Building a National Strategy for High-Cost Drugs for Rare Diseases: A Discussion Paper for Engaging Canadians
TABLE OF CONTENTS
Download the alternative format
(777 KB, 16 pages)
- What is a rare disease?
- How are high-cost drugs for rare diseases accessed and paid for?
- Why are drugs for rare diseases so expensive?
- How do public and private drug plans decide which drugs to cover?
- Why do we need a national strategy for high-cost drugs for rare diseases?
- Three key issues in creating a national strategy:
- How to improve patient access to high-cost drugs for rare diseases, and ensure access is consistent across the country.
- How to ensure decisions on covering high-cost drugs for rare diseases are informed by the best evidence available.
- How to ensure spending on high-cost drugs for rare diseases does not put pressure on the sustainability of the Canadian health care system.
- Next steps: Engaging Canadians to help build a national strategy
What is a rare disease?
Every so often you hear a personal story, or see an item on the news, about someone living with a rare disease. The patient in the story is often a child, even an infant, but people can be affected at any age. The disease itself is unfamiliar to most people, although the story is not: there is a drug that might save the patient's life or dramatically improve its quality, but it costs hundreds of thousands of dollars, sometimes millions, and is not covered by health insurance. The family can't afford it.
Rare diseases are not rare as a group: it is estimated there are between 6,000 and 8,000 in the world.Footnote 1 They are considered rare because they each affect small numbers of people, ranging from a handful of individuals to a few thousand.
Rare diseases are often chronic, can be seriously debilitating and potentially life-threatening. There is no common internationally or even nationally accepted definition of what a rare disease is. In Canada, the Canadian Organization for Rare Disorders defines a rare disease as one that affects fewer than one in 2,000 people Footnote 2, which is also the figure the European Union uses Footnote 3. The United States' National Institutes of Health and Human Services defines a rare disease as any condition that affects fewer than 200,000 people in the U.S. Footnote 4 Because of that lack of definition, and the challenge of getting a correct diagnosis, it is difficult to say how many people in Canada are living with a rare disease. Estimates vary widely; one estimate is that one in 12 Canadians live with a rare diseaseFootnote 1 while another puts the number at one million people overall. Footnote 5
Even without a broadly agreed on definition, rare diseases have several things in common:
- Approximately 80 percent of rare diseases have a genetic basis.
- About half the people with rare diseases are children whose disease is recognized through pre-natal screening or at birth or shortly thereafter.
- Many children with rare diseases die before their first birthday.
- People with rare diseases often rely on a great deal of medical care and may have limited life expectancy.
How are high-cost drugs for rare diseases accessed and paid for?
Having timely access to safe and effective treatments can significantly improve the health and overall quality of life of a person with a rare disease; in some cases, it can even mean the difference between life and death. Currently, Canadians with rare diseases can access the drugs they need in several ways:
- Through government drug plans: These tend to be focused on coverage for certain populations, such as seniors, people on social assistance, or people with specific diseases.
- Through private drug plans: The majority of these are employer-sponsored plans, although it is possible for individuals to pay for private drug insurance. Private insurance may limit the amount covered for a particular drug each year or over the life of a patient.
- By paying out of pocket : Because drugs for rare diseases are so expensive, patients generally cannot afford to pay by themselves. Some manage through community fundraising.
- Through a patient-support program: These programs may be offered by pharmaceutical companies on a compassionate basis to provide access to a drug in cases where it is not covered by public or private drug plans.
- By enrolling in a clinical trial or through Health Canada's Special Access Program: Clinical trials and the Special Access Program give access to drugs that are still in development or are not yet authorized for sale in Canada.
Why are drugs for rare diseases so expensive?
There are few or no treatments available for rare diseases. When treatments are available, they tend to be extremely expensive, ranging from $100,000 to more than $2 million per patient per year, often for life. In 2019, more than half (56 percent) of all high-cost drugs for rare diseases available in Canada cost more than $200,000 per patient per year. Over the past five years, the number of high-cost drugs on the market that cost more than $200,000 per year has been increasing: in 2015 and 2016, 36 percent of high-cost drugs approved in Canada cost more than $200,000 per year; in 2017 it was 55 percent, in 2018, 82 percent and 73 percent in 2019. Footnote 6 This challenge is not unique to Canada, as all countries pay extremely high prices for drugs for rare diseases.
A number of reasons have been put forward to explain why these drugs cost so much, including small market size, high research and development costs, and limited treatment options. However, there is no clear information on how much it costs to bring a drug to market and how drugs are priced, although some evidence suggests these costs are less for rare-disease drugs compared to the costs for conventional drugs. Footnote 7
How do public and private drug plans decide which drugs to cover?
Before any drug is allowed to be sold in Canada, it must be assessed by Health Canada , to ensure that it meets high standards of safety, efficacy and quality. Footnote 8 Health Canada works with pharmaceutical manufacturers and regulatory partners to simplify regulatory processes, improve performance targets and adopt new agile regulatory pathways for novel technologies. These efforts are working to ensure that drugs like orphan drugs can get to the Canadian market faster.
Once a drug is authorized by Health Canada, it can be sold and purchased, and both public and private drug plans can decide if they will cover the drug for their members. Every public or private drug plan decides for itself whether it will pay for the drug for their members, and under what conditions.
The Patented Medicine Prices Review Board (PMPRB) , a regulatory agency, plays a role in pricing of drugs for sale in Canada, when it independently assesses prices to determine whether Canadians are being charged excessive prices.
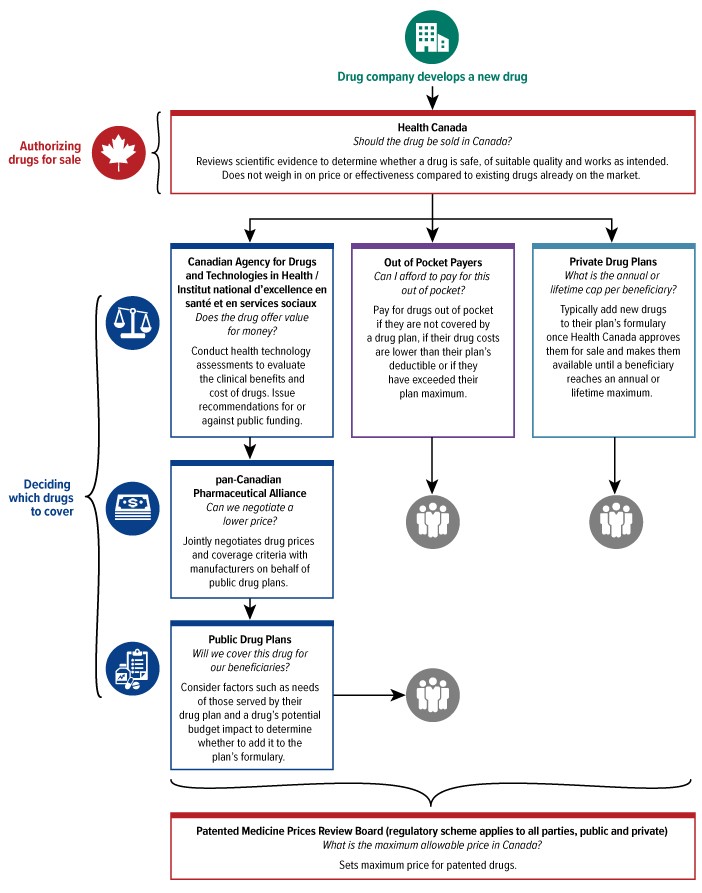
A tree diagram showing the steps for determining which drugs would be covered under a drug plan
Public Drug Plans
There are more than 100 drug plans run by federal, provincial and territorial governments, aimed at improving access to prescription drugs primarily for people who might otherwise not be able to afford them. Each plan is different but tailored for specific groups such as seniors, children, those with low incomes, or people with serious medical conditions. Footnote 9 Each province and territory has its own public drug plan that covers defined populations. The federal government also administers drug plans for various populations, including registered First Nations and recognized Inuit populations, federal inmates, members of the Canadian forces, veterans, resettled refugees and refugee claimants.
Public drug plans make decisions about coverage with the help of expert guidance from organizations including the Canadian Agency for Drugs and Technology in Health (CADTH) , the Institut national d'excellence en santé et services sociaux (INESSS) , and sometimes from provincial expert committees. These organizations evaluate how well a drug (or device) works and whether it is cost effective. They may also look at "the ethical, legal, and social implications of health technologies on patient health and the health-care system." Footnote 10 These complex review processes can take a number of years, which is a concern for patients seeking treatment. To speed up access to new drugs, Health Canada has been working with CADTH and INESSS to do aligned reviews of new drugs simultaneously rather than sequentially. Once CADTH and INESSS have made their recommendations, public drug plans review the recommendations and decide whether and under what conditions to cover a drug.
If public drug plans decide to pay for a drug, the pan-Canadian Pharmaceutical Alliance (pCPA) negotiates the price and terms of coverage for the drug with the manufacturer.
When the assessments and negotiations are complete, the federal, provincial and territorial public drug plans make their final decisions on whether to pay for the drug, including whether to set conditions for its use (sometimes, for example, a doctor must provide additional clinical information for the government to authorize coverage for a patient). Rather than giving general approval for a high-cost drug for a rare disease, plans may look at each individual patient's case.
Private Drug Plans
Private drug plans also make their own decisions on what drugs they will cover, which may be different from public drug plans and from other private plans, depending on the clients served and agreements with plan sponsors (which are generally employers).
Special Access Program
In a case where a patient might benefit from a drug not authorized for sale in Canada, doctors can submit a request to Health Canada's Special Access Program . Footnote 11 The program lets physicians prescribe and access drugs that are not on the market in Canada (but have been approved in other jurisdictions) to treat patients with serious or life-threatening conditions when other therapies have failed, are unsuitable or unavailable in this country. Under the program, the manufacturer determines the cost of the drug. In some cases, the drugs are free; if there is a charge, it is covered either by public or private drug plans or, in some cases, patients and their families.
Why do we need a national strategy on high-cost drugs for rare diseases?
Rare diseases can cause a great deal of suffering for the people who have them and anguish for their families. For generations, there were virtually no treatments for rare diseases; even today fewer than 10 percent of rare diseases have any treatment. However, policies to encourage research on rare diseases and genetic and biomedical breakthroughs over the past two decades are starting to change that, bringing hope for patients and their families. At the same time, these innovations are raising the difficult issue of cost for governments and private insurers, researchers, pharmaceutical manufacturers and other stakeholders.
The key challenge is the high cost of most drugs for rare diseases, which as mentioned above, can be between $100,000 and more than $2-million per person per year. While pharmaceutical manufacturers are taking risks in developing innovative rare-disease therapies and need a return on their investment, these costs are far beyond what patients or their families can afford, and they put significant pressure on both public and private drug plans and the health care system.
A national strategy on high-cost drugs for rare diseases, with agreed on priorities and processes, will help patients better access the drugs they need. By consolidating efforts, a strategy should also allow governments and funders to make more timely decisions about coverage, plan more effectively and ease the strain on public and private drug plans and the health care system across the country.
Another important goal of a national strategy would be to promote more cooperation among federal, provincial and territorial governments along the lines of the pan-Canadian Pharmaceutical Alliance's work, to further reduce costs and increase access. By making common decisions based on pre-determined principles, governments could strengthen their negotiating power while at the same time simplifying the process for pharmaceutical manufacturers to get their products funded by drug plans. A common process for monitoring and assessing the real-world impact of new drugs would give governments information to help determine whether a drug was meeting expectations. In the longer term, a national strategy could stimulate innovative research for breakthroughs in rare-disease treatments.
Closer cooperation could also improve consistency of access to rare disease drugs: if all funders agreed on which drugs to cover, Canadians would worry less about differences in access based on where they live and what kind of drug plan they have.
Another benefit of a national strategy could be to address some of the challenges faced by rare-disease treatments as they are developed, from discovery to domestic research and development to clinical translation and finally commercialization. Actions such as increasing domestic knowledge and expertise, gathering real-world evidence of how the drugs affect patients once they're in use, facilitating data and information sharing, and supporting early-stage researchers or companies to develop and manufacture their drugs in Canada, could significantly improve access and reduce costs.
The Advisory Council on the Implementation of National Pharmacare Footnote 9 (Box 1) and the House of Commons Standing Committee on Health Footnote 5 (Box 2) both recently concluded a new approach is needed for providing and paying for high-cost drugs for rare diseases, based on long-standing calls for a different way of doing things from patients, patient groups, physicians, researchers, industry representatives and other stakeholders. A national strategy will be the basis for that change.
Box 1: Advisory Council on the Implementation of National Pharmacare
Announced in Budget 2018, the Advisory Council on the Implementation of National Pharmacare led a national dialogue on how to implement affordable national pharmacare in Canada. The Council’s final report was delivered in June 2019 and includes recommendations for the Canadian government to develop a formal national strategy for expensive drugs for rare diseases and to establish a distinct pathway and a national expert panel to work with patients and their clinicians to determine which rare disease drugs should be funded for which patients.
Box 2: Standing Committee on Health
In 2018-2019, the Standing Committee on Health carried out a study of the barriers to access to treatment and drugs for Canadians affected by rare diseases and disorders. The final report, which was presented to the House of Commons in 2019, acknowledged that the biggest barrier limiting patients’ access to these drugs is their affordability. There are 19 recommendations in the report, including calls to establish coordinated processes and options for covering the costs of drugs for rare diseases.
Three key issues in creating a national strategy
Arrangements to fund and provide access to high-cost drugs require a different approach than with other medications. Drugs for rare diseases are not just another option for treatment. For many people with rare diseases, a drug may be the only option, whether it offers incremental improvement in quality of life or is a life-saver. The effectiveness and risks of rare-disease drugs must be balanced against their high price tags, which are using an increasing proportion of government health-care budgets. For that reason, a national strategy will inevitably involve different considerations than those typically associated with new drugs for illnesses where there is a range of treatment options. How to judge benefit for patients and the amount of risk it is reasonable to take will also be different.
To improve the odds of success in the search for rare-disease treatments and to do the best possible assessment of their safety and effectiveness, cooperative work and sharing of knowledge should be encouraged at every level. Health Canada, for instance, already has strong relationships with international regulators and will continue to build new partnerships and work sharing agreements. Canadian researchers, clinicians and scientists could work with their international colleagues to share data and pool the results of research at different stages of drug development; the federal, provincial and territorial governments could pool and coordinate their resources and investments in biomanufacturing and research and development, in addition to negotiating together to build bargaining power. These efforts to build capacity in Canada to support the development of rare-disease drugs could help spur innovation and improve access to therapies for rare diseases that affect Canadians. It might even be possible to collaborate with other countries to share non-confidential information and inform negotiations with pharmaceutical manufacturers.
Building on existing efforts helps to increase collective impact
A range of efforts is underway across federal, provincial and territorial governments, in collaboration with many partners, to address key issues related to high-cost drugs for rare diseases. This includes work by Health Canada to make regulatory processes more efficient and better able to meet the needs of the health-care system, including meeting the needs of people with rare diseases . Footnote 12 For example, since 2017, Health Canada has strengthened the way it collaborates with regulators around the world on the scientific review of drugs and medical devices, including therapies for rare diseases. This has facilitated approval of three novel treatments.
In response to the Government of Canada's targeted review of the health and biosciences sector in 2018, Health Canada is also reducing regulatory barriers to increase the availability of treatment options, including advanced therapies and personalized medicines, that may benefit those with rare diseases. Together, these efforts will help give people better access to the drugs and devices they need.
In 2019, the Government of Canada announced amendments to the Patented Medicines Regulations, which provide the Patented Medicine Prices Review Board with new information and tools to protect Canadians from excessive prices for patented drugs, including those for rare diseases. These amendments will come into force on July 1, 2021.
Finally, a provincial/territorial working group on Expensive Drugs for Rare Diseases has been discussing a supplemental process for these treatments over the last few years.
To increase collective impact, the national strategy for high-cost drugs for rare diseases will not duplicate these existing initiatives, instead focusing on areas that complement those already underway.
This discussion paper on a national strategy for high-cost drugs for rare diseases explores three key issues:
- How to improve patient access to high-cost drugs for rare diseases and ensure that access is consistent across the country.
- How to ensure decisions on funding high-cost drugs for rare diseases are informed by the best available evidence.
- How to ensure spending on high-cost drugs for rare diseases does not put pressure on the sustainability of the Canadian health care system.
The following sections explore these issues and some options for addressing them. These are intended as starting points for discussion - if you have other ideas, please tell us about them.
Issue 1: How to improve patient access to high-cost drugs for rare diseases and ensure that access is consistent across the country.
Patients with rare diseases live in every part of the country, but access to high-cost drugs to treat them varies greatly depending on where they live and how their drugs are paid for.
Federal, provincial and territorial governments and private drug plans make separate - and often different - decisions about what rare disease treatments they will pay for. This results in patients having different chances of getting a drug based on where they live or how they are insured. Patient-support programs sponsored by industry and clinical trials fill part of that gap, but they provide drugs for select patients only and offer no guarantees on how long that access will last.
Consistent coverage is difficult to achieve when there are so many different payers, no national framework or guidelines for assessing the value and effectiveness of drugs for rare diseases, and when many funding decisions are made on a case-by-case basis. These inconsistencies make it difficult for patients, their families and doctors to know where and how to get access to these drugs.
Options for improving access and improving national consistency for people with rare diseases include:
- A single framework for decision making on high-cost drugs - Getting federal, provincial and territorial governments to agree on a single principled approach for deciding 1) which high-cost drugs for rare diseases to cover and 2) which patients should be covered under what conditions would create greater consistency across the country as well as predictability for patients.
- A transparent co-ordinating body - Creating a co-ordinating body to improve communication and collaboration among key players (including private and public drug plans) would create consistency in decisions on what drugs to pay for, ensure agreed-on conditions for consistent access to drugs were followed and clearly communicate rationale and decisions, as well as information on process and timelines.
- Patient and clinician engagement - Improving engagement with patients, patient groups, and clinicians to increase awareness of policies and programs related to accessing high-cost drugs for rare diseases.
- Co-ordinated support for research on rare diseases in Canada - While each rare disease is different, there are common challenges and factors affecting research and discovery of treatments. Nationally co-ordinated research support could increase knowledge of rare diseases affecting Canadians and lead to new discoveries, thus bringing these treatments to Canadian patients.
Questions for Discussion
1a. How can access to high-cost drugs for rare diseases be made consistent in order to improve patient access to these treatments?
1b. Which of the proposed options, or combination of options, would be the most effective for improving access and improving consistency?
1c. Please explain the option(s) that you selected above.
Issue 2: How to ensure decisions on covering high-cost drugs for rare diseases are informed by the best evidence available.
Evidence on how well high-cost drugs for rare diseases work is often limited, which makes it harder to decide which drugs should be covered.
Over the decades, clinicians and scientists have developed robust standards for testing new drugs to ensure safety and efficacy. Randomized controlled trials give hundreds or even thousands of volunteers a new drug and carefully observe its effects. But with so few patients affected by rare diseases, large trials are often impossible. Additionally, if there is a reasonable expectation the drug will make a big difference for severely ill people, it may not be considered ethical to make patients wait for enough evidence to be gathered. As a result, approval and funding decisions for these drugs may have to be based on limited evidence and uncertain health benefits and risks.
The limitations in the quality and availability of evidence for rare disease therapies have led regulators to adopt more tailored oversight approaches for them.Footnote 12 This includes accelerated and exceptional review pathways through the regulatory process, incentives, and access to early regulatory guidance in designing trials for small populations. Health Canada uses its Notice of Compliance with conditions policy to accelerate reviews for a sub-set of drugs for serious, life-threatening or severely debilitating conditions where there are no available treatments. For example, when early evidence shows a drug is promising, the regulator can grant a market authorization with conditions that require the manufacturer to monitor the product once it is in use and to do additional testing. Health Canada is also planning regulatory changes that will expand use of terms and conditions on approvals for drugs and devices, allowing more precise information to be gathered, which could be used to support further research and coverage decisions down the road.
Even with measures in place to bring these products to market quickly, uncertain data can make it difficult to determine if new treatments provide significant enough benefit to the patient to merit the price - leaving public and private drug plans in the difficult position of paying for expensive treatments that may not work very well. But when no other treatment is available, patients understandably want the opportunity to try anything that could help improve their quality of life. The lack of solid evidence about the benefits of a rare-disease drug, can therefore result in both great pressure to pay for high-cost drugs, even when the benefits are minimal or evidence is limited, and, at the same time, delay or limit access to therapies that could be beneficial.
Options for addressing the challenge of covering drugs with limited evidence include:
- Innovative approval and coverage models - Approval of and funding for drugs for rare diseases could be tied to how well they work, meaning payment would depend on patients having specified outcomes, which manufacturers would track through long-term studies and regular reporting on the effectiveness and safety of their drug. This would require an agreement on what level of benefit would be sufficient as well as clear, objective indicators for measuring benefit (which could be difficult to develop). If data showed poor performance in the longer term, payments would be reduced or discontinued.
- A national expert panel - The panel would have the authority to 1) study data and evidence to make informed recommendations on who should be treated with high-cost drugs for rare diseases and 2) monitor how the drugs are being used, how well they are working and making recommendations on continued funding.
- A national data system - This system would capture more comprehensive and consistent information about the prevalence of rare diseases in Canada, how treatments for rare diseases are used by Canadians and how they are working.
- Independent national and international networks - These networks would build on existing partnerships to facilitate knowledge and data sharing on real-world experience of patients in a way that is independent and free from conflict of interest.
Questions for Discussion:
2a. How can decisions on covering high-cost drugs for rare diseases be made when the evidence is limited?
2b. Which of the proposed options, or combination of options, would be most effective for strengthening the evidence base?
2c. Please explain the option(s) that you selected above.
Issue 3: How to ensure spending on high-cost drugs for rare diseases does not put pressure on the sustainability of the Canadian health care system.
Most drugs for rare diseases are very expensive, and can pose a challenge to the long-term sustainability of government- and employer-sponsored drug plans.
Drugs for rare diseases are the fastest growing segment of the pharmaceutical market in Canada, increasing by an annualized rate of 32 percent and accounting for nearly one-tenth of Canadian pharmaceutical sales. Footnote 6 Sales are estimated to have increased multiple-fold in recent years, one of many factors putting health care budgets (which have many competing priorities) under increasing strain.
The pan-Canadian Pharmaceutical Alliance was created to negotiate fair prices for drugs on behalf of federal, provincial and territorial governments. However, even with that joint buying power behind it, negotiating fair prices for drugs for rare-disease drugs is extremely difficult: the prices are high, there is rarely competition in the market, the diseases are severe and patients and caregivers are anxious for quick access.
Because of the lack of treatment options for most rare diseases, investing in innovation, research and development of effective treatments for them is important. Innovation and discovery should provide pharmaceutical companies with a reasonable return on investment without affecting patient access or driving spending so high it threatens the overall sustainability of the health care system.
Options for controlling the impact of high-cost drugs on health-system budgets include:
- Sharing of costs and pooling of risks - Working together with other payers to help everyone negotiate better agreements with pharmaceutical manufacturers (and possibly help bring drugs to market that would otherwise not be commercially viable).
- Investments up front to reduce the risk in early development - Rather than relying solely on price to compensate drug manufactures for research and development, the cost of developing new drugs for rare diseases could be shared among research funders and companies to reduce risk and lower expenses. This could include up-front investment by governments and health charities for research, development and production of rare-disease treatments. Negotiated agreements with manufacturers could limit prices later on.
- Pay for performance - Exploration of innovative funding models that are tied to how well a drug works, including defunding drugs that offer only marginal or unproven benefits.
- Supports for "made-in-Canada" innovation - Development of domestic innovative or generic capacity to sustain all elements of drug discovery, research and development, through to manufacturing, trials, approval and sale. This would help keep costs lower than if researchers sell their discovery to a multinational company to be brought to market.
- International collaboration - Working together with other countries to share non-confidential information to inform negotiations and leverage better pricing.
Questions for Discussion:
3a. Which of the proposed options, or combination of options, would be most effective for getting rare-disease treatments to patients?
3b. Please explain the option(s) that you selected above.
General Questions for Discussion:
4. Do you have other ideas that might help improve access and lower costs for drugs for rare diseases?
Next steps: Engaging Canadians to help build a national strategy
As Canada considers how best to create a national strategy on high-cost drugs for rare diseases, it is important that Canadians have a voice in helping to shape it.
If you have any views on any of the questions in this discussion paper, you can share them in three ways:
- Complete the online questionnaire
- Participate in a virtual public town hall
- Send feedback by email or mail
In addition to collecting feedback from Canadians, Health Canada is working closely with provincial and territorial representatives to build a strategy that will work in the context of Canada's health system and respect the role of provinces and territories in health care delivery.
Health Canada will also engage with Indigenous organizations and health stakeholders over the coming months to gather their views about what works best for their communities.
Over the coming weeks, virtual meetings will be held with key stakeholders to collect their input, including:
- Patients, their families and caregivers
- Patient groups
- Health care providers
- Other health care system partners (e.g., pCPA, CADTH, INESSS, PMPRB, Canadian Institutes for Health Information, Canada Health Infoway)
- Academics and researchers (including the Canadian Institute of Health Research)
- Pharmaceutical industry
- Private insurers
- Business and labour unions
All of this input will inform the development and implementation of a national strategy for high-cost drugs for rare diseases, which the government intends to launch by 2022-2023.
REFERENCES
- Footnote 1
-
Health Canada. “About orphan drugs and rare diseases.” Retrieved December 30, 2020 from: https://www.canada.ca/en/health-canada/services/licences-authorizations-registrations-drug-health-products/regulatory-approach-drugs-rare-diseases/about-drugs-rare-diseases.html
- Footnote 2
-
Canadian Organization for Rare Diseases. “About CORD Key Facts.” Retrieved December 30, 2020 from: https://www.raredisorders.ca/about-cord/
- Footnote 3
-
European Union. “Health Research and Innovation: Rare Diseases.” Retrieved December 30, 2020 from: https://ec.europa.eu/info/research-and-innovation/research-area/health-research-and-innovation/rare-diseases_en#:~:text=In%20the%20EU%2C%20a%20rare,million%20people%20in%20the%20EU
- Footnote 4
-
National Institute of Health, Genetic and Rate Diseases Information Center. “FAQs about Rare Diseases.” Retrieved December 30, 2020 from: https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
- Footnote 5
-
The Standing Committee on Health, (2019). Canadians Affected by Rare Diseases and Disorders: Improving Access to Treatment. Retrieved December 30, 2020 from: https://www.ourcommons.ca/Content/Committee/421/HESA/Reports/RP10349306/hesarp22/hesarp22-e.pdf
- Footnote 6
-
Patented Medicine Prices Review Board, (2020). “Insight into the spending on expensive drugs for rare diseases” (presentation). Retrieved December 30, 2020 from: https://www.canada.ca/content/dam/pmprb-cepmb/documents/consultations/draft-guidelines/2020/Research-Webinar1-EDRD-Market-Size-EN.pdf
- Footnote 7
-
Berdud et al. Establishing a reasonable price for an orphan drug. Cost Eff Resour Alloc (2020) 18:31
https://resource-allocation.biomedcentral.com/articles/10.1186/s12962-020-00223-x - Footnote 8
-
Health Canada. “Our role in bringing drugs to patients.” Retrieved December 30, 2020 from: https://www.canada.ca/en/health-canada/services/licences-authorizations-registrations-drug-health-products/regulatory-approach-drugs-rare-diseases/our-role.html
- Footnote 9
-
Advisory Council on the Implementation of National Pharmacare, (2019). A Prescription for Canada: Achieving Pharmacare for All. Retrieved December 30, 2020 from: https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/implementation-national-pharmacare/final-report.html
- Footnote 10
-
Canadian Agency for Drugs and Technologies in Health. “Health Technology Assessment Service” Retrieved December 30, 2020 from: https://www.cadth.ca/about-cadth/what-we-do/products-services/hta
- Footnote 11
-
Health Canada. “Special Access Program.” Retrieved December 30, 2020 from: https://www.canada.ca/en/health-canada/services/drugs-health-products/special-access/drugs.html
- Footnote 12
-
Health Canada. “Canada’s regulatory approach to drugs for rare diseases.” Retrieved December 30, 2020 from: https://www.canada.ca/en/health-canada/services/licences-authorizations-registrations-drug-health-products/regulatory-approach-drugs-rare-diseases.html
