Notice to industry: Aligned reviews between Health Canada and health technology assessment organizations
Effective immediately, the option for aligned reviews is available to all biological and pharmaceutical new drug submissions. This includes:
- biosimilars
- supplemental new drug submissions for new indications made to Health Canada (HC) where the sponsor intends to seek a coverage recommendation from health technology assessment organizations (HTAs), on a pre Notice of Compliance (NOC) basis
This notice formalizes the timelines, process, and considerations for sponsors with qualifying drug submissions. This is for sponsors who are interested in taking part in aligned reviews between HC and HTAs.
On this page
- About aligned reviews
- Why we are offering the option to align reviews
- Goals and objectives of aligned reviews
- Benefits of aligned reviews
- Maximizing benefits of aligned reviews
- Principles of alignment
- Contacts
About aligned reviews
Sponsors of qualifying submissions will be able to opt in to an aligned review pathway at any stage of the review process. To do so, they must complete the consent letter template authorizing the sharing of information and submit it to HC.
Sponsors are encouraged to opt in and consent to information sharing as early as possible in the review process. This is to help maximize the benefits of alignment between HC and the HTA reviews. Sponsors must also comply with the advance notice requirements of HTAs.
Why we are offering the option to align reviews
Health Canada's Regulatory Review of Drugs and Devices initiative aims to provide more timely access to drugs and devices. We are therefore giving the option to sponsors to formally align reviews of all qualifying submissions in partnership with:
- Canada's Drug Agency (CDA) (formerly the Canadian Agency for Drugs and Technologies in Health [CADTH])
- l'Institut national d'excellence en santé et en services sociaux (INESSS) (hereinafter referred to as HTAs)
Health Canada, HTAs and industry have recognized the need for greater coordination of the review processes within the drug approval system. HTA processes allow for early submission intake, up to 180 days before an expected NOC from HC.
For additional details, please consult the CDA and INESSS web sites.
In a 2017 pilot project, opportunities to better align reviews and enhance information-sharing were explored by HC's Bureau of Metabolism, Oncology and Reproductive Sciences, and CADTH's pan-Canadian Oncology Drug Review program.
Health Canada and CADTH used the pilot project to establish a foundation for setting out the process for aligned reviews.
Goals and objectives of aligned reviews
Health Canada and HTAs are committed to:
- enhancing Canada's drug reviews
- finding efficiencies to support Canadians' timely access to effective new therapies
Alignment of the HTA reviews with the HC review is expected to:
- reduce duplication
- reduce time lags between HC market authorization and HTA recommendations, where possible (refer to Table 1)
An aligned review will not alter the independent requirements, decision making, or respective processes maintained by HC and HTAs.
Benefits of aligned reviews
Table 1 outlines the potential benefits of aligning HC and HTA reviews.
| Reduces time | Helps to reduce time between issuing an NOC (including those issued under the NOC/c Guidance) and HTA recommendations. |
|---|---|
| Improved communication | Allows for real time discussions between HC and HTAs, greater ability to share information, more efficient resolution of review issues and reduction of duplication, where possible. |
Opting into an aligned review pathway and consenting to information sharing will help to minimize potential delays between issuing an NOC (including those issued under the Notice of Compliance with Conditions [NOC/c] Guidance) by HC and the recommendations by HTAs.
Maximizing the benefits of aligned reviews
The timing of the sponsor's submission to the HTAs affects how much the benefits of an aligned review are maximized. Sponsors are strongly encouraged to file with each of the HTAs around the same time. They are also encouraged to provide consent as early as possible.
To ensure the shortest possible interval between HC regulatory approval and issuing HTA recommendations, sponsors should note that:
- Health Canada's priority review submission review target is 180 calendar days. Therefore, the HTA submissions should be filed as early as possible from the date the submission was accepted for review by HC.
- Health Canada's advance consideration for NOC/c pathway submission review target is 200 calendar days. Therefore, the HTA submissions should be filed as early as 3 weeks from the date the submission was accepted for review by HC.
- A standard HC submission review target is 300 calendar days. Therefore, submissions to the HTAs should be filed as early as 4 months into HC's review.
Aligned reviews are possible for different regulatory review pathways. For this to occur, the sponsor must file submissions to the HTAs before the targeted date of the NOC being issued (including those issued under the NOC/c Guidance).
Figure 1 shows the timing of HTA submissions to maximize benefits of aligned reviews.
Figure 1
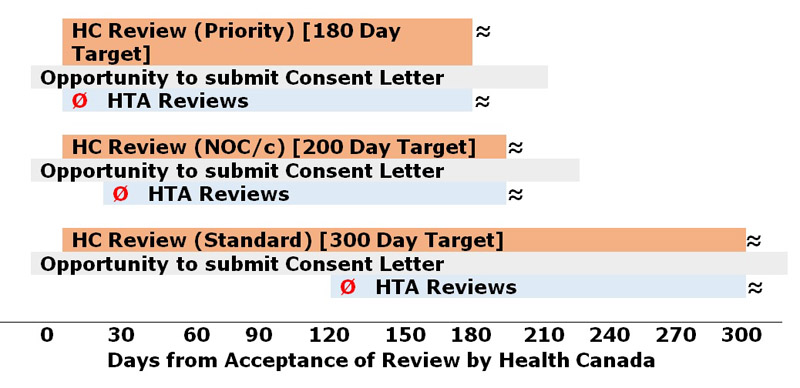
NOC/c = Advance consideration under the NOC/c Guidance; Ø = Submission filing to HTAs for optimal alignment
Text Description
This table shows 3 different examples of time lines for HC submission review in parallel with the HTA 180-day submission review. The start of the HC 180-day priority review can overlap with the start of the HTA review. Both end about 180 days later. The HC 200-day NOC/c review can start about 20 days earlier than the HTA review. Both end around the same time. Finally, the HC standard 300-day review can start about 120 days earlier than the HTA review. Both end around the same time. All 3 of the examples shown in Figure 1 include a parallel chance to submit a Consent Letter by the sponsor. Sponsors can choose to provide consent for information sharing between HC and HTAs. This can occur at any point during the HC review process and up to 30 days after market authorization has been issued.
Sponsors can choose to provide consent for information sharing between HC and HTAs. This can occur at any point during a review process and up to 30 days after market authorization.
Consenting to information sharing between HC and HTAs may save processing time and reduce duplication of effort. However, sponsors must continue to comply with the requirements of both HC and HTAs.
Interested sponsors should opt-in to the aligned review pathway and consent to information sharing at the time of, or before filing their submission at HC. This will help ensure the best alignment of the regulatory and HTA reviews. It will also help to minimize the time between issuance of an NOC and HTAs' recommendations.
Sponsors interested in this opportunity should complete these steps:
- complete the consent letter authorizing sharing of information with health technology assessment organizations and submit it to HC in section 1.2.6 of the drug submission:
- at the time of submission filing to HC or
- during submission screening or review by HC
- notify the HTA agencies of their willingness to permit HC to share information and documents at the time of providing advance notification of the pending HTA submission
- file their submissions to HTAs as close to 180 days pre-NOC as possible
- comply with existing consent requirements from HTAs
Principles of alignment
These principles will apply to all aligned reviews and can serve as a guide for the application of aligned reviews for industry. Aligned reviews will be:
- continuously improving: HC and HTAs will monitor the uptake rate by sponsors and adjust the aligned review process as needed to ensure that it provides benefits to HC, HTAs, and industry. They will also ensure the process supports the goal of collaborating more with our health partners.
- consent based: participation in an aligned review is voluntary and dependent on the submission of consent using the consent letter template authorizing the sharing of information. The template must be completed in full and any revisions, deletions or additions may make it invalid and disqualify a sponsor from taking part in aligned reviews.
- limited in the sharing of confidential information (including confidential business information as defined in the Food and Drugs Act): although the sponsor authorizes sharing of information, HC and HTAs will make best efforts to limit the sharing of information to those they deem relevant to the alignment of their reviews. For example, manufacturing process information would generally not be shared as it is typically not needed by the HTAs. In addition, HC will respect existing Memoranda of Understanding (MOUs) with foreign regulators as it pertains to further sharing of information.
- respectful of independent processes and decision-making: independent HC and HTAs processes, timelines, decision making and regulatory requirements will be respected. For example, the 300-day HC standard review target or 180-day priority review target will not change. Neither will the timelines for the HTAs' reviews. Participation in an aligned review will not affect the outcome of the market authorization decision nor the HTAs' recommendations.
- transparent: HC and HTAs are committed to transparency. Information about which sponsors participate in aligned reviews may be made public. This is also true of information about participation in the aligned reviews in general. Beginning in the Fall of 2018, HC will indicate which submissions on the Submissions Under Review lists are undergoing an aligned review.
Contacts
For further information, or if you have questions on this initiative, please contact:
Health Canada
Office of Planning, Performance and Review Services (OPPRS)
Therapeutic Products Directorate
Health Products and Food Branch
Address Locator: 3002C
Ottawa, Ontario
K1A 0K9
Email: OPPRS_enquiries@hc-sc.gc.ca
Telephone: 613-941-1248
Fax: 613-957-1483
Canada's Drug Agency (CDA) (formerly Canadian Agency for Drugs and Technology in Health [CADTH])
865 Carling Avenue, Suite 600
Ottawa, Ontario
K1S 5S8
Email: requests@cda-amc.ca
Telephone: 613-226-2553
Fax: 613-226-5392
l'Institut national d'excellence en santé et en services sociaux (INESSS)
2535 boulevard Laurier, 5th Floor
Quebec City, Quebec
G1V 4M3
Email: inscription@inesss.qc.ca
Phone: 418-643-1339
Fax: 418-646-8349