Regulatory cooperation - Canadian Guidance on Veterinary Drug Simultaneous Reviews with the United States: Crosswalk example
This is a fictitious example of a Canadian table of contents that is cross-walked to the U.S. dossier for a submission undergoing simultaneous reviews with the U.S. Center for Veterinary Medicine (CVM). The example focuses on “Part IV: Requirements for efficacy” of the technical section.
Sponsors must provide a complete table of contents, fill out the relevant sections and ensure each submitted technical section is complete. Contact VDD for the complete crosswalk template and requirements for completion.
The crosswalk to the table of contents is similar to Appendix V, “Master Index” of the Guidance for industry preparation of veterinary new drug submissions. However, the crosswalk to the table of contents includes subsections that apply to submissions undergoing simultaneous reviews only, for example:
- “Acknowledgement receipt from other regulatory jurisdictions” for Part I, “Master Volume”
- “Protocol concurrence letters” for some technical sections
In the following example, the date of submission refers to the date on which the sponsor submits the information to Health Canada's Veterinary Drugs Directorate (VDD).
Note the following:
- date of submission should be identical to the date of the cover letter submitted to VDD in the data package
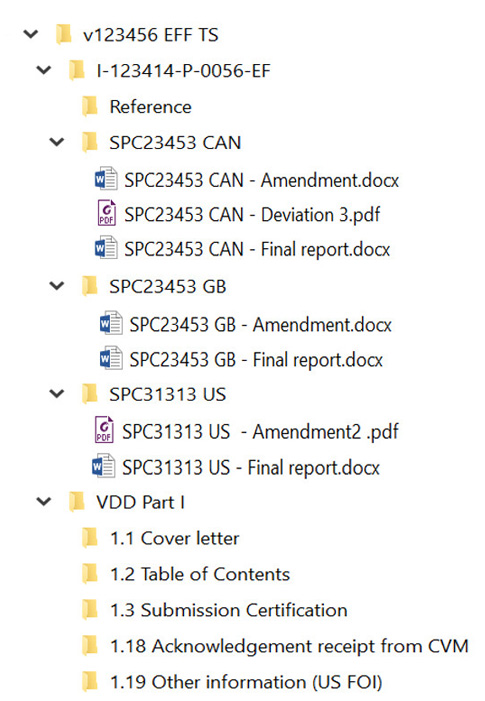
- last column, location of information, should include the folder name, file name and location in submission
- if not applicable, provide a justification such as “not applicable”, “to be provided in the future” or “see previously approved submission”
| Table of content | |||
|---|---|---|---|
| Information | Date of submission to VDD (Month DD, YYYY) | Location of information (Folder level 1 / Folder level 2 / Folder level 3), file names or other justification | |
| Part IV: Requirements for efficacy (Please include the provided study numbers and titles under each subsection.) |
|||
| 4.2 | Sectional reports | ||
| 4.2.1 | Microbiology studies | ||
| 4.2.2 | Laboratory studies | ||
| SPC12345-PK: Pharmacokinetics in rats following oral administration | April 1, 2022 | v123456 HFS TS / I-123414-P-0052-HFS / SPC12345-PK | |
| SPC23453 GB: Non-pivotal laboratory efficacy study in dogs for 5 days | September 1, 2022 | v123456 EFF TS / I-123414-P-0056-EF / SPC23453 GB
|
|
| 4.2.3 | Animal model efficacy studies | ||
| SPC23453 CAN: Pharmacokinetics in dogs after topical administration | September 1, 2022 | v123456 EFF TS / I-123414-P-0056-EF / SPC23453 CAN
|
|