Screening assessment - Triarylmethanes Group
Official title: Screening assessment - Triarylmethanes Group
Chemical Abstracts Service Registry Numbers
548-62-9, 569-64-2, 1324-76-1, 2390-59-2, 2390-60-5, 3844-45-9
Environment and Climate Change Canada
Health Canada
Cat. No.: En14-423/2020E-PDF
ISBN 978-0-660-35887-1
October 2020
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of six substances referred to collectively under the Chemicals Management Plan as the Triarylmethanes Group. These six substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other concerns. A seventh substance was initially included in the group; however, it was determined to be of low concern through other approaches, and the conclusion for this substance is provided in a separate report.Footnote 1 Accordingly, this screening assessment addresses the six substances listed in the table below. The six substances addressed in this screening assessment will hereinafter be referred to as the Triarylmethanes Group.
| CAS RNa | Domestic Substances List name | Common name |
|---|---|---|
| 548-62-9b | Methanaminium, N-[4-[bis[4-(dimethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-methyl-, chloride | Basic Violet 3 |
| 569-64-2 | Methanaminium, N-[4-[[4-(dimethylamino)phenyl]phenylmethylene]-2,5-cyclohexadien-1-ylidene]-N-methyl-, chloride | Malachite Green |
| 1324-76-1b | Benzenesulfonic acid, [[4-[[4-(phenylamino)phenyl][4-(phenylimino)-2,5-cyclohexadien-1-ylidene]methyl]phenyl]amino]- | Pigment Blue 61 |
| 2390-59-2 | Ethanaminium, N-[4-[bis[4-(diethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, chloride | Basic Violet 4 |
| 2390-60-5 | Ethanaminium, N-[4-[[4-(diethylamino)phenyl][4-(ethylamino)-1-naphthalenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, chloride | Basic Blue 7 |
| 3844-45-9b | Benzenemethanaminium, N-ethyl-N-[4-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl](2-sulfophenyl)methylene]-2,5-cyclohexadien-1-ylidene]-3-sulfo-, hydroxide, inner salt, disodium salt | Brilliant Blue FCF |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society, and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior written permission of the American Chemical Society.
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
Triarylmethanes are primarily used as colouring agents and do not occur naturally in the environment. They are used as dyes and/or pigments in inks, toners, and colourants, in paper products and manufactured items, and potentially in food packaging materials, for commercial and consumer use. Substances in this group are also used in other products available to consumers, including cosmetics (e.g., body cream, hair products, hair dyes, makeup, perfume), cleaning products, and water treatment products for aquarium fish, as well as in additional industrial and laboratory products. Brilliant Blue FCF is also used in food, natural health products, pest control products, prescription and non-prescription drugs, and a range of additional products available to consumers. According to information submitted for the reporting years of either 2008 or 2011, all six substances were imported into Canada, each in quantities ranging from 1000 to 100 000 kg, and Brilliant Blue FCF was manufactured in Canada in a quantity ranging from 100 to 1000 kg.
Substances in the Triarylmethanes Group may be released to the Canadian environment from their use in Canada in paper dyeing and deinking, as well as from the formulation, manufacture and consumer use of products containing these substances. Releases are expected to the aquatic environment from both diffuse and point sources. Releases of some of these substances to terrestrial environments are also possible. If released to the aquatic environment, Pigment Blue 61 is likely to behave like a particle and settle to bed sediment. The other triarylmethane substances will be charged at environmentally relevant pH and will tend to sorb to dissolved and suspended solids. Therefore, these substances may potentially be transported in the water column or settle to bed sediment. Substances in the Triarylmethanes Group tend to persist in water, sediment and soil. They have a low potential to bioaccumulate in the lipids of aquatic organisms; however, the non-sulfonated dyes (i.e., Basic Violet 3, Malachite Green, Basic Violet 4 and Basic Blue 7) instead may bind to proteins and accumulate in other types of fish tissue.
Experimental acute toxicity data for the non-sulfonated dyes show they have the potential to cause adverse effects to aquatic organisms at low concentrations. Adverse effects in aquatic organisms were observed for Brilliant Blue FCF at relatively higher concentrations, whereas no effects were observed at the solubility limit for Pigment Blue 61. Ecological exposure scenarios were developed for down-the-drain releases from uses of products containing these substances and for releases from industrial sites. Risk quotient analyses were conducted to compare estimated aquatic concentrations to adverse effect concentrations in aquatic organisms for different exposure scenarios. Scenarios for paper dyeing and paper deinking indicated that the non-sulfonated triarylmethane dyes pose a risk to aquatic organisms, whereas the scenarios for general formulation/product handling and consumer uses did not. Exposure scenarios for Brilliant Blue FCF did not show a risk to aquatic organisms and Pigment Blue 61 is not expected to pose a risk to aquatic organisms as it is expected to behave more like a particle and is not likely to be bioavailable.
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7. It is concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. It is also concluded that Pigment Blue 61 and Brilliant Blue FCF do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the general population of Canada, the predominant sources of exposure to dye substances in the Triarylmethanes Group are from use of products available to consumers that contain these substances and from environmental media (e.g., drinking water). Potential oral exposures to Basic Violet 3, Malachite Green, Basic Violet 4, Basic Blue 7 and Brilliant Blue FCF were estimated based on potential levels in drinking water. Potential dermal and oral exposures to Brilliant Blue FCF were derived from use of natural health products and cosmetics, as well as oral exposures from its use as a food additive. Potential exposures to Malachite Green, Basic Violet 4 and Basic Blue 7 were derived from use of cosmetics (hair dyes). Potential inhalation exposure to Brilliant Blue FCF from use of perfume was also characterized. Inhalation exposure to the remaining substances in the Triarylmethanes Group was not considered to be of concern due to their negligible volatility, as well as their potential uses. Given its physical and chemical properties and identified uses, exposure to Pigment Blue 61 for the general population of Canada is not expected.
In laboratory studies, Basic Violet 3 is not observed to cause developmental or reproductive toxicity, but is genotoxic and carcinogenic. On the basis of health effects information for a structurally-related substance, the critical health effect for Malachite Green is developmental toxicity. Pigment Blue 61 was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Basic Violet 4 and the structurally-related substance Basic Blue 7 are not genotoxic. On the basis of health effects information on structurally-related substances, Basic Violet 4 and Basic Blue 7 are not considered to be developmental or reproductive toxicants, but may be carcinogenic. Brilliant Blue FCF is poorly absorbed orally and dermally, is not a developmental or reproductive toxicant, is not genotoxic, and is not carcinogenic.
For Basic Violet 3, Basic Violet 4, Basic Blue 7, and Brilliant Blue FCF, comparisons of levels of exposure to the general population and levels at which critical health effects were observed result in margins of exposure considered adequate to address uncertainties in the health effects and exposure databases for both non-cancer and cancer effects. In contrast, similar comparisons of exposure from use of Malachite Green in hair dye resulted in margins of exposure that are considered potentially inadequate to address uncertainties in the health effects and exposure databases, particularly since the critical health effects were observed at the lowest tested dose.
On the basis of the information presented in this screening assessment, it is concluded that Malachite Green meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. It is also concluded that Basic Violet 3, Pigment Blue 61, Basic Violet 4, Basic Blue 7, and Brilliant Blue FCF do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 meet one or more of the criteria set out in section 64 of CEPA. It is concluded that Pigment Blue 61 and Brilliant Blue FCF do not meet any of the criteria set out in section 64 of CEPA. It is also concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 meet the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on six of seven substances, referred to collectively under the Chemicals Management Plan as the Triarylmethanes Group, to determine whether these six substances present or may present a risk to the environment or to human health. These six substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other concerns (ECCC, HC [modified 2017]).
The seventh substance, CAS RNFootnote 2 632-99-5, was originally included in the Triarylmethanes Group. However, it was considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016), and via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018), and it was identified as being of low concern to both human health and the environment. As such, it is not further addressed in this report. Conclusions for this substance are provided in the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018). The six substances addressed in this screening assessment will hereinafter be referred to as the Triarylmethanes Group.
While all the substances in the Triarylmethanes Group have common structural features and similar functional uses as pigments or dyes in multiple sectors, there is notable diversity within the group with respect to overall structure and physical-chemical properties. This diversity has been taken into account through the individual assessment of each substance.
Certain substances within the Triarylmethanes Group were reviewed internationally through the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the European Food Safety Authority (EFSA), the US Environmental Protection Agency (US EPA), the European Commission (EC, and the National Toxicology Program (NTP), and there are existing assessments available. These assessments undergo rigorous review. Health Canada and Environment and Climate Change Canada consider these assessments to be reliable.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to April 2017. Additional data were submitted by stakeholders up to June 2019. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Isabel Beauchesne, Mr. Geoff Granville (GCGranville Consulting Corp.), and Dr. Jarai Mon. Comments on the technical portions relevant to human health were received from Ms. Theresa Lopez, Ms. Jennifer Flippin, and Dr. Joan Garey at Tetra Tech. Additionally, the draft of this screening assessment (published December 8, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 3 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names and common names for the substances in the Triarylmethanes Group are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 548-62-9 | Methanaminium, N-[4-[bis[4-(dimethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-methyl-, chloride (Basic Violet 3b) | ![[Cl-].CN(C)c1ccc(C(c2ccc(N(C)C)cc2)=C2C=CC(=[N+](C)C)C=C2)cc1](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig21a.jpg) C25H30N3.Cl
C25H30N3.Cl | 407.99 |
| 569-64-2 | Methanaminium, N-[4-[[4-(dimethylamino)phenyl]phenylmethylene]-2,5-cyclohexadien-1-ylidene]-N-methyl-, chloride (Malachite Green) | ![[Cl-].CN(C)c1ccc(C(c2ccccc2)=C2C=CC(=[N+](C)C)C=C2)cc1](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig21b.jpg) C23H25N2.Cl C23H25N2.Cl | 364.92 |
| 1324-76-1 | Benzenesulfonic acid, [[4-[[4-(phenylamino)phenyl][4-(phenylimino)-2,5-cyclohexadien-1-ylidene]methyl]phenyl]amino]- (Pigment Blue 61) | ![O=S(C(C=C1)=CC=C1NC2=CC=C(/C(C3=CC=C(NC4=CC=CC=C4)C=C3)=C5C=C/C(C=C\5)=N/C6=CC=CC=C6)C=C2)([O-])=O](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig21c.jpg) C37H29N3O3S C37H29N3O3S | 595.72 |
| 2390-59-2 | Ethanaminium, N-[4-[bis[4-(diethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, chloride (Basic Violet 4) | ![[Cl-].CCN(CC)c1ccc(C(c2ccc(N(CC)CC)cc2)=C2C=CC(=[N+](CC)CC)C=C2)cc1](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig21d.jpg) C31H42N3.Cl C31H42N3.Cl | 492.15 |
| 2390-60-5 | Ethanaminium, N-[4-[[4-(diethylamino)phenyl][4-(ethylamino)-1-naphthalenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-ethyl-, chloride (Basic Blue 7) | ![[Cl-].CCNc1ccc(C(c2ccc(N(CC)CC)cc2)=C2C=CC(=[N+](CC)CC)C=C2)c2ccccc12](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig21e.jpg) C33H40N3.Cl C33H40N3.Cl | 514.15 |
| 3844-45-9 | Benzenemethanaminium, N-ethyl-N-[4-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl](2-sulfophenyl)methylene]-2,5-cyclohexadien-1-ylidene]-3-sulfo-, hydroxide, inner salt, disodium salt (Brilliant Blue FCF) | ![[Na+].[Na+].CCN(Cc1cccc(S(=O)(=O)[O-])c1)c1ccc(C(c2ccccc2S(=O)(=O)[O-])=C2C=CC(=[N+](CC)Cc3cccc(S(=O)(=O)[O-])c3)C=C2)cc1](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig21f.jpg) C37H34N2O9S3.Na2 C37H34N2O9S3.Na2 | 792.86 |
a Colour index (C.I.) numbers (Sigma-Aldrich c2017): Basic Violet 3, 42555; Malachite Green, 42000; Basic Violet 4, 42600; Basic Blue 7, 42595; Pigment Blue 61, 42765:1; Brilliant Blue FCF, 42090.
b Basic Violet 3 (CAS RN 548-62-9) is commonly referred to in the literature as crystal violet, and gentian violet. The term “gentian violet” originally was used to describe a mixture of methyl pararosaniline dyes (methyl violet), but is now commonly used to refer to the single component Basic Violet 3. Toxicology studies in this document using the term “gentian violet” were commercial preparations of at least 96% CAS RN 548-62-9, with the remainder being mainly methyl violet or pentamethylpararosaniline (Aidoo et al. 1990).
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected that were structurally similar and/or functionally similar to substances within this group (similar physical-chemical properties, toxicokinetics) and that had relevant empirical data that could be used to read across to substances with limited empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen are further discussed in the Ecological Effects Assessment and Health Effects Assessment sections of this report. Information on the identities of the analogues used to inform this assessment is presented in Table 2‑2.
| CAS RN | Common name | Chemical structure and molecular formula | Molecular weight (g/mol) | Target substance(s) for analogue |
|---|---|---|---|---|
| 6417-46-5 | Pigment Blue 56 | 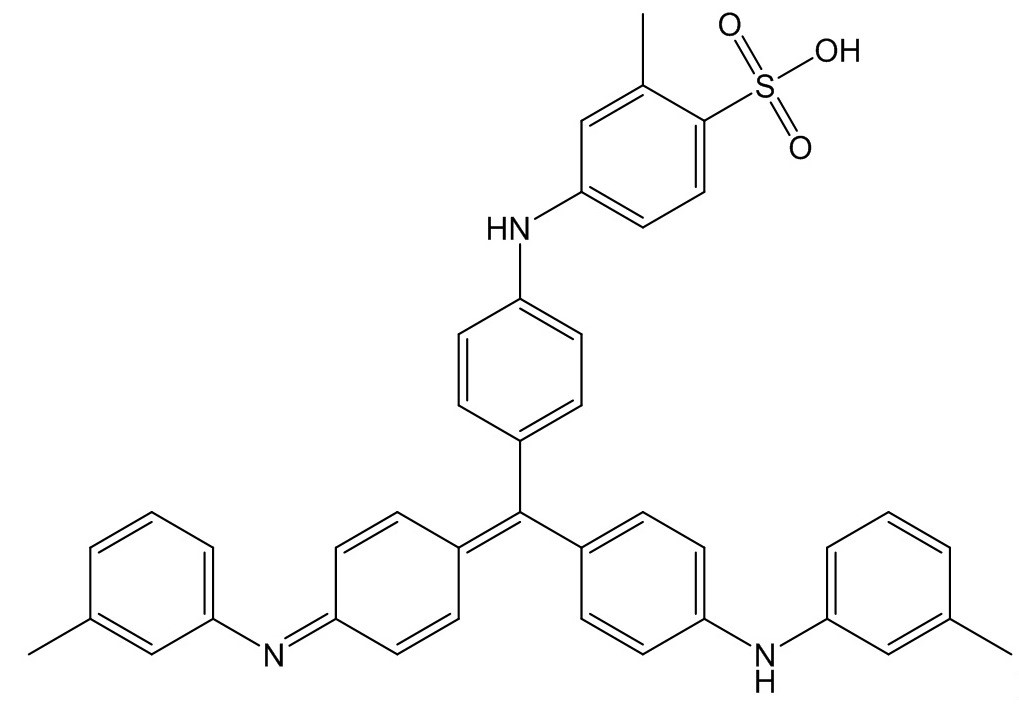 C40H35N3O3S C40H35N3O3S | 637.80 | Pigment Blue 61 |
| 2437-29-8 | Malachite Green Oxalate | /C.O=C(O)C(O)=O.O=C([O-])C([O-])=O.O=C([O-])C([O-])=O.CN(C)C(C=C4)=CC=C4/C(C5=CC=CC=C5)=C6C=C/C(C=C/6)=[N+](C)/C](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig22b.jpg) C46H50N4.C2H2O4.2C2HO4 C46H50N4.C2H2O4.2C2HO4 | 927.02 | Malachite Green |
| 63157-72-2 | Ethyl Violet Acetate | /CC.O=C([O-])C](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig22c.jpg) C31H42N3.C2H3O2 C31H42N3.C2H3O2 | 515.74 | Basic Violet 4, Basic Blue 7 |
| 2580-56-5 | Basic Blue 26 | /C.[Cl-]](/content/dam/eccc/images/pded/triarylmethanes/20200818-fig22d.jpg) C33H32N3.Cl C33H32N3.Cl | 506.09 | Basic Blue 7 |
a Additional substances within the Triarylmethanes Group (e.g., Basic Violet 3, Malachite Green) were also used as analogues. Their use is identified in the applicable section.
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Triarylmethanes Group is presented in Table 3‑1 and Table 3‑2.
When experimental information was limited or not available, (Q)SAR models were used to generate data for vapour pressure, Henry’s law constant, octanol-water partition coefficient (Kow), organic carbon-water partition coefficient (Koc) and octanol-air partition coefficient (Koa). Many of these models are mainly based on fragment addition methods (i.e., they rely on the structure of the chemical) and typically accept only the neutral (i.e., un-ionized) form of a chemical as input (in a simplified molecular-input line-entry system (SMILES) form). As such, the un-ionized form was used as model input where required. Where more than one appropriate model or valid empirical result was available for a given property, the mean was taken as the key value for that parameter. The selected key values for the estimation of vapour pressure, water solubility, log Kow, air-water partition coefficient (log Kaw), and log Koa were adjusted using the least-squares adjustment procedure (Cole and Mackay 2000; Schenker et al. 2005) and represent internally consistent partitioning properties considering thermodynamic constraints.
The four dyes Basic Violet 3, Malachite Green, Basic Violet 4 and Basic Blue 7 are chloride salts. As salts, they will dissociate in water to produce the corresponding cationic organic dye moiety and chloride counterion. These substances are oxidized to charged species during their synthesis and are expected to retain this positive charge in the environment. The pKa values have not been reported as they are not applicable to these permanent charges, which would be present over the whole pH range. In addition to the permanent charge, the speciation of these substances in biological fluids will also be dependent on pH (ACD/Percepta c1997-2015).
Brilliant Blue FCF is a disodium salt with three sulfonate groups in its structure. At pH 4 and above, greater than 95% of the molecules will have a single amine group that will be positively charged and all three sulfonic acid groups will be negatively charged, resulting in a net negative charge (ACD/Percepta c1997-2015).
In contrast to the other triarylmethanes, Pigment Blue 61 is expected to behave more like a particle similar to other organic pigments. The substance has a median particle diameter size of 294 µm (ECHA c2007-2017a). The physical and chemical properties of many of the structural classes of pigments are often not amenable to model prediction because they are typically considered out of the model domain of applicability (e.g., structural and/or property parameter domains). Due to the molecular structure features, organic pigments tend to have very low solubility. Therefore, the octanol-water partition coefficient for sparingly soluble substances is reasonably represented by the quotient of solubilities in octanol and in water (Soct/Sw) (ECHA 2017). For Pigment Blue 61, the log (Soct/Sw) value was estimated to be 3.99 based on a water solubility of 2.5 µg/L and octanol solubility of 23.8 mg/L.
| Property | Basic Violet 3 | Malachite Green | Basic Violet 4 | Basic Blue 7 | Reference(s) |
|---|---|---|---|---|---|
| Adjusted water solubility (mg/L) | 1.8×104 | 1.3×104 | 6.7×103 | 2.1×104 | ACD/Percepta c1997-2015; Baughman et al. 1994; Green 1990; SCBT c2007-2017; WATERNT 2010; WSKOWWIN 2010 |
| Experimental water solubility (mg/L) | 4.0×103 | 4.0×104 | 9.0×103 | 2.0×104 | Baughman et al. 1994; Green 1990; SCBT c2007-2017 |
| Vapour pressure (Pa)a | 1.8×10-10 | 3.6×10-9 | 3.3×10-11 | 4.0×10-13 | MPBPWIN 2008 |
| Henry’s law constant (Pa·m3/mol) | 2.0×10-12 | 1.2×10-10 | 1.1×10-11 | 3.9×10-14 | HENRYWIN 2008 |
| log Kaw (dimensionless) | -14.8 | -13.4 | -15.0 | -17.4 | Schenker et al. 2005 |
| Adjusted log Kow (dimensionless) | 0.7 | 0.6 | 4.7 | 4.8 | ACD/Percepta, c1997-2015; KOWWIN 2010 |
| Experimental log Kow (dimensionless) | 0.5 | 0.6 | 2.4 | NA | ECHA c2007-2017b; Hansch et al. 1995; Tsai et al. 1991 |
| log Koc (dimensionless) | 1.2 | 1.2 | 3.4 | 3.5 | KOCWIN 2010 |
| log Koa (dimensionless) | 15.5 | 14.0 | 19.7 | 22.3 | KOAWIN 2010 |
Abbreviation: NA, not available
a As triarylmethane substances are solids at room temperature, subcooled liquid vapour pressures are calculated.
| Property | Pigment Blue 61 | Brilliant Blue FCF | Reference(s) |
|---|---|---|---|
| Adjusted water solubility (mg/L) | N/A | 1.8×103 | ECHA c2007-2017a; Green 1990; ACD/Percepta c1997-2015; WATERNT 2010; WSKOWWIN 2010 |
| Experimental water solubility (mg/L) | 2.5×10-3 | 3.0×104 | ECHA c2007-2017a; Green 1990 |
| Solubility in octanol (mg/L) | 23.8 | NA | ECHA c2007-2017a |
| Vapour pressure (Pa)a | N/A | 3.0×10-42 | MPBPWIN 2008 |
| Henry’s law constant (Pa·m3/mol) | N/A | 1.4×10-31 | HENRYWIN 2008 |
| log Kaw (dimensionless) | N/A | -38.4 | Schenker et al. 2005 |
| Adjusted log Kow (dimensionless) | N/A | 0.3 | ACD/Percepta c1997-2015; KOWWIN 2010 |
| log Kow (dimensionless) | 3.99b | 0.02 | ACD/Percepta c1997-2015; ECHA c2007-2017a; KOWWIN 2010 |
| log Koc (dimensionless) | < 1.3–4.9 | 0.3 | ECHA c2007-2017a; KOCWIN 2010 |
| log Koa (dimensionless) | N/A | 37.3 | KOAWIN 2010 |
Abbreviations: N/A, not applicable; NA, not available
a As triarylmethane substances are solids at room temperature, the subcooled liquid vapour pressures are calculated.
b The log Kow for pigments is estimated by calculating the ratio between the empirical solubility in octanol and the empirical solubility in water.
4. Sources and uses
None of the substances in the Triarylmethanes Group occur naturally. These substances have been included in surveys issued pursuant to section 71 of CEPA for the reporting years 2008 (Basic Violet 3, Malachite Green) or 2011 (Pigment Blue 61, Basic Violet 4, Basic Blue 7, Brilliant Blue FCF) (Canada 2009, 2012). Brilliant Blue FCF was reported to be manufactured in Canada in a quantity ranging from 100 to 1000 kg in the 2011 calendar year (Environment Canada 2013). According to section 71 surveys for reporting years of either 2008 or 2011, all six substances were imported into Canada, each in quantities ranging from 1000 to 100 000 kg (Environment Canada 2009, 2013).
In Canada and globally, the six substances of the Triarylmethanes Group are primarily used as colourants, spanning a wide variety of potential applications. Table 4‑1 and Table 4‑2 present a summary of the uses of these substances in Canada.
| Major usesa | BV3 | MG | PB61 | BV4 | BB7 | BBFCF |
|---|---|---|---|---|---|---|
| Agricultural substances (non-pesticidal) | N | N | N | N | N | Y |
| Arts, crafts and hobby materials (including children’s uses) | N | N | N | N | N | Y |
| Cleaning and furnishing care | N | N | N | N | N | Y |
| Food and beverage | N | N | N | N | N | Y |
| Ink, toner and colourants | N | Y | Y | N | N | Y |
| Laundry and dishwashing | N | N | N | N | N | Y |
| Lawn and garden care | N | N | N | N | N | Y |
| Medical devices | Yb | N | N | N | N | N |
| Paint and coatings | N | N | Nc | N | Nc | Y |
| Paper products, mixtures or manufactured items | Y | Y | N | Y | N | N |
| Personal care products | N | N | N | N | N | Y |
Abbreviations: BV3, Basic Violet 3; MG, Malachite Green; PB61, Pigment Blue 61; BV4, Basic Violet 4; BB7, Basic Blue 7; BBFCF, Brilliant Blue FCF; Y = yes this use was reported for this substance and N = no this use was not reported for this substance
a Non-confidential uses reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2009, 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
b Reported as a laboratory substance for use in medical devices.
c Although not reported in section 71 surveys, this substance is known to be used in Canada in paints and coatings, but at volumes below the CEPA section 71 survey reporting threshold of 100 kg/year (personal communication, emails from the Canadian Paints and Coatings Association to Environment and Climate Change Canada, 2016-2017; unreferenced).
| Use | BV3 | MG | PB61 | BV4 | BB7 | BBFCF |
|---|---|---|---|---|---|---|
| Arts, crafts and hobby materials (including children’s uses) | N | Na | N | N | N | N |
| Food additiveb | N | N | N | N | N | Yc |
| Food packaging materialsb,d | Y | N | Y | N | N | Y |
| Incidental additivesb | N | N | N | N | N | Ye |
| Formulant in pest control products registered in Canadaf | N | N | N | N | N | Yg |
| Internal Drug Product Database as medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug products in Canadah | Ni | N | N | N | N | Yj |
| Natural Health Products Ingredients Databasek | Yl | N | N | N | N | Ym |
| Licensed Natural Health Products Database as medicinal or non-medicinal ingredients in natural health products in Canadak | N | N | N | N | N | Yn |
| Licensed medical devices in Canadao | Yp | N | N | N | N | N |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadaq | N | Yr | N | Yr | Ys | Yt |
| Inks (including printing ink)u | Y | Y | Y | Y | Y | Y |
| Water treatment for aquarium fish | N | Yv | N | N | N | N |
Abbreviations: BV3, Basic Violet 3; MG, Malachite Green; PB61, Pigment Blue 61; BV4, Basic Violet 4; BB7, Basic Blue 7; BBFCF, Brilliant Blue FCF; Y = yes this use was indicated for this substance and N = no this use was not indicated for this substance
a Previously found in craft markers based on publicly available information, but the product has since been confirmed to no longer be available in Canada; MSDS 2014.
b Personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016-2017; 2019; unreferenced.
c Health Canada [modified 2015a].
d Potentially used in food packaging materials.
e Identified as a possible component in incidental additives (e.g., cleaners and dish detergents) used in food processing plants.
f Personal communication, email from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2017; unreferenced.
g PMRA 2010.
h Personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced.
i Previously identified as a medicinal ingredient (i.e., active ingredient (AI)); DPD [modified 2015], but the associated drug identification numbers (DINs) have been cancelled and any products that were on the Canadian market have been recalled (Health Canada 2019).
j Identified as a non-medicinal ingredient (NMI) in a wide variety of prescription and non-prescription drugs, including allergy medications and cold medications; Canada 1978.
k Personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016-2017; unreferenced.
l Listed in the Natural Health Products Ingredients Database (NHPID) with a non-NHP role because it is not a naturally occurring substance included in Schedule 1 of the Natural Health Products Regulations (NHPID [modified 2017]).
m Listed in the NHPID with a non-medicinal role for use as a colour additive in natural health products (NHPID [modified 2017]). Also associated with an acceptable daily intake of up to 6 mg/kg bw/day based on JECFA (2017).
n Listed in the Licensed Natural Health Products Database as being present as a non-medicinal ingredient in a variety of currently licensed natural health products, including workout supplements, multi-vitamin/mineral supplements, acne therapy products, and toothpastes (LNHPD [modified 2016]).
o Personal communication, email from the Medical Devices Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2019; unreferenced.
p Identified in certain licensed sterile wound dressings made from polyurethane foam (Health Canada 2019).
q Personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016-2017; unreferenced.
r Identified in semi-permanent hair dye(s).
s Identified in semi-permanent hair dye and hair conditioner.
t Identified in a wide variety of cosmetics including body cream, various hair products, makeup, and perfume.
u General use assumed to be in Canada; Herbst and Hunger 2004; Hunger 2003.
v May be available in Canada from use in water treatment products for aquarium fish based on publicly available information; Hikari USA 2016.
Brilliant Blue FCF is a formulant used in currently registered pest control products in Canada (as indicated above in Table 4-2) and is listed on the PMRA List of Formulants (PMRA 2010).
Internationally, Basic Violet 3 has previously been reported as a colourant in cosmetics (Diamante et al. 2009; AGDH 2014; EWG c2007-2017). However, in Canada it is currently listed as prohibited on the Cosmetic Ingredient Hotlist (Health Canada [modified 2015b]),Footnote 4 and in Europe, it is now prohibited from use in cosmetics, including hair dyes (EC 2009). In Europe, Malachite Green is also prohibited from use in cosmetics, and Basic Violet 4 and Basic Blue 7 are prohibited from use in hair dye products (EC 2009). Triarylmethane dyes and pigments are generally recognized for their use internationally in the printing inks industry, particularly for use in packaging (Herbst and Hunger 2004), for the dyeing of paper and textiles, and for their use in cosmetics, drugs, and food (Hunger 2003). Basic Violet 3 and Malachite Green are also used in laboratories as pH indicators and biological stains (Hunger 2003).
5. Releases to the environment
Malachite Green was reported to the National Pollutant Release Inventory by a single company involved in chemical manufacturing, with ≤ 0.004 tonnes per year released to all environmental media between 2003 and 2007 (NPRI 1993-2015).
There are potential releases of substances in the Triarylmethanes Group to water from industrial facilities involved in paper deinking and paper dyeing (for substances associated with these uses), as well as the formulation or manufacture of products and consumer use of products containing these substances. Down-the-drain releases to wastewater treatment systemsFootnote 5 (WWTSs), and eventually to surface water, could result from various uses of products available to consumers that contain these substances.
Intentional applications of agricultural products containing Brilliant Blue FCF could result in releases to surface water through run-off.
6. Environmental fate and behaviour
6.1 Environmental distribution
The substances in the Triarylmethanes Group are not expected to be released to air given their intended uses and physical-chemical properties. These substances have very low vapour pressures and Henry’s law constants, and they exist in a cationic form in the environment (with the exception of Brilliant Blue FCF and Pigment Blue 61). These properties indicate that volatilization would be negligible from soil surfaces and surface waters. Long-range atmospheric transport is therefore not expected to occur.
Given the reported uses of substances in the Triarylmethanes Group, it is expected that these substances may end up in surface water. The characteristics of the WWTS and the affinity of the triarylmethane substances for dissolved and suspended solids will determine the degree to which they end up in surface water. Generally, most of the substances in the Triarylmethanes Group are expected to be associated with dissolved and suspended solids to a large degree. Although the characteristics of Brilliant Blue FCF make it less likely to sorb to particles in the environment, some sorption may occur depending on the conditions of the media (German-Heins and Flury 2000).
Pigment Blue 61 has very low solubility, while the other substances are all soluble in water. Within the aquatic environment, Pigment Blue 61 is likely to behave like a particle and settle to bed sediment, whereas the other substances will dissociate, releasing the ionic triarylmethane molecule and the associated counterion. The non-sulfonated dyes will exist as cations at environmentally relevant pH, and thus will have an affinity for negatively charged particles in the water column (e.g., humic and fulvic acids, clay materials), although a fraction of them may remain in the water column. The sorption processes would be dominated by electrostatic interactions as a result of the negatively charged sorption sites on dissolved organic carbon and suspended solids, although organic carbon may also play a small role (Kah and Brown 2006; Droge and Goss 2012, 2013). Brilliant Blue FCF will also exist in a charged state (having a net negative charge); therefore, its environmental fate will also be dictated by electrostatic interactions. Transport of these dyes in water may occur and suspended solids may eventually settle to bed sediment, where the sorbed dyes are likely to remain unless mixing and transport of the bed sediment occurs. Therefore, the non-sulfonated triarylmethane dyes will likely bind to particulate matter and settle to sediment, whereas Brilliant Blue FCF, given its high solubility and anionic character, is more likely to remain in the water.
The fate of the triarylmethane substances in soils will also be determined by their sorption characteristics. Because of the high solubility and anionic character of Brilliant Blue FCF, it could, under certain conditions, move through the soil pore water. Pigment Blue 61 is not expected to be mobile given its poor water solubility, nor are the non-sulfonated triarylmethane dyes. While ion exchange is complex and not fully understood (Droge and Goss 2012, 2013), it is expected that triarylmethane dyes would have an affinity for charged particles and may or may not be mobile depending on the moisture content, soil type, and amount of soil erosion or runoff. For example, the non-sulfonated triarylmethane dyes would likely be less mobile in soils with high organic matter or high clay content (Droge and Goss 2012, 2013; Kah and Brown 2006). In addition, for organic cations such as the non-sulfonated triarylmethane dyes, the sorption affinity further depends on competition with other organic cations present in soils (Droge and Goss 2012). Also, Brilliant Blue FCF adsorption appears to be influenced by the ionic strength of soil solution (German-Heins and Flury 2000).
6.2 Environmental persistence
ETAD (1995) states that, with some exceptions, dyes may be considered essentially non-biodegradable under aerobic conditions. Repeated evaluation of ready- and inherent-biodegradability of over 80 different dyes using accepted screening tests (e.g., OECD tests) have confirmed this characteristic (Pagga and Brown 1986; ETAD 1992). Although there is some evidence that triarylmethane dyes will degrade over time (Bumpus and Brock 1988; Andrews et al. 1990; Perez-Estrada et al. 2008; Ogugbue and Sawidis 2011), modelling information (BIOWIN 2008) indicates that the biodegradation of triarylmethanes will be relatively slow, with extrapolated half-lives (where applicable) ranging from weeks to months and some being considered to be recalcitrant.
Like other organic pigments, Pigment Blue 61 is not expected to biodegrade in aquatic systems (ECHA c2007-2017a).
Therefore, the substances in the Triarylmethanes Group are expected to be persistent in environmental media (water, sediment and soil), with predicted half-lives greater than 182 days for water and soil, and greater than 365 days for sediment (BIOWIN 2008). The potential for organisms to be exposed both spatially and temporally to these substances in the environment is thus increased.
6.3 Potential for bioaccumulation
The empirical information on Malachite Green and the modelled information for the other substances in the group indicate that these triarylmethanes are not likely to bioaccumulate in aquatic organisms (Table 6‑1). However, due to the cationic nature of the non-sulfonated triarylmethane dyes, the Kow and octanol solubility values, which are used to estimate the bioconcentration factor (BCF) and bioaccumulation factor (BAF) from models such as EPIWIN’s BCFBAF (2010), may not be appropriate predictors of bioaccumulation for these substances. For these triarylmethane dyes, partitioning to proteins in the cell membrane is more likely to occur than partitioning to lipids, the latter being estimated using Kow and octanol solubility values.
| Common name | Test organism | Experimental concentrationmg/L (duration) | BCF (L/kg) | BAF (L/kg) | Reference |
|---|---|---|---|---|---|
| Basic Violet 3 | Fish | NA | 3.2 | 1.4 | BCFBAF 2010 |
| Malachite Green | Carp | 0.002–0.02 (56 days) | 75– 91 | NA | NITE 2002 |
| Basic Violet 4a | Fish | NA | 1091 | 1191 | BCFBAF 2010 |
| Basic Blue 7a | Fish | NA | 2104 | 2717 | BCFBAF 2010 |
| Pigment Blue 61 | Fish | NA | 476b | NA | ECHA c2007-2017a |
| Brilliant Blue FCF | Fish | NA | 3.2 | 0.9 | BCFBAF 2010 |
Abbreviations: BCF, bioconcentration factor; BAF, bioaccumulation factor; NA, not available
a Estimated mid-trophic BCF/BAF including biotransformation rate estimates (Arnot-Gobas method) as estimated log Kow values are greater than 4.
b The BCF is calculated as the concentration in biota (1.19 mg/L) divided by the concentration in water (0.0025 mg/L), following the method of Gobas and Morrison (2000).
There is indication that the potential accumulation of triarylmethanes in fish tissue would likely be through binding to protein and DNA (Docampo and Moreno 1990; Mani and Bharagava 2016). This is supported by the fact that some of the substances in the Triarylmethanes Group are commonly used as biological stains in laboratories because they easily stain amino acids within proteins (Mani and Bharagava 2016).
When solubilized in natural water systems, the non-sulfonated triarylmethane dyes could bind to the surface of various tissues (e.g., fish gills, algae, dermal surfaces) or to food items. Although it is unclear if these triarylmethane dyes would cross the gastrointestinal tract to the bloodstream, it is reasonable to estimate that a fraction of them could bind to plasma protein (Enoch et al. 2011). Malachite Green has been shown to persist in fish tissue (Lanzing 1965; Poe and Wilson 1983; Srivastava et al. 2004; Xie et al. 2012).
Although triarylmethane substances (specifically, Malachite Green) have been measured in fish tissue (Jiang et al. 2009; Zhijun et al. 2011), they are likely to be depurated from the body due to their physical-chemical properties (Bergwerff et al. 2004; Niska et al. 2009).
Brilliant Blue FCF is likely to behave differently than the non-sulfonated triarylmethane dyes as it is expected to be negatively charged (anionic) at environmentally relevant pH and thus is less likely to partition to cell membranes as cationic substances can. Moreover, being highly soluble in water, it is not expected to partition to storage lipids in fish or, therefore, to bioaccumulate in aquatic organisms.
In contrast to the triarylmethane dyes in the group, Pigment Blue 61 is a neutral substance at environmentally relevant pH and is thus not expected to interact electrostatically with various media. An estimate of the log Kow can therefore reliably be calculated and used to estimate a BCF value. An average fish lipid content of 5% (Geyer et al. 1985) is commonly used to normalize whole-body lipid content in recognized guidelines (OECD TG 305). The solubility of Pigment Blue 61 in octanol of 23.8 mg/L (Table 3‑2) and the average fish lipid content of 5% were used to estimate a maximum concentration of the substance in fish of 1.19 mg/L. The BCF value for Pigment Blue 61, calculated following the method of Gobas and Morrison (2000), is 476 (Table 6‑1), and it is therefore not expected to bioaccumulate in fish (Anliker and Moser 1987).
Overall, information on Brilliant Blue FCF and Pigment Blue 61 indicates that these substances have low potential for bioaccumulation. Available experimental data and modelled results show that the non-sulfonated triarylmethane dyes may have some potential for bioaccumulation, though at levels well below the criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA. The non-sulfonated triarylmethane dyes are not expected to bioaccumulate significantly in the lipid tissues of aquatic organisms. However, they can interact and bind with proteins, which may result in some bioaccumulation in other tissues.
7. Potential to cause ecological harm
7.1 Ecological effects assessment
7.1.1 Mode/mechanism of action
Using the OECD QSAR Toolbox for the mode of action (MOA) classification, the triarylmethane substances were classified as “reactive unspecified.” In addition, some outcomes of the ToxCast AR binding model indicate the potential for endocrine-type effects. Substances in the Triarylmethanes Group seem to cause three main types of cellular effects.
First, there is evidence that they cause mitochondrial disturbance. Basic Violet 3 appears to concentrate in animal mitochondria, where it disrupts these organelles by acting as an uncoupler of oxidative phosphorylation (Docampo and Moreno 1990). It can increase ATPase activity, release respiratory control, and interfere with ATP synthesis (Docampo and Moreno 1990). Similarly, studies have found that Malachite Green can damage mitochondria and cause nuclear alteration (Gerundo et al. 1991), which can result in increased glycolysis, a situation that is common in cancer cells (Xu et al. 2005).
Second, triarylmethanes may act through binding to DNA and proteins. Studies have reported that Basic Violet 3 binds with DNA and interacts with two adjacent A-T base pairs, causing kinking, severe bending or unwinding of the DNA double helix. The result is chromosomal alteration and damage (Docampo and Moreno 1990). Triarylmethanes are commonly used as biological stains in laboratories since they easily stain amino acids within proteins (Mani and Bharagava 2016).
Third, triarylmethanes may cause adverse effects through free radical damage in cells. Basic Violet 3 can be photoreduced into a carbon-centred reactive oxygen species (ROS) through exposure to visible light (Docampo et al. 1988). Under aerobic conditions, a photodynamic action occurs, during which the free radical auto-oxidizes. It is believed that this photoreduction action is mediated by oxygen reduction products (Docampo et al. 1988). Malachite Green has also been shown to trigger depletion of intracellular iron pools in organisms and, like Basic Violet 3, to enhance ROS levels (Dhamgaye et al. 2012). There is evidence to suggest that excessive accumulation of ROS can lead to necrosis (Xu et al. 2005).
Triarylmethanes may also cause adverse effects in organisms through physical effects. Malachite Green has been shown to increase ventilation and respiration rates in fish, as it clogs the gills with particulates, increases production of mucus, and causes epithelial damage (Ross et al. 1985).
In general, due to the similarity in physical-chemical characteristics, the four non-sulfonated triarylmethane dyes are expected to have a common mechanism of action pertaining to ecological harm.
While there is a lack of data on the effects of Basic Violet 4 and Basic Blue 7, they are expected to act similarly to Basic Violet 3 and Malachite Green, given their similarity in structure. Brilliant Blue FCF and Pigment Blue 61, however, are expected to have lower toxicity due to the presence of sulfonic acid (SO3) groups. Studies have observed that increasing the number of SO3 groups on a molecule make the molecules more hydrophilic and will decrease the substance’s toxicity (Mon et al. 2006).
7.1.2 Effects on aquatic organisms
While the acute toxicity of Basic Violet 3 and Malachite Green to aquatic organisms has been well characterized, limited data are available for Brilliant Blue FCF, and there is a lack of aquatic toxicity data for Basic Violet 4, Basic Blue 7, and Pigment Blue 61. In light of the similarities in the non-sulfonated triarylmethane dyes, the toxicity information available on Basic Violet 3 and Malachite Green was used as read-across to assess the toxicity of Basic Violet 4 and Basic Blue 7.
Ecological effects studies available for these substances include data for fish, invertebrates, and algae. Based on the available data, which are primarily from acute studies, the most sensitive organisms appear to be freshwater fish. The key studies for aquatic organisms can be found in Appendix A (Table A-1).
The critical toxicity value selected for aquatic organisms for the four non-sulfonated dyes (i.e., Basic Violet 3, Malachite Green, Basic Violet 4 and Basic Blue 7) was a 96-hour LC50 of 0.03 mg/L from a study that examined the toxicity of Malachite Green to Bluegill fish (Bills et al. 1977). An assessment factor of 30 was applied to account for the acute to chronic extrapolation (factor of 10) and to address uncertainty around substances that are expected to have a reactive MOA (factor of 3). No extrapolation to account for interspecies variation was required because there are effects data available for a large number of species (i.e., more than 10) from several different taxonomic groups. The predicted no effect concentration (PNEC) for non-sulfonated dyes was therefore calculated as 0.001 mg/L. This value indicates that Basic Violet 3, Malachite Green, Basic Violet 4 and Basic Blue 7 (both individually and collectively) have the potential to cause adverse effects to aquatic organisms at low concentrations.
Although no reproduction or developmental data were found for aquatic organisms, there is evidence that Malachite Green Oxalate is a developmental toxicant in mammals (see section 8.2).
For Brilliant Blue FCF, one empirical aquatic toxicity study was available. The most sensitive endpoint in the study was a 96-hour LC50 of 180 mg/L for sockeye salmon (Wan et al. 1991). As the dye tested in the study was 50% Brilliant Blue FCF, it is estimated that the LC50 for Brilliant Blue FCF would be half this value, i.e., an LC50 of 90 mg/L, assuming toxicity is expected to increase linearly and that the other components (surfactant and other formulants) did not contribute to the overall toxicity of the tested substance. An assessment factor of 600 was applied to extrapolate from short-term lethal median effects to long-term sub-lethal low effects (factor of 10), to account for interspecies variation because the dataset consisted of six species from one taxonomic group (factor of 20) and to account for the reactive MOA for this substance (factor of 3). The resulting PNEC value derived for this substance was 0.15 mg/L.
The aquatic studies for both Pigment Blue 61 and its analogue, Pigment Blue 56, demonstrated no effects at concentrations that are well above their solubility limits (2.5 µg/L), indicating that at its most dissolved state, Pigment Blue 61 would not be expected to cause any adverse effects. Therefore, a PNEC for Pigment Blue 61 was not derived.
7.1.3 Effects on sediment and soil organisms
Data for soil and sediment toxicity of substances in the Triarylmethanes Group are very limited. A soil toxicity study available for Malachite Green reported a 14-day LC50 value of 1.45 mg/kg for earthworms (Gopinathan et al. 2015). Triarylmethane dyes that are used as biological stains (such as Malachite Green and Basic Violet 3) can act as a DNA binder and protein binder. This action could potentially cause adverse effects leading to death in skin-breathing organisms, like earthworms, by hindering the respiratory functions of the skin. This was shown to be the case for earthworms exposed to a xanthene dye, which is a substance that also binds to DNA and protein (Princz et al. 2014).
Given the limited ecotoxicity data and exposure characterization of these substances (discussed in the next section), PNECs for soil and sediment were not derived for any of the substances in the Triarylmethanes Group.
7.2 Ecological exposure assessment
Potential environmental exposure of organisms to substances in the Triarylmethanes Group will occur mainly through surface water. No environmental monitoring data were available for any of these substances. Therefore, exposures were estimated for key scenarios. Exposure characterization was focused on the scenarios that represent the most probable ecological exposure situations for the four non-sulfonated dyes collectively and for Brilliant Blue FCF. These scenarios included paper dyeing, paper deinking, general formulation, and consumer uses resulting in down-the-drain releases to WWTSs. For all four scenarios, a probabilistic approach was used.
| Scenario | Description of scenario | Substances included |
|---|---|---|
| 1 | Paper dyeing | 4 non-sulfonated substances (Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7) |
| 1 | Paper dyeing | Brilliant Blue FCF |
| 2 | Paper deinking | 4 non-sulfonated substances, as above |
| 2 | Paper deinking | Brilliant Blue FCF |
| 3 | General formulation | 4 non-sulfonated substances, as above |
| 4 | Consumer uses | Brilliant Blue FCF |
Releases of the non-sulfonated dyes to surface water are expected to occur via industrial WWTSs for scenarios 1 and 2 and via both industrial and off-site WWTSs for scenario 3. Given their physical-chemical properties, they will partition to sludge to a large degree. As sludge from on-site WWTSs would not be applied to agricultural or pasture lands, an exposure scenario for soil was not developed for the non-sulfonated dyes. Brilliant Blue FCF is found in products available to consumers, and releases to surface water are expected to occur via WWTSs. However, given its physical-chemical properties (lower affinity for suspended solids) and the lack of soil toxicity data with which to derive a soil PNEC, an exposure scenario for soil was not developed for this substance. Exposure scenarios were not developed for Pigment Blue 61 because it has very low water solubility, effects on aquatic organisms were only observed well above its water solubility limit in the presence of an emulsifier, and there were no data available for soil-dwelling organisms. It is expected to behave as a particle given its median particle size and low water solubility, and hence it likely would not be bioavailable.
7.2.1 Calculation of PECs and general assumptions
Predicted environmental concentrations (PECs) were calculated collectively for the four non-sulfonated dyes combined (Basic Violet 3, Malachite Green, Basic Violet 4, Basic Blue 7) and for Brilliant Blue FCF for relevant exposure scenarios identified. The industrial release scenarios were based on the maximum production capacities of the facilities, rather than use quantities reported for individual substances. It was assumed that any one of the non-sulfonated triarylmethane dyes could be substituted for another. Therefore, no distinction was made between the different substances, and a single collective PEC range for non-sulfonated triarylmethane dyes was generated. The PECs represent potential concentrations of these substances in the receiving water body near the discharge point of a WWTS. The PEC values are presented in each exposure scenario, and a summary of key assumptions is provided in Appendix B.
In all cases, aquatic PECs were derived using a range of removal rates applicable to the type of treatment technologies employed at the WWTS and based on the physical-chemical properties of the triarylmethane substances. All aquatic PECs were also derived using a dilution factor based on the 10th percentile flow rate of the receiving water body and capped at 10.
7.2.2 Scenario 1: paper dyeing
In this scenario, 32 pulp and paper mills in Canada that have the capability to dye paper were considered. Two PECs were developed: one for paper dyeing using any of the four non-sulfonated triarylmethane dyes and the other for paper dyeing using Brilliant Blue FCF.
Information was compiled for each of these facilities, including site data for known paper production capacities, operating days, water discharge rates, receiving water body flow rates, and on-site wastewater treatment technologies employed. Information on whether facilities were discharging to another (off-site) WWTS was also considered. Other key parameters that are not specific to a particular site were estimated as distributional ranges and applied to all sites. These parameters included dye product use rate, fraction of the chemical in the dye product, the retention rate, and removal rate for a given wastewater treatment type. Refer to Appendix B (Table B-1) for a summary of parameters and assumptions for non-sulfonated triarylmethanes and for Brilliant Blue FCF. These parameters, along with the information for each site, were used in a Monte Carlo analysis resulting in a range of PECs. They were then compared to the PNEC for non-sulfonated dyes and the PNEC for Brilliant Blue FCF respectively.
For any of the non-sulfonated triarylmethane dyes, the calculated PECs in receiving water bodies near the point of discharge range from 1.45×10-9 to 425µg/L, with 28% of iterations yielding PECs greater than the PNEC (1 µg/L).
For Brilliant Blue FCF, the calculated PECs in receiving water bodies near the point of discharge range from 2.41×10-8 to 479 µg/L. In this case, fewer than 1% of iterations yield PECs greater than the PNEC (150 µg/L).
7.2.3 Scenario 2: paper deinking
This scenario considered the removal of inks containing triarylmethane dyes from recycled paper. Two PECs were calculated: one for the four non-sulfonated dyes and one for Brilliant Blue FCF, with the assumption that some of the recycled paper was printed with ink containing one or more of the four non-sulfonated dyes or Brilliant Blue FCF.
Recycled paper may contain triarylmethane dyes both in the paper fibre from dyes used to colour the paper itself, as well as in the inks printed on the paper. It was assumed that the deinking process removes just the ink (and the dye it contains) affixed to the surface of the paper and not the dye bound to the paper fibre that was used to colour the paper (Liu et al. 2007). A key assumption is the mass of the substance in the paper to be recycled, which was estimated using the capacity of a given deinking plant. Additional assumptions about the composition of recycled paper included the average coverage of ink, the average paper density, and the ink millage or coverage (see Table B-2). The resulting fractional ink content of a given pile of paper bound for recycling was estimated at between 0.004 and 0.01 gram of ink per gram of paper (g ink/g paper). This estimate is supported by European estimates of ink content in paper for recycling, which range from 0.003 to 0.07 g ink/g paper (OECD 2009).
Thirteen pulp and paper recycling plants were used in the scenario, along with their individual known recycling capacities, effluent flow rates, on-site WWTSs, and dilution factors. Assumptions included a fractional emission factor of ink (0.02 to 0.2; Beatson 2012) and the fraction of triarylmethane substance in the ink (1×10-4 and 2×10-2) on a weight basis. A key uncertainty in the PEC calculations is the actual proportion of triarylmethane dyes in the ink. A summary of key assumptions is provided in Appendix B (Table B-2). These parameters were used in a Monte Carlo analysis resulting in a range of PECs.
For any of the non-sulfonated triarylmethane dyes, the calculated PECs in receiving water bodies near the point of discharge range from 5.5×10-5 to 231 µg/L, with 50% of iterations yielding PECs greater than the PNEC (1 µg/L).
For Brilliant Blue FCF, the calculated PECs in receiving water bodies near the point of discharge range from 1.8×10-6 to 136 µg/L. In this case, none of the iterations yield PECs greater than the PNEC (150 µg/L).
7.2.4 Scenario 3: general formulation
A probabilistic analysis was conducted to determine PECs for release of triarylmethane dyes from facilities that use these substances in the formulation of products. Given the greater hazard of the four non-sulfonated dyes, this exposure scenario focused on estimating a PEC for general formulation of products containing any of these four substances. Site-specific details of the formulator that reported the largest use quantities of the non-sulfonated triarylmethane dyes in Canada were used. This scenario included details such as mass balance-based emission factors ranging from 0.5% to 1.1%. Allowance was given for on-site and off-site secondary removal and high dilution in the environment. If any of these conditions were not present at a facility processing large quantities of non-sulfonated triarylmethane dyes, then refinement of this scenario could impact the outcome, and there could be a concern. A summary of assumptions is provided in Appendix B (Table B-3). These parameters were used in a Monte Carlo analysis resulting in a range of PECs.
For any of the non-sulfonated triarylmethane dyes, the calculated PECs in receiving water bodies near the point of discharge range from 3.6×10-3 to 2.4 µg/L. Fewer than 2% of iterations yield PECs greater than the PNEC (1 µg/L).
7.2.5 Scenario 4: consumer uses
This scenario was developed for Brilliant Blue FCF, given its use in products available to consumers. Consumer release of Brilliant Blue FCF during its use in products is expected to occur throughout Canada. As such, the PEC is estimated using the Consumer Release Aquatic Model (CRAM; Environment and Climate Change Canada internal model). CRAM is a Canadian, population-based probabilistic model used to estimate environmental exposure resulting from wastewater treatment facility releases of chemicals present in products available to consumers that are released down the drain. Distribution information, including dilution factors (derived from the 10th percentile flow rate of receiving water bodies), WWTS treatment type and per capita water discharge, was used. A wide range of potential chemical usage was used to account for importation of manufactured items containing these substances.
The calculated PECs for Brilliant Blue FCF in receiving water bodies near the point of discharge range from 3.0×10-9 to 455 µg/L. Fewer than 0.01% of PECs are greater than the PNEC (150 µg/L).
For a worst-case scenario, quantities beyond what was reported under the CEPA section 71 survey were used to account for maximum uses of Brilliant Blue FCF. Relatively high simulated aquatic PECs (above 150 µg/L) were largely associated with conditions of consumer usage reaching 3 g of Brilliant Blue FCF per day per person, or the total mass of Brilliant Blue FCF reaching 45 million kg per year, which greatly exceeds the total reported mass per year in the section 71 survey. As an example, given known concentrations of Brilliant Blue FCF in shampoo, even if 100% of Canadians used shampoo containing this substance, the 95th percentile PEC would not exceed the PNEC of 150 µg/L. Although there are other products used by consumers that would also contribute to environmental releases, it is not expected that these would result in significant environmental concentrations, given the low concentrations of Brilliant Blue FCF in these products.
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop conclusions based on a weight of evidence approach and using precaution. Evidence was gathered to determine the potential for substances in the Triarylmethanes Group to cause harm in the Canadian environment. Various direct lines of evidence were considered to support the characterization of ecological risk.
7.3.1 Risk quotient analysis
Risk quotient analyses were performed by comparing estimates of exposure (PECs; see section 7.2, Ecological Exposure Assessment) with ecological toxicity information (PNECs; see section 7.1, Ecological Effects Assessment) to determine whether there is potential for ecological harm in Canada. Risk quotients (RQs) were calculated by dividing the PEC by the PNEC for relevant environmental compartments and associated exposure scenarios. RQs were not calculated for Pigment Blue 61. Table 7‑2 presents RQs for the other five triarylmethane dyes for releases to water via wastewater.
| Exposure scenario | PEC range (µg/L) | Aquatic PNEC (µg/L) | RQ range | Percentage of iterations with RQ greater than 1 |
|---|---|---|---|---|
| Paper dyeing (non-sulfonated triarylmethane dyes) | 1.45×10-9 to 425a | 1a | 1.45×10-9 to 425 | 28 |
| Paper dyeing (Brilliant Blue FCF) | 2.41×10-8 to 479b | 150b | 1.61×10-10 to 3.19 | < 1 |
| Paper deinking (non-sulfonated triarylmethane dyes) | 5.5×10-5 to 231a | 1a | 5.5×10-5 to 231 | 50 |
| Paper deinking (Brilliant Blue FCF) | 1.8×10-6 to 136b | 150b | 1.2×10-8 to 0.91 | 0 |
| General formulation (non-sulfonated triarylmethane dyes) | 3.6×10-3 to 2.4a | 1a | 3.6×10-3 to 2.4 | 2 |
| Consumer uses (Brilliant Blue FCF) | 3.0×10-9 to 455 | 150b | 2.0×10-11 to 3.0 | 0.01 |
a Any of the non-sulfonated triarylmethane dyes (Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7)
b Brilliant Blue FCF
As shown in Table 7-2, with respect to the paper dyeing scenario for any of the four non-sulfonated dyes, 28% of iterations had RQs greater than 1. Simulated RQs vary according to the combination of parameters, such as dye product use rate, retention rate, concentration of triarylmethane dye substance in dye product, and removal rate. For instance, a higher dye product use rate (> 0.2 kg dye product per tonne of paper) will lead to a higher probability of the RQ exceeding 1. However, the dye product use rate is often lower than this (personal communication, email from the Forest Products Association of Canada to Environment and Climate Change Canada, 2017; unreferenced).
For the paper deinking scenario for the non-sulfonated dyes, 50% of iterations had RQs greater than 1. The simulations with non-sulfonated dyes began exceeding the PNEC when these dyes reached 1% of the dyes used in inks on printed paper bound for recycling. Simulated PECs for the paper deinking scenario with Brilliant Blue FCF began to exceed the PNEC when Brilliant Blue FCF was assumed to account for more than 60% of the dyes used in inks on printed paper bound for recycling. However, this situation is unlikely to occur.
The scenario developed for general formulation and product handling based on the formulator that reported the largest use quantities did not exceed the PNEC due to refinement of site-specific factors, such as the mass balance based emission factors, on-site and off-site secondary removal, and high dilution in the environment. However, general formulation and product handling simulations for a generic scenario, considering chemical formulation facilities in Canada that could fill this market demand, showed some potential for exceedances of the PNEC for the non-sulfonated triarylmethane dyes.
7.3.2 Consideration of the lines of evidence
To characterize the ecological risk of substances in the Triarylmethanes Group, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion for the four non-sulfonated triarylmethane dyes are presented in Table 7‑3, with an overall discussion of the weight of evidence provided in section 7.3.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. Relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Similarity in chemical structure for read-across | high | high | high |
| Environmental fate and behaviour (ionic nature) | moderate | high | moderate-high |
| Persistence in the environment (i.e., water, sediment, soil) | moderate | moderate | moderate |
| Bioaccumulation in aquatic organisms | low | moderate | low-moderate |
| Mode of action (reactive) | moderate | moderate | moderate |
| PNEC for aquatic organisms | high | high | high |
| PECs in paper dyeing scenario | moderate | high | moderate-high |
| PECs in paper deinking scenario | low | low | low |
| PECs in general formulation and product handling scenario | moderate | high | moderate-high |
| RQs for paper dyeing | moderate | high | moderate-high |
| RQs for paper deinking | low | low | low |
| RQs for general formulation and product handling | moderate | high | moderate-high |
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
A moderate to high level of confidence would be assigned to the data evaluated for Brilliant Blue FCF and Pigment Blue 61, but with low relevance to demonstrating a potential to cause harm in the Canadian environment. These key lines of evidence included their environmental fate and behaviour, persistence, potential for bioaccumulation, potential to cause adverse effects in aquatic organisms, and RQs that were calculated.
7.3.3 Weight of evidence for determining potential to cause harm to the Canadian environment
The four non-sulfonated triarylmethane dyes (Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7) have similar chemical structures and molecular weights. They are water soluble and have negligible vapour pressure. As such, they are assumed to behave similarly in the environment with respect to both fate in the environment and effects on organisms. If released to the aquatic environment, a fraction of the amount released may reside in the water column given their water solubility. At environmentally relevant pH, these substances are likely to partition to negatively charged, dissolved and suspended solids. This adsorption may or may not be irreversible. Therefore, these substances may be transported in water far from sources or they may settle to bed sediment. Considering the uses of these non-sulfonated triarylmethane dyes, a high degree of removal during on-site industrial wastewater treatment is expected due to partitioning of these dyes to organic matter. As sludge from these on-site WWTSs would not be applied to agricultural or pasture lands, the non-sulfonated triarylmethane dyes would not end up in soil, and exposure via this medium would not be a concern.
These four dyes are expected to be persistent in environmental media (water, sediment and soil). Thus, the potential for organisms to be exposed both spatially and temporally to these dyes in the environment is increased. These substances are expected to have a low potential to bioaccumulate in lipid tissues of aquatic organisms based on limited experimental data and modelled results. However, Basic Violet 3 and Malachite Green can interact and bind with proteins and DNA, as evidenced by their functional uses as laboratory stains. Using the OECD QSAR Toolbox for the MOA classification, the triarylmethane substances were classified as “reactive unspecified.” In addition, outcomes of the ToxCast AR binding model indicate the potential for endocrine-type effects. However, no empirical information demonstrating such effects was available. The acute toxicity of Basic Violet 3 and Malachite Green to a variety of aquatic organisms is well documented, and these substances have been shown to have the potential to cause adverse effects to aquatic organisms at low concentrations (µg/L). The most sensitive organisms appear to be freshwater fish.
According to information reported in response to section 71 surveys under CEPA, these triarylmethane substances are mainly used in paper dyeing, inks and toners, and are formulated for use in a wide range of products for consumer and commercial use. Although there is no information on environmental concentrations in the Canadian environment, PECs were calculated for relevant exposure scenarios on the basis of their uses. In comparing PECs with the PNEC for the four non-sulfonated dyes (Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7), the results for paper dyeing and paper deinking scenarios indicate that these substances pose a risk to aquatic organisms.
Pigment Blue 61 is expected to behave more like a particle, similar to other organic pigments. Considering its physical and chemical properties, it is expected to be persistent in the environment and have a low potential to bioaccumulate in aquatic organisms. The aquatic toxicity studies available for both Pigment Blue 61 and its analogue, Pigment Blue 56, demonstrated no effects at concentrations up to the water solubility limit, with one study showing effects only at concentrations well above the solubility limit with the use of an emulsifier. Therefore, an aquatic PNEC was not developed. This substance is not expected to pose a risk to aquatic organisms. No data existed for soil-dwelling organisms. However, considering the properties of pigments, Pigment Blue 61 is expected to be sorbed to soil particles and would not likely be bioavailable.
Brilliant Blue FCF is very soluble in water and is expected to be persistent in the environment. As an anion, it is less likely to bind to sediment or organic matter, it has a low potential to bioaccumulate in aquatic organisms, and its toxicity to aquatic organisms may be mitigated by its sulfonic acid groups (see Table 7.1). However, the PNEC developed is below 1 mg/L due to the relatively high assessment factor used as a result of the limited dataset. The PECs derived for the exposure scenarios show that there is a potential for risk depending on the assumptions used. However, the conditions that would result in RQs above 1 are not likely to be reached. In considering this information, Brilliant Blue FCF is not expected to pose a risk to aquatic organisms.
In summary, the information evaluated for the four non-sulfonated dyes (Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7) demonstrates that they have the potential to cause ecological harm in Canada, and the information available for Pigment Blue 61 and Brilliant Blue FCF shows they have low potential to cause ecological harm in Canada.
It has also been determined that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 meet the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
7.3.4 Sensitivity of conclusion to key uncertainties
No aquatic toxicity data were available for Basic Violet 4 or Basic Blue 7, and the data available for Basic Violet 3 indicated that adverse effects may occur at slightly higher concentrations than those for Malachite Green. The chemical similarities of these four substances warrant the use of a read-across approach for toxicity to aquatic organisms, and the potential for Malachite Green to cause adverse effects in aquatic organisms is well documented. Therefore, additional empirical toxicity studies for the other substances would not likely change the conclusion. Although there are limited bioaccumulation data for these four dyes, modelled results along with physical-chemical property data corroborate the expected low potential for these substances to bioaccumulate in aquatic organisms. Additional information on bioaccumulative potential would also have a low impact on the conclusion.
There is uncertainty regarding the potential for these substances to cause chronic, reproductive or developmental effects in aquatic species and the concentrations at which those effects would occur. Although there is evidence that certain triarylmethane dyes bind to proteins and DNA, it is unknown if this could lead to reproductive and/or developmental effects, and empirical information on these types of effects was not available for aquatic organisms. Discussion on the developmental toxicity of Malachite Green to mammals can be found in section 8.2 of this assessment. Depending on the mechanism by which developmental effects occur in mammals, it might be reasonable to expect similar effects in other types of organisms. This uncertainty is addressed through the use of additional assessment factors in deriving PNECs to account for the reactive MOA of these triarylmethane dyes. Availability of empirical developmental toxicity studies on aquatic organisms could result in refinement of the PNECs.
The exposure scenarios identified for substances in the Triarylmethanes Group are developed on the basis of information obtained through CEPA section 71 surveys, follow-up with stakeholders, and data from the literature. In the absence of particular data, realistic assumptions are made in order to estimate PECs. For the paper dyeing and deinking scenarios, refinement of the dye mass used at sites, the usage rates of the substance(s), and emission factors would help to increase the confidence in the PECs. In particular for the deinking scenario, better knowledge of how paper recycling plants filter their feedstock (to help estimate the mass of substance) and more reliable information about the emission factors for water-based dyes used in inks could lead to adjustments in this scenario. Sufficient refinement of these factors could impact these scenarios as they may lead to an understanding that exposure is significantly reduced. However, current information is sufficient to support the conclusion that there is a potential to cause ecological harm as a result of the use of the non-sulfonated triarylmethane dyes in paper dyeing and inks.
8. Potential to cause harm to human health
8.1 Exposure assessment
Potential exposures to substances in the Triarylmethanes Group from environmental media, food, and products available to consumers are presented in this section. For each substance, exposure scenarios resulting in the highest exposures were selected to characterize risk.
Environmental media
Substances in the Triarylmethanes Group were not identified or measured in any environmental media in Canada.
The uses of the substances in the Triarylmethanes Group are based on information submitted pursuant to a CEPA section 71 notice (Environment Canada 2009, 2013), information on products submitted to Health Canada, and publicly available data. The information indicates that releases of these substances to the Canadian environment may result from the consumer use of products containing these substances (i.e., down-the-drain releases) and from various industrial processes. As described in the ecological exposure assessment (see section 7.2), such releases are expected to occur primarily to WWTSs, but treatment technologies may only partially degrade these substances, with a portion partitioning to biosolids. Therefore, environmental releases of these substances could contribute to general population exposure through drinking water. Brilliant Blue FCF would be expected to potentially contribute to general population exposure through drinking water as a result of consumer down-the-drain releases, whereas the use of the other substances in Canada would not be expected to result in such releases because of the limited or absence of use of these substances in products that would be expected to be poured or washed down the drain by consumers. In addition, use of all five dye substances in Canada would be expected to potentially contribute to exposure through drinking water as a result of industrial releases (e.g., from paper dyeing, paper deinking, and general formulation, as noted in section 7.2).
Given the absence of surface or drinking water monitoring data for substances in the Triarylmethanes Group in Canada or elsewhere, theoretical concentrations of each triarylmethane dye in surface water, used as a surrogate for drinking water, were derived from the predicted environmental concentration (PEC) distributions calculated in section 7.2. PEC distributions for potential industrial and consumer release scenarios for the non-sulfonated triarylmethane dyes and Brilliant Blue FCF were considered, where relevant.
The ranges of PECs described in section 7.2 for each of the four release scenarios represent the potential concentrations of the triarylmethane dye substances in a receiving body of water near the discharge point of a WWTS. These scenarios, which were developed for the purpose of the ecological exposure assessment, are anticipated to be overly-conservative within the context of assessing drinking water exposures that would be expected to occur downstream rather than at the point of discharge. One of the key input parameters into the scenarios in section 7.2 was the application of a dilution factor cap of 10; application of such a cap, however, would likely not be representative of a realistic scenario for assessing drinking water exposures further downstream and may result in an overestimate of the concentration of each substance that would be potentially present in drinking water. As such, the 50th percentile PECs for the paper dyeing, paper deinking, and general formulation scenarios were selected (i.e., rather than the upper bound of the ranges described in section 7.2). These scenarios are considered to be more realistic for assessing drinking water exposures while still being conservative.
The 50th percentile PEC for the estimated surface water concentration of Brilliant Blue FCF from consumer releases was used.
The theoretical intake estimates for drinking water for formula-fed infants (0 to 0.5 years) were calculated. The resulting surface water concentrations and theoretical intake estimates for the triarylmethane dyes in drinking water are provided in Table 8‑1. Theoretical concentrations were not estimated for the triarylmethane pigment, Pigment Blue 61, as it is not expected to partition into water.
| Exposure scenario | Surface water concentration as described in section 7.2 (mg/L) | Exposure from environmental releases, formula-fed infants (mg/kg bw/day)b |
|---|---|---|
| Paper dyeing (non-sulfonated triarylmethane dyes)a | 3.2×10-4 (50th percentile) | 3.4×10-5 |
| Paper dyeing (Brilliant Blue FCF) | 2.1×10-3 (50th percentile) | 2.2×10-4 |
| Paper deinking (non-sulfonated triarylmethane dyes)a | 9.5×10-4 (50th percentile) | 1.0×10-4 |
| Paper deinking (Brilliant Blue FCF) | 3.7×10-4 (50th percentile) | 3.2×10-5 |
| General formulation (non-sulfonated triarylmethane dyes)a | 2.1×10-4 (50th percentile) | 2.2×10-5 |
| Consumer uses (Brilliant Blue FCF) | 4.0×10-5 (50th percentile) | 4.3×10-6 |
Abbreviation: N/A, not applicable
a Non-sulfonated triarylmethane dyes = Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7
b A drinking water intake rate of 0.8 L/day and a body weight of 7.5 kg were used (Health Canada 1998).
To estimate potential cancer risk from daily exposure of Basic Violet 3, a lifetime average daily dose (LADD) was calculated (Appendix C). Drinking water intake rates and body weights from Health Canada (1998) were used for formula-fed infants, toddlers, children, teenagers and adults. The LADD for daily intake of Basic Violet 3 from drinking water was estimated to be 2.3 × 10‑5 mg/kg bw/day (for the paper deinking scenario, as it represents the highest exposure). As other exposures were identified for Malachite Green, Basic Violet 4, Basic Blue 7 and Brilliant Blue FCF that were higher than those from environmental media, LADDs were not calculated for those substances.
For Pigment Blue 61, due to a combination of its limited commercial quantities in Canada, negligible volatility, very low water solubility, and expected removal by water treatment systems, exposure from environmental media is not expected.
Food
With the exception of Brilliant Blue FCF, substances in the Triarylmethanes Group were not reported to be present in food. JECFA has reported that Basic Violet 3 and Malachite Green may be present in animal by-products/meats and/or fish as residues from continued use in veterinary drugs and aquaculture (EFSA 2016; JECFA 2009, 2014). Neither of these substances is currently approved for use in food-producing aquatic animals or in livestock feed in Canada (CFIA 2015; Health Canada [modified 2010]). Exposure of the general population to trace amounts of these substances in food due to non-compliant use or as a result of imported fish was determined to not pose a safety concern to consumers (CFIA 2012; personal communication, email from the Risk Management Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016; unreferenced). Three substances—Basic Violet 3, Pigment Blue 61, and Brilliant Blue FCF—are potentially used in food packaging materials in contact with food. Brilliant Blue FCF may also be a component in incidental additives (e.g., cleaners and dish detergents) used in food processing plants. Exposure to these substances due to the presence in food packaging and/or as a component of an incidental additive (e.g., due to the food manufacturing process) is expected to be negligible (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016-2017, 2019; unreferenced).
In Canada, Brilliant Blue FCF is permitted for use as a food additive in a number of foods at a maximum level of use of 100 ppm, singly, or in combination with Fast Green FCF in accordance with Item 4 of the List of Permitted Food Colouring Agents (Health Canada [modified 2015a]). It is also permitted for use in feta cheese at a maximum level of 0.10 ppm and in lumpfish caviar at a maximum level of 450 ppm in accordance with Items 4 and 9, respectively, of the List of Permitted Food Colouring Agents (Health Canada [modified 2015a]). Dietary exposure to Brilliant Blue FCF was estimated using the levels measured by the Canadian Food Inspection Agency from its targeted surveys on food colours (CFIA 2010, 2011) and one-day recall food consumption data from the Canadian Community Health Survey Cycle 2.2 on Nutrition (Statistics Canada 2004). Where data were not available for a particular food category, the level of Brilliant Blue FCF measured in a similarly coloured food was applied. At the 90th percentile, the highest estimated dietary exposure of Brilliant Blue FCF as a result of its use as a food additive, on a body weight basis, was for children aged 4 to 8 years, at 330 µg/kg bw/day (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, 2016-2017; unreferenced).
Based on consideration of the above information, the potential exposure of the general population to Brilliant Blue FCF through the dietary intake of food and drinking water is expected to be significantly lower than potential exposure through use of products available to consumers. Exposure of Basic Violet 3 and Pigment Blue 61 through food and food packaging in Canada is expected to be negligible. Exposure of the remaining three substances of the Triarylmethanes Group through food in Canada is not expected.
Products available to consumers
Exposures from the use of products available to consumers containing substances in the Triarylmethanes Group were evaluated. Product scenarios that result in the highest levels of potential exposure for each substance by the oral, dermal, and inhalation routes are presented in Table 8‑2, Table 8‑3, and Table 8‑4, respectively. Potential exposures were estimated based on conservative assumptions and using default values from sentinel exposure scenarios (see Appendix C for further details).
To estimate potential cancer risk, daily systemic exposures on an age group-specific basis were used, except where lifetime averaging was undertaken (see section 8.3, Characterization of Risk to Human Health). For those scenarios where such an adjustment was required, lifetime average daily doses (LADDs) were calculated.
| Substance | Product scenario | Age group | Per event systemic exposure (mg/kg bw) | Daily systemic exposure (mg/kg bw/ day) |
|---|---|---|---|---|
| Brilliant Blue FCF | Non-medicinal use in a natural health product | Adult | NA | 2.82 |
Abbreviation: NA, not applicable.
Basic Violet 3’s reported use as or in a pigment in paper products, mixtures, or manufactured items is limited to commercial applications in Canada. Malachite Green’s reported use as a dye in ink, toner, or colourants is also limited to commercial applications, and its reported use in paper products, mixtures, or manufactured items is not likely to result in oral exposures. Therefore, potential oral exposures to these substances from products used by consumers are not expected. Similarly, given its reported use as a pigment in printing ink limited to commercial applications in Canada, potential oral exposure to Pigment Blue 61 from products used by consumers is also not expected.
For estimated potential exposures via the dermal route, the maximum flux (Jmax) approach (Williams et al. 2016) was used for Malachite Green, Basic Violet 4, and Basic Blue 7 to characterize systemic doses as a refinement (See Appendix C). A dermal flux study conducted by Lucová et al. (2013) showed the skin penetration of Brilliant Blue FCF in an oil-in-water emulsion to be practically negligible. In this study, the cumulative amount of Brilliant Blue FCF (oil-in-water emulsion) absorbed into the epidermis applied to an ex vivo sample of intact full thickness porcine ear skin (1 ± 0.07 mm) at a dermal load of 250 ng/cm2 over 24 hours is 14 ng/cm2 (with standard deviation, or SD, of 3 ng/cm2). Any amount of Brilliant Blue FCF that may have been present in the receptor fluid or the dermis was below the limit of quantitation. Since the stratum corneum was not separated from the viable epidermis, the total amount absorbed into the epidermis of 17 ng/cm2 (14 ng/cm2 plus 1 SD) was conservatively used to estimate dermal absorption of Brilliant Blue FCF in this assessment. An in vitro percutaneous absorption study on Brilliant Blue FCF was also available (SCCNFP 2004), which similarly showed no measurable permeation through skin (i.e., in the receptor fluid) but conservatively estimated a maximum potential absorption of 6.2 µg/cm2 for a hair colour gel formulation and 35.2 µg/cm2 for the pure dye based on the limit of detection and skin-bound residues. On the basis of formulation considerations, the study by Lucová et al. (2013) was deemed more relevant to the body cream scenario and was used accordingly.
| Substance | Product scenario | Age group | Per event systemic exposure (mg/kg bw) | Daily systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Malachite Green | Hair dye (semi-permanent) | Teenager | 0.337 | 0.0033 |
| Malachite Green | Hair dye (semi-permanent) | Adult | 0.282 | 0.0102 |
| Basic Violet 4 | Hair dye (semi-permanent) | Teenager | 0.0589 | 0.00058 |
| Basic Violet 4 | Hair dye (semi-permanent) | Adult | 0.0494 | 0.00178 |
| Basic Blue 7 | Hair dye (semi-permanent) | Teenager | 0.0177 | 0.000175 |
| Basic Blue 7 | Hair dye (semi-permanent) | Adult | 0.0148 | 0.000533 |
| Brilliant Blue FCF | Body cream | Infant | NA | 0.0068 |
| Brilliant Blue FCF | Body cream | Adult | NA | 0.0041 |
Abbreviation: NA, not applicable
To estimate the potential cancer risk to intermittent per event dermal exposure, a lifetime average daily dose (LADD) of 0.00134 mg/kg bw/day was calculated for Basic Violet 4 for the use of hair dye by teenagers and adults. For all other dermal exposures scenarios where potential cancer risks were estimated, the daily systemic exposures on an age group-specific basis were used (see section 8.3, Characterization of Risk to Human Health).
Although dermal contact with Pigment Blue 61 from printing ink is possible, the solubility of this pigment is very low and hence it will not readily solubilize in perspiration (BfR 2007). As a result, it cannot readily penetrate intact skin and therefore systemic exposure is not expected. Similarly, potential dermal contact with Basic Violet 3 based on its reported use as or in a pigment in paper products, mixtures or manufactured items is not expected to result in systemic exposure; migration of the substance from paper as a pigment component of an ink (e.g., printing ink) and subsequent absorption by intact skin is not expected. Pigments used in printing inks are frequently dyes (e.g., Basic Violet 3) rendered insoluble by complexing with a metal ion (IARC 1996). Further, systemic exposure to Basic Violet 3 would also not be expected from use as a paper dye because dyes contained within the matrix of the paper would be anticipated to exhibit minimal migration in a dermal scenario.
The estimated exposure to Brilliant Blue FCF in a perfume spray is presented below.
| Substance | Product scenario | Age group | Per event systemic exposure (mg/kg bw) | Daily systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Brilliant Blue FCF | Perfume | Teenager | NA | 0.00064 |
| Brilliant Blue FCF | Perfume | Adult | NA | 0.00054 |
Abbreviation: NA, not applicable
Because of the very low vapour pressures (10-13 mmHg or lower at 25°C) of all six members of the Triarylmethanes Group, any potential non-aerosol exposures via the inhalation route were considered to be limited.
8.2 Health effects assessment
There were limited chemical-specific hazard data for some substances in the Triarylmethanes Group. Analogues were considered on the basis of similarities in their physical and chemical properties, metabolism, and structure. The chemical-specific data is presented first, followed by data on analogues used to inform the health effects characterization of substances in the Triarylmethanes Group.
Basic Violet 3
Basic Violet 3 toxicity was reviewed by JECFA (2014) and Diamante et al. (2009). Basic Violet 3 was classified by the European Commission as Carc. 2 (or 1B if there is more than or equal to 0.1% of Michler’s ketone) (EC 2008). In rats or mice, a single or one-week daily gavage exposure of 4 up to 7 mg/kg bw(/day) Basic Violet 3 was rapidly but poorly absorbed, then distributed primarily to fat, liver, kidneys, and to a lesser extent to muscle, ovaries and testes (McDonald et al. 1984; McDonald 1989). It is metabolized through reduction and demethylation pathways, with primarily reduced metabolites such as leucogentian violet identified in tissues (McDonald and Cerniglia 1984; McDonald 1989). Basic Violet 3 was mostly (66% to 73%) excreted in feces, and to a lesser extent in urine (less than 8%) (McDonald et al. 1984; McDonald 1989; Docampo and Moreno 1990). The developmental toxicity of Basic Violet 3 was assessed in both rabbits and rats. In rabbits gavaged from gestation days (GDs) 6 to 19 with 0, 0.5, 1, or 2 mg/kg bw/day gentian violet and sacrificed on GD 30, at the lowest observed adverse effect level (LOAEL) of 0.5 mg/kg bw/day, there was both decreased fetal body weight and maternal toxicity (increased mortality, decreased body weight and body weight gain, and clinical signs, such as wheezing, diarrhea, and congestion) (NTP 1983). No no observed adverse effect level (NOAEL) was determined since this was the lowest tested dose (JECFA 2014). In comparison, no effects were observed at the lowest tested dose of 2.5 mg/kg bw/day in rats gavaged from GDs 6 to 15 and sacrificed on GD 20 (NTP 1982). Maternal toxicity (clinical signs of toxicity and decreased body weight gain) was only observed at 5 mg/kg bw/day, with developmental toxicity (increased incidences of short 13th rib, hydronephrosis, and hydroureter) observed at 10 mg/kg bw/day (NTP 1982).
In a three-generation reproductive toxicity study in rats exposed to Basic Violet 3 in the diet, no reproductive effects were observed up to the highest tested dose of 30 mg/kg bw/day (Littlefield 1988). Parents were exposed to 0, 5, 15, or 30 mg/kg bw/day for 80 days pre-mating exposure, then mated twice to make F1a and b litters. F1a rats (2/sex/litter) were used for the chronic carcinogenicity study (Littlefield et al. 1989). F1b rats (1/sex/litter) were then mated after 100 to 140 days to generate F2a litters. This mating was repeated to make F2b litters, which then similarly reproduced F3a litters. The parental NOAEL was 15 mg/kg bw/day based on decreased body weight at 30 mg/kg bw/day (Littlefield 1988). There was no NOAEL for offspring due to increased focal dilatation of the renal cortex and tubules and necrosis of the thymus in F3a weanlings (2/sex/litter) at the LOAEL of 5 mg/kg bw/day and above (Littlefield 1988). In 13-week dietary studies, there were also slight decreased body weights in rats fed 25 mg/kg bw/day and increased liver weight in dogs fed up to 16 mg/kg bw/day, both of which were the highest tested doses (Littlefield et al. 1989).
There were mixed results for the genotoxicity of Basic Violet 3, but overall it was considered genotoxic in vitro, based on positive cell mutation (Aidoo et al. 1990), chromosome aberration (Au et al. 1978, Au and Hsu 1979) and DNA binding and repair assays (Müller and Gautier 1975; Wakelin et al. 1981) reviewed in Mani and Bharagava (2016). In contrast, Basic Violet 3 did not affect the incidence of chromosomal aberrations in mice exposed via drinking water up to 8 mg/kg bw/day for 1 month, nor did it damage spleen lymphocyte DNA in mice exposed intravenously to up to 6 mg/kg bw for 1 hour (Au and Hsu 1979; Aidoo et al. 1990). In a 2-year carcinogenicity study in mice fed approximately 0, 11/14, 32/36, and 64/71 mg/kg bw/day (males/females) Basic Violet 3, increased erythropoiesis in the spleen and atrophy of ovaries in females were observed at the lowest tested dose of 14.3 mg/kg bw/day (Littlefield 1984; Littlefield et al. 1985). At approximately 36 mg/kg bw/day and above, there were increased liver adenomas and carcinomas in females, both of which were also increased in males at 64 mg/kg bw/day, along with increased mortality in females. In a 2-year carcinogenicity study in rats exposed to Basic Violet 3 via the diet, no adverse effects were observed at the lowest tested dose of 30/40 mg/kg bw/day (males/females). At the LOAEL of 80/100 mg/kg bw/day (males/females), there was increased mortality (males) and increased follicular cell adenoma of the thyroid gland (females), as well as increased hepatocellular adenoma in both sexes (Littlefield et al. 1989).
The incidence of increased hepatocellular carcinomas in female mice at 2 years was 7/185, 5/93, 30/93, or 73/95 for 0, 14, 36, or 71 mg/kg bw/day, respectively. The dose-response relationship for the two tumour types was similar (the incidence of hepatocellular adenomas in female mice at 2 years was 8/185, 8/93, 36/93, 20/95 for 0, 14, 36, or 71 mg/kg bw/day, respectively) (JECFA 2014). To determine the increased risk of hepatocellular carcinomas, JECFA (2014) used the US EPA’s benchmark dose software (BMDS, version 2.2) to establish that use of the multistage model had an acceptable fit, had the lowest Akaike’s Information Criterion value, and had the lowest benchmark dose value for 10% extra risk (19.9 mg/kg bw/day) and benchmark dose lower 95% confidence limit for 10% extra risk (BMDL10 of 16.8 mg/kg bw/day) among the models.
Malachite Green
Malachite Green was reviewed as part of an NTP carcinogenicity study (2005) and by EFSA (2016). When rats or mice were fed 5 or 30 mg/kg bw/day Malachite Green for 1 month, it remained mostly unmetabolized in the liver, but reduced to leucomalachite green, and to a lesser extent to mono- and di-desmethyl malachite green or mono- and di-desmethyl leucomalachite green (Culp et al. 1999). Malachite Green Oxalate was considered an analogue of Malachite Green based on physical-chemical and structural similarities (e.g., the structure of Malachite Green Oxalate is identical to that of Malachite Green except that the anionic moiety of the salt is oxalate instead of chloride). In a developmental study in rabbits gavaged with 0, 5, 10, or 20 mg/kg bw/day Malachite Green Oxalate from GDs 6 to 18 and sacrificed on GD 30, the maternal NOAEL was 5 mg/kg bw/day (Meyer and Jorgenson 1983). At 10 mg/kg bw/day, there was decreased food consumption, body weight, and body weight gain in dams. At 5 mg/kg bw/day and above, there were increased pre-implantation losses, increased early fetal resorptions, decreased fetal survival, decreased fetal body weight, and increased skeletal deviations. Although there were limitations in this developmental study in rabbits with Malachite Green Oxalate (e.g., an unknown number of developmental incidences per litter) and a lack of clear dose-response in fetal effects, adverse developmental effects were observed at the lowest dose tested. On the basis of this study, Malachite Green is expected to have developmental effects at 5 mg/kg bw/day, based on increased pre-implantation losses, increased early fetal resorptions, decreased fetal survival, decreased fetal body weight, and increased skeletal deviations at the lowest tested dose of 5 mg/kg bw/day.
Malachite Green was not genotoxic in vitro in cell mutation assays, chromosomal aberration assays, or Comet assays (Au and Hsu 1979; Ferguson and Baguley 1988; Panandiker et al. 1994; Fessard 1999; NTP 2004; Bose et al. 2005; Stammati et al. 2005). On the other hand, it also inhibited polymerase I catalyzed DNA replication, induced single strand DNA breaks, and was cytotoxic in vitro (Wolfe 1977; Panandiker et al. 1994; Stammati et al. 2005). There was mixed evidence of genotoxicity in vivo, with negative micronucleus, Hprt or cII mutant assays in mice fed 0 or 43 mg/kg bw/day for 4 or 6 weeks (Mittelstaedt et al. 2004), but evidence of chromosomal aberrations, DNA fragmentation, sister chromatid exchange, and DNA adduct formation in mice or rats gavaged or fed with approximately 4 to 78 mg/kg bw/day Malachite Green for 4 weeks (Culp et al. 1999; Donya et al. 2012; Kasem et al. 2016).
In a 2-year dietary carcinogenicity study in female mice, no systemic toxicity or tumours were observed up to the highest tested dose of 67 mg/kg bw/day Malachite Green (NTP 2005). In contrast, female rats fed 0, 7, 21, or 43 mg/kg bw/day Malachite Green for 2 years had a NOAEL of 7 mg/kg bw/day, based on 9% decreased body weight and slightly increased tumour incidences at the LOAEL of 21 mg/kg bw/day and above (NTP 2005). Thyroid follicular cell adenoma and carcinomas [combined incidence: 0/46, 0/48, 3/47 (6%), 2/46 for control to high doses, respectively, historical control range up to 3%], and hepatocellular adenomas [1/48, 1/48, 3/48 (6%), 4/48 for control to high doses, respectively, historical control range up to 0.6%] were both supported by slight non-neoplastic changes (cystic follicles in thyroid gland, eosinophilic foci in liver) at the LOAEL of 21 mg/kg bw/day (NTP 2005). At 43 mg/kg bw/day, there was a slight increase in mammary gland carcinomas [2/48, 2/48, 1/48, 5/48 (10%) for control to high doses, respectively, historical control range up to 4%] and increased relative liver weight in female rats. There were no thyroid hormone changes observed in a satellite 21-day study in rats fed 60 mg/kg bw/day Malachite Green (NTP 2005). In a 4-month oral study in rats, increased hepatocellular eosinophilic foci with 13 mg/kg bw/day Malachite Green in drinking water were transformed with diethylnitrosamine initiation into hepatocellular carcinomas accompanied by increased relative liver weight and cell cycle changes (Sundarrajan et al. 2000). NTP consider there to be equivocal evidence of carcinogenicity in female rats based on increased combined thyroid tumours and marginal increases in hepatocellular adenoma and mammary gland carcinomas.
Pigment Blue 61
Pigment Blue 61 was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. It is also not on the European Chemicals Agency’s Candidate List of Substances of Very High Concern for Authorisation (ECHA [modified 2016]). Further investigation of health effects is not warranted at this time given the low expected exposure of the general Canadian population.
Basic Violet 4
Basic Violet 4 was not genotoxic in in vitro bacterial reverse mutation assays nor in a mammalian gene mutation assay (mouse lymphoma L5178Y cells) (Seifried et al. 2006). The toxicity of Basic Violet 4 was informed by its analogue Ethyl Violet Acetate (CAS RN 63157-72-2) (ECHA c2007-2017b). Similar to Malachite Green and its analogue Malachite Green Oxalate, the structure of Ethyl Violet Acetate is identical to that of Basic Violet 4 except that the anionic moiety of the salt is acetate instead of chloride. When no Ethyl Violet Acetate data were available, Basic Violet 3 was also used as an analogue, since it is also similar to Basic Violet 4 with respect to physical-chemical properties and chemical structure.
In a developmental study with Ethyl Violet Acetate, there was no maternal or developmental effect up to the highest tested dose of 12 mg/kg bw/day in rats (ECHA c2007-2017b). This was consistent with a 4-week gavage study in rats with Ethyl Violet Acetate, in which there were no adverse effects at 10 mg/kg bw/day, but there was a steep dose-response curve since severe toxicity was observed in both sexes at 20 to 30 mg/kg bw/day (including mortality, decreased body weight and body weight gain, decreased food consumption, and poor general condition) (ECHA c2007-2017b).
On the basis of a 3-generation reproductive toxicity study in rats exposed to 0, 5, 15, or 30 mg/kg bw/day Basic Violet 3 by diet (Littlefield 1988), Basic Violet 4 may result in offspring effects (focal dilatation of the renal cortex and tubules and necrosis of the thymus in F3a weanlings) and reduced maternal body weight gain at 5 mg/kg bw/day, but no reproductive effect is expected.
Based on the structural similarity between Basic Violet 3 and Basic Violet 4, it is assumed that up to 6 or 8 mg/kg bw(/day) Basic Violet 4 will be unlikely to increase chromosomal aberrations or damage DNA in mice (Au and Hsu 1979; Littlefield et al. 1985; Aidoo et al. 1990; Diamante et al. 2009). A 2-year carcinogenicity study in mice exposed to dietary Basic Violet 3 indicates that potential effects may include increased erythropoiesis in the spleen and atrophy of the ovaries in females at 14 mg/kg bw/day and above, and increased hepatocellular tumours at 36 mg/kg bw/day and above, with a BMDL10 of 16.8 mg/kg bw/day for increased risk of hepatocellular carcinomas in female mice (Littlefield 1984; Littlefield et al. 1985; JECFA 2014). In addition to similarities in the chemical structure and physical-chemical properties of Basic Violet 3 and Basic Violet 4, the applicability of Basic Violet 3 data to characterize the carcinogenic potential of Basic Violet 4 was supported by similarities in their chemical profiles and (Q)SAR model predictions (Derek Nexus 2016; Leadscope Model Applier 2016; OECD QSAR Toolbox 2013; Times 2016). Further, metabolic pathways predicted by Times 2016 indicate that Basic Violet 4 may undergo the same metabolic transformations as Basic Violet 3 (Docampo and Moreno 1990), which further supports the read-across from Basic Violet 3 to Basic Violet 4 in the absence of a substance-specific carcinogenicity study.
Basic Blue 7
In the absence of any substance-specific hazard data, the toxicity of Basic Blue 7 was based on analogues with physical-chemical and structural similarities, with Basic Violet 4 and Ethyl Violet Acetate being more similar to Basic Blue 7 than Basic Blue 26 or Basic Violet 3. On the basis of a 4-week study and a developmental study in rats administered Ethyl Violet Acetate by gavage, up to 12 mg/kg bw/day Basic Blue 7 by gavage is not expected to result in any adverse developmental effect, with systemic toxicity including mortality expected at 20 mg/kg bw/day and above (ECHA c2007-2017c). Up to 30 mg/kg bw/day Basic Blue 7 by diet is not expected to result in reproductive effects, based on a 3-generation study with Basic Violet 3 in rats (Littlefield 1988; JECFA 2014). The in vitro and in vivo genotoxicity of Basic Blue 7 is expected to be negative, given the negative in vitro genotoxicity of Basic Violet 4 (Seifried et al. 2006; ECHA c2007-2017b) and Basic Blue 26 (Nagai 1959; Janik-Spiechowicz et al. 1997) and the negative in vivo genotoxicity of Basic Violet 3 or Basic Blue 26 (Janik-Spiechowicz et al. 1997). On the basis of a 2-year carcinogenicity study in mice exposed to dietary Basic Violet 3, Basic Blue 7 is expected to increase erythropoiesis in the spleen and atrophy of the ovaries in females at 14 mg/kg bw/day and above and to increase the incidence of hepatocellular tumours at 36 mg/kg bw/day and above, with a BMDL10 of 16.8 mg/kg bw/day for increased risk of hepatocellular carcinomas in female mice (Littlefield 1984; Littlefield et al. 1985; JECFA 2014). In addition to similarities in the chemical structure and physical-chemical properties of Basic Violet 3 and Basic Blue 7, the applicability of Basic Violet 3 data to characterize the carcinogenic potential of Basic Blue 7 was supported by similarities in their chemical profiles and (Q)SAR model predictions (Derek Nexus 2016; Leadscope Model Applier 2016; OECD QSAR Toolbox 2013; Times 2016). As in the case of Basic Violet 4, the metabolic pathways predicted by Times 2016 indicate that Basic Blue 7 may undergo the same metabolic transformations as Basic Violet 3 (Docampo and Moreno 1990), which further supports the read-across from Basic Violet 3 to Basic Blue 7 in the absence of a substance-specific carcinogenicity study.
Brilliant Blue FCF
Brilliant Blue FCF was reviewed by JECFA (1969), EFSA (2010), and US EPA (2013). It was poorly absorbed orally (2% in bile-duct ligated female rats) and excreted almost entirely (95.5% to 99.99%) as the parent compound in feces within three days (Brown et al. 1980; Phillips et al. 1980; EFSA 2010). There were two in vitro dermal absorption studies with Brilliant Blue FCF, as discussed in section 8.2 (SCCNFP 2004; Lucová et al. 2013). No adverse effects were observed in rats administered Brilliant Blue FCF in the diet for 75 weeks, establishing a NOAEL of 1500 mg/kg bw/day at the highest tested dose (Mannell et al. 1962; US EPA 2013). It was not mutagenic and it did not induce DNA damage in vitro (Borzelleca et al. 1990) nor micronuclei or DNA damage in mice in vivo (EFSA 2010). Positive in vitro genotoxicity results were attributed to purity of test materials (Borzelleca et al. 1990), which may also account for increased micronuclei in human lymphocytes with Brilliant Blue FCF in vitro (Kus and Eroglu 2015).
In a 2-year dietary study with a reproductive study and in utero phase in rats, there was decreased terminal mean body weight and survival in females at the LOAEL of 1318 mg/kg bw/day and a NOAEL of 631 mg/kg bw/day, with no reproductive effect (Borzelleca et al. 1990; EFSA 2010; US EPA 2013). The NOAEL for males was 1072 mg/kg bw/day. Consistent with such findings, no reproductive effects were observed in a dietary three-generation reproductive toxicity study in rats (US EPA 2013), although there was decreased body weight [nursing offspring and F1 and F2 rats (details regarding age not stated in EFSA review)] at the highest tested dose of 1000 mg/kg bw/day in this limited study) (Bio/dynamics Inc. 1972, 1973). In a reproductive/developmental study in mice offspring, there were no adverse effects at 347 to 1287 mg/kg bw/day in males and females, with potential indications of offspring neurotoxicity only evidenced at high doses of 1032 to 3856 mg/kg bw/day (decreased surface righting reflex at postnatal day 4, decreased horizontal activity at 8 weeks, and increased spontaneous activity in females) (Tanaka et al. 2012).
No tumours were observed at up to the highest tested dose of 2500 mg/kg bw/day in 2-year dietary rat studies or 7354/8966 mg/kg bw/day (males/females) in mice which were limited by a lack of detail including protocol description) (Wilheim and Ivy 1953; Klinke 1955; Hansen et al. 1966; US EPA 2013). It may affect the endocrine system based on Toxcast and Tox21data searches (JMPR 2016). No developmental effects were identified in rat or rabbit studies with Brilliant Blue FCF (Burnett et al. 1974) in studies limited in detail (EFSA 2010; US EPA 2013), nor was developmental neurotoxicity observed in vitro (Lau et al. 2006). On the basis of available information, health effects of concern were not identified for Brilliant Blue FCF.
8.3 Characterization of risk to human health
The points of departure (PODs) selected for risk characterization are summarized in Appendix D. Tables 8-5 to 8-8 provide all the relevant exposure estimates and critical effect level PODs for the substances in the Triarylmethanes Group, as well as resultant margins of exposure. Oral studies are used to characterize hazard following dermal or inhalation exposure, in the absence of route-specific hazard data. For the per event exposure scenarios (for Malachite Green, Basic Violet 4, and Basic Blue 7), a POD from a developmental study is considered relevant to both prenatal and postnatal young, since effects in prenatal young suggest sensitivity of the young.
For daily exposures to Malachite Green, taking into consideration that its genotoxicity profile is mixed, and no carcinogenic effects were observed below 21 mg/kg bw/day, use of the LOAEL of 5 mg/kg bw/day from the developmental toxicity study was considered protective of these effects observed at higher levels of exposure.
Environmental media
| Exposure scenario | Systemic exposure (mg/kg bw /day) | Critical effect level (mg/kg bw /day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Drinking water, Daily,Basic Violet 3 | 0.00010 | LOAEL 0.5 (LTD) | Maternal toxicity (increased mortality, decreased body weight and body weight gain, clinical signs) and decreased fetal body weight | 5000 |
| Drinking water, LADD,Basic Violet 3 | 0.000023 | BMDL10 of 16.8 | Increased hepatocellular carcinomas | 730 000 |
| Drinking water, Daily,Malachite Green | 0.00010 | LOAEL 5 (LTD) | Increased pre-implantation loss, increased early fetal resorptions, decreased fetal survival, decreased fetal body weight, and increased skeletal deviations | 50 000 |
Abbreviations: BMDL10, benchmark dose lower 95% confidence limit for 10% extra risk; LOAEL, lowest observed adverse effect level; LTD, lowest tested dose; MOE, margin of exposure; NOAEL, no observed adverse effect level; POD, point of departure.
These margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases for both non-cancer and cancer effects.
Products available to consumers
| Exposure scenario | Systemic exposure (mg/kg bw/day) | Critical effect level (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Non-medicinal use in a natural health product, Adult, Daily, Brilliant Blue FCF | 2.82 | NOAEL 631 | Decreased body weight and survival in females at 1318 mg/kg bw/day | 220 |
Abbreviations: LOAEL, lowest observed adverse effect level; LTD, lowest tested dose; MOE, margin of exposure; NOAEL, no observed adverse effect level; POD, point of departure.
Other potential oral exposures to Brilliant Blue FCF including through lipstick or lip balms (toddlers, adults), cold or allergy medication (children or adults), and dietary intake through food ranged from 0.1 to 1 mg/kg bw (/day), with MOEs of 630 to 6300. These margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases for non-cancer effects. Furthermore, the estimated oral exposures to Brilliant Blue FCF (including its use in a natural health product) are below the upper bound of the acceptable daily intake (ADI) of 6 mg/kg bw/day established by EFSA, and subsequently adopted by JECFA in 2017 at which time the previous upper bound of the ADI of 12.5 mg/kg bw/day was withdrawn, which is based on a 100-fold uncertainty factor applied to the NOAEL of 631 mg/kg bw/day (EFSA 2010; JECFA 2017). A qualitative risk assessment approach as used by the US EPA (2013) was also considered, since no adverse effects were observed in chronic dietary studies up to 2500 mg/kg bw/day in rats and above 7354 mg/kg bw/day in mice, nor in a 3-generation reproductive toxicity study in rats up to 1000 mg/kg bw/day. However, a quantitative risk assessment was conducted since these other studies were limited in detail, the LOAEL of 1318 mg/kg bw/day was based in part on decreased survival, and the point of departure was in agreement with EFSA (2010).
| Exposure scenario | Systemic exposure (mg/kg bw /day) | Critical effect level (mg/kg bw /day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Hair dye (semi-permanent), Teenager and adult, Daily, Malachite Green | 0.0033 (teen);0.0102 (adult) | LOAEL 5 (LTD) | Increased pre-implantation loss, increased early fetal resorptions, decreased fetal survival, decreased fetal body weight, and increased skeletal deviations | 1500 (teen); 490 (adult) |
| Hair dye (semi-permanent), Teenager and adult, Per Event, Malachite Green | 0.337 (teen);0.282 (adult) | LOAEL 5 (LTD) | Increased pre-implantation loss, increased early fetal resorptions, decreased fetal survival, decreased fetal body weight, and increased skeletal deviations | 15 (teen); 18 (adult) |
| Hair dye (semi-permanent), Teenager and adult,Per Event, Basic Violet 4 | 0.0589 (teen);0.0494 (adult) | NOAEL 12 (HTD) | No observed maternal or developmental effects | 200 (teen); 240 (adult) |
| Hair dye (semi-permanent), Teenager and adult,Per Event, Basic Blue 7 | 0.0177 (teen);0.0148 (adult) | NOAEL 12 (HTD) | No observed maternal or developmental effects | 680 (teen); 810 (adult) |
| Body cream, Infant and adult,Daily,Brilliant Blue FCF | 0.0068 (infant);0.0041 (adult) | NOAEL 12.6a | Decreased body weight and survival in females at 1318 mg/kg bw/day | 1900 (infant); 3100 (adult) |
Abbreviations: HTD, highest tested dose; LOAEL, lowest observed adverse effect level; LTD, lowest tested dose; MOE, margin of exposure; NOAEL, no observed adverse effect level; POD, point of departure.
a NOAEL 631 mg/kg bw/day x 2% oral absorption (Brown et al. 1980), which estimates the internal dose at which the critical health effects were observed.
| Exposure scenario | Systemic exposure (mg/kg bw/day) | Critical effect level (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Hair dye (semi-permanent), LADD,Basic Violet 4 | 0.00134 | BMDL10 16.8 | Increased hepatocellular carcinomas | 13 000 |
| Hair dye (semi-permanent), Teenager and adult, Daily, Basic Blue 7 | 0.000175 (teen);0.000533 (adult) | BMDL10 16.8 | Increased hepatocellular carcinomas | 96 000 (teen)a; 32 000 (adult)a |
Abbreviations: BMDL10, benchmark dose lower 95% confidence limit for 10% extra risk; MOE, margin of exposure; POD, point of departure
a The MOEs presented are considered to be conservative, as the exposures have not been adjusted to lifetime average daily doses (LADDs). Such adjustments were not performed as the MOEs for each individual age group are considered adequate, and the adjustments would result in higher MOEs due to a presumed typical lack of use by younger age groups.
Inhalation risks from daily exposure to Brilliant Blue FCF in perfume (0.00054 or 0.00064 mg/kg bw/day, for adults or teenagers, respectively) would result in MOEs of 1 200 000 and 990 000, respectively, to the NOAEL of 631 mg/kg bw/day. These margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases for non-cancer effects.
The MOEs from dermal exposure to Malachite Green ranged from 15 to 1500 (hair dye) for all age groups. These margins are potentially inadequate to account for uncertainties in the health effects and exposure databases for non-cancer effects, which includes consideration that the LOAEL selected as the point of departure was the lowest tested dose.
Comparison of estimated systemic dermal and oral exposures with the range of critical effect levels results in MOEs as follows: Basic Violet 4 ranged from 200 to 240; Basic Blue 7 ranged from 680 to 810; and Brilliant Blue FCF ranged from 220 to 3100. The potential cancer risk from daily exposures to Basic Violet 4 or Basic Blue 7 resulted in MOEs ranging from 13 000 to 96 000. The MOE for inhalation risk of Brilliant Blue FCF was above 990 000. These MOEs are considered adequate to address uncertainties in the exposure and health effect databases for both non-cancer and cancer effects.
While exposures of the general population to Basic Violet 3, Basic Violet 4, and Basic Blue 7 are not of concern at current levels, these substances are considered to have a health effect of concern based on their potential carcinogenicity. Basic Violet 3 was classified by the European Commission as Carc 2 (or 1B if there is more or equal to 0.1% of Michler’s ketone) (EC 2008). Therefore, there may be a concern for human health if exposures were to increase.
8.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key sources of uncertainty | Impact |
|---|---|
| No chemical-specific empirical dermal absorption data were available, with the exception of Brilliant Blue FCF. | +/- |
| There is a lack of Canadian monitoring data for triarylmethanes in ambient environmental media (e.g., surface water) or drinking water | +/- |
| There are no sub-chronic or chronic animal studies via the dermal or inhalation routes, and limited chronic animal studies via the oral route, for substances in the Triarylmethanes Group. | +/- |
| There are limited reproductive toxicity or developmental studies for substances in the Triarylmethanes Group; for example, no reproductive toxicity study for Malachite Green was identified. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
9. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7. It is concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from Brilliant Blue FCF and Pigment Blue 61. It is concluded that Brilliant Blue FCF and Pigment Blue 61 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that Malachite Green meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this screening assessment, it is concluded that Basic Violet 3, Pigment Blue 61, Basic Violet 4, Basic Blue 7, and Brilliant Blue FCF do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 meet one or more of the criteria set out in section 64 of CEPA. It is concluded that Pigment Blue 61 and Brilliant Blue FCF do not meet any of the criteria set out in section 64 of CEPA. It is also concluded that Basic Violet 3, Malachite Green, Basic Violet 4, and Basic Blue 7 meet the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
ACD/Percepta [prediction module]. c1997-2015. Toronto (ON): Advanced Chemistry Development, Inc.
Aidoo A, Gao N, Neft RE, Schol HM, Hass BS, Minor TY, Heflich RH. 1990. Evaluation of the genotoxicity of Gentian Violet in bacterial and mammalian cell systems. Teratog Carcinog Mutagen. 10:449-462.
[AGDH] Australian government Department of Health. 2014. Crystal Violet and related dyes. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS). [accessed 2016 Apr 19].
Andrews JJ, Johnston RV, Bee DE, Arens JF. 1990. Photodeactivation of ethyl violet: A potential hazard of Sodasorb. Anesthesiology. 72(1):59-64.
Anliker R, Moser P. 1987. The limits of bioaccumulation of organic pigments in fish: their relation to the partition coefficient and the solubility in water and octanol. Ecotoxicol Environ Saf. 13(1):43-52.
Au W, Hsu TC. 1979. Studies on the clastogenic effects of biologic stains and dyes. Environ Mutagen. 1:27-35. [cited in Aidoo et al. 1990].
Au W, Pathak S, Collie CJ, Hsu TC. 1978. Cytogenetic toxicity of Gentian Violet and Crystal Violet on mammalian cells in vitro. Mutat Res. 58:269-276. [cited in Aidoo et al. 1990].
Ballantyne B, Gazzard MF, Swanston DW. 1973. Eye damage caused by Crystal Violet. Proc Br Pharmacol Soc. 49(1):181P-182P. [cited in Diamante et al. 2009].
Baughman GL, Weber EJ. 1994. Transformation of dyes and related compounds in anoxic sediment: kinetics and products. Environ Sci Technol. 28(2):267-76. [cited in EpiSuite c2000-2012].
[BCFBAF] Bioaccumulation Program for Microsoft Windows [estimation model]. 2010. Ver. 3.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Beatson RP. 2012. Retention of MAPBAP acetate on mechanical and de-inked pulp. Unpublished report prepared by Rodger Beatson & Associates. 26 p.
Bergwerff AA, Kuiper RV, Scherpenisse P. 2004. Persistence of residues of malachite green in juvenile eels (Anguilla anguilla). Aquaculture. 233(1-4):55-63.
[BfR] Federal Institute for Risk Assessment. 2007. Introduction to the problems surrounding garment textiles. BfR Information No. 018/2007, 1 June 2007. Berlin (GR):BrR. Available upon request.
Bills TD, Chandler Jr. JH, Marking LL. 1977. Malachite green: its toxicity to aquatic organisms, persistence, and removal with activated carbon. Investigations in Fish Control. 75:1-6.
Bio/dynamics Inc., 1972, 1973. (Unpublished reports cited in catalogue of Food Colours Volume 2. Colour Committee of the International Life sciences Institute). Bio/dynamics Inc., 1972, 1973. Unpublished reports cited in catalogue of Food Colours Volume 2. [cited in EFSA 2010].
[BIOWIN] Biodegradation Probability Program for Microsoft Windows [estimation model]. 2008. Ver. 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Borzelleca JF, Depuka K, Hallagan JB. 1990. Lifetime toxicity/carcinogenicity studies of FD & C Blue No. 1 (Brilliant Blue FCF) in rats and mice. Food Chem Toxicol. 28(4):221-234.
Bose B, Motiwale L, Rao KV. 2005. DNA damage and G2/M arrest in Syrian hamster embryo cells during Malachite green exposure associated with elevated phosphorylation of ERK1 and JNK1. Cancer Lett. 230(2):260-70.
Brown JP, Dorsky A, Enderlin FE, Hale RL, Wright VA, Parkinson TM. 1980. Synthesis of 14C-labelled FD & C Blue No. 1 (Brilliant Blue FCF) and its intestinal absorption and metabolic fate in rats. Food Cosmet Toxicol. 18(1):1-5.
Burnett C, Agersborg H, Borzelleca J, Eagle E, Ebert A, Pierce E, Kirschman J, Scala R. 1974. Teratogenic studies with certified colors in rats and rabbits. Toxicol Appl Pharmacol. 29:121. [cited in Borzelleca et al. 1990].
Bumpus JA, Brock BJ. 1988. Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 54(5):1143-50.
Burnett C, Opdyke D. 1971. Chronic eye irritation and staining properties of some organic colors and lakes. CTFA Cosmet J. 3:18-20. [cited in Borzelleca et al. 1990].
Canada. [1978]. Food and Drug Regulations. C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[CFIA] Canadian Food Inspection Agency. 2010. Food Safety Action Plan report. 2009-2010 targeted surveys chemistry. Food colours used in the production of manufactured foods. Ottawa (ON): CFIA. Report No.: TS-CHEM-09/10-05.
[CFIA] Canadian Food Inspection Agency. 2011. Food Safety Action Plan report. 2010-2011 targeted surveys chemistry. Food colours used in the production of manufactured foods. Ottawa (ON): CFIA. Report No.: TS-CHEM-10/11.
[CFIA] Canadian Food Inspection Agency. 2012. Malachite Green: questions and answers. Chemical hazards and potential carcinogens - Fact sheets. [accessed 2017 Mar 29].
[CFIA] Canadian Food Inspection Agency. 2015. Gentian Violet for use in livestock feeds. RG-8 regulatory guidance: Contaminants in feed. [accessed 2017 Mar 29].
Ciba Ltd., Basel, safety data sheet No. T5150/A, 1989. [cited in Flury and Fuhler 1994].
[CITI] Chemicals Inspection & Testing Institute Japan. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (JP): Japan Chemical Industry Ecology-Toxicology & Information Center.
Cole JG, Mackay D. 2000. Correlating environmental partitioning properties of organic compounds: The three solubility approach. Environ Toxicol Chem. 19(2):265-270.
[ConsExpo] Consumer Exposure Model. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. [cited 2017 Apr 25].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[CPMA] Color Pigments Manufacturers Association. 2016. Unpublished confidential studies submitted to Environment and Climate Change Canada (ECCC) under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division. Submission received on 2016-11-04.
Culp SJ, Blankenship LR, Kusewitt DF, Doerge DR, Mulligan LT, Beland FA. 1999. Toxicity and metabolism of Malachite Green and Leucomalachite green during short-term feeding to Fischer 344 rats and B6C3F1 mice. Chem Biol Interact.122(3):153-170.
Das JK, Sarkarb S, Hossain Sk U, Chakraborty P, Das RK, Bhattacharya. Indian S. J Med Res 137, June 2013, pp 1163-1173. [cited in ECHA 2016].
Derek Nexus [toxicity prediction module]. 2016. Ver. 5.0.2. Leeds (UK): Lhasa Limited.
Dhir SP, Sharma Sk, Munjal VP, Gupa A. 1982. Keratoconjunctivitis sicca following instillation of Gentian Violet. Indian J Ophthalmol. 30:21-22. [cited in Diamante et al. 2009].
Dhamgaye S, Devaux F, Manoharlal R, Vandeputte P, Shah AH, Singh A, Prasad R. 2012. In vitro effect of malachite green on Candida albicans involves multiple pathways and transcriptional regulators UPC2 and STP2. Antimicrob Agents Chemother. 56(1):495-506.
Diamante C, Bergfeld WF, Belsito DV, Klaassen CD, Marks Jr JG, Shank RC, Slaga TJ, Snyder PW, Andersen FA. 2009. Final report on the safety assessment of Basic Violet 1, Basic Violet 3, and Basic Violet 4. Int J Toxicol. 28(suppl 3):193S-204S.
Docampo R, Moreno SN, Gadelha FR, De Souza W, Cruz, FS. 1988. Prevention of Chagas' disease resulting from blood transfusion by treatment of blood: toxicity and mode of action of gentian violet. Biomed Environ Sci. 1(4):406-413.
Docampo R, Moreno SNJ. 1990. The metabolism and mode of action of Gentian Violet. Drug Metab Rev. 22:161-178. [cited in JECFA 2014].
Donya SM, Farghaly AA, Abo-Zeid MA, Aly HF, Ali SA, Hamed MA, El-Rigal NS. 2012. Malachite green induces genotoxic effect and biochemical disturbances in mice. Eur Rev Med Pharmacol Sci. 16(4):469-482. [cited in ECHA 2016].
Droge S, Goss K. 2012. Effect of sodium and calcium cations on the ion-exchange affinity of organic cations for soil organic matter. Environ Sci Technol. 46:5894-5901.
Droge ST, Goss K. 2013. Development and evaluation of a new sorption model for organic cations in soil: contributions from organic matter and clay minerals. Environ Sci Technol. 47:14233-14241.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada. [accessed 2017 April 21].
[EC] European Commission. 2008. Regulation (EC) No 1272/2008 of the European Parliament and of the Council [PDF], of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Off J Eur Union L. 353:1-1355.
[EC] European Commission. 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products [PDF]. Official Journal of the European Union. 342/59-209.
[ECCC] Environment and Climate Change Canada. 2016. Science approach document: ecological risk classification of organic substances. Ottawa (ON) : Government of Canada.
[ECCC] Environment and Climate Change Canada. 2019. Data collected following public comments from the Forest Products Association of Canada (FPAC) on the triarylmethanes draft screening assessment under the Chemicals Management Plan (February-June 2019). Data analyzed by ECCC; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 Apr 25].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2017a. Registered substances database. Search results for CAS RN [1324-76-1]. Helsinki (FI): ECHA. [updated 2015 Dec 27; accessed 2017 Apr 20].
[ECHA] European Chemicals Agency. c2007-2017b. Registered substances database. Search results for CAS RN [63157-72-2]. Helsinki (FI): ECHA. [updated 2015 Dec 27; accessed 2016 Dec 28].
[ECHA] European Chemicals Agency. c2007-2017c. Registered substances database. Search results for CAS RN [6441-82-3]. Helsinki (FI): ECHA. [updated 2015 Dec 27; accessed 2016 Dec 28].
[ECHA] European Chemicals Agency. 2017. Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.11: PBT/vPvB assessment [PDF]. Draft version 3. Helsinki (FI): ECHA.
[ECHA] European Chemical Agency. [modified 2016 Dec 20]. Candidate list of substances of very high concern for authorisation. Helsinki (FI): ECHA. [accessed 2017 Sep 27].
[EFSA] European Food Safety Authority, Panel on Food Additives and Nutrient Sources Added to Food (ANS). 2010. Scientific opinion on the re-evaluation of Brilliant Blue FCF (E 133) as a food additive. EFSA Journal. 8(11):1853.
[EFSA] European Food Safety Authority, Panel on contaminants in the food chain (CONTAM). 2016. Scientific opinion on malachite green in food. EFSA Journal. 14(7):4530.
Enoch SJ, Ellison CM, Schultz TW, Cronin MTD. 2011. A review of the electrophilic reaction chemistry involved in covalent protein binding relevant to toxicity. Crit Rev Toxicol 41(9):783-802.
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1992. Draft guidelines for the assessment of environmental exposure to dyestuffs.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1995. Health & environmental information on dyes used in Canada. An overview to assist in the implementation of the New Substances Notification Regulation under the Canadian Environmental Protection Act. Prepared by the ETAD Canadian Affiliates. July 1995. Report 7/21/95.
[EWG] Environmental Working Group. SkinDeep Cosmetics Database [database]. c2007-2017. Washington (DC): EWG. [accessed 2017 Apr 21].
Federal Registry. 1988. Acid Blue 9. Toxic chemical release reporting, community right-to-know. Fed. Regist. 53:12035-12037. [cited by Flury and Fuhler 1994].
Fessard V, Godard T, Heut S, Mourot A, Poul JM. 1999. Mutagenicity of Malachite Green and Leucomalachite Green in in vitro tests. J Appl Toxicol. 19:421-430. [cited in NTP 2005].
Ferguson LR, Baguley BC. 1988. Verapamil as a co-mutagen in the Salmonella/mammalian microsome mutagenicity test. Mutat Res. 209:57-62. [cited in NTP 2005].
Flury M, Flühler H. 1994. Brilliant Blue FCF as a dye tracer for solute transport studies - a toxicological overview. J Environ Qual. 23:1108-1112.
German-Heins J, Flury M. 2000. Sorption of Brilliant Blue FCF in soils as affected by pH and ionic strength. Geoderma. 97:87-101.
Gerundo N, Alderman DJ, Clifton-Hadely RS, Feist SW. 1991. Pathological effects of repeated doses of malachite green: a preliminary study. J Fish Dis. 14(5):521-532.
Geyer H, Scheunert I, Korte F. 1985. Relationship between the lipid content of fish and their bioconcentration potential of 1,2,4-trichlorobenzene. Chemosphere. 14(5):545-555.
Gobas FA, Morrison HA 2000. Bioconcentration and biomagnification in the aquatic environment. In: Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals, environmental and health sciences. Boca Raton (FL): CRC Press. pp 189-231.
Gopinathan R, Kanhere J, Banerjee J. 2015. Effect of malachite green toxicity on non-target soil organisms. Chemosphere. 120:637-644.
Green FJ. 1990. The Sigma-Aldrich handbook of stains, dyes and indicators. Milwaukee (WI): Aldrich Chemical Company, Inc. [cited in EpiSuite c2000-2012].
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR hydrophobic, electronic and steric constants. Washington (DC): ACS. [cited in EpiSuite c2000-2012].
Hansen WH, Fitzhugh OG, Nelson AA, Davis KJ. 1966. Chronic toxicity of two food colors, Brilliant blue FCF and Indigotine. Toxicol Appl Pharmacol. 8:29-36. [cited in EFSA 2010].
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Government of Canada.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. [modified 2010 Jun 4]. List of veterinary drugs that are authorized for sale by Health Canada for use in food-producing aquatic animals. Ottawa (ON): Government of Canada. [accessed 2017 Mar 29].
Health Canada. [modified 2015a Oct 7]. List of permitted colouring agents. Ottawa (ON): Government of Canada. [accessed 2016 Sep 27].
Health Canada. [modified 2015b Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Government of Canada. [accessed 2017 Apr 21].
Health Canada. 2019. Health Canada warns Canadians of potential cancer risk associated with gentian violet. Ottawa (ON): Government of Canada. [accessed 2019 Jun 25].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2008. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Herbst W, Hunger K. 2004. Industrial organic pigments: production, properties, applications. 3rd ed. Weinheim (DE): Wiley-VCH.
Hernando MD, De Vettori S, Bueno MM, Fernández-Alba AR. 2007. Toxicity evaluation with Vibrio fischeri test of organic chemicals used in aquaculture. Chemosphere. 68(4):724-730.
Hikari USA. 2016. Betta Revive. Hayward (CA): Hikari Sales USA Inc. [accessed 2016 May 27].
Hinton MJ, Eversole AG. 1978. Toxicity of ten commonly used chemicals to American eels [Includes herbicides copper sulfate and Diquat]. Proc Ann Conf S E Assoc Fish & Wildl Agencies. 32:599-604.
Hinton MJ, Eversole AG. 1979. Toxicity of ten chemicals commonly used in aquaculture to the black eel stage of the American eel. J World Aquac Soc. 10(1-4):554-560.
Hodge HC, Indra J, Drobeck HP, Duprey LP, Tainter ML. 1972. Acute oral toxicity of methylrosaniline chloride. Toxicol Appl Pharmacol. 22:1-5. [cited in JECFA 2014].
Hunger K. 2003. Industrial dyes: chemistry, properties, applications. Weinheim (DE): Wiley-VCH.
[IARC] IARC Working Group on the evaluation of carcinogenic risks to humans. 1996. Printing Processes and Printing Inks [PDF]. IARC Monogr Eval Carcinog Risks Hum. 65:33-147.
IHS Markit. 2018. Dyes: CEH Chemical Economic Handbook. London (UK): IHS Markit.
Janik-Spiechowicz E, Dziubałtowska E, Wyszyńska K. 1997. Mutagenic and genotoxic activity detected by the Ames, micronucleus and SCE tests under the influence of samples of dyes manufactured in Poland. Int J Occup Med Environ Health. 10(1):55-65.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1969. Toxicological evaluation of some food colours, emulsifiers, stabilizers, anti-caking agents and certain other substances. Brilliant Blue FCF. WHO technical report series, no. 46A. Prepared by the thirteenth meeting of JECFA. Geneva (CH): World Health Organization.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2009. Evaluation of certain veterinary drug residues in food [PDF]. Malachite Green. WHO technical report series, no. 954. Prepared by the seventieth meeting of JECFA. Geneva (CH): World Health Organization.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2014. Toxicological evaluation of certain veterinary drug residues in food [PDF]. Gentian violet. WHO Food Additives Series, No. 69. Prepared by the seventy-eighth meeting of JECFA. Geneva (CH): World Health Organization.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2017. Toxicological information, dietary exposures and information on specifications. Eighty-fourth meeting, Rome, 6-15 June 2017. Summary and Conclusions. Geneva (CH): World Health Organization.
Jiang Y, Xie P, Liang G. 2009. Distribution and depuration of the potentially carcinogenic malachite green in tissues of three freshwater farmed Chinese fish with different food habits. Aquaculture. 288(1-2):1-6.
[JMPR] Joint FAO/WHO Meeting on Pesticide Residues. 2016. Pesticide residues in food, evaluations. Part II: Toxicological. Geneva (CH): World Health Organization. Jointly sponsored by the Food and Agriculture Organization of the United Nations and the World Health Organization.
Kah M, Brown CD. 2006. Adsorption of ionisable pesticides in soils. Rev Environ Contam Toxicol. 188:149-217.
Kanhere J, Gopinathan R, Banerjee J. 2014. Cytotoxicity and genotoxicity of malachite green on non-target aquatic organisms: Chlorella pyrenoidosa and Daphnia magna. Water Air Soil Pollut. 225(9):2134.
Kasem H, Ibrahim AE, Rania HA, El Hady KA. 2016. In vivo toxicity study of malachite green in mice: Estimation of hepatotoxicity, oxidative stress and genotoxicity. Int. J PharmTech Res. 9(3):58-67.
Klinke. 1955. Referred to by EFSA 2010 but not included in reference list.
[KOAWIN] Octanol-Air Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[KOCWIN] Organic Carbon Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kus E, Eroglu HE. 2015. Genotoxic and cytotoxic effects of sunset yellow and brilliant blue, colorant food additives, on human blood lymphocytes. Pak J Pharm Sci. 28(1):227-230.
Lanzing WJR. 1965. Observations on malachite green in relation to its application to fish diseases. Hydrobiologia. 25(3):426-441.
Lau K, McLean WG, Williams DP, Howard CV. 2006. Synergistic interactions between commonly used food additives in a developmental neurotoxicity test. Toxicol Sci. 90:178-187. [cited in EFSA 2010].
Leadscope Model Applier [prediction module]. 2016. Ver. 2.1. Columbus (OH): Leadscope, Inc. [restricted access].
Littlefield NA. 1984. Chronic toxicity and carcinogenicity studies of Gentian Violet in mice. Final report. Jefferson (AR): National Center for Toxicological Research, Division of Chemical Toxicology (NCTR technical report for experiment 304). [cited in JECFA 2014].
Littlefield NA. 1988. Three-generation reproduction and toxicity studies of Gentian Violet in Fischer 344 rats. Jefferson (AR): National Center for Toxicological Research (NCTR technical report for experiments 305, 354, 355). [cited in JECFA 2014].
Littlefield NA, Blackwell BN, Hewitt CC, Gaylor DW. 1985. Chronic toxicity and carcinogenicity studies of Gentian Violet in mice. Fundam Appl Toxicol. 5(5):902-912. [cited in JECFA 2014].
Littlefield NA, Gaylor DW, Blackwell BN, Allen RR. 1989. Chronic toxicity/carcinogenicity studies of Gentian Violet in Fischer 344 rats: two-generation exposure. Food Chem Toxicol. 27:239-47. [cited in JECFA 2014].
Liu H, Yang S, Ni Y. 2007. Using dyes for improving the optical properties of high yield pulps. Pulp & Paper Canada. 108(10):25-29.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2018 Jan 15].
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279-291.
Lucová M, Hojerová J. Pažoureková S, Klimová A. 2013. Absoprtion of triphenylmethane dyes Brilliant Blue and Patent Blue through intact skin, shaven skin and lingual mucosa from daily life products. Food Chem Toxicol. 52:19-27.
Lu FC, Lavallée A. 1964. The acute toxicity of some synthetic colours used in drugs and food. Can Pharm J. 97:30. [cited in Borzelleca et al. 1990].
Mani S, Bharagava RN. 2016. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev Environ Contam Toxicol. 237:71-104.
Mannell WA, Grice H, Allmar M. 1962. Chronic toxicity studies on food colours. V. Observations on the toxicity of Brilliant Blue FCF, Guinea Green B and Benzyl Violet 4B in rats. J Pharm Pharmac. 14:378-384. [cited in Borzelleca et al. 1990].
McDonald JJ. 1989. Metabolism of Gentian Violet in Fischer 344 rats and B6C3F1 mice. Jefferson (AR): National Center for Toxicological Research (NCTR technical report for experiments 302, 303). [cited in JECFA 2014].
McDonald JJ, Cerniglia CE. 1984. Biotransformation of Gentian Violet to Leucogentian Violet by human, rat and chicken intestinal microflora. Drug Metab Dispos. 12(3):330-336. [cited in JECFA 2014].
McDonald JJ, North CR, Breeden CR, Lai CC, Roth RW. 1984. Synthesis and disposition of 14C-labelled Gentian Violet in F344 rats and B6C3F1 mice. Food Chem Toxicol. 22:331-336. [cited in JECFA 2014].
Meyer FP, Jorgenson TA. 1983. Teratological and other effects of Malachite Green on development of rainbow trout and rabbits. Trans Am Fish Soc. 112:818-824.
Mittelstaedt RA, Mei N., Webb PJ, Shaddock JG, Dobrovolsky VN, McGarrity LJ, Morris SM, Chen T, Beland FA, Greenlees KJ, Heflich RH. 2004. Genotoxicity of malachite green and leucomalachite green in female Big Blue B6C3F1 mice. Mutat Res. 561:127-138.
Mon J, Flury M, Harsh JB. 2006. A quantitative structure-activity relationships (QSAR) analysis of triarylmethane dye tracers. J Hydrol. 316(1-4):84-97.
Müller W, Gautier F. 1975. Interactions of heteroaromatic compounds with nucleic acids. A- T-specific non-intercalating DNA ligands. Eur J Biochem. 54(2):385-394. [cited in Docampo and Moreno 1990].
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2008. Ver. 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[MSDS] Material Safety Data Sheet. 2014. Elmer’s Color Change Markers. Columbus (OH): Elmer’s Products Inc. [accessed 2016 May 27].
Nagai S. 1959. Induction of the respiration-deficient mutation in yeast by various synthetic dyes. Science. 130:118-1189. [cited in Janik-Spiechowicz et al. 1997].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 Oct 23]. Ottawa (ON): Government of Canada. [accessed 2018 Jan 15].
Niska K, Korkea-aho T, Lindfors E, Kiuru T, Tuomainen M, Taskinen J, Peltonen K. 2009. Disappearance of malachite green residues in fry of rainbow trout (Oncorhynchus mykiss) after treatment of eggs at the hatching stage. Aquaculture. 297(1-4):25-30.
[NITE] National Institute of Technology and Evaluation. 2002. [cited in OECD QSAR Toolbox v3.4.0.17].
[NPRI] National Pollutant Release Inventory. 1993-2015. NPRI Dataset: bulk data, substance releases normalized since 1993. Gatineau (QC): Environment and Climate Change Canada. Search results for CAS RN 569-64-2. [accessed 2017 Apr 3].
[NTP] National Toxicology Program (US). 1982. Teratologic evaluation of Gentian Violet (CAS No. 548-62-9) in CD rats. Final report. Research Triangle Park (NC): United States Department of Health and Human Services, Public Health Service, National Toxicology Program. Report No.: TER82079. [cited as Wolkowski-Tyl in JECFA 2014].
[NTP] National Toxicology Program (US). 1983. Teratologic evaluation of Gentian Violet (CAS No. 548-62-9) in New Zealand white rabbits. Final report. Research Triangle Park (NC): United States Department of Health and Human Services, Public Health Service, National Toxicology Program. Report No.: TER82080. [cited as Wolkowski-Tyl in JECFA 2014].
[NTP] National Toxicology Program. 2004. NTP Technical Report on the toxicity studies of Malachite Green Chloride and Leucomalachite Green (CAS Nos. 569-64-2 and 129-73-7) in F344/N rats and B6C3F1 mice (feed studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Report No.: 04-4416.
[NTP] National Toxicology Program. 2005. NTP Technical Report on the toxicology and carcinogenesis studies of Malachite Green Chloride and Leucomalachite Green (CAS Nos. 560-64-2 and 129-73-7) in F344/N rats and B6C3F1 mice (feed studies). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Report No.: TR 527.
[OECD] Organisation for Economic Co-operation and Development. 2009. Emission scenario document on pulp, paper and board industry. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 23). Report No.: ENV/JM/MONO(2009)24.
OECD QSAR Toolbox. [read across tool]. 2013. Ver. 3.2. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ogugbue CJ, Sawidis T. 2011. Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent. Biotechnol Res Int. 2011:1-11.
Panandiker A, Mauru GB, Rao KVK. 1994. Dose-response effects of Malachite Green on free radical formation, lipid peroxidation and DNA damage in Syrian hamster embryo cells and their modulation nby antioxidants. Carcinogen. 15(11):2445-2448.
Pagga U, Brown D. 1986. The degradation of dyestuffs: Part II behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere. 15(4):479-491.
Perez-Estrada LA, Aguera A, Hernando MD, Malato S, Fernandez-Alba AR. 2008. Photodegradation of malachite green under natural sunlight irradiation: Kinetic and toxicity of the transformation products. Chemosphere. 70(11):2068-75.
Phillips JC, Mendis D, Eason CT, Gangolli SD.1980. The metabolic disposition of 14C-labelled Green S and Brilliant Blue FCF in the rat, mouse and guinea-pig. Food Cosmet Toxicol. 18(1):7-13.
[PMRA] Pest Management Regulatory Agency. 2010. PMRA list of formulants [PDF]. Ottawa (ON): Government of Canada. HC Pub. No.: 100460, Cat. No.: H114-22/2010E. [accessed 2017 Apr 7].
Poe WE, Wilson RP. 1983. Absorption of malachite green by channel catfish. Prog Fish Cult. 45(4):45228-229.
Princz J, Bonnell M, Ritchie E, Velicogna J, Robidoux P, Scroggins R. 2014. Estimation of the bioaccumulation potential of a non-chlorinated bisphenol and an ionogenic xanthene dye to Eisenia andrei in field-collected soils, in conjunction with predictive in silico profiling. Environ Toxicol Chem. 33(2):308-316.
Ross LG, Ward KMH, Ross B. 1985. The effects of formalin, malachite green and suspended solids on the respiratory activity of rainbow trout, Salmo gairdneri Richardson. Aquac Res. 16(2):129-138.
[SCCNFP] Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. 2004. Opinion of the SCCNFP concerning Acid Blue 9. COLIPA No. C40. SCCNFP/0787/04. Brussels (BE): European Commission.
[SCCS] Scientific Committee on Consumer Safety. 2016. Revision of the SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation, 9th revision [PDF]. Brussels (BE): European Commission.
[SCBT] Santa Cruz Biotechnology. c2007-2017. Victoria Blue BO (CAS 2390-60-5). Dallas (TX).
Schenker U, Macloed M, Cheringer M, Hungerbühler K. 2005. Improving data quality for environmental fate models: A lest-squares adjustment procedure for harmonizing physicochemical properties of organic compounds. Environ Sci Technol. 39(21):8434-8441.
Seifried HE, Seifried RM, Clarke JJ, Junghans TB, San RHC. 2006. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol. 19:627-644.
[Sigma-Aldrich]: Sigma-Aldrich Co. LLC. c2017. Oakville (Ont). [accessed 2017 Aug 28].
Srivastava S, Sinha R, Roy D. 2004. Toxicological effects of malachite green. Aquat Toxicol. 66(3):319-329.
Statistics Canada. 2004. Canadian Community Health Survey - Nutrition (CCHS). Detailed information for 2004 (Cycle 2.2) [Internet]. Ottawa (ON): Statistics Canada.
Statistics Canada. 2012. Canadian Health Measure Survey (CHMS) - Cycle 1. Ottawa (ON): Statistics Canada.
Stammati A, Nebbia C, De Angelis I, Albo AG, Carletti M, Rebecchi C, Zampaglioni F, Dacasto M. 2005. Effects of Malachite Green (MG) and its major metabolite, Leucomalachite Green (LMG), in two human cell lines. Toxicol in Vitro. 19(7):853-858.
Sundarrajan M, Fernandis AZ, Subrahmanyam G, Prabhudesai S, Krishnamurthy SC, Rao KV. 2000. Overexpression of G1/S cyclins and PCNA and their relationship to tyrosine phosphorylation and dephosphorylation during tumor promotion by Metanil Yellow and Malachite Green. Toxicol Lett. 116: 119-130.
Tanaka T, Takahasi O, Inomata A, Ogata A, Nakae D. 2012. Reproductive and neurobehavioral effects of Brilliant Blue FCF in mice. Birth Defects Res B Dev Reprod Toxicol. 95(6):395-409.
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2016. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
Tonogai Y, Ogawa S, Ito Y, Iwaida M. 1982. Actual survey on TLm (median tolerance limit) values of environmental pollutants, especially on amines, nitriles, aromatic nitrogen compounds and artificial dyes. J Toxicol Sci. 7(3):193-302.
Tsai RS, El Tayar N, Testa B. 1991. Toroidal coil centrifugal partition chromatography, a method for measuring partition coefficients. J Chromatogr. 538(1):119-123. [cited in EpiSuite c2000-2012].
[US EPA] US Environmental Protection Agency. 2002. Flexographic ink options: A cleaner technologies substitutes assessment [PDF]. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
[US EPA] US Environmental Protection Agency. 2011. Age dependent Adjustment Factor (ADAF) application. Final Report. Washington (DC): US EPA, Office of Water Policy.
[US EPA] US Environmental Protection Agency. 2013. FD&C Blue No. 1; exemption from the requirement of a tolerance. Federal Register. 78(186):58886-58890.
Van Heerden E, Van Vuren JHJ, Steyn GJ. 1995. LC50 determination for malachite green and formalin on rainbow trout (Oncorhynchus mykiss) juveniles. Water SA. 21(1):87-94.
Wakelin LPG, Adams A, Hunter C, Waring MJ. 1981. Interaction of Crystal Violet with nucleic acids. Biochemistry. 20:5779-5787. [cited in Docampo and Moreno 1990].
Wan MT, Watts RG, Moul DJ. 1991. Acute toxicity to juvenile pacific northwest salmonids of basacid blue NB755 and its mixture with formulated products of 2,4-D, Glyphosate, and Triclopyr. Bull Environ Contam Toxicol. 47(3):471-478.
[WATERNT] Water Solubility Program [estimation model]. 2010. Ver. 1.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
White CR, Davies SJ, Henry TB. 2012. Malachite green toxicity and effects on reproductive success in zebrafish Danio rerio. Zebrafish. 9(3):135-139.
Willheim R, Ivy AC. 1953. A preliminary study concerning the possibility of dietary carcinogenesis. Gastroenterology. 23:1-19. [cited in EFSA 2010].
Williams FM, Rothe H, Barrett G, Chiodini A, Whyte J, Cronin MTD, Monteiro-Riviere NA, Plautz J, Roper C, Westerhout J. 2016. Assessing the safety of cosmetic chemicals: consideration of a flux decision tree to predict dermally delivered systemic dose for comparison with oral TTC (Threshold of Toxicological Concern). Regul Toxicol Pharmacol. 76:174-186.
Wolfe AD. 1977. Influence of cationic triphenylmethane dyes upon DNA polymerization and product hydrolysis by Escherichia coli polymerase I. Biochemistry. 16(1):30-33. [cited in NTP 2005].
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. 2006. What are the sources of exposure to eight frequently used pthalic acid esters in Europeans? Risk Anal 26(3):803-824.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [estimation model]. 2010. Ver. 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Xie J, Peng T, Chen DD, Zhang QJ, Wang GM, Wang X, Guo Q, Jiang F, Chen D, Deng JJ. 2012. Determination of malachite green, crystal violet and their leuco-metabolites in fish by HPLC-VIS detection after immunoaffinity column clean-up. J Chromatogr B Analyt Technol Biomed Life Sci. 913-914:123-128.
Xu R, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. 2005. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 65(2):613-621.
Yasuhiro, N. 1984. Toxicity of agrochemicals to freshwater organisms. CV. Dyes. (in Japanese) Suisan Zoshoku. 32:173-175. [cited in Flury and Fuhler 1994].
Zhijun T, Lihong X, Mengmeng G, Hongyan W, Yanhua J, Zhaoxin L, Yuxiu Z. 2011. Persistence of malachite green and leucomalachite green in perch (Lateolabrax japonicus). Chin J Oceanol Limnol. 29(3):647-655.
Appendix A. Aquatic toxicity
| Common name | Test organism | Endpoint | Effect | Value (mg/L) | Reference |
|---|---|---|---|---|---|
| Basic Violet 3 | Japanese rice fish (Oryzias latipes) | 48-h LC50 | Mortality | 0.1 | Tonogai et al. 1982 |
| Malachite Green | Rainbow trout (Oncorhynchus mykiss) | 96-h LC50 | Mortality | 0.25 | Bills et al. 1977 |
| Malachite Green | Bluegill (Lepomis macrochirus) | 96-h LC50 | Mortality | 0.03 | Bills et al. 1977 |
| Malachite Green | Brown trout (Salmo trutta morpha lacustris) | 96-h LC50 | Mortality | 0.22 | Bills et al. 1977 |
| Malachite Green | Smallmouth bass (Micropterus dolomieu) | 96-h LC50 | Mortality | 0.045 | Bills et al. 1977 |
| Malachite Green | American eel (Anguilla rostrata) | 96-h LC50 | Mortality | 0.27 | Hinton and Eversole 1978 |
| Malachite Green | American eel (Anguilla rostrata) | 96-h LC50 | Mortality | 2.86 | Hinton and Eversole 1978 |
| Malachite Green | Japanese rice fish (Oryzias latipes) | 48-h LC50 | Mortality | 0.32 | CITI 1992 |
| Malachite Green | Bacteria (Vibrio fischeri) | 30-min EC50 | Reduction in biolumines-cence | 0.031 | Hernando et al. 2007 |
| Malachite Green | Rainbow trout (Oncorhynchus mykiss) | 96-h LC50 | Mortality | 0.267 | Van Heerden et al. 1995 |
| Malachite Green | Japanese rice fish (Oryzias latipes) | 48-h LC50 | Mortality | 0.3 | Tonogai et al. 1982 |
| Malachite Green | American eel (Anquilla rostrata) | 96-h LC50 | Mortality | 0.54 | Hinton and Eversole 1979 |
| Malachite Green | Zebrafish embryos(Danio rerio) | 96-h LC50 | Mortality | 0.042 | White et al. 2012 |
| Malachite Green | Zebrafish larvae(Danio rerio) | 96-h LC50 | Mortality | 0.376 | White et al. 2012 |
| Malachite Green Oxalate | Zebrafish embryo(Danio rerio) | 96-h LC50 | Mortality | 0.331 | White et al. 2012 |
| Malachite Green Oxalate | Zebrafish embryo(Danio rerio) | 96-h LC50 | Mortality | 0.264 | White et al. 2012 |
| Malachite Green | Water flea (Daphnia magna) | 48-h EC50 | Growth | 0.77 | Kanhere et al. 2014 |
| Pigment Blue 61 | Fish | Acute | NA | <70 | US EPA 2002 |
| Pigment Blue 61 | Invertebrates | Acute | NA | <70 | US EPA 2002 |
| Pigment Blue 61 | Algae | Acute | NA | <10 | US EPA 2002 |
| Pigment Blue 61 | Fish | Chronic | NA | <7 | US EPA 2002 |
| Pigment Blue 61 | Invertebrates | Chronic | NA | <7 | US EPA 2002 |
| Pigment Blue 61 | Algae | Chronic | NA | <1 | US EPA 2002 |
| Pigment Blue 61 | Water flea (Daphnia magna) | 48-h EC50 | Immobiliza-tion | >0.048 | CPMA 2016 |
| Pigment Blue 61 | Algae (Pseudokirchneriella subcapitata) | 72-h NOEC | Growth rate reduction | 0.422 | CPMA 2016 |
| Pigment Blue 61 | Algae (Pseudokirchneriella subcapitata) | 72-h NOEC | Yield inhibition | <0.422 | CPMA 2016 |
| Pigment Blue 56 (analogue for Pigment Blue 61) | Zebrafish(Brachydanio rerio) | 96-h LC50 | Mortality | >500 | CPMA 2016 |
| Brilliant Blue FCF | Japanese rice fish(Oryzias latipes) | 48-h LC0 | Mortality | >3000 | Tonogai et al. 1978 |
| Brilliant Blue FCF | Daphnia sp. | 48-h LC50 | Mortality | >1000 | Federal Registry 1988 |
| Brilliant Blue FCF | Sewage bacteria | NA | Reduction of activity | >300 | Ciba 1989 |
| Brilliant Blue FCF | Guppy(Poecilia reticulata) | 48-h LC0 | Mortality | >500 | Ciba 1989 |
| Brilliant Blue FCF | Snail(Indoplanorbis exustus) | 48-h LC50 | Mortality | >100 | Yasuhiro 1984 |
| Brilliant Blue FCF | Daphnia carinata | 3-h LC50 | Mortality | >100 | Yasuhiro 1984 |
| Brilliant Blue FCF | Nagoya daruma pond frog(Rana brevipoda porosa) | 48-h LC50 | Mortality | >100 | Yasuhiro 1984 |
| Brilliant Blue FCF | Japanese common toad(Bufo bufo japanicus) | 48-h LC50 | Mortality | >100 | Yasuhiro 1984 |
| Brilliant Blue FCF | Common carp(Cyprinus carpio) | 48-h LC50 | Mortality | >100 | Yasuhiro 1984 |
| Brilliant Blue FCF | Coho salmon(Oncorhynchus kisutch) | 96-h LC50 | Mortality | 116 | Wan et al. 1991 |
| Brilliant Blue FCF | Chinook salmon(Oncorhynchus tshawytscha) | 96-h LC50 | Mortality | 185 | Wan et al. 1991 |
| Brilliant Blue FCF | Chum salmon (Oncorhynchus keta) | 96-h LC50 | Mortality | 213.5 | Wan et al. 1991 |
| Brilliant Blue FCF | Pink salmon (Oncorhynchus gorbuscha) | 96-h LC50 | Mortality | 119.5 | Wan et al. 1991 |
| Brilliant Blue FCF | Rainbow trout (Oncorhynchus mykiss) | 96-h LC50 | Mortality | 206 | Wan et al. 1991 |
| Brilliant Blue FCF | Sockeye salmon(Oncorhynchus nerka) | 96-h LC50 | Mortality | 90 | Wan et al. 1991 |
Abbreviations: NA, not available; NOEC: no observed effect concentration; LCx, lethal concentration for x% of the population; ECx, effect concentration for x% of the population.
Appendix B. Assumptions used in ecological exposure scenarios
| Variable name | Information type | Value | Unit | Data source |
|---|---|---|---|---|
Paper production capacity | Mill specific values | CBI | t/day | ECCC compiled data |
Dye product use rate | Distribution | P10 = 0.03 P50 = 0.09 P90 = 0.33 | kg/t | OECD 2009 and personal communication, e-mail from supplier, to the Environmental Stewardship Branch, Environment Canada, 2013; unreferenced. |
Fraction of a paper product containing the substance | Single value | 1 | Fraction | Assumption. In a worst-case scenario, assuming a facility uses the chemical to dye all its paper to some extent. |
Fraction of a mill’s feedstocks that are from recycled paper | Mill specific values | CBI | Fraction | ECCC compiled data |
Fraction of the non-sulfonated triarylmethane dye substance in a dye product | Distribution | P10 = 0.27 P50 = 0.47 P90 = 0.64 | Fraction | ECCC 2019, IHS Markit 2018 |
Fraction of the chemical (Brilliant Blue FCF) in a dye product | Distribution | P10 = 0.29 P50 = 0.51 P90 = 0.75 | Fraction | IHS Markit 2018 and professional assumptions. |
Retention rate | Distribution | P10 = 0.89 P50 = 0.95 P90 = 0.99 | Fraction | Beatson 2012, OECD 2009 |
Removal rate of a secondary WWTSa | Distribution | P10 = 0.82 P50 = 0.88 P90 = 0.92 | Fraction | SimpleTreat models were run for applicable removal rates for secondary treatment systems. |
Removal rate of a lagoon WWTSa | Distribution | P10 = 0.84 P50 = 0.86 P90 = 0.88 | Fraction | STP-EX models were run for applicable removal rates for lagoon systems. |
Removal rate of a primary WWTSa | Distribution | P10 = 0.51 P50 = 0.59 P90 = 0.67 | Fraction | SimpleTreat models were run for applicable removal rates for primary treatment systems. |
Effluent flow rate | Mill specific values | CBI | m3/s | ECCC compiled data |
Flow rate for the receiving water body | Values based on mill locations | CBI | m3/s | ECCC compiled data. 10th percentile flow rate is used. |
Dilution factor | Values based on mill locations | Maximum 10 | Unitless | Actual dilution factor is used when DF is below 10. Maximum DF of 10 is used when actual DF is greater than 10. |
Abbreviations: CBI, confidential business information; DF, dilution factor; ECCC, Environment and Climate Change Canada; P10, P50, and P90, values at the 10th percentile, 50th percentile, and 90th percentile of a distribution; WWTS, wastewater treatment system.
a Treatment type used in the calculation is dependent on the type of treatment system that is associated with each mill, whether on-site or off-site treatment or both, as relevant.
| Variable name | Information type | Range of values | Units | Data source |
|---|---|---|---|---|
| Capacity of a facility to de-ink paper | Mill specific values | CBI | t/day | ECCC compiled data |
| Fraction of paper that has been covered in ink | Uniform distribution | 5–50 | % | Assumptions |
| Amount of coverage that can be expected on a piece of paper for a given amount of Ink | Uniform distribution | 150 000–350 000 | in2 [paper] /lbs [ink] | Assumptions |
| Density of paper | Uniform distribution | 75–105 | g/m2 | ECCC compiled data |
| Concentration fraction of chemical in ink | Uniform distribution | 0.0001–0.02 | Fraction | ECCC compiled data |
| Fraction of ink with chemical of interest in it | Uniform distribution | 0.01–1.0 | Fraction | Assumptions |
| Emission factor | Uniform distribution | 0.1–0.2 | Fraction | ECCC compiled data |
| Effluent flow rate | Mill specific values | CBI | m3/s | ECCC compiled data |
| Flow rate for the receiving water body | Values based on mill locations | CBI | m3/s | ECCC compiled data; 10th percentile flow rate is used. |
| Dilution factor | Values based on mill locations | Maximum 10 | Unitless | Actual dilution factor is used when DF is below 10. Maximum DF of 10 is used when actual DF is greater than 10. |
Abbreviations: CBI, confidential business information; DF, dilution factor; ECCC, Environment and Climate Change Canada.
Appendix C. Estimated potential human exposures to triarylmethanes from products used by consumers
All assumptions (Table C-2) were ConsExpo default assumptions (ConsExpo 2006) unless otherwise noted. For dermal exposure estimates, an overall retention factor (RF) of 1 was used, unless otherwise specified. Inhalation absorption was assumed to be 100%.
Maximum Flux Approach
As a refinement, the maximum flux (Jmax) approach as conducted in Williams et al. (2016) was used to estimate dermal exposures for Malachite Green, Basic Violet 4 and Basic Blue 7.
The equations used are provided below. Values for water solubility, log Kow, and molecular weight (MW) were obtained from Table 3-1 of this screening assessment report (where available, experimental values were used). A mass balance check was also done for each scenario; see Table C-2 below.
Kp (Potts & Guy equation, based on aqueous vehicle):
log Kp (in cm/h) = -2.71 + (0.71)(log Kow) - (0.0061)(MW, in g/mol)
Jmax:
Jmax (in mg/cm2/h) = Kp (in cm/h) x Water solubility (in mg/cm3)
Maximum theoretical amount absorbed per day (Qabs):
Qabs (in mg) = Jmax (in mg/cm2/h) x Surface area of skin contact (in cm2) x Exposure duration (in h)
Dermal Systemic Exposure = Qabs/BW
The resulting dermal systemic exposure estimate represents a “per event” estimate where exposure frequency “F” is < 1/day or represents a “daily” estimate where “F” is ≥ 1/day. However, it should be noted that there are no exposure scenarios for Malachite Green, Basic Violet 4, or Basic Blue 7 that fall into the latter category. Amortization of a “per event” estimate to generate a daily systemic exposure estimate was performed where relevant.
A mass balance check was conducted by comparing the Qabs to the total amount of the substance on the skin (Qapp; which is referred to in Table C-2 as the “dermal load”)
For mass balance check:
Qapp = Conc x Product Amount x RF (see individual exposure scenarios in Table C-2 for specific values; note that F is not applicable in the mass balance check since there are no exposure scenarios where exposure frequency exceeds once per day)
If the Qabs > Qapp, then Qapp (equivalent to 100% dermal absorption) was used to characterize the amount absorbed. Otherwise, Qabs was used.
| Substance and sentinel exposure scenario | Age group(s) | Jmax (mg/cm2/h) | Qabs (mg) |
|---|---|---|---|
| Malachite Green, semi-permanent hair dye | Teenager, Adult | 0.0013 | 20 |
| Basic Violet 4, semi-permanent hair dye | Teenager, Adult | 8.7x10-4 | 13 |
| Basic Blue 7, semi-permanent hair dye | Teenager, Adult | 0.072 | 1.1 x 103 |
a See exposure scenarios in Table C-2 for frequency (F), if relevant.
b See Table C-2 for details on the per event and daily exposure scenarios.
Lifetime average daily dose (LADD)
The LADD was calculated as a refinement for dermal exposure to semi-permanent hair dye exposures to Basic Violet 4 to account for use of this product by teenagers and adults, as well as to estimate the potential cancer risk from daily exposure to Basic Violet 3 from drinking water. The assumptions and equation are provided below:
DSE: daily systemic exposure
Average lifetime (AL): 70 years (US EPA 2011)
Age group durations (AD): 0.5 years for infants (0 to 0.5 years), 4.5 years for toddlers (0.5 to 4 years), 7 years for children (5 to 11 years), 8 years for teenagers (12 to 19 years) and 50 years for adults (20+ years) (Health Canada 1998)
LADD = [[(DSEinfant x ADinfant) + (DSEtoddler x ADtoddler) + (DSEchild x ADchild) + (DSEteen x ADteen) + (DSEadult x ADadult)] / [AL]
Dermal exposures to Brilliant Blue FCF
The potential absorbable dose of Brilliant Blue FCF from the dermal flux study by Lucová et al. (2013) was used to characterize systemic exposures for each dermal scenario. The following parameters, algorithms and considerations were used.
AV: skin surface area exposed
PAA: potential absorbable dose (over 24 hours of exposure)
F: exposure frequency
Conc: concentration
RF: Retention factor
Per Event Systemic Exposure = (AV x PAA)/BW
For mass balance check:
(Total) Dermal Load = Conc x Product Amount x RF x F
(where “F” is only incorporated if >1)
If the per event systemic exposure was less than the (total) dermal load, the per event systemic exposure was used to characterize systemic exposure given the lack of full dose depletion, otherwise the (total) dermal load was used (due to full dose depletion). Where “F” is >1/day, the per event systemic exposure can be used as a daily systemic exposure estimate as “PAA” represents the cumulative amount absorbed over 24 hours.
| Substance | Sentinel exposure scenario | Assumptionsa |
|---|---|---|
Malachite Green, Basic Violet 4, and Basic Blue 7 | Hair dye (semi-permanent) | Concentration: £1% for Malachite Green, £0.1% for Basic Violet 4, and £0.03% for Basic Blue 7 (2016 email from Consumer Product Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) Age group: Teenager and adult Body weight (BW): 59.4 kg for teenager and 70.9 kg for adult Frequency (F): 0.0099/day for teenager (Statistics Canada 2012)b and 0.036/day for adult (SCCS 2016) Product amount: 35 g/application (SCCS 2016) Retention factor (RF): 0.1 (SCCS 2016) Surface area of skin contact (SA): 637.5 cm2 (Based on ½ surface area of adult head; Health Canada 1995) Exposure duration: 24 h/day
For Per Event and Daily Dermal Exposure, see Jmax approach above (Williams et al. 2016)
For mass balance check (Per Event and Daily Dermal Exposure): Dermal load = Conc x Product Amount x RF |
Brilliant Blue FCF | Non-medicinal use in a natural health product | Amount per dose (Amt): 200 mg/scoop (2017 email from Natural and Non-prescription Health Products Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) Maximum Daily Dose (MDD) = 1 scoop (2017 email from Natural and Non-prescription Health Products Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) Age group: Adult Body weight (BW): 70.9 kg Frequency (F) = 1/day
For Estimated Daily Oral Exposure: = (Amt x MDD x F)/(BW) |
Brilliant Blue FCF | Body cream | Concentration (Conc): £10% (2016 email from Consumer Product Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) Age group: Infant and Adult Body weight (BW): 7.5 kg for Infant and 70.9 kg for Adult
For Estimated Daily Dermal Exposure: Surface area of skin contact (AV)c: 3020 cm2 for Infant and 16925 cm2 for Adult (Health Canada 1995) Product amount per application = 1.4 g for Infant and 4.4 g for Adult (Wormuth et al. 2006; Loretz et al. 2005) PAA: 17 ng/cm2 (Lucová et al. 2013) Frequency (F) = 1.7/day for Infant and 1.1/day for Adult (Wormuth et al. 2006; Loretz et al. 2005)
See potential absorbable dose approach above |
Brilliant Blue FCF | Perfume (aerosol spray) | Concentration: £30% (2016 email from Consumer Product Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) Age group: Teenager and adult Body weight: 59.4 for teenager and 70.9 kg for adult
For Estimated Daily Inhalation Exposure, default parameters for spray model, eau de toilette fragrance product (ConsExpo Web 2016)d unless noted otherwise: Frequency: 1.7/day (Loretz et al. 2005) Mode of release: Spraying (towards person) Spray duration: 0.08 min Exposure duration: 5 min Room volume: 10 m3 Room height: 2.5 m Ventilation rate: 2h-1 Cloud volume: 0.0625 m3 Mass generation rate: 0.1 g/s Airborne fraction: 0.02 g/g Density non-volatile: 1.5 g/cm3 Median particle diameter: 2.7 µm Maximum particle diameter: 50 µm Inhalation cut-off diameter: 10 µm Inhalation rate (adult; used for both adult and teenager as it is protective): 16.2 m3/day (Health Canada 1998) |
a The age ranges for an infant, a toddler, a child, a teenager, and an adult were assumed to be newborn to 6 months, 0.5 to 4 years, 5 to 11 years, 12 to 19 years, and 20 to 59 years, respectively. Default body weights were obtained from Health Canada (1998).
b Statistics Canada (2012) survey question referred to generic “Hair Dyes” and did not specify specific type (e.g., Permanent, Semi-Permanent, Temporary). Used dataset pertaining to 12 to 19 year old group for Semi-Permanent Hair Dyes (used median value).
c Total body surface area minus surface of head.
d The default scenario for Application, Exposure to Spray - Spraying using the factsheet for Eau-de-toilette in ConsExpo Web (2016) was applied.
Appendix D. Points of departure for human health risk characterization
| Substance | Per event exposure | Daily exposure (systemic) |
|---|---|---|
Basic Violet 3 | Developmental study (rabbits, gavage) Maternal toxicity (increased mortality, decreased body weight and body weight gain, clinical signs) and decreased fetal body weight at the LOAEL of 0.5 mg/kg bw/day (LTD) | Default to the per event point of departure. [The LOAEL (LTD) of 14.3 mg/kg bw/day (increased erythropoiesis in the spleen, atrophy of the ovaries) from a dietary carcinogenicity study in female mice would not be protective of potential effects at the LOAEL of 0.5 mg/kg bw/day.] |
Malachite Green | Developmental study (rabbits, gavage, Malachite Green Oxalate) Increased pre-implantation losses and early fetal resorptions, decreased fetal survival, decreased fetal body weight, and increased skeletal deviations at the LOAEL of 5 mg/kg bw/day (LTD) | Default to the per event point of departure. [The NOAEL of 7 mg/kg bw/day from a dietary carcinogenicity study in rats would not be protective of potential developmental effects at the LOAEL of 5 mg/kg bw/day.] |
Pigment Blue 61 | NA | NA |
Basic Violet 4 | Developmental study (rats, gavage, Ethyl Violet Acetate)
No observed maternal or developmental effects at the NOAEL of 12 mg/kg bw/day (HTD) | NA |
Basic Blue 7 | Developmental study (rats, gavage, Ethyl Violet Acetate) No observed maternal or developmental effects at the NOAEL of 12 mg/kg bw/day (HTD) | NA |
Brilliant Blue FCF | NA | 2-year study with a reproductive study and an in utero phase (rats, dietary) NOAEL of 631 mg/kg bw/day based on decreased body weight and survival in females at 1318 mg/kg bw/day. |
Abbreviations: HTD, highest tested dose; LOAEL, lowest observed adverse effect level; LTD, lowest tested dose; NA, not applicable; NOAEL, no observed adverse effect level
The BMDL10 of 16.8 mg/kg bw/day was used to estimate the cancer risk for all dermal and oral daily exposure scenarios of Basic Violet 3, Basic Violet 4, and Basic Blue 7. It was based on increased hepatocellular carcinomas in female mice fed Basic Violet 3 in a carcinogenicity study.
