Evaluation of the Health Portfolio Tobacco and Vaping Activities 2016-17 to 2020-21
Download the alternative format
(PDF format, 933.4 KB, 57 pages)
Organization: Health Canada and the Public Health Agency of Canada
Published: December 2021
Table of Contents
- Executive Summary
- Program Description
- Evaluation Description
- Evaluation Findings
- Conclusions and Recommendations
- Management Response and Action Plan
- Annex
- Endnotes
List of Acronyms
- CPAB
- Communications and Public Affairs Branch
- CCPSA
- Canada Consumer Product Safety Act
- CSCB
- Controlled Substances and Cannabis Branch
- CTS
- Canada's Tobacco Strategy
- FDA
- Food and Drugs Act
- FTCS
- Federal Tobacco Control Strategy
- FCTC
- Framework Convention on Tobacco Control
- G&Cs
- Grants and Contributions
- HCCF
- Healthy Canadians and Communities Fund
- HECSB
- Healthy Environments and Consumer Safety Branch
- MSP
- Multi-sectoral Partnerships to Promote Healthy Living and Prevent Chronic Disease
- OIA
- Office of International Affairs for the Health Portfolio
- PHAC
- Public Health Agency of Canada
- ROEB
- Regulatory, Operations and Enforcement Branch
- SUAP
- Substance Use and Addictions Program
- TCD
- Tobacco Control Directorate
- TVCEP
- Tobacco and Vaping Compliance and Enforcement Program
- TVPA
- Tobacco and Vaping Products Act
- US
- United States
- WHO
- World Health Organization
Executive Summary
Program Profile
Canada has a strong history of tobacco control. The launch of the Federal Tobacco Control Strategy (FTCS) in 2001 was a significant accomplishment that earned Canada global recognition as a leader in tobacco control and contributed to important declines in tobacco-use prevalence. Despite a decline in tobacco use since 2001, tobacco remains a significant public health issue in Canada. There are close to five million smokers in Canada with an estimated 48,000 people dying from smoking-related causes each year.
In May 2018, Canada's Tobacco Strategy (CTS) replaced the Federal Tobacco Control Strategy. The CTS has a goal of less than 5% tobacco use by 2035 (from the 2017 baseline of 18%). It commits more than $330 million over five years to help Canadians who smoke to quit or reduce the harms of their addiction to nicotine, and protect the health of young persons and non-users of tobacco products from the health hazards of tobacco use. The main themes of Canada's Tobacco Strategy are to:
- Help Canadians Quit Tobacco
- Protect Young People and Non-Tobacco-Users from Nicotine Addiction
- Strengthen our Foundations in Science, Surveillance, and Partnerships
- Co-Develop Distinctions-Based Approaches with Indigenous peoples.
Health Canada activities contribute to these themes and comprise policy and regulatory development, compliance and enforcement, science, research and surveillance, public education, and funded and non-funded collaborations with partners, including the provinces and territories, Indigenous organizations, municipalities, non-governmental organizations, health care professionals and the academic sector. Health Canada's spending on tobacco and vaping activities for the period of the evaluation was approximately $191M.
The Public Health Agency of Canada (PHAC) contributes to the first theme of the CTS by providing funding for tobacco prevention and cessation initiatives. The Office of International Affairs for the Health Portfolio (OIA), as a shared service to PHAC and Health Canada, supports Canada's membership in the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) and provides strategic advice to advance Canada's engagement on international tobacco control issues. PHAC's spending for tobacco activities over the period of the evaluation was approximately $13M.
What we found
Progress on results
- Tobacco prevalence rates have declined, from 17.7% in 2015 to 14.8% in 2019. This decline has largely been driven by a reduction in initiation, primarily in teen smoking, and, unfortunately, deaths.
- Over the last five years, Health Canada has made significant progress on the development, implementation and enforcement of the Tobacco and Vaping Products Act (TVPA), which was enacted in May 2018.
- In collaboration with its partners, including PHAC, Health Canada also developed and implemented the new Canada's Tobacco Strategy (CTS). Although they are not harmless, the CTS recognized vaping products containing nicotine as being a source of nicotine that is a less harmful option than smoking if smokers switch completely to vaping, and integrated this view into the broader approach for dealing with tobacco use. The CTS also acknowledged that tobacco use is not equally spread across the population, with groups who experience higher rates of smoking being a key priority.
- Health Canada and PHAC have funded a variety of prevention and cessation projects, and available performance data for some of the more mature projects show that they have supported participants to quit smoking. Newer PHAC projects are aiming to reach groups that experience health inequalities and face higher rates of tobacco use than the general population, in line with the CTS priority.
Recent Health Canada and PHAC initiatives, such as new regulations and funded tobacco cessation projects, should help contribute to planned tobacco and vaping outcomes. However, more work is required to move towards the goal of less than 5% tobacco use by 2035 and to address the high rates of youth vaping, which have doubled in recent years.
Challenges to effectiveness and efficiency
- While Health Canada has internal planning and performance measurement documents for tobacco and vaping activities, Health Canada has not communicated these plans, and associated performance measurement information, to stakeholders in an integrated way. This has led stakeholders to wonder about Health Canada's specific contributions to the long-term goal of less than 5% tobacco use by 2035 and to addressing the issue of youth vaping.
- Some Health Canada information technology systems are outdated while others are non-existent. This has led to extra time required to process and access information, and sometimes to missed opportunities to collect and analyze information that could inform the program.
- Health Canada's financial and human resources have been largely focused on vaping activities in recent years. While the importance of addressing youth vaping was not in question, there was some concern among internal and external key informants that decreasing the focus on tobacco-related issues for an extended period of time might result in lost ground in that area.
- While several regulatory initiatives have been made to help address the rapid rise in youth vaping, implementing these recent regulations will take time. The current legal regime for vaping under the TVPA provides a range of enforcement tools that should be expanded to better support efficiency and effectiveness of compliance activities. Research and surveillance activities could also benefit from industry reporting requirements.
- Health Canada's website includes information regarding health hazards arising from vaping use, and it is important that the most up-to-date information continue to be reflected. In addition, some of Health Canada's statements in relation to vaping products appear to be consistent with a therapeutic claim, even though no vaping products are licensed as a smoking cessation aid in Canada.
- Challenges facing PHAC's tobacco activities included inconsistent performance measurement and limited systematic knowledge exchange. However, the implementation of the Healthy Canadians and Communities Fund, which is replacing the Multi-sectoral Partnerships to Promote Healthy Living and Prevent Chronic Disease program (MSP), includes plans to address these concerns.
Recommendations
The findings from this evaluation have resulted in the four recommendations listed below, all of which are directed at Health Canada. In addition to the recommendations below, the evaluation findings highlighted several other areas that the program could consider. These include options to address challenges such as, high rates of youth vaping, identifying regulated parties in order to conduct vaping retail and online inspections, determining vaping product ingredients to support surveillance and science activities, and ensuring non-compliant vaping products are quickly removed from the market. However, given that a legislative review was underway at the time this report was written, the program is encouraged to consider the results of that review alongside evaluation findings in order to explore potential improvements.
There are no recommendations for PHAC, given that actions to address knowledge exchange and performance measurement issues are already underway.
Recommendation 1: Communicate to partners and stakeholders, Health Canada's action plan for the Department's contribution to achieving CTS goals and for addressing youth vaping.
Health Canada and its partners have an ambitious target of less than 5% tobacco use by 2035. In addition, youth vaping rates remain a concern. While Health Canada does have internal planning and performance measurement documents related to both these issues, these plans have not been communicated to stakeholders in an integrated way. As a result, some key stakeholders have the impression that Health Canada has not defined a coordinated, department-wide plan. Several key informants indicated that having knowledge of Health Canada's plan could help inform their own work and the work of other groups, and help assess progress on the issues of tobacco use and youth vaping. In communicating this plan, Health Canada should coordinate with Health Portfolio partners and key stakeholders to facilitate cooperation and buy-in from relevant groups.
Recommendation 2: Enhance information technology systems and data analytics capacity to support program activities.
Current information systems do not allow the program to access and analyse information from various sources related to tobacco and vaping in support of program activities. This is in part due to antiquated systems and in part because commitments for delivering a new tobacco and vaping compliance and enforcement system are still in progress. The program should work with relevant partners to complete and implement the new system, including providing appropriate training. In addition, opportunities to update and enhance information technology systems to support other activity areas should also be identified.
Recommendation 3: Examine resource distribution between tobacco and vaping activities.
The rise of youth vaping and the high level of non-compliance of the vaping industry (particularly specialty vaping establishments) meant the program reallocated internal resources from tobacco to support the needed level of activities related to vaping. Recognizing that limited resources are available, the program should continue to use a risk-based approach to allocate resources to deal with priorities.
Recommendation 4: Review and update the Health Canada website to reflect the most up-to-date science and public health advice on health risks and benefits of vaping.
Health Canada's website includes information regarding health hazards arising from vaping use, and it is important that the most up-to-date information continue to be reflected. Furthermore, Health Canada does not clearly indicate that there are no vaping products that have been licensed as smoking cessation aids when stating that these products may reduce the health risks for adult smokers who cannot or do not want to quit using nicotine, if they completely switch to vaping. As evidence of health risks and benefits of vaping evolves, it is important for the success and credibility of the harm reduction messaging and the protection of Canadians, to keep Health Canada's message current and in line with the most up-to-date science.
Program Description
Context
Canada has a strong history of tobacco control. The launch of the Federal Tobacco Control Strategy (FTCS) in 2001 was a significant accomplishment that earned Canada global recognition as a leader in tobacco control and contributed to important declines in tobacco-use prevalence.
Despite a decline in tobacco use since 2001, it remains a significant public health issue in Canada, with 48,000 Canadians dying annually because of tobacco-related illness. Health and economic costs associated with tobacco use in Canada are estimated at $12.3 billion annually, based on 2017 data.
Canada's Tobacco Strategy
In May 2018, Canada's Tobacco Strategy (CTS) replaced the Federal Tobacco Control Strategy. The CTS has a goal of less than 5% tobacco use by 2035, down from the 2017 baseline of 18%. It commits more than $330 million over five years to help Canadians who smoke to quit or reduce the harms of their addiction to nicotine, and protect the health of young persons and non-users of tobacco from the health hazards of tobacco use.
Health Canada and the Public Health Agency of Canada (the Health Portfolio) are not the only federal departments playing a role in CTS. Departments, like Indigenous Services Canada, Public Safety Canada, and others, also have roles. However, this evaluation only covers the Health Portfolio activities.
What does "Less than 5% by 2035" mean?
In 2019, 14.8% of Canadians aged 12 and older, roughly 4.7 million people, smoked cigarettes either daily or occasionally. Based on population projections, reducing the rate of tobacco use to less than 5% means there would be fewer than 1.8 million Canadians using tobacco.
Health Canada activities in support of CTS
Within Health Canada, the Tobacco Control Division (TCD), which is part of the Controlled Substances and Cannabis Branch (CSCB), leads activities in support of the CTS. TCD works with a variety of partners from other branches, as described below.
Policy and Regulatory Development
Led by the Tobacco Control Directorate (TCD) within the Controlled Substances and Cannabis Branch (CSCB), in collaboration with the Healthy Environments and Consumer Safety Branch (HECSB) for elements falling under the Canada Consumer Product Safety Act (CCPSA). The program leads policy and regulatory actions related to tobacco and vaping.
Science and Surveillance
TCD leads research activities to support evidence-based decisions, such as:
- Studying new and unique ways to address tobacco use in Canada;
- Studying the health impacts, market use patterns, and populations who use new nicotine products like vaping and heated tobacco products; and
- Informing Canadians and aiding research from academics and health charities by publicly releasing industry reports, research findings, and surveillance results.
Public Education
TCD collaborates with the Communications and Public Affairs Branch (CPAB) to deliver public education activities related to tobacco cessation and youth vaping prevention. This includes developing resources and marketing campaigns to educate youth and their parents of the health hazards associated with vaping products, as well as to educate adults of the health hazards associated with tobacco, and support cessation efforts.
External collaborations
TCD works with the Office of International Affairs for the Health Portfolio (OIA), a shared service of the Health Portfolio, to advance international cooperation on tobacco control. Nationally, CSCB's Substance Use and Addictions Program (SUAP) offers funding to eligible Canadian organizations for time-limited projects that aim to support evidence-informed and innovative nicotine and tobacco prevention, harm reduction and treatment initiatives across Canada at the community, regional and national levels. The program also collaborates with other stakeholders as part of their efforts to achieve CTS goals. These include provinces and territories, NGOs, community agencies, health care professionals, academia, private sector groups, and international partners.
Compliance and enforcement
TCD works with the Tobacco and Vaping Compliance and Enforcement Program (TVCEP) of the Regulatory Operations and Enforcement Branch (ROEB) for compliance and enforcement activities. TCD also works with the Healthy Environments and Consumer Safety Branch (HECSB) for regulating aspects of tobacco and vaping products that fall under the CCPSA. Compliance and enforcement activities help ensure that manufacturers, importers and retailers of tobacco and vaping products comply with legislative and regulatory requirements under all relevant Acts. Health Canada inspectors conduct inspections of tobacco and vaping product retailers, manufacturers, on-line establishments, and any other place, including a conveyance, where tobacco and vaping products are manufactured, tested, stored, transported, furnished, or promoted.
Health Canada's planned spending on tobacco and vaping activities for the period of the evaluation was approximately $189M.
| Fiscal Year | Planned Spending | |
|---|---|---|
| G&CsFootnote * | Total | |
| 2016-17 | N/A | $29,027,999 |
| 2017-18 | N/A | $34,846,701 |
| 2018-19 | N/A | $40,202,944 |
| 2019-20 | $2,500,000 | $44,366,709 |
| 2020-21 | $4,500,000 | $40,640,467 |
| TOTAL | $7,000,000 | $189,084,820 |
Source: Chief Financial Officer Branch |
||
PHAC activities in support of CTS
Within PHAC, the Health Promotion and Chronic Disease Prevention Branch (HPCDP) provides funding for tobacco prevention and cessation initiatives. The Office of International Affairs for the Health Portfolio (OIA), a shared service of PHAC and Health Canada, supports Canada's international engagement on tobacco control initiatives. It also facilitates the payment of Canada's assessed contributions to the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) through OIA's international Health Grants Program (IHGP). The IHGP also serves as a funding mechanism for providing grants for WHO FCTC projects supported by Health Canada and PHAC.
Grants and contributions
PHAC has funded tobacco prevention and cessation initiatives through the Multi-sectoral Partnerships to Promote Healthy Living and Prevent Chronic Disease (MSP) program. In 2021, the Healthy Canadians and Communities Fund (HCCF) was launched to replace the MSP Program as the new funding mechanism for PHAC's tobacco initiatives.
International engagement
OIA, in close coordination with TCD, supports Canada's membership in the WHO FCTC and provides strategic advice to advance Canada's engagement on international tobacco control issues. OIA primarily leads on the governance aspects of the WHO FCTC. Furthermore, OIA's IHGP supports WHO FCTC projects and is the mechanism through which Canada's assessed contributions are paid.
PHAC's planned spending for tobacco activities for the period of the evaluation was approximately $15.8M. PHAC did not receive funding for vaping activities.
| Fiscal Year | Planned Spending | |
|---|---|---|
| G&CsFootnote * | Total | |
| 2016-17 | $2,455,000 | $2,455,000 |
| 2017-18 | $2,205,000 | $2,205,000 |
| 2018-19 | $2,705,000 | $2,881,393 |
| 2019-20 | $3,455,000 | $3,636,845 |
| 2020-21 | $4,455,000 | $4,636,895 |
| TOTAL | $15,275,000 | $15,815,132 |
| Source: Chief Financial Officer and Corporate Management Branch | ||
Evaluation description
Evaluation scope
The purpose of the evaluation was to examine the performance and efficiency of Health Canada and the Public Heath Agency of Canada (Health Portfolio) activities in support of the tobacco control program. The evaluation covers activities of the Health Portfolio for the period 2016-17 to 2020-21. The evaluation used multiple lines of evidence, both qualitative and quantitative, to ensure triangulation of findings (see Annex A for detailed methodology, limitations and mitigation strategy).
Excluded from the scope:
Activities linked to the vaping-associated lung illness (VALI) were excluded, as were activities related to the safety of vaping products as consumer products (e.g. child-resistant containers, device batteries). These activities will be covered in separate evaluations.
Evaluation questions
Given that the need to address tobacco and nicotine use is well established, along with Health Canada's and PHAC's roles in doing so, the evaluation did not focus on program relevance. Instead, attention was given to achievement of results and efficiency.
The key objective of the CTS is reducing smoking in Canada to less than 5% by 2035 by helping Canadians quit tobacco or reduce the harms of their addiction to nicotine, as well as protecting the health of young persons and non-users of tobacco from the health hazards of tobacco use.
1: Results
In support of the CTS objectives, are the following activities effective in advancing Health Canada's overall goals?
- Policy and regulatory development;
- Compliance and enforcement;
- Science and surveillance;
- Public education; and
- External collaborations (funded and non-funded).
2: Efficiency
Are there ways to improve these activities to enhance the efficiency of Health Canada's approach in terms of the following areas?
- Program design (e.g., governance, roles and responsibilities, IT systems and tools); and
- Resource allocation across activities and between tobacco and vaping.
To reflect PHAC's context and information needs better, Question 1 was adjusted for PHAC's activities to focus on the outcome aligned with their contribution (helping Canadians quit smoking) and on their external collaborations. Question 2 was also adjusted to take a deeper dive into PHAC's activities in terms of program design, and clarity of roles and responsibilities between PHAC and Health Canada.
Evaluation Findings
Question 1: Progress on Results
Over the years, tobacco prevalence rates have declined, from 17.7% in 2015 to 14.8% in 2019. This decline has largely been driven by a reduction in initiation, primarily in teen smoking, and, unfortunately, deaths.
Recent program initiatives, such as the introduction of plain and standardized appearance requirements for tobacco products and their packaging, may lead to further reductions in smoking rates, but more work is required to move towards the goal of less than 5% tobacco use by 2035. There are close to five million smokers in Canada, with an estimated 48,000 people dying from smoking-related causes each year.
While the prevalence rate for youth tobacco use has decreased over the years, the rise in youth vaping rate represents a focus for the program in the future.
To assess progress on results, the evaluation looked at trends in tobacco prevalence rates and at how Health Canada and PHAC activities have supported the goal of less than 5% tobacco use by 2035 through achievement of the following results:
- Implementing a new legislative and policy framework;
- Helping Canadians quit tobacco, especially smoking, or reduce the harms of their addiction to nicotine;
- Protecting youth and non-users of tobacco products from nicotine addiction;
- Increasing awareness of the health hazards of vaping product use for youth and non-users of tobacco products;
- Supporting decision making through data access; and
- Ensuring industry compliance.
Tobacco use in Canada
There are close to five million smokers in Canada with an estimated 48,000 people dying from smoking-related causes each year. However, the prevalence of tobacco product use has decreased over time. Canadian surveys on tobacco use indicate a decrease of smoking rate over the years. For example, the Canadian Community Health Survey (CCHS) shows a decrease in daily or occasional smoking among Canadians from 17.7% in 2015 to 14.8% in 2019.

Source: Canadian Community Health Survey
Figure 1 - Text description
Graph showing Daily or occasion smoking rates in the general population by year for the years 2015 to 2019.
| Annual year | Percentage |
|---|---|
| 2015 | 17.7 |
| 2016 | 16.9 |
| 2017 | 16.2 |
| 2018 | 15.8 |
| 2019 | 14.8 |
According to experts interviewed, this decline has largely been driven by a reduction in initiation, primarily in teen smoking, and, unfortunately, deaths. According to the Canadian Tobacco and Nicotine Survey (CTNS), the smoking rate in 2019 among youth aged 15 to 19 was about 5%.Endnote 1
This survey also showed an overall decline in reported cigarette smoking from 2019. The most significant decrease observed was among those aged 20 to 24, with 8% reporting that they smoked in 2020 compared with 13% in 2019.
However, smoking rates remain disproportionately higher in some population groups, such as:
- Canadians diagnosed with a mood and/or an anxiety disorder (25%);
- First Nations, Inuit and Métis (37%, 59%, and 29%, respectively);
- Certain occupational groups, such as construction, manufacturing, etc. (ranging from 20 to 27%);
- people identifying as LGBTQ+ (ranging from 21% to 27%); and
- Canadians in low income brackets (23%).
Implementing a legislative and policy framework
To move further in addressing the public health problem of tobacco use, Bill S-5, An Act to amend the Tobacco Act and the Non-smokers' Health Act and to make consequential amendments to other Acts, amended the Tobacco Act in May 2018 to regulate the manufacture, sale, labelling and promotion of vaping products and to change the title to the Tobacco and Vaping Products Act (TVPA). The Bill also amended certain provisions of the Act relating to tobacco products, including with respect to product standards, disclosure of product information, product sale, sending and delivery, and product promotion. Furthermore, new authorities were provided to allow the making of new regulations to require plain and standardized packaging for tobacco products. At the same time, the Government of Canada committed through the new Canada's Tobacco Strategy (CTS) to a goal of less than 5% tobacco used by 2035.
Health Canada, in collaboration with its other federal partners in tobacco control, including PHAC, led the development and implementation of the CTS. Although these products are not harmless, the strategy recognized vaping products containing nicotine as a source of nicotine that is a less harmful option than smoking for adult tobacco users, especially adult smokers, if they switch completely to vaping. It integrated this view into the broad approach for dealing with tobacco use. The strategy also acknowledged that tobacco use is not equally spread across the population and makes groups who experience higher rates of smoking a key priority. The main themes of Canada's Tobacco Strategy are to:
- Help Canadians Quit Tobacco
- Protect Young People and Non-Tobacco-Users from Nicotine Addiction
- Strengthen our Foundations in Science, Surveillance, and Partnerships.
- Co-Develop Distinctions-Based Approaches with Indigenous peoples.
Health Canada also led the development and implementation of regulations. In support of the TVPA and CTS implementation, five new regulations were made by Health Canada since 2018:
- Regulations Excluding Certain Vaping Products Regulated Under the Food and Drugs Act (FDA) from the Application of the Tobacco and Vaping Products Act (made in 2018)
- Tobacco Products Regulations (Plain and Standardized Appearance) (made in 2019)
- Vaping Products Labelling and Packaging Regulations (made in 2019)
- Vaping Products Promotion Regulations (made in 2020)
- Nicotine Concentration in Vaping Products Regulations (made in 2021).
Health Canada also worked on amendments, for example, the Order Amending the Schedule to the Tobacco Act (Menthol) (2017) and the Regulations Amending the Tobacco Products Reporting Regulations (2019).
Canada's new Tobacco Products Regulations (Plain and Standardized Appearance) are seen to be some of the most comprehensive in the world. Several internal and external key informants pointed to these plain packaging regulations as a key success for Health Canada. Research has shown that plain and standardized packaging reduces the appeal and attractiveness of tobacco products, especially to youth.Endnote 2Endnote 3Endnote 4
This important work required significant investment in staff training and development, such as the Tobacco Control Program National Training and Development Session. This session was held in 2019 and brought together 139 staff from TCD and TVCEP over two days to share information and knowledge related to Leadership, Program Cohesion, Program Consistency and Effectiveness, and Technical and Regulatory Excellence.
Seeking Feedback on Policy and Regulatory Initiatives
While amending legislation and developing new regulations and policies, the program has demonstrated its commitment to stakeholder engagement and input. Below are key examples:
- In 2017, the program collaborated with stakeholders via The National Forum on the Future of Tobacco Control (2017). This Forum engaged 150 stakeholders in broad discussions pertinent to the development of the CTS.
- Also in 2017, the program, in collaboration with the Canadian Institutes of Health Research, established the Scientific Advisory Board on Vaping Products to review the scientific literature on the potential health benefits and harms of vaping products and provide recommendations on the federal legislative framework for vaping products.
- In 2019, Health Canada began hosting quarterly round tables with NGOs to share information and obtain their views on issues related to tobacco and vaping.
- In May 2021, Health Canada held a symposium with stakeholders and individuals on public education and awareness for substance use, including nicotine. The symposium focused on the impact that the COVID-19 pandemic has had on substance use and how organizations have adapted public education efforts on substance use during the pandemic.
Overall, most external interviewees indicated that collaborations between the program and NGOs, both formal and informal, were positive.
Health Canada engagement with the industry is subject to Canada's international commitment to the WHO FCTC. In support of Article 5.3 of the WHO FCTC, the program must ensure to protect public health policies from commercial and other vested interests of the tobacco industry. Thus, engagement with the tobacco industry is limited to instances where it is necessary to regulate the industry and tobacco products effectively. In full support of transparency, information on industry meetings is posted online regularly.Endnote 5 Several key informants mentioned that they appreciated this transparency regarding the public disclosure of information on meetings between Health Canada staff and tobacco and vaping industry representatives.
Helping Canadians quit tobacco or reduce the harms of their addiction to nicotine
Quitting tobacco is a difficult and complex process. For instance, persons who smoke and are trying to quit tobacco use many methods and approaches to achieve cessation. It may take them up to 30 attempts before quitting for good.Endnote 6 Still, each attempt brings them closer to their ultimate goal.Endnote 7
The program aims to help Canadians quit tobacco in many ways including:
- Giving information to adults who use tobacco on, and access to, how to quit or less harmful sources of nicotine;
- Funding for cessation initiatives;
- Collaborating with provinces and territories; and
- Engaging in public education activities.
Detailed action plan to reach long-term CTS goal
Despite progress being made in support of tobacco cessation, the CTS has set an ambitious goal of less than 5% tobacco use by 2035, to which Health Canada and other CTS partners contribute. While Health Canada does have internal plans and a Performance Information Profile to outline and track progress on objectives, those plans have not been communicated to stakeholders in an integrated manner that sets out an overarching path for Health Canada's contributions. Furthermore, several internal and external key informants noted that the CTS goal is a national one, and P/Ts and other partners were not involved in setting it.
A number of external key informants also raised concerns about the lack of a clearly communicated Health Canada integrated plan. While they acknowledged that Health Canada is one of many federal partners involved in the CTS, they indicated that they are not aware of Health Canada's plans for the Department's specific contribution to the CTS. This was seen as a potential impediment to national cooperation and coordination on the issue.
Harm reduction approach to smoking
Based on the direction in the CTS, Health Canada has taken a harm reduction approach for helping Canadians to quit smoking or for adults who smoke who cannot quit, to reduce the harms of their addiction to nicotine by completely switching to vaping. In other words, moving adult smokers away from tobacco and towards vaping is considered a success since the relative harm of vaping is less than that of combustible tobacco products, such as cigarettes.
Data from the 2019 Canadian Tobacco and Nicotine Survey (CTNS), indicated that among the group aged 25 and older who currently vape, 86% currently or formerly have smoked cigarettes. Furthermore, 56% reported smoking cessation and avoiding returning to smoking as the main reasons for use. Daily vaping (the behaviours associated with smoking cessation) was reported by 54% of which 64% were males and 36% were females.
Funding of cessation initiatives
Both Health Canada and PHAC provide funding in support of smoking cessation initiatives. For Health Canada, this is done through the Substance Use and Addictions Program (SUAP), and for PHAC, through the Multi-sectoral Partnerships to Promote Healthy Living and Prevent Chronic Disease (MSP) program.
As stated earlier, reaching groups of Canadians that face health inequalities and have higher rates of tobacco use is a CTS priority. Within the Health Portfolio, this objective is mainly achieved through PHAC's MSP program.
Spotlight on Inequality
Many external key informants praised the program for its focus on populations with higher rates of smoking than the general population. This focus on addressing health inequalities was seen as an essential component in achieving the goal of less than 5% tobacco use by 2035.
PHAC's MSP funding
During the evaluation period, PHAC funded eight multi-year tobacco-related projects under the MSP Program. Two of these projects are complete, while six are still active. Funding was provided to a variety of different groups, such as non-profits, private sector organizations, academic institutions, and other levels of government. By the end of 2019-20, 12,457 participants had been enrolled in PHAC-funded tobacco cessation interventions and 740 health professionals had been trained throughout projects funded by PHAC.
- All Together Now LGBTQ2+, which provides tobacco cessation support services through social media messaging and experiential events targeted to the LGBTQ2+ populations in Toronto, Montreal and Thunder Bay.
- Build Smoke-Free is a workplace tobacco cessation intervention for construction workers in Alberta, Ontario and British Columbia that provides individually customized and locally adapted cessation support services and resources.
Several internal and external interviewees were supportive of this approach, indicating that it is a positive step as it focuses on the target populations. This is seen to be important, given the challenge of reaching the remaining individuals who smoke.
Several projects funded after the 2018 implementation of the CTS were too new to have measureable results in terms of behavioural change. However, evidence regarding changes in smoking behaviour was available for four MSP projects, which are more mature.
The Walk or Run to Quit project had substantial data available regarding behaviour change. For example:
- A 2017 study looking at 168 male and female smokers found that 91% had reported reducing their smoking, and there was a significant decrease in participants' carbon monoxide levels.Endnote 8
- A 2020 study, which evaluated the project over three years, found that carbon monoxide levels significantly decreased from weeks 1 to 10, there was an increase in physical activity and, at the six-month follow-up point, 28.9% of participants who were contacted self-reported a six-month abstinence.Endnote 9
Following the completion of a first cohort of participants in Spring 2019, the Build Smoke-Free project reported a significant decrease in current smoking status between the baseline and six-month follow-up among intervention participants (93% of individuals who currently smoke at baseline vs. 68% of individuals who currently smoke at follow-up). Furthermore, scores on the Heaviness of Smoking Index decreased between the baseline and follow-up, where 49% of intervention participants were moderately or highly addicted at baseline compared to only 34% at follow up.
Build Smoke-Free participants who were interviewed as part of a project-level evaluation, reported that the program was effective in helping them address their tobacco use. Smoking reduction was the most commonly observed impact, but they also noted that the program helped with attempts to quit and, for some, staying smoke-free.
Over the years, some projects have successfully been scaled-up, or expanded to additional areas across the country. For example, Walk or Run to Quit was piloted at a single site in 2013. The project started to receive PHAC funding in 2015, and by 2018-19, it had been expanded to 98 unique locations across Canada. Furthermore, Build Smoke-Free started at approximately 12 workplace sites in year one (2018- 19), and now plans to expand to 102 construction sites by year five (2022-23).
Good relationships between funding recipients and PHAC, as well as between funding recipients and their other funding partners, have been key in developing successful projects. Funding recipients interviewed described PHAC as being collaborative and transparent, supportive of their programming, and willing to work alongside them. Internal key informants indicated that the ability of funded projects to reach target populations, especially those at risk, is facilitated by positive relationships with external partners. An example of this is the Build Smoke-Free project where a partnership with a construction firm allowed the initiative to reach construction workers more directly.
PHAC funding recipients praised PHAC's flexibility in program design, stating that PHAC's willingness to adapt and make improvements "on the go" was particularly useful in adapting to unexpected events. This was particularly important over the last year within the context of the COVID-19 pandemic.
In 2021, the Healthy Canadians and Communities Fund (HCCF) replaced the MSP Program as the funding mechanism for PHAC's new tobacco cessation initiatives. As part of the move towards the HCCF, health equity is being reinforced as a program priority.
Health Canada's SUAP funding
Since 2019-20, when project funding became available through the implementation of CTS, nine projects have been funded. As there were no SUAP funds dedicated to youth vaping, the Department decided to reallocate some of its SUAP funds to help address this issue. SUAP funding is now used for smoking cessation initiatives, activities that aim to reduce youth vaping, and research activities to inform tobacco and vaping policy development. The details of the nine funded projects are as follows:
- Two on youth smoking cessation;
- One on adult smoking cessation in hospital patients;
- Two on youth vaping (one on cessation and one on research); and
- Four on research related to smoking and vaping for youth or general audience.
Data specific to impacts of SUAP-funded projects on cessation behaviours was not available at the time this report was written, as all projects were relatively new.
Collaboration with provinces and territories
Health Canada also collaborates with provincial and territorial (P/T) government partners in support of cessation activities. An example of this is the pan-Canadian Quitline, a toll-free phone service aimed at those attempting to quit smoking. While the Quitline is delivered by P/T governments, Health Canada provides funding to support service enhancements and promotion of the Quitline. While phone calls to the Quitline have not been as high as their peak in 2012, web registration for Quitline services has increased, based on 2017 data. Health Canada has sought renewal of the Memorandum of Agreement (MOA) with P/Ts to continue, and most recently in the 2020-21 renewal, to modernize the Pan-Canadian Quitline Initiative.
Such services are recognized as an effective population-based approach to motivate attempts to quit and increase smoking cessation.Endnote 10
Public education
In addition to providing funding for cessation projects, Health Canada's CPAB worked with the TCD to support Canadians in quitting tobacco use through public education. A key piece of work was the development and implementation of a national public awareness campaign for smoking cessation called Break It Off. This campaign was delivered in collaboration with the Canadian Cancer Society and aimed to increase awareness, knowledge and uptake of quit-smoking tools and resources for young adult smokers (20-24 years old).
This campaign began in 2014 and has included such tactics as: web and social media advertising, experiential events, use of digital influencers for video content, organic social media, website content, and a mobile application.
Break It Off campaign: Outputs 2019-20 and 2020-21
- Advertising (22.2M impressions,Endnote i mixed media)
- Experiential events (74.1K impressions, 21.1K engagements (53% with individuals who smoke)
- Digital influencers (6 young adult influencers)
- Social Media (Facebook/Instagram)
- Campaign efforts resulted in:
- 730K visits to the Break It Off microsite
- Almost 3K followers to social media channels
In 2020-21, Health Canada launched a national smoking cessation public awareness campaign to reach adults aged 50-64 years, for the first time since 2003. The "It's never too late" campaign's goal was to reach the audience with motivational messaging that it's not too late to quit and provided resources to help improve the efficacy of their quit attempts.
It's Never Too Late campaign: Outputs 2020-21
- Advertising (web, mobile and social media) that generated:
- 9.7M impressions
- 1.5M video views
- 16K sessions to the campaign web landing page
Awareness of the health hazards of vaping product use among youth and non-users of tobacco products
Public education was not limited to supporting Canadians to quit smoking, but was also used to help raise awareness amongst youth and non-users of tobacco products about the health hazards of vaping products. CPAB worked with the TCD on the design and delivery of the Consider the Consequences of Vaping campaign that aims to increase awareness of the health hazards of vaping product use. It is targeted toward youth (13-18 years old) and their parents.
Experiential events in schools and communities across the country were a highlight of the Consider the Consequences campaign. Participants were able to navigate a visually and physically engaging 35' x 25' maze that delivered key messages about the risks of vaping at various points as they move through it. The majority of the participants in the maze mentioned on the survey that they would probably not vape in the future following their participation in this event (60% percent of teens claimed a committed intention to change behaviour, 72% indicated that they are likely to not start or to stop vaping due to the hidden health hazards of vaping). Unfortunately, the COVID-19 pandemic paused in-person events, however, activities shifted to a virtual platform beginning in spring 2020.

Figure 2 - Text description
Photo of students sitting in a school gymnasium, listening to a presentation, with the Consider the Consequences campaign maze in the background.
The campaign also used other public education strategies to reach the target groups. Key outputs are described in the box that follows.
Consider the Consequences: Outputs 2019 - March 2021
- Advertising (894 M impressions, mixed media)
- Experiential events (64K engagements with youth through 515 schools visits and 22 community events)
- Digital influencers (eight parent influencers and nine youth influencers)
- Outreach materials (15K parent tip sheets and posters to doctors' offices, 12K teen awareness kits to schools)
- Campaign activities resulted in:
- 677K visits to Canada.ca/vaping-info
- 34K views of four vaping videos on YouTube
- 29K visits to the Consider the Consequences microsite
In addition to the public awareness campaign, Health Canada also provided information to the public in other ways. For example:
- through SUAP funding for 64 micro-grants with a target on creating awareness about the health hazards of vaping product use among youth;
- by responding to requests for information from Canadians through the public enquiry service, which saw a significant increase in requests following the enactment of the TVPA.
Internal and external key informants noted that a key challenge for Health Canada was trying to reach youth as a target audience particularly within the constraints of a government organization. For example, the need to use approved messages and language meant that it was not always possible to respond to questions from students. Suggestions to improve youth outreach included using more peer-to-peer strategies, and working through schools and community groups.
How aware of the health hazards of vaping are youth and non-users of tobacco?
There is evidence that most Canadian youth and non-users of tobacco products are aware of health hazards associated with vaping nicotine products. However, both groups are less likely to be aware that non-nicotine vaping also has health hazards.
According to a recent survey, 42% of Canadian students in grades 7 to 12 indicated that using an e-cigarette with nicotine on a regular basis posed a "great risk" of harm, while 32% believed it posed a "moderate risk".Endnote 11 Another recent survey showed that just over 81% of adult non-users of tobacco believe that nicotine vaping is harmful (moderately, very or extremely harmful).Endnote 12
However, far fewer students thought the use of vaping without nicotine on a regular basis posed serious health concerns, with only 14% reporting such behaviour posed a "great risk" and 27% indicating it posed a "moderate risk".Endnote 13
While health risks associated with tobacco products are widely studied, evidence related to the health risks of vaping products with or without nicotine is still emerging and long-term effects are not known. Although it is too early to provide a clear answer on the long-term impacts of using vaping products or being exposed to them, evidence shows that they are harmful to health.Endnote 14 Negative health risks associated with nicotine are, however, more widely recognized.Endnote 15Endnote 16Endnote 17
Web content on health hazards and benefits of vaping
CTS recognized the potential of switching completely to vaping as a harm reduction approach for adults who use tobacco products, especially adults who smoke, while also acknowledging that the approach to addressing tobacco use must protect youth and non-users of tobacco products from nicotine addiction and inducements to use tobacco. Health Canada's public outreach activities and website share both a harm reduction message with adults who smoke and a cautionary message to youth and non-users of tobacco products regarding the health hazards of vaping product use.
Many external and internal interviewees discussed the challenges associated with this "double messaging", indicating that it was potentially conflicting or confusing. Some were of the view that having a message focusing on the relative harm of vaping products compared to cigarettes could lead the population to overlook the harm associated with vaping. The 2019 Canadian Tobacco and Nicotine Survey (CTNS) showed that just under half (48.5%) of individuals who currently smoke think vaping is about as harmful or more harmful than cigarettes, while about 60% of individuals who currently vape think that vaping is less harmful than smoking.Endnote 18 A few Health Canada interviewees did not perceive there to be issues with the harm reduction messaging; however, they acknowledged that different groups may have misinterpreted the messaging.
In terms of communications related to the health hazards of vaping, Health Canada's vaping website includes statements regarding potential harms,Endnote 19 and it is important that the most up-to-date information continue to be reflected. For example, one web page links to a relative harm estimateEndnote 20 that some experts now contest.Endnote 21Endnote 22
While the content of the Health Canada website regarding vaping products shares many similarities to the websites of other public health organizations, some of these appear to refer to research that is more recent and comprehensive. For example, there is limited information on the Health Canada website on the potential risks associated with vaping, such as those related to dual use (smoking and vaping at the same time) and those for pregnant women. The United States (US) Center for Disease Control (CDC) has a web page dedicated to e-cigarettes and pregnancy that indicates, "e-cigarettes and other products containing nicotine are not safe to use during pregnancy. Nicotine is a health danger for pregnant women and developing babies and can damage a developing baby's brain and lungs. Also, some of the flavorings used in e-cigarettes may be harmful to a developing baby."Endnote 23 When discussing the use of e-cigarettes in adult smoking cessation, the CDC also provides information on the danger of dual use and the importance of completely switching to fully reduce smoking related health risks.Endnote 24Endnote 25
In terms of the harm reduction messaging for adult smokers on Health Canada's website, there may also be a lack of clarity related to vaping products for cessation. In Canada, products with therapeutic claims (e.g., "this product will help you quit smoking") require authorization under the FDA prior to sale. No vaping products have been licensed for therapeutic use. However, some of the materials that Health Canada shares in relation to vaping products appear to give a message that is consistent with a therapeutic claim. For example, the Health Canada website has a section entitled, "Vaping to help quit smoking". Although this website lists other potential cessation aids, such as medication and approved nicotine replacement therapies, it does not explain that vaping devices are not licensed cessation tools. In contrast, while the website for the US CDC indicates that vaping may help smokers, it clearly identifies that e-cigarette are not approved as a smoking aid in the US.Endnote 26 The same is true for Public Health England in their latest "vaping in England: 2021 evidence update summary".Endnote 27
Some external key informants indicated that Health Canada's messages regarding vaping could be construed as promoting vaping products as cessation devices even though they are not licensed as a cessation aid. Some indicated that they would like Health Canada to facilitate the approval of vaping devices as cessation tools, while others wanted Health Canada to adopt more of a precautionary principle and wait for strong evidence to emerge.
Protection of youth and non-users of tobacco products from nicotine addiction
In terms of nicotine from tobacco use, especially from cigarettes, in 2019, the prevalence of current cigarette smoking among youth aged 15 to 19 was 5%.Endnote 28 This corresponds to an all-time low and is an important milestone in tobacco control.
On the other hand, the Canadian Student Tobacco, Alcohol and Drug Survey showed that the percentage of high school students who had used an e-cigarette in the past 30 days doubled from 10% in 2016-17 to 20% in 2018-19. Furthermore, it found that among students who vaped in the last 30 days, 40% reported daily or almost daily use. Among students who had used a vaping product, 90% had used a product with nicotine.
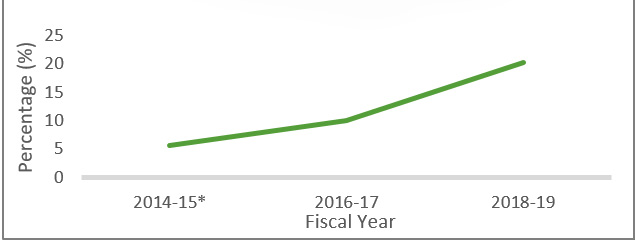
*Grade 6 included for 2014-15
Source: Canadian Student Tobacco, Alcohol and Drugs Survey
Figure 3 - Text description
Graph showing past 30 days use of e-cigarettes in students, grades 7 to 12, for the years 2014-15, 2016-17 and 2018-19.
| Fiscal year | Percentage |
|---|---|
| 2014-15 | 5.7 |
| 2016-17 | 10 |
| 2018-19 | 20.2 |
Data from the Canadian Tobacco and Nicotine Survey (CTNS)Footnote ii also shows changes in vaping use. It found that the vaping rate for youth (aged 15 to 19) increased dramatically between 2017 and 2019 (from 6% to 15%), but did not show an increase from 2019 to 2020 (14% in 2020). For young adults (aged 20 to 24), the prevalence of vaping was 6% in 2017, 15% in 2019, and 13% in 2020.Footnote iii
The 2019 survey found that most vaping was done using nicotine products (approximately 80%). This proportion was even higher among users aged 15 to 24, with almost 90% vaping nicotine products. The same survey found that, of the students who had vaped (with or without nicotine) in the past 30 days, 42% indicated that they had never smoked a cigarette.Endnote 29
Concerns were raised by key informants about the potential for vaping leading to tobacco use, as there is recent evidence linking the use of vaping among youth and young adults, to an "increased risk" of ever using tobacco cigarettes.Endnote 30 However, this "increased risk" has not been seen at the population level in terms of surveillance data.
The Minister of Health's Mandate Letter from December 2019 included the direction to address the rapid rise in youth vaping, starting with regulations to reduce the promotion and appeal of vaping products to young persons. It also encouraged Health Canada to explore additional measures.Endnote 31
Regulatory work to address youth vaping
Starting early in 2019, Health Canada began taking regulatory action to further protect youth and the Department is currently moving to introduce additional regulations under the TVPA that should help address youth vaping. Actions include:
- Banning promotion and advertising of vaping products in public spaces and at points of sale (including online) that can be seen or heard by youth: implemented in 2020;
- Change to concentration of nicotine in vaping products: came into force July 2021;
- Restrictions on flavours: consultation on regulatory proposal launched in June 2021;
- Age Verification for Online Sales: consultation on regulatory proposal expected in fall 2022; and
- Proposed Vaping Products Reporting Regulations: consultation on the regulatory proposal expected in spring 2022.
These regulatory actions were informed by extensive public consultations undertaken by Health Canada, for example, related to potential measures to reduce the impact of vaping products advertising on youth and non-users of tobacco products and potential regulatory measures to reduce youth access and appeal of vaping products.
One of the primary challenges noted by Health Canada internal key informants related to policy and regulatory development was that evidence related to vaping is continuing to emerge, given that it is a relatively new product. Key informants also identified a number of other issues that were seen as hindering progress: resource constraints, workload associated with the relatively high number of new regulations developed recently, human resources challenges (for example, in terms of large numbers of new staff), and information technology system-related barriers.
Exploring additional regulatory measures
In January 2020, the Council of Chief Medical Officers of Health released a statement on Nicotine Vaping in Canada, in which they provided regulatory and policy recommendations for federal, provincial/territorial, and municipal governments to address youth vaping. At the same time, they acknowledged that governments have already taken steps to implement some of their recommendations, which included regulations to reduce attractiveness and accessibility for young people, for example, related to packaging, advertising, flavouring, nicotine concentration, on-line sales and minimum sales age.Endnote 32
Feedback from internal and external interviewees on the potential to broaden regulations was consistent with the recommendations from the Council of Chief Medical Officers of Health. Many internal and external interviewees indicated that more could be done to protect young people and non-users of tobacco products from nicotine addiction, citing particular concerns regarding the increase in youth vaping. A few elaborated on concerns for youth, related particularly to their vulnerability to the negative health effects of nicotine and their susceptibility to developing a dependence on nicotine. Several external interviewees believed that while Health Canada had intended to shift adults who smoke from tobacco products to vaping products, an unintended consequence was young people who were not smokers starting to vape nicotine.
Youth vaping goal
Health Canada has internal plans and strategies for helping to address youth vaping, some of which have been communicated to the public, for example, through news releases and public consultations. However, an integrated plan does not appear to have been communicated to interested parties. Many internal and external key informants indicated that to lower youth vaping rates, a clear path should be identified. Ideas for such a plan included setting short-, medium- and long-term targets, and ensuring that it is aligned with the CTS. Both internal and external key informants noted the importance of working collaboratively with stakeholders to help address this issue.
Use of data generated or supported by Health Canada in decision making
Activities in support of this intended result aimed to strengthen science and surveillance, and to support partnerships, including the WHO FCTC.
Science and surveillance
In support of tobacco and vaping, Health Canada has generated and supported the development of a wide variety of science and surveillance data and information. Research projects consisted of surveillance and public opinion research (POR) reports, evidence syntheses to support risk management for vaping, lab testing to characterize the constituents and emissions of vaping and tobacco products, and business intelligence reports on tobacco and vaping product markets. Completed projects were either shared directly with decision makers or published on the canada.ca website to allow access to the wider stakeholder groups involved in the implementation of the CTS.
Surveillance tools like the Canadian Tobacco Alcohol and Drugs Survey (CTADS), Canadian Community Health Survey (CCHS), and Canadian Tobacco and Nicotine Survey (CTNS) were implemented in collaboration with Statistics Canada and helped capture trends in tobacco and vaping products use prevalence within various population groups. The school based Canadian Student Tobacco, Alcohol and Drugs Survey (CSTADS) and POR like the Vapers Online Survey helped assess uptake on smoking cessation strategies, as well as attitudes and behaviours regarding vaping products.
The program also undertook a number of evidence reviews, including on health effects of vaping, tobacco enforcement best practices, e-cigarettes retailer behaviour, flavours and flavour chemicals in vaping products, and harm reduction approach to smoking cessation. Social media monitoring and vaping-associated lung illness (VALI) incident reports were used to inform the vaping risk management framework. Research on trends in tobacco sales was also conducted and was distributed to internal and external stakeholders, including P/T partners. External interviewees who were familiar with Health Canada's research outputs were positive about their usefulness.
Additionally, a newly acquired piece of laboratory equipment, a 'pyrolizer', was used to perform chemical tests to identify the composition of the thermal degradation of vaping liquids with nicotine salts. Testing results were summarized and shared with other program areas so that they could be used to inform program decision making.
The program also participated in bilateral research collaborations with the United States and the Netherlands to increase capacity for surveillance and testing of vaping products. The project with the US CDC involved standardization around interpretation of biomonitoring data, whereas the project with the Dutch National Institute for Public Health and the Environment consisted of a collaboration on developing a test bed for e-cigarettes. Investments were made in increasing capacity for performing scientific analyses of vaping products in-house and in collaborations with other government departments to conduct product analysis at national and provincial levels. Collaborative research agreements were signed between the Tobacco Products Regulatory Office, Compliance and Enforcement Division and the Health Products Laboratories at ROEB to perform analyses of samples of tobacco products provided by the Tobacco Products Regulatory Office. In addition to supporting science and surveillance activities, the laboratories also support compliance and enforcement work.
Existing gaps
Several key informants noted that, unlike for tobacco products, there are no regulatory reporting requirements for the vaping industry under the TVPA. This has implications for Health Canada's research and surveillance work as it leads to information gaps that must be addressed internally, for example, the program had to design and implement studies to obtain such information.
This gap was also identified in the 2020 statement from the Council of Chief Medical Officers of Health, which included recommendations related to vaping industry reporting, as well as other areas to consider for research and surveillance, such as:
- Require product manufacturers to disclose all ingredients of vaping devices to Health Canada as a condition of being marketed, including establishing consistency in reporting nicotine levels in both open and closed vaping systems;
- Enhance surveillance and reporting of vaping product use and population health impacts;
- Monitor and research the short and long-term health effects of vaping products;
- Research the effectiveness of vaping products in supporting smokers to end or reduce their use of all nicotine-containing products; and
- Research the effectiveness of policy approaches to address youth vaping.Endnote 33
International engagement
International engagement plays an important role in providing access to data and Canadian expertise as it relates to tobacco control, to other jurisdictions and international organizations, including the WHO, the Pan American Health Organization (PAHO), and the WHO FCTC.
The Office of International Affairs for the Health Portfolio (OIA), a shared service of Health Canada and PHAC, supports Canada's participation in the WHO FCTC to bring the Canadian perspective and contribute to shaping the international tobacco control agenda. This includes Canada's engagement and participation in the WHO FCTC's Conference of Parties (COP) meetings and the WHO FCTC Working Group to Strengthen the Implementation of the Convention through Coordination and Cooperation. OIA, in close coordination with TCD, provides strategic policy analysis and advice on governance issues to ensure accountability and transparency in the management of the Convention and the global efforts to advance tobacco control.
In addition, TCD and OIA contributed to the WHO FCTC Surveillance Knowledge Hub, which supports the Parties to the Convention in their implementation of the FCTC in areas of tobacco surveillance and health-in-all-policies approach through analysis, synthesis and dissemination of knowledge and information.Endnote 34 They also led Canada's engagement efforts with the Global Tobacco Regulators Forum (GTRF), which included hosting the first ever GTRF meeting and co-chairing the Forum.
Other recent highlights related to international engagement include attending and actively participating in the eighth session of the COP (COP8), which took place in 2018, where Canada successfully:
- championed a proposal to have the plenary session of COP8 webcast live;
- helped to ensure a key position within the Secretariat remained apolitical; and
- contributed to the development and adoption of the "Global Strategy to Accelerate Tobacco Control: Advancing Sustainable Development through the Implementation of the WHO FCTC 2019-2025".
The most recent Global Progress Report on the Implementation of the WHO FCTC noted that, in terms of contributing to international cooperation, Canada had provided a grant to the WHO FCTC Convention Secretariat to support work on the development of the Medium-term Strategic Framework, now called the Global Strategy to Accelerate Tobacco Control. Canada also funded a staff member to support the Secretariat's work in the areas of reporting and knowledge management.Endnote 35
External interviewees who were knowledgeable about Canada's international engagement noted that they worked well with contacts within both OIA and TCD, and that the relationships were open, transparent and constructive. One noted, however, that the differences in mandates of Health Canada and PHAC were not always clear, and they weren't always sure which organization to contact for certain issues.
Relevant external interviewees were also positive about Canada's contributions to the issue of tobacco control globally. This included Canada leading work in the development of the Global Strategy to Accelerate Tobacco Control, which is intended to guide the implementation of the WHO FCTC for the period 2019 to 2025. Also of note is Canada's contribution to the development of guidelines for Articles 9 and 10 of the FCTC (which relate to regulation of the contents of tobacco products and regulation of tobacco product disclosures, respectively).
Internal key informants working with international partners indicated that key challenges include financial and human resource limitations, such as high turnover of staff and limited representation at key international engagements such as those related to the Conference of the Parties to the FCTC. In addition, interviewees noted that there is no agreement among parties of the FCTC regarding vaping.
Is the information used for decision making?
The evaluation found that data generated or supported by Health Canada in the context of tobacco and vaping products is being used in decision making, both within and outside of the Department. While indicators collected through the program Performance Information Profile report the number of times research is used in decision making, details outlining where/how research was used were more challenging to find. This is not an issue specific to this program; it is often difficult to find an abundance of information directly associated with how research feeds into policy decisions or changes.
Available evidence showed that the results from research and surveillance projects are summarized and then disseminated internally using a variety of means, such as meetings, seminars and science newsletters. Data from these projects was used as an evidence base to support Health Canada web content, marketing campaigns, litigation, and provision of science advice on regulatory initiatives, including identifying emerging issues and informing policy and regulatory development within Health Canada. Specific examples include the use of research regarding plain packaging to inform the Tobacco Products Regulations (Plain and Standardized Appearance), and the use of laboratory data to propose vaping product standards. Some internal interviewees noted that information technology systems limit the usability of some information, for example, tobacco reports and sales data.
There was also evidence that external groups, including NGOs, researchers, PTs, other government departments and international partners, have used Health Canada data. Many external interviewees noted that they use Health Canada research and data from surveys, POR, surveillance, and business intelligence studies to inform their work. Examples include the use of business intelligence data by international partners for reporting exercises, and the use of data by national partners to inform their research and communication materials, as well as to help them identify target groups. In addition, Finance Canada reportedly used Health Canada research to inform a decision on taxation.
Suggestions to consider for the future to make Health Canada data even more useful to external partners included:
- developing a more interactive platform for data so that it's possible for others to generate customized reports;
- conducting surveys more frequently and including more detailed questions related to vaping;
- making data relevant to sub-groups, such as municipalities; and
- creating a mailing list for sharing research updates.
In terms of making data relevant to sub-groups, Health Canada has worked with Statistics Canada to include vaping as core content in the CCHS nationally, which is reportable by health region.
Industry compliance with legislative and regulatory requirements
Health Canada undertakes compliance and enforcement activities to help ensure that manufacturers, importers, and retailers of tobacco products and vaping products comply with legislative and regulatory requirements. The Tobacco and Vaping Compliance and Enforcement Program (TVCEP) of the Regulatory Operations and Enforcement Branch (ROEB) has undertaken extensive compliance and enforcement activities over the period of the evaluation. Under the Tobacco Act, these activities included collecting and analyzing samples from the tobacco manufacturing and importing sector, inspecting retail establishments, and, reviewing reports from tobacco manufacturers and importers, which are required under the Tobacco Reporting Regulations.Endnote 36 With the enactment of the TVPA on May 23, 2018, compliance and enforcement responsibilities were expanded to include vaping products, in addition to tobacco.
Tobacco Inspections
For the period of the evaluation, inspection activities were as follows.
| Fiscal Year | Tobacco Retail Inspections | Tobacco Manufacturer Inspections | ||
|---|---|---|---|---|
| # of Completed Inspections | % of Planned Inspections | # of Completed Inspections | % of Planned Inspections | |
| 2016-17 | 6,792 | 101% | 66 | 99% |
| 2017-18 | 4,109 | 100% | 67 | 102% |
| 2018-19 | 6,716 | 99% | 73 | 101% |
| 2019-20 | 691 | 33% | 2 | 4% |
A lower number of inspections were planned in 2017-18, as it was a transition year due to anticipated Tobacco Act amendments related to tobacco and vaping products coming into force later in the year. In 2019-20, fewer tobacco inspections were completed due to the prioritization of vaping product inspections caused by the recent coming into force of the TVPA and the increase in youth vaping rates. The target of 2,000 gas station and convenience store inspections for 2019-20 was intended to include inspections of both tobacco and vaping products. However, the inspection approach was revised in October 2019 to focus solely on vaping products at those gas station and convenience stores. As a result, 2094 such inspections were completed, but only 691 inspections assessed tobacco products (33% of those completed).
The COVID-19 pandemic forced TVCEP onsite tobacco inspections to be put on hold in 2020-21. Recognizing that the traditional approach to inspections would be difficult during the pandemic, processes and tools were developed to initiate remote inspection activities for the collection and analysis of tobacco products. Thus, in Q4 of 2020-21, TVCEP conducted 10 remote inspections of tobacco product manufacturers/distributors, and received 124 tobacco product samples for assessment against new product and package requirements under the Tobacco Products Regulations (Plain and Standardized Appearance).
Enforcement actions, following the identification of non-compliant tobacco products, included warning letters, negotiated compliance, and where appropriate, seizures of non-compliant tobacco products. For example, in 2019, enforcement actions included 22 warning letters, 13 negotiated compliances, and 60 seizures, including 67 cigarette packages, 695 cigarettes, 2011 other tobacco packages, and 35,719 other tobacco individual units (e.g., cigars).
Is the tobacco industry compliant?
Evidence showed that there have been high levels of industry compliance with tobacco legislative and regulatory requirements (see table 3). For the years, 2016-17 to 2019-20, there was a 92% average compliance rate for inspected retail establishments.Footnote iv The average compliance rate for inspected manufacturers was 95%.
However, in response to the rise in youth vaping, the number of tobacco inspections decreased starting in 2019-20 as the program shifted its compliance and enforcement activities towards the vaping industry. Some internal and external interviewees expressed concern that tobacco compliance rates may start to drop if tobacco inspections remain low for an extended period of time. In 2020-21, TVCEP did conduct some remote inspections of tobacco product manufacturers/importers.Footnote v The compliance rates for the remote inspections were high (94% for cigarettes and 100% for little cigars).
| Fiscal Year | Tobacco Inspection Compliance Rates | |||
|---|---|---|---|---|
| Inspected Retail establishments | Inspected Manufacturers | |||
| Labelling Regulations | Prohibition of Promotion of Prohibited Additives | Packaging and Content for Prohibition of Additives | ||
| 2016-17 | 89% | 94% | 85% | 99% |
| 2017-18 | 91% | 84% | 100% | 98% |
| 2018-19 | 91% | 96% | 100% | 99% |
| 2019-20 | 96% | n/a | 100% | 100% |
Vaping Inspections
The implementation of the new legislative framework regulating vaping products brought some challenges for compliance and enforcement activities as inspectors were required to undertake work under three Acts:
- Tobacco and Vaping Products Act
- Canada Consumer Product Safety Act
- Food and Drugs Act
To help address these challenges, TVCEP was redesigned to deliver activities under the vaping regime with cross-designated inspectors (i.e., who have the authority to inspect and take appropriate enforcement action under multiple legislations). TVCEP worked with other implicated Health Canada branches to develop a cross-designation model for vaping inspection, which covers the three relevant Acts.
To assist with its processes, the program also created a streamlined method to more efficiently deal with inquiries and complaints, as well as a dedicated on-line compliance and enforcement oversight for vaping products.
Given that there are no federal regulatory requirements under the TVPA or CCPSA for regulated parties that manufacture, import or sell vaping products to become licensed, registered, or provide information to Health Canada on their business, it was necessary for the program to identify these regulated parties in order to conduct vaping retail and online inspections. In addition to identifying the regulated parties and associated retail establishments and online sites and pages, this work included the development of new tools and procedures. According to program representatives, work to identify this range of business intelligence is ongoing today.
In 2019-20, TVCEP surpassed their target of 3,000 vaping product retail inspections, conducting 3,187 on-site inspections of retail establishments in total. This included 2,094 retail inspections at convenience stores and 1,093 inspections at specialty vaping establishments. However, due to the observed high level of major non-compliances of vaping products with the TVPA, which increased the time required, the program had to amend its inspection approach by reviewing only a selection of products under particular legislative sections. Five of these sections were under the TVPA (furnishing to young persons, appealing to youth, life-style advertising, prohibited flavours, and testimonials or endorsements), and the other three were under the CCPSAFootnote vi (classification labelling, child resistant packaging, nicotine concentration). This allowed inspectors to cover more retail locations and inform retailers of any non-compliance through additional compliance promotion.
TVCEP also conducted inspections at 40 festivals and events, and issued letters to vaping manufacturers and importers to help increase awareness of their obligations under the TVPA, CCPSA and associated regulations.Footnote vii
Enforcement actions in response to observed instances of non-compliance for vaping productsFootnote viii included warning letters, negotiated compliance seizures of vaping products under the TVPA and the issuance of stop sale orders under the CCPSA. For example, in 2019, compliance actions included 370 warning letters, 30 negotiated compliances, 114 stop sales, and 923 seizures (including 78,460 e-liquid bottles and 5,739 vaping devices/parts).
In the interest of openness and transparency, Health Canada began publishing quarterly Vaping Product Enforcement Reports. These reports provided information about compliance and enforcement activities, including lists of non-compliant retail establishments and online stores. Two such reports were published (July to September 2019 and October to December 2019) before the COVID-19 pandemic emerged. The program published its third report in August 2021, which lists non-compliant regulated parties identified during the 2020-21 online inspections of Instagram accounts. Several external interviewees were positive about these reports, as they provide key information on compliance and corrective measures used. They indicated a desire to see them continue to be published in the future.
In 2020-21, within the constraints of the COVID-19 pandemic and similarly to the approach taken for tobacco inspections, the program moved away from in-person inspections, instead initiating on-line and remote inspections. Online inspections included two sections of the TVPA (those related to testimonials and endorsements, and to the promotion of prohibited flavours). The program completed 304 online inspections and conducted monitoring for 1007 websites and social media accounts for age-gating mechanisms. Inspectors sent a total of 161 warning letters in response to issues of non-compliance.
Internal key informants noted some challenges in their work, including the high workload associated with the commitment to conduct 3,000 inspections, human resource issues, in particular a high turnover at the director level and contention over the different job classifications of employees doing similar work, information technology system-related barriers, and issues related to the COVID-19 pandemic.
Is the vaping industry compliant?
In 2019-20, Health Canada found that 83% of inspected specialty vaping establishments did not fully comply with the TVPA. In contrast, only 13% of inspected convenience stores were found to be non-compliant. Online inspections conducted in 2020-21 continued to see high levels (about 50%) of non-compliance among specialty vaping establishments, based on their online presence (Instagram accounts).
Some internal and external key informants, also noted that vaping product sales to teenagers were a continuing problem. Program representatives indicated that the data collected through online monitoring of age-gating mechanisms done in 2020-21, which was still under analysis at the time of this report, will provide additional information on the issues and support planning of further actions.
Exploring potential improvement
The 2020 statement from the Council of Chief Medical Officers of Health included a recommendation to enhance compliance and enforcement of the provisions of the TVPA.Endnote 37 Feedback from some internal interviewees was consistent with this recommendation. Several internal key informants noted that a broader range of compliance and enforcement tools could potentially facilitate effectiveness and efficiency.
Question 2: Efficiency
Overall, the program has clear governance with well-defined roles and responsibilities, which allows for efficient information sharing and coordination.
Challenges to the efficiency of activities were observed. For Health Canada, these were related to unbalanced resource allocation between tobacco and vaping and gaps in the information systems. For PHAC, challenges were related to the collection and use of performance data from funded initiatives.
The evaluation explored if efficiency could be enhanced in terms of:
- Governance, including role and responsibilities;
- IM/IT systems and tools (Health Canada specific);
- Collection and use of performance data from funded initiatives (PHAC specific);
- Resource allocation between vaping and tobacco (Health Canada specific); and
- Resource use.
Governance, roles, and responsibilities
Within Health Canada
There are several formal committees and working groups for coordinating and overseeing Health Canada CTS implementation, including at the Director General, Director and Manager level.
The Tobacco Strategy Steering Committee aims to provide leadership and oversight for Canada's Tobacco Strategy. It is a Director General level committee that includes representation from participating federal departments, and is chaired by the Director General of Health Canada's TCD.
Within Health Canada, there are both formal and informal mechanisms in place for governance and collaboration. Examples of formal committees include:
- Vaping Director Steering Committee, which is led by TCD and includes representatives from other implicated directorates and branches. It was created to provide a coordination role for vaping activities within Health Canada.
- National Compliance Management Committee, which was created to strengthen inter-regional working relationships and ensure consistency in addressing compliance and enforcement issues and practices by developing and implementing national strategies, and sharing information and advice with various regional offices. It includes participants from ROEB, TCD and HECSB.
- National Tobacco Committee and National Vaping Committee. These committees include staff members at all levels from ROEB, TCD and HECSB, and reports to the National Compliance Management Committee to deal with tobacco or vaping issues, policies and procedures for greater efficiency.
- National Specialist Committee, which has a mandate to ensure consistent, effective and timely application and delivery of the tobacco and vaping program through the application of the TVPA, CCPSA, and FDA. This committee includes specialists from Health Canada regions and headquarters, and reports to the National Compliance Management Committee. This committee was discontinued in 2020-21.
In addition, there are working groups that support compliance and enforcement activities (for example, the Compliance and Enforcement Vaping Working Group), as well as website and social media activities (for example, the Website Inspections Working Group and the Social Media Inspections Pilot Project Working Group).
Along with formal mechanisms for collaboration, several internal interviewees discussed informal engagement between program staff related to tobacco and vaping, indicating that there are positive relationships and cooperation between groups.
While program representatives were generally positive about working relationships, coordination and collaboration internally within Health Canada, views on the effectiveness of the current governance structure were somewhat mixed. While some were positive about the way the Department had organized tobacco and vaping activities, others indicated that the governance structure could benefit from being updated to better include the issue of vaping.
Overall, internal interviewees were positive about the clarity of roles and responsibilities related to tobacco and vaping activities. However, compliance and enforcement was one area that was seen to have faced particular challenges in this regard. For example, several internal key informants indicated that the multi-act approach to vaping (which includes the TVPA, CCPSA, Cannabis Act and FDA), had introduced complexities for compliance and enforcement staff, that led to a lack of clear roles and responsibilities in some instances, duplication of effort and a need for enhanced internal coordination. Further, ROEB was seen to be inadequately resourced to conduct compliance and enforcement for vaping products and, as a result, had to leverage all available resources. This approach is not considered to be sustainable and the program indicated a need to review its complement of inspector resources. These challenges for compliance and enforcement were seen to have been exacerbated by the ambitious target of conducting 3,000 inspections of vaping establishments in 2019-20, which led to an increased workload. However, internal key informants were positive about ROEB's performance in the face of these challenges.
Within the Heath Portfolio
Both Health Canada and PHAC internal interviewees agreed that there is regular and effective communication between Health Canada and PHAC on grants and contributions programs. Program representatives were aware of the other organization's funding objectives and indicated that they work collaboratively to review some of the proposals received or to share information on proposals that might be a better fit with the other organization. While internal informants recognized the good working collaboration and open communication between both organizations, there was a suggestion made by some internal key informant that the two groups could share more project performance results.
Most external key informants who were knowledgeable about funding programs agreed that the distinction between the two organizations' funding opportunities and objectives were clear, well understood, and did not create any a duplication.
F/P/T collaboration
The Tobacco Control Liaison Committee (TCLC) is the formal mechanism for Health Canada's engagement with F/P/T governments on issues of tobacco and vaping. The TCLC provides a forum to plan, promote and advance tobacco control with the goal of improving the policy coherence and programming efficiency of tobacco controls across all levels of government. Areas of activity include cessation programs, enforcement of youth access restrictions, promotion and display restrictions, smoke-free spaces, public education, research and policy and regulatory development.
Key informants knowledgeable about the TCLC had positive views about it, stating that it was a good mechanism to share information and advance collaboration on files. For example, in 2019, the TCLC created a sub-working group on youth vaping to identify opportunities for multi-jurisdictional collaboration and action to address youth vaping rates in Canada, and to provide programming, policy and regulatory recommendations to the TCLC.
IM/IT systems (specific to Health Canada)
As part of the funding received for the Federal Regime to Address the Benefits and Harms of Vaping Products, there were commitments to establish national business requirements and support testing, implementation, and administration of an information management and information technology (IM/IT) system for compliance and enforcement of vaping measures. However, to date this has not been completed as planned. According to program representatives, delays resulted from the COVID-19 pandemic and issues related to procurement and contracting, which were outside the control of the program.
Program documentation over the period of the evaluation identified challenges and risks related to information systems, noting that they need to be modernized to better support current and emerging program needs. For example, some plans and reports noted that regulatory tools had not been modified to reflect amendments to the Tobacco Reporting Regulations and that updated information system infrastructure was required in order to meet operational requirements. Issues with information systems were also identified as a key factor that may impede delivery of the program's mandate.
Internal key informants, across all activity areas identified the inadequacy of information systems and tools as a key challenge. Some systems are considered antiquated while others are non-existent. Gaps in information technology systems have led to extra time for accessing and analyzing information, and have sometimes resulted in missed opportunities to use program data or to collect information that could inform the program.
Collection and use of performance data from funded initiatives (specific to PHAC)
One of the MSP Program's main objectives is to provide evidence of what intervention does or does not work, for whom and in what contexts. This is done through collection of performance measurement information and knowledge exchange.
The evaluation found that, while performance measurement data was collected for all PHAC projects throughout the period of this evaluation, there were some issues with the data collected, including lack of targets, inconsistent data collection, and lack of systematic compilation of project results.
In terms of knowledge exchange, while the evaluation was able to find occurrences of experience sharing between projects and with external stakeholders, there was no systematic approach to sharing project results. Such a systematic approach would aid not only in terms of designing and improving projects, but also for decision making.
These issues were already well known by the program as they were previously identified in the 2020 evaluation of the MSP program. As such, the evaluation found that actions have already been taken to address the shortcomings in the collection and use of performance data. More specifically, the program implemented in 2020 the following mechanisms to further standardize and strengthen performance measurement:
- New MSP funding recipients have been provided with systematic guidance by the Partnership and Strategies Division (PSD) performance team as recipients develop and finalize their evaluation plan with their third party evaluator. They received a Performance Measurement and Evaluation Guidance document, which includes a menu of outcome indicators and associated measurement tools for tobacco and other MSP or HCCF projects. All projects are expected to choose from these common indicators. This has resulted in a more standardized method of measuring and reporting results.
- Tobacco baselines and targets were developed in 2020-21. These are grounded in the literature and based on the results from tobacco projects to date.
Furthermore, knowledge mobilization is set to be a key component of the HCCF, which has replaced the MSP program. This includes a plan to roll up lessons learned across projects. Both MSP and HCCF results will be captured in a knowledge mobilization strategy that is planned for 2021-22.
The success of these new measures, which are expected to improve program efficiency, will need to be examined at the program's next evaluation cycle.
Resource allocation between vaping and tobacco (specific to Health Canada)
In terms of resource stability and forward planning, a few internal key informants discussed the importance of having secured ongoing funding for the issues of tobacco and vaping, which was achieved through the Federal Regime to Address the Benefits and Harms of Vaping Products and the CTS.
There was, however, general agreement among internal key informants that despite the specific funding having been allocated to tobacco and to vaping, the majority of resources had been focused on vaping in recent years. In addition to increased financial and human resources being dedicated to vaping, there was a perception that this was also the case for the attention of decision makers whose 'bandwidth' was monopolized by vaping.
For some, this was perceived to be appropriate given that youth vaping had increased rapidly. Others expressed concerns that a continued focus on vaping over an extended period of time could impact the program's goal of reaching less than 5% tobacco use by 2035.
Compliance and enforcement activities were seen to have been particularly affected by resources being reallocated towards vaping. As discussed earlier in the report, starting in 2019-20, the number of tobacco inspections waned as the program focused on inspections related to vaping products. This was necessary to meet the target of 3,000 vaping product retail inspections in 2019-20, but led to a much lower numbers of completed tobacco inspections during that timeframe. While compliance rates for tobacco products are typically high, some internal and external interviewees expressed concerns that tobacco compliance rates may start to drop if the number of tobacco inspections remains low for an extended period of time. Some interviewees also questioned whether policy and regulatory work related to tobacco would be affected by a continued focus on vaping.
Program planning documentation shows that the program plans to continue to reallocate some tobacco resources towards the issue of vaping, although specific amounts were not identified.
Resource use
Overall, Health Canada and PHAC spent almost 97% of planned resources on tobacco and vaping activities from 2016-17 to 2020-21. Health Canada spent approximately $2M more than planned, for a variance of about 1% (see Table 4). Health Canada spending by branch, outlined in Table 5, shows a significant increase in actual spending over planned spending within CPAB on public education and outreach activities (366% of planned). From 2017-18 to 2020-21 CPAB received additional funding from CSCB to support activities aimed at preventing youth from vaping and, in 2019-20, CPAB activities were augmented based on a new funding allocation.
Spending was also higher than planned for ROEB, which overspent by 22%. This was in part due to the commitment to inspect 3,000 vaping product retail establishments. HECSB spent 138% of planned resources, largely due to a variety of more complex than anticipated science-based activities and analyses completed in response to CSCB's growing needs for analyzing additional substances (for example, related to biomarkers of exposure in users of tobacco and vaping products, identification of unknown substances in e-cigarette fluids and cigarette ignition propensity testing). However, the absolute dollar value of this overspending was small (less than $2M). Conversely, Health Canada only spent 76.4% of planned G&C funding, although the dollar value was small again (less than $2M in unspent funds).
Over the period of the evaluation, PHAC spent approximately 83% of its funds, including 82% of its G&Cs funds (Table 6). Notable variances were in 2017-18 and 2020-21. The program spent 134% of planned resources in 2017-18 due to a transfer from the Integrated Strategy for Healthy Living and Chronic Disease fund (ISHLCD) to support projects addressing tobacco prevention and cessation. The ISHLCD fund supports initiatives that address multiple risk factors for chronic disease prevention including tobacco use. In contrast, PHAC spent only 64.2% of planned funding in 2020-21, because the COVID-19 pandemic prevented funded projects from conducting certain planned activities. As a result, funding recipients requested amendments to shift funds to subsequent fiscal years.
| Fiscal Year | Planned Spending | Actual Spending | Variance | |||||
|---|---|---|---|---|---|---|---|---|
| G&Cs | Total | G&Cs | Total | G&Cs | Actual/ Planned G&Cs | Total | Actual/ Planned Total | |
| 2016-17 | -- | $29,027,999 | -- | $28,507,425 | -- | -- | $520,574 | 98.2% |
| 2017-18 | -- | $34,846,701 | -- | $34,119,853 | -- | -- | $726,848 | 97.9% |
| 2018-19 | -- | $40,202,944 | -- | $39,412,415 | -- | $790,529 | 98.0% | |
| 2019-20 | $2,500,000 | $44,366,709 | $1,926,430 | $50,863,385 | $573,570 | 77.1% | $-6,496,676 | 114.6% |
| 2020-21 | $4,500,000 | $40,640,467 | $3,410,080 | $38,130,805 | $1,089,920 | 75.8% | $2,509,662 | 93.8% |
| TOTAL | $7,000,000 | $189,084,820 | $5,336,510 | $191,033,883 | $1,663,490 | 76.2% | $-1,949,063 | 101% |
| Source: Chief Financial Officer Branch | ||||||||
| Branch | Planned | Actual | Varianc | Actual/Planned |
|---|---|---|---|---|
| CSCBFootnote * | $126,145,271 | $ 110,855,444 | $15,690,311 | 87.6% |
| CSCB (SUAP) | $7,275,265 | $5,556,722 | $1,718,543 | 76.4% |
| ROEB | $18,833,443 | $ 23,016,599 | $-4,183,156 | 122.2% |
| CPAB | $5,000,000 | $18,282,628 | $-13,282,628 | 365.7% |
| HECSB | $5,045,459 | $6,937,591 | $-1,892,132 | 137.5% |
Source: Chief Financial Officer Branch / Does not include Internal Services |
||||
|
||||
| Fiscal Year | Planned Spending | Actual Spending | Variance | |||||
|---|---|---|---|---|---|---|---|---|
| G&Cs | Total | G&Cs | Total | G&Cs | Actual/ Planned G&Cs | Total | Actual/ Planned Total | |
| 2016-17 | $2,455,000 | $2,455,000 | $1,883,722 | $1,883,722 | $571,278 | 76.7% | $571,278 | 76.7% |
| 2017-18 | $2,205,000 | $2,205,000 | $2,972,169 | $2,972,169 | $-767,169 | 134.8% | $-767,169 | 134.8% |
| 2018-19 | $2,705,000 | $2,881,393 | $1,903,464 | $2,108,093 | $801,536 | 70.4% | $773,299 | 73.2% |
| 2019-20 | $3,455,000 | $3,636,845 | $2,950,080 | $3,145,329 | $504,920 | 85.4% | $491,516 | 86.5% |
| 2020-21 | $4,455,000 | $4,636,895 | $2,850,275 | $ 2,975,598 | $1,604,725 | 64.0% | $1,661,297 | 64.2% |
| TOTAL | $15,275,000 | $15,815,132 | $12,559,710 | $13,084,911 | $2,715,290 | 82.2% | $2,730,221 | 82.7% |
| Source: Chief Financial Officer Branch | ||||||||
Conclusions and Recommendations
Conclusions
Year after year, tobacco prevalence rates have been decreasing, from 17.7% in 2015 to 14.8% in 2019. This decline has largely been driven by a reduction in initiation, primarily teen smoking, and, unfortunately, deaths.
Recent Health Canada and PHAC initiatives, such as the introduction of plain and standardized appearance requirements for tobacco products and their packaging, as well as funded tobacco cessation projects targeting high-risk groups, may lead to further reductions in smoking rates. However, more work is required to move towards the goal of less than 5% tobacco use by 2035, as there are close to five million smokers in Canada with an estimated 48,000 people dying from smoking-related causes each year.
Furthermore, new challenges have arisen since the inception of the program, the foremost of which is youth vaping. While the prevalence rate for youth tobacco use is at an all-time low, the rapid rise in youth vaping represents a focus for the program in the future.
While several initiatives to address youth vaping were made under the TVPA, implementing these new regulations will take time. Furthermore, resource capacity and the current range of enforcement tools available under the same Act are seen as potential limitations to the program's ability to address non-compliance issues in a timely manner in certain circumstances.
What worked well
Over the last five years, Health Canada achieved a great deal in the development, implementation, and enforcement of the new federal regime to address tobacco and vaping products. This includes five new regulations in support of the TVPA and CTS since 2018. Among these, Canada's new Tobacco Products Regulations (Plain and Standardized Appearance) are seen to be some of the most comprehensive in the world. In 2019-20, TVCEP surpassed their target of 3,000 vaping product retail inspections. This work has been supported by research and surveillance to inform decision making, external collaborations with a variety of partners, and public outreach and education. For example, Health Canada undertook two national public awareness campaigns for smoking cessation, and a vaping campaign in schools and communities across the country to increase awareness of the health hazards of vaping product use.
Health Canada and PHAC program areas, in close coordination with OIA, brought the Canadian perspective forward in global discussions and contributed to shaping the international tobacco control agenda. This was done through Canada's participation in the World Health Organization Framework Convention on Tobacco Control (FCTC) and engaging in related activities such as the Conference of Parties (COP) meetings and the Working Group to Strengthen the Implementation of the Convention through Coordination and Cooperation. External interviewees were positive about Canada's contributions to the issue of tobacco control globally.
There is evidence that some more mature projects funded by PHAC have supported participants to quit smoking. Newer projects are aiming to reach groups that experience health inequalities and face higher rates of tobacco use than the general population, a priority under Canada's Tobacco Strategy. Strong relationships with both external and internal stakeholders and effective program design were seen to have enabled successful outcomes for PHAC-funded projects.
What the challenges were
As a result of the rapid rise in youth vaping, Health Canada's financial and human resources have been largely focused on vaping activities in recent years. While the importance of addressing youth vaping was not in question, there was some concern that decreasing the focus on tobacco-related issues for an extended period of time might result in lost ground in that area.
Related to the CTS goal of less than 5% tobacco use by 2035, while the program has internal planning and performance measurement documents, a clear path for Health Canada's contributions towards goal has not been shared with stakeholders.
Vaping industry compliance with legislative and regulatory requirements varies widely between types of retailers, with low levels of compliance noted at specialty vaping establishments in particular. The current legal regime for vaping under the TVPA provides a range of enforcement tools that should be expanded to better support efficiency and effectiveness of compliance and enforcement activities. It could be worthwhile considering the full range of enforcement tools available more carefully in light of the expressed concerns. The current regulatory regime may also affect the efficiency and effectiveness of other areas of activity, for example, in terms of the increased work load to identify retail establishments manufacturing and selling vaping products, and to study the contents of vaping products.
Another key challenge for tobacco and vaping activities at Health Canada relates to information technology systems. Some systems are antiquated while others are non-existent, for example, commitments for an information technology system for vaping compliance and enforcement were not completed as planned. These issues with information technology systems have led to extra time for processing and accessing information, and sometimes opportunities to collect and analyze information that could inform the program have been missed.
Health Canada's website includes extensive information and resources on the issue of vaping. Although this includes information on health hazards arising from vaping use, it is important that the most up-to-date science continue to be reflected on the website. Furthermore, certain risks may not be fully articulated on the website, such as, the health hazards of dual use and risks to pregnant women. Finally, some of Health Canada's statements in relation to vaping products appear to be consistent with a therapeutic claim even though no such vaping products are licensed as a cessation aid in Canada.
Challenges facing PHAC's tobacco cessation activities included inconsistent performance measurement and limited systematic knowledge exchange. However, the implementation of the Healthy Canadians and Communities Fund, which is replacing the MSP program, includes plans to address these concerns.
Recommendations
The findings from this evaluation have resulted in the four recommendations listed below, all of which are directed at Health Canada. In addition to the recommendations below, the evaluation findings highlighted several other areas that the program could consider. These include options to address challenges such as, high rates of youth vaping, identifying regulated parties in order to conduct vaping retail and online inspections, determining vaping product ingredients to support surveillance and science activities, and ensuring non-compliant vaping products are quickly removed from the market. However, given that a legislative review was underway at the time this report was written, the program is encouraged to consider the results of that review alongside evaluation findings in order to explore potential improvements.
There are no recommendations for PHAC, given that actions to address knowledge exchange and performance measurement issues are already underway.
Recommendation 1: Communicate to partners and stakeholders, Health Canada's action plan for the Department's contribution to achieving CTS goals and for addressing youth vaping.
Health Canada and its partners have an ambitious target of less than 5% tobacco use by 2035. In addition, youth vaping rates remain a concern. While Health Canada does have internal planning and performance measurement documents related to both these issues, these plans have not been communicated to stakeholders in an integrated way. As a result, some key stakeholders have the impression that Health Canada has not defined a coordinated, department-wide plan. Several key informants indicated that having knowledge of Health Canada's plan could help inform their own work and the work of other groups, and help assess progress on the issues of tobacco use and youth vaping. In communicating this plan, Health Canada should coordinate with Health Portfolio partners and key stakeholders to facilitate cooperation and buy-in from relevant groups.
Recommendation 2: Enhance information technology systems and data analytics capacity to support program activities.
Current information systems do not allow the program to access and analyse information from various sources related to tobacco and vaping in support of program activities. This is in part due to antiquated systems and in part because commitments for delivering a new tobacco and vaping compliance and enforcement system are still in progress. The program should work with relevant partners to complete and implement the new system, including providing appropriate training. In addition, opportunities to update and enhance information technology systems to support other activity areas should also be identified.
Recommendation 3: Examine resource distribution between tobacco and vaping activities.
The rise of youth vaping and the high level of non-compliance of the vaping industry (particularly specialty vaping establishments) meant the program reallocated internal resources from tobacco to support the needed level of activities related to vaping. Recognizing that limited resources are available, the program should continue to use a risk-based approach to allocate resources to deal with priorities.
Recommendation 4: Review and update the Health Canada website to reflect the most up-to-date science and public health advice on health risks and benefits of vaping.
Health Canada's website includes information regarding health hazards arising from vaping use, and it is important that the most up-to-date information continue to be reflected. Further, Health Canada does not clearly indicate that there are no vaping products that have been licensed as smoking cessation aids when stating that these products may reduce the health risks for adult smokers who cannot or do not want to quit using nicotine, if they completely switch to vaping. As evidence of health risks and benefits of vaping evolves, it is important for the success and credibility of the harm reduction messaging and the protection of Canadians, to keep Health Canada's message current and in line with the most up-to-date science.
Management Response and Action Plan
Recommendation 1
Communicate to partners and stakeholders Health Canada's action plan for the Department's contribution to achieving CTS goals and for addressing youth vaping.
| Action Plan | Deliverables | Expected Completion Date | Accountability | Resources |
|---|---|---|---|---|
|
|
April 30, 2023 June 30, 2023 September 30, 2023 |
Lead: Controlled Substances and Cannabis Branch-Tobacco Control Directorate Supporting branch: Communications and Public Affairs Branch |
NA (Existing resources will be used) |
Recommendation 2
Enhance information technology systems and data analytics capacity to support program activities.
| Action Plan | Deliverables | Expected Completion Date | Accountability | Resources |
|---|---|---|---|---|
|
1. Implement NICEMS solution: Gate 2: Prototype evaluated and funding approved Gate 3: Solution implemented and available to end users Gate 4: Project closed NICEMS training completed |
July 15, 2022 July 31, 2023 September 15, 2023 September 25, 2023 |
Lead: Controlled Substances and Cannabis Branch-Tobacco Control Directorate Support: Corporate Services Branch-Information Management Services Directorate, and Regulatory Operations and Enforcement Branch (Consumer Products and Controlled Substances Directorate and Planning and Operations Directorate) |
NA (Existing resources will be used) |
|
2.a Critical updates to legacy systems (TCIMS and TRRS) | As required until NICEMS – IP 626 is deployed in September 2023 | Lead: Corporate Services Branch-Information Management Services Directorate Support: Controlled Substances and Cannabis Branch-Tobacco Control Directorate |
NA (Existing resources will be used) |
|
2.b Interim system is officially launched and used until NICEMS IP 626 has been deployed in August 2023. | April 2022 | Lead: Regulatory Operations and Enforcement Branch -Consumer Products and Controlled Substances Directorate Support: Corporate Services Branch-Information Management Services Directorate and Regulatory Operations and Enforcement Branch - Planning and Operations Directorate |
NA (Existing resources will be used) |
|
2.c Learning and Development plans on data analytics and data literacy for program staff, workshops, seminars and training boot camps on data topics. | December 31st 2022 | Lead: Regulatory Operations and Enforcement Branch and Controlled Substances and Cannabis Branch-Tobacco Control Directorate Support: Chief Data Office |
Recommendation 3
Examine resource distribution between tobacco and vaping activities.
| Action Plan | Deliverables | Expected Completion Date | Accountability | Resources |
|---|---|---|---|---|
Develop a risk-based approach for compliance and enforcement activities for tobacco products and vaping products to inform resource allocation (within the Tobacco and Vaping Compliance and Enforcement Program's existing budget), with the objective of outlining:
|
Draft risk-based approach for compliance and enforcement activities for tobacco and vaping products presented to senior management. Resource allocation proposal presented to senior management. Final risk-based approach for compliance and enforcement activities for tobacco and vaping products and resource allocation approved by senior management. |
October 2022 November 2022 December 2022 |
Lead: Regulatory Operations and Enforcement Branch: Consumer Products and Controlled Substances Directorate Support branches: Regulatory Operations and Enforcement Branch: Labs Directorate, |
NA (Existing resources will be used) |
Recommendation 4
Review and update the Health Canada website to reflect the most up-to-date science and public health advice on health risks and benefits of vaping.
| Action Plan | Deliverables | Expected Completion Date | Accountability | Resources |
|---|---|---|---|---|
|
|
April 30, 2022 | Lead: Controlled Substances and Cannabis Branch-Tobacco Control Directorate Support: Healthy Environments and Consumer Safety Branch – Consumer and Hazardous Products Safety Directorate |
NA (Existing resources will be used) |
|
|
August 31st, 2022 | Lead: Controlled Substances and Cannabis Branch-Tobacco Control Directorate Support: Healthy Environments and Consumer Safety Branch – Consumer and Hazardous Products Safety Directorate and Communications and Public Affairs Branch |
|
|
|
December 31st, 2022 | Lead: Controlled Substances and Cannabis Branch-Tobacco Control Directorate and Communications and Public Affairs Branch Support: Healthy Environments and Consumer Safety Branch – Consumer and Hazardous Products Safety Directorate |
Annex
Annex A: Evaluation Methodology
The scope of the evaluation included all FTCS and CTS activities from April 2016 to March 2021. Evaluation data was collected using various sources and methods, including:
Document and File Review
Program staff at Health Canada and PHAC provided documents for evaluators for review. For Health Canada, documents were provided in the areas of policy and regulatory development, compliance and enforcement, science and surveillance, public education, and collaboration. Documents were also provided for PHAC's G&Cs and collaborations. A total of 508 internal files were reviewed.
Key Informant Interviews
Evaluators conducted interviews with a total of 46 key informants (17 internal to PHAC and Health Canada, and 29 external):
- P/T partners: 6
- NGOs: 10
- International: 2
- Academics and experts: 4
- SUAP funding recipients: 4
- PHAC funding recipients: 3
Emerging themes from interviews were identified and quantified using NVIVO qualitative analysis software.
Financial Data
Health Canada's Chief Financial Officer Branch and PHAC's Chief Financial Officer and Corporate Management Branch provided financial data on planned and actual program expenditures.
Limited Jurisdictional Comparison
A focused comparative analysis of tobacco and vaping systems and messaging was conducted within Canadian P/T jurisdictions, as well as with the United States and the United Kingdom.
Academic and Grey Literature
A focused review of academic and grey literature was conducted to inform evaluation findings.
Performance Measurement Data
Health Canada and PHAC provided performance measurement data, which was analyzed to look for key trends and outcomes.
Data collected by these various methods was analyzed by triangulation to increase the reliability and credibility of the evaluation findings and conclusions.
Still, most evaluations face constraints that may affect the validity and reliability of evaluation findings and conclusions.
| Limitation | Impact | Mitigation Strategy |
|---|---|---|
| Key informant interviews are retrospective in nature, providing only a recent perspective on past events. | This can affect the validity of assessments of activities or results that may have changed over time. | Triangulation with other lines of evidence substantiated or provided further information on data captured in interviews. Document review also provided corporate knowledge. |
| PHAC performance measurement data was limited and heavily reliant on project participants' self-reported data. Furthermore, not all projects collected the same information. | A significant portion of project data presented is captured through self-reported data that participants provide. It was difficult to aggregate performance data to reach conclusions on the achievement of program outcomes due to differences in data collection methods. | Triangulation of other lines of evidence was used to substantiate or provide further information on performance measurement data received from the Program. |
| Financial data structure is not linked to outputs or outcomes. | There is a limited ability to assess efficiency and economy quantitatively. | Used other lines of evidence, including key informant interviews and document reviews, to assess efficiency and economy qualitatively. |
| The evaluation was not able to obtain input from industry stakeholders. | Qualitative information related to industry compliance was not available. | The evaluation focused on quantitative industry compliance data. |
| There are a variety of surveys that include questions on tobacco and vaping product use. | Results from different surveys may not be comparable and survey findings may not be consistent. | Results from surveys that were most relevant to the evaluation were used. |
Endnotes
- Footnote i
-
Impressions are the number of times content is displayed, no matter if it was clicked or not.
- Footnote ii
-
The CTNS replaced the Canadian Tobacco, Alcohol and Drugs Survey (CTADS) in 2019.
- Footnote iiii
-
While it is positive that vaping rates have not increased from 2019 to 2020, it is important for the program to continue monitoring to determine whether this is a trend.
- Footnote iv
-
For all applicable regulations except Sales to Youth provisions.
- Footnote v
-
A total of 119 cigarette primary packages (individual cigarette packages) and 113 cigarette secondary packages (cartons) were collected from 7 manufacturers/importers and a total 8 little cigar primary packages were sampled from 3 importers. These were considered representative of approximately 80% of the Canadian market for cigarettes and little cigars respectively.
- Footnote vi
-
CCPSA inspections were outside the scope of this evaluation.
- Footnote vii
-
Letters were issued regarding vaping product promotions (December 2019), to vaping product retailers (June 2019), and to vaping and retail associations to remind them of their responsibilities under the TVPA and the CCPSA (December 2019).
- Footnote viii
-
The section entitled 'Industry compliance with vaping requirements', below, includes information on compliance rates.
- Footnote 1
-
Health Canada, Canadian Tobacco and Nicotine Survey (CTNS). Retrieved from:
https://www.canada.ca/en/health-canada/services/canadian-tobacco-nicotine-survey/2019-summary/2019-detailed-tables.html - Footnote 2
-
Moodie C. et al. 2012. Plain tobacco packaging: a systematic review. London: University of London, Institute of Education, Social Science Research Unit, EPPI-Centre. EPPI Report.
- Footnote 3
-
Hammond D. 2014. Standardized packaging of tobacco products - Evidence review: Prepared on behalf of the Irish Department of Health.
- Footnote 4
-
White V, Williams T, Wakefield M. 2015. Has the introduction of plain packaging with larger graphic health warnings changed adolescents' perceptions of cigarette packs and brands? Tobacco Control. 24:ii42–ii49.
- Footnote 5
-
Health Canada, Health Canada Tobacco Control Directorate and tobacco and vaping industry meetings, Retrieved from:
https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/meeting-summaries-tobacco-vaping-industry.html - Footnote 6
-
Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, Schwartz R. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016 Jun 9;6(6):e011045.
https://pubmed.ncbi.nlm.nih.gov/27288378/ - Footnote 7
-
US Food and Drug Administration: Quitting Smoking: Closer with Every Attempt. Retrieved from
https://www.fda.gov/tobacco-products/health-information/quitting-smoking-closer-every-attempt - Footnote 8
-
Priebe,C., Atkinson J.,and Faulkner G., Run to Quit: An evaluation of a scalable physical activity-based smoking cessation intervention, Mental Health and Physical Activity, Volume 13, Pages 15-21.
https://doi.org/10.1016/j.mhpa.2017.08.001 - Footnote 9
-
Priebe,C. Wunderlich k., Atkinson J., G. Faulker (2020), Walk or Run to Quit: a 3-year evaluation of a physical activity-based smoking cessation intervention, Journal of Smoking Cessation, Volume 15, Issue 4, pp. 181 – 188.
https://doi.org/10.1017/jsc.2020.24 - Footnote 10
-
U.S. Department of Health and Human Services (2020). Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020.
https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf - Footnote 11
-
Health Canada, Summary of results for the Canadian Student Tobacco, Alcohol and Drugs Survey 2018-19, Retrieved from:
https://www.canada.ca/en/health-canada/services/canadian-student-tobacco-alcohol-drugs-survey/2018-2019-summary.html - Footnote 12
-
Earnscliffe Strategy Group (2020), Social Values and Psychographic Segmentation of Tobacco and Nicotine, Retrieved from:
https://publications.gc.ca/collections/collection_2020/sc-hc/H14-345-2020-1-eng.pdf - Footnote 13
-
Health Canada, Summary of results for the Canadian Student Tobacco, Alcohol and Drugs Survey 2018-19, Retrieved from:
https://www.canada.ca/en/health-canada/services/canadian-student-tobacco-alcohol-drugs-survey/2018-2019-summary.html - Footnote 14
-
World Health Organization (2020), Tobacco: E-cigarettes, Retrieved from:
https://www.who.int/news-room/q-a-detail/tobacco-e-cigarettes - Footnote 15
-
US Public Health Service (2014), The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Retrieved from:
https://www.hhs.gov/sites/default/files/consequences-smoking-exec-summary.pdf - Footnote 16
-
McGrath-Morrow, Sharon A. et al., The Effects of Nicotine on Development. Pediatrics Official Journal of The American Academy Of Pediatrics, March 2020, 145 (3).
https://pediatrics.aappublications.org/content/145/3/e20191346) - Footnote 17
-
Mishra, A., Chaturvedi, P., Datta, S., Sinukumar, S., Joshi, P., & Garg, A. (2015). Harmful effects of nicotine. Indian journal of medical and paediatric oncology: Official Journal of Indian Society of Medical & Paediatric Oncology, 36(1), 24–31. https://doi.org/10.4103/0971-5851.151771
- Footnote 18
-
Health Canada, Canadian Tobacco and Nicotine Survey (CTNS). Retrieved from:
https://www.canada.ca/en/health-canada/services/canadian-tobacco-nicotine-survey/2019-summary/2019-detailed-tables.html - Footnote 19
-
Health Canada, Risks of vaping, Retrieved from: https://www.canada.ca/en/health-canada/services/smoking-tobacco/vaping/risks.html
- Footnote 20
-
Public Health England (2018), PHE publishes independent expert e-cigarettes evidence review, Retrieved from
https://www.gov.uk/government/news/phe-publishes-independent-expert-e-cigarettes-evidence-review - Footnote 21
-
Wilson N., Summers J., Ouakrim D., Hoek J., EdwardsR., Blakely T. (2020). Improving on estimates of the potential relative harm to health from using modern ENDS (vaping) compared to tobacco smoking, MedRxiv.
https://www.medrxiv.org/content/10.1101/2020.12.22.20248737v2 - Footnote 22
-
Eissenberg, Thomas et al. Invalidity of an Oft-Cited Estimate of the Relative Harms of Electronic Cigarettes. American Journal of Public Health. 2020 February; 110(2): 161–162.Retrieved from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6951374/ - Footnote 23
-
Center for Disease Control and Prevention, E-Cigarettes and Pregnancy, Retrieved from:
https://www.cdc.gov/reproductivehealth/maternalinfanthealth/substance-abuse/e-cigarettes-pregnancy.htm - Footnote 24
-
Center for Disease Control and Prevention, About Electronic Cigarettes (E-Cigarettes), Retrieved from:
https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html - Footnote 25
-
Center for Disease Control and Prevention, Adult Smoking Cessation—The Use of E-Cigarettes, Retrieved from:
https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/fact-sheets/adult-smoking-cessation-e-cigarettes-use/index.html - Footnote 26
-
Center for Disease Control and Prevention, Adult Smoking Cessation—The Use of E-Cigarettes, Retrieved from:
https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/fact-sheets/adult-smoking-cessation-e-cigarettes-use/index.html - Footnote 27
-
Public Health England (2021), Vaping in England: 2021 evidence update summary, Retrieved from:
https://www.gov.uk/government/publications/vaping-in-england-evidence-update-february-2021/vaping-in-england-2021-evidence-update-summary - Footnote 28
-
Health Canada, Canadian Tobacco and Nicotine Survey (CTNS). Retrieved from:
https://www.canada.ca/en/health-canada/services/canadian-tobacco-nicotine-survey/2019-summary/2019-detailed-tables.html - Footnote 29
-
Health Canada, Canadian Tobacco and Nicotine Survey (CTNS) : 2019 detailed tables, Retrieved from:
https://www.canada.ca/en/health-canada/services/canadian-tobacco-nicotine-survey/2019-summary/2019-detailed-tables.html - Footnote 30
-
National Academies of Sciences, Engineering, and Medicine. 2018. Public Health Consequences of E-Cigarettes. Washington, DC: The National Academies Press.
- Footnote 31
-
PMO (2019), Minister of Health Mandate Letter, Retrieved from:
https://pm.gc.ca/en/mandate-letters/2019/12/13/minister-health-mandate-letter - Footnote 32
-
PHAC (2020), Statement from the Council of Chief Medical Officers of Health on Nicotine Vaping in Canada, Retrieved from:
https://www.canada.ca/en/public-health/news/2020/01/statement-from-the-council-of-chief-medical-officers-of-health-on-nicotine-vaping-in-canada.html - Footnote 33
-
PHAC (2020), Statement from the Council of Chief Medical Officers of Health on Nicotine Vaping in Canada, Retrieved from:
https://www.canada.ca/en/public-health/news/2020/01/statement-from-the-council-of-chief-medical-officers-of-health-on-nicotine-vaping-in-canada.html - Footnote 34
-
WHO FCTC, WHO FCTC Secretariat's Knowledge Hub on Surveillance, Retrieved from
https://untobaccocontrol.org/kh/surveillance/about-us/what-we-do/ - Footnote 35
-
WHO FCTC (2018), 2018 Global Progress Report, Retrieved from
https://fctc.who.int/publications/m/item/2018-global-progress-report - Footnote 36
-
Health Canada (2017), Annual Report on Compliance and Enforcement Activities (Tobacco Control) 2016-2017, Retrieved from:
https://www.canada.ca/en/health-canada/services/publications/healthy-living/annual-report-on-compliance-enforcement-activities-tobacco-control-2016-2017.html - Footnote 37
-
PHAC (2020), Statement from the Council of Chief Medical Officers of Health on Nicotine Vaping in Canada, Retrieved from:
https://www.canada.ca/en/public-health/news/2020/01/statement-from-the-council-of-chief-medical-officers-of-health-on-nicotine-vaping-in-canada.html
