to 15 (NOAEL = 2520 mg/kg bw/day). However, decreased body weight gain in the dams was observed at 1680 mg/kg bw/day (EC, HC 2011). In addition, the lowest dermal LOAEL of 834 mg/kg bw/day for 2-EH was derived from a short-term study based on the health effects of lymphopenia, reduced spleen weights and histopathological effects on the skin in both sexes of rats exposed to 0, 0.5, or 1.0 mL of 2-EH (equivalent to 0, 417 or 834 mg/kg bw/day) 9 times within 12 days (EC, HC 2011).
2-EH was known to be rapidly and extensively metabolized into 2-ethylhexanoic acid (2- EHA, CAS RN 149-57-5) via oxidation in vivo. The risk to human health of 2-EHA was previously assessed by Health Canada (EC, HC 2011). Developmental toxicity was identified as a critical effect for 2-EHA by HC (EC, HC 2011). The lowest oral LOAEL of 100 mg/kg bw/day for 2-EHA was determined by Health Canada (EC, HC 2011) on the basis of dose-dependent increase in skeletal malformations in fetuses observed in an oral developmental toxicity study in which pregnant rats were dosed at 0, 100, 300 or 600 mg/kg bw/day of 2-EHA via drinking water from gestation day 6 to 19 of (EC, HC 2011). There is an uncertainty regarding the mode of action for developmental toxicity following 2-EHA oral exposure. 2-EHA has been shown to induce peroxisome proliferation in rodents (mediated by the peroxisome proliferator-activated receptor alpha or PPARα) (EC, HC 2011). However, this is less likely to be relevant to humans and the role of PPARα in developmental and reproductive toxicity remains to be established (EC, HC 2011).
The equivalent critical effect level for the oral route of exposure to ethylhexyl cocoate converted from that of 2-EH is approximately 120 mg/kg bw/day (NOAEL) for systemic effects following 24 months of exposure in rats. The equivalent critical effect levels for dermal route of exposure to ethylhexyl cocoate converted from those of 2-EH are approximately 6045 mg/kg bw/day (NOAEL) for developmental effects and 2015 mg/kg bw/day (NOAEL) for maternal health effects following 9 days of exposure in rats. The lowest dermal NOAEL for ethylhexyl cocoate converted from the 12-day repeated dose study of 2-EH is 1000 kg/bw/day for increased incidences of lymphopenia, reduced spleen weights and histopathological effects on the skin at the next dose level.
There were no international health effect assessments identified for coconut fatty acids that may release from ethylhexyl cocoate upon esterase hydrolysis. However, the major components of coconut fatty acids—i.e., lauric acid (CAS RN 143-07-7), myristic acid (CAS RN 544-63-8), oleic acid (CAS RN 112-80-1) and palmitic acid (CAS RN 57-10-3)—were not identified by OECD (2014) as possessing properties indicating a hazard for human health for systemic health effects.
Toxicity data of the major component of ethylhexyl cocoate as UVCB
Ethylhexyl laurate (CAS RN 20292-08-4), a major component of ethylhexyl cocoate (45% to 52%), was negative in the Ames S. typhimurium test in the presence and absence of metabolic activation (Belsito et al. 2013). In an in vivo assay, it did not induce genotoxicity in mice at an oral dose of up to 5.0 mL/kg (Fiume et al. 2015). In a 28-day study, ethylhexyl laurate did not induce adverse effects in SD rats administered ethylhexyl laurate via gavage at doses of up to 1000 mg/kg bw/day (NOAEL = 1000 mg/kg bw/day, Fiume et al. 2015).
6.4.2.3 Characterization of risk to human health
The hydrolyzed product of ethylhexyl cocoate, 2-EH, did not impact the development of fetuses of rats after dermal exposure during gestation. However, it reduced maternal body weight gain in dams. Body and organ weights were also impacted by 2-EH after chronic oral exposure in rats. In addition, a dermal absorption of 6% derived from an in vivo rat study for 2-EH is used to estimate systemic exposures for ethylhexyl cocoate.
Exposure of the general population to ethylhexyl cocoate is expected to occur mainly from cosmetics. Table 6‑5 provides all relevant exposure and hazard values from daily use of products containing ethylhexyl cocoate. Table 6‑6 provides all relevant exposure and hazard values from intermittent use of products containing ethylhexyl cocoate.
In addition to the dermal scenarios presented in Table 6‑5 and Table 6-6, a comparison of the oral exposures for adults and toddlers, using lipsticks/lip gloss containing ethylhexyl cocoate, with the critical oral effect level of 2-EH (converted NOAEL = 120 mg/kg bw/day) results in margins of exposures ranging from 184 615 to 352 941. The oral critical effect level of 2-EH is considered protective of the development effects induced by 2-EHA (converted LOAEL = 218 mg/kg bw/day) via the oral route of exposure.
Exposure scenario |
Systemic exposure (mg/kg bw/day)a |
Critical effect level (converted)b |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Body moisturizer (adult) |
0.041 – 0.41 |
NOAEL = 120 mg/kg bw/day
|
24-month oral study, derived from 2-EH for systemic effect |
293 – 2 927 |
Face moisturizer |
0.55 |
NOAEL = 120 mg/kg bw/day |
24-month oral study, derived from 2-EH for systemic effect |
200 |
Facial make-up |
1.44E-05 – 0.56 |
NOAEL = 120 mg/kg bw/day |
24-month oral study, derived from 2-EH for systemic effect |
196 – >7 million |
Hair oil |
0.12 – 0.37 |
NOAEL = 120 mg/kg bw/day |
24-month oral study, derived from 2-EH for systemic effect |
297 – 917 |
Abbreviations: NOAEL, no-observed adverse effect level.
a Estimated exposures using a dermal absorption of 6%.
b NOAEL of 50 mg/kg bw/day for 2-EH converted to 120 mg/kg bw/day for ethylhexyl cocoate using molecular weight of its major component ethylhexyl laurate.
Exposure scenario |
External exposure (mg/kg bw/day) |
Critical effect level |
Critical health effect endpoint |
MOE |
|---|---|---|---|---|
Massage oil for body (adult) |
0.045 – 0.14 mg/kg per event |
NOAEL = 1 000 kg/bw/day |
12-day dermal, study, derived from 2-EH for systemic effect |
7 143 – 22 222 |
Abbreviations: NOAEL, no-observed adverse effect level.
The above-noted margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
6.4.2.4 Uncertainties in Evaluation of Risk to Human Health
The key sources of uncertainty are presented in the table below.
Key source of uncertainty |
Impact |
|---|---|
Maximum concentrations were used to estimate cosmetic exposures, which likely result in overestimates. |
+ |
There is some uncertainty regarding the dermal absorption value used to estimate exposures from cosmetics; however, confidence is high that dermal absorption is likely low given the physical and chemical properties of the substance and existing dermal absorption data from breakdown products. |
+ |
There is no genotoxicity study or repeated dose toxicity study identified for ethylhexyl cocoate. Toxicity studies of major components and potential hydrolyzed products of ethylhexyl cocoate were used to inform the health effects of ethylhexyl cocoate. |
+/- |
As the capacity of ethylhexyl cocoate to hydrolyze to 2-EH on the skin is unknown, the use of critical effect levels derived from the dermal and oral studies of 2-EH to characterize the risk from exposures to ethylhexyl cocoate, based on the assumption of 100% of hydrolysis, is likely an overestimate. |
+ |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from undecylenic acid; ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid and ethylhexyl cocoate. It is concluded that undecylenic acid; ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid; and ethylhexyl cocoate do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that undecylenic acid; ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid; and ethylhexyl cocoate do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that undecylenic acid; ALA; tung oil; tall oil fatty acid; tall oil fatty acids, potassium salts; evening primrose oil; dimer acid; trimer acid; and ethylhexyl cocoate do not meet any of the criteria set out in section 64 of CEPA.
References
[AGDH] Australian Government Department of Health. 2017. Dimer acid. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme. [last updated 2017 May 3].
Alternative Medicine Review. 2002. Undecylenic Acid, Monograph [PDF]. Alternative Medicine Review. 7(1):68-70. [accessed 2017 June 29].
Beisson F, Aoubala M, Marull S, Moustacas-Gardies A-M, Voultoury R, Verger R, Arondel V. 2001 Use of the tape stripping technique for directly quantifying esterase activities in human stratum corneum. Anal Biochem. 290:179-185.
Belsito MD, Hill RA, Klaassen CD, Liebler DC, Marks Jr JG, Ronald C. 2013. Amended Safety Assessment of Alkyl Esters as Used in Cosmetics [PDF]. [accessed 2017 June 29].
Bookstaff RC, PaiBir S, Bharaj SS, Kelm GR, Kulick RM, Balm TK, Murray JV. 2003. The safety of the use of ethyl oleate in food is supported by metabolism data in rats and clinical safety data in humans. Regul Toxicol Pharmacol. 37(1):133-48.
Buist HE, van Burgsteden JA, Freidig AP, Maas WJ, van de Sandt JJ. 2010. New in vitro dermal absorption database and the prediction of dermal absorption under finite conditions for risk assessment purposes. Regul Toxicol Pharmacol. 57:200-209.
Burdock GA, editor. 2010. Fenaroli’s Handbook of Flavor Ingredients. 6th ed. 10-Undecenoic acid. Boca Raton (FL): CRC Press, Taylor & Francis Group.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement .
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [updated 2012 Nov 26; accessed 2017 June 29]. CHEMPRO. Date unknown. Top-notch technology in production of oils and fats. Gujarat (India): CHEMPRO [date updated: not specified; accessed 2016 Dec 14].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Danish (Q)SAR Database [database]. 2015. Division of Diet, Disease Prevention and Toxicology, National Food Institute, Technical University of Denmark. [accessed 2017 September 13].
Deisinger PJ, Boatman RJ, Guest D. 1994. Metabolism of 2-ethyhexanol administered orally and dermally to the female Fisher 344 rat. Xenobiotica 24:429–440 [cited in EC, HC 2011].
Dietitians of Canada. 2017. Food Sources of Omega-3 Fats. [posted 2016 Oct 28; accessed 2017 May 15].
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada. [accessed 2016 April 22].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[EC, HC] Environment Canada, Health Canada. 2011. Screening assessment for the Challenge: 2-Ethylhexanoic Acid: Chemical Abstracts Service Registry Number 149-57-5. Ottawa (ON): Government of Canada. [accessed 2017 May 20].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2016].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018a. Rapid screening of substances with limited general population exposure. Ottawa (ON):Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018b. Screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON):Government of Canada.
[ECHA] European Chemicals Agency. c2007-2017a. Registered substances database; search results for CAS RN 112-38-9. Helsinki (FI): ECHA. [accessed 2017 May 20]. [ECHA] European Chemicals Agency. c2007-2017b. Registered substances database; search results for CAS RN 61788-89-4. Helsinki (FI): ECHA. [accessed 2017 May 20].
[ECHA] European Chemicals Agency. c2007-2017c. Registered substances database; search results for CAS RN 68937-90-6. Helsinki (FI): ECHA. [accessed 2017 May 20].
[ECHA] European Chemicals Agency. c2007-2017d. Registered substances database; search results for CAS RN 92044-87-6. Helsinki (FI): ECHA. [accessed 2017 May 20].
[ECHA] European Chemicals Agency. c2007-2017e. Registered substances database; search results for CAS RN 68955-98-6. Submission from Pine Chemicals Association. Helsinki (FI): ECHA. [accessed 2017 May 20
[ECHA] European Chemicals Agency. c2007-2017f. Registered substances database; search results for CAS RN 91051-05-7. Submission from Pine Chemicals Association. Helsinki (FI): ECHA. [accessed 2017 May 20].
[EFSA ] European Food Safety Authority. 2010. Scientific opinion on the safety of “conjugated linoleic acid (CLA)-rich oil” (Clarinol®) as a Novel Food ingredient [PDF]. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), European Food Safety Authority (EFSA), Parma, Italy. [accessed 2017 June 12].
[EMA] European Medicines Agency. 2011. Assessment report on Oenothera biennis L., Oenothera. lamarckiana L., oleum. Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use). Committee on Herbal Medicinal Products (HMPC), European Medicines Agency. 16 December 2011, EMA/HMPC/277791/2009.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Esoteric Oils. (c1998-2019). Fatty acids found in evening primrose oil. Cape Town (South Africa): Sallamander Concepts (Pty) Ltd. [last updated 2019 April 17; accessed 2016 Dec 14].
Everett DJ, Greenough RJ, Perry CJ, McDonald P, Bayliss P. 1988a. Chronic toxicity studies of Efamol evening primrose oil in rats and dogs. Med Sci Res. 16(16):863-864. [cited in NTP 2009].
Everett DJ, Perry CJ, Bayliss P. 1988b. Carcinogenicity studies of Efamol evening primrose oil in rats and mice. Med Sci Res. 16(16):865-866. [cited in NTP 2009].
[FAO] Food and Agriculture Organization of the United Nations. 2010. Fats and fatty acids in human nutrition: Report of an expert consultation 10 – 14 November 2008 [PDF]. Geneva. FAO Food and Nutrition Paper 91. Rome (Italy): Food and Agriculture Organization of the United Nations. [accessed 2017 June 12].
Ficheux AS, Morisset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food Chem Toxicol. 66:36-43.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90:130-141.
Fiume MM, Heldreth BA, Wilma FB. 2015. Safety assessment of alkyl esters as used in cosmetics. Int J Toxicol. 34(2 Suppl):5S-69S.
Fukushima M, Ohhashi T, Ohno S, Saitoh H, Sonoyama K, Shimada K, Sekikawa M, Nakano M. 2001. Effects of diets enriched in n-6 or n-3 fatty acids on cholesterol metabolism in older rats chronically fed a cholesterol-enriched diet. Lipids 36(3):261-6.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Health Canada, Great Lakes Health Effects Program.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2010. Dietary Reference Intakes Tables. [Internet]. [updated 2010 Nov 29; accessed 2017 May 20].
Health Canada. 2015. Environmental Assessment Unit drinking water spreadsheets. [Excel format]. Ottawa (ON): Health Canada. [accessed 2017 June 29].
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada. [accessed 2017 March 20].
Hegsted DM, Gotsis A, Stabe FJ. 1957. The effect of various fats upon experimental hypercholesteremia in the rat. J Nutr. 63(3):377-391.
[HERA] Human & Environmental Risk Assessment. 2002. Human & Environmental Risk Assessment on ingredients of European household cleaning products: Fatty Acid Salts, Human Health Risk Assessment, Draft for Public Comment [PDF]. Brussels (Belgium): HERA. [cited accessed 2017 June 29].
Howes D. 1975. The percutaneous absorption of some anionic surfactants. J Soc Cosmet Chem. 26:47-63.
International Organization of the Flavor Industry. 1995. European inquiry on volume of use. Unpublished report to the Flavor Manufacturers’ Association of the United States, Washington DC, United States. [cited in WHO 1999].
[IOM] Institute of Medicine. 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Chapter 8 and Summary Tables. Washington (DC): The National Academies Press. [accessed 2017 June 29].
Kezutyte T. Desbenoit N. Brunelle A. Briedis V. 2013. Studying the penetration of fatty acids into human skin by ex vivo TOF-SIMS imaging. Biointerphases. 8(1): 3.
Kim K, Nam YA, Kim HS, Hayes AW, Lee BM. 2014. Alpha-linolenic acid: Nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol. 70:163-78.
Leaver HA, Lytton FD, Dyson H, Watson ML, Mellor DJ. 1986. The effect of dietary ω3 and ω6 polyunsaturated fatty acids on gestation, parturition and prostaglandin E2 in intrauterine tissues and the kidney. Prog Lipid Res. 25:143-146. [cited in NTP 2009].
[LNHPD] Licensed Natural Health Products Database [database] [modified 2018]. Ottawa (ON): Health Canada. [accessed 2017 June 29].
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43: 279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, Renskers K, Scrafford C, Vater S. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44: 2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46: 1516-1524.
McGuire JM, Powis PJ. 1998. Gas chromatographic analysis of tall oil fractionation products after methylation with Ν,Ν-dimethylformamide dimethylacetal. J Chromatogr Sci. 36:104-108.
McPherson Jr JC. 1973. Suppression of food intake in the rat by tung oil. Can J Physiol Pharmacol 51(10):733-6.
[NCI] National Chemical Inventories [database on a CD-ROM]. 2015. Issue 2. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [accessed 2017 June 29].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2019]. Ottawa (ON): Health Canada. [accessed 2017 Dec 20].
[NTP] National Toxicology Program (US). 2009. Chemical Information Review Document for Evening Primrose Oil (Oenothera biennis L.) [CAS No. 90028-66-3][PDF]. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. [accessed 2017 June 29].
[OECD] Organisation for Economic Co-operation and Development. 2014. SIDS Initial Assessment Profile (SIAP): Aliphatic Acids Category. CoCAM [Cooperative Chemicals Assessment Meeting] 6, 2014 September 30 – October 3. [accessed 2017 June 29].
OECD QSAR Toolbox. [read across tool]. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ramírez M, Amate L, Gil A. 2001. Absorption and distribution of dietary fatty acids from different sources. Early Hum Dev. 65 Suppl. (2001) S95 – S101.
Robinson V, Bergfeld WF, Belsito DV, Klaassen CD, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Alan Andersen F. 2009. Amended safety assessment of tall oil acid, sodium tallate, potassium tallate, and ammonium tallate. Int J Toxicol. 28(6 Suppl 2):252S-258S.
[SDS] Safety Data Sheet. 2016. No-Toil Biodegradable Foam Filter Oil. Gastonia (NC) [PDF]: Mann+Hummel Filtration Technology US LLC. [accessed 2017 June 29].
Seppanen 1969 as cited in: Anon. 1989. Final report on the safety assessment of tall oil acid. J. Amer. Coil. Toxicol. 8:769-776.
Shockey J, Rinehart T, Chen Y, Yangdong W, Zhiyong Z, Lisong H. 2016. Chapter 10: Tung (Vernicia Fordii and Vernicia montana). In: McKeon T, Hayes D, Hildebrand D, Weselake R, editors. Industrial Oil Crops. Amsterdam (the Netherlands): AOCS Press, Elsevier Inc.
SimpleTreat [sewage treatment plant removal model]. 1997. Ver. 3.0. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (RIVM) [National Institute for Public Health and the Environment]. Laboratory for Ecological Risk Assessment.
Tokudome Y, Katayanagi M, Hashimoto F. 2015. Esterase activity and intracellular localization in reconstructed human epidermal cultured skin models. Ann Dermatol. 27(3):269-274.
Tsuzuki T, Tokuyama Y, Igarashi M, Miyazawa T. 2004a. Tumor growth suppression by alpha-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation. Carcinogenesis. 25(8):1417-1425.
Tsuzuki T, Igarashi M, Miyazawa T. 2004b. Conjugated eicosapentaenoic acid (EPA) inhibits transplanted tumor growth via membrane lipid peroxidation in nude mice. J Nutr. 134:1162-1166.
Univar. c2019. Dimer acids and trimer acids. Twinsburg (OH): Univar’s Oleochemicals Team [formerly Chemical Associates] . [accessed 2016 Aug 17].
[US EPA] US Environmental Protection Agency. 2005. Detail Query Results for Fatty Acid Dimers and Trimer Category. Posting dates of documents from HPV Challenge web site from which data have been entered into the HPVIS: February 2, 2005. [accessed 2017 07 02].
US National Academy of Sciences. 1989. Evaluating the Safety of Food Chemicals. Washington DC, United States [cited in WHO 1999].
[WHO] World Health Organization. 1999. Safety evaluation of certain food additives: fifty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additive Series, No. 42. Geneva (CH): World Health Organization. [accessed 2017 May 20].
Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. 2006. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Analysis. 26(3):803-824.
Zhuo RJ, Wang F, Zhang XH, Zhang JJ, Xu J, Dong W, Zou ZQ. 2014. α-eleostearic acid inhibits growth and induces apoptosis in breast cancer cells via HER2/HER3 signaling. Mol Med Rep. 9(3):993-998.
Appendices
Appendix A. Substance identity information
Major fatty acid constituents as single component |
Single component fatty acid (%) |
UVCB fatty acid major components (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
CAS RN |
OECD 2014 assessed FAs (IUPAC or CAS Name) |
Common name |
Structure feature |
α-linolenic acid (ALA) 463-40-1 |
Undecylenic acid 112-38-9 |
Tung oil 8001-20-5a |
Tall oil fatty acid 61790-12-3b |
Tall oil fatty acids, potassium salts 61790-44-1 |
evening primrose oil 90028-66-3c |
Ethylhexyl cocoate 92044-87-6a |
Trimer acid 68937-90-6d Dimer acid 61788-89-4 |
124-07-2 |
Octanoic acid |
Caprylic acid |
C8:0 |
- |
- |
- |
- |
- |
- |
5-10 |
- |
334-48-5 |
Decanoic acid |
Decanoic acid |
C10:0 |
- |
- |
- |
- |
- |
- |
8 |
- |
463-40-1 |
(9Z,12Z,15Z)-Octadeca-9,12,15-trienoic acid; 9,12,15-Octadecatrie |
Alpha-linolenic acid |
C18:3 ; cis-9,12,15 |
>99 |
- |
- |
- |
- |
- |
- |
- |
112-38-9 |
n/a |
10-Undecylenic acid |
C11:1 ; cis 10 |
- |
>99 |
- |
- |
- |
- |
- |
- |
143-07-7 |
Dodecanoic acid |
Lauric acid |
C12:0 |
- |
- |
- |
- |
- |
- |
45-52 |
- |
544-63-8 |
Tetradecanoic acid |
Myristic |
C14:0 |
- |
- |
- |
- |
- |
- |
16-21 |
- |
57-10-3 |
Hexadecanoic acid |
Palmitic acid |
C16:0 |
- |
- |
~4 |
~6.3 |
~6.3 |
5.8-7 |
7-10 |
- |
57-11-4 |
Octadecanoic acid |
Stearic acid |
C18:0 |
- |
- |
- |
- |
- |
1.5-2.5 |
- |
predominant source |
112-80-1 |
(Z)-Octadec-9-enoic acid; 9-Octadecenoic acid, (Z)- |
Oleic acid |
C18:1; cis 9 |
- |
- |
~8 |
39-48.2 |
39-48.2 |
5-11 |
5-8 |
- |
60-33-3 |
(9Z,12Z)-Octadeca-9,12-dienoic acid; 9,12-Octadecadienoic acid |
Linoleic acid |
C18 :2; cis 9, cis 1 |
- |
- |
~4 |
34-35.9 |
34-35.9 |
70-77 |
- |
- |
26764-25-0 |
n/a |
Octadecadienoic acid |
C18:2; trans 7, cis 9 |
- |
- |
- |
2.3-10 |
2.3-10 |
- |
- |
- |
506-23-0 |
n/a |
Alpha eleostearic acid |
C18:3; cis 9, trans 11, trans 13 |
- |
- |
~80 |
- |
- |
- |
- |
- |
506-26-3 |
n/a |
Gamma-linolenic acid |
C18:3; cis-6,9,12 |
- |
- |
- |
- |
- |
9-10.9 |
- |
- |
a CHEMPRO (date unknown)
b McGuire and Powis 1998
c Esoteric Oils (c1998-2019)
d Univar (c2019)
Appendix B. Parameters used to estimate human exposures
Cosmetic exposures were primarily estimated using ConsExpo Web (2016). Exposure estimates were calculated on the basis of default body weights of 70.9 kg, 15.5 kg, and 7.5 kg for adults (20 years and older), toddlers (6 months to 4 years old), and infants (0 to 6 months old), respectively (Health Canada 1998). The estimated dermal and oral exposure parameters for cosmetics are described in Table B-1 and Table B-2, respectively. Details on the dermal absorption rate used to derive systemic exposures can be found in section 6.4.1 for undecylenic acid and 6.4.2 for ethylhexyl cocoate.
Product (substance) |
Assumptionsa |
|---|---|
Body moisturizer (Undecylenic acid and ethylhexyl cocoate)
|
Adults: Product amount (g/use): 4.4 (Loretz et al. 2005) Frequency (use/day): 1.1 (Loretz et al. 2005) Surface area: whole body – head = 16 925 cm2 (Health Canada 1995)
Infants (for undecylenic acid only): Product amount (g/use): 1.4 (Wormuth et al. 2006) Frequency (use/day): 1.7 (Wormuth et al. 2006) Surface area: whole body – head = 3 020 cm2 (Health Canada 1995) |
Specialized body moisturizer |
Adults:Product amount (g/use): 2.2 (assume use half the amount of body lotion) Frequency (use/day): 1 Surface area: Assume used on legs and feet only (based on personal communication, e-mail from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 22, 2016; unreferenced)
|
Face moisturizer (Undecylenic acid and ethylhexyl cocoate)
|
Adults: Product amount (g/use): 1.2 (Loretz et al. 2005) Frequency (use/day): 1.8 (Loretz et al. 2005) Surface area: Half area of head = 637.5 cm2 (Health Canada 1995)
|
Facial make-up (Undecylenic acid and ethylhexyl cocoate) |
Adults: Product amount (g/use): 0.54 (Loretz et al. 2006) Frequency (use/day): 1.24 (Loretz et al. 2006) Surface area: Half area of head = 637.5 cm2 (Health Canada 1995) |
Hair oil (Ethylhexyl cocoate) |
Adults: Product amount (g/use): 13.1 (Loretz et al. 2008) Frequency (use/day): 1.24 (Loretz et al. 2006) Retention (or transfer) factor: 0.1 (amount on the scalp available for absorption) Surface area: Half area of head = 637.5 cm2 (Health Canada 1995) |
Nail conditioner (Undecylenic acid) |
In the absence of specific data for this exposure scenario, adjusted product amount for hand cream using a surface area adjustment factor (hands to fingertips).
Adults: Product amount (g/use): 0.1 g (adjusted product amount for hand cream 1.7 g by 0.058 (surface area adjustment) Frequency is less than once a day, exposure estimates are per event Surface area: Hands = 910 cm2 (Health Canada 1995), surface area of fingernails ~ 50 cm2 (Ficheux et al. 2014) |
Massage oil (body) (Ethylhexyl cocoate)
|
Adults: Product amount (g/use): 3.2 (Ficheux et al. 2016) Frequency is less than once a day, exposure estimates are per event Surface area: Total body surface area - half area of head – half area of trunk = 14 380 cm2 (Health Canada 1995) |
a Unless specified, a retention factor of 1 was used
Product (substance) |
Assumptions |
|---|---|
Non-medicinal ingredient in natural health producta (Undecylenic acid) |
2-3 capsules once a day. 15 mg of undecylenic acid per capsule.
Adults: Estimated dose = 15 mg/capsule x 2 or 3 capsules/day 70.9 kg Estimated dose = 0.42 to 0.63 mg/kg bw/day
|
Lipstick/lip glossb (Ethylhexyl cocoate)
|
Adults: Product amount (g/use): 0.01 (Loretz et al. 2005) Frequency (use/day): 2.4 (Loretz et al. 2005)
Toddler: Product amount (g/use): 0.01 (assumed to be the same as adults from) Frequency is less than once a day, exposure estimates are per event |
a Product is associated with the recommended use or purpose “Garlic is traditionally used in Herbal Medicine to help relieve the symptoms associated with upper respiratory tract infections and catarrhal conditions,” The duration of use statement “Consult a health care practitioner for use beyond 6 weeks,” and other recommended conditions of use, such as cautions and warnings, contra-indications, and known adverse reactions (LNHPD [modified 2018]; personal communication, e-mail from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 28, 2016; unreferenced).
b Assume amount applied is completely ingested, no dermal exposure
