Draft guidance document on the collection and analysis of disaggregated data in clinical trials: Disaggregated data decision tree
The disaggregated data decision tree outlines key questions to guide clinical trial design, based on the population that will use the drug being tested. The decision tree summarizes the content in "Considerations in protocol design".
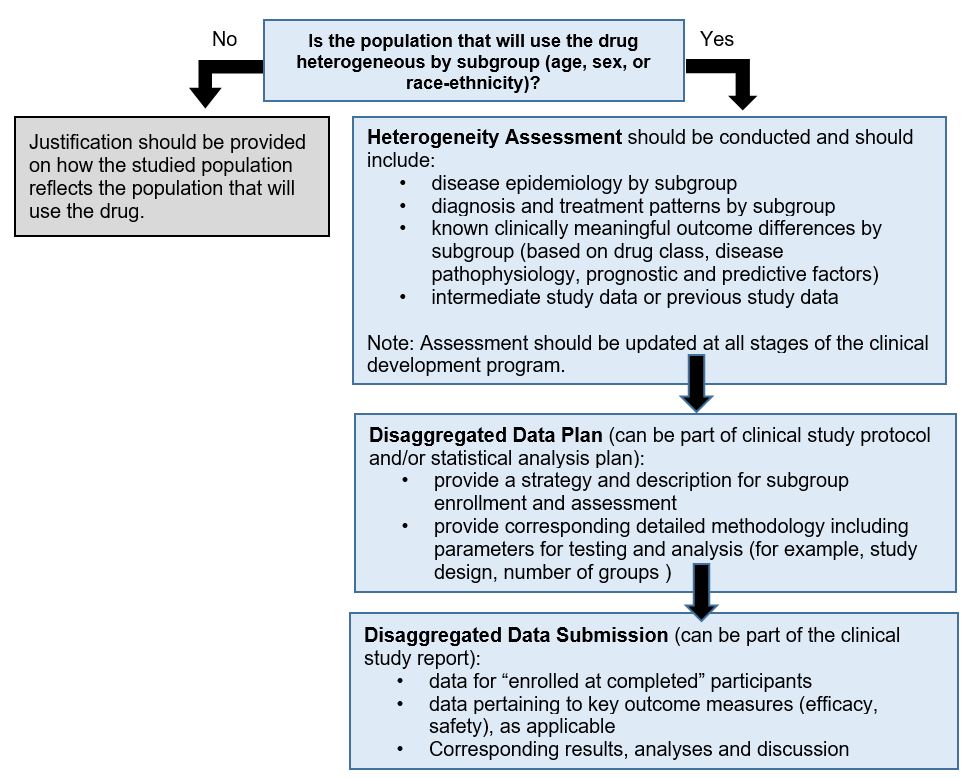
Figure 1 - Text description
The decision tree starts with a question about whether the population that will use the drug is expected to be heterogeneous. The yes/no question then leads to a series of sub-questions that lead to 2 outcomes. The first is a justification, if the clinical population of use is not expected to be heterogeneous. The second pathway summarizes what is needed in the heterogeneity assessment and disaggregated data plan. This information is also communicated in the “Data collection and analysis” section of this guidance.